Abstract
We have compared the signalling mechanisms involved in the pertussis toxin-sensitive and -insensitive contraction of rat isolated mesenteric microvessels elicited by sphingosylphosphorylcholine (SPC) and noradrenaline (NA), respectively.
The phospholipase D inhibitor butan-1-ol (0.3%), the store-operated Ca2+ channel inhibitor SK>F 96,365 (10 μM), the tyrosine kinase inhibitor genistein (10 μM), and the src inhibitor PP2 (10 μM) as well as the negative controls (0.3% butan-2-ol and 10 μM diadzein and PP3) had only little effect against either agonist.
Inhibitors of phosphatidylinositol-3-kinase (wortmannin and LY 294,002, 10 μM each) or of mitogen-activated protein kinase kinase (PD 98,059 and U 126, 10 μM each) did not consistently attenuate NA- and SPC-induced contraction as compared to their vehicles or negative controls (LY 303,511 or U 124).
The phospholipase C inhibitor U 73,122 (10 μM) markedly inhibited the SPC- and NA-induced contraction (70% and 88% inhibition of the response to the highest NA and SPC concentration, respectively), whereas its negative control U 73,343 (10 μM) caused only less than 30% inhibition.
The rho-kinase inhibitors Y 27,632 (10 μM) and fasudil (30 μM) caused a rightward-shift of the NA concentration-response curve by 0.7–0.8 log units and reduced the response to 10 μM SPC by 88% and 83%, respectively.
These data suggest that SPC and NA, while acting on different receptors coupling to different G-protein classes, elicit contraction of rat mesenteric microvessels by similar signalling pathways including phospholipase C and rho-kinase.
Keywords: Sphingosylphosphorylcholine, α1A-adrenoceptor, vasoconstriction, phospholipase C, rho-kinase
Introduction
Multiple cellular mechanisms have been implicated in receptor-mediated smooth muscle contraction but all of them ultimately converge on increased phosphorylation of myosin light chain (Allen & Walsh, 1994; Somlyo & Somlyo, 1994). Classically this has been attributed to activation of a phospholipase (PL) C leading to mobilization of Ca2+ from intracellular stores and/or to activation of voltage-operated Ca2+ channels. In recent years a variety of additional signalling pathways has been implicated in receptor-mediated contraction of vascular and other smooth muscle, including activation of a PLD (Aburto et al., 1995; Jinsi et al., 1996), of store-operated Ca2+ channels (Low et al., 1994; Lagaud et al., 1996), of protein kinase C (Aburto et al., 1995; Dessy et al., 1998), of various types of tyrosine kinases (Jinsi et al., 1996; di salvo et al., 1997) including the src family (Roberts, 2001), of phosphatidylinositol-3-kinases (PI-3-kinases) (Ibitayo et al., 1998), of extracellular signal-regulated kinase (ERK) forms of the mitogen-activated protein kinases (MAPK) (Dessy et al., 1998; Fetscher et al., 2001) and of rho-associated protein kinases (Fukata et al., 2001; Mukai et al., 2001).
Lysosphingolipids are a newly emerging class of biological mediators which can serve as intracellular as well as intercellular mediators, with the latter effects often being mediated by G-protein-coupled receptors (Spiegel & Milstien, 2000; Pyne & Pyne, 2000; Chun et al., 2002). Lysosphingolipids, including sphingosine 1-phosphate, sphingosylphosphorylcholine (SPC), sphingosine and glucopsychosine, cause vasoconstriction in vitro (Sugiyama et al., 2000; Bischoff et al., 2000a; 2001a; Shirao et al., 2002) and in some cases also in vivo (Bischoff et al., 2000b; 2001a, 2001b). SPC was the most potent and effective vasoconstrictor lysosphingolipid in rat isolated mesenteric microvessels (Bischoff et al., 2000a). Stereo-selectivity and pertussis toxin (PTX)-sensitivity suggested that this response is receptor-mediated. This SPC-induced vasoconstriction was abolished by chelation of extracellular Ca2+ and partly inhibited by a dihydropyridine-type Ca2+ entry blocker. SPC-induced vasoconstriction was endothelium-independent but slightly attenuated by a cyclo-oxygenase inhibitor; the latter finding, however, was not confirmed in a more recent study (unpublished observations). Taken together, these data suggest that SPC-induced rat mesenteric microvessel contraction is mediated by a receptor located on the vascular smooth muscle cells with couples to a PTX-sensitive G-protein to promote the influx of extracellular Ca2+, partly via L-type Ca2+ channels. The detailed signal transduction pathways underlying SPC-induced vasoconstriction, however, remain unclear.
In rat mesenteric microvessels, vasoconstriction can also be elicited by noradrenaline (NA) acting on α1A-adrenoceptors via PTX-insensitive G-proteins; this also involves the influx of extracellular Ca2+ and possibly also tyrosine kinases and ERK forms of the MAPK (Chen et al., 1996; Bischoff et al., 2000a; Fetscher et al., 2001). Therefore, the present study was performed to characterize potential pathways in the SPC-induced contraction of rat mesenteric microvessels, and specifically to determine whether the PTX-sensitive SPC response and the PTX-insensitive NA response use distinct signal transduction pathways. Our data for the first time characterize in detail the signal transduction underlying vasoconstriction by SPC, which represents a novel class of endogenous vasoactive agents. Moreover, these data shed light on the question how extracellular signals acting via distinct G-protein classes converge on a single down-stream effector mechanism, i.e. vascular smooth muscle contraction.
Methods
Contraction experiments
Adult Wistar rats (males 300–450 g, females 200–350 g) were obtained from the breeding facility at the University of Essen. They were killed by decapitation under ether anaesthesia and mesenteric microvessels were prepared from these rats according to Mulvany and Halpern as previously described (Chen et al., 1996). Briefly, mesenteric vessels adjacent to the gut were isolated. While vessel size had not consistently been protocoled for the present study, in a separate series of vessels prepared identically vessel diameter was 256±4 μm (n=99). Up to four vessels from each animal were used, but only 1–2 vessel per animal were tested for each experimental condition. A stainless-steel wire was inserted into the vessel lumen, and the vessel was mounted in the myograph chamber. A second wire was inserted and connected to a force transducer for isometric recording of tension development. In the myograph, the vessels were bathed in Krebs-Henseleit buffer of the following composition (mM): NaCl 119, NaHCO3 25, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, CaCl2 2.5, EDTA 0.026, HEPES 10, glucose 5.5 at 37°C. The chamber was gassed continuously with 5% CO2/95% O2 to maintain pH at 7.4. Before generating NA concentration-response curves, 1 μM propranolol and 5 μM cocaine were added to the buffer to block β-adrenoceptors and neuronal catecholamine uptake, respectively. Each preparation was used to generate three concentration-response curves (with intermittent wash-outs): The first curve was generated with NA and was used as reference for the subsequent curves. The second curve was also generated with NA but in the presence of various inhibitors, their negative controls or their vehicles. The third curve was generated with SPC in the presence of inhibitors, their negative controls or their vehicles. Whenever possible, an inhibitor and its negative control or vehicle were tested in parallel with vessels from the same animal. Force of contraction of the second and third curve were expressed as per cent of the maximum NA response in the first curve.
