Abstract
We investigated the adrenergic mechanisms of the two venous systems that drain the nasal mucosa, thereby their exact role in eliciting nasal decongestion. The action of endogenously released noradrenaline and exogenous adrenergic agonists on different segments of the nasal venous systems, i.e. collecting (LCV, SCV) and outflow (SPV) veins of posterior venous system, collecting (ACV) and outflow (DNV) veins of anterior venous system and venous sinusoids of the septal mucosa (SM), were studied. In vitro isometric tension of the vascular segments was measured.
Transmural nerve stimulation (TNS) produced constriction in ACV, DNV and SM, primary constriction followed by secondary dilatation in LCV and SCV and dilatation in SPV. Tetrodotoxin (10−6 M) abolished all responses. Phentolamine (10−6 M), prazosin (10−6 M) and rauwolscine (10−7 M) inhibited the constriction in all venous vessels. Propranolol (10−6 M), atenolol (10−6 M) and ICI 118,551 (10−6 M) inhibited the relaxation in SPV but not in LCV and SCV. Phenylephrine and clonidine constricted whereas dobutamine and terbutaline relaxed all venous vessels dose-dependently.
These results indicate α1-, α2-, β1- and β2-adrenoceptors are present in both venous systems. TNS causes constriction of anterior venous system, venous sinusoids and posterior collecting veins primarily via postjunctional α2-adrenoceptors but relaxation of posterior outflow vein equally via postjunctional β1- and β2-adrenoceptors. The combined action of the two adrenergic mechanisms can reduce nasal airway resistance in vivo by decreasing vascular capacitance and enhancing venous drainage via the posterior venous system.
Keywords: Isometric tension, nasal, vein, alpha-adrenoceptor, beta-adrenoceptor, transmural nerve stimulation, agonist, antagonist
Introduction
In man and experimental animals, a layer of highly vascular mucosa, comprising of sinusoidal venous plexuses, lines the nasal cavity (Cauna, 1982; Dawes & Prichard, 1953; Lung & Wang, 1989a). Blood from the nasal cavity is drained via the high-flow and high-pressure dorsal nasal vein while blood from the posterior nasal cavity is drained via the low-flow and low-pressure sphenopalatine vein (Lung & Wang, 1987; 1989a). It is conventionally believed that when the venous sinusoids are distended with blood the mucosa will swell and this must be a major factor in nasal blockage. As the collecting veins of both systems are located within the nasal cavity, their dilatation (especially that of the posterior collecting veins because of their large size and highly muscular nature) can increase considerably mucosal blood volume (Lung & Wang, 1989a). In contrast, the outflow veins (dorsal nasal vein and sphenopalatine vein) are located outside the nasal cavity and their dilatation favours venous drainage (Lung & Wang, 1989a). Hence, mucosal congestion may be caused by dilatation of venous sinusoids and/or collecting veins and constriction of outflow veins. Opposite changes in the mechanisms would lead to mucosal decongestion. The vasomotor activity of each vascular segment is of unique importance in the control of nasal airway resistance.
The nasal vascular bed is under sympathetic nervous controls (Eccles, 1978; 1982; Lung & Wang, 1989b). Both resistance and capacitance vessels receive adrenergic nerve supply, with the supply to the former being richer than the latter (ånggård & Densert, 1974; Dahlström & Fuxe, 1965). In the dog, sympathetic nerve stimulation causes constriction of the resistance vessels via an α-adrenergic mechanism and constriction of capacitance vessels via α-adrenergic as well as non-adrenergic and non-cholinergic mechanisms (Lung & Wang, 1989b). Other studies have demonstrated that both postjunctional α1- and α2-adrenoceptors are involved in mediating the nasal blood flow and airway patency responses (Berridge & Roach, 1986). Similar results have also been obtained in pigs (Lacroix & Lundberg, 1989) and humans (Andersson & Bende, 1984). Apart from α-adrenoceptors, β-adrenoceptors have been shown to influence nasal blood flow and mucosal volume. β-adrenergic agonists increase arterial blood flow and mucosal volume in the nasal mucosa of the pig and dog (Lacroix et al., 1995; Lung et al., 1984), suggesting that β-adrenoceptors are vasodilatatory in action.
Both α- and β-adrenoceptors seem to exist in the nasal mucosa, modifying not only blood flow but also vascular capacitance. Where do they act on the two nasal venous systems in eliciting change in nasal airway resistance? What is their relative contribution to the sympathetic control of nasal vascular capacitance and hence airway resistance? These questions prompted us to examine the action of endogenously released noradrenaline (NA) and exogenous adrenergic agonists on the different segments of nasal venous vascular bed so as to identify their site(s) of action and to characterize the receptors involved.
Methods
Tissue preparation
Mongrel dogs of either sex (weighing 15–25 kg) were first anaesthetized with pentobarbitone sodium (30 mg kg−1, i.v.) and then killed by an overdose of the same anaesthetic agent (200 mg kg−1, i.v.). Dorsal nasal vein (DNV), anterior collecting vein (ACV), sphenopalatine vein (SPV), lateral collecting vein (LCV), septal collecting vein (SCV), and the septal mucosa (SM) were isolated from the nasal cavity (Figure 1). All isolated segments were immediately immersed in chilled modified Krebs–Ringer bicarbonate solution of the following composition (in mM): NaCl 119, KCl 4.7, CaCl2 (CaCl2-6H2O) 2.5, KH2PO4 1.2, MgSO4 (MgSO4-7H2O) 1.2, NaHCO3 25, calcium disodium EDTA 0.026 and glucose 11.1. The solution was previously aerated with a gas mixture of 95% O2 and 5% CO2. With the aid of a dissecting microscope, the isolated tissues were cleaned of extraneous tissue. Only one vascular ring of 4 mm in length from each blood vessel and a strip of 6 mm long and 6 mm wide from the septal mucosa were cut off for experimentation. Care was taken to minimize rubbing of the internal surface when preparing rings so as to keep an intact and functional endothelium. The diameters of various collecting and outflow veins were: 3.5–4.5 mm for LCV, 1.5–2.5 mm for SCV, 0.5–1 mm for ACV, 2–3 mm for DNV, 0.5–1.5 mm for SPV. The septal mucosa contained a network of venous sinusoidal vessels of diameters ranging from 0.1–0.5 mm (Lung & Wang, 1989a).
Figure 1.
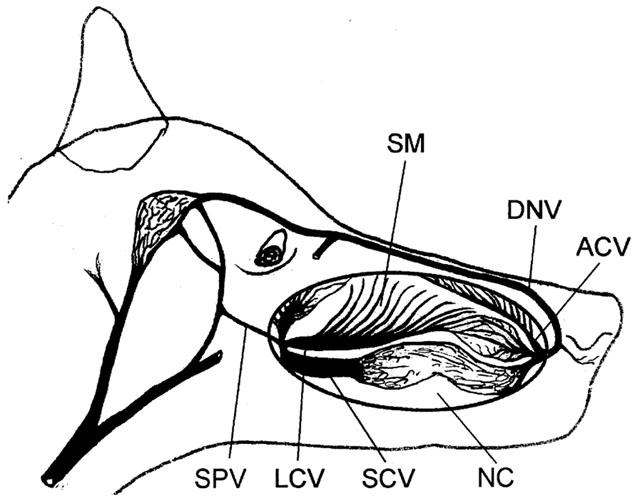
Diagrammatic illustration of location of the nasal venous vessels. ACV, anterior collecting vein; DNV, dorsal nasal vein; SM, septal mucosa; LCV, lateral collecting vein; SCV, septal collecting vein; SPV, sphenopalatine vein; NC, nasal cavity.
