Abstract
In the hippocampus, axon collaterals of CA1 pyramidal cells project locally onto neighbouring CA1 pyramidal cells and interneurones, forming a local excitatory network which, in disinhibited conditions, feeds polysynaptic epscs (poly-epscs). 5-hydroxytryptamine (5-HT) has been shown to inhibit poly-epscs through activation of a presynaptic receptor. The aim of the present work was the pharmacological characterization of the 5-HT receptor involved in this 5-HT action.
Poly-epscs, evoked by electrical stimulation of the stratum radiatum and recorded in whole-cell voltage-clamp from CA1 pyramidal neurones, were studied in mini-slices of the CA1 region under pharmacological block of GABAA, GABAB, and 5-HT1A receptors.
The 5-HT1B receptor selective agonist 1,4-dihydro-3-(1,2,3,6-tetrahydro-4-pyridinyl)-5H-pyrrolo[3,2-b]pyridin-5-one dihydrochloride (CP 93129) inhibited poly-epscs (EC50=55 nM), an effect mimicked by the 5-HT1B ligands 5-carboxamidotryptamine (5-CT; EC50=14 nM) and methylergometrine (EC50=78 nM), but not by 1-(3-chlorophenyl)piperazine dihydrochloride (mCPP; 10 μM) or 7-trifluoromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline dimaleate (CGS 12066B; 10 μM).
The effects of CP 93129 and 5-CT were blocked by the selective 5-HT1B receptor antagonist 3-[3-(dimethylamino)propyl]-4-hydroxy-N-[4-(4-pyridinyl)phenyl]benzamide dihydrochloride (GR 55562; KB≈100 nM) and by cyanopindolol (KB=6 nM); methiothepin (10 μM) and dihydroergotamine (1 μM). For both GR 55562 and methiothepin, application times of at least two hours were required in order to achieve their full antagonistic effects.
Our results demonstrate that 5-HT1B receptors are responsible for the presynaptic inhibition of neurotransmission at CA1/CA1 local excitatory synapses exerted by 5-HT.
Keywords: Electrophysiology, hippocampus, CA1, epsc, 5-HT, 5-HT1B receptor, presynaptic inhibition, CP 93129, GR 55562, CGS 12066B
Introduction
The CA1 region of the hippocampus receives dense innervation originating from the raphe nuclei (Segal, 1975) and the released 5-hydroxytryptamine (5-HT) exerts multiple actions on CA1 neurones, acting through a number of distinct receptor types located at pre- and/or postsynaptic levels (for review, see Hoyer et al. (1994) and Barnes & Sharp (1999)). The resulting changes in synaptic transmission produced by endogenous 5-HT are a likely consequence of simultaneous activation of more than one 5-HT receptor.
Direct postsynaptic actions exerted by 5-HT through changes in somatodendritic excitability on CA1 neurones are fairly well known. In CA1 pyramidal cells, the 5-HT1A receptor-mediated increase and the 5-HT4 receptor-mediated block in K+ conductances produce inhibitory and excitatory effects, respectively (Andrade & Nicoll, 1987; Colino & Halliwell, 1987). Furthermore, CA1 interneurones may be excited by activation of their somatodendritic 5-HT2 (Shen & Andrade, 1998) and 5-HT3 (Ropert & Guy, 1991) receptors.
Possible presynaptic modulation of CA1 neurotransmission by 5-HT remains still largely unexplored, in spite of evidence that 5-HT inhibits CA1 synaptic transmission at concentrations lower than those required for eliciting changes in cell excitability. In particular, stimulation of the principal excitatory input to the CA1 region, Schaffer collaterals (CA3/CA1), evokes polysynaptic responses in the CA1 region, which are potently inhibited by 5-HT (Segal, 1990; Mlinar et al., 2001).
Both inhibitory and excitatory polysynaptic responses in the CA1 region are generated by the activation of a local excitatory network formed by collaterals of axonal branches of CA1 pyramidal neurones which projects to basal dendrites of other CA1 pyramidal cells as well as of CA1 interneurones (Knowles & Schwartzkroin, 1981; Freund & Buzsaki, 1996). These local excitatory recurrent connections between pyramidal neurones have been electrophysiologically characterized (Christian & Dudek, 1988; Thomson & Radpour, 1991; Deuchars & Thomson, 1996) and local polysynaptic responses have been elicited following blockade of A1 adenosine receptors (Klishin et al., 1995) and GABAA receptors (Crepel et al., 1997; Mlinar et al., 2001). This local CA1/CA1 network appears to be implicated in physiological CA1 signal processing (Thomson & Radpour, 1991; Radpour & Thomson, 1991).
We have recently demonstrated (Mlinar et al., 2001) that 5-HT decreases the polysynaptic excitatory and inhibitory responses by presynaptic modulation of local excitatory connections formed by axon collaterals of CA1 pyramidal cells. In that study, an unexpected lack of sensitivity to several broad spectrum 5-HT receptor antagonists, together with the ineffectiveness of some agonists (i.e.: CGS 12066B), suggested unconventional pharmacological properties of the 5-HT receptor involved in the presynaptic inhibition of CA1 axon collateral transmission.
From the pharmacological profile of the effective agonists, a putative receptor, named provisionally 5-HT1G, with nM affinity for 5-HT, 5-CT, and 5-MeOT and insensitive to several antagonists (Castro et al., 1997) could not be dismissed. Furthermore, an ‘atypical' 5-HT1A receptor showing high-affinity for 5-CT and 8-OH-DPAT, while relatively insensitive to antagonists has been described in the CA1 region of the rat hippocampus (Waeber & Moskowitz, 1995). Finally, the anatomical consideration that 5-HT1B were likely to be expressed on axons of pyramidal cells in CA1 (Bruinvels et al., 1994; Boschert et al., 1994) prompted us to re-appraise the issue regardless of the ineffectiveness of the putative 5-HT1B receptor agonist CGS 12066B.
In addition to 8-OH-DPAT, to investigate the possibility of the involvement of an atypical 5-HT1A receptor, we used a wider range of 5-HT receptor ligands, including rodent 5-HT1B receptor selective agonist CP 93129 (Macor et al., 1990) or antagonists such as CR 55562 (Walsh et al., 1995; Lamothe et al., 1997) and cyanopindolol (Maura et al., 1987; Hoyer et al., 1994).
In our investigation, we used whole cell voltage-clamp recording of the polysynaptic excitatory response evoked in CA1 pyramidal cells by electrical stimulation of the stratum radiatum of rat dorsal hippocampal slices under block of GABA receptor-mediated responses and ablation of the CA3 region. The effects of 5-HT at the presynaptic level within the CA1/CA1 local network may be difficult to study in isolation. However, a favourable feature of the CA1 local network arrangement and of the selective sensitivity of CA1/CA1 synapses to 5-HT is that the poly-epscs originating from the temporal summation of CA1/CA1 excitatory connections can be easily separated from the monosynaptic component of epscs (mono-epsc) originating from CA3 afferents. This allows for quantitative measurement of the action of 5-HT agonists on CA1 pyramidal cell axons.