In light of possible non-specific effects of some inhibitors of signal transduction pathways (Davies et al., 2000), we have used compounds with high specificity within their class. The inhibitor concentrations were chosen based on their reported effectiveness (or ineffective in case of negative controls) in other smooth muscle preparations as listed below. PLC: U 73,122 and U 73,344 10 μM (Lang et al., 2002); PLD: butan-1-ol and butan-2-ol 0.3% (Hinton et al., 1999); store-operated Ca2+ channels: SK>F 96,365 10 μM (Low et al., 1994); tyrosine kinases: genistein and daidzein 10 μM (Roberts, 2001); src-like protein kinases: PP2 and PP3 10 μM (Roberts, 2001); PI-3-kinase: wortmannin, LY 294,002 and LY 303,511 10 μM (Hong & Chang, 1998); ERK-type MAPK: PD 98,095, U 126 and U 124 10 μM (Roberts, 2001); rho-kinase: Y27,632 10 μM and fasudil 30 μM (Batchelor et al., 2001).
Radioligand binding
Competition radioligand binding to a membrane preparation from Chinese hamster ovary cells expressing the cloned human α1A-adrenoceptor was performed as previously described using a [3H]-prazosin concentration of 110–140 pM (Eltze et al., 2001). Briefly, experiments were performed in binding buffer consisting of 50 mM Tris, 10 mM MgCl2 and 0.5 mM EDTA at pH 7.5 in a total assay volume of 1000 μl. The protein content typically was 40–60 μg assay−1. The mixtures were incubated at 25°C for 45 min unless otherwise indicated. Incubations were terminated by rapid vacuum filtration over Whatman GF/C filters followed by two washes of the filters each with 10 ml ice-cold incubation buffer. Non-specific binding was defined as binding in the presence of 10 μM phentolamine. Each inhibitor was tested in quadruplicates within each experiment.
Chemicals
Carbachol HCl, (−)-NA bitartrate, PD 98,059 (2′-amino-3′-methoxyflavone), (±)-propranolol HCl, SK&F 96,365 (1-[β-[3-(4-methoxyphenyl)propoxy]- 4] methoxyhphenethyl] -1H -imidazole HCl), U 73,122, (1-(6-[([17β]-3-methoxyestra-1,3,5[10]-trien-17-yl)-amino]hexyl)-1H-pyrrole-2,5-dione), U 73,343 (1-(6-[-([17β]-3-methoxyestra-1,3,5[10]-trien-17-yl)-amino] hexyl-2,5-pyrrolidinedione) and wortmannin were obtained from Sigma-Aldrich (Taufkirchen, Germany). SPC was from Biomol (Hamburg, Germany). Fasudil dihydrochloride (previously known as HA-1077), LY 294,002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one), LY 303,511 (2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one), PP2 (4-amino-5-)4-chlorophenyl)-7-(t-butyl)pyrazolol[3,4]pyrimidine), PP3 (4-amino-7-phenylpyrazolol[3,4]pyrimidine, U 124 (1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene) and U 126 (1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene) were obtained from Calbiochem (Bad Soden, Germany). Y 27,632 (trans - 4 - [(1R) - 1 - aminoethyl]-N- 4-pyridinylcyclohexanecarboxamide) was from Tocris (Bristol, U.K.).
SPC was dissolved in methanol, dried in a speed-vac concentrator and, thereafter, dissolved at 10 mM in a solution of bovine serum albumin (1 mg ml−1). Daidzein (at 10 mM), genistein (at 10 mM), PP2 (at 10 mM), PP3 (at 10 mM), U 73,122 (at 3 mM) and U 73,343 (at 3 mM) were dissolved in DMSO. Y 27,632 and fasudil (at 10 mM each) were dissolved in water.
Data analysis
Data are shown as means±s.e.mean of n vessels. Unless otherwise noted, all data on force of contraction are expressed as per cent of the maximum response to NA in the first concentration-response curve, i.e. prior to addition of any inhibitor or its vehicle. This allows the variability of inhibitor effects to be looked at without confusion by the biological variability in the agonist responses. It has been used in our previous studies (Bischoff et al., 2000a; 2001a) and is supported by an excellent reproducibility of NA responses in this preparation (Chen et al., 1996), as further documented by the correlation analysis in the present study (see Results).
To test for statistical significance of inhibitor effects, our primary analysis was two-way ANOVA of the entire concentration-response curves, using main treatment effect and agonist concentration as the explanatory variables; Bonferroni post-tests were performed for individual agonist concentrations. As a secondary outcome parameter for inhibitor effects, agonist potency and maximum effects were analysed by fitting sigmoidal curves to pooled data from all experiments; in this analysis statistical significance was assumed if 95% confidence intervals of parameter estimates for pEC50 or Emax in the presence of the inhibitor did not overlap with those in the presence of its negative control and/or vehicle. Statistical significance of inhibitor effects in the radioligand binding assay was determined by one-sample t-tests. All curve fitting procedures and statistical calculations were performed using the Prism program, and P<0.05 was considered significant (GraphPad, San Diego, CA, U.S.A.).
Results
The first NA concentration-response curve in the mesenteric microvessels had a mean pEC50 of 6.88±0.13 and a mean maximum effect of 19.8±0.4 mN (n=238 vessels), with similar responses in male (pEC50 6.84±0.23, maximum 21.9±0.4 mN, n=90 vessels) and female rats (pEC50 6.91±0.15, maximum 18.6±0.5 mN, n=148 vessels). To further validate the expression of contractile effects of NA and SPC in the presence of inhibitors, their negative controls and/or their vehicles as per cent of the maximum response in the first NA concentration-response curve within the same vessel, we have compared the maximum response of the second NA curve and of the SPC curve in the pooled vehicle groups with the maximum response in the first NA curve (Figure 1). While the response to the first and second NA addition exhibited an excellent correlation, the correlation between NA and SPC effects was statistically significant but considerably weaker. In confirmation of our previous studies (Bischoff et al., 2000a), SPC-induced contraction of rat mesenteric microvessels consistently peaked at an agonist concentration of 30 μM and yielded approximately 50% smaller maximal effects than NA (Figures 2,3,4,5,6,7,8).
Figure 1.
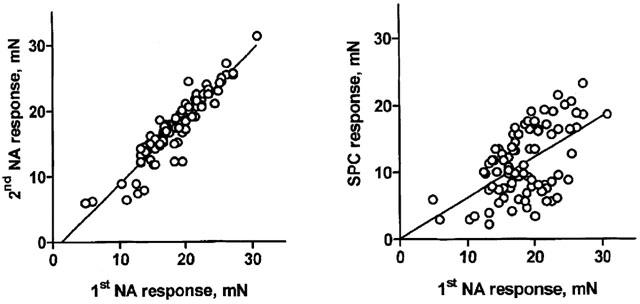
Maximum contraction response to NA and SPC relative to the first NA effect within the same preparation (n=83 vessels each). The linear regression slopes were 1.02±0.04 and 0.61±0.10, respectively, and the r2 was 0.87 and 0.31, respectively, yielding P-values of <0.0001 in both cases.
