Abstract
RT–PCR and Western blots were used to detect bradykinin B2 receptors in testis and isolated peritubular cells of pre-pubertal rats. RT–PCR demonstrated expression of a single transcript, whereas Western blots showed up to three specific bands that were in accordance with the described native, glycosylated and dimeric form of B2 receptor proteins, respectively.
Fura-2-loaded peritubular cells responded with an instantaneous, linear and transient rise in [Ca2+]i after adding bradykinin. Stimulation of cells with bradykinin concentrations between 1 μM and 1 pM showed a dose dependent increase of [Ca2+]i. The calcium response to bradykinin was diminished after stimulation of peritubular cells in calcium-free buffer. After blocking the SERCA-pumps by thapsigargin and subsequent stimulation with bradykinin, no rise of [Ca2+]i was appreciated.
Multiple stimulation of a single peritubular cell by local perfusion with a brief addition of BK (10 nM) resulted in a fast and immediate response. However, the second and third stimuli had slower rise rates and diminished [Ca2+]i peaks, showing desensitization of the kinin receptor.
Addition of the bradykinin B1 receptor agonist [des-Arg9]-bradykinin (100 nM) to Fura-2-loaded peritubular cells did not change the [Ca2+]i. However, the B2 receptor antagonist HOE 140 (100 nM) strongly inhibited the bradykinin-induced calcium response.
We conclude that the bradykinin-induced increase in [Ca2+]i, in testicular peritubular cells is mediated by the stimulation of kinin receptors of the B2 subtype.
Keywords: Kinin B2 receptor, bradykinin, intracellular calcium, rat, testis, peritubular cells, male reproduction
Introduction
Bradykinin receptors are part of the tissue kallikrein kinin system (tKKS) that triggers a variety of physiological functions. Pharmacologically, two subtypes of bradykinin receptors can be distinguished. The B2 receptor (B2R) exhibits high affinity for bradykinin and kallidin (lys-bradykinin), whereas the B1 receptor (B1R) shows high affinity for the carboxyl terminally truncated kinins, [des-Arg9]-bradykinin and [des-Arg9]-kallidin. The B2R is constitutively expressed in many tissues, whereas the B1R is almost undetectable but is up-regulated under certain pathological conditions such as inflammation or tissue injury. Both subtypes belong to the G protein-coupled receptor family characterized by seven membrane-spanning helices (Marceau, 1997; Müller-Esterl, 1997; Regoli et al., 2001). The signalling pathways of kinin receptors include the activation of G proteins that stimulate the activity of phospholipase C (PLC) resulting in phosphatidylinositol (PI) turnover and a transient increase in intracellular free calcium ion concentration [Ca2+]i (Lee et al., 1993). The rise in [Ca2+]i increases the levels of nitric oxide (NO) and cGMP. A second signalling cascade includes phospholipase A2 (PLA2), which releases the prostaglandin precursor, arachidonic acid (Burch & Axelrod, 1987).
Peritubular cells are contractile myoid cells that are embedded within the lamina propria surrounding the seminiferous tubules of the testis. In a sexually mature adult, each seminiferous tubule has a central lumen lined by an actively replicating germinal epithelium mixed with a population of non-dividing, supporting cells–the Sertoli cells. Peritubular cells produce several factors that are involved in seminiferous tubule contractility and extracellular matrix formation (Holstein et al., 1996). It has been suggested that these cells also secrete the peritubular modifying substance (PmodS) which influences the biosynthesis of products from Sertoli cells (Skinner, 1993). In the rat, expression of B2 receptor mRNA occurs in testis (Mceachern et al., 1991) and isolated testicular cells (Monsees et al., 1999). Recently, we detected B2R protein in the testis of pre-pubertal rats by immune histochemistry (Monsees et al., 2002). However, there is limited knowledge on the presence of kinin receptors in testicular peritubular cells. Thus, the aim of this study was to determine whether or not these cells express a functional B2 receptor.
Methods
Isolation and culture of primary peritubular cells
Peritubular cells were isolated from pre-pubertal Sprague–Dawley rats (18–21 days postnatal) as described previously (Wennemuth et al., 2000) with some modifications. In brief, testes were rinsed twice in DHM (Dulbecco modified Eagle medium+Ham's F-12 medium; 1 : 1) containing 1 ml of gentamycin solution. After decapsulation, the tissue was minced into small fragments and incubated in DHM containing collagenase (1 mg ml−1) and DNase (20 μg ml−1) for 15 min at 37°C with constant shaking.
Thereafter, the tubule fragments were allowed to settle for 7 min at room temperature. The pellet was resuspended in DHM containing soybean trypsin inhibitor (400 μg ml−1 DHM, supplemented with 2 mg ml−1 bovine serum albumin) to stop enzymatic cleavage and the tubule fragments again were allowed to settle for 7 min. Then the pellet was resuspended in a solution of collagenase (2 mg ml−1), hyaluronidase (2 mg ml−1), and DNase (20 μg ml−1) in DHM and incubated for 30 min at 37°C. Thereafter, the cell suspension was centrifuged for 45 s at 43×g. The supernatant containing the peritubular cells was removed and diluted with the 1.5 times its volume of 10 mM phosphate buffered saline. Peritubular cells were collected by centrifugation for 10 min at 50×g. The pellet was resuspended in Nutrient Mixture F-10 supplemented with 15% horse serum, 3% foetal calf serum, 2 mM L-glutamine, and 1% penicillin/streptomycin. Peritubular cells were seeded in 250 ml culture flasks and incubated at 37°C. After two passes, the purity of the peritubular cells was >98% as estimated by histochemical staining for α-smooth muscle isoactin (Tung & Fritz, 1990) and morphological examination for germ cells.
Detection of B2 receptor mRNA by RT–PCR
Samples of rat testes were removed, mechanically crushed in liquid nitrogen, and total RNA were extracted using the Qiagen RNeasy Midi Kit according to the manufacturer's instruction. Additionally total RNA from peritubular cells in primary culture were obtained using the Qiagen RNeasy Mini Kit. RNA from each sample were reverse transcribed into cDNA using oligo dT15-primers. Briefly, each reaction tube consisted of 2 μg of total RNA, 1 μg of oligo dT15-primers, 5 μl of 5×RT-buffer (250 mM Tris, pH 8.3, 375 mM KCl, 15 mM MgCl2, 50 mM DTT), 1.25 μl of 10 mM dNTP, 25 U RNAsin ribonuclease inhibitor, 200 U of Moloney murine leukaemia virus reverse transcriptase, and sterile distilled water to a final volume of 25 μl. This was incubated at 36°C for 1 h to synthesize the first strand of cDNA. The RT enzyme was inactivated by heating to 70°C for 10 min.