Methods
Experiments were carried out as previously described (Mlinar et al., 2001). All animal manipulations were performed according to the European Community guidelines for animal care (DL 116/92, application of the European Communities Council Directive 86/609/EEC) and approved by the Committee for Animal Care and Experimental Use of the University of Florence.
Experimental procedure
Twenty- to 28-day old Wistar rats (Harlan Italy, Udine, Italy) were anaesthetised with ether and decapitated with a guillotine. The brain was quickly removed and cooled in partially frozen oxygenated artificial cerebrospinal fluid (aCFS) which consisted of (in mM): NaCl 126; KCl 1.5; KH2PO4 1.25; NaHCO3 26; MgSO4 1.5; CaCl2 2; D-glucose 10 and was bubbled with 95% O2/5% CO2 gas mixture (pH 7.4). Transverse dorsal hippocampus slices of 350 μm nominal thickness were cut with a vibroslicer (VSL, WPI, Sarasota, FL, U.S.A.), and transferred to an incubation chamber containing aCFS at room temperature. After at least 1 h of incubation, one slice was transferred to a Petri dish, where, under the magnifying glass, the CA1 region was disconnected from the CA3 region by surgical cut. Following this procedure, the slice was returned to the incubation chamber and after at least one additional hour of recovery, was transferred to a recording chamber (volume 0.7 ml). Slices were maintained submerged with a U-shaped platinum wire and were continuously superfused with oxygenated aCFS at a rate of 2 ml·min−1. All experiments were performed at room temperature (21–24°C).
In all experiments bath solutions contained: (−)-bicuculline methiodide or (−)-bicuculline methochloride (10 μM) to block GABAA receptors, 3-N[1-(S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)-hydroxypropyl-P-benzyl-phosphinic acid (CGP 55845A; 1.5 μM) to block GABAB receptors (Davies et al., 1993), and N-(2-(-4(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide (WAY 100635; 10 nM), to block 5-HT1A receptors. In some early experiments P-[3-aminopropyl]-P-diethoxymethylphosphinic acid (CGP 35348, 300 μM) was used instead of CGP 55845A as GABAB receptor antagonist. Epileptiform activity was not observed under these experimental conditions.
In experiments where 5-HT1B antagonists were applied for 2 h or longer, slices were exposed to the antagonist for the required time in an incubation chamber containing oxygenated aCFS at room temperature. After this pre-incubation, the slice was transferred to the recording chamber, in which the antagonist was present throughout the experiment.
Experiments were made on an upright microscope (Axioskop; Zeiss, Göttingen, Germany) with a ×40 water immersion objective lens. Visual guidance was achieved by infrared differential interference contrast videomicroscopy (Stuart et al., 1993), utilizing a Newvicon camera (C2400-07; Hamamatsu, Hamamatsu City, Japan). All acquisition was done with the use of an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, U.S.A.) controlled with Clampex 6 software (Axon) through a PC equipped with a Digidata 1200 interface (Axon). Patch pipettes were pulled from borosilicate glass (1.5 mm O.D., 1.17 mm I.D.; Clark Electromedical Instruments, Reading, U.K.) and had resistance of 1.8 to 2.6 MΩ. The pipette solution contained (in mM): KCH3SO3 130; KCl 2; NaCl 2.5; MgCl2 1; MgATP 3; Na3GTP 0.5; HEPES 10 and NaOH ≈5 to pH 7.3, ≈295 mosmol l−1.
The pipette capacitive transient was compensated before obtaining the whole-cell configuration with the compensation circuits of Axopatch amplifier. The cell membrane capacitance and the series resistance (RS), which typically ranged from 5 to 10 MΩ, were not compensated. The input resistance (RIN) of recorded cells was in the range of 90 to 200 MΩ. The stability of recording conditions was monitored through the experiments by applying a 5 mV hyperpolarising pulse shortly before each stimulation pulse. Filtering was at 2–5 kHz and sampling was 20–40 kHz. Data traces were acquired every 10 s.
In most experiments, shortly after establishing the whole-cell configuration, we switched in current clamp mode to determine the resting membrane potential (r.m.p.) and voltage-current relationship to obtain a ‘signature' of the recorded neurone. Neurones included in this study had r.m.p. more negative than −60 mV, did not spontaneously fire action potentials (a.p.-s) and had overall typical appearance of CA1 pyramidal neurone current-clamp recordings.
Following this procedure, about 5 min after the whole-cell configuration was established, the amplifier was switched back to voltage clamp mode and holding potential was set to −75 mV or −80 mV. epscs were evoked by stimulation of the stratum radiatum with computer driven stimulus isolation unit (DS2, Digitimer, Welwyn Garden City, U.K.) through a concentric bipolar electrode (Clark Electromedical Instruments, Pangbourne, U.K.) positioned in the middle of the layer 1–1.2 mm from the recorded cell. Stimulation intensity was selected to give epsc amplitude from 50 to 200 pA. Following several minutes of settling time, synaptic currents were routinely stable throughout recording (up to 100 min). Slow run-down of polysynaptic currents, likely due to the degradation of slices, was rarely observed and these experiments were discarded.
With these stimulation parameters almost ‘pure' poly-epscs were obtained in approximately 20% of the cases (e.g. Figure 6A) and compound epscs in which mono- and poly-epscs were kinetically separable in the rest of experiments. Low resistance of patch electrodes and consequently good access to the cell interior were crucial for separating mono- and poly-epsc components. Poly-epscs were measured as average currents over a 30–50 ms wide time window, beginning at least 30 ms after the stimulus (see Figure 1B). The preparations showing a detectable residual mono-epsc component of the response in the region of poly-epsc measurement (⩾30 ms following the stimulus artefact) were discarded.
Figure 6.
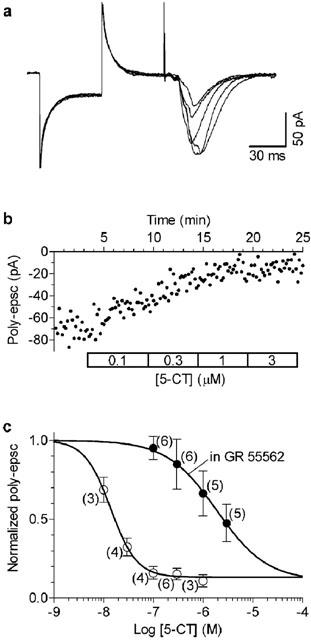
Long applications of GR 55562 antagonise the inhibitory effect of 5-CT. Slices were incubated in 10 μM GR 55562 for at least 2 h and then transferred in the recording chamber where increasing concentrations (100 nM–3 μM, 5 min each) of 5-CT were applied in the presence of the antagonist. Whole-cell recordings (A) and time-course of the effect on poly-epsc amplitudes (B) show that in the presence of GR 55562 the effect of 5-CT was antagonised. (C) Individual points correspond to mean values of poly-epsc amplitudes normalised compared to values before agonist application. Solid lines are best least square fits to the logistic equation (see Figure 2E). The data in the presence of GR 55562 were fitted with the bottom (b; fraction of poly-epsc remaining at the maximal agonist effect) constrained to 0.13 (the value determined from CP 93129 concentration-response curve in the absence of antagonist). In the presence of GR 55562 (open circles), the concentration-response curve of 5-CT appeared shifted to the right in comparison with that obtained in control (filled circles). Note that the antagonism by GR 55562 was apparently surmountable. The number of neurones is given in parentheses.