Figure 2.
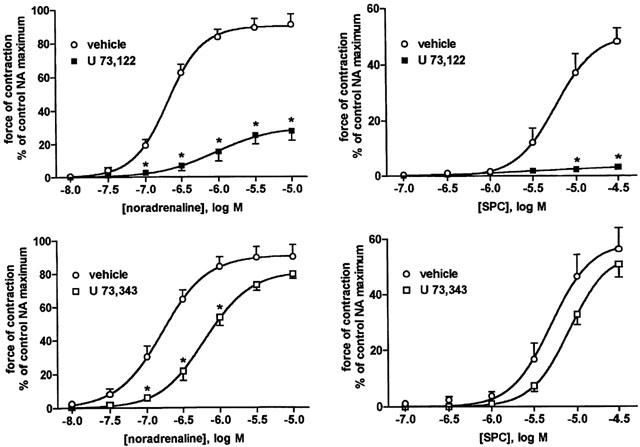
Effect of the phospholipase C inhibitor U 73,122 and its negative control U 73,343 (10 μM) each on NA- and SPC-induced vasoconstriction. Data are means±s.e.mean of eight experiments. P=0.0151 for overall treatment effect of U 73,343 against SPC in a two-way ANOVA, and P<0.0001 for all other overall treatment effects; *P<0.05 for individual agonist concentrations vs vehicle in Bonferroni post-tests.
Figure 3.
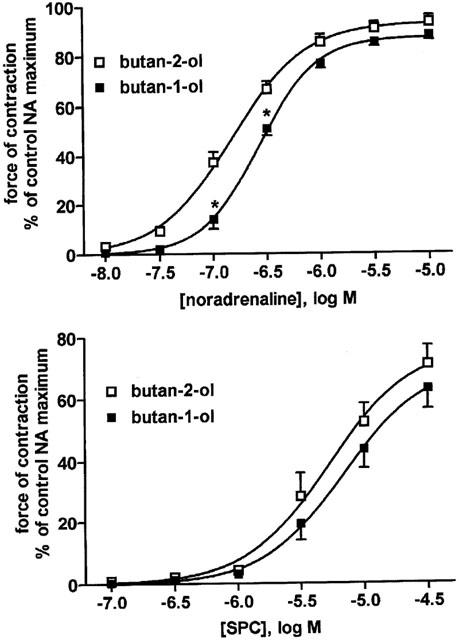
Effects of the phospholipase D inhibitor butan-1-ol and its negative control butan-2-ol (0.3% each) on NA- and SPC-induced vasoconstriction. Data are means±s.e.mean of six experiments. P<0.0001 for overall treatment effects of butan 1-ol-vs butan-2-ol against NA but not SPC effects in a two-way ANOVA; *P<0.05 for individual agonist concentrations vs butan-2-ol in Bonferroni post-tests.
Figure 4.
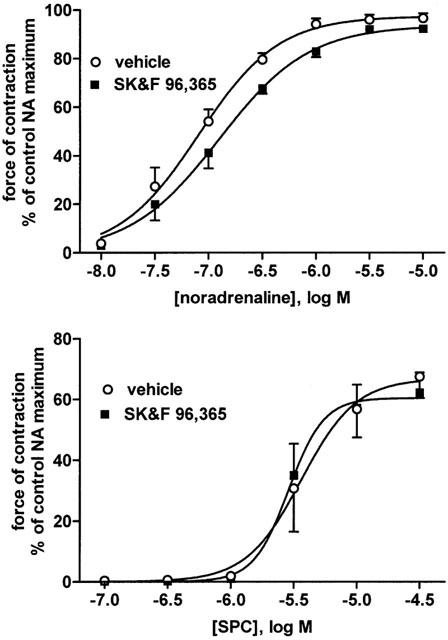
Effects of the SK&F 96,365 (10 μM), an inhibitor of store-operated Ca2+ channels, on NA- and SPC-induced vasoconstriction. Data are means±s.e.mean of six experiments. P=0.0006 for overall treatment effect of SK&F 96,365 against NA but not SPC effects in a two-way ANOVA.
Figure 5.
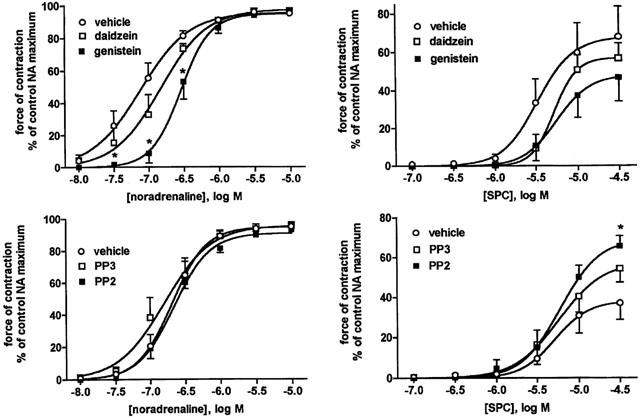
Effects of the tyrosine kinase inhibitor genistein and the src kinase inhibitor PP2 and their negative controls daidzein and PP3, respectively (10 μM each), on NA- and SPC-induced vasoconstriction. Data are means±s.e.mean of 5–6 experiments. The following P-values for overall treatment effect were obtained for NA (P<0.0001 genistein vs vehicle, P=0.0017 genistein vs daidzein) and SPC (P=0.0248 genistein vs vehicle, P=0.0030 PP2 vs vehicle) in a two-way ANOVA, *P<0.05 for individual agonist concentrations vs vehicle in Bonferroni post-tests.
Figure 6.
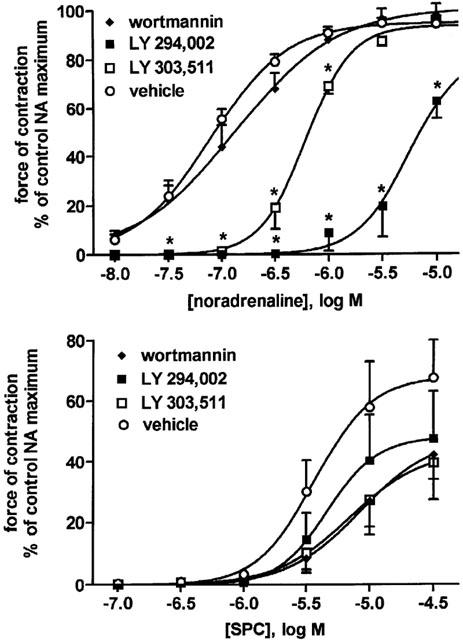
Effects of phosphatidylinositol-3-kinase inhibitors wortmannin and LY 294,002 and the negative control LY 303,511 (10 μM) each on NA- and SPC-induced vasoconstriction. Data are means±s.e.mean of 5–7 experiments. The following P-values for overall treatment effect were obtained for NA (P<0.0001 for LY 294,002 and LY 303,511 vs vehicle and LY 294,002 vs LY 303,511) and SPC (P=0.0018 for wortmannin vs vehicle and P=0.0060 for LY 303,511 vs vehicle) in a two-way ANOVA; *P<0.05 for individual agonist concentrations in the presence of LY 294,002 vs LY 303,511 and presence of LY 303,511 vehicle in Bonferroni post-tests.