One-fourth (6 μl) of this reaction product was used as a template for PCR in combination with 1 μM of each of the specific bradykinin B2 receptor primers, described next. The upstream primer was 5′-CCA TCT CTC CAC CTG CAT TG-3′ (B2R3/98). It spanned intron2 and corresponded to position 2075–2090 on exon2 and 2892–2896 on exon3 according to the rat B2R gene sequence (Wang et al., 1994). The downstream primer was 5′-CGT CTG GAC CTC CTT GAA CT-3′ (B2R10/99). It derived from position 3615–3596 on exon 3 (El-Dahr et al., 1997). Primers for β-actin (loading control) were as follows: upstream primer 5′-GGC CAA CCG TGA AAA GAT GAC-3′; downstream primer 5′-ATT GCC GAT AGT GAT GAC CTG-3′ (Macdonald et al., 1996). Six μl RT product and 2 μl of each of the 25 μM specific primers described above were mixed with 5 μl of 10×PCR buffer (100 mM Tris-HCl, pH 9.0, 500 mM KCl, 1% Triton), 3 μl of 25 mM MgCl2, 1 μl of each of the 10 mM dNTP, 1.25 U of Taq-Polymerase, and sterile RNAse-free distilled water to a final volume of 50 μl. After denaturation at 94°C for 5 min, the following cycling parameters were used for amplification of the bradykinin B2 receptor: denaturation at 94°C for 45 s, annealing at 58°C for 1 min, and extension at 72°C for 1 min. A total of 40 cycles were performed. A final extension step at 72°C for 7 min followed. Amplification of β-actin was performed using the same procedure with the following adjustments: denaturation for 1 min, annealing at 57°C for 1 min, and extension at 72°C for 1.5 min. A total of 25 cycles were applied.
A 9 μl aliquot from each amplified sample tube was analysed in a 2% agarose gel in TBE buffer (90 mM Tris-Borate, 0.2 mM EDTA, pH 8.4) and visualized by ethidium bromide staining. The expected sizes of the PCR products were 739 bp and 412 bp for the bradykinin B2 receptor and β-actin, respectively. Negative controls were included for each set of RT–PCR by omitting the sample in the RT reaction and the RT product in the PCR step.
DNA sequencing
Representative RT–PCR products of the bradykinin B2 receptor gene were excised from the agarose gel and purified using the QIAquick Gel Extraction Kit according to the manufacturer's instructions. Purified RT–PCR products were sequenced using B2R3/98 and B2R10/99 primers and the ABI PRISM Big Dye Terminator Cycle Sequencing Kit on an automated ABI PRISM 310 Genetic Analysis Sequencer (PE Applied Biosystems, Weiterstadt, Germany). Sequences were analysed using the Sequencing Analysis program (PE Applied Biosystems) and compared with the published B2R gene sequence (Wang et al., 1994) with the Sequence Navigator program (PE Applied Biosystems).
Immunoblotting of bradykinin B2 receptor
Rat testis were mechanically crushed in liquid nitrogen, dissolved in five times its volume of lysis buffer (1% SDS, 1 mM sodium ortho-vanadate, 10 mM Tris-HCl, pH 7.4) and heated for 15 s at 900 W in a microwave oven. Peritubular cells were washed with cold phosphate-buffered saline (PBS, 10 mM, pH 7.3). Thereafter, lysis buffer at 90°C was added and the cells were scraped from the dish. Protein content from tissue or cell extracts was determined using BioRad's protein assay. Aliquots of the protein (250 μg) or cell (125 μg) extracts were freeze dried and dissolved in sample buffer (125 mM Tris-HCl, pH 6.8, 10% glycerol, 4% SDS, 0.006% bromophenol blue, 1.8% β-mercaptoethanol). These protein extracts were heated for 5 min at 95°C, run in each lane of a 12% polyacrylamide gel and then electrophoretically transferred to polyvinylidene difluoride membranes (PVDF, Applied Biosystems, CA, U.S.A.) using transfer buffer (25 mM Tris-HCl, 190 mM glycine, and 20% methanol).
Detection and visualization of the B2R protein was performed using a monoclonal primary antibody and the Amplified Opti 4 CN Detection Kit according to the manufacturer's instructions. In brief, the PVDF membranes underwent the following series of room-temperature incubations, all washing steps lasted 5 min unless otherwise stated: (1) incubation for 5 min in methanol, (2) two washes in PBST (PBS plus 0.1% Tween 20), (3) preincubation for 1 h in PBST containing 3% blocking solution (BioRad), (4) two washes in PBST, (5) incubation with a 1 : 1000 dilution of B2R antisera in PBST containing 1% bovine serum albumin (BSA) for 1 h, (6) two rinses in PBST, (7) incubation with a 1 : 3000 dilution of the secondary antibody (goat anti-mouse) in PBST containing 1% BSA for 1 h, (8) two rinses in PBST, (9) incubation with amplification reagent (BioRad) for 10 min, (10) four rinses in PBST containing 20% dimethyl sulphoxide of 5 min each, (11) two rinses in PBST, (12) incubation with streptavidin-HRP in PBST (BioRad) containing 1% BSA, (13) two rinses in PBST, (14) incubation with staining solution (BioRad) for 5–10 min and (15) four 15 min rinses in water.
Dye loading and photometry
For single cell measurements, cells grown as a monolayer on coverslips were washed three times with HEPES-buffered salt solution (HBSS, pH 7.4; 10 mM HEPES, 135 mM NaCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 5 mM KCl, 0.8% dextrose) before incubation at 32°C for 30 min with Fura-2 AM. Three μl of a 3 mM stock solution of Fura-2 AM (in dimethylsulphoxide) was mixed with 3 μl of Pluronic F-127 (10%) and adjusted with HBSS to a final volume of 1 ml. After incubation with Fura-2 AM for 30 min, cells were washed again twice with HBSS as described above. The intracellular Fura-2 AM was hydrolyzed by incubating the cells an additional 30 min at 32°C with 5% CO2. For cuvette measurements, cells grown in cell culture flasks were washed three times with HBSS before incubation with trypsin (0.05%) for 30 s. The reaction was stopped with RPMI medium. The cell suspension was placed in a 50 ml Falcon tube and washed twice by centrifugation at 500×g for 1 min and resuspended in HBSS before it was loaded with Fura-2 AM. Loading was accomplished by resuspending the pellet in 1 ml of a 3 μM Fura-2 AM solution. After 30 min of incubation at 32°C with 5% CO2, cells were washed again twice by centrifugation at 500×g for 1 min. Cells were adjusted to a concentration of 1–2×106 cells ml−1 with HBSS and were stored at 32°C with 5% CO2 for 30–60 min prior to performing photometric measurements.