Figure 1.
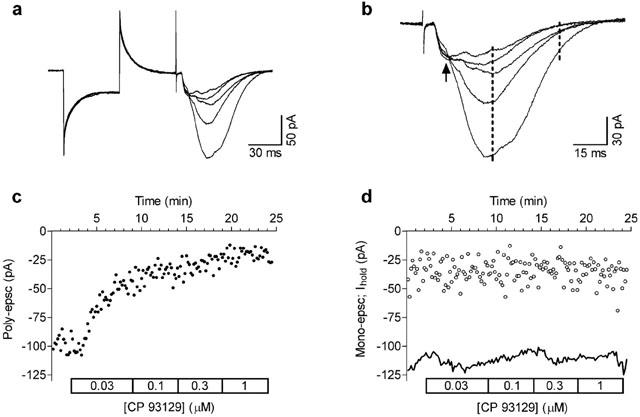
Activation of 5-HT1B receptors inhibits poly-epscs evoked by stimulation of stratum radiatum in the CA1 hippocampal region. (A) Whole-cell recordings show the concentration-dependent decrease in poly-epscs produced by the selective 5-HT1B agonist CP 93129 (30 nM–1 μM; see D). In this and in the following figures the initial part of traces is the response evoked by 5 mV hyperpolarising pulses used to monitor stability of the recording. The following fast, transient, upward deflections are stimulus artefacts. Traces shown are the average of seven consecutive responses obtained in control and at the end of application of each concentration of CP 93129. (B) Same traces as in (A) are shown with expanded time base to illustrate the separation between mono-epscs (arrow) and poly-epscs. Broken lines indicate the time-window used for measuring the amplitude of poly-epscs (see Methods). (C) Time-course of the effects of increasing concentrations of CP 93129 on the amplitude of poly-epsc. (D) Mono-epscs (open circles) were not significantly affected by CP 93129. Continuous line shows the current at holding potential (Ihold) during the experiment. All experiments were performed under block of GABAA, GABAB, and 5-HT1A receptors.
Concentration-response relationships
To obtain data for concentration-response relationships, for each preparation, 4–5 concentrations of an agonist were applied with a cumulative protocol. Only one concentration-response curve, either in the presence or in the absence of an antagonist, was constructed per slice. The duration of the individual agonist concentrations applied was chosen on the basis of the 5-HT action time-course studied in a previous work (see Figure 5D, Mlinar et al., 2001), and of the preliminary results obtained with 5-HT1B agonists. Thus, in order to reach at least 95% of the steady-state (maximum) effect for each concentration applied, the low concentrations (10–30 nM) of agonists were applied for 7–8 min and higher concentrations for at least 5 min. For each concentration of agonist, steady-state values were obtained by averaging seven consecutive responses taken at the last minute of the application.
Figure 5.
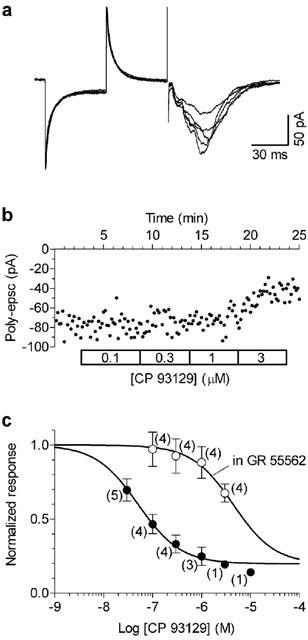
Long applications of GR 55562 antagonise the inhibitory effect of CP 93129. Slices were incubated in 10 μM GR 55562 for at least 2 h and were then transferred in the recording chamber where increasing concentrations (100 nM–3 μM, 5 min each) of CP 93129 were applied in the presence of the antagonist. Whole-cell recordings (A) and time-course of the effect on poly-epsc amplitudes (B) show that in the presence of GR 55562 the effect of CP 93129 was antagonised in an apparently surmountable manner. (C) Individual points correspond to mean values (mean±s.e.mean) of poly-epsc amplitudes normalised compared to values before agonist application. Solid lines are best least squares fits to the logistic equation (see Figure 2E). The data in the presence of GR 55562 were fitted with the bottom (b; fraction of poly-epsc remaining at the maximal agonist effect) constrained to 0.17 (the value determined from CP 93129 concentration-response curve in the absence of antagonist). In the presence of GR 55562 (open circles), the concentration-response curve of CP 93129 appeared shifted to the right in comparison with that obtained in control conditions (filled circles). The number of neurones is given in parentheses.
Data analysis
Data were analysed using Clampfit (Axon) and Prism 3.02 softwares (GraphPad Software, San Diego, CA, U.S.A.). Fitting of data points to functions was done by non-linear least squares algorithms. All values are expressed as mean±s.e.mean. Data were tested for statistical significance with one-sample, unpaired or paired two-tailed Student t-test, as appropriate. A value of P<0.05 was considered significant.
For the analysis of the action of competitive antagonists the Gaddum-Schild equation, based on the assumption that equal responses rely on equal fractional occupancy of a given receptor, was applied. Under this assumption, the ratio (dose ratio : dr) between the concentration of agonist A in the presence of antagonist B ([A′]) and that giving the same response obtained in the absence of antagonist ([A]) is related to the affinity constant of the antagonist (KB) by the equation:
Alternatively, double reciprocal plots of equiactive concentrations of agonist in the absence and the presence of antagonist were used. This type of calculation does not assume any particular type of non-competitive antagonism and may be applied in almost all cases with the exclusion of irreversible antagonism (Gaddum et al., 1955; Kenakin, 1997).
It can be demonstrated (Kenakin, 1997) that, when the antagonist can completely block the response to agonist, equiactive concentrations of the agonist in the absence ([A]) and in the presence ([A′]) of antagonist (B) are related to each other in the following double reciprocal relationship:
where KB and KA are the equilibrium dissociation constants of the antagonist- and agonist-receptor complex, respectively.
The slope of the straight line resulting from the double reciprocal plot is related to KB by the equation:
and therefore KB can be calculated from the slope of the line obtained with regression of the equiactive concentrations obtained experimentally.
It should be noted that while equation 2 also takes into account possible changes in agonist affinity produced by the antagonist (factor α), the calculation of KB is not affected by the allosteric effects of the antagonist on agonist affinity, since the slope term does not contain α. Double reciprocal plots were constructed with values of equiactive concentrations obtained from nonlinear regression of concentration-response curves.