Figure 7.
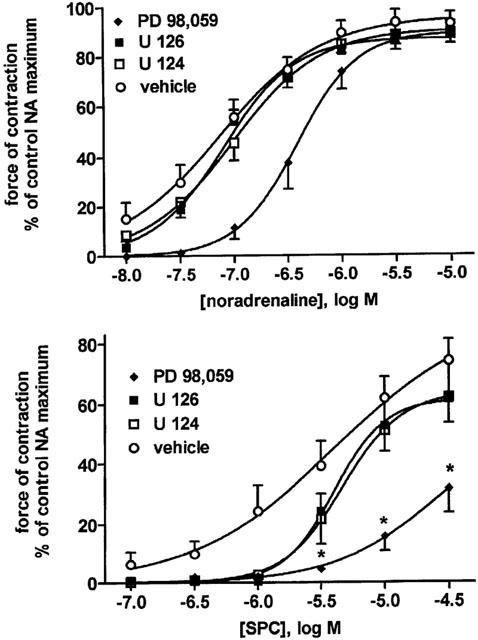
Effects of the ERK-type mitogen-activated protein kinase inhibitor PD 98,059, U 126 and its negative control U 124 (10 μM each) and their vehicle on NA- and SPC-induced vasoconstriction. Data are means±s.e.mean of 6–11 experiments. In a two-way ANOVA the overall treatment effects were significant for inhibition of NA and SPC by PD 98,059 vs vehicle (P<0.0001) and for inhibition of SPC by U 126 vs vehicle (P=0.0042; *P<0.05 for individual agonist concentrations vs vehicle in Bonferroni post-tests.
Figure 8.
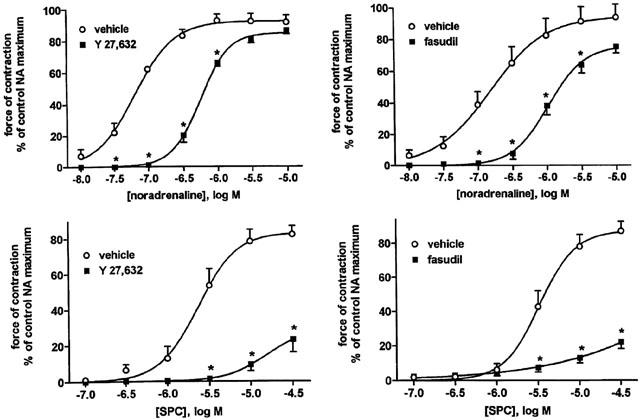
Effects of the rho-kinase inhibitors Y 27,632 (10 μM) and fasudil (30 μM) and their vehicles on NA- and SPC-induced vasoconstriction. Data are means±s.e.mean of 6–8 experiments. P<0.0001 for overall treatment effect of both Y 27,632 and fasudil vs vehicle in a two-way ANOVA; *P<0.05 for individual agonist concentrations vs vehicle in Bonferroni post-tests.
The PLC inhibitor U 73,122 significantly inhibited NA-induced constriction, i.e. reduced maximum NA responses by more than 60% and concomitantly reduced the sensitivity of the remaining response by more than 0.6 log units (Table 1, Figure 2). U 73,122 almost completely inhibited SPC-induced constriction (Table 2, Figure 2). The supposed negative control U 73,343 inhibited NA and SPC responses significantly but to a much weaker extent (Tables 1 and 2, Figure 2).
Table 1.
Potency (pEC50) and maximum effects (Emax) of NA in the presence of signal transduction inhibitors
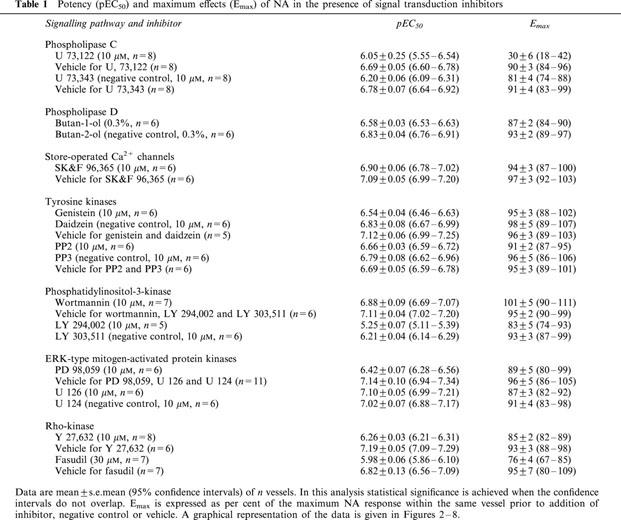
Table 2.
Potency (pEC50) and maximum effects (Emax) of NA in the presence of signal transduciton inhibitors
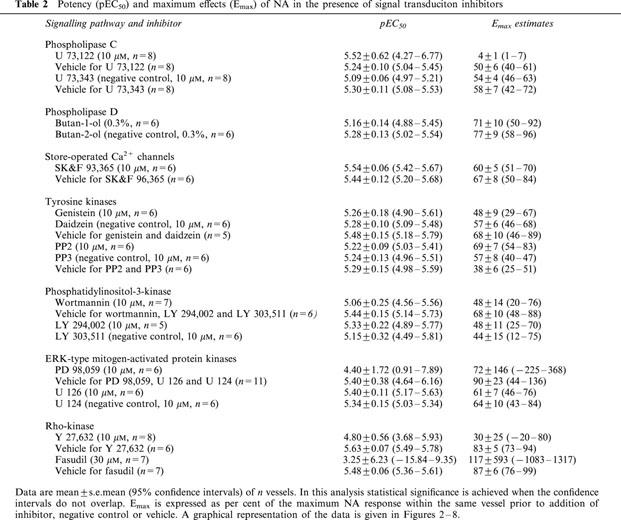
A possible involvement of a PLD was tested by comparing vasoconstriction in the presence of the inhibitor butan-1-ol relative to its negative control butan-2-ol. Butan-1-ol caused a small but statistically significant inhibition of NA but not the SPC responses (Tables 1 and 2, Figure 3).
SK>F 96,365, an inhibitor of store-operated Ca2+ channels, relative to its vehicle caused a very small but statistically significant inhibition of the response to NA but not to SPC (Tables 1 and 2, Figure 4).
