Abstract
Opioid agonists have been used for many years to treat all forms of headache, including migraine. We sought to characterize opioid receptors involved in craniovascular nociceptive pathways by in vivo microiontophoresis of μ-receptor agonists and antagonists onto neurons in the trigeminocervical complex of the cat.
Cats were anaesthetized with α-chloralose 60 mg kg−1, i.p. and 20 mg kg−1, i.v. supplements after induction and surgical preparation using halothane. Units were identified in the trigeminocervical complex responding to supramaximal electrical stimulation of the superior sagittal sinus, and extracellular recordings of activity made.
Seven- or nine-barrelled glass micropipettes incorporating tungsten recording electrodes in their centre barrels were used for microiontophoresis of test substances onto cell bodies.
Superior sagittal sinus (SSS)-linked cells whose firing was evoked by microiontophoretic application of L-glutamate (n=8 cells) were reversibly inhibited by microiontophoresis of H2N-Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol (DAMGO) (n=12), a selective μ-receptor agonist, in a dose dependent manner, but not by control ejection of sodium or chloride ions from a barrel containing saline.
The inhibition by DAMGO of SSS-linked neurons activated with L-glutamate could be antagonized by microiontophoresis of selective μ-receptor antagonists D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) or D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), or both, in all cells tested (n=4 and 6, respectively).
Local iontophoresis of DAMGO during stimulation of the superior sagittal sinus resulted in a reduction in SSS-evoked activity. This effect was substantially reversed 10 min after cessation of iontophoresis. The effect of DAMGO was markedly inhibited by co-iontophoresis of CTAP.
Thus, we found that μ-receptors modulate nociceptive input to the trigeminocervical complex. Characterizing the sub-types of opioid receptors that influence trigeminovascular nociceptive transmission is an important component to understanding the pharmacology of this synapse, which is pivotal in primary neurovascular headache.
Keywords: Headache, migraine, opioid, nociception, craniovascular, trigeminal nucleus caudalis, microiontophoresis
Introduction
Opioid receptor agonists are widely used in various forms to treat pain of all types. Head pain is a common human experience (Rasmussen, 1995), while primary headaches, such as migraine and cluster headache, in their most severe forms can be among the most disabling forms of human disease (Menken et al., 2000). It has been known for some time that opioid receptors are found in the trigeminal nucleus caudalis of the cat (Henry et al., 1980; Wong et al., 1986), and rat (Wang et al., 2000; Zhang et al., 1996), although it has not been established that they exist on neurons with intracranial trigeminovascular input. Opioid receptor agonists, such as morphine, codeine and pethidine (meperidine), have been used for many years to treat all forms of headache, including migraine (Gowers, 1888; Ziegler, 1997). Simple and effective for some headaches, even favoured in some settings (Silberstein & McCrory, 2000), detailed studies of the possible loci of action of opiate receptor agonists in primary headaches have not been conducted. Studies of the action of opioids in headache may increase understanding of their clinical role in these disorders.
The neurobiology of the commonest form of disabling primary headache, migraine, essentially involves three components (Goadsby et al., 2002). First, the inherited headache diathesis, best characterized by the mis-sense mutations seen in familial hemiplegic migraine (Ophoff et al., 1996), and most commonly illustrated by the common clinical finding of an affected first degree relative (Russell & Olesen, 1995). Secondly, migraine involves activation of brainstem regions (Bahra et al., 2001; Weiller et al., 1995) that uniquely distinguish the condition when compared to other forms of primary headache. Thirdly, the pain component of migraine seems to involve activation (Goadsby et al., 1990), or at least the perception of activation, of the trigeminal innervation of the pain-producing intracranial contents (Wolff, 1948). Animal models of migraine have exploited the later feature of the disorder by studying various aspects of the innervation of these structures, the trigeminovascular system (Edvinsson, 1999). One such model system involves the electrical or chemical stimulation of the superior sagittal sinus, an intracranial structure that produces pain in humans when stimulated (Feindel et al., 1960).
During stimulation of the superior sagittal sinus (SSS) neurons can be studied using cell population-based anatomical techniques, such as measurement of Fos with immunohistochemistry (Kaube et al., 1993), or metabolic activity with 2-deoxyglucose (Goadsby & Zagami, 1991), or single neurons can be more closely tracked using electrophysiological techniques (Hoskin et al., 1996). Electrophysiological methods, combined with microiontophoresis facilitates characterization of the pharmacology of neurons of interest by repeated local application of appropriate agonists and antagonists (Bloom, 1974). It has been shown that opioid receptor systems play a role in meningeal vascular change associated with local stimulation of perivascular nerves that are branches of the trigeminal nerve (Williamson et al., 2001b). Specifically, opioid agonists inhibit neurogenic meningeal vasodilation (Williamson et al., 2001b), as do agents useful in the acute treatment of migraine, such as triptans, which are 5-HT1B/1D agonists (Williamson et al., 1997a,b; 2001a). Similarly, it has been shown that many acute anti-migraine agents have inhibitory actions at second order neurons in the trigeminal nucleus caudalis activated by stimulation of trigeminovascular nociceptive afferents (Goadsby, 2000).
Given that agents active in migraine are inhibitory within the trigeminal nucleus caudalis, the actions of opiate receptor agonists and antagonists at this site are important in building a comprehensive understanding of this potentially pivotal synapse in headache biology. We therefore sought to characterize opioid receptors involved in craniovascular nociceptive pathways by in vivo microiontophoresis of aμ-receptor agonist and antagonists onto neurons in the trigeminocervical complex of the cat.