Drugs
(−)-bicuculline methiodide, (−)-bicuculline methchloride, methiothepin maleate, GR 55562, cyanopindolol hemifumarate, CP 93129, methylergometrine maleate and 8-OH-DPAT were from Tocris (Bristol, U.K.). 5-CT, CGS 12066B, mCPP and dihydroergotamine methanesulphonate were from Sigma-RBI (Milano, Italy). Inorganic salts were Aristar grade (BDH, Poole, U.K.) and all other drugs were from Sigma-Aldrich (Steinheim, Germany) or Fluka (Buchs, Switzerland). CGP 55845A and CGP 35348 were kindly provided by Dr Wolfgang Froestl (Novartis, Basel, Switzerland) and WAY 100635 was a gift of Dr Michel Hamon (INSERM U-288, Paris, France).
Serotonergic drugs were prepared as stock solutions in distilled water or in up to 0.1 M HCl, at one thousand times the highest experimental concentration, aliquoted and stored at −20°C until use. Methiothepin (10 mM) was dissolved by gentle heating in equimolar HCl, and was used on the same day to avoid precipitation. Cyanopindolol was prepared as 20 mM stock solutions in DMSO.
Results
The experiments were carried out on 104 slices taken from 23 rats. In all experiments the GABAergic inhibition was blocked by 10 μM bicuculline and 1.5 μM CGP 55845A. 5-HT1A receptors were blocked by 10 nM WAY 100635.
The stimulation of the stratum radiatum evoked compound epscs comprising an early monosynaptic (mono-epsc) component and a late polysynaptic (poly-epsc) response (Figure 1A,B). In similar experimental conditions we have previously demonstrated that the poly-epscs evoked by the stimulation of the stratum radiatum are the summation of epscs originating from CA1 axon collaterals (Mlinar et al., 2001).
Effects of 5-HT1B receptor agonists
As illustrated in Figure 1, application of the selective 5-HT1B receptor agonist CP 93129, decreased the amplitude of poly-epscs in a concentration-dependent manner without affecting membrane conductance of the recorded cell. In contrast, the mono-epscs and the membrane current at the holding potential were unaffected (Figure 1D).
Similar results were obtained with 5-CT (Figure 2A,B) and with methylergometrine (Figure 2C,D), two non-selective, 5-HT1B agonists. The concentration-response curves obtained with 5-CT and CP 93129 are shown in Figure 2E. The resulting EC50 values were 55 nM (95% Confidence Limits: 38–59 nM) for CP 93129, 14 nM (95% C.L.: 12–16 nM) for 5-CT, and 78 nM (95% C.L.: 64–95 nM) for methylergometrine (not shown).
Figure 2.
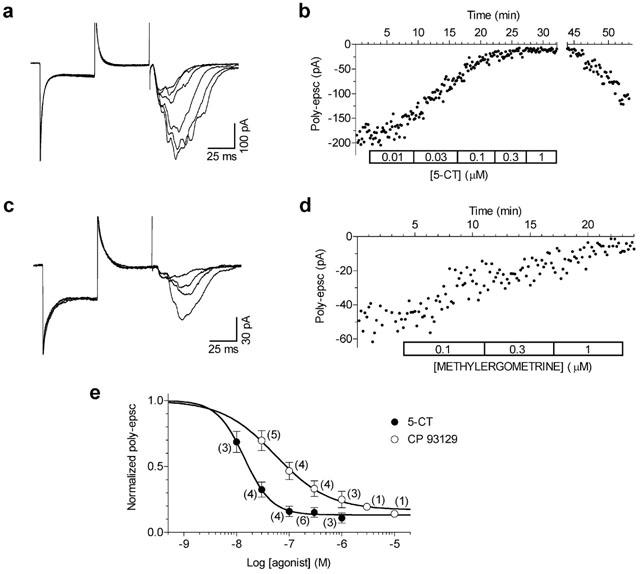
5-CT and methylergometrine inhibition of poly-epsc. (A) Whole-cell recordings show the concentration-dependent decrease in poly-epscs produced by 5-CT (10 nM–1 μM; see B). (B) Time-course of the effects of increasing concentrations of 5-CT on the amplitude of poly-epscs. Note the gradual recovery of responses after the washout. Whole-cell recordings (C) and the time-course (D) of the effects of increasing concentrations of methylergometrine (100 nM–1 μM) on the amplitude of poly-epscs. (E) Individual points correspond to mean values (mean±s.e.mean) of poly-epsc amplitudes normalised by taking the control as unity. Numbers of experiment replication are given in parentheses. The solid lines are the best least squares fits to the logistic equation, b+(1-b)/(1+(EC50/[A])nH), where EC50 is the half-maximally effective concentration, [A] is the agonist concentration, nH is the Hill coefficient, and b is the fraction of poly-epsc remaining at the maximal agonist effect.
The putative 5-HT1B receptor agonist CGS 12066B did not significantly affect poly-epsc responses at a concentration (10 μM) which should have ensured high occupancy of 5-HT1B receptors (Figure 3A,B). In addition, CGS 12066B did not prevent the effect of 5-CT on poly-epscs (Figure 3A,B). Similarly, m-CPP (10 μM) and dihydroergotamine (1 μM), known to bind to 5-HT1B receptors, were ineffective in decreasing the poly-epsc response (Figure 3C). Figure 3C also shows that superfusion with 1 μM 8-OH-DPAT, a 5-HT1A receptor ligand, did not significantly affect the epscs, indicating that the effect of 5-CT could not be ascribed to 5-HT1A receptor activation.
Figure 3.
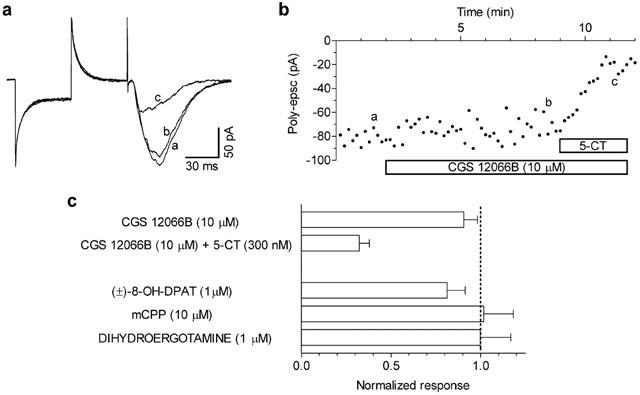
The selective 5-HT1B receptor ligand CGS 12066B neither mimicks nor antagonises the 5-CT-mediated inhibition of poly-epscs. (A) Whole-cell recordings in control (a), in the presence of 10 μM CGS 12066B (b), and of CGS 12066B+300 nM 5-CT. Traces are averages of seven responses taken at times indicated by corresponding letters in (B). (B) Time-course of the poly-epsc amplitude in the presence of CGS 12066B and of CGS 12066B+5-CT. (C) Responses to CGS 12066B (n=7), CGS 12066B+5-CT (n=4), 8-OH-DPAT (n=4), mCPP (n=6), and dihydroergotamine (n=3) are normalised compared to the pre-drug values. All compounds were applied for 10–15 min.