Tissue bath studies
The venous ring or mucosal strip was suspended in an organ chamber filled with 5 ml of the modified Krebs–Ringer bicarbonate solution, which was aerated with a mixture of 95% O2 and 5% CO2 and maintained at 37°C. The tissue segment was connected to a Grass force FT 03 displacement transducer and changes in isometric force were recorded on PICO model and fed on-line to a computer. After a 30-min equilibrium period, the tissue segment was stretched to its optimal resting tension. The optimal resting tension was determined in preliminary experiments by constructing the length-tension relationship of the isolated tissue segment. The optimal tension used to set up various tissue segments were as follows: 2.0 g for LCV; 1.5 g for SCV; 1.0 g for ACV; 1.5 g for DNV; 1.0 g for SPV and 1.0 g for SM. The tissue was allowed to equilibrate for another 60 min under the optimal resting tension and was washed every 20 min during the equilibration period. The tissue segment was then exposed to 80 mM KCl until two reproducible contractions (with variation less than 10%) developed. After washout, cocaine (3×10−5 M) and hydrocortisone (3×10−5 M), inhibitors of neuronal and extra-neuronal uptakes (O'rourke & Vanhoutte, 1990), were added to the tissue bath 30 min before a protocol began. At the end of experiments, the maximal relaxation or passive tone of the vessel was determined by the addition of NaNO2 (5×10−2 M). The total active tone (TAT) in the present study was defined as the difference between the tone at 80 mM KCl and the tone at 5×10−2 M NaNO2.
Transmural nerve stimulation (TNS)
The tissue segments were subjected to an electric current to stimulate nerves. The electrodes, two 1-mm platinum wires, were placed 3 mm from each side of the tissue so as to establish an electrical field in the longitudinal direction of the tissue. Rectangular constant-current pulses were delivered between the electrodes through a Grass S48 stimulator at supramaximal voltage (100 V), varying frequency (0.5–64 Hz), 0.3 ms impulse duration, and duration of 30 s. A recovery period of 10 min was given between stimulations. In preliminary studies, the preparation was subjected to full range stimulation several times at 30 min intervals. The responses were reproducible, showing minimal changes in tissue sensitivity or the amount of released neurotransmitters over the time course of the experiment.
Experimental protocol
To examine the action of endogenously released NA on the nasal venous vessels, TNS was used to activate the sympathetic nerve and the frequency-response (0.5–64 Hz) curves were constructed. To confirm if the responses were neurogenic in nature, action of tetrodotoxin (10−6 M) was studied after the control frequency-response curve in a group of vessels. To characterize the α-adrenoceptors involved, after the control frequency-response curve, the frequency-response curve was repeated in the presence of one of the following: phentolamine (10−6 M), prazosin (10−6 M), rauwolscine (10−7 M) or prazosin (10−6 M) plus rauwolscine (10−6 M). Some vascular segments demonstrated a vasodilatatory response to TNS after pretreatment with phentolamine. To investigate if β-adrenoceptors were involved and the receptor subtypes responsible, the phentolamine-treated vascular segments were stimulated in the presence of one of the following: propranolol (10−6 M), (±)-atenolol (10−6 M), ICI 118,551 (10−6 M), or atenolol (10−6 M) plus ICI 118,551 (10−6 M). All antagonists were added to the tissue bath 30 min before nerve stimulation for incubation of the tissues. The concentration of the adrenoceptor antagonists was effective in blocking the effect of the corresponding exogenous agonists given at a dose that caused a response comparable to the maximal response evoked by TNS in the same tissue. The agonists used in testing the adequacy of α1-, α2-, β1- and β2-adrenoceptor blockades were phenylephrine, clonidine, dobutamine and terbutaline respectively.
To assess the effects of α-adrenoceptor agonists, phenylephrine or clonidine was cumulatively added to the organ bath in 10 fold increments until a maximum contractile response was attained. To assess the effects of β-adrenoceptor agonists, the preparation was first contracted with phenylephrine (at a dose ranging from 10−7 M to 10−5 M) so as to raise the tension to approximately 70% of TAT. After a stable contraction had been obtained, dobutamine or terbutaline was cumulatively added to the organ bath in 10 fold increments until the relaxation had reached a maximum and stabilized. Only one cumulative dose-response study was carried out on each tissue segment.
Drugs
The following drugs were used: (±)-atenolol (RBI, U.S.A.), clonidine (Boehringer Ingelheim, Germany), cocaine hydrochloride (May & Baker, U.K.), dobutamine hydrochloride (Eli Lilly, U.S.A.), hydrocortisone sodium succinate (Sigma, U.S.A.), ICI 118,551 (RBI, U.S.A.), noradrenaline hydrochloride (Sigma, U.S.A.), pentobarbitone sodium (Rhone Merieux, Australia), phenylephrine hydrochloride (Sigma, U.S.A.), phentolamine mesylate (Ciba, U.K.), prazosin hydrochloride (Pfizer, U.S.A.), DL-propranolol hydrochloride (Sigma, U.S.A.), rauwolscine hydrochloride (RBI, U.S.A.), sodium nitrite (Sigma, U.S.A.), terbutaline sulphate (Astra, Sweden) and tetrodotoxin (Sigma, U.S.A.).
All solutions were freshly prepared before each experiment by dissolving the compounds in distilled water. All drugs were added in volumes of 0.015 ml, i.e. 0.3% of the organ bath volume. Vehicle experiments were performed in preliminary studies. Identical volume of drug vehicle was found to have no effect on all tissue preparations. Concentration of an agent was expressed as the final concentration in the organ bath.
Analysis of data
All responses were expressed as a percentage of TAT of the same tissue. The data are the means±s.e.mean and n refers to the number of animals. The dose-response curve was computer-fitted using nonlinear regression and the maximal response elicited by the agonist (MR), the concentration required to achieve half response (EC50) and pD2 value (pEC50=−log EC50) were calculated (Graphpad prism, Version 2.1, U.S.A.). Comparison of MR and pD2 values between various groups was performed with one-way analysis of variance, followed by Student-Neuman-Keuls test. Comparison of frequency-response curves was performed using GLM repeated measures analysis of variance. When the F-test was significant, the Bonferroni test was carried out to determine the frequencies for which the responses were statistically different. P values of less than 0.05 were considered statistically significant.
Results
TNS-induced responses
In DNV, ACV and SM, TNS produced frequency dependent constriction; in LCV and SCV, TNS produced primary constriction followed by secondary dilatation; in SPV, TNS produced dilatation. Similar responses were obtained after the addition of drug vehicle (0.015 ml of distilled water) (Figure 2A). Figure 2B shows the typical tracings obtained in LCV, DNV and SPV. The maximal constrictive response induced by TNS in LCV, SCV, ACV, DNV and SM was reached at 32 Hz while the maximal relaxant response in LCV, SCV, and SPV occurred at 8-16 Hz. The responses at all frequencies were completely blocked by tetrodotoxin.