A possible role for tyrosine kinases was initially tested using the inhibitor genistein relative to its negative control daidzein and their vehicle. Relative to vehicle, genistein caused significant inhibition of the NA response, which mainly consisted of a rightward shift of the concentration-response curve; relative to the negative control daidzein, the inhibitory effect of genistein was less pronounced but remained statistically significant (Table 1, Figure 5). Inhibitory effects of genistein on SPC-induced microvessel contraction were small and reached statistical significance only relative to vehicle but not relative to daidzein (Table 2, Figure 5). To test specifically for a role of src-like tyrosine kinases, we have investigated possible inhibitory effects of PP2. However, PP2 neither relative to its negative control PP3 nor relative to their vehicle significantly affected the NA concentration-response curve (Table 1, Figure 5). SPC-induced contraction was not at all inhibited by PP2 and if anything it was slightly enhanced relative to vehicle but not relative to PP3 (Table 2, Figure 5).
Studies with PI-3-kinase inhibitors yielded equivocal results. Thus, wortmannin relative to its vehicle did not significantly affect the NA response (Table 1, Figure 6). The negative control LY 303,511 alone also caused significant inhibition of the NA response relative to vehicle (Table 1, Figure 6). The PI-3-kinase inhibitor LY 294,002 relative to LY 303,511 caused further marked inhibiton of the NA response, and this inhibition consisted largely of a right shift of the NA concentration-response curve by about 1 log unit (Table 1, Figure 6). SPC-induced microvessel contraction was not significantly inhibited by LY 294,002 relative to LY 303,511 or vehicle (Table 2, Figure 6), but wortmannin and the supposed negative control LY 303,511 caused a very minor but statistically significant inhibition of the SPC effect relative to vehicle (Table 2, Figure 6).
Studies regarding a possible role of ERK-type MAPK also yielded inconclusive results. Thus, PD 98,095 caused marked inhibition which mainly consisted of a right shift of the NA concentration-response curve by approximately 0.7 log units (Table 1, Figure 7). In contrast, U 126 did not cause significant inhibition of the NA response relative to its negative control U 124 or to vehicle (Table 1, Figure 7). PD 98,059 also significantly inhibited the SPC response, whereas U 126 caused only very minor if any inhibition (Table 2, Figure 7).
The rho-associated protein kinase inhibitors Y 27,632 and fasudil caused marked inhibition of the NA response, which consisted mainly of a right shift of about 0.7–0.8 log units (Table 1, Figure 8). Both Y 27,632 and fasudil also strongly inhibited SPC-induced microvessel contraction (Table 2, Figure 8).
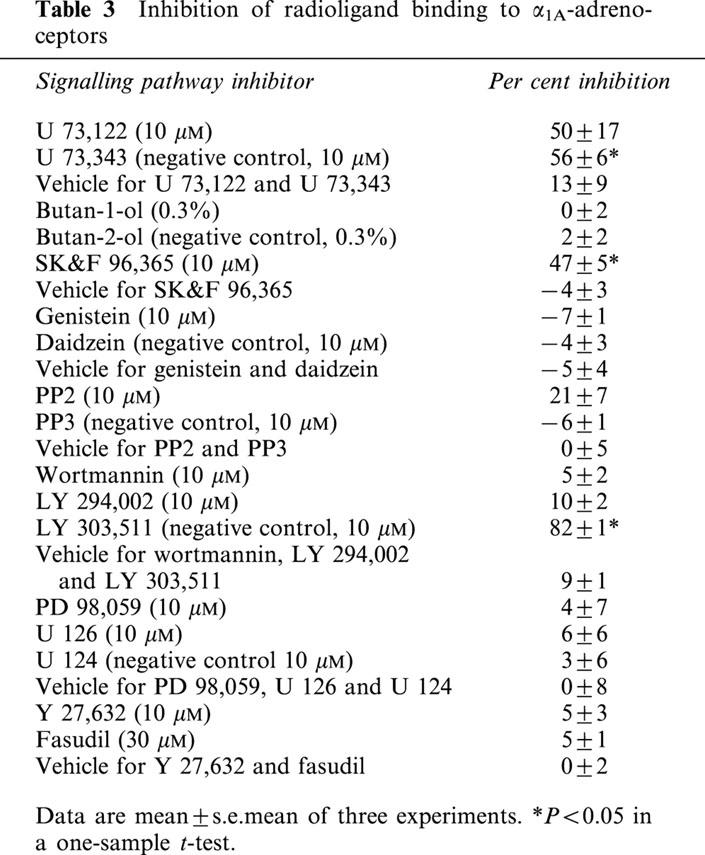
To exclude that some of the observed inhibitory effects are due to direct interaction of the signal transduction inhibitors with the receptors mediating the response, we have performed radioligand binding experiments with cloned human α1A-adrenoceptors. These experiments demonstrated that in the concentrations employed in the present study, butan-1-ol, butan-2-ol, genistein, daidzein, PP3, wortmannin, LY 294,002, PD 98,059, U 126, U 124, Y 27,632 or fasudil did not inhibit radioligand binding (Table 3). A small inhibitory effect was observed for PP2, and an approximately 50% inhibition was seen with U 73, 122, U 73,344 and SK&F 96,365; the inhibitory effect of LY 303,511 was approximately 80% (Table 3).
Table 3.
Inhibition of radioligand binding to α1A-adrenoceptors
Discussion
Recent years have brought considerable progress in the understanding of the cellular and molecular mechanisms leading to the contraction of vascular and other smooth muscle by receptor agonists. Smooth muscle contraction can be elicited by receptors coupling to both Gq/11-like PTX-insensitive and Gi/o-like PTX-sensitive G-proteins, although both G-protein classes typically activate different signalling responses (Neer, 1995). In rat mesenteric microvessels the former class is represented by NA and the latter by SPC (Bischoff et al., 2000a). In the present study we have observed that SPC-induced microvessel contraction is correlated significantly to that upon a first NA addition, but the correlation is considerably weaker than that between the effects of the first and second NA addition. These data imply that the tissue responsiveness to the two agonists involves distinct and shared components. Obviously, the receptors and G-proteins mediating the two responses are distinct, but whether and to what extent down-stream signalling pathways are distinct is unknown. Therefore, the present study was designed to investigate for the first time the signal transduction pathways underlying the SPC-induced PTX-sensitive contraction of rat mesenteric microvessels and to compare it to the NA-induced PTX-insensitive contraction. Our overall data suggest that NA and SPC use largely similar signalling mechanisms to elicit vasoconstriction despite acting via different types of G-proteins.