Methods
All studies reported were conducted and terminated under general anaesthesia in accordance with a project license issued by the Home Office of the United Kingdom under the Animals (Scientific Procedures) Act, 1986. Eight cats weighing 3.01±0.36 kg (mean±s.d.) were anaesthetized with α-chloralose (60 mg kg−1 i.p.; Sigma, St Louis, MO, U.S.A.) and prepared for physiological monitoring. Halothane (Rhone Merieux, Essex, U.K.) (0.5–3% in a 40% oxygen/air mixture) was administered from an anaesthetic machine during surgical procedures and then discontinued during experimental protocols. A catheter was placed in the femoral artery for arterial blood sampling, and continuous measurement of blood pressure (DTXplus transducer, Ohmeda, Madison, WI, U.S.A.; PM-1000 amplifier CWE Instruments). A second catheter placed in the femoral vein allowed for fluid and drug administration. Supplementary doses of α-chloralose in 2-hydroxypropyl-β-cyclodextrin (RBI, Natick, MA, U.S.A.) were given i.v. at a rate of 5–10 mg kg−1 h−1 (Storer et al., 1997). The cats were intubated after local anaesthesia with lignocaine hydrochloride (Intubeaze, Arnolds, Shrewsbury, U.K.) and fixed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, U.S.A.).
Jackson/Foley urethral catheters were inserted to drain the bladder, providing more even temperature regulation, more stable control of blood pressure through control of bladder distension, and monitoring of urine output. Core temperature was monitored and maintained between 37–39°C using a rectal thermistor probe and a low radio noise-emitting homeothermic heater blanket system (Harvard Apparatus, Holliston, MA, U.S.A.). Cats were ventilated with a 40% oxygen/air mixture (665 controlled volume ventilator, Harvard Apparatus), end-tidal CO2 was maintained between 2–4% and expired oxygen continuously monitored (Datex-Ohmeda, Helsinki, Finland). Heart rate was monitored by electrocardiogram (CT-1000; CWE Instruments). The depth of anaesthesia was monitored periodically throughout the experiment by testing for sympathetic (pupillary and cardiovascular) responses to noxious stimulation and withdrawal reflexes in the absence of neuromuscular blockade.
Surgery
A midline craniotomy (20-mm diameter) and C1/C2 laminectomy were performed allowing access to the superior sagittal sinus and the recording site in the spinal cord. The sinus was isolated by dissecting the dura and falx cerebri adjacent to the sinus over approximately 15 mm. A small polyethylene sheet was inserted under the isolated sinus and tucked under the outlying dura. To prevent dehydration and to provide electrical insulation to the cortex, a polypropylene dam was sealed to the bone around the craniotomy with dental acrylic (Vertex, Zeist, Netherlands) and filled with liquid paraffin (BDH Laboratory Supplies, Poole, U.K.). Possible artefacts from arterial pulsation and respiratory movement were reduced by: bilateral pneumothoraces, held patent with polypropylene tubes; immobilization of the spine by clamping a thoracic spinous process to the stereotaxic frame; clamping the C1 transverse processes to auxiliary ear bar holders on the frame, and clamping the remaining caudal portion of the dorsal C2 spinous process to the frame.
Stimulation and recording
The isolated SSS was gently lifted onto a pair of bipolar platinum hook electrodes connected to a stimulus isolation unit (SIU5A; Grass Instruments, West Warwick, RI, U.S.A.). To activate primary trigeminal afferents, the SSS was supramaximally stimulated with stimulus-isolated (Grass SIU) square wave pulses from a Grass S88 stimulator (120–150 V, 250 μs, 0.1–1.0 Hz) after neuromuscular blockade with gallamine triethiodide (Concord, Essex, U.K.) (initially 10–15 mg kg−1 i.v. and maintained with 5–10 mg kg−1 h−1). Extracellular recordings were made using microiontophoretic combination electrodes consisting of seven- or nine-barrelled glass pipettes incorporating central tungsten recording electrodes with an exposed recording tip length of approximately 12 μm (Hellier et al., 1990). Recording electrode impedances were typically 400 kΩ–1 MΩ when measured at 1 kHz in 0.9% saline. The dura above the recording regions on the surface of the spinal cord was reflected and held to the edges of the laminectomy with N-butyl-cyanoacrylate. After local removal of the pia mater the electrode was lowered into the cord substance near to the C2 roots in the area of the dorsal root entry zone. The electrode was advanced or retracted in the cord substance in 5 μm steps using a microelectrode positioner consisting of a piezoelectric motor (IW-711, Burleigh Instruments, Harpenden, U.K.) and ultra low noise controller (6000 ULN) attached to a micromanipulator (Kopf 1760-61). Tissue culture grade agar (3% (w v−1) in saline; Sigma, St Louis, MO, U.S.A.) was set over the exposed cord after electrode insertion to further reduce cardiovascularly related movements. Signal from the recording electrode attached to a high impedance headstage preamplifier (NL100AK; Neurolog, Digitimer, Herts, U.K.) was fed via an AC preamplifier (Neurolog NL104, gain×1000) through filters (Neurolog NL125; bandwidth approximately 300 Hz to 20 kHz) and a 50 Hz noise eliminator (Humbug, Quest Scientific, North Vancouver, BC, Canada) to a second stage amplifier (Neurolog NL106) providing variable gain (×20–×90). This signal (total gain approximately ×20,000–×90,000) was fed to a gated amplitude discriminator (Neurolog NL201) and analogue-to-digital converter (Labmaster DMA, Scientific Solutions, Mentor, OH, U.S.A.) in an 80486 microprocessor-based computer where the signal was processed and stored. Filtered and amplified action potentials were fed to a loudspeaker via a power amplifier (Neurolog NL120) for audio monitoring and displayed on an oscilloscope to assist the isolation of single unit activity from adjacent cell activity and noise.
In order to record the response of single units to stimulation, post-stimulus histograms were constructed on-line and saved to disk. Free-running neuronal activity, such as stimulated by local L-glutamate microiontophoresis, was analysed as cumulative rate histograms, where activity gated through the amplitude discriminator was collected into successive bins. Averaged action potentials were constructed to discriminate between somatic and axonal recordings, using an averaging routine and an analogue signal delay unit (NL202), setting the NL125 filter bandwidth from d.c. to approximately 30 kHz. During experiments, electrophysiological data, blood pressure, heart rate and end-tidal CO2 were processed and recorded on VHS magnetic tape (Pulse Code Modulator; Vetter, Rebersburgh, PA, U.S.A.) for documentation and latter review.