5-HT1B receptor antagonists
In a first set of experiments we tested the effectiveness of the selective 5-HT1B antagonist GR 55562 on the inhibition of poly-epscs exerted by CP 93129 or 5-CT. As shown in Figure 4, application of GR 55562 (10 μM) for 30–60 min did not greatly decrease the responses to CP 93129 (300 nM) and to 5-CT (300 nM). Nevertheless, the antagonist appeared to hasten the recovery from CP 93129 action indicating that GR 55562 was not completely ineffective. Taking into account the possibility of a slow onset of antagonist action, we preincubated the slices for longer than 2 h before application of the agonists. As illustrated in Figure 4C, when applied for a long time GR 55562 was able to significantly antagonise the effects of both CP 93129 (300 nM) and 5-CT (300 nM).
Figure 4.
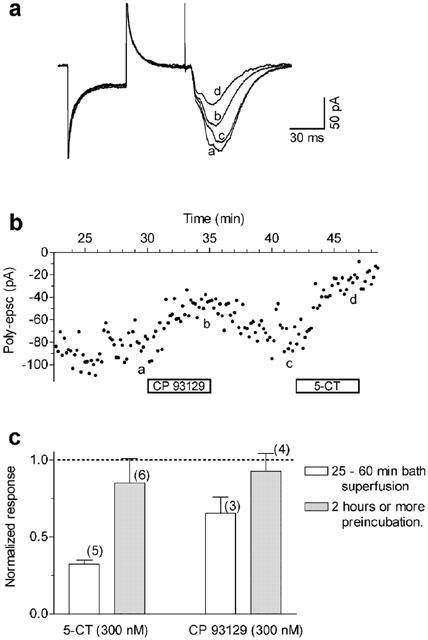
The antagonism of responses to CP 93129 or 5-CT by the selective 5-HT1B antagonist GR 55562 has slow onset. (A) Whole-cell recordings in the presence of GR 55562 (10 μM, 30 min application) (a), after the addition of CP 93129 (300 nM, 5 min) (b), after recovery from CP 93129 effect (c), and after the addition of 300 nM 5-CT (d). Note that 45 min of GR 55562 application did not fully antagonise the effect of 5-CT on poly-epscs (see Figure 6C). Traces are averages of seven responses taken at times indicated by corresponding letters in (B). (B) Time-course of the poly-epsc amplitude inhibition by CP 93129 and 5-CT in the presence of GR 55562. Times on ordinate are given in respect to the beginning GR 55562 application. (C) Superfusion of GR 55562 (10 μM) for ⩾2 h substantially antagonised the action of both agonists. Values are mean±s.e.mean, responses are normalised compared to the pre-drug values. The number of neurones is given in parentheses.
Long preincubation times (>2 h) were therefore used for constructing concentration-response curves to agonist action in the presence of CR 55562 (10 μM). As shown in Figure 5, the concentration-response curve to CP 93129 in the presence of GR 55562 was displaced to the right in an apparently competitive manner. The apparent KB of GR 55562 calculated from the shift to the right of CP 93129 concentration-response curve was 104 nM (see Methods). Similar results were obtained using 5-CT as agonist (Figure 6) and the calculated KB of GR 55562 was 72 nM.
The same experimental protocol (i.e. >2 h drug application) was used to investigate the effects of other known 5-HT1B receptor antagonists (Figure 7). The potent 5-HT1B receptor antagonist cyanopindolol (10 μM) completely blocked the effects of CP 93129 and 5-CT. Similarly, methiothepin, a non-selective 5-HT1B antagonist (10 μM), significantly blocked the effects of CP 93129 and 5-CT. We also tested dihydroergotamine, a high affinity 5-HT1B ligand. At the concentration of 1 μM, which did not affect poly-epscs (Figure 3C), the drug significantly antagonised the effects of 5-CT within 30 min of superfusion (Figure 7C).
Figure 7.
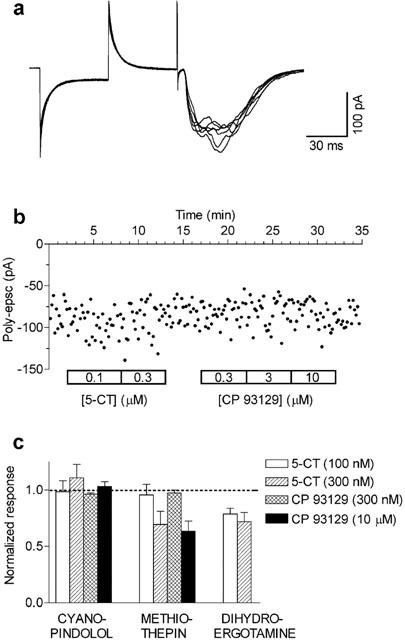
Long applications of cyanopindolol, methiothepin, or dihydroergotamine antagonise the effects of CP 93129 and 5-CT on poly-epscs. Whole-cell recordings (A) and time-course of the effect on poly-epsc amplitudes (B) show that in the presence of cyanopindolol (10 μM; n=3) applied for longer than 2 h the effects of 5-CT and of CP 93129 were fully antagonised. In similar conditions, also methiothepin (10 μM; n=3) or dihydroergotamine (1 μM; n=4) antagonised CP 93129 and/or 5-CT effects on poly-epscs. Bars correspond to mean values (mean±s.e.mean) of poly-epsc amplitudes normalised to the respective values before agonist application.
Figure 8A illustrates that, when applied in the presence of 1 μM CP 93129, cyanopindolol (10 μM) almost fully antagonised the effect of the agonist within 5 min of combined application. The effect of cyanopindolol reversed within 30 min of washout. This indicated that cyanopindolol was effective in antagonising the action of CP 93129 with faster onset action when compared with GR 55562.
Figure 8.
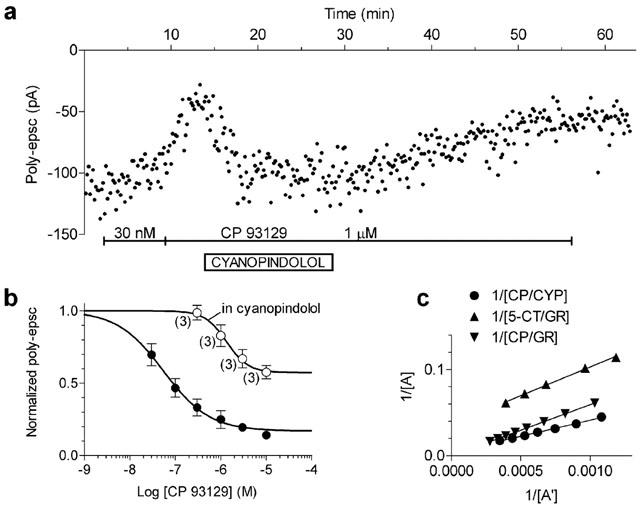
Properties of cyanopindolol antagonism of CP 93129. (A) The time-course of the amplitude of poly-epscs shows effects of 30 nM and 1 μM CP 93129 comparable to those expected from the control concentration-response curve. Addition of cyanopindolol 10 μM rapidly and reversibly antagonised the effect of CP 93129 1 μM. (B) In slices incubated in cyanopindolol 200 nM (open circles), the concentration-response curve of CP 93129 was shifted to the right and the maximum was depressed in comparison with control (filled circles; see Figure 5C), suggesting a non-competitive component of the antagonism. (C) Double reciprocal plot of equiactive concentrations of CP 93129 in the presence of cyanopindolol 200 nM. For comparison the same plot of CP 93129 and 5-CT in the presence of GR 55562 10 μM is shown (see text for details).