The dye-loaded cells were placed into a measuring chamber and examined with a 40× oil objective and a 10× ocular lens in an inverted microscope (Nikon, Diaphot 400, Düsseldorf, Germany) or 2 ml of the cell suspension was placed in a cuvette and fluorescence was monitored by a spectrofluorometer (PTI, Wedel, Germany). Emission intensities were recorded as a ratio (340–380 nm−1) at 510 nm with a photomultiplier (PTI, Wedel, Germany). The background-corrected signal (R) was calibrated by applying the standard equation [Ca2+]=Kd(R-Rmin) (Rmax-R)−1 where Rmin and Rmax were determined empirically from cells (Grynkiewicz et al., 1985).
Test solutions were added directly to the bath or the cuvette. In some experiments, drugs were applied with a solenoid-controlled, gravity-fed, multibarrelled local perfusion system as previously described (Wennemuth et al., 1998, 2000).
Materials
Dulbecco modified Eagle medium: Ham's F-12 medium, Nutrient Mixture F-10, RPMI medium, penicillin/streptomycin solution, horse serum and foetal calf serum were purchased from Gibco BRL (Karlsruhe, Germany). Gentamycin, bovine serum albumin, collagenase, hyaluronidase, DNase, soybean trypsin inhibitor, Pluronic F-127, bradykinin, [des-ArgH]-bradykinin and HOE 140 were obtained from Sigma (Deisenhofen, Germany). L-glutamine originated from Biochrome (Berlin, Germany). Qiagen RNeasy Midi Kit, Qiagen RNeasy Mini Kit and QIAquick Gel Extraction Kit were purchased from Qiagen (Hilden, Germany). Oligo dT15-primers, dNTP Promega, RNAsin ribonuclease inhibitor, Moloney murine leukaemia virus reverse transcriptase and Taq-Polymerase were obtained from Promega (Mannheim, Germany). BioRad protein assay, Amplified Opti 4 CN Detection Kit and secondary antibody (goat antimouse) were purchased from BioRad (Hercules, U.S.A.). The B2R monoclonal primary antibody (mouse) was obtained from Transduction Laboratories (Lexington, KY, U.S.A.). Fura-2 AM was purchased from Molecular Probes (Leiden, Netherlands). RPMI medium originated from Greiner (Frickenhausen, Germany).
Data analysis
Calibration and kinetic analysis of digital photometric records were performed in Igor Pro Vers. 4.0.2.1 (Wavemetrics, Lake Oswego, OR, U.S.A.). Statistical calculations were performed in Excel (Microsoft, Redmond, WA, U.S.A.).
Results
Expression of the bradykinin B2 receptor mRNA
A 739-bp PCR product corresponding to the expected size of bradykinin B2 receptor mRNA was detected in testis and peritubular cells isolated from immature rats (Figure 1). The 739-bp PCR product was subsequently confirmed to be authentic B2R by direct nucleotide sequence analysis. The negative controls (C1, no RNA; C2, no template) displayed no bands, indicating the accuracy of the reaction conditions. To ensure that equal amounts of RNA were reverse-transcribed and amplified in each reaction tube, β-actin was also amplified in these experiments to yield the expected band at 412 bp.
Figure 1.
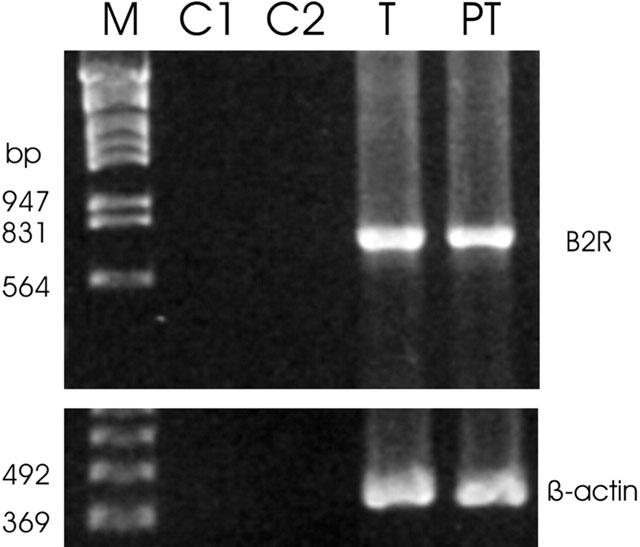
RT–PCR analysis of bradykinin B2 receptor (B2R) mRNA expression in testis (T) and isolated peritubular cells (PT) from immature rats (18 days). The expected size of the PCR products is 739 bp for B2R and 412 bp for β-actin, respectively. Negative controls: C1, no RNA; C2, no template; M, marker.
Immunoblotting of the bradykinin B2 receptor
Western blots, using a monoclonal antibody raised against the C-terminal peptide 350–364 of the bradykinin B2 receptor, detected two proteins with relative molecular weights of approximately 42,000 and 45,000 in immature testis (Figure 2). Peritubular cells displayed a major band at approximately 42,000 Mr and two minor bands at approximately 45,000 and 132,000 Mr.
Figure 2.
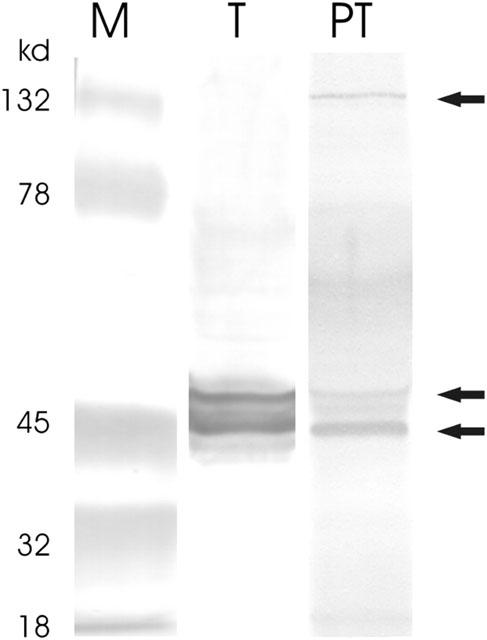
Western blot analysis of the bradykinin B2 receptor in testis (T) and isolated peritubular cells (PT) from immature rats (18 days). Proteins were separated by electrophoresis using a 12% polyacrylamide gel (SDS–PAGE) and blotted on a PVDF membrane. Detection and visualization of the B2R protein was performed using a monoclonal B2R-antibody (mouse, Transduction Laboratories, Lexington, KY, U.S.A.) and the Amplified Opti 4 CN Detection Kit (BioRad, Hercules, U.S.A.). Arrows indicated three B2R protein bands with relative molecular masses of approximately 42, 45 and 132 kDa. M=molecular weight marker.