The validity of studies into signal transduction pathways underlying functional responses is limited by the efficacy and selectivity of the available inhibitors. Recent work has highlighted the problem, that many supposedly specific inhibitors may be less selective and/or less effective than previously believed (Davies et al., 2000). To minimize this problem, the present study has employed multiple and structurally distinct inhibitors of a given pathway in many cases. Whenever possible, we have in addition compared the active inhibitors not only to their vehicle but also to negative control compounds, i.e. structurally similar drugs which have much less, if any, effect on the signal transduction pathway under investigation. Inhibitor concentrations, which combine efficacy and selectivity to the greatest possible extent, were chosen based on published data in other tissues (see Methods). To investigate possible direct inhibitor effects at the receptor level, radioligand binding studies have been performed with cloned α1A-adrenoceptors, i.e. the receptor subtype which mediates noradrenaline-induced contraction in rat mesenteric microvessels (Williams & Clarke, 1995; Chen et al., 1997). According to these radioligand binding data, LY 303,511 (the negative control for the PI-3-kinase inhibitor LY 294,002) and the PLC inhibitor U 73,122, its negative control U 73,344 and SK&F 96,365, an inhibitor of store-operated Ca2+ channels, have direct effects on α1A-adrenoceptors whereas the other tested inhibitors inhibit receptor binding by 10% of less. These receptor effects of some inhibitors must be considered in interpreting their functional effects. While lack of effect of a given inhibitor in appropriate concentrations strongly argues against involvement of the corresponding pathway, its effectiveness will not prove involvement of that pathway in all cases. This can be partly be overcome by testing multiple inhibitor concentrations, but the use of full concentration-response curves for the inhibitors was not feasible in the present study because only one SPC concentration-response curve can be obtained per preparation. These limitations must be kept in mind in the interpretation of our data.
Smooth muscle contraction depends on elevations of intracellular Ca2+ concentrations. Elevation of intracellular Ca2+ upon stimulation of Gq/11-coupled receptors in general and of α1A-adrenoceptors in particular typically involves PLC activation (Garcia-Sainz et al., 1999), whereas that by Gi/o-coupled receptors in general and by SPC in particular in most cases does not (Meyer zu Heringdorf et al., 2002).
PLC involvement in physiological responses is most frequently tested using its inhibitor, U 73,122. In the present study this PLC inhibitor blunted vasoconstriction by both, NA and SPC, whereas its negative control U 73,343 only slightly inhibited microvessel contraction. Direct α1A-adrenoceptor antagonism may contribute to the inhibition of NA response by both U 73,122 and U 73,343, but the inhibition of vasoconstriction by U 73,122 was considerably greater than by U 73,343 despite similar inhibition by both in the binding experiments, which indicates that inhibition by U 73,122 involves more than direct α1A-adrenoceptor antagonism. However, U 73,122 has various PLC-independent effects, some of which can be shared by U 73,343 (Muto et al., 1997; Mogami et al., 1997; Hughes et al., 2000). Nevertheless, our data indicate the involvement of PLC in SPC-induced microvessel contraction since U 73,122 inhibited the SPC-response at least as much as that to the bona fide PLC-activator NA.
α1A-Adrenoceptors can activate PLD (Taguchi et al., 1998), and at least in some preparations PLD activation may contribute to vasoconstriction (Aburto et al., 1995). However, in the present study the PLD inhibitor butan-1-ol relative to its negative control butan-2-ol had only little if any effect against NA or SPC suggesting that PLD does not play a major role in NA-or SPC-induced vasoconstriction.
The contraction of rat mesenteric microvessels by both NA and SPC requires the presence of extracellular Ca2+ and is partly inhibited by dihydropyridine-type Ca2+ entry blockers (Chen et al., 1996; Bischoff et al., 2000a; Fetscher et al., 2001). In some blood vessels, store-operated Ca2+ channels can contribute to α-adrenoceptor-mediated vasoconstriction (Low et al., 1994). However, SK&F 96,365, an inhibitor of these channels (McFadzean & Gibson, 2002), did not affect NA- or SPC-induced vasoconstriction in the present study despite its binding to α1A-adrenoceptors, suggesting the lack of involvement of store-operated Ca2+ channels.
Further experiments were designed to elucidate the protein kinases which may contribute in the pathways leading to vascular smooth muscle contraction. Whether protein kinase C activation can contribute to smooth muscle contraction is the matter of a lively debate. While this controversy may partly relate to biological heterogeneity, it should be considered that none of the available protein kinase C inhibitors is truly specific (Davies et al., 2000). According to our previous data, protein kinase C does not play a major role in the contraction of rat mesenteric microvessels since inhibitors such as bisindolymaleimide I (up to 1 μM) caused only little if any inhibition of the NA response and also since even strong stimulation of protein kinase C by a phorbol ester failed to cause vasoconstriction (Fetscher et al., 2001).
In recent years tyrosine kinases have emerged as potential mediators of receptor-induced smooth muscle contraction (Hollenberg, 1994; di salvo et al., 1997), and have been proposed to link receptor activation to elevation of intracellular Ca2+ (di salvo et al., 1997). Our previous studies had shown that two tyrosine kinase inhibitors, genistein and tyrphostin 23, relative to vehicle concentration dependently inhibited NA-induced contraction of rat mesenteric microvessels (Fetscher et al., 2001). While the present data confirm these observations, they also show that at least a part of this inhibition is shared by the negative control daidzein leaving only a small ‘specific' inhibition by genistein. Relative to daidzein, genistein did not cause significant inhibition of the SPC response. Tyrosine kinases of the src type have been implicated in α2-adrenoceptor-mediated vasoconstriction of the porcine palmar lateral vein (Roberts, 2001) and in ceramide-induced contraction of colon smooth muscle (Ibitayo et al., 1998), but the src inhibitor PP2, relative to its negative control PP3, did not inhibit NA- or SPC-induced vasoconstriction in the present study. Taken together these data indicate that tyrosine kinases in general and those of the src type in particular contribute only little to NA- and SPC-induced contraction of rat mesenteric microvessels.
PI-3-kinases have also been implicated as mediators of receptor-induced smooth muscle contraction (Takayama et al., 1996; Ibitayo et al., 1998). In the present study wortmannin caused little if any inhibition of NA- or SPC-induced microvessel contraction, whereas LY 294,002 relative to vehicle and, to a smaller extent, relative to its negative control LY 303,511 inhibited NA- but not SPC-induced contraction. The inhibitory effect of LY 303,511 could be explained by its strong inhibition of radioligand binding to α1A-adrenoceptors, but it should be noted that neither LY 294,002 nor wortmannin significantly inhibit receptor binding. Since the high wortmannin concentration of the present study undoubtedly causes effective PI-3-kinase inhibition (Davies et al., 2000), it must be considered that the inhibitory effects of LY 294,002 despite being only partially shared by LY 303,511 may not relate to PI-3-kinase inhibition (Davies et al., 2000). Therefore, the present data do not provide conclusive evidence regarding a role for PI-3-kinase in NA- or SPC-induced vasoconstriction.
Previous studies with α1A-adrenoceptors in rat mesenteric microvessels (Fetscher et al., 2001) and in ferret aorta (Dessy et al., 1998) have demonstrated that PD 98,059, an inhibitor of ERK activation, can attenuate α1-adrenoceptor-mediated vasoconstriction. These finding were confirmed in the present study and extended to SPC. Although PD 98,059 is considered to be a highly specific inhibitor of ERK activation (Davies et al., 2000), its effects were not shared by another inhibition of ERK activation, U 126. Thus, NA- and SPC-induced microvessel contraction in the presence of U 126 was similar as that in the presence of its inactive control U 124 or vehicle. While these data do not allow definitive conclusion regarding a role of MAPK of the ERK type in NA- or SPC-induced vasoconstriction, they certainly do not point towards differential signal transduction underlying the NA and SPC effects.
