Abstract
The role of individual residues in the 8-18 helix of CGRP8-37 in promoting high-affinity binding to CGRP1 receptors expressed on rat L6 and human SK-N-MC cells has been examined. The relative potencies of various derivatives were estimated from their ability to inhibit the human αCGRP-mediated increase in cyclic AMP production and the binding of [125I]-human αCGRP.
Arg11 and Arg18 were replaced by serines to give [Ser11,18]CGRP8-37. These bound with pKi values <6 to SK-N-MC cells and had apparent pA2 values of 5.81±0.04 and 5.31±0.11 on SK-N-MC and L6 cells. CGRP8-37 had a pKi of 8.22 on SK-N-MC cells and pKb values on the above cell lines of 8.95±0.04 and 8.76±0.04.
The arginines were replaced with glutamic acid residues. [Glu11]CGRP8-37 had a pKb of 7.14±0.14 on SK-N-MC cells (pKi=7.05±0.05) and 6.99±0.08 on L6 cells. [Glu18]CGRP8-37 had a pKb of 7.10±0.0.08 on SK-N-MC cells (pKi=6.91±0.23) and 7.12±0.09 on L6 cells.
Leu12, Leu15 and Leu16 were replaced by benzoyl-phenylalanine (bpa) residues. On SK-N-MC cells, the apparent pA2 values of [bpa12]-, [bpa15]- and [bpa16]CGRP8-37 were respectively 7.43±0.23, 8.34±0.11 and 5.66±0.16 (pKi values of 7.14±0.17, 7.66±0.21 and <6): on L6 cells they were 7.96±0.36, 8.28±0.21 and 6.09±0.04 (all n=3).
It is concluded that the Arg11 and Arg18 are involved in specific electrostatic interactions with other residues, either on the CGRP1 receptors or elsewhere on CGRP8-37. Leu16 is in a conformationally restricted site when CGRP8-37 binds to CGRP1 receptors, unlike Leu12 and Leu15.
Keywords: CGRP, CGRP8-37, CGRP1 receptor, L6 cells, SK-N-MC cells, structure-activity studies, bpa-CGRP8-37
Introduction
Calcitonin gene related peptide (CGRP) is an abundant 37 amino acid neuropeptide. It forms a family with calcitonin, amylin and adrenomedullin (Poyner et al., 2002). Whilst these have only modest sequence homology, they share a number of structural features in common; a N-terminal disulphide bonded ring required for receptor activation, an amphipathic α-helix and a C-terminal amide (Poyner et al., 2002). Removal of the N-terminus of CGRP gives CGRP8-37, an antagonist (Chiba et al., 1989). This discriminates between different CGRP-activated receptors (Quirion et al., 1992). The best characterized of these is the CGRP1 receptor, formed of a complex between the heptahelical calcitonin receptor- like receptor (CRLR) and receptor activity modifying protein 1 (RAMP1), a protein with a single transmembrane helix (McLatchie et al., 1998). The CGRP1 receptor has a high affinity for CGRP8-37 i.e. pKb>7 (Quirion et al., 1992; Poyner et al., 2002). By contrast, the CGRP2 receptor as identified by Quirion and co-workers has a pKb about an order of magnitude lower. The molecular nature of the CGRP2 receptor is unclear and it is possible that several distinct entities may give CGRP-responsive receptors that have low affinity for CGRP8-37 (Poyner et al., 2002). However, the nomenclature is useful in indicating that there is heterogeneity within receptors that are activated by CGRP.
Within CGRP, the characteristic amphipathic α-helix stretches between residues 8 and 18; thus it is present in both the full-length peptide and CGRP8-37. The importance of this structure in CGRP binding was first demonstrated by Lynch & Kaiser (1988). Deletion of residues 8-18 leads to a 50–100 fold decrease in affinity at the CGRP1 receptor (Rovero et al., 1992; Howitt & Poyner, 1997; Poyner et al., 1998). Introduction of a bend in the helix at position 16 also causes a large decrease in affinity (Wisskirchen et al., 1999; 2000). The contribution of individual amino acids to the role of 8-18 in promoting high affinity binding has not been fully elucidated. An alanine scan of the first four amino acids identified Arg11 as the most significant residue, but its substitution produced barely a 5 fold potency change (Mimeault et al., 1991; 1992). Much of the amphipathic character of the region comes from Arg11 and Arg18, the other fully charged residue that is present in the helix. Whilst replacement of either of these alone by alanine produces only modest potency decreases, the double alanine substitution reduces affinity by 100 fold (Mimeault et al., 1992; Howitt & Poyner, 1997). However, it is not known whether this reduction is due to a decrease in the overall amphipathicity of the peptide or whether it implies that the arginines are involved in specific charge–charge interactions with other residues, either on the receptor or elsewhere on the ligand. Little work has been done to investigate the role of individual amino acids on the hydrophobic face of the peptide.