BK induced [Ca2+]i elevation in peritubular cells
Fura-2-loaded peritubular cells responded with an instantaneous, linear and transient rise in [Ca2+]i after adding BK. In Figure 3, BK was added to the cuvette at a final concentration of 10 nM. To test the need for extracellular Ca2+ we used Ca2+-deficient HBSS (HBSS0). Cells were placed in HBSS0 only a few seconds (<5 s) before stimulation with BK to prevent depletion of internal stores. The addition of BK showed a diminished maximal [Ca2+]i response with a mean value of 124.23 nM (±22.55 SE; n=7; Figure 4A). Subsequent addition of 2 mM Ca2+ led to a high increase in [Ca2+]i (Figure 4B). Pretreatment of cells with 5 μM thapsigargin showed a transient rise in [Ca2+]i, but subsequent stimulation with BK did not increase [Ca2+]i (Figure 4C). Stimulation of cells with BK concentrations between 1 μM and 1 pM showed a dose dependent rise of [Ca2+]i. Figure 5 depicts the averaged Δ[Ca2+]i signals as a function of BK–where Δ[Ca2+]i is defined as the maximal Ca2+ response to BK minus the resting [Ca2+]i. We used local perfusion to stimulated single peritubular cells with BK multiple times. Between the stimuli, cells were perfused with HBSS. Figure 6A shows a typical example of the outcome of eight independent experiments. The cell was stimulated three times with a brief (12–40 s) perfusion of BK (10 nM). All stimuli showed a fast and immediate response. The second and third stimuli had slower rise rates and diminished [Ca2+]i peaks, pointing to a receptor desensitization process.
Figure 3.
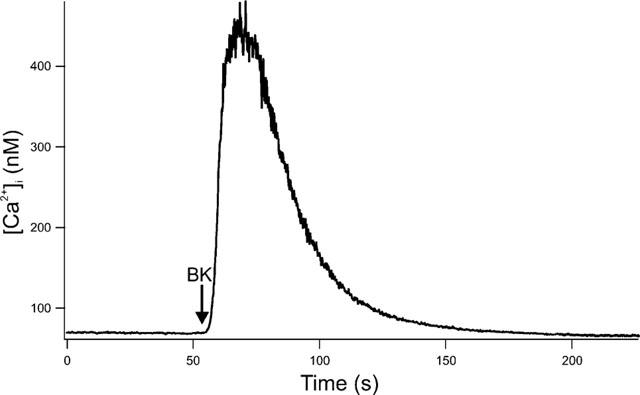
Effect of BK on [Ca2+]i in Fura-2-loaded peritubular cells. Original recording of peritubular cells stimulated with BK in a final concentration of 10 nM. 200,000 cells ml−1 were stimulated in a cuvette-based system. The arrow marks the point of BK addition to the cuvette. Subsequently the cells responded with a transient rise in [Ca2+]i. The picture shows one typical experiment out of seven.
Figure 4.
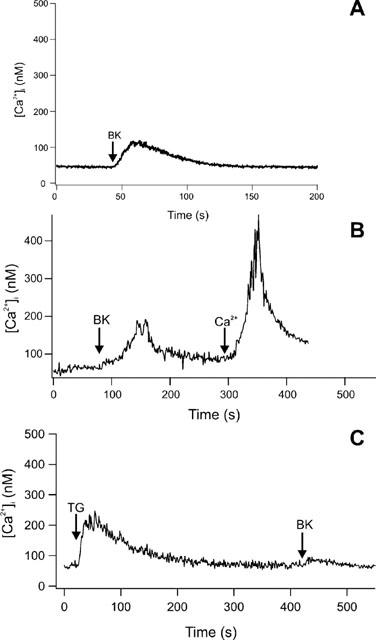
(A) Increase of [Ca2+]i in peritubular cells by stimulation with BK at a final concentration of 10 nM in a nominally Ca2+-free external HBSS. The stimulus was followed by a diminished calcium response compared to the stimulation in the presence of external calcium. (B) Addition of Ca2+ (2 mM) after BK stimulation in nominally Ca2+-free external HBSS led to an instantaneous and linear rise of [Ca2+]i (C) The SERCA-pumps of the endoplasmic reticulum were blocked by 5 μM thapsigargin. After a transient rise in [Ca2+]i cells were stimulated with BK (10 nM). No substantial rise in [Ca2+]i was detectable. All figures show one typical experiment out of at least seven.
Figure 5.
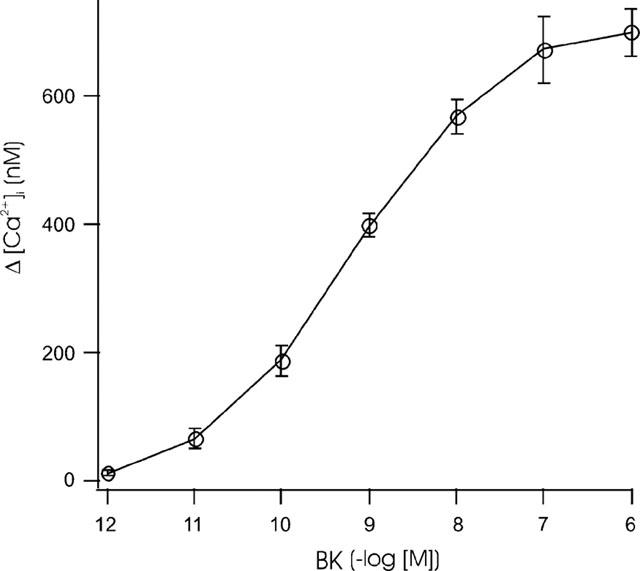
Response of Fura-2-loaded peritubular cells to increasing concentrations of BK. BK concentrations of 1 μM to 1 pM were used to stimulate the cells in a cuvette-based system and monitored by a photomultiplier. The basal [Ca2+]i was subtracted from the maximal Ca2+ responses (Δ[Ca2+]i). At least five experiments were averaged for each BK concentration and are graphed with their standard errors.