Figure 2.
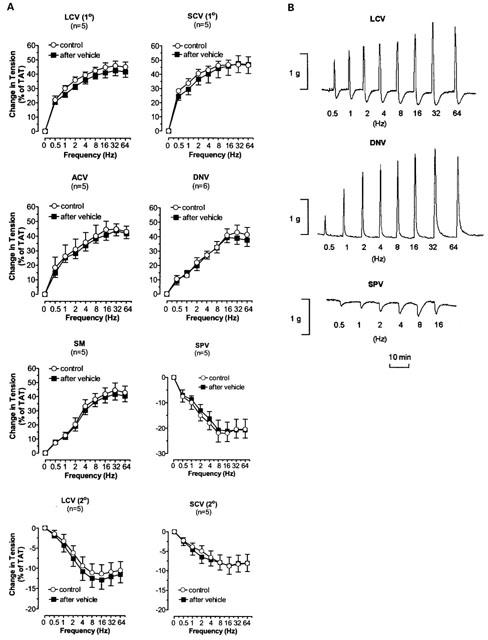
(A) The TNS frequency-response curves of nasal venous vessels and the action of drug vehicle on the curves. Each point represents the mean±s.e.mean. n is the number of animals. O, normal response. ▪&, response at 30 min after addition of drug vehicle (0.015 ml of distilled water). LCV (1°) and SCV (1°), primary response of LCV and SCV respectively. LCV (2°) and SCV (2°), secondary response of LCV and SCV respectively. (B) Experimental tracings illustrating the effects of TNS at different frequencies (Hz) on LCV, DNV and SPV.
The effects of α-adrenoceptor antagonists on the TNS-induced responses
Phentolamine, prazosin and rauwolscine significantly decreased the TNS-induced constriction in LCV, SCV, ACV, DNV, and SM (Figures 3, 4 and 5). Phentolamine and rauwolscine were stronger than prazosin in inhibiting the constriction (P<0.05). The residual response to TNS in the presence of prazosin was markedly reduced by the addition of rauwolscine (P<0.05) (Figure 6).
Figure 3.
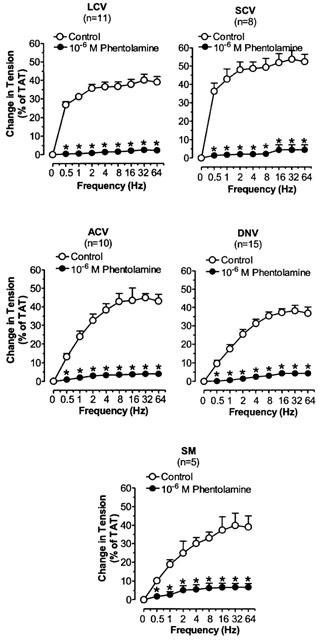
The effects of phentolamine on TNS frequency-constriction curves in nasal venous vessels. Each point represents the mean±s.e.mean. n is the number of animals. *P<0.05, vs corresponding control.
Figure 4.
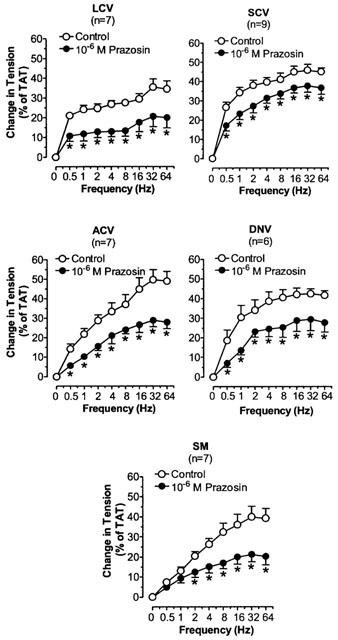
The effects of prazosin on TNS frequency-constriction curves in nasal blood vessels. Each point represents the mean±s.e.mean. n is the number of animals. *P<0.05 vs corresponding control.
Figure 5.
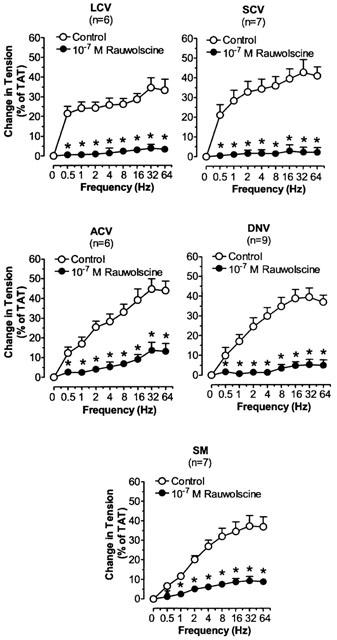
The effects of rauwolscine on TNS frequency-constriction curves in nasal blood vessels. Each point represents the mean±s.e.mean. n is the number of animals. *P<0.05 vs corresponding control.
Figure 6.
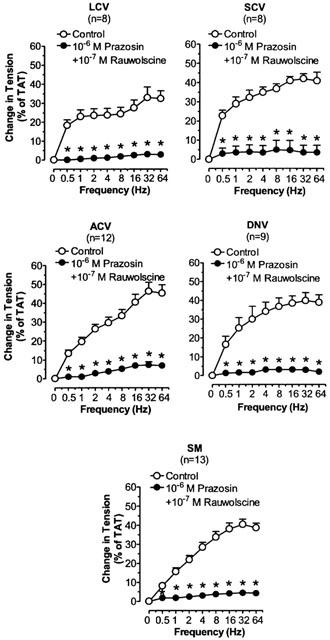
The effects of prazosin plus rauwolscine on TNS frequency-constriction curves in nasal blood vessels. Each point represents the mean±s.e.mean. n is the number of animals. *P<0.05 vs corresponding control.
Phentolamine and prazosin but not rauwolscine produced significant increases of the TNS-induced relaxation in SPV (P<0.05). Combined treatment with prazosin and rauwolscine produced no additional influence with respect to the isolated effect of prazosin (Figure 7). Phentolamine and prazosin exerted similar potentiating effect on the relaxation response in SPV (P=NS).
Figure 7.
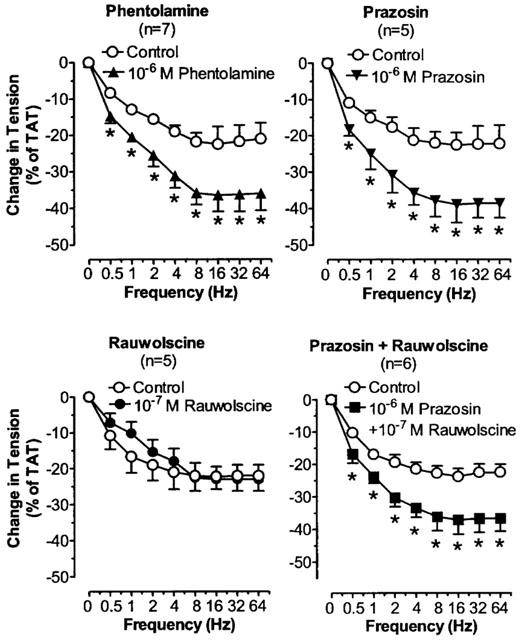
The effects of α-adrenergic antagonists on TNS frequency-relaxation curves in SPV. Each point represents the mean±s.e.mean. n is the number of animals. *P<0.05 vs corresponding control.