In the present study, both the hydrophilic and hydrophobic surfaces of the α-helix have been probed. The effects of substituting the positively charged Arg11 and Arg18 with either serine (uncharged but hydrophilic) and glutamic acid (negatively charged) has been examined, to evaluate whether these make specific contacts with other residues or are simply involved in hydrogen bonding to solvent water molecules. Steric constraints on the hydrophobic side of the α-helix have been investigated by substituting Leu12, Leu15 and Leu16 with the large benzoyl-phenylalanine (bpa) moiety. These have been incorporated into CGRP8-37. The abilities of these derivatives to antagonize CGRP binding and stimulation of cyclic AMP production on human SK-N-MC cells and rat L6 cells, both of which express CGRP1 receptors, has been used to estimate their potency compared with CGRP8-37.
A portion of this work has previously appeared in abstract form (Wang et al., 2001).
Methods
Cell culture
SK-N-MC human neuroblastoma cells were a gift from Prof. S. Nahorski, University of Leicester. L6 cells were purchased from the European Collection of Animal Cell Cultures (Porton Down, U.K.). Cells were cultured essentially as described previously (Poyner et al., 1992; 1998). Briefly L6 cells were grown in Dulbecco's Modified Eagle Medium supplemented with 10% foetal calf serum. SK-N-MC cells were grown in a 1 : 1 mixture of Dulbecco's Modified Eagle Medium/F12 medium supplemented with 10% foetal calf serum. Cells were passaged at confluency with trypsin/EDTA (Sigma) and grown for experiments in 48-well plates at 37°C in 5%CO2/air in a humidified atmosphere.
Measurement of cyclic AMP
An hour prior to experiments, the medium on the cells was replaced with Kreb's solution supplemented with 0.1% bovine serum albumin and 1 mM isobutyl methyl xanthine. The cells were preincubated with antagonists for 30 min before addition of increasing concentrations of human (h) αCGRP. Incubations were terminated 5 min after addition of agonist by aspiration of the medium and addition of 0.1 ml of absolute ethanol. This was allowed to dry down at room temperature and the cyclic AMP was extracted by addition of 0.25 ml of assay buffer containing (in mM): EDTA 5, HEPES 20, pH 7.5. The samples were agitated for 5 min before 50 μl samples were withdrawn and cyclic AMP measured by a radioreceptor assay as described previously (Poyner et al., 1992).
Radioligand binding
This was carried out essentially as described previously (Poyner et al., 1998). Briefly, SK-N-MC cell membranes were homogenized in buffer containing (in mM): HEPES 20; EDTA 1; pH 7.5 and stored frozen at −70°C until required. They were then resuspended at approximately 0.25 mg protein per ml containing 50 mM HEPES, 0.1% bovine serum albumin, pH 7.5 and incubated at room temperature for 30 min with 100 pM [125I]-iodohistidyl8-human αCGRP with various concentrations of displacing ligands in a final volume of 0.5 ml. Non-specific binding was estimated with 1 μM CGRP. Incubations were terminated by centrifugation, pellets washed twice with tap water and counted to determine bound radioactivity.
Analysis of data
For cyclic AMP studies, the data from each concentration-response curve was fitted to a sigmoidal concentration-response curve to obtain the maximum response, Hill coefficient and EC50 using the fitting routine PRISM Graphpad. Where data was normalised, the maximum fitted cyclic AMP response to CGRP in the absence of antagonist was taken as 100%. From the individual curves, dose-ratios were calculated. Where several antagonist concentrations were used, a Schild plot was constructed; after confirming that the slope was not significantly different from unity, it was constrained to 1 to obtain the pKb. Where only a single antagonist concentration was used, an apparent pA2 was calculated from the formula, log[antagonist]-log(dose ratio-1). For radioligand binding, curves were fitted to obtain Hill coefficient, IC50 and non-specific binding. The pKi values were calculated from the Cheung-Prussoff equation, log[IC50]-log([radioligand].Kdradioligand+1).
Statistical analysis was either by Student's t-test or by one-way ANOVA followed by Tukey's test where several values were being compared with each other. Significance was accepted at P<0.05; two-tailed tests were used throughout. All values are quoted as means±s.e.mean.
Drugs and materials
HαCGRP and hαCGRP8-37 were purchased from Calbiochem. [Ser11,18]-hαCGRP8-37 was a gift of Merck, Sharpe and Dohme U.K. (Harlow). Other peptides were synthesized and purified as described previously (Poyner et al., 1998). All radiolabelled compounds were purchased from Amersham Biosciences (Little Chalfont, Buckinghamshire, U.K.). Isobutyl methyl xanthine was purchased from Sigma (Sigma-Aldrich, Gillingham, Dorset, U.K.). Cell culture medium and foetal calf serum were purchased from GIBCO BRL (Life Technologies, Paisley, Renfrewshire, U.K.). Other reagents were purchased from Sigma or Fisher. Peptides were dissolved and stored as frozen aliquots before use as previously described (Poyner et al., 1998).