Figure 6.
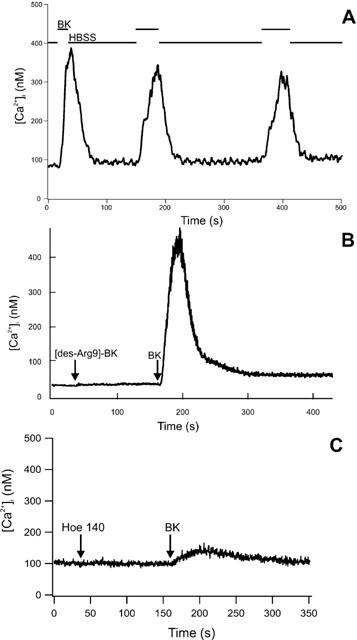
(A) Local perfusion of peritubular cells with BK. Fluorescence was recorded from a single Fura-2-loaded peritubular cell perfused with HBSS. The lines above the recording indicate the change between HBSS and BK perfusion. The recording shows three brief stimulations with 10 nM BK. BK was applied with a local, solenoid-controlled perfusion device with an exchange time between different buffers of <1 s. All three stimulations led to an increase in [Ca2+]i. (B) [Ca2+]i response to BK following stimulation of bradykinin B1 receptor (B1R). In cuvette experiments, the specific B1R agonist [des-Arg9]-BK had no effect on [Ca2+]i when added to Fura-2-loaded peritubular cells 2 min prior to stimulation with BK. No inhibition of the [Ca2+]i response was noticed after adding BK (10 nM). (C) Using the same protocol described in the legend to Figure 6B with the specific B2R antagonist HOE 140 instead of [des-Arg9]-BK showed a strong inhibition of the [Ca2+]i response after BK stimulation. HOE 140 itself did not alter the [Ca2+]i.
To evaluate what subtype of kinin receptors are required for peritubular cells to respond to BK, we used a specific agonist of the bradykinin B1 receptor (B1R) and a specific antagonist of the bradykinin B2 receptor, respectively. Stimulation of B1R, by addition of [des-Arg9]-BK (100 nM) to the cuvette containing Fura-2-loaded peritubular cells, showed no influence on the [Ca2+]i (Figure 6B). Also a 1 μM concentration of [des-Arg9]-BK did not evoke a rise in calcium levels (not shown). Application of BK (10 nM) after the addition of [des-Arg9]-BK did not show any difference when compared to stimulation with BK alone. The average value of the [Ca2+]i peak after BK stimulation was 606.95 nM (±39.55 s.e.; n=6). Preincubation (2 min) with the specific inhibitor of B2R, HOE 140 (100 nM), did not change the [Ca2+]i level (Figure 6C). However, subsequent stimulation with BK (10 nM) resulted in a dramatic decrease of the [Ca2+]i peak (17.74 nM±6.13 s.e.; n=9) when compared to the uninhibited reaction.
Discussion
The major finding of the present study is the observation that the bradykinin-induced increase in [Ca2+]i in testicular peritubular cells is mediated via B2 receptors. Local production of kinins occurs on the surface of the target cell by the action of the protease tissue kallikrein on the inactive precursor low molecular weight kininogen (Hess, 1997). The main source for kininogens are liver and kidney tissue. However, at least in rodents, small amounts of low molecular weight kininogen are also expressed in other tissues including the testis (Takano et al., 1997), but their precise cellular location is still unknown. Tissue kallikrein is also expressed in rat testis (Clements, 1997). Using immune histochemistry we located tissue kallikrein on the acrosomal cap of round and elongated spermatids of pubertal and mature rats (Monsees et al., 1999) and–to a lesser extent–on peritubular cells of pre-pubertal rats (unpublished observation). Thus, formation of kinins in rodent testis, especially in the seminiferous tubule, is likely. The liberated kinins act on the B2 receptor or are rapidly inactivated by specific peptidases which are called kininases and are also located on the plasma membrane. Kininases are present in Sertoli cells of the rat testis (Monsees et al.,1996a, b; 1998). Recently we also detected activities of several kininases in peritubular cells isolated from pre-pubertal rat testis (unpublished observations).
The present study demonstrates for the fist time a functional bradykinin B2 receptor in peritubular myoid cells of the pre-pubertal rat testis. Previously, Mceachern et al. (1991) showed expression of B2 receptor mRNA in different tissues of the rat, including kidney, vas deferens and testis. By immune histochemistry, we recently located the B2 receptor in different testicular cells, including peritubular and endothelial cells, pachytene spermatocytes and spermatids (Monsees et al., 2002). Here, we showed expression of the B2 receptor gene by RT–PCR and identified three B2 receptor proteins using immune blot technique. The first protein band consistent with cDNA data of rat B2R, i.e. an unmodified protein of 366 residual amino acid with an Mr of 41,696 (Mceachern et al., 1991). The two proteins with higher molecular weights may correspond to glycosylated structures of the bradykinin B2 receptor, that likely form oligomers after activation (Abd Alla et al., 1996; Blaukat et al., 1999).
Bradykinin was found to increase intracellular calcium levels, [Ca2+]i, in different cell types, including fibroblasts, tracheal, epithelial and adrenal cells (Farmer et al., 1991; Houchi et al., 1993; Issandou & Rozengurt, 1990; Ricciardolo et al., 1998). The present study demonstrated, that bradykinin also stimulated [Ca2+]i in testicular myoid peritubular cells and that bradykinin B2 receptors are involved in this mechanism. This conclusion is supported by several lines of evidence. On the molecular level, we showed expression of the B2R mRNA using primers (Monsees et al., 2002) and B2R using a monoclonal antibody specific for the B2-subtype of the kinin receptor (Ju et al., 1998). On the pharmacologic level, we observed that an increase in [Ca2+]i is induced by physiological concentrations of BK, which had a high affinity to the B2-subtype kinin receptor (Hall & Morton, 1997). In contrast, even μM concentrations of [des-Arg9]-bradykinin, a typical B1-subtype receptor agonist (Hall & Morton, 1997), were totally inactive. Furthermore, the selective B2 receptor antagonist HOE 140 (Hall & Morton, 1997) strongly inhibited the increase in [Ca2+]i induced by bradykinin. These findings indicate that the rise in [Ca2+]i in testicular peritubular cells is mediated by the stimulation of kinin receptors of the B2 subtype. The pharmacological profile of the agonists and antagonists used in this study have been studied in detail previously (Regoli & Barabé, 1980; Regoli et al., 1998; 2001). Based on the results of different bioassays, rank orders of potency were described for agonists and antagonists of the B1R and B2R respectively. The selective and specific B2R antagonist HOE 140 blocks with high affinity (IC50=6 10−10 M) the effect of agonists on the B2R but shows almost no antagonistic properties (IC50>10−5 M) on the B1R. Similarly, [des-Arg9]-bradykinin binds with high affinity (EC50=5 10−8 M) to the B1R but is almost inactive (EC50>10−5 M) towards the B2R. Corresponding EC50 values for bradykinin are >10−5 M and 3 10−9 M, respectively (Regoli et al., 2001).