Phentolamine did not affect the TNS-induced relaxation in LCV and SCV (P=NS (Figure 8).
Figure 8.
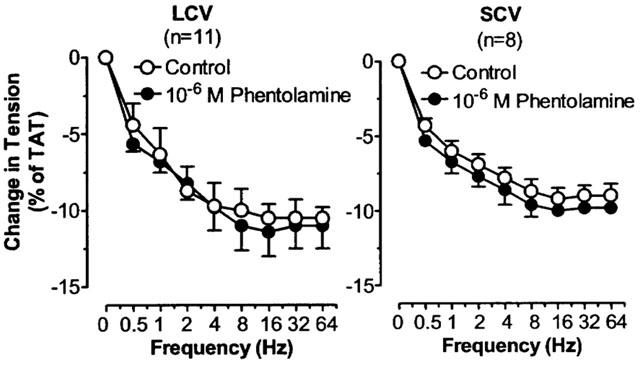
The effects of phentolamine on TNS frequency-relaxation curves in LCV and SCV. Each point represents the mean±s.e.mean. n is the number of animals.
The effects of β-adrenoceptor antagonists on theTNS-induced relaxation in LCV, SCV and SPVafter blockade of α-adrenoceptors
In the presence of phentolamine, TNS produced frequency dependent relaxation in LCV, SCV and SPV. Maximum relaxation was achieved at 8–16 Hz. The maximal relaxation in LCV and SCV was similar in magnitude (P=NS) but both were less prominent than the maximal relaxation in SPV (P<0.05). The relaxant responses were effectively antagonized by propranolol in SPV (P<0.05), but not in LCV and SCV (P=NS) (Figure 9). Both atenolol and ICI 118,551 suppressed to a similar extent the TNS-induced relaxation in SPV (P=NS) and combined treatment with atenolol and ICI 118,551 produced greater suppression of the relaxation (P<0.05) (Figure 10).
Figure 9.
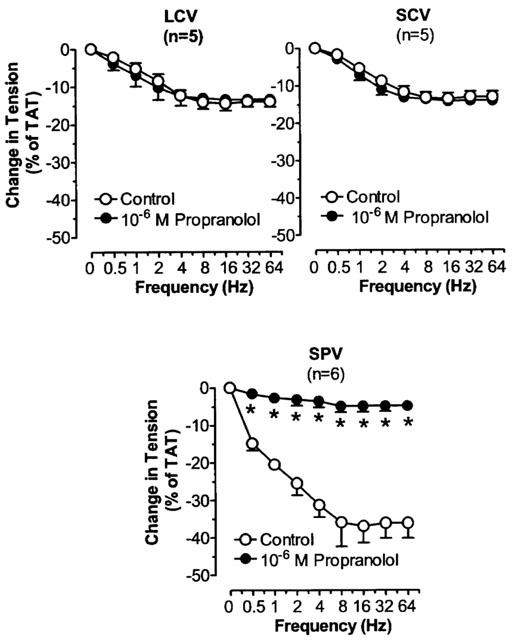
The effects of propranolol on TNS frequency-relaxation curves in LCV, SCV and SPV after blockade of α-adrenoceptors. Each point represents the mean±s.e.mean. n is the number of animals. *P<0.05, vs corresponding control.
Figure 10.
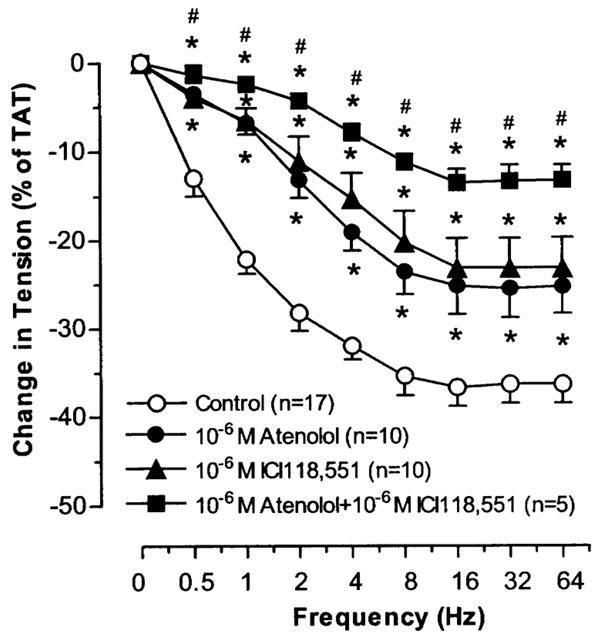
The effects of atenolol and ICI118,551 on TNS frequency-relaxation curves in SPV after blockade of α-adrenoceptors. Each point represents the mean±s.e.mean. n is the number of animals. *P<0.05, vs corresponding control. #P<0.05, vs atenolol or ICI118,551.
Effects of extraneous adrenoceptor agonists
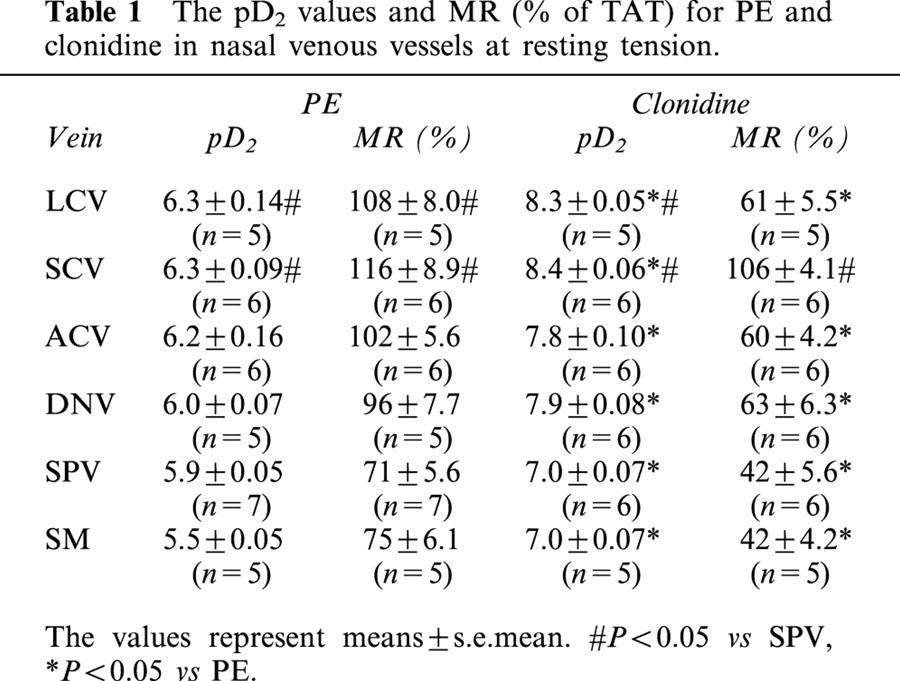
Phenylephrine and clonidine caused dose-dependent constriction in all vascular segments studied (Figure 11). The pD2 value for clonidine was greater than that for phenylephrine in all venous segments (P<0.05), whereas the maximal response to phenylephrine was larger than that to clonidine in all venous segments (P<0.05) except SCV (Table 1). For the blood vessels of the posterior venous system, the pD2 values for phenylephrine and clonidine were higher in LCV and SCV than in SPV (P<0.05); the maximal responses to these agonists were also greater in LCV and SCV than in SPV (P<0.05). For the blood vessels of the anterior venous system, pD2 values for phenylephrine and clonidine and the maximal responses to these agonists were similar in ACV and DNV.