In three cells, we tested the antagonism of cyanopindolol (200 nM) applied for longer than 2 h on increasing concentrations of CP 93129. As shown in Figure 8B, cyanopindolol shifted the concentration-response curve of CP 93129 to the right and decreased the maximal response to the agonist. Given the non-competitive behaviour of the antagonist, its apparent KB was calculated using the double reciprocal plot of equieffective agonist concentrations (see Methods). Figure 8C shows the plot which gave a KB value of 6 nM. Although the GR 55562 antagonism appeared to be competitive, a possible non competitive component cannot be ruled out completely. Therefore, Figure 8C also shows the double reciprocal plot for CP 93129 and 5-CT responses in the presence of GR 55562. The resulting KB values of GR 55562 were 171 nM for CP 93129 and 152 nM for 5-CT effects.
Discussion
The major result of our investigation is the pharmacological identification of the receptor responsible for the inhibition of excitatory neurotransmission exerted by 5-HT at the level of axon collaterals of CA1 pyramidal cells. The inhibition of poly-epscs by the selective 5-HT1B agonist CP 93129 and its block by cyanopindolol and by the selective 5-HT1B antagonist GR 55562 identified 5-HT1B receptor as responsible receptor type. These findings, together with the ineffectiveness of 8-OH-DPAT to inhibit poly-epscs rule out the possible involvement of ‘atypical' 5-HT1A receptors (Waeber & Moskowitz, 1995), and/or of the putative 5-HT1G receptors (Castro et al., 1997).
CP 93129 recognises the rat 5-HT1B receptor with high affinity and selectivity (Macor et al., 1990). In functional studies, CP 93129, acting on presynaptic 5-HT1B receptors, inhibited glutamatergic field potentials in subiculum (Boeijinga & Boddeke, 1993), as well as glutamatergic epscs in caudal raphe neurones (Li & Bayliss, 1998). In our study, CP 93129 mimicked the previously described effect of 5-HT on poly-epscs with an EC50 value (55 nM) consistent with the affinity of the compound for 5-HT1B receptors (Macor et al., 1990) and with a potency similar to that found for inhibition of glutamatergic epscs in caudal raphe neurones (Li & Bayliss, 1998). In addition, the non-selective agonists 5-CT and methylergometrine potently inhibited CA1 poly-epscs. The EC50 of 5-CT in the present investigation is closely consistent with that obtained with sharp-electrode recordings and reported in our previous work (i.e.: ∼15 nM; Mlinar et al., 2001) where also 5-HT (EC50=100–200 nM) and 5-MeOT (EC50=320 nM) were tested. The resulting rank of potency of the agonists active in inhibiting poly-epsc/ps in CA1 in similar conditions is 5-CT>CP 93129⩾methylergometrine⩾5-HT>5-MeOT, which closely fits with the pharmacological profile of 5-HT1B receptors.
GR 55562, a selective 5-HT1B antagonist (Walsh et al., 1995; Lamothe et al., 1997), completely antagonised the actions of CP 93129 and 5-CT on poly-epscs. The GR 55562 antagonism appeared competitive and the calculated KB values (104–171 nM for CP 93129 and 72–152 for 5-CT) are fairly well in agreement with those described in binding studies (39–54 nM; Lamothe et al., 1997; Pauwels et al., 1999). Moreover, the KB values obtained using either CP 93129 or 5-CT as agonists were virtually the same. This independence of KB from the agonist used further ensured that both compounds inhibited poly-epscs through activation of 5-HT1B receptors.
Cyanopindolol and methiothepin, two recognised 5-HT1B antagonists (Hoyer et al., 1994), also fully antagonised the actions of CP 93129 and 5-CT on poly-epscs. Interestingly, we found a non-competitive component in the antagonism exerted by cyanopindolol. It is becoming increasingly clear that substances showing fully competitive behaviour in binding studies may display additional non-competitive actions in functional assays (Kenakin, 1997). Given the paucity of functional data obtained using concentration-response curves in the presence of cyanopindolol it appears difficult to ascertain whether this is a general characteristic of the drug or if it depends on particular characteristics of the studied receptor.
It should be noted that methiothepin and GR 55562 showed an unusually slow onset of action (e.g. Figure 4). Importantly, this unexpected property of antagonists action on 5-HT1B receptors expressed on axon collaterals of CA1 pyramidal cells appears sufficient to explain the lack of antagonist effects reported in our previous work, in which 25–30 min application of 10–100 μM methiothepin or 100 μM methysergide failed to antagonise the inhibition of polysynaptic responses produced by 5-CT or 5-HT, both in patch-clamp and sharp-electrode recordings (Mlinar et al., 2001).
A slow penetration of antagonists in the slice, due to their solubility into membrane lipids, seems not to be a convincing explanation, because cyanopindol, the most lipophilic among the antagonists tested, had faster action than the highly hydrophilic GR 55562.
Furthermore 30 min application of methiothepin (10 μM) exerted the expected block of 5-HT1A receptor-mediated hyperpolarisation in CA1 pyramidal cells produced by 100 nM 5-CT (−61.5±8.3%; n=4; Giachi & Corradetti, unpublished observation; see also Andrade & Nicoll, 1987). Although methiothepin and GR 55562 have been shown to behave as inverse agonists (Pauwels et al., 1999) it is difficult to ascertain whether the slow onset of the response could be ascribed to this property or is merely coincidental.
In conclusion, these antagonist peculiarities, while not challenging the selectivity of the 5-HT1B block, may deserve further investigation directed to full understanding of the mechanisms underlying the unexpectedly slow kinetics of effects shown by these substances. CGS 12066B has been claimed to be a selective 5-HT1B receptor agonist (Neale et al., 1987), although it was later demonstrated to have a higher affinity for 5-HT1D and 5-HT1A than for 5-HT1B receptors (Macor et al., 1990; Adham et al., 1992). Nevertheless, CGS 12066B was found to inhibit 5-HT release (Neale et al., 1987) and to act as a full 5-HT1B agonist in some functional assays in vitro (Schoeffter & Hoyer, 1989).
In our hands, CGS 12066B was ineffective in inhibiting polysynaptic responses both in sharp electrode (Mlinar et al., 2001) and in patch-clamp recordings (Figure 3). In addition, the agonist action of 300 nM 5-CT was not antagonised by 10 μM CGS 12066B, indicating lack of partial agonist/antagonist activity of CGS 12066B at 5-HT1B receptors in CA1 pyramidal cells. Another putative 5-HT1B agonist, mCPP (Schoeffter & Hoyer, 1989), was similarly ineffective in inhibiting poly-epscs (Figure 3C). The observed lack of agonism by CGS 12066B, together with its poor receptor selectivity, challenges the use of CGS 12066B as a tool in characterizing 5-HT1B receptor-mediated responses.
