Abstract
It has long been thought that death adder venoms are devoid of myotoxic activity based on studies done on Acanthophis antarcticus (Common death adder) venom. However, a recent clinical study reported rhabdomyolysis in patients following death adder envenomations, in Papua New Guinea, by a species thought to be different to A. antarcticus. Consequently, the present study examined A. rugosus (Irian Jayan death adder) venom for myotoxicity, and isolated the first myotoxin (acanmyotoxin-1) from a death adder venom.
A. rugosus (10–50 μg ml−1) and acanmyotoxin-1 (MW 13811; 0.1–1 μM) were screened for myotoxicity using the chick directly (0.1 Hz, 2 ms, supramaximal V) stimulated biventer cervicis nerve-muscle (CBCNM) preparation. A significant contracture of skeletal muscle and/or inhibition of direct twitches were considered signs of myotoxicity. This was confirmed by histological examination.
High phospholipase A2 (PLA2) activity was detected in both A. rugosus venom (140.2±10.4 μmol min−1 mg−1; n=6) and acanmyotoxin-1 (153.4±11 μmol min−1 mg−1; n=6). Both A. rugosus venom (10–50 μg ml−1) and acanmyotoxin-1 (0.1–1 μM) caused dose-dependent inhibition of direct twitches and increase in baseline tension (n=4–6). In addition, dose-dependent morphological changes in skeletal muscle were observed.
Prior incubation (10 min) of CSL death adder antivenom (5 units ml−1; n=4) or inactivation of PLA2 activity with 4-bromophenacyl bromide (1.8 mM; n=4) prevented the myotoxicity caused by acanmyotoxin-1 (1 μM).
Acanmyotoxin-1 (0.1 μM; n=4) displayed no significant neurotoxicity when it was examined using the indirectly (0.1 Hz, 0.2 ms, supramaximal V) stimulated CBCNM preparation.
In conclusion, clinicians may need to be mindful of possible myotoxicity following death adder envenomation in Irian Jaya.
Keywords: Death adder, Acanthophis, antarcticus, rugosus, myotoxic, Phospholipase A2, acanmyotoxin-1, rhabdomyolysis, antivenom, venom
Introduction
Death adders (genus Acanthophis) are unique among Australian snakes in both morphology and behaviour. Although classified into the Elapidae family of snakes they are viper-like in appearance and habit (Campbell, 1966). They are characterized by a somewhat flattened, almost triangular head and a short, stout body terminating to a thin rat-like tail (Cogger, 2000). This makes them among the most specialized of all elapids and closely convergent in many respects with members of the family Viperidae.
Death adders are the widest ranging of the Australian elapids, being found not only in continental Australia, but North throughout the Torres Straight Islands, Papua New Guinea, Irian Jaya and the Indonesian islands of Seram, Halmahera, Obi and Tanimbar. Although there have been up to 12 species and three subspecies of death adders described thus far (Hoser, 1998), considerable debate remains about species identification (Wuster et al., 1999). Of these, only the venoms of the common (A. antarcticus) and northern (A. praelongus) death adders have been studied in some detail. However, recently the venoms of the major species and regional variants have been investigated by liquid chromatography/mass spectrometry (Fry et al., 2002). This study revealed a great diversity in the venoms.
Previously, using the chick isolated stimulated biventer cervicis nerve-muscle (CBCNM) preparation, we have shown that Irian Jayan death adder (A. rugosus) venom (1–10 μg ml−1) caused time-dependent inhibition of indirect twitches and blocked contractile responses to exogenous acetylcholine and carbachol (Fry et al., 2001). Thus, suggesting the presence of postsynaptic neurotoxins. In addition, the efficacy of CSL death adder antivenom against the in vitro neurotoxicity of A. rugosus venom was studied. It was found that CSL death adder antivenom (1 unit ml−1), raised against A. antarcticus venom, was markedly less effective against A. rugosus venom compared to A. hawkei, A. praelongus and A. pyrrhus venoms (Fry et al., 2001). However, a higher concentration of antivenom (5 units ml−1) completely neutralized the in vitro neurotoxicity of A. rugosus venom (Fry et al., 2001). To date, no components have been studied from A. rugosus venom.