Figure 11.
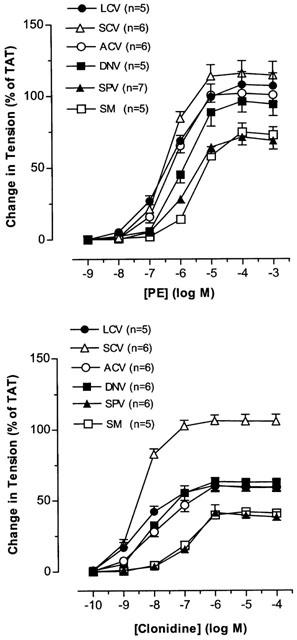
The effects of PE and clonidine on nasal venous vessels. Each point represents the mean±s.e.mean. n is the number of animals.
Table 1.
The pD2 values and MR (% of TAT) for PE and clonidine in nasal venous vessels at resting tension
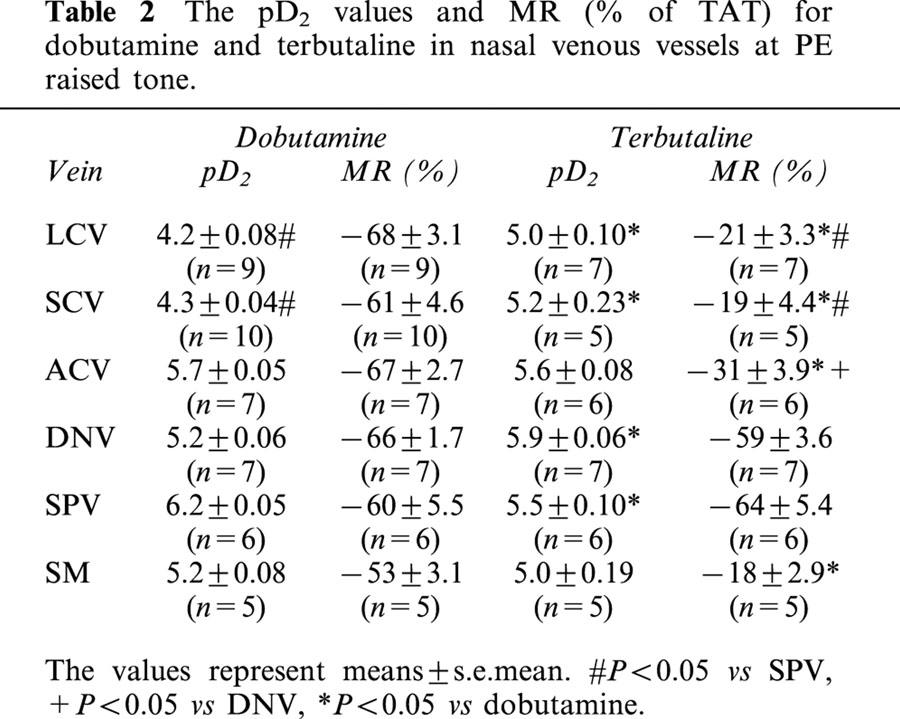
In phenylephrine-precontracted nasal venous vessels, dobutamine and terbutaline caused dose-dependent relaxation in all vascular segments studied (Figure 12). The pD2 values for dobumatine and terbutaline were similar in ACV and SM. The pD2 value for dobutamine was greater than that for terbutaline in SPV (P<0.05), while the pD2 value for dobutamine was lower than that for terbutaline in LCV, SCV and DNV (P<0.05). The maximal response to dobutamine was larger than to terbutaline in LCV, SCV, ACV and SM (P<0.05). The maximal responses induced by dobutamine and terbutaline were similar in DNV and SPV (Table 2).
Figure 12.
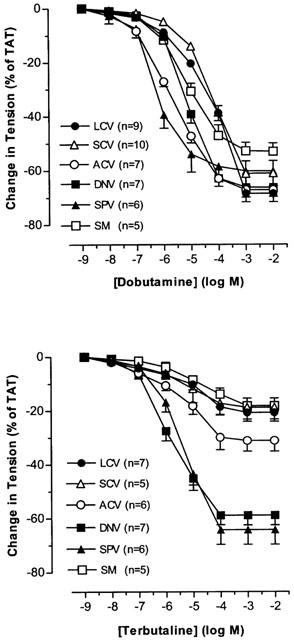
The effects of dobutamine and terbutaline on PE-precontracted nasal venous vessels. Each point represents the mean±s.e.mean. n is the number of animals.
Table 2.
The pD2 values and MR (% of TAT) for dobutamine and terbutaline in nasal venous vessels at PE raised tone
Discussion
In the present study, we found that TNS caused contraction in ACV, DNV and SM, contraction followed by dilatation in LCV and SCV, but dilatation in SPV. The drug vehicle did not affect the responses. All the responses were abolished by TTX, indicating the TNS-induced responses are neurogenic in nature. The contractile responses in LCV, SCV, ACV, DNV and SM were markedly inhibited by phentolamine whereas the relaxant responses in SPV were markedly inhibited by propranolol. These results strongly suggest that the contraction in LCV, SCV, ACV, DNV and SM and the dilatation in SPV be mediated via the release of NA from adrenergic nerve endings in the wall of the blood vessels. As the dilatation in LCV and SCV was resistant to propranolol, further studies are required to identify the mechanism(s) involved.
Alpha-adrenoceptors
We found that both prazosin and rauwolscine inhibited the TNS-induced contraction in LCV, SCV, ACV, DNV and SM. We also demonstrated that the effect of rauwolscine in inhibiting the TNS-induced constriction was much stronger than that of prazosin. In addition, rauwolscine markedly reduced or almost abolished the prazosin-resistant response to nerve stimulation. These findings suggest that the role of α2-adrenoceptors is predominant over that of α1-adrenoceptors in the TNS-induced responses. Based on experiments with local infusion of α1-agonist and α2-agonist to the nasal mucosa, other investigators have also found that α2-adrenoceptors predominate over α1-adrenoceptors in controlling nasal airway patency in dogs (Berridge & Roach, 1986) or nasal blood flow in pigs (Lacroix & Lundberg, 1989). However, their studies cannot specify the exact vascular segment(s) involved. In this study, using TNS-induced contraction, we are able to demonstrate that, in the control of the nasal venous vasculature by endogenous NA, the α2-adrenergic mechanism is predominant over α1-adrenergic mechanism in all nasal venous vessels except the posterior outflow vein (SPV).