The characterization of 5-HT1B receptor-mediated responses has suffered for a long time from poor selectivity of compounds. Nevertheless, convincing evidence indicates that 5-HT1B receptors mediate presynaptic inhibition in many areas of the central nervous system including glutamatergic neurotransmission in the subiculum (Boeijinga & Boddeke, 1993), caudal raphe (Li & Bayliss, 1998) and nucleus accumbens (Muramatsu et al., 1998), serotonergic transmission in the dorsal raphe (Morikawa et al., 2000) and GABAergic transmission in the ventral midbrain (Johnson et al., 1992). However, the availability of novel selective agonists and antagonists, makes it increasingly clearer that additional variability in pharmacological sensitivity of 5-HT1B receptor-mediated responses may derive from the different characteristics of 5-HT1B receptors expressed in various neurone populations. For instance, in neurochemical assays 5-HT1B receptors have been shown to inhibit the release of various neurotransmitters, e.g. acetylcholine (Maura & Raiteri, 1986), and 5-HT1B autoreceptors have been demonstrated to have higher sensitivity to 5-HT and CP 93129 than heteroreceptors on dopaminergic and cholinergic terminals (Sarhan & Fillion, 1999).
Additional pharmacological complexity may arise from the finding that 5-HT1B and 5-HT1D receptors form homodimers when expressed alone, but form heterodimers when co-expressed (Xie et al., 1999). If physiological dimerisation occurs, 5-HT1B homodimers would be formed in neurones expressing only 5-HT1B receptor (CA1 pyramidal cells) and 5-HT1B/5-HT1D heterodimers would be present in neurones expressing both receptors (e.g. dorsal raphe). Since there are differences in the pharmacology of rodent 5-HT1B and 5-HT1D receptors, responses to 5-HT1B agonists and antagonists in different assays may additionally depend on whether 5-HT1B homodimers or 5-HT1B/D heterodimers are present in the presynaptic terminals.
In the hippocampus, 5-HT1B receptors are abundant on axons of CA1 pyramidal cells (Boschert et al., 1994; Bruinvels et al., 1994) and 5-HT, acting through 5-HT1B receptors, potently inhibits both local excitatory transmission (Mlinar et al., 2001) and CA1 projections to subiculum (Boeijinga & Boddeke, 1993; 1996). Functionally, local CA1 excitatory connections are involved in the induction of long-term potentiation (Radpour & Thomson, 1991) and therefore 5-HT1B receptors on axon collaterals of CA1 pyramidal cells are suitably located for controlling hippocampus-dependent learning and/or memory. Moreover, the fact that 5-HT activates 5-HT1B receptors and reduces polysynaptic responses at concentrations lower than those required to produce its postsynaptic effects in hippocampus, together with strategic localisation of 5-HT1B receptors for control of CA1 output suggests that activation of these receptors represents an important physiological mechanism of 5-HT action in the hippocampal formation.
Acknowledgments
Supported by grants from EC (QLG3-CT-2002-00809), Ente Cassa di Risparmio di Firenze (2000-1073), and University of Florence (ex 60%).
Abbreviations
- 5-CT
5-carboxamidotryptamine
- 8-OH-DPAT
(±)-8-hydroxy-2-dipropylaminotetralin hydrobromide
- a.p.
Action potential
- CGP 35348
P-[3-aminopropyl]-P-diethoxymethylphosphinic acid
- CGP 55845A
3-N[1-(S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)-hydroxypropyl-P-benzyl-phosphinic acid
- CGS 12066B
7-trifluoromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline dimaleate
- CP 93129
1,4-dihydro-3-(1,2,3,6-tetrahydro-4-pyridinyl)-5H-pyrrolo[3,2-b]pyridin-5-one dihydrochloride
- GR 55562
3-[3-(dimethylamino)propyl]-4-hydroxy-N-[4-(4-pyridinyl)phenyl]benzamide dihydrochloride
- mCPP
1-(3-chlorophenyl)piperazine dihydrochloride
- RIN
Input resistance
- r.m.p.
Resting membrane potential
- RS
Series resistance
- WAY 100635
N-(2-(-4(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide
References
- ADHAM N., ROMANIENKO P., HARTIG P., WEINSHANK R.L., BRANCHEK T. The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol. Pharmacol. 1992;41:1–7. [PubMed] [Google Scholar]
- ANDRADE R., NICOLL R.A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J. Physiol. (Lond). 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BOEIJINGA P.H., BODDEKE H.W. Serotonergic modulation of neurotransmission in the rat subicular cortex in vitro: a role for 5-HT1B receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:553–557. doi: 10.1007/BF00167229. [DOI] [PubMed] [Google Scholar]
- BOEIJINGA P.H., BODDEKE H.W. Activation of 5-HT1B receptors suppresses low but not high frequency synaptic transmission in the rat subicular cortex in vitro. Brain Res. 1996;721:59–65. doi: 10.1016/0006-8993(96)00149-7. [DOI] [PubMed] [Google Scholar]
- BOSCHERT U., AMARA D.A., SEGU L., HEN R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- BRUINVELS A.T., LANDWEHRMEYER B., GUSTAFSON E.L., DURKIN M.M., MENGOD G., BRANCHEK T.A., HOYER D., PALACIOS J.M. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- CASTRO M.E., ROMON T., CASTILLO M.J., DEL OLMO E., PAZOS A., DEL ARCO C. Identification and characterization of a new serotonergic recognition site with high affinity for 5-carboxamidotryptamine in mammalian brain. J. Neurochem. 1997;69:2123–2131. doi: 10.1046/j.1471-4159.1997.69052123.x. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN E.P., DUDEK F.E. Electrophysiological evidence from glutamate microapplications for local excitatory circuits in the CA1 area of rat hippocampal slices. J. Neurophysiol. 1988;59:110–123. doi: 10.1152/jn.1988.59.1.110. [DOI] [PubMed] [Google Scholar]
- COLINO A., HALLIWELL J.V. Differential modulation of three separate K-conductances in hippocampal CA1 neurones by serotonin. Nature. 1987;328:73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- CREPEL V., KHAZIPOV R., BEN ARI Y. Blocking GABA(A) inhibition reveals AMPA- and NMDA-receptor-mediated polysynaptic responses in the CA1 region of the rat hippocampus. J. Neurophysiol. 1997;77:2071–2082. doi: 10.1152/jn.1997.77.4.2071. [DOI] [PubMed] [Google Scholar]
- DAVIES C.H., POZZA M.F., COLLINGRIDGE G.L. CGP 55845A: a potent antagonist of GABAB receptors in the CA1 region of rat hippocampus. Neuropharmacology. 1993;32:1071–1073. doi: 10.1016/0028-3908(93)90073-c. [DOI] [PubMed] [Google Scholar]