The position of the recording electrodes was controlled by use of a stereotaxic micropositioner (Kopf 1760-61) with reference to the mid-point of the C2 dorsal roots. Together with the depth of the recording electrode tip with respect to the surface of the spinal cord at the dorsal root entry zone, as determined by the distance travelled display on the ULN6000 piezoelectric motor controller (Burleigh Instruments), provided the coordinates of the recording sites. The location of selected recording sites were marked with Pontamine Sky Blue dye using a −2.00 μA current for 5–10 min. Animals were euthanased with sodium pentobarbital (400 mg), followed by 10% KCl (5 ml). After termination of experiments the sections of spinal cord containing the recording sites were removed, fixed with neutral buffered 10% formalin, and sectioned (40 μm) Pontamine Sky Blue was counterstained with neutral red, a Nissl procedure which allowed identification of the laminae of the grey matter. The position of the recording sites within the cord were determined from histologically identified dye marks and unmarked recording sites located by reference to dye marks and coordinates of recording electrode positions.
Receptive fields
Cells responding to superior sagittal sinus (SSS) stimulation were characterized as receiving low threshold mechanoreceptor (LTM) input if they responded to non-noxious input such as brush or stroke on cutaneous receptive fields on the face or forepaws. They were characterized as nociceptive specific (NS) if they responded to noxious mechanical stimuli, such as pinch or pricking with a needle, or wide dynamic range (WDR) if they responded to both (Hu et al., 1981). These cells usually had increased firing in response to noxious stimuli.
Test compounds
Micropipette barrels used for iontophoresis of test substances were filled with 1.0 M L-glutamate, monosodium, pH 8.0 (Sigma); saline (controls); H3N-Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol acetate ([D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin; DAMGO; Sigma); D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 [disulphide bridge 2–7] (CTOP; Sigma); D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 [disulphide bridge 2–7] (CTAP; Sigma or Peninsula Laboratories, Merseyside, U.K.). DAMGO was ionized as a cation and retained in its iontophoretic barrel with a small negative current (∼−5 nA). L-Glu, CTOP and CTAP were ionized as anions and retained with small positive currents (∼5 nA). Ejection currents in directions opposite to the retaining currents were used (2–200 nA). Chloride or sodium ions ejected from the barrel containing saline were used as controls. Current balancing was provided through a barrel containing 1 M NaCl.
Microiontophoresis
After filling, the iontophoretic electrode micropipette barrels had resistances between 60–150 MΩ. A microiontophoresis current generator (Dagan 6400, Dagan Corporation, Minneapolis, MN, U.S.A.) provided the current for ejecting test substances from the barrels. Retaining and balancing currents were used routinely as described above (Bloom, 1974). The L-Glu ejection current was adjusted so cells had free running activity at a rate of around 20 Hz, such that inhibition of the cell activity could be distinguished from random firing. Where L-Glu was applied in pulses the evoked firing rate was often higher. Ejection currents were adjusted such that cell activity inhibited by the agonist could be reversed by the antagonists.
Statistical analysis
Neuronal firing was distinguished from noise using an amplitude discriminator. Statistical evaluations were made using the average rate of firing in Hz evoked during each epoch of microiontophoretic application of L-glutamate. The background neuronal discharge was calculated by averaging the period of ongoing activity immediately preceding each epoch of excitation and subtracting this value from the evoked responses. For each unit a minimum of five-paired baseline-response data were collected. For the control responses to L-glutamate an analysis of variance (ANOVA) with repeated measures was carried out. When this was not significant the reliability of the measurements was tested using Cronbach's alpha. If there was no difference across the baseline data, and there was high reliability, the data were pooled and compared to further treatments with a t-test for independent samples. Where there was difference across repetitions for a treatment an ANOVA with repeated measures design was used to compare to the baseline responses. The P values were assessed at the 0.05 level and the Bonferroni test applied to comparisons between drugs (SPSS v.10). Summary data are presented as the mean±standard error of the mean unless otherwise stated.
Results
Animals from which data was recorded had cardio-respiratory parameters that were normal for the anaesthetized cat. Blood gas parameters were measured at intervals throughout the experiment and were within normal limits: arterial blood pH 7.40±0.03 and pCO2 3.22±0.19 kPa.
Localization and neuronal characteristics
Extracellular recordings were made from neurons in the trigeminocervical complex (Kaube et al., 1993). Cells were located +4 mm rostral to −4 mm caudal to the midpoint of the C2 rootlets, 0–150 μm lateral to the dorsal root entry zone at a depth of approximately −700 μm to around −2000 μm below the (dorsal) cord surface (Figure 1). Units responding to electrical stimulation of the superior sagittal sinus had latencies consistent with A-δ fibres [fibre input/afferents] (typically 8–25 ms; Figure 2). Recordings were made from units that were both linked to SSS stimulation and that could be identified as cell bodies, as characterized by their unfiltered biphasic action potential morphology (Fussey et al., 1970) and the reversible excitatory effect of L-glutamate on cell firing. Studied units received wide dynamic range or nociceptive specific mechanoreceptor input from cutaneous receptive fields on the face and forepaws. Cell firing was not significantly inhibited by control ejection of sodium or chloride ions from a barrel containing saline at similar currents.
Figure 1.
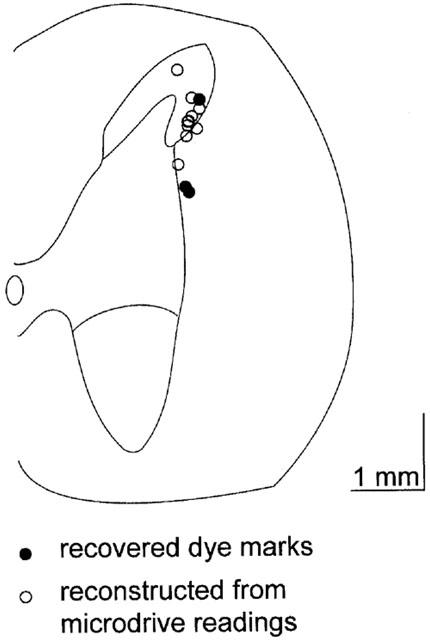
Localization of recording sites. A transverse section through the spinal cord at the level of C2 is represented. Pontamine Sky Blue dye marked recording sites (filled circles) from experiments were identified histologically. The findings closely matched microdrive estimates of dye mark placement. The positions of unmarked sites were identified by reference to the position of dye marks at recording sites and at the end of electrode tracts, and electrode tip coordinates (open circles). Although the recorded units are only mapped to one side of the cord in the figure, they represent the results obtained from both the left-hand-side and right-hand-side of the spinal cord. Scale bar represents a distance of 1 mm in both directions.