In contrast to A. rugosus venom, A. antarcticus venom has previously been examined for lethality, neurotoxicity, myotoxicity and its effects on blood coagulation, both experimentally and clinically (Kellaway, 1929a, b; Campbell, 1966; Broad et al., 1979; Mebs & Samejima, 1980, Sutherland et al., 1981). In addition, five postsynaptic neurotoxins have been isolated and sequenced from A. antarcticus venom (Sheumack et al., 1979; Kim & Tamiya, 1981a, b; Sheumack et al., 1990; Tyler et al., 1997). In terms of phospholipase A2 (PLA2) components, acanthin I and II, both potent inhibitors of platelet aggregation have been isolated from A. antarcticus venom (Chow et al., 1998). In addition, acanthoxin A1 and A2, two PLA2 isoforms with weak neurotoxic activity, have been isolated from A. antarcticus venom (van der weyden et al., 1997; 2001). Three PLA2 isoenzymes, praelongins 2bIII, 2cII and 2cIV, with antiplatelet activity have also been isolated from A. praelongus venom (Sim, 1998). However, no myotoxic components have been isolated from any death adder venom to date.
It has long been thought that death adder venoms are devoid of myotoxic activity based on studies done on A. antarcticus venom. This venom had no myotoxic activity in Rhesus monkeys (Macaca fascicularis) (Sutherland et al., 1981). Mebs & Samejima (1980) fractionated A. antarcticus venom by size exclusion chromatography. None of the isolated fractions were capable of causing myoglobinuria in mice after subcutaneous injection. Furthermore, A. antarcticus venom (30 μg ml−1) had no myotoxic activity in vitro in the directly stimulated CBCNM preparation (Wickramaratna & Hodgson, 2001). However, a recent clinical study reported myotoxic activity in vivo following death adder envenomations, in Papua New Guinea, by a species thought to be different to A. antarcticus (Lalloo et al., 1996). In this study there was one patient who developed renal failure following delayed presentation after a suspected death adder bite. There were significantly elevated creatine kinase levels (median of 411 IU l−1, range of 164–4220 IU l−1) in two thirds of envenomed patients (Lalloo et al., 1996). However, these levels may not be clinically important in terms of causing renal failure (GK Isbister, personal communication 2002). Renal failure and elevated creatine kinase levels suggest rhabdomyolysis and the presence of myotoxic activity in the venom (Sutherland et al., 1981).
The first aim of this study was to examine the venom from death adders (A. rugosus) found in Irian Jaya (West Papua) to determine any possible myotoxic activity. Secondly, to isolate and pharmacologically characterize myotoxins from this venom. Thirdly, to determine the effectiveness of CSL death adder antivenom, which has been raised against A. antarcticus venom, in neutralizing myotoxic activity.
Methods
Venom preparation and storage
A. rugosus venom was purchased from Venom Supplies Pty. Ltd., South Australia. Freeze dried venom and stock solutions of venom prepared in 0.1% bovine serum albumin in 0.9% saline (BSA) were stored at −20°C until required.
Fractionation of venom
Freeze dried venom was dissolved in distilled water and filtered through a 0.45 μm Millipore (Bedford, MA, U.S.A.) filter. Reverse phase high performance liquid chromatography (RP-HPLC) separations were performed on the BIOCAD Perfusion Chromatography Workstation (Applied Biosystems, CA, U.S.A.) using Phenomenex Jupiter preparative (250×21.2 mm, 10 μ, 300 Å) and semi-preparative (250×10 mm, 5 μ, 300 Å) C18 columns. The column was equilibrated with solvent A (0.1% trifluoroacetic acid - TFA) and the sample then eluted with the following gradient conditions of solvent B (90% acetonitrile in 0.09% TFA) and solvent A at a flow rate of 10 ml min−1: 0 to 60% over 60 min (1% gradient) and then 60 to 80% in 5 min (4% gradient). The eluant was monitored at 214 and 280 nm.
The purified component was re-run on a Hewlett Packard series 1100 ChemStation (Agilent Technologies, CA, U.S.A.) using a Phenomenex Jupiter analytical (150×2 mm, 5 μ, 300 Å) C18 column. The column was equilibrated with solvent A (0.1% TFA) and loaded with 100 μl of 100 μg ml−1 isolated component. The sample was then eluted with the following gradient conditions of solvent B (90% acetonitrile in 0.09% TFA) and solvent A at a flow rate of 0.2 ml min−1: 0 to 20% over 5 min (4% gradient), 20 to 60% in 40 min (1% gradient) and then 60 to 80% over 5 min (4% gradient). The eluant was monitored at 214 nm.
Molecular mass determination by electrospray mass spectrometry
The sample was dissolved in 50% acetonitrile and analysed using a Perkin-Elmer Sciex API 300 (PE-Sciex, Thronton, Canada) triple quadrupole instrument equipped with an ionspray interface. The ionspray voltage was set at 4600 V and the orifice potential at 30 V. Nitrogen gas was used as a curtain gas with a flow rate of 0.6 l min−1 while compressed air was the nebulizer gas. The sample (10 μl) was injected manually into the LC-MS system and analysed in positive ion mode. Data processing was performed with the aid of the software package Biomultiview (PE-Sciex, Thronton, Canada).