Results
[Ser11,18]CGRP8-37
[Ser11,18]CGRP8-37 was investigated for its ability to antagonize CGRP on L6 and SKN-MC cells. In its absence, CGRP increased cyclic AMP in both cell lines (Figure 1, 2: pEC50 of 9.41±0.08 in L6 cells; 9.89±0.12 in SK-N-MC cells; n=3 in both cases). It had a pKi of 10.07 measured by radioligand binding on SK-N-MC membranes (Table 2, Figure 3). Schild analysis confirmed that the parent antagonist, CGRP8-37 behaved competitively (slopes of 0.90±0.07 for SK-N-MC cells and 0.89±0.07 for L6 cells, neither significantly different from 1). When the slopes were constrained to unity, pKb values of 8.95 in the L6 cells and 8.76 in SK-N-MC cells were obtained (Table 1, Figures 1, 2) CGRP8-37 had a pKi of 8.22 on SK-N-MC membranes, in good agreement with the pKb (Table 2, Figure 3). Only when [Ser11,18]CGRP8-37 was present at 10 μM did it produce a significant shift in the concentration-response curve in any of the cell lines (Table 1, Figures 1, 2). The Hill slopes of these curves in the presence of antagonist (2.0±0.2 for SK-N-MC cells and 2.0±0.2 for L6 cells) were not significantly different from the corresponding control curves, suggesting that it was a very weak competitive antagonist. Compared to CGRP8-37, in functional assays [Ser11,18]CGRP8-37 was over 1000 fold less potent on both cells. Consistent with this data, it had a pKi of below 6 on SK-N-MC membranes (Table 2, Figure 3). Thus the replacement of both arginines with serines caused a very substantial reduction in affinity.
Figure 1.
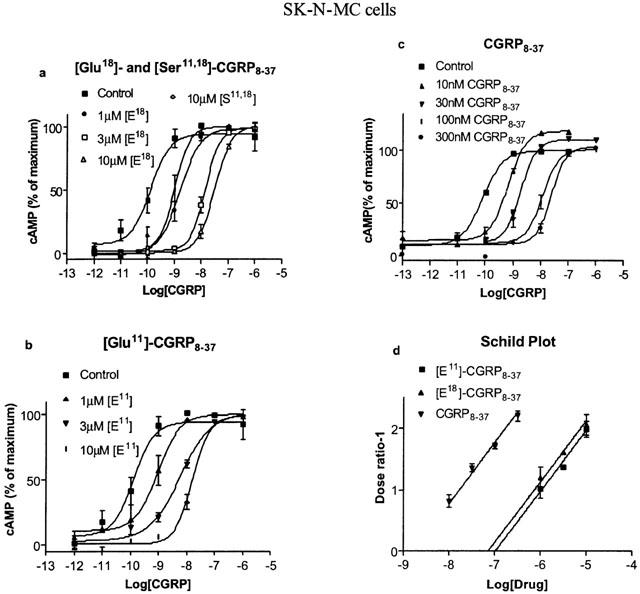
Effects of [Ser11,18] hαCGRP8-37, [Glu11] hαCGRP8-37, [Glu18] hαCGRP8-37 and hαCGRP8-37 on the stimulation of cyclic AMP production by hαCGRP in SK-NMC cells. (a) [Ser11,18] hαCGRP8-37 and [Glu18] hαCGRP8-37; (b) [Glu11] hαCGRP8-37; (c) hαCGRP8-37 (d) Schild plot for data in parts a, b and c. Parts a, b and c are representative of experiments carried out three or four times. Points were measured in duplicate in each experiment. Data are expressed as percentage of maximum cyclic AMP production caused by CGRP alone, estimated by fitting each line to a logistic Hill equation as described in the Methods. Maximum cyclic AMP values were as follows: (a) 260±20 pmol per 106 cells; (b) 250±20 pmol per 106 cells; (c) 160±15 pmol per 106 cells. Basal values were all below 10 pmol per 106 cells. Part (d) includes the data from all experiments.
Figure 2.