Figure 2.
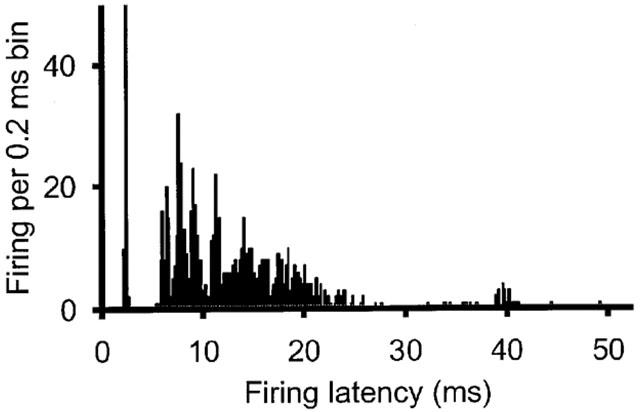
Post-stimulus histogram of neuron firing linked to electrical SSS stimulation. Recordings were made from neurons in the trigeminal nucleus caudalis using tungsten-in-glass electrodes surrounded by microiontophoretic pipette barrels. The superior sagittal sinus was electrically stimulated with 250 μs pulses of 120–150 V at 0.3–0.5 Hz using a bipolar platinum wire hook electrode. Typically resistances along the sinus between the two poles of the stimulating electrode were around 2.0 MΩ giving a suprathreshold stimulus current of 60–75 μA. The histogram shows the results from 100 such stimuli.
Baseline L-glutamate firing
Only neurons identified as linked to stimulation of the superior sagittal sinus were tested for the stability of their baseline response to L-glutamate application. The firing rate for trigeminal nucleus caudalis neurons was 21.6±2.9, 21.5±2.5, 21.1±2.4, 21.5±2.7, and 22.1±2.6 Hz for each of five consecutive applications of L-glutamate (−30 to −50 nA; n=15). There was no difference across these responses (F4,56=0.38, P=0.82). The reliability of these responses across time was excellent with an alpha of 0.99. We thus pooled these data for further comparison with other drug applications.
Effect of μ-receptor agonist DAMGO
L-glutamate evoked firing
Neuronal firing evoked by local microiontophoresis of L-glutamate onto neurons in the trigeminocervical complex that were linked to sagittal sinus stimulation was reversibly and significantly (t20=3.3, P=0.003, n=12) inhibited by the potent and selective μ-receptor agonist, DAMGO (Suh & Tseng, 1990). At a current of −30 nA DAMGO inhibition (n=7) was consistent (F4,20=0.57, P=0.69; alpha=0.99) across five epochs tested, with residual L-glutamate-evoked responses of 8.8±1.4, 8.2±1.4, 8.6±1.4, 8.7±1.2, and 8.5±1.4 Hz, respectively. Considering the pooled control and DAMGO effects at −30 nA the L-glutamate evoked response was reduced by 60%. The inhibition was dose-dependent (Figure 3) with a more substantial inhibition at higher currents (Figure 5).
Figure 3.
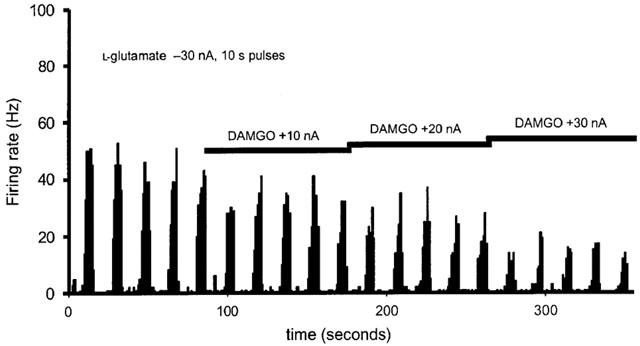
Effect of DAMGO. Neuronal firing evoked by local microiontophoretic application of L-glutamate onto sagittal sinus linked neurons in the trigeminocervical complex in the cat spinal dorsal horn was inhibited by the local microiontophoretic application of the potent and selective μ-receptor agonist, DAMGO, in a dose dependent manner.
Figure 5.
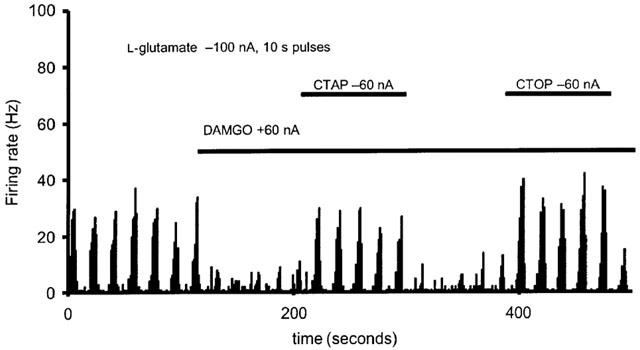
Effect of μ-receptor antagonists, CTAP and CTOP. Representative histogram showing inhibition of the L-glutamate evoked firing of sagittal sinus linked neurons by local microiontophoretic application of DAMGO reversed by local microiontophoretic application of the μ-receptor selective antagonists, CTAP and CTOP through a multibarrelled microiontophoretic pipette and tungsten electrode recording assembly.
Superior sagittal sinus (SSS) stimulation
Stimulation of the SSS resulted in activation of units at latencies of 8–25 ms. Local microiontophoresis of DAMGO (120 nA for 5 min) resulted in a 59±17% reduction (n=8; P<0.05) in SSS-evoked activity (Figure 4). This effect was reversible to 79±13% of control 10 min after cessation of iontophoresis. The effect of DAMGO was markedly inhibited by co-iontophoresis of CTAP (−120 to −150 nA) with a response of 95±25% (n=5) of control.