Amino acid sequence determination
Pure peptide (400 μg) was dissolved in 400 μl of 6 M guanidinium hydrochloride and then 8 μl of 2-mercaptoethanol was added. The sample was then vortexed and briefly centrifuged. Subsequently, 80 μl of 4-vinylpyridine was then added, nitrogen gas passed over the sample for 2 min, the sample sealed airtight and then incubated at 37°C for 2 h. The reduced/alkylated peptide was N-terminally sequenced using Edman degradation chemistry on an Applied Biosystems 494 pulsed-liquid-phased sequencer (Applied Biosystems, CA, U.S.A.).
Determination of phospholipase A2 activity
The PLA2 activity of whole venom and isolated component was determined using a secretory PLA2 colourimetric assay kit (Cayman Chemical, U.S.A.). The assay uses the 1,2-dithio analogue of diheptanoyl phosphatidylcholine as a substrate. Free thiols generated upon hydrolysis of the thio ester bond at the sn-2 position by PLA2 are detected using DTNB (5,5′- dithiobis(2-nitrobenzoic acid)). Colour changes were monitored by the CERES900C microplate reader (Bio-Tek Instruments, U.S.A.) at 405 nm, sampling every min for a 5 min period. PLA2 activity was expressed as micromoles of phosphatidylcholine hydrolysed per min per milligram of enzyme.
Inactivation of PLA2 activity with 4-bromophenacyl bromide
PLA2 activity of acanmyotoxin-1 was inhibited by alkylation with 4-bromophenacyl bromide (4-BPB). Acanmyotoxin-1 (0.1 mM) was made up in sodium cacodylate-HCl buffer (50 μl, 0.1 M, pH 6.0), and 4-BPB made up in acetone was added to give a final concentration of 1.8 mM (Abe et al., 1977; Bell et al., 1998; Crachi et al., 1999a). Each vial containing the above solution was then incubated at 30°C for 16 h. As a positive control, acanmyotoxin-1 (0.1 mM) made up in sodium cacodylate-HCl buffer was incubated with acetone. As a negative control, sodium cacodylate-HCl buffer was incubated with 1.8 mM 4-BPB in acetone.
Chick isolated biventer cervicis nerve-muscle preparation
Male White leg horn chicks aged between 5 and 9 days were killed with CO2 and both biventer cervicis nerve-muscle preparations were removed. These were mounted under 1 g resting tension in organ baths (5 ml) containing Krebs solution of the following composition (mM): NaCl, 118.4; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 2.5; NaHCO3, 25 and glucose, 11.1. The Krebs solution was bubbled with carbogen (95% O2 and 5% CO2) and maintained at 34°C. Indirect twitches were evoked by stimulating the motor nerve every 10 s with pulses of 0.2 ms duration at a supramaximal voltage (Harvey et al., 1994) using a Grass S88 stimulator. After a 30 min equilibration period, d-tubocurarine (10 μM) was added. Subsequent abolition of twitches confirmed selective stimulation of nerves. Twitches were then re-established by thorough washing. Contractile responses to acetylcholine (ACh; 1 mM for 30 s), carbachol (CCh; 20 μM for 60 s) and potassium chloride (KCl; 40 mM for 30 s) were obtained in the absence of stimulation (Harvey et al., 1994). Electrical stimulation was then recommenced and the preparations were allowed to equilibrate for a further 30 min period before commencement of the experiment. Venom or toxin was left in contact with the preparations until complete twitch blockade occurred, or for a 3 h period. Contractile responses to ACh, CCh and KCl were then obtained as previously described.
In experiments determining myotoxicity, direct twitches were evoked by stimulating the muscle directly every 10 s with pulses of 2 ms duration at a supramaximal voltage (Harvey et al., 1994). To achieve selective stimulation of muscle, d-tubocurarine (10 μM) was added and left in the organ bath for the duration of the experiment. A. rugosus venom (10–50 μg ml−1), acanmyotoxin-1 (0.1–1 μM), 4-BPB modified acanmyotoxin-1 (1 μM) or relevant controls were left in contact with the preparations for a 3 h period. Where indicated, CSL death adder antivenom (5 units ml−1) was added 10 min prior (Barfaraz & Harvey, 1994; Crachi et al., 1999b; Fry et al., 2001; Wickramaratna & Hodgson, 2001) to the addition of acanmyotoxin-1 (1 μM). A significant contracture of skeletal muscle (i.e. a rise in baseline) and/or inhibition of direct twitches were considered signs of myotoxicity (Harvey et al., 1994).