The rho/rho-kinase pathway has also emerged as an important mediator of smooth muscle contraction, and inhibitors of rho-kinase such as Y 27,632 and fasudil have been shown to attenuate smooth muscle contraction in many tissues (Fukata et al., 2001). This appears to involve both, Ca2+-dependent enhanced myosin light chain phosphorylation and Ca2+-independent attenuated myosin light chain dephosphorylation, the latter resulting in Ca2+-sensitization of the tissue. In the present study, both Y 27, 632 and fasudil markedly inhibited the NA- and SPC-induced microvessel contraction, suggesting a similar role of rho-kinase for both agonists. This is in line with previous findings from permeabilized porcine coronary artery smooth muscle cells, in which SPC caused contraction at least partly via Ca2+ sensitization and that was abolished by Y 27,632 (Todoroki-Ikeda et al., 2000). Moreover, in a very recent study SPC caused contraction of bovine middle cerebral artery in a similar concentration range as in the present study, and this response was also sensitive to Y 27,632 (Shirao et al., 2002). Taken together these data point to an important role of rho-kinase in NA- and SPC-induced vasoconstriction.
Taken together, NA and SPC induce contraction of rat mesenteric microvessels via different receptors signalling through PTX-insensitive and PTX-sensitive G-proteins, respectively (Bischoff et al., 2000a). This differentiation was also reflected by the finding that NA- and SPC-induced contractile responses were only loosely correlated. The present study has characterized the signalling pathways which are involved in SPC-induced vasoconstriction. Based on the present and previous data (Bischoff et al., 2000a; Fetscher et al., 2001), both SPC and NA strongly depend on the influx of extracellular Ca2+ to elicit vasoconstriction, and this occurs partly via voltage-operated channels. Moreover, PLC and rho-kinase also appear important in the mesenteric microvessel contraction by both agonists. The role of rho-kinase in the SPC effects is further supported by recent study in other vascular smooth muscle preparations (Todoroki-Ikeda et al., 2000; Shirao et al., 2002). In contrast, PLD, store-operated Ca2+ channels, PI-3-kinase, tyrosine kinases and MAPK of the ERK type are not involved to a major extent in either response. Thus, two receptors acting via different G-proteins appear to converge early on shared signalling pathways to elicit the same physiological response, i.e. vasoconstriction. We speculate that rho-kinase effects on phosphatidylinositol phosphate kinases and hence increased substrate pools for PLC may represent a point of convergence (Anderson et al., 1999). Such signalling pathways may also be shared by other vasoconstrictor agents including those which act receptor-independently.
Acknowledgments
Ms Veronica Steenpaß was the recipient of a thesis stipend by the intramural grant programme of the University of Essen Medical School (IFORES). The skilful assistance by Ms Charlotte Fetscher is gratefully acknowledged.
Abbreviations
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- NA
noradrenaline
- PI-3-kinase
phosphatidylinositol-3-kinase
- PL
phospholipase
- PTX
pertussis toxin
- SPC
sphingosylphosphorylcholine
References
- ABURTO T., JINSI A., ZHU Q., DETH R.C. Involvement of protein kinase C activation in α2-adrenoceptor-mediated contractions of rabbit saphenous vein. Eur. J. Pharmacol. 1995;277:35–44. doi: 10.1016/0014-2999(95)00054-o. [DOI] [PubMed] [Google Scholar]
- ALLEN B.G., WALSH M.P. The biochemical basis of the regulation of smooth-muscle contraction. Trends Biochem. Sci. 1994;19:362–368. doi: 10.1016/0968-0004(94)90112-0. [DOI] [PubMed] [Google Scholar]
- ANDERSON R.A., BORONENKOV I.V., DOUGHMAN S.D., KUNZ J., LOIJENS J.C. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J. Biol. Chem. 1999;274:9907–9910. doi: 10.1074/jbc.274.15.9907. [DOI] [PubMed] [Google Scholar]
- BATCHELOR T.J.P., SADABA J.R., ISHOLA A., PACAUD P., MUNSCH C.M., BEECH D.J. Rho-kinase inhibitors prevent agonist-induced vasospasm in human internal mammary artery. Br. J. Pharmacol. 2001;132:302–308. doi: 10.1038/sj.bjp.0703809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., FETSCHER C., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br. J. Pharmacol. 2000a;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner. Br. J. Pharmacol. 2000b;130:1878–1883. doi: 10.1038/sj.bjp.0703516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., FINGER J., MICHEL M.C. Nifedipine inhibits sphingosine-1-phosphate-induced renovacular contraction in vitro and in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001a;364:179–182. doi: 10.1007/s002100100446. [DOI] [PubMed] [Google Scholar]
- BISCHOFF A., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Lysosphingolipid receptor-mediated diuresis and natriuresis in anaesthetised rats. Br. J. Pharmacol. 2001b;132:1925–1933. doi: 10.1038/sj.bjp.0703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN H., BISCHOFF A., SCHÄFERS R.F., WAMBACH G., PHILIPP T., MICHEL M.C. Vasoconstriction of rat renal interlobar arteries by noradrenaline and neuropeptide Y. J. Auton. Pharmacol. 1997;17:137–146. doi: 10.1046/j.1365-2680.1997.00452.x. [DOI] [PubMed] [Google Scholar]
- CHEN H., FETSCHER C., SCHÄFERS R.F., WAMBACH G., PHILIPP T., MICHEL M.C. Effects of noradrenaline and neuropeptide Y on rat mesenteric microvessel contraction. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:314–323. doi: 10.1007/BF00168634. [DOI] [PubMed] [Google Scholar]
- CHUN J., GOETZL E.J., HLA T., IGARASHI Y., LYNCH K.R., MOOLENAAR W., PYNE S., TIGYI G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESSY C., KIM I., SOUGNEZ C.L., LAPORTE R., MORGAN K.G. A role for MAP kinase in differentiated smooth muscle contraction evoked by α-adrenoceptor stimulation. Am. J. Physiol. 1998;275:C1081–C1086. doi: 10.1152/ajpcell.1998.275.4.C1081. [DOI] [PubMed] [Google Scholar]
- DI SALVO J., NELSON S.R., KAPLAN N. Protein tyrosine phosphorylation in smooth muscle: a potential coupling mechanism between receptor activation and intracellular calcium. Proc. Soc. Exp. Biol. Med. 1997;214:285–301. doi: 10.3181/00379727-214-44097. [DOI] [PubMed] [Google Scholar]
- ELTZE M., BOER R., MICHEL M.C., HEIN P., TESTA R., ULRICH W.-R., KOLASSA N., SANDERS K.H. In vitro and in vivo uroselectivity of B8805-033, an antagonist with high affinity at prostatic α1A- vs. α1B- and α1D-adrenoceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:649–662. doi: 10.1007/s002100100413. [DOI] [PubMed] [Google Scholar]