Figure 4.
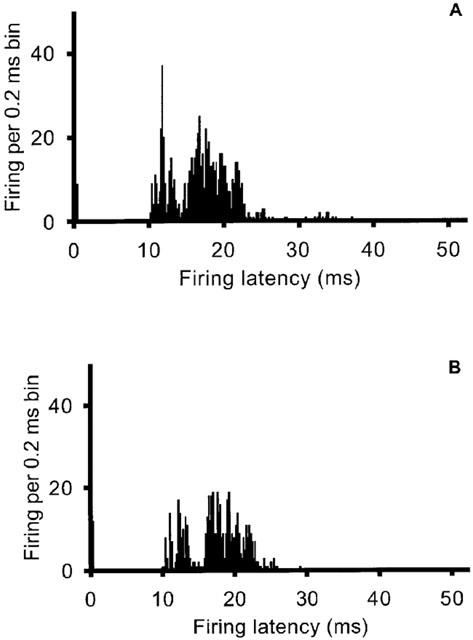
Post-stimulus histogram of superior sagittal sinus (SSS)-linked units. Stimulation of the SSS recruits linked units (A) that are inhibited after iontophoresis of the potent and selective μ-receptor agonist DAMGO (120 nA; B).
Effect of μ-receptor antagonists
The inhibition of neuronal activity by DAMGO could be reversed by the μ-receptor selective antagonists CTAP or CTOP (Gulya et al., 1986; Kramer et al., 1989; Pelton et al., 1986), or both (Figure 5), in all cells tested. CTAP produced a consistent reversal of the effects of DAMGO (F4,8=1.8, P=0.22; alpha 0.99). The effect of DAMGO on L-glutamate evoked responses was significantly reversed with a mean L-glutamate evoked response of 22.1±4.0 Hz across the cells tested (t10=3.7, P=0.004). CTOP also produced a consistent reversal of the effects of DAMGO (F4,20=0.41, P=0.53; alpha 0.99). The effect of DAMGO on L-glutamate evoked responses was significantly reversed with a mean L-glutamate evoked response of 17.7±2.2 Hz across the cells tested (t12=3.6, P=0.004).
The duration and time of these currents were adjusted to sufficiently antagonize the inhibition caused by the DAMGO. Sometimes there was a lag in the effect of the antagonists. No ordering effects were noted. When the antagonists were applied alone no significant alteration of the response of the SSS linked neurons to L-glutamate was detected.
Discussion
In conclusion, we found that μ-opioid receptors modulate nociceptive input to the trigeminocervical complex of the cat. The inhibition of trigeminovascular nociceptive afferent activation is potent, reproducible and reversible with specific antagonists of the μ-receptor. The data are consistent with broad clinical evidence that μ-receptor activation can be useful in treatment of acute migraine, notwithstanding the adverse clinical consequences of regular, frequent use of opioid receptor agonists. The data do not address, and therefore, do not exclude a role for κ- and δ-opioid (Raynor et al., 1994) or the ‘opioid-like' orphanin receptor (ORL-1) now known as the NOP1 receptor (Mogil & Pasternak, 2001) in the trigeminocervical complex; these questions require further specific studies. It is noteworthy in this regard that we have recently shown evidence for a trigeminovascular inhibitory effect of the NOP1 receptor on peripheral trigeminovascular neurons (Bartsch et al., 2002).
The term opioid applies to all agonists or antagonists with activity at one of the opioid receptor types, such as derivatives of opium (formerly called opiates) or endogenous or synthetic opioid peptides (Cox, 2000; Reisine & Pasternak, 1996). At least four distinct opioid receptor subtypes are recognized: μ, κ and δ (Raynor et al., 1994) and the opioid-like orphan in receptor (ORL-1) now known as the NOP1 receptor (Mogil & Pasternak, 2001). Morphine and other morphine-like opioid agonists produce analgesia primarily through μ-receptor activation (Pasternak, 1993) with a range of other transmitter systems being modulated. Opioids close N-type and low threshold T-type voltage gated calcium channels (Rhim & Miller, 1994; Schroeder et al., 1991) and inhibit P/Q-type voltage gated calcium channels through the μ-receptor (Piros et al., 1996; Rhim & Miller, 1994). In addition opioids inhibit adenylyl cyclase, decreasing cAMP concentration and modulating release of other neurotransmitters (Bovill, 1997). The selective μ-receptor agonist [D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin; H2N-Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol (DAMGO) has been shown to inhibit the activity of sensory neurons in the trigeminal nucleus caudalis, suggesting a modulatory role of opioids at this site (Wang & Mokha, 1996). Our data are consistent with such an action, providing clear evidence of inhibition of trigeminal second order neurons that receive input from a normally pain-producing intracranial vascular structure (Feindel et al., 1960).
Microiontophoresis allows delivery of specific agonists or antagonists with a very restricted anatomical locus of action (Bloom, 1974). By ejecting L-glutamate we could mimic the activation of the trigeminovascular nociceptive system that has been shown to have excitatory transmission involving N-methyl-D-aspartate (NMDA) (Classey et al., 2001; Goadsby & Classey, 2000; Mitsikostas et al., 1998), α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid (AMPA) (Storer & Goadsby, 1999), and kainate (Mitsikostas et al., 1999) mediated components. This post-synaptic activation by-passes the first order-second order synapse, such that its subsequent inhibition with the μ-receptor agonist DAMGO demonstrates the presence of what is likely to be a post-synaptic effect. A similar effect can be seen with local 5-HT1B/1D agonist iontophoresis (Goadsby et al., 2001). Moreover, the lack of an effect on baseline firing by the μ-receptor antagonists CTAP or CTOP suggest there is little resting opioid-mediated inhibition of this synapse, at least in the anaesthetized cat. This is consistent with observations in humans that endogenous opioid changes in cerebrospinal fluid are limited to patients with primary headache disorders that are active (Nappi et al., 1985).