The effects of α2-adrenoceptor antagonists on responses to sympathetic nerve stimulation may be complicated by enhancement of neurotransmitter release produced by blockade of inhibitory prejunctional α2-adrenoceptors (Langer, 1974; Starke, 1977). In LCV, SCV, ACV, DNV and SM, the α2-adrenoceptor antagonist, rauwolscine, which should enhance neurotransmitter release, potently reduced contractile responses to nerve stimulation. In addition, rauwolscine was more effective than prazosin in antagonizing the contractile responses to nerve stimulation. These results suggest that rauwolscine most probably acts primarily on the postjunctional receptors which might have outnumbered the prejunctional receptors. That rauwolscine or yohimbine is more effective than prazosin when tested against contractile responses to nerve stimulation has also been found in other venous preparations, e.g., dog saphenous vein and rabbit portal vein (Starke & Docherty, 1982; Flavahan et al., 1984). It has been hypothesized that both the α1- and α2-adrenoceptors are differentially located in relation to the nerve terminals in blood vessels; in veins, the former in extrajunctional sites whereas the latter in the vicinity of the sympathetic nerve terminals (Guimaraes & Moura, 2001). Hence, like other systemic veins, nasal veins might have the α2-adrenoceptors situated closer to the sympathetic nerve terminals than α1-adrenoceptors, resulting in preferential stimulation by the endogenous NA. However, further experiments are required to verify this.
In SPV, the TNS-induced relaxant response was potentiated by phentolamine, indicating a hidden constrictive component mediated via α-adrenoceptors. Prazosin also enhanced the relaxant response to TNS in SPV. Rauwolscine alone had no effect on the response and also additional rauwolscine did not cause the further enhancement with respect to the isolated effect of prazosin. These results suggest that the hidden constrictive response to TNS in SPV be related to the α1-adrenoceptors rather than the α2-adrenoceptors and that SPV may have mainly α1-adrenoceptors. As aforementioned, the α1-adrenoceptors in veins might not be strategically situated to be preferentially activated by endogenous NA. However, we cannot rule out the possibility that they might be stimulated by the same endogenous NA which have diffused away from the nerve terminals. As the amount of NA diffused away would be small, the effect elicited by α1-adrenoceptors will be masked by the effect due to α2-adrenoceptors activation. However, if there is a deficiency or insufficiency of functional α2-adrenoceptors, the α1-adrenoceptors mediated effect may be unmasked. That SPV has insufficient receptor density/coupling efficiency of postjunctional α2-adrenoceptors may lead to the manifestation of the constrictive response via the α1-adrenoceptors. This is supported by the finding that both pD2 value and maximal response for clonidine in SPV were the lowest among all nasal veins. An insufficiency of postjunctional α2-adrenoceptors may also explain why rauwolscine had no effect on the TNS-induced dilatation as the antagonist's actions on the prejunctional and postjunctional α2-adrenoceptors might have cancelled each other.
We also studied the action of selective α-adrenoceptor agonists on the nasal veins. PE is selective for the α1-adrenoceptors and clonidine is selective for α2-adrenoceptors (Starke, 1981). Both PE and clonidine contracted all the nasal venous vessels studied. PE is an efficacy driven agonist of low affinity (full agonist) while clonidine is an affinity driven agonist of low efficacy (partial agonist) (Kenakin, 1997). If both agonists act on the same receptor in the same tissue, PE would have higher pD2 value and maximal response than clonidine. When the responses of the two agonists are compared, PE has lower pD2 values (5.5–6.3) and higher maximal responses (71–116% of TAT) while clonidine shows higher pD2 values (7–8.4) and lower maximal responses (42–106% of TAT). The results indicate that the two agonists may act on different receptors in all nasal veins, presumably α1-adrenoceptors for PE and α2-adrenoceptors for clonidine. It had been found in preliminary studies that prazosin and rauwolscine antagonized their effects respectively. However, further radioligand saturation binding and displacement experiments are required to verify the receptor type(s) involved.
Tissue factors, i.e. receptor density and coupling efficiency, vary dramatically from tissue to tissue. The pD2 value and maximal response to an agonist are dependent on not only intrinsic efficacy of the drug but also tissue factors. A partial agonist can behave as a full agonist if receptor density/coupling efficiency is high (Kenakin, 1997). That pD2 values and maximal responses to PE and clonidine vary greatly in various nasal veins indicates that there is considerable regional variation in the density or coupling efficiency of both α1- and α2-adrenoceptors in the nasal venous vasculature. That SCV has the biggest pD2 and largest maximal response for PE and clonidine as compared with other veins implies that the vessel might have the highest receptor density/coupling efficiency for both α1- and α2-adrenoceptors among all nasal veins.
The potency of clonidine relative to PE (as revealed by pD2 value for clonidine and PE) is about 100 in LCV, SCV and DNV; 30 in ACV and SM; 10 in SPV, showing that the nasal veins are more sensitive to clonidine. However, clonidine induced a lower maximum in most vessels when compared to PE. This may be due to clonidine being a partial agonist for α2-adrenoceptors or differences in the excitation-contraction coupling mechanisms utilized by α1- and α2-adrenoceptors, as demonstrated in other systemic venous vessels (Roach et al., 1983; Ruffolo, 1986).
Taking together the results of the TNS studies and of exogenous application of agonists, it is likely that the response of the nasal venous vasculature to low and moderate levels of α-adrenergic stimulation, e.g. physiological or low pharmacological doses of NA, represents primarily the effects of α2-adrenergic activation. With maximal α-adrenergic stimulation, e.g. large pharmacological doses of NA, α1-adrenergic mechanism will contribute more to the response, as the maximal response of PE on the nasal venous vessels is greater than that of clonidine.
Beta-adrenoceptors
In this study, TNS caused primary relaxation in SPV, secondary relaxation in LCV and SCV, and no relaxation in ACV, DNV and SM. If both α- and β-adrenoceptors were present in the same tissue, the endogenously released NA will elicit the β-adrenoceptor effect when the α-adrenoceptor influence is removed. The relaxant response in SPV was enhanced after phentolamine, suggesting that the β-adrenoceptors present were responsive to endogenously released NA. However, the relaxation in LCV and SCV was not enhanced and there was unmasked relaxation in ACV, DNV and SM after phentolamine. Since all these vessels were responsive to dobutamine and terbutaline, the results imply that the β-adrenoceptors present were not responsive to the endogenously released NA. Hence, it seems likely that β-adrenoceptors are favourably located in relation to the sympathetic nerve endings in SPV but not in other nasal veins. Like α-adrenoceptors, β-adrenoceptors are not evenly distributed in the blood vessels (Guimaraes & Moura, 2001). Propranolol, a β-adrenoceptor antagonist, markedly inhibited the relaxant response in SPV but did not affect the relaxant responses in LCV and SCV, confirming that the relaxation in SPV is related to β-adrenoceptor activation but not in LCV and SCV. One may think that the relaxation in LCV and SCV, though elicited through other mechanism(s), should be enhanced after the removal of the vasoconstrictor response. As the vasoconstriction is before the vasodilatation (biphasic), it is likely that the vasoconstriction is a fast event while the dilatation is a slower process. The dilatation maximum may occur at the time when the constriction is subsiding. Hence, removal of the constriction may not be able to influence appreciably the subsequent dilatation.