- FETSCHER C., CHEN H., SCHÄFERS R.F., WAMBACH G., HEUSCH G., MICHEL M.C. Modulation of noradrenaline-induced microvascular constriction by protein kinase inhibitors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:57–65. doi: 10.1007/s002100000338. [DOI] [PubMed] [Google Scholar]
- FUKATA Y., AMANO M., KAIBUCHI K. Rho-rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- GARCIA-SAINZ J.A., VAZQUEZ-PRADO J., VILLALOBOS-MOLINA R. α1A-Adrenoceptors: subtypes, signaling, and roles in health and disease. Arch. Med. Res. 1999;30:449–458. doi: 10.1016/s0188-0128(99)00059-7. [DOI] [PubMed] [Google Scholar]
- HINTON J.M., ADAMS D., GARLAND C.J. 5-Hydroxytryptamine stimulation of phospholipase D activity in the rabbit isolated mesenteric artery. Br. J. Pharmacol. 1999;126:1601–1608. doi: 10.1038/sj.bjp.0702484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLENBERG M.D. Tyrosine kinase pathways and the regulation of smooth muscle contractility. Trends Pharmacol. Sci. 1994;15:108–114. doi: 10.1016/0165-6147(94)90046-9. [DOI] [PubMed] [Google Scholar]
- HONG S.J., CHANG C.C. Novel inhibition of contractility by wortmannin in skeletal muscle. Br. J. Pharmacol. 1998;124:849–856. doi: 10.1038/sj.bjp.0701898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES S.-A.C.F., GIBSON W.J., YOUNG J.M. The interaction of U-73122 with the histamine H1 receptor: implications for the use of U-73122 in defining H1 receptor-coupled signalling pathways. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:555–558. doi: 10.1007/s002100000326. [DOI] [PubMed] [Google Scholar]
- IBITAYO A.I., TSUNODA Y., NOZU F., OWYANG C., BITAR K.N. Src kinase and PI-3-kinase as a transduction pathway in ceramide-induced contraction of colonic smooth muscle. Am. J. Physiol. 1998;275:G705–G711. doi: 10.1152/ajpgi.1998.275.4.G705. [DOI] [PubMed] [Google Scholar]
- JINSI A., PARADISE J., DETH R.C. A tyrosine kinase regulates α-adrenoceptor-stimulated contraction and phospholipase D activation in the rat aorta. Eur. J. Pharmacol. 1996;302:183–190. doi: 10.1016/0014-2999(96)00049-0. [DOI] [PubMed] [Google Scholar]
- LAGAUD G.J.L., STOCLET J.-C., ANDRIANTSITOHAINA R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. J. Physiol. (London) 1996;492:689–703. doi: 10.1113/jphysiol.1996.sp021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG R.J., HASHITANI H., KELLER S., TAKANO H., MULHOLLAND E.L., FUKUTA H., SUZUKI H. Modulators of internal Ca2+ stores and the spontaneous electrical and contracile activity of the guinea-pig renal pelvis. Br. J. Pharmacol. 2002;135:1363–1374. doi: 10.1038/sj.bjp.0704609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW A.M., BOWDISH D.M., PRASHAD T.R., GASPAR V. Interactions of chloroethylclonidine with rauwolscine- and prazosin-sensitive adrenoceptors in dog saphenous vein. Br. J. Pharmacol. 1994;113:1263–1268. doi: 10.1111/j.1476-5381.1994.tb17134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFADZEAN I., GIBSON A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., HIMMEL H.M., JAKOBS K.H. Sphingosylphosphorylcholine – biological functions and mechanisms of action. Biochim. Biophys. Acta. 2002;1582:178–189. doi: 10.1016/s1388-1981(02)00154-3. [DOI] [PubMed] [Google Scholar]
- MOGAMI H., LLOYD MILLS C., GALLACHER D.V. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem. J. 1997;324:645–651. doi: 10.1042/bj3240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKAI Y., SHIMOKAWA H., MATOBA T., KANDABASHI T., SATOH S., HIROKI J., KAIBUCHI K., TAKESHITA A. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 2001;10.1096/fj.00-0735fje doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- MUTO Y., NAGAO T., URUSHIDANI T. The putative phospholipase C inhibitor U73122 and its negative control, U73343, elicit unexpected effects on the rabbit parietal cell. J. Pharmacol. Exp. Ther. 1997;282:1379–1388. [PubMed] [Google Scholar]
- NEER E.J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- PYNE S., PYNE N.J. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 2000;349:395–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS R.E. Role of the extracellular signal-regulated kinase (Erk) signal transduction cascade in α2 adrenoceptor-mediated vasoconstriction in porcine palmar lateral vein. Br. J. Pharmacol. 2001;133:859–866. doi: 10.1038/sj.bjp.0704149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRAO S., KASHIWAGI S., SATO M., MIWA S., NAKAO F., KUROKAWA T., TODOROKI-IKEDA N., MOGAMI K., MIZUKAMI Y., KURIYAMA S., HAZE K., SUZUKI M., KOBAYASHI S. Sphingosylphosphorylcholine is a novel messenger for rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery. Unimportant role for protein kinase C. Circ. Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., MILSTIEN S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim. Biophys. Acta. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., YATOMI T., OZAKI Y., HASHIMOTO K. Sphingosine 1-phosphate induces sinus tachycardia and coronary vasoconstriction in the canine heart. Cardiovasc. Res. 2000;46:119–125. doi: 10.1016/s0008-6363(00)00013-4. [DOI] [PubMed] [Google Scholar]
- TAGUCHI K., YANG M., GOEPEL M., MICHEL M.C. Comparison of human α1A-adrenoceptor subtype coupling to protein kinase C activation and related signalling pathways. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;358:100–110. doi: 10.1007/pl00005143. [DOI] [PubMed] [Google Scholar]
- TAKAYAMA M., OZAKI H., KARAKI H. Effects of a myosin light chain kinase inhibitor, wortmannin, on cytoplasmic Ca2+ leels, myosin light chain phosphorylation and force in vascular smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:120–127. doi: 10.1007/BF00178711. [DOI] [PubMed] [Google Scholar]
- TODOROKI-IKEDA N., MIZUKAMI Y., MOGAMI K., KUSADA T., YAMAMOTO K., MIYAKE T., SATO M., SUZUKI S., YAMAGATA H., KOKAZONO Y., KOBAYASHI S. Sphingosylphosphorylcholine induces Ca2+-sensitization of vascular smooth muscle contraction: possible involvement of rho-kinase. FEBS Lett. 2000;482:85–90. doi: 10.1016/s0014-5793(00)02046-9. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., CLARKE D.E. Characterization of α1A-adrenoceptors mediating vasoconstriction to noradrenaline and nerve stimulation in the isolated perfused mesentery of rat. Br. J. Pharmacol. 1995;114:531–536. doi: 10.1111/j.1476-5381.1995.tb13259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