An important limitation of the use of CTOP derives from the fact that it has structural similarities to somatostatin (Gulya et al., 1986; Pelton et al., 1986). CTOP has in addition to μ-receptor effects, antagonist effects at somatostatin receptors (Chieng et al., 1996). Recent data demonstrate the localization of somatostatin (SST) receptors in the trigeminal nucleus (Schindler et al., 1998). These are inhibitory since expression of Fos immunoreactivity elicited by corneal stimulation is reduced by somatostatin analogues (Bereiter, 1997). Although CTOP has both μ and SST receptor actions, CTAP does not have SST receptor effects, so that the combination of the two allows a firm conclusion that the μ-receptor has functional effects in the trigeminal nucleus in our experiments on neurons with nociceptive trigeminovascular input.
The strength of this study is the combination of a well-characterized model of trigeminovascular nociception, stimulation of the superior sagittal sinus (SSS), with iontophoresis, which facilitates the study of agonist/antagonist combinations in the same animal with the same neurons. Stimulation of the large venous sinuses is pain-producing in humans (Feindel et al., 1960; McNaughton & Feindel, 1977). It is likely that a substantial component of the pain of migraine (Goadsby et al., 2002) and cluster headache (Goadsby, 2002) results from activation of ophthalmic division afferents with substantial intracranial ramifications. Neurons in the trigeminocervical complex in cat (Kaube et al., 1993) and monkey (Goadsby & Hoskin, 1997) are activated by stimulation of the SSS. Triptans, serotonin-5-HT1B/1D receptor agonists, and ergotamine-derivatives, inhibit activation of neurons in the trigeminocervical complex (de vries et al., 1999; Goadsby, 2000), and are effective agents in the acute treatment of migraine and cluster headache. The new data demonstrate that just as triptans (Goadsby et al., 2001), and ergotamine derivatives (Lambert et al., 1992; Storer & Goadsby, 1997) have inhibitory actions localized to the trigeminocervical complex, μ-receptor activation also inhibits these neurons. Neurons linked to SSS stimulation, whether being activated subsequently by L-glutamate or by further SSS stimulation, are inhibited by the potent μ-receptor agonist DAMGO, an effect reversed by the specific μ-receptor antagonist CTAP. It is general clinical experience that while effective in some part (Silberstein & McCrory, 2000), opioids are not usually as effective as triptans.
In conclusion, we have demonstrated that activation of the μ-receptor with a specific agonist, DAMGO, can inhibit trigeminovascular nociceptive transmission in the anaesthetized cat. This inhibition can be reversed by the specific μ-receptor antagonists CTAP or CTOP. Taken with the effect of this inhibition upon locally iontophoresed L-glutamate it is likely that μ-receptors exist post-synaptically in the trigeminocervical complex. This site is a likely site of action for clinically-employed opioids, further emphasizing the importance of this synapse in the overall understanding of migraine.
Acknowledgments
The authors thank Paul Hammond and Michele Lasalandra for their excellent technical assistance. The Wellcome Trust supported this work. P. J. Goadsby is a Wellcome Trust Senior Research Fellow. This work has been presented in preliminary form at the 10th Congress of the International Headache Society, June 29–July 2, 2001, New York City, New York, USA (Storer et al., 2001).
Abbreviations
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- CTOP
D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2
- DAMGO
H2N-Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol
- LTM
low threshold mechanoreceptor
- NS
nociceptive specific
- SSS
superior sagittal sinus
- WDR
wide dynamic range
References
- BAHRA A., MATHARU M.S., BUCHEL C., FRACKOWIAK R.S.J., GOADSBY P.J. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- BARTSCH T., AKERMAN S., GOADSBY P.J. The ORL-1 (NOP1) receptor ligand nociceptin (NCE/orphanin FQ) inhibits trigeminovascular activation in the meningeal circulation of the rat. Cephalalgia. 2002;22:572. [Google Scholar]
- BEREITER D.A. Morphine and somatostatin analogue reduce c-FOS expression in trigeminal subnucleus caudalis produced by corneal stimulation in the rat. Neuroscience. 1997;77:863–874. doi: 10.1016/s0306-4522(96)00541-6. [DOI] [PubMed] [Google Scholar]
- BLOOM F.E. To spritz or not to spritz: the doubtful value of aimless iontophoresis. Life Sci. 1974;14:1819–1834. doi: 10.1016/0024-3205(74)90400-7. [DOI] [PubMed] [Google Scholar]
- BOVILL J.G. Mechanisms of actions of opioids and non-steroidal anti-inflammatory drugs. Eur. J. Anaesthesiol. 1997;15:9–16. doi: 10.1097/00003643-199705001-00003. [DOI] [PubMed] [Google Scholar]
- CHIENG B., CONNOR M., CHRISTIE M.J. The mu-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) [but not D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP)] produces a nonopioid receptor-mediated increase in K+ conductance of rat locus ceruleus neurons. Mol. Pharmacol. 1996;50:650–655. [PubMed] [Google Scholar]
- CLASSEY J.D., KNIGHT Y.E., GOADSBY P.J. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity within the trigeminocervical complex following superior sagittal sinus stimulation in the cat. Brain Res. 2001;907:117–124. doi: 10.1016/s0006-8993(01)02550-1. [DOI] [PubMed] [Google Scholar]
- COX B.M. The Compendium of Receptor Characterization and Classification. London: IUPHAR Media; 2000. Opioid receptors; pp. 321–333. [Google Scholar]