Effects of [Ser11,18] hαCGRP8-37, [Glu11] hαCGRP8-37, [Glu18] hαCGRP8-37 and hαCGRP8-37 on the stimulation of cyclic AMP production by hαCGRP L6 cells. (a) [Ser11,18] hαCGRP8-37, [Glu18] hαCGRP8-37; (b) [Glu11] hαCGRP8-37; (c) hαCGRP8-37 (d) Schild plot for data in parts a, b and c. Parts a, b and c are representative of experiments carried out three or four times. Points were measured in duplicate in each experiment. Data are expressed as percentage of maximum cyclic AMP production caused by CGRP alone, estimated by fitting each line to a logistic Hill equation as described in the Methods. Maximum cyclic AMP values were as follows: (a), 205±15 pmol per 106 cells; (b) 230±30 pmol per 106 cells; (c) 215±20 pmol per 106 cells. Basal values were all below 10 pmol per 106 cells. Part (d) includes the data from all experiments.
Table 2.
Binding parameters for ligands on SK-N-MC cells

Figure 3.
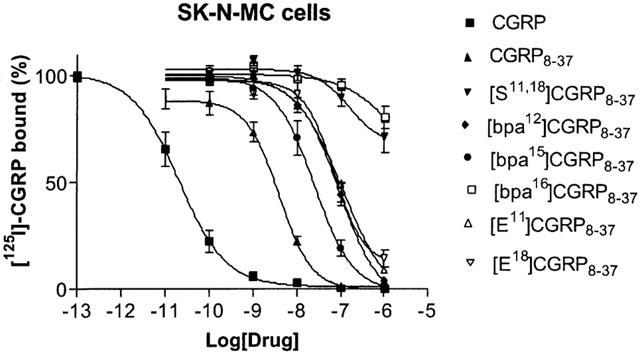
Displacement of [125I]-hαCGRP by CGRP and derivatives. Each point represents mean±s.e.mean of three or four experiments. Maximum specific binding represents 0.5 fmol ligand bound mg−1 membrane protein.
Table 1.
pA2 estimates for antagonists

[Glu11]CGRP8-37 and [Glu18]CGRP8-37
A second series of derivatives replaced the individual arginines with glutamic acid residues to give [Glu11]CGRP8-37 and [Glu18]CGRP8-37 (Tables 1, 2, Figures 1,2,3). In both cell lines, these derivatives were less potent than CGRP8-37. Due to their modest potencies, only limited Schild analysis could be undertaken, but the slope of the Schild plots was not significantly different from 1, consistent with competitive inhibition (0.97±0.18 Glu11, 0.87±0.21 Glu18 for SK-N-MC cells; 0.97±0.24 Glu11, 0.67±0.22 Glu18 for L6 cells). From these plots, pKb values were calculated (Table 1, Figures 1, 2). Based on these values [Glu18]CGRP8-37 was approximately 70 and 90 fold less potent on the L6 and SK-N-MC cells respectively; for [Glu11]CGRP8-37 the potency reductions were 40 and 70 fold. The pKi values measured for the two derivatives on SK-N-MC membranes showed somewhat smaller potency differences (15 fold for [Glu11]CGRP8-37 and 20 fold for [Glu18]CGRP8-37; Table 2, Figure 3). However, all the measurements demonstrated a significant decrease in affinity for both derivatives compared to CGRP8-37.
Bpa-substitutents
The ability of [bpa12]-, [bpa15]- and [bpa16]CGRP8-37 to inhibit CGRP action on SK-NMC (Figure 3) and L6 cells (Tables 1, 2, Figures 3,4,5) were examined. [Bpa12]CGRP8-37 was selected for detailed analysis in SK-N-MC cells (Table 1, Figure 4). The slope of the Schild plot was not significantly different from 1 (0.85±0.10); when constrained to unity it allowed a pKb estimate of 7.43. This was in good agreement with the pKi estimated from binding studies of 7.14. On L6 cells, the apparent pA2 estimated from a single antagonist concentration was 7.96 (Figure 5), in line with the values found on SK-N-MC cells. Based on the results with this compound, it was concluded that the bpa derivatives were likely to be competitive antagonists and that it should be possible to estimate their affinities from apparent pA2's calculated from single dose-ratios and pKi's measured in radioligand binding assays. Neither the maximum responses (92% to 103%) nor the Hill slopes of the concentration-response curves in the presence of the antagonists (0.67±0.23 to 1.68±0.32) were significantly different from the corresponding controls (0.58±0.04 to 1.20±0.20), consistent with this assumption. The pA2/pKb and pKi estimates for [bpa12]CGRP8-37 were all significantly different from those for CGRP8-37. [Bpa15]CGRP8-37 appeared slightly more potent than [bpa12]CGRP8-37; neither the apparent pA2 on L6 cells nor the pKi on SK-N-MC membranes were significantly different from the corresponding values for CGRP8-37 (Tables 1,2 Figures 3,45). By contrast, all the affinity estimates for [bpa16]CGRP8-37 on both cell lines showed that it was much reduced in potency compared to CGRP8-37, being ∼500 fold less potent than CGRP8-37 in the L6 cells and ∼2000 fold less potent in the SK-N-MC cells.
Figure 4.