We demonstrated that the TNS frequency-relaxation in SPV after blockade of α-receptor was decreased to similar extent by atenolol (β1-selective antagonist) and ICI 118,551 (β2-selective antagonist). This suggests that the relaxation of SPV to TNS be mediated via both postjunctional β1- and β2-adrenoceptors. Combined treatment with atenolol and ICI 118,551 produced greater decrease of the TNS frequency-relaxation than the isolated effect of atenolol or ICI 118,551, providing additional support for the co-involvement of β1- and β2-adrenoceptors in the relaxation.
In the present study, dobutamine (a selective β1-adrenoceptor agonist) and terbutaline (a selective β2-adrenoceptor agonist) relaxed all the nasal venous segments. In preliminary studies, we found that atenolol and ICI 118,551 blocked their effects respectively. Hence, the results imply that both β1- and β2-adrenoceptors are present in the nasal venous vascular bed. However, further radioligand saturation binding and displacement experiments are required to confirm their presence. Early investigators doubted the existence of β-adrenoceptors in the nasal mucosa (Andersson & Bende, 1984). However, more recent studies demonstrated the presence of both β1- and β2-adrenoceptors in the resistance and capacitance vessels of the nasal mucosa of pigs, humans and dogs (Lacroix et al., 1995; Lung et al., 1984). These authors measured changes in nasal blood flow, perfusion pressure or nasal airway patency to define the effects of the extraneously administered β-agonists. Actually, nasal blood flow or nasal patency refers to the overall function of the whole vascular bed and does not reflect the actual situation in different vascular components. In this study, we directly applied the β1- and β2-adrenoceptor agonists onto the different components of nasal venous systems to study their effects. The results suggest the presence of both β1- and β2-adrenoceptors in all segments of nasal venous systems. Most beta-receptors in vascular tissue are β2-adrenoceptors. But β1-adrenoceptor dilatatory mechanism has been described in rat pulmonary artery (O'donnell & Wanstall, 1981) coronary artery of several species (Baron et al., 1972; Toda, 1981), feline cerebral artery (Edvinsson & Owman, 1974), rat femoral artery (Fujimoto et al., 1988) and rat jugular and portal veins (Cohen & Wiley, 1978).
Dobutamine showed great variations in the pD2 value but not of the maximal response whereas terbutaline had much less varying pD2 value but bigger fluctuating maximal response between nasal veins. Dobutamine is a full agonist while terbutaline is a partial agonist (Hoffman & Lefkowotz, 1991; Johnson, 1998). Changes in tissue factors will affect the dose-response curve of full and partial agonists in different ways. As full agonists are efficacy driven, changes in receptor density/coupling efficiency will shift the location of the curve affecting greatly the pD2 value without significant effect on the maximal response. However, for the partial agonists, which are affinity driven, similar changes in tissue factors will alter the maximal response with a slight change in the pD2 value (Kenakin, 1997). Hence, differences in tissue factors can account for the great variation of pD2 value for dobutamine and large fluctuation of maximal response for terbutaline between vessels. That SPV had the highest pD2 value for dobutamine and the biggest maximal response to terbutaline suggests that receptor density/coupling efficiency for both β1- and β2-adrenoceptors is highest in SPV among the nasal veins. This is in line with what was found in TNS studies that β-adrenoceptor vasodilatation was manifested in SPV and not other veins.
When compared to dobutamine, terbutaline was found to be more potent in LCV and SCV but of similar potency in ACV and SM (as reflected by pD2 values). The results imply that β2-adrenoceptors will be preferentially activated in the posterior collecting veins while the β1- and β2-adrenoceptors will be equally activated in the anterior collecting veins and venous sinusoids if exposed to low and moderate levels of β-adrenergic stimulation. However, the maximal response to dobutamine in these vascular segments was larger when compared to terbutaline, indicating that the action of β1-adrenoceptors is predominant over that of β2-adrenoceptors during maximal β-adrenergic stimulation in these venous segments. For the outflow veins, dobutamine was less potent than terbutaline in DNV but more potent than terbutaline in SPV, suggesting that β2-adrenoceptors will be preferentially activated in DNV whereas β1-adrenoceptors preferentially activated in SPV when exposed to low and moderate levels of β-adrenergic stimulation. The maximal responses of DNV and SPV to dobutamine and terbutaline are similar in magnitude. Hence both β1- and β2-adrenoceptors contribute equally to the effects in the outflow veins during maximal β-adrenergic stimulation.
Role of adrenergic mechanisms in sympathetic control of nasal venous vasculature
In the present study, we found that more than 80% of TNS induced constriction in LCV, SCV, ACV, DNV and SM was inhibited by phentolamine and about 80% of TNS induced relaxation in SPV was inhibited by propranolol. These results suggest that adrenergic mechanisms play a very important role in the sympathetic control of nasal venous systems. Although both α- and β-adrenoceptors were identified in all nasal venous vessels, upon sympathetic nerve stimulation the α-adrenergic constrictive effect was seen in LCV, SCV, ACV, DNV and SM whereas the β-adrenergic relaxant effect was seen in SPV. According to the vascular arrangements in the nasal mucosa, constriction of the venous sinusoids (SM) and collecting veins (ACV, LCV, and SCV) of both venous systems will decrease mucosal blood volume. Relaxation of posterior outflow vein (SPV), coupled with constriction of anterior outflow vein (DNV), will favour venous drainage through the posterior venous system.
The results of this study clearly demonstrate, for the first time, the exact role of adrenergic mechanisms in the sympathetic nervous control of nasal venous vasculature. Both α-adrenoceptors and β-adrenoceptors are concurrently involved, the former responsible for the fall in vascular capacitance of both venous systems while the latter favouring venous outflow from the posterior nasal cavity. The combined effect of the two mechanisms will lead to a fall in nasal airway resistance. The action of the α2-adrenoceptors is predominant over that of α1-adrenoceptors in causing the vasoconstrictive effect whereas both β1- and β2-adrenoceptors are equally important in causing the vasodilatatory action.
Acknowledgments
The present study was supported by RGC Grant (HKU373/94M) and HKU/CRCG Grant (335/034/0067) to Mary A.K.Y. Lung. The authors thanked LAU/HKU and AFCD/HKSAR Government for supplying experimental animals.