Our data clearly showed that the specific B1R agonist [des-Arg9]-bradykinin did not influence [Ca2+]i in isolated peritubular cells. In many tissues, B1 receptors co-exist and mediate similar effects, including transient increases in [Ca2+]i (Marceau, 1997). As this and our previous study (Monsees et al., 2002) investigated only the expression and transcription of the B2R, the expression and involvement of the bradykinin subtype 1 receptor in peritubular cell or testis physiology was not quantified. The B1R is generally absent from healthy tissues and animals but can be induced under pathological conditions such as injury or inflammation. The agonists for B1R are the carboxyl truncated kinins, [des-Arg9]-kallidin and [des-Arg9]-bradykinin. Thus, B1R upregulation by injury may extend the tissue response to kinin metabolites. In some pathological systems such as chronic inflammation, the B1R-mediated response becomes more evident with time as the effect mediated by B2R fades (Marceau, 1997).
In the present study we used peritubular cells which were isolated from pre-pubertal rats (18 days). At this age the tight junctions of the Sertoli cell barrier are not developed which simplifies tissue dissociation. Also, spermatogonia and pachytene spermatocytes are the only germ cells present, which makes it easier to get a pure preparation of peritubular cells. Preliminary data may point to possible development-dependent expression of the B2R (Monsees et al., 2002). Although semiquantitative RT–PCR analysis did not show significant differences in testicular B2R mRNA levels, immune histochemistry demonstrated variations in the location of the B2R protein in rats of different ages. Positive immune staining was observed in peritubular cells of testis slices from pre-pubertal (18 days) but not from sexually mature rats (53 days), which paralleled our results of the immune reaction for tissue kallikrein. However, further studies are needed to clarify development-dependent expression and the influence of androgens on the B2R expression in testicular cells.
In various cell types, both the influx of Ca2+ and the mobilization of sequestered intracellular Ca2+ have been demonstrated (Houchi et al., 1993; Yang et al., 1994). In our study, removal of external Ca2+ by use of a calcium-free buffer decreased the [Ca2+]i response of peritubular cells to bradykinin exposure. This indicates the possible release of Ca2+ from internal stores. The addition of Ca2+ after stimulating cells with BK in a Ca2+-free medium led to an [Ca2+]i increase. In another experiment the endoplasmic reticulum, a major internal Ca2+ store, was emptied by thapsigargin treatment. Subsequently, no [Ca2+]i rise was detectable after BK stimulation. This finding is an indicator that in the presence of external Ca2+, the initial rise in [Ca2+]i might lead to an opening of store operated channels (Icrac) localized in the plasma membrane. Repeated or continuous exposure to kinins alters the response of bradykinin receptors to agonists in a way that the cellular response is dampened or completely switched off. This phenomenon is called adaptation or desensitization and occurs in all biological systems (Böhm et al., 1997; Yang et al., 1994). We observed adaptation of the kinin receptor in peritubular cells of the rat testis. The desensitization process may result from receptor endocytosis preceded by agonist-induced dimerization and phosphorylation of the B2 receptor (Abd Alla et al., 1999; Blaukat et al., 1996; Pizard et al., 1999). Internalization of the B2 receptor seems to be a prerequisite for resensitization and receptor recycling to the plasma membrane (Lamb et al., 2001; Zhang et al., 1997).
Little is known about calcium responses induced by hormones in peritubular cells of the testis. Endothelin-1 (ET-1), produced by Sertoli cells, stimulates increases in [Ca2+]i in peritubular cells via ETA-subtype receptors (Santiemma et al., 1996). Recently, we showed that the macrophage migration inhibitory factor (MIF) induced a Ca2+ response in peritubular cells (Wennemuth et al., 2000). Small peptides such as oxytocin are regulators of peritubular myoid cell contraction (Niemi & Kormano, 1965). Thus, bradykinin may also be involved in triggering the contractility of these cells. This view is supported by the fact that cGMP or prostaglandins, which are involved in the second messenger cascade of oxytocin as well as bradykinin receptors, also stimulate peritubular cell contractions (Burch & Axelrod, 1987; Buhrley & Ellis, 1975; Farr & Ellis, 1980).
Bradykinin is known to induce cell growth and differentiation (Walsh & Fan, 1997), which are essential processes in testicular and peritubular cell development. Within the germ cell epithelium, bradykinin B2 receptors seem to be involved in the local regulation of spermatogenesis. In organ cultures of immature rat testis, a significant stimulation of prespermatogonial cell proliferation after exposure to bradykinin was reported (Atanassova et al., 1998). We recently demonstrated that certain germ cells express the B2R protein, which pointed to a possible involvement of the tissue kallikrein kinin system in regulating growth of pachytene spermatocytes and differentiation of round spermatids into highly elongated spermatozoa (Monsees et al., 2002).
The results of this study confirm that the bradykinin-induced increase in [Ca2+]i in testicular peritubular cells is mediated by stimulation of bradykinin B2 receptors. Thus, the tKKS may act as a local modulator of peritubular cell function in the rat testis.
Acknowledgments
The skillful technical assistance of Gabriele Thiele, Andrea Dersch and Wega-Maria Gutschank are gratefully acknowledged. Thanks to Drs Frank Heidorn and Guenter Weiler (Institute of Legal Medicine, JLU Giessen) for sequencing PCR products. This study was supported by the DFG (2344–4) and the Fonds der Chemischen Industrie. We thank JR Drew for critical reading of the manuscript.