Effects of bpa derivatives on the stimulation of cyclic AMP production by hαCGRP in SK-N-MC cells. (a) [bpa12]-hαCGRP8-37, (b) Schild plot for [bpa12]-hαCGRP8-37, (c) [bpa15]-hαCGRP8-37 and (d) [bpa16]-hαCGRP8-37. Data represent mean±s.e.mean of 3–8 experiments. Points were measured in duplicate in each experiment. Data are expressed as percentage of maximum cyclic AMP production caused by CGRP alone, estimated by fitting each line to a logistic Hill equation as described in the Methods. Maximum cyclic AMP values were as follows: (a) 220±25 pmol per 106 cells; (c) 255±30 pmol per 106 cells; (d) 240±35 pmol per 106 cells; basal values were all below 10 pmol per 106 cells.
Figure 5.

Effects of: (a) [bpa12]-, (b) [bpa15]- and (c) [bpa16]-hαCGRP8-37 on the stimulation of cyclic AMP production by human αCGRP in L6 cells. Data represent mean±s.e.mean of 3–6 experiments. Points were measured in duplicate in each experiment. Data are expressed as percentage of maximum cyclic AMP production caused by CGRP alone, estimated by fitting each line to a logistic Hill equation as described in the Methods. Maximum cyclic AMP values were as follows: (a) 245±40 pmol per 106 cells; (b) 265±30 pmol per 106 cells; (c) 255±45 pmol per 106 cells; basal values were all below 10 pmol per 106 cells.
Discussion
The amphipathic helix formed by residues 8-18 in CGRP is an important factor in high affinity binding of the peptide to its receptor (Lynch & Kaiser, 1988). This paper provides new detail on how the helix fulfils this function.
The characteristics of the CGRP receptors expressed on L6 and SK-N-MC cells in this study are in agreement with previous work by ourselves and others (van valen et al., 1990; Muff et al., 1992; Semark et al., 1992; Longmore et al., 1994; Poyner et al., 1992; 1998; Howitt & Poyner, 1997). In radioligand binding studies, CGRP shows high affinity binding; the Hill slopes for it and its derivatives suggest they interact predominantly with a single class of sites. The pKb values for CGRP8-37 on SK-N-MC cells is higher than we have previously reported (7.49, Poyner et al., 1998) but it is in line with values found by other groups (8.7 to 8.9, Semark et al., 1992; Longmore et al., 1994). In the current study we have used a longer pre-incubation with antagonist (30 min as opposed to 5 min); this may be significant although other studies have suggested that CGRP8-37 reaches equilibrium at its receptor within 5 min (Wisskirchen et al., 1998). We confirmed that CGRP8-37, the glutamate-substituted analogues and [bpa12]CGRP8-37 all behaved as competitive antagonists.
Previous studies have shown that the Arg11 and Arg18 collectively play a key role in the high affinity binding of CGRP (Howitt & Poyner, 1997; Poyner et al., 1998). However, those studies did not address how the arginines worked in promoting high affinity binding. At least two models are possible. On the one hand, the arginines could act to reinforce the amphipathic nature of residues 8-18. This might be expected if the hydrophilic face of the helix faced the aqueous solvent, with the hydrophobic face making peptide–peptide or protein contact. In this case, replacement with other hydrophilic residues such as serine or glutamic acid would not be expected to cause a reduction in affinity. Indeed, the fact that 8-18 can be replaced by other amphipathic peptides with relatively little loss in affinity (Lynch & Kaiser, 1988; Poyner et al., 1998) might be considered as supporting this proposal. The alternative is that the arginines interact electrostatically with groups carrying either a full or partial negative charge, either elsewhere on the CGRP molecule or on the receptor. Replacement with the negatively charged glutamic acid will result in electrostatic repulsion and hence a decrease in affinity; serines, lacking full charges will also not fully promote these interactions. It is this latter pattern that is observed, suggesting that Arg11 and Arg18 are involved in well-defined electrostatic interactions.
Previously, it had been shown that the single replacement of Arg11 with alanine produced, at most, only a 6 fold decrease in affinity; replacement of Arg18 alone had a negligible effect on affinity (Mimeault et al., 1991; Howitt & Poyner, 1997; Poyner et al., 1998). The most likely explanation for this pattern of activity is that, whilst both arginines are involved in charge–charge interactions, there is redundancy so that one or other of these can be disrupted with minimal loss of affinity. When the amphipathic peptide mastoparan is substituted for residues 8-18, the two arginines are replaced by lysines, suggesting the reason why this derivative remains active (Poyner et al., 1998). The introduction of electrostatic repulsion at either site is sufficient to produce larger changes in affinity, so that marked affinity decreases are seen with either of the singly substituted derivatives.