Abbreviations
- ACV
anterior collecting vein
- DNV
dorsal nasal vein
- LCV
lateral collecting vein
- MR
maximal response
- NA
noradrenaline
- PE
phenylephrine
- SCV
septal collecting vein
- SM
septal mucosa
- SPV
sphenopalatine vein
- TAT
total active tone
- TNS
transmural nerve stimulation
- TTX
tetrodotoxin
References
- ÅNGGÅRD A., DENSERT O. Adrenergic innervation of the nasal mucosa in cat. Acta Otolaryngol. 1974;78:232–241. doi: 10.3109/00016487409126349. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E., BENDE M. Adrenoceptors in the control of human nasal mucosal blood flow. Ann. Otol. Rhinol. Laryngol. 1984;93:179–182. doi: 10.1177/000348948409300216. [DOI] [PubMed] [Google Scholar]
- BARON G.D., SPEDEN R.N., BOHR D.F. Beta-adrenergic receptors in coronary and skeletal muscle arteries. Am. J. Physiol. 1972;223:878–881. doi: 10.1152/ajplegacy.1972.223.4.878. [DOI] [PubMed] [Google Scholar]
- BERRIDGE T.L., ROACH A.G. Characterization of α-adrenoceptors in the vasculature of the canine nasal mucosa. Br. J. Pharmacol. 1986;88:345–354. doi: 10.1111/j.1476-5381.1986.tb10210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUNA N.Blood and nerve supply of the nasal lining The Nose: Upper Airway Physiology and the Atmospheric Environment 1982Amsterdam: Elsevier; 45–69.ed. Proctor, D.R. & Andersen, I.B. [Google Scholar]
- COHEN M.L., WILEY K.S. Beta1 and beta2 receptor mechanisms in rat jugular veins: Differences between norepinephrine and isoproterenol-induced relaxation. Life Sci. 1978;23:1997–2006. doi: 10.1016/0024-3205(78)90231-x. [DOI] [PubMed] [Google Scholar]
- DAHLSTRÖM A., FUXE K. The adrenergic innervation of the nasal mucosa of certain mammals. Acta Otolaryngol. 1965;59:65–72. doi: 10.3109/00016486509128547. [DOI] [PubMed] [Google Scholar]
- DAWES J.D.K., PRICHARD M. Studies of the vascular arrangement of the nose. J. Anat. 1953;87:311–322. [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. The domestic pig as an experimental model for studies on the nasal cycle. Acta Otolaryngol. (Stockholm) 1978;85:431–436. doi: 10.3109/00016487809121472. [DOI] [PubMed] [Google Scholar]
- ECCLES R.Innervation of the nose The Nose: Neurological and pharmacological considerations 1982Amsterdam: Elsevier; 191–214.ed. Proctor, D.R. & Andersen, I.B. [Google Scholar]
- EDVINSSON L., OWMAN C. Pharmacological characterization of adrenergic alpha and beta receptors in mediating the vasomotor responses of cerebral arteries in vitro. Circ. Res. 1974;35:835–849. doi: 10.1161/01.res.35.6.835. [DOI] [PubMed] [Google Scholar]
- FLAVAHAN N.A., RIMELE T.J., COOKE J.P., VANHOUTTE P.M. Characterization of alpha-1 and alpha-2 adrenoceptors activated by exogenous and nerve-released noradrenaline in the canine saphenous vein. J. Pharmacol. Exp. Ther. 1984;230:699–705. [PubMed] [Google Scholar]
- FUJIMOTO S., DOHI Y., AOKI K., MATSUDA T. Altered vascular beta adrenoceptor-mediated relaxation in deoxycorticosterone-salt hypertensive rats. J. Pharmacol. Exp. Ther. 1988;244:716–723. [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HOFFMAN B.B., LEFKOWOTZ R.J.Catecholamines and sympathomimetic drugs. In: The Pharmacological Basis of Therapeutics 1991volume 1New York: Pergamon Press; 202ed. Gilman, A.G., Rall, T.W., Nies, A.S. and Taylor, P., 8th ed.p [Google Scholar]
- JOHNSON M. The β-adrenoceptor. Am. J. Respir. Crit. Care Med. 1998;158:S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.Efficacy Pharmacologic Analysis of Drug-Receptor Interaction 1997Philadelphia: Lippincott; 289–330.3rd ed., p [Google Scholar]
- LACROIX J.S., KURT A.M., AUBERSON S., BRETTON C. Beta-adrenergic mechanisms in the nasal mucosa vascular bed. Eur. Arch. Otorhinolaryngol. 1995;252:298–303. doi: 10.1007/BF00185393. [DOI] [PubMed] [Google Scholar]
- LACROIX J.S., LUNDBERG J.M. Sympathetic vascular control of the pig nasal mucosa: adrenoceptor mechanisms in blood flow and volume control. Br. J. Pharmacol. 1989;97:1075–1084. doi: 10.1111/j.1476-5381.1989.tb12564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGER S.Z. Presynaptic regulation of catecholamine release. Biochem. Pharmacol. 1974;23:1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- LUNG M.A., PHIPPS R.J., WANG J.C.C., WIDDICOMBE J.G. Control of nasal vasculature and airway resistance in the dog. J. Physiol. Lond. 1984;349:535–551. doi: 10.1113/jphysiol.1984.sp015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNG M.A., WANG J.C.C. Arterial supply, venous drainage and collateral circulation in the nose of the anaesthetized dog. J. Physiol. 1987;391:57–70. doi: 10.1113/jphysiol.1987.sp016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNG M.A., WANG J.C.C. An anatomical investigation of the nasal venous vascular bed in the dog. J. Anat. 1989a;166:113–119. [PMC free article] [PubMed] [Google Scholar]
- LUNG M.A., WANG J.C.C. Autonomic nervous control of nasal vasculature and airflow resistance in the anaesthetized dog. J. Physiol. 1989b;419:121–139. doi: 10.1113/jphysiol.1989.sp017864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'DONNELL S.R., WANSTALL J.C. Demonstration of both β1- and β2-adrenoceptors mediating relaxation of isolated ring preparations of rat pulmonary artery. Br. J. Pharmacol. 1981;74:547–552. doi: 10.1111/j.1476-5381.1981.tb10463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'ROURKE S.T., VANHOUTTE P.M. Adrenergic and cholinergic responsiveness of isolated canine bronchial arteries. Am. J. Physiol. 1990;259:H156–H161. doi: 10.1152/ajpheart.1990.259.1.H156. [DOI] [PubMed] [Google Scholar]
- ROACH A.G., DOXEY J.C., STRACHAN D.A., CAVERAO I. Sleeping times evoked by alpha adrenoceptor agonists in two-day chicks: An experimental model to evaluate full and partial agonists at central alpha-2-adrenoceptors. J. Pharmacol. Exp. Ther. 1983;227:421–428. [PubMed] [Google Scholar]
- RUFFOLO R.R. Spare α adrenoceptors in the peripheral circulation: excitation-contraction coupling. Federation Proc. 1986;45:2341–2346. [PubMed] [Google Scholar]
- STARKE K. Regulation of noradrenaline release by presynaptic receptor systems. Rev. Physiol. Biochem. Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- STARKE K. α-adrenoceptor subclassification. Rev. Physiol. Biochem. Pharmacol. 1981;88:199–236. [PubMed] [Google Scholar]
- STARKE K., DOCHERTY J.R. Types and functions of peripheral α-adrenoceptors. J. Cardiovasc. Pharmacol. 1982;4:S3–S7. doi: 10.1097/00005344-198200041-00002. [DOI] [PubMed] [Google Scholar]
- TODA N. Response of isolated monkey coronary arteries to catecholamines and to transmural electrical stimulation. Circ. Res. 1981;49:1228–1236. doi: 10.1161/01.res.49.6.1228. [DOI] [PubMed] [Google Scholar]