- DE VRIES P., VILLALON C.M., SAXENA P.R. Pharmacological aspects of experimental headache models in relation to acute antimigraine therapy. Eur. J. Pharmacol. 1999;375:61–74. doi: 10.1016/s0014-2999(99)00197-1. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L. Experimental Headache Models in Animals and Man. London: Martin Dunitz; 1999. [Google Scholar]
- FEINDEL W., PENFIELD W., MCNAUGHTON F. The tentorial nerves and localization of intracranial pain in man. Neurology. 1960;10:555–563. doi: 10.1212/wnl.10.6.555. [DOI] [PubMed] [Google Scholar]
- FUSSEY I.F., KIDD C., WHITWAM J.G. The differentiation of axonal and soma-dendritic spike activity. Pflügers Arch. 1970;321:283–292. doi: 10.1007/BF00588643. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J. The pharmacology of headache. Prog. Neurobiol. 2000;62:509–525. doi: 10.1016/s0301-0082(00)00010-1. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1:37–43. doi: 10.1016/s1474-4422(02)00104-7. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., AKERMAN S., STORER R.J. Evidence for post junctional serotonin (5-HT1) receptors in the trigeminocervical complex. Ann. Neurol. 2001;50:804–807. doi: 10.1002/ana.10066. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., CLASSEY J.D. Glutamatergic transmission in the trigeminal nucleus assessed with local blood flow. Brain Res. 2000;875:119–124. doi: 10.1016/s0006-8993(00)02630-5. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., HOSKIN K.L. The distribution of trigeminovascular afferents in the non-human primate brain Macaca nemestrina: a c-fos immunocytochemical study. J. Anat. 1997;190:367–375. doi: 10.1046/j.1469-7580.1997.19030367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOADSBY P.J., LIPTON R.B., FERRARI M.D. Migraine – current understanding and treatment. N. Engl. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., ZAGAMI A.S. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain. 1991;114:1001–1011. doi: 10.1093/brain/114.2.1001. [DOI] [PubMed] [Google Scholar]
- GOWERS W.R. A Manual of Diseases of the Nervous System. London: J&A Churchill; 1888. [Google Scholar]
- GULYA K., PELTON J.T., HRUBY V.J., YAMAMURA H.I. Cyclic somatostatin octapeptide analogues with high affinity and selectivity toward mu opioid receptors. Life Sci. 1986;38:2221–2229. doi: 10.1016/0024-3205(86)90574-6. [DOI] [PubMed] [Google Scholar]
- HELLIER M., BOERS P., LAMBERT G.A. Fabrication of a metal-cored multi-barrelled microiontophoresis assembly. J. Neurosci. Meth. 1990;32:55–61. doi: 10.1016/0165-0270(90)90071-m. [DOI] [PubMed] [Google Scholar]
- HENRY J.L., SESSLE B.J., LUCIER G.E., HU J.W. Effects of substance P on nociceptive and non-nociceptive trigeminal brain stem neurons. Pain. 1980;8:33–45. doi: 10.1016/0304-3959(80)90088-3. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., KAUBE H., GOADSBY P.J. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiology study. Brain. 1996;119:249–256. doi: 10.1093/brain/119.1.249. [DOI] [PubMed] [Google Scholar]
- HU J.W., DOSTROVSKY J.O., SESSLE B.J. Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). I. Responses to oral-facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. J. Neurophysiol. 1981;45:173–192. doi: 10.1152/jn.1981.45.2.173. [DOI] [PubMed] [Google Scholar]
- KAUBE H., KEAY K.A., HOSKIN K.L., BANDLER R., GOADSBY P.J. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993;629:95–102. doi: 10.1016/0006-8993(93)90486-7. [DOI] [PubMed] [Google Scholar]
- KRAMER T.H., SHOOK J.E., KAZMIERSKI W., AYRES E.A., WIRE W.S., HRUBY V.J., BURKS T.F. Novel peptidic mu opioid antagonists: pharmacologic characterization in vitro and in vivo. J. Pharmacol. Exp. Ther. 1989;249:544–551. [PubMed] [Google Scholar]
- LAMBERT G.A., LOWY A.J., BOERS P., ANGUS-LEPPAN H., ZAGAMI A. The spinal cord processing of input from the superior sagittal sinus: pathway and modulation of ergot alkaloids. Brain Res. 1992;597:321–330. doi: 10.1016/0006-8993(92)91489-2. [DOI] [PubMed] [Google Scholar]
- MCNAUGHTON F.L., FEINDEL W.H.Innervation of intracranial structures: a reappraisal Physiological aspects of Clinical Neurology. 1977Oxford: Blackwell Scientific Publications; 279–293.ed. Rose, F.C. [Google Scholar]
- MENKEN M., MUNSAT T.L., TOOLE J.F. The global burden of disease study–implications for neurology. Arch. Neurol. 2000;57:418–420. doi: 10.1001/archneur.57.3.418. [DOI] [PubMed] [Google Scholar]
- MITSIKOSTAS D.D., SANCHEZ DEL RIO M., WAEBER C., HUANG Z., CUTRER F.M., MOSKOWITZ M.A. Non-NMDA glutamate receptors modulate capsaicin induced c-fos expression within trigeminal nucleus caudalis. Br. J. Pharmacol. 1999;127:623–630. doi: 10.1038/sj.bjp.0702584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSIKOSTAS D.D., SANCHEZ DEL RIO M., WAEBER C., MOSKOWITZ M.A., CUTRER F.M. The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain. 1998;76:239–248. doi: 10.1016/s0304-3959(98)00051-7. [DOI] [PubMed] [Google Scholar]
- MOGIL J.S., PASTERNAK G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- NAPPI G., FACCHINETTI F., MARTIGNONI E., PETRAGLIA F., MANZONI G.C., SANCES G., SANDRINI G., GENAZZANI A.R. Endorphin patterns within the headache spectrum disorders. Cephalalgia. 1985;5:201–210. doi: 10.1177/03331024850050S240. [DOI] [PubMed] [Google Scholar]