In principle, Arg11 and Arg18 may be involved in ionic interactions or hydrogen bonds. [Ser11,18]CGRP8-37 has the potential to form hydrogen bonds and this binds much more weakly than CGRP8-37. However, the hydrogen bonds formed by serine are likely to be weaker than those formed by arginine. Not only will there be a decrease in coulombic interactions when replacing the positively charged arginine by the neutral serine, but as the side chain of serine is much smaller than that of arginine, it is very unlikely that the serines will be as favourably placed sterically as the arginines to maintain a hydrogen bond interaction. The decrease in free energy of binding seen when [Ser11,18]CGRP8-37 is used instead of CGRP8-37 is about 12KJ per mole. This value is consistent with either loss of hydrogen bonds or weak ionic interactions (where the charges are shielded by water molecules and other ions in solution) (Ferscht, 1977; Howell et al., 1986). Thus the arginines may be involved in either of these interactions. If they are involved in hydrogen bonds, these could be between acceptors either on the receptor or elsewhere on CGRP8-37. There is evidence for interactions between the 8-18 helix and the C-terminus of CGRP8-37; replacement of Ser17 by alanine increases potency 2 fold but this can be correlated with changes in the structure of residues 28–37 (Boulanger et al., 1995; 1996). If however ionic interactions are involved, these must be between 8-18 and the receptor, as there is no negatively charged residue in CGRP8-37.
The bpa derivatives give information on the hydrophobic face of the 8-18 helix. Although care must be taken not to over-interpret this data, as apparent pA2 values were estimated from single dose-ratio measurements, there is agreement between these values measured on both L6 and SK-N-MC cells and pKi values estimated from radioligand binding. Three leucine residues were replaced by the larger bpa moiety.
The most dramatic changes were seen with [bpa16]CGRP8-37, with over a 100 fold decrease in potency. This suggests that Leu16 sits in a conformationally restricted space. No such constraints were apparent for the adjacent residue Leu15. A small decrease was seen when Leu12 was substituted, but this was much less marked than that for Leu16. If Leu16 is substituted by alanine there is little change in affinity (Wisskirchen et al., 1999; 2000), indicating that any contribution from the packing energy of the side chain is not by itself crucial to the binding of CGRP8-37. As with the arginines, it is difficult to say whether bpa16 interacts with either the receptor or another portion of CGRP8-37. However, it is difficult to envisage how both faces of the 8-18 helix could simultaneously interact with the rest of CGRP8-37, and so it is likely that either the arginines or Leu16 are in proximity to the receptor.
All the derivatives show similar potency reductions on the CGRP1 receptor of the SKN-MC and L6 cells. Thus the 8-18 helix is not particularly sensitive to species differences between human and rat. This is consistent with the observation that CGRP8-37 itself has a similar affinity at both rat and human CGRP receptors. By contrast the low molecular weight CGRP antagonist BIBN4096BS shows well over 100 fold higher affinity for the human CGRP1 receptors compared to that of the rat (Doods et al., 2000). Human and rat CRLR have over a 91% identity (Njuki et al., 1993; Flühmann et al., 1995) whereas the two RAMP1s show only 71% identity (Foord et al., 2000). It has been shown that the species selectivity of BIBN4096BS is due to a single residue in RAMP1 (Mallee et al., 2002). The 8-18 helix must be promoting high affinity CGRP binding by (directly or indirectly) interacting with a more conserved part of the CRLR/RAMP1 heterodimer.
It is possible to integrate the results of the present study with other work to build a fuller picture of the key features of the 8-18 helix. Alanine scans have revealed that replacement of any of the first three residues (Val8, Thr9, His10) causes no more than a 3 fold potency reduction; however, their complete removal causes a 10 fold reduction in affinity (Mimeault et al., 1991; 1992). Thus within the first turn of the helix, no single residue is of particular importance, but collectively they are significant, perhaps in stabilising a helical conformation (Mimeault et al., 1992). Wisskirchen et al., (1999; 2000) demonstrated that substitution of His10 and Gly14 with aspartic acid residues caused a large drop in the affinity of CGRP8-37. As it has also been shown that Gly14 can be replaced by aspartic acid with less than a 2 fold affinity shift (Li et al., 1997), it seems that the replacement of the electropositive His10 was mainly responsible for the affinity decrease. The pattern of activity seen with His10 is similar to that seen with Arg18, suggesting that the two may have similar roles in interacting with electronegative residues. Arg11 is involved in a coulombic interaction but Leu12 is not subject to stringent steric constraints. A study on Gly14 suggests that it can be substituted freely by charged and non-polar amino acids (Li et al., 1997). Leu15 is not subject to spatial constraints but Leu16 is in a conformationally restricted environment. Ser17 plays a minor role, perhaps in stabilising the C-terminus of CGRP (Boulanger et al., 1995; 1996) but Arg18 is involved in a charge–charge interaction. Thus, it appears that the residues highlighted in this study, Arg11, Leu16 and Arg18 are of particular significance. It will be important to discover how they interact with either the receptor or with other parts of CGRP.