Abbreviations
- BK
bradykinin
- B2R
bradykinin subtype 2 receptor
- [Ca2+]i
intracellular calcium ion concentration
- HBSS
HEPES-buffered salt solution
- HOE 140
DArg-[Hyp3, Thi5, DTic7, Oic8]-bradykinin
- tKKS
tissue kallikrein kinin system
References
- ABD ALLA S., QUITTERER U., GRIGORIEV S., MAIDHOFF A., HAASEMANN M., JARNAGIN K., MÜLLER-ESTERL W. Extracellular domains of the bradykinin B2 receptor involved in ligand binding and agonist sensing defined by anti-peptide antibodies. J. Biol. Chem. 1996;271:1748–1755. doi: 10.1074/jbc.271.3.1748. [DOI] [PubMed] [Google Scholar]
- ABD ALLA S., ZAKI E., LOTHER H., QUITTERER U. Involvement of the amino terminus of the B2 receptor in agonist-induced receptor dimerization. J. Biol. Chem. 1999;274:26079–26084. doi: 10.1074/jbc.274.37.26079. [DOI] [PubMed] [Google Scholar]
- ATANASSOVA N., KANCHEVA L., SOMLEV B. Bradykinin stimulates prepubertal germ cell proliferation in vitro. Immunopharmacology. 1998;40:173–178. doi: 10.1016/s0162-3109(98)00037-x. [DOI] [PubMed] [Google Scholar]
- BLAUKAT A., ABD ALLA S., LOHSE M.J., MÜLLER-ESTERL W. Ligand-induced phosphorylation/dephosphorylation of the endogenous bradykinin B2 receptor from human fibroblasts. J. Biol. Chem. 1996;271:32366–32374. doi: 10.1074/jbc.271.50.32366. [DOI] [PubMed] [Google Scholar]
- BLAUKAT A., HERZER K., SCHROEDER C., BACHMANN M., NASH N., MÜLLER-ESTERL W. Overexpression and functional characterization of kinin receptor reveal subtype-specific phosphorylation. Biochemistry. 1999;38:1300–1309. doi: 10.1021/bi981727r. [DOI] [PubMed] [Google Scholar]
- BÖHM S.K., GRADY E.F., BUNNETT N.W. Regulatory mechanism that modulate signaling by G protein-coupled receptors. Biochem. J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUHRLEY L.E., ELLIS L.C. Contractility of at testicular seminiferous tubules in vitro: Prostaglandin F1 and indomethacin. Prostaglandins. 1975;10:151–162. doi: 10.1016/0090-6980(75)90101-x. [DOI] [PubMed] [Google Scholar]
- BURCH R.M., AXELROD J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts. Evidence for a G protein regulation of phospholipase A2. Proc. Natl. Acad. Sci. U.S.A. 1987;84:6374–6381. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTS J.A.The molecular biology of kallikreins and their roles in inflammation The handbook of immunopharmacology. The kinin system. 1997San Diego: Academic Press; 71–98.ed. Farmer, S.G. [Google Scholar]
- EL-DAHR S.S., FIGUEROA C.D., GONZALES C.B., MÜLLER-ESTERL W. Ontogeny of bradykinin B2 receptors in the rat kidney: implications for segmental nephron maturation. Kidney Int. 1997;51:739–749. doi: 10.1038/ki.1997.105. [DOI] [PubMed] [Google Scholar]
- FARMER S.G., ENSOR J.E., BURCH R.M. Evidence that cultured airway smooth muscle cells contain bradykinin B2 and B3 receptors. Am. J. Respir./Cell. Mol. Biol. 1991;4:273–277. doi: 10.1165/ajrcmb/4.3.273. [DOI] [PubMed] [Google Scholar]
- FARR C.H., ELLIS L.C. In vitro contractility of rat seminiferous tubules in response to prostaglandins, cyclic GMP, testosterone and 2,4′-dibromoacetophenone. J. Reprod. Fertil. 1980;58:37–42. doi: 10.1530/jrf.0.0580037. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biochem. Sci. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HALL J.M., MORTON I.K.M.The pharmacology and immunopharmacology of kinin receptors The kinin system. 1997San Diego: Academic Press; 9–44.ed. Farmer, S.G. [Google Scholar]
- HESS J.F.Molecular pharmacology of kinin receptors The kinin system. 1997San Diego: Academic Press; 45–56.ed. Farmer, S. G. [Google Scholar]
- HOLSTEIN A.F., MAEKAWA M., NAGANO T., DAVIDOFF M.S. Myofibroblasts in the lamina propria of human seminiferous tubules are dynamic structures of heterogeneous phenotype. Arch. Histol. Cytol. 1996;59:109–125. doi: 10.1679/aohc.59.109. [DOI] [PubMed] [Google Scholar]
- HOUCHI H., MASUDA Y., ISHIMURA Y., OHUCHI T., MURAKUMO Y., OKA M. Calcium efflux from cultured bovine adrenal chromaffin cells induced by bradykinin. Biochem. Pharmcol. 1993;47:1309–1313. doi: 10.1016/0006-2952(94)90328-x. [DOI] [PubMed] [Google Scholar]
- ISSANDOU M., ROZENGURT E. Bradykinin transiently activates protein kinase C in Swiss 3T3 cells: distinction from activation by bombesin and vasopressin. J. Biol. Chem. 1990;265:11890–11896. [PubMed] [Google Scholar]
- JU H., VENEMA V.J., MARRERO M.B., VENEMA R.C. Inhibitory interactions of the bradykinin B2 receptor with endothelial nitric-oxide synthase. J. Biol. Chem. 1998;273:24025–24029. doi: 10.1074/jbc.273.37.24025. [DOI] [PubMed] [Google Scholar]
- LAMB M.E., DEWEERD W.F.C., LEEB-LUNDBERG L.M.F. Agonist-promoted trafficking of human bradykinin receptors: arrestin- and dynamin-independent sequestration of the B2 receptor and bradykinin in HEK293 cells. Biochem. J. 2001;355:741–750. doi: 10.1042/bj3550741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K.M., TOSCAS K., VILLEREAL M.L. Inhibition of bradykinin- and thapsigargin-induced Ca2+ entry by tyrosine kinase inhibitors. J. Biol. Chem. 1993;268:9945–9948. [PubMed] [Google Scholar]
- MACDONALD R.J., SOUTHARD-SMITH E.M., KROON E. Disparate tissue-specific expression of members of the tissue kallikrein multigene family. J. Biol. Chem. 1996;271:13684–13690. doi: 10.1074/jbc.271.23.13684. [DOI] [PubMed] [Google Scholar]