- OPHOFF R.A., TERWINDT G.M., VERGOUWE M.N., VAN EIJK R., OEFNER P.J., HOFFMAN S.M.G., LAMERDIN J.E., MOHRENWEISER H.W., BULMAN D.E., FERRARI M., HAAN J., LINDHOUT D., VAN OMMEN G.-J.B., HOFKER M.H., FERRARI M.D., FRANTS R.R. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- PASTERNAK G.W. Pharmacological mechanisms of opioid analgesics. Clin. Neuropharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- PELTON J.T., KAZMIERSKI W., GULYA K., YAMAMURA H.I., HRUBY V.J. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for μ opioid receptors. J. Med. Chem. 1986;29:2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- PIROS E.T., HALES T.G., EVANS C.J. Functional analysis of cloned opioid receptors in transfected cell lines. Neurochem. Res. 1996;21:1277–1285. doi: 10.1007/BF02532368. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN B.K. Epidemology of headache. Cephalalgia. 1995;15:45–68. doi: 10.1046/j.1468-2982.1995.1501045.x. [DOI] [PubMed] [Google Scholar]
- RAYNOR K., KONG H., CHEN Y., YASUDA K., YU L., BELL G.I., REISINE T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol. Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- REISINE T., PASTERNAK G.Opioid analgesics and antagonists The Pharmacological Basis of Therapeutics. 1996New York: McGraw-Hill; 521–556.ed. Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W. & Gilman, A.G. [Google Scholar]
- RHIM H., MILLER R.J. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J. Neurosci. 1994;14:7608–7615. doi: 10.1523/JNEUROSCI.14-12-07608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL M.B., OLESEN J. Increased familial risk and evidence of genetic factor in migraine. BMJ. 1995;311:541–544. doi: 10.1136/bmj.311.7004.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER M., HOLLOWAY S., HATHWAY G., WOOLF C.J., HUMPHREY P.P., EMSOM P.C. Identification of somatostatin sst2(a) receptor expressing neurones in central regions involved in nociception. Brain Res. 1998;798:25–35. doi: 10.1016/s0006-8993(98)00361-8. [DOI] [PubMed] [Google Scholar]
- SCHROEDER J.E., FISCHBACH P.S., ZHENG D., MCCLESKEY E.W. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991;6:13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- SILBERSTEIN S.D., MCCRORY D.C. Opioids. Cephalalgia. 2000;20:854–864. doi: 10.1046/j.1468-2982.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- STORER R.J., AKERMAN S., GOADSBY P.J. Opioid receptors modulate nociceptive neurotransmission in the trigeminocervical complex. Cephalalgia. 2001;21:354. doi: 10.1038/sj.bjp.0705034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORER R.J., BUTLER P., HOSKIN K.L., GOADSBY P.J. A simple method, using 2-hydroxypropyl-β-cyclodextrin, of administering α-chloralose at room temperature. J. Neurosci. Meth. 1997;77:49–53. doi: 10.1016/s0165-0270(97)00110-6. [DOI] [PubMed] [Google Scholar]
- STORER R.J., GOADSBY P.J. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain. 1997;120:2171–2177. doi: 10.1093/brain/120.12.2171. [DOI] [PubMed] [Google Scholar]
- STORER R.J., GOADSBY P.J. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience. 1999;90:1371–1376. doi: 10.1016/s0306-4522(98)00536-3. [DOI] [PubMed] [Google Scholar]
- SUH H.H., TSENG L.F. Different types of opioid receptors mediating analgesia induced by morphine, DAMGO, DPDPE, DADLE and beta-endorphin in mice. Naunyn Schmiedebergs Arch. Pharmacol. 1990;342:67–71. doi: 10.1007/BF00178974. [DOI] [PubMed] [Google Scholar]
- WANG X.-M., MOKHA S.S. Opioids modulate N-methyl-D-aspartic acid (NMDA)-evoked responses of trigeminothalamic neurons. J. Neurophysiol. 1996;76:2093–2096. doi: 10.1152/jn.1996.76.3.2093. [DOI] [PubMed] [Google Scholar]
- WANG X.-M., ZHANG K.M., LONG L.O., FLORES C.A., MOKHA S.S. Endomorphin-1 and endomorphin-2 modulate responses of trigeminal neurons evoked by N-methyl-D-aspartic acid and somatosensory stimuli. J. Neurophysiol. 2000;83:3570–3574. doi: 10.1152/jn.2000.83.6.3570. [DOI] [PubMed] [Google Scholar]
- WEILLER C., MAY A., LIMMROTH V., JUPTNER M., KAUBE H., SCHAYCK R.V., COENEN H.H., DIENER H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat–intravital microscope studies. Cephalalgia. 1997a;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HILL R.G., SHEPHEARD S.L., HARGREAVES R.J. The anti-migraine 5-HT1B/1D agonist rizatriptan inhibits neurogenic dural vasodilation in anaesthetized guinea-pigs. Br. J. Pharmacol. 2001a;133:1029–1034. doi: 10.1038/sj.bjp.0704162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., COOK D.A., HARGREAVES R.J., HILL R.G., CUMBERBATCH M.J. Role of opioid receptors in neurogenic dural vasodilation and sensitization of trigeminal neurones in anaesthetized rats. Br. J. Pharmacol. 2001b;133:807–814. doi: 10.1038/sj.bjp.0704136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., HILL R.G., HARGREAVES R.J. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur. J. Pharmacol. 1997b;328:61–64. doi: 10.1016/s0014-2999(97)83028-2. [DOI] [PubMed] [Google Scholar]
- WOLFF H.G. Headache and Other Head Pain. New York: Oxford University Press; 1948. [Google Scholar]
- WONG C.L., CHAN Y.S., CHEUNG Y.M., HWANG J.C., POON P.W., WONG T.M. Effects of superfusion of morphine and enkephalins on the activity of single units in the spinal trigeminal nucleus and cuneate nucleus of cat. Methods Find. Exp. Clin. Pharmacol. 1986;8:351–355. [PubMed] [Google Scholar]
- ZHANG K.-M., WANG X.-M., MOKHA S.S. Opioids modulate N-methyl-D-aspartic acid (NMDA)-evoked responses of neurons in the superficial and deeper dorsal horn of the medulla (trigeminal nucleus caudalis) Brain Res. 1996;719:229–233. doi: 10.1016/0006-8993(96)00123-0. [DOI] [PubMed] [Google Scholar]
- ZIEGLER D.K.Opioids in headache treatment. Is there a role Neurol. Clin. North Am. 1997Philadelphia: WB Saunders; 199–207.ed. Mathew, N.T. [DOI] [PubMed] [Google Scholar]