Acknowledgments
This work was supported by the British Heart Foundation and the Wellcome Trust. We thank Dr J. Longmore of Merck, Sharpe and Dohme (U.K.) for the gift of [Ser11,18]CGRP8-37 and Dr C.H. Schwalbe and Prof. I.L. Martin for discussions about the results.
Abbreviations
- bpa
benzoyl-phenylalanine
- CGRP
calcitonin gene-related peptide
- CRLR
calcitonin receptor-like receptor
- h
human
- RAMP1
receptor activity modifying protein 1
References
- BOULANGER Y., KHIAT A., CHEN Y., SENECAL L., TU Y., ST-PIERRE S., FOURNIER A. Structure of human calcitonin gene-related peptide (hCGRP) and of its antagonist hCGRP8-37 as determined by NMR and molecular modelling. Peptide Res. 1995;8:206–213. [PubMed] [Google Scholar]
- BOULANGER Y., KHIAT A., LAROCQUE A., FOURNIER A., ST-PIERRE S. Structural comparison of alanine substituted analogues of the calcitonin gene-related peptide 8-37. Int. J. Peptide Protein Res. 1996;47:477–483. doi: 10.1111/j.1399-3011.1996.tb01098.x. [DOI] [PubMed] [Google Scholar]
- CHIBA T., YAMAGUCHI A., YAMATANI T., NAKAMURA A., MORISHITA T., INUI T., FUKASE M., NODA T., FUJITA T. Calcitonin gene-related peptide antagonist CGRP(8-37) Am. J. Physiol. 1989;256:E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., WU D., ENTZEROTH M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERSCHT A. Reading: W.H. Freedman and Co; 1977. Enzyme structure and mechanism; pp. 226–243. [Google Scholar]
- FLÜHMANN B., MUFF R., HUNZIKER W., FISCHER J.A., BORN W. A human orphan calcitonin receptor-like structure. Biochem. Biophys. Res. Commun. 1995;206:341–347. doi: 10.1006/bbrc.1995.1047. [DOI] [PubMed] [Google Scholar]
- FOORD S.M., FRASER N.J., MAIN M.J., WISE A., MCLATCHIE L.M., BROWN J., THOMPSON N., SOLARI R., LEE M.G.Receptor activity modifying proteins (RAMPs) define the receptors for CGRP and adrenomedullin The CGRP Family: Calcitonin gene related peptide (CGRP), Amylin and Adrenomedullin 2000Texas: Landes Biosciences; 55–65.ed. Poyner, D.R., Marshall, I., & Brain, S.D. [Google Scholar]
- HOWELL E.E., VILLAFRANCA J.E., WARREN M.S., OATLEY S.J., KRAUT J. Functional role of aspartic acid-27 in dihydrofolate reductase revealed by mutagenesis. Science. 1986;231:1123–1128. doi: 10.1126/science.3511529. [DOI] [PubMed] [Google Scholar]
- HOWITT S.G., POYNER D.R. The selectivity and structural determinants of peptide antagonists at the CGRP receptor of rat, L6 myocytes. Br. J. Pharmacol. 1997;121:1000–1004. doi: 10.1038/sj.bjp.0701212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z., MATSUURA J.E., WAUGH D.J.J., ADRIAN T.E., ABEL P.W., MANNING M.C., SMITH D.D. Structure-activity studies on position 14 of human α-calcitonin gene-related peptide. J. Med. Chem. 1997;40:3071–3076. doi: 10.1021/jm9608164. [DOI] [PubMed] [Google Scholar]
- LONGMORE J., HOGG J.E., HUTSON P.H., HILL R.G. Effects of two truncated forms of the human calcitonin gene-related peptide: implications for receptor classification. Eur. J. Pharmacol. 1994;265:53–59. doi: 10.1016/0014-2999(94)90222-4. [DOI] [PubMed] [Google Scholar]
- LYNCH B., KAISER E.T. Biological properties of two models of calcitonin gene-related peptide with idealized amphiphilic alpha helices of different lengths. Biochemistry. 1988;27:7600–7607. doi: 10.1021/bi00420a005. [DOI] [PubMed] [Google Scholar]
- MALLEE J.J., SALVATORE C.A., LEBOURDELLES B., OLIVER K.R., LONGMORE J., KOBLAN K., KANE S.A. RAMP1 determines the species selectivity of non-peptide CGRP receptor antagonists. J. Biol. Chem. 2002;277:14294–14298. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor- like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MIMEAULT M., FOURNIER A., DUMONT Y., ST-PIERRE S., QUIRION R. Comparative affinities and antagonistic potencies of various human calcitonin gene-related peptide fragments on calcitonin gene-related peptide receptors in brain and periphery. J. Pharmacol. Exp. Ther. 1991;258:1084–1090. [PubMed] [Google Scholar]