- MARCEAU F.Kinin B1 receptor induction and inflammation The handbook of immunopharmacology. The kinin system. 1997San Diego: Academic Press; 143–156.ed. Farmer, S.G. [Google Scholar]
- MCEACHERN A.E., SHELTON E.R., BHAKTA S., OBERNOLTE R., BACH C., ZUPPAN P., FUJISAKI J., ALDRICH R.W., JARNAGIN K. Expression cloning of the rat B2 receptor. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONSEES T.K., BLÖCHER S., HEIDORN F., WINKLER A., SIEMS W.E., MÜLLER-ESTERL W., HAYATPOUR J., MISKA W., SCHILL W.B.Expression and location of the bradykinin B2 receptor in rat testis Biol. Reprod. 200267(in press) [DOI] [PubMed] [Google Scholar]
- MONSEES T.K., GÖRNIG M., SCHILL W.-B., MISKA W. Possible involvement of proteases in the regulation of spermatogenesis. Andrologia. 1998;30:185–191. doi: 10.1111/j.1439-0272.1998.tb01160.x. [DOI] [PubMed] [Google Scholar]
- MONSEES T.K., MISKA W., BLÖCHER S., WINKLER A., SIEMS W.E. Elements of the kallikrein kinin system are present in rat seminiferous epithelium. Immunopharmacol. 1999;45:107–114. doi: 10.1016/s0162-3109(99)00062-4. [DOI] [PubMed] [Google Scholar]
- MONSEES T.K., MISKA W., SCHILL W.-B. Enzymatic digestion of bradykinin by rat Sertoli cell cultures. J. Androl. 1996a;17:375–381. [PubMed] [Google Scholar]
- MONSEES T.K., MISKA W., SCHILL W.-B. Characterization of kininases in testicular cells. Immunopharmacol. 1996b;32:169–171. doi: 10.1016/0162-3109(95)00084-4. [DOI] [PubMed] [Google Scholar]
- MÜLLER-ESTERL W.Immunological probes for the bradykinin B2 receptor. A toolbox The handbook of immunopharmacology. The kinin system. 1997San Diego: Academic Press; 99–110.ed. Farmer, S.G. [Google Scholar]
- NIEMI M., KORMANO M. Contractility of the seminiferous tubule of the postnatal rat testis, and its response to oxytocin. Ann. Med. Ep. Fenn. 1965;43:40–42. [PubMed] [Google Scholar]
- PIZARD A., BLAUKAT A., MÜLLER-ESTERL W., ALHENC-GELAST F., RAJERISON R.M. Bradykinin-induced internalization of the human B2 receptor requires phosphorylation of three serine and two threonine residues at its carboxyl tail. J. Biol. Chem. 1999;274:12738–12747. doi: 10.1074/jbc.274.18.12738. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- REGOLI D., NSA ALLOGHO S., RIZZI A., GOBEIL F., JR Bradykinin receptors and their antagonists. Eur. J. Pharmacol. 1998;348:1–10. doi: 10.1016/s0014-2999(98)00165-4. [DOI] [PubMed] [Google Scholar]
- REGOLI D., RIZZI A., PERRON S., GOBEIL F., JR Classification of kinin receptors. Biol. Chem. 2001;382:31–35. doi: 10.1515/BC.2001.005. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L.M., LOVETT M., HALLIDAY D.A., NADEL A., KANEKO T., BUNNETT N.W., GEPPETTI P. Bradykinin increases intracellular calcium levels in a human bronchial epithelial cell line via the B2 receptor subtype. Inflamm. Res. 1998;47:231–235. doi: 10.1007/s000110050322. [DOI] [PubMed] [Google Scholar]
- SANTIEMMA V., BELIGOTTI F., MAGNANTI M., PALLESCHI S., SILVESTRONI L., FABBRINI A. Endothelin-1 stimulates desoxyribonucleic acid synthesis and contraction in testicular peritubular myoid cells. Biol. Reprod. 1996;54:583–590. doi: 10.1095/biolreprod54.3.583. [DOI] [PubMed] [Google Scholar]
- SKINNER M.K.Secretion of growth factors and other regulatory products The Sertoli Cell. 1993Clearwater: Cache River Press; 237–248.ed. Russel, L.D. & Griswold, M.D. [Google Scholar]
- TAKANO M., KONDO J., YAYAMA K., OTANI M., SANO K., OKAMOTO H. Molecular cloning of cDNAs for mouse low molecular-weight and high-molecularweight prekininogens. Biochim. Biophys. Acta. 1997;30:222–230. doi: 10.1016/s0167-4781(97)00018-3. [DOI] [PubMed] [Google Scholar]
- TUNG P.S., FRITZ I.B. Characterization of rat testicular peritubular myoid cells in culture: alpha-smooth muscle isoactin is a specific differentiation marker. Biol. Reprod. 1990;42:351–360. doi: 10.1095/biolreprod42.2.351. [DOI] [PubMed] [Google Scholar]
- WALSH D.A., FAN T.P.D.Bradykinin as a growth factor The handbook of immunopharmacology. The kinin system. 1997San Diego: Academic Press; 301–314.ed. Farmer, S.G. [Google Scholar]
- WANG D.Z., MA J.X., CHAO L., CHAO J. Molecular cloning and sequence analysis of rat bradykinin B2 receptor gene. Biochim. Biophys. Acta. 1994;1219:171–174. doi: 10.1016/0167-4781(94)90264-x. [DOI] [PubMed] [Google Scholar]
- WENNEMUTH G., AUMÜLLER G., BACHER M., MEINHARDT A. Macrophage migration inhibitory factor-induced Ca2+ response in rat testicular peritubular cells. Biol. Reprod. 2000;62:1632–1639. doi: 10.1095/biolreprod62.6.1632. [DOI] [PubMed] [Google Scholar]
- WENNEMUTH G., EISOLDT S., BODE H.P., RENNEBERG H., SCHIEMANN P., AUMÜLLER G. Measurement of calcium influx in surface-fixed single sperm cells: efficiency of different immobilization methods. Andrologia. 1998;30:141–146. doi: 10.1111/j.1439-0272.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- YANG C.M., HSIA H.C., HSIEH J.T., ONG R., LUO S.F. Bradykininstimulated calcium mobilization in cultured canine tracheal smooth muscle cells. Cell Calcium. 1994;16:59–70. doi: 10.1016/0143-4160(94)90001-9. [DOI] [PubMed] [Google Scholar]
- ZHANG J., BARAK L.S., WINKLER K.E., CARON M.G., FERGUSON S.S.G. A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta(2)-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J. Biol. Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]