- DEUCHARS J., THOMSON A.M. CA1 pyramid-pyramid connections in rat hippocampus in vitro: dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- FREUND T.F., BUZSAKI G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- GADDUM J.H., HAMEED K.A., HATHWAY D.E., STEPHENS F.F. Quantitative studies of antagonists for 5-hydroxytryptamine. Q. J. Exp. Physiol. 1955;40:49–74. doi: 10.1113/expphysiol.1955.sp001097. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- JOHNSON S.W., MERCURI N.B., NORTH R.A. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J. Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T. Pharmacologic analysis of drug-receptor interaction. Philadelphia: Lippencott-Raven; 1997. [Google Scholar]
- KLISHIN A., TSINTSADZE T., LOZOVAYA N., KRISHTAL O. Latent N-methyl-D-aspartate receptors in the recurrent excitatory pathway between hippocampal CA1 pyramidal neurons: Ca(2+)-dependent activation by blocking A1 adenosine receptors. Proc. Natl. Acad. Sci. U.S.A. 1995;92:12431–12435. doi: 10.1073/pnas.92.26.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOWLES W.D., SCHWARTZKROIN P.A. Axonal ramifications of hippocampal Ca1 pyramidal cells. J. Neurosci. 1981;1:1236–1241. doi: 10.1523/JNEUROSCI.01-11-01236.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMOTHE M., PAUWELS P.J., BELLIARD K., SCHAMBEL P., HALAZY S. Differentiation between partial agonists and neutral 5-HT1B antagonists by chemical modulation of 3-[3-(N,N-dimethylamino)propyl]-4-hydroxy-N-[4-(pyridin-4-yl)phenyl]benzamide (GR-55562) J. Med. Chem. 1997;40:3542–3550. doi: 10.1021/jm9702948. [DOI] [PubMed] [Google Scholar]
- LI Y.W., BAYLISS D.A. Presynaptic inhibition by 5-HT1B receptors of glutamatergic synaptic inputs onto serotonergic caudal raphe neurones in rat. J. Physiol. 1998;510:121–134. doi: 10.1111/j.1469-7793.1998.121bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACOR J.E., BURKHART C.A., HEYM J.H., IVES J.L., LEBEL L.A., NEWMAN M.E., NIELSEN J.A., RYAN K., SCHULZ D.W., TORGERSEN L.K., KOE B.K. 3-(1,2,5,6-Tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one: a potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1,2,5,6-tetrahydropyrid-4-yl)indole. J. Med. Chem. 1990;33:2087–2093. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- MAURA G., RAITERI M. Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur. J. Pharmacol. 1986;129:333–337. doi: 10.1016/0014-2999(86)90443-7. [DOI] [PubMed] [Google Scholar]
- MAURA G., ULIVI M., RAITERI M. (−)-Propanol and (+/−)-cyanopindolol are mixed agonists-antagonists at serotonin autoreceptors in the hippocampus of the rat brain. Neuropharmacology. 1987;26:713–717. doi: 10.1016/0028-3908(87)90232-2. [DOI] [PubMed] [Google Scholar]
- MLINAR B., PUGLIESE A., CORRADETTI R. Selective inhibition of local excitatory synaptic transmission by serotonin through an unconventional receptor in the CA1 region of rat hippocampus. J. Physiol. 2001;534:141–158. doi: 10.1111/j.1469-7793.2001.t01-2-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIKAWA H., MANZONI O.J., CRABBE J.C., WILLIAMS J.T. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol. Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- MURAMATSU M., LAPIZ M.D., TANAKA E., GRENHOFF J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur. J. Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- NEALE R.F., FALLON S.L., BOYAR W.C., WASLEY J.W., MARTIN L.L., STONE G.A., GLAESER B.S., SINTON C.M., WILLIAMS M. Biochemical and pharmacological characterization of CGS 12066B, a selective serotonin-1B agonist. Eur. J. Pharmacol. 1987;136:1–9. doi: 10.1016/0014-2999(87)90772-2. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., GOUBLE A., WURCH T. Activation of constitutive 5-hydroxytryptamine(1B) receptor by a series of mutations in the BBXXB motif: positioning of the third intracellular loop distal junction and its G(o)alpha protein interactions. Biochem. J. 1999;343 Pt 2:435–442. [PMC free article] [PubMed] [Google Scholar]
- RADPOUR S., THOMSON A.M. Coactivation of local circuit NMDA receptor mediated epsps induces lasting enhancement of minimal schaffer collateral epsps in slices of rat hippocampus. Eur. J. Neurosci. 1991;3:602–613. doi: 10.1111/j.1460-9568.1991.tb00846.x. [DOI] [PubMed] [Google Scholar]
- ROPERT N., GUY N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J. Physiol. 1991;441:121–136. doi: 10.1113/jphysiol.1991.sp018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARHAN H., FILLION G. Differential sensitivity of 5-HT1B auto and heteroreceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360:382–390. doi: 10.1007/s002109900067. [DOI] [PubMed] [Google Scholar]
- SCHOEFFTER P., HOYER D. Interaction of arylpiperazines with 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors: do discriminatory 5-HT1B receptor ligands exist. Naunyn Schmiedebergs Arch. Pharmacol. 1989;339:675–683. doi: 10.1007/BF00168661. [DOI] [PubMed] [Google Scholar]
- SEGAL M. Physiological and pharmacological evidence for a serotonergic projection to the hippocampus. Brain Res. 1975;94:115–131. doi: 10.1016/0006-8993(75)90881-1. [DOI] [PubMed] [Google Scholar]
- SEGAL M. Serotonin attenuates a slow inhibitory postsynaptic potential in rat hippocampal neurons. Neuroscience. 1990;36:631–641. doi: 10.1016/0306-4522(90)90006-p. [DOI] [PubMed] [Google Scholar]
- SHEN R.Y., ANDRADE R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J. Pharmacol. Exp. Ther. 1998;285:805–812. [PubMed] [Google Scholar]
- STUART G.J., DODT H.U., SAKMANN B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- THOMSON A.M., RADPOUR S. Excitatory connections between CA1 pyramidal cells revealed by spike triggered averaging in slices of rat hippocampus are partially NMDA receptor mediated. Eur. J. Neurosci. 1991;3:587–601. doi: 10.1111/j.1460-9568.1991.tb00845.x. [DOI] [PubMed] [Google Scholar]
- WAEBER C., MOSKOWITZ M.A. Autoradiographic visualisation of [3H]5-carboxamidotryptamine binding sites in the guinea pig and rat brain. Eur. J. Pharmacol. 1995;283:31–46. doi: 10.1016/0014-2999(95)00275-p. [DOI] [PubMed] [Google Scholar]
- WALSH D.M., BEATTIE D.T., CONNOR H.E. The activity of 5-HT1D receptor ligands at cloned human 5-HT1D alpha and 5-HT1D beta receptors. Eur. J. Pharmacol. 1995;287:79–84. doi: 10.1016/0014-2999(95)00612-1. [DOI] [PubMed] [Google Scholar]
- XIE Z., LEE S.P., O'DOWD B.F., GEORGE S.R. Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett. 1999;456:63–67. doi: 10.1016/s0014-5793(99)00918-7. [DOI] [PubMed] [Google Scholar]