- MIMEAULT M., QUIRION R., DUMONT Y., ST-PIERRE S., FOURNIER A. Structure-activity study of hCGRP8-37, a calcitonin gene-related peptide receptor antagonist. J. Med. Chem. 1992;35:2163–2168. doi: 10.1021/jm00090a003. [DOI] [PubMed] [Google Scholar]
- MUFF R., STANGL D., BORN W., FISCHER J.A. Comparison of a calcitonin gene-related peptide receptor in a human neuroblastoma cell line (SK-N-MC) with a calcitonin receptor in a human breast carcinoma cell line (T47D) Ann. N.Y. Acad. Sci. 1992;657:106–116. doi: 10.1111/j.1749-6632.1992.tb22760.x. [DOI] [PubMed] [Google Scholar]
- NJUKI F., NICHOLL C.G., HOWARD A., MAK J.C., BARNES P.J., GIRGIS S.I., LEGON S. A new calcitonin-receptor- like sequence in rat pulmonary blood vessels. Clin. Sci. Colch. 1993;85:385–388. doi: 10.1042/cs0850385. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., ANDREW D.P., BROWN D., BOSE C., HANLEY M.R. Pharmacological characterization of a receptor for calcitonin gene-related peptide on rat, L6 myocytes. Br. J. Pharmacol. 1992;105:441–447. doi: 10.1111/j.1476-5381.1992.tb14272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POYNER D.R., SEXTON P.M., MARSHALL I., SMITH D.M., QUIRION R., BORN W., MUFF R., FISCHER J.A., FOORD S.M. International Union of Pharmacology. XXXII. The mammalian CGRP, adrenomedullin, amylin and calcitonin receptors. Pharm. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., SOOMETS U., HOWITT S.G., LANGEL U. Structural determinants for binding to CGRP receptors expressed by human SK-N-MC and Col-29 cells: studies with chimeric and other peptides. Br. J. Pharmacol. 1998;124:1659–1666. doi: 10.1038/sj.bjp.0702032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIRION R., VAN ROSSUM D., DUMONT Y., ST PIERRE S., FOURNIER A. Characterization of CGRP1 and CGRP2 receptor subtypes. Anal. N.Y. Acad. Sci. 1992;657:88–105. doi: 10.1111/j.1749-6632.1992.tb22759.x. [DOI] [PubMed] [Google Scholar]
- ROVERO P., GIULIANI S., MAGGI C.A. CGRP antagonist behaviour of short C-terminal fragments of human αCGRP, CGRP(19-37) and CGRP(23-37) Peptides. 1992;13:1025–1027. doi: 10.1016/0196-9781(92)90067-d. [DOI] [PubMed] [Google Scholar]
- SEMARK J.E., MIDDLEMISS D.N., HUTSON P.H. Comparison of calcitonin gene-related peptide receptors in rat brain and a human neuroblastoma cell line, SKN-MC. Mol. Neuropharmacol. 1992;2:311–317. [Google Scholar]
- VAN VALEN F., PEICHOT G., JURGHENS H. Calcitonin gene-related peptide receptors are related to cyclic adenosine monophosphate production in SK-NMC neuroblastoma cells. Neurosci. Lett. 1990;119:195–198. doi: 10.1016/0304-3940(90)90832-t. [DOI] [PubMed] [Google Scholar]
- WANG Y., HOWITT S.G., POYNER D.R. A comparison of the antagonist actions of [(11,18)Ser]CGRP(8-37) with CGRP(8-37) on the CGRP receptors expressed on L6, SK-N-MC and COL 29 cells. Br. J. Pharmacol. 2001;133 Suppl.:P149. [Google Scholar]
- WISSKIRCHEN F.M., DOYLE P.M., GOUGH S.L., HARRIS C.J., MARSHALL I. Bioactive beta-bend structures for the antagonist halpha CGRP(8-37) at the CGRP(1) receptor of the rat pulmonary artery. Br. J. Pharmacol. 2000;129:1049–1055. doi: 10.1038/sj.bjp.0703152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSKIRCHEN F.M., BURT R.P., MARSHALL I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br. J. Pharmacol. 1998;123:1673–1683. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSKIRCHEN F.M., DOYLE P.M., GOUGH S.L., HARRIS C.J., MARSHALL I. Conformational restraints revealing bioactive beta-bend structures for halpha CGRP8-37 at the CGRP2 receptor of the rat prostatic vas deferens. Br. J. Pharmacol. 1999;126:1163–1170. doi: 10.1038/sj.bjp.0702432. [DOI] [PMC free article] [PubMed] [Google Scholar]