Morphological studies
After the conclusion of the functional myotoxic experiments the tissues were quickly placed in Tissue Tek and frozen with liquid nitrogen. The tissues were stored at −80°C until required. Using a Leica CM1800 cryostat, tissues were cut into transverse sections (14 μm) and placed onto gelatin-coated slides. Tissue sections were post fixed for 15 min in a solution containing 4% paraformaldehyde in phosphate buffered saline (PBS; (mol l−1) NaCl, 0.137; KH2PO4, 0.002; and Na2HPO4, 0.008). Tissue sections were routinely stained with haematoxylin and eosin and examined under a light microscope (Olympus BH-2, Olympus Optical Co., Japan). Areas exhibiting typical pathological changes were photographed using an Olympus C-35AD (Olympus Optical Co., Japan) camera and Kodak film (Ektachrome P1600).
Chemicals and drugs
The following drugs and chemicals were used: acetonitrile (Fisher Scientific, U.K.); acetylcholine chloride, 4-bromophenacyl bromide (4-BPB), bovine serum albumin (BSA), cacodylic acid (sodium cacodylate), carbamylcholine chloride (carbachol), d-tubocurarine chloride; eosin, Mayer's Haemalum (Sigma Chemical Co., St. Louis, MO, U.S.A.); trifluoroacetic acid, 4-vinylpyridine (Fluka Chemika-Biochemika, Buchs, Switzerland). Sequencing grade chemicals were obtained from Applied Biosystems (Singapore). Except where indicated, stock solutions were made up in distilled water. 4-BPB was made up in acetone. Death adder antivenom, which is raised against A. antarcticus venom in horses, was obtained from CSL Ltd (Melbourne, Australia). All reagents were of analytical grade.
Analysis of results and statistics
For isolated tissue experiments, responses were measured via a Grass force displacement transducer (FT03) and recorded on a MacLab System. For both neurotoxicity and myotoxicity studies, twitch height was expressed as a percentage of the pre-treated twitch height. Statistical difference was determined by a one-way analysis of variance (ANOVA) on the twitch height at the 180 min time point. Likewise, a one-way ANOVA was performed on the contractile response induced by the venom and acanmyotoxin-1 at the 180 min time point. Contractile responses to ACh, CCh and KCl were expressed as a percentage of the respective initial response. These were analysed using either Student's paired t-tests or, where stated, compared against the control response via a one-way ANOVA. All ANOVAs were followed by a Bonferroni post hoc test. Statistical significance was indicated when P<0.05.
Results
Isolation and purification of acanmyotoxin-1
Acanmyotoxin-1 was isolated from A. rugosus venom by successive RP-HPLC separations. The initial fractionation of A. rugosus venom using a Phenomenex Jupiter preparative column produced eleven major peaks. The eleventh peak was subjected to further purification by RP-HPLC. In order to determine homogeneity and location of acanmyotoxin-1 in relation to other peaks of the whole venom both A. rugosus venom and acanmyotoxin-1 were run on the same conditions using a Phenomenex Jupiter analytical column (Figure 1a,b). Acanmyotoxin-1 eluted as a clean peak separating away from minor contaminants at about 47.5% acetonitrile or at 32.5 min.
Figure 1.
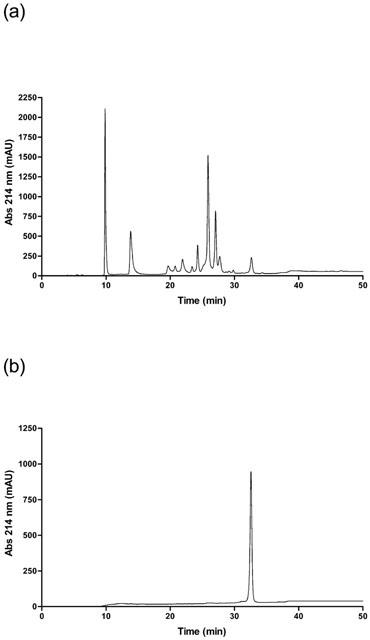
RP-HPLC chromatograph of (a) A. rugosus venom or (b) acanmyotoxin-1 run on a Jupiter analytical C18 column, equilibrated with solvent A (0.1% TFA) and eluted with the following gradient conditions of solvent B (90% acetonitrile in 0.09% TFA) and solvent A: 0 to 20% over 5 min, 20 to 60% in 40 min and then 60 to 80% over 5 min.
Purity and molecular mass determination
Homogeneity and molecular mass of acanmyotoxin-1 were determined by electrospray mass spectrometry (Figure 2). The mass spectra of purified acanmyotoxin-1 displayed several charged states and these could be reconstructed into a single molecular mass of 13811.38±0.81 daltons.
Figure 2.
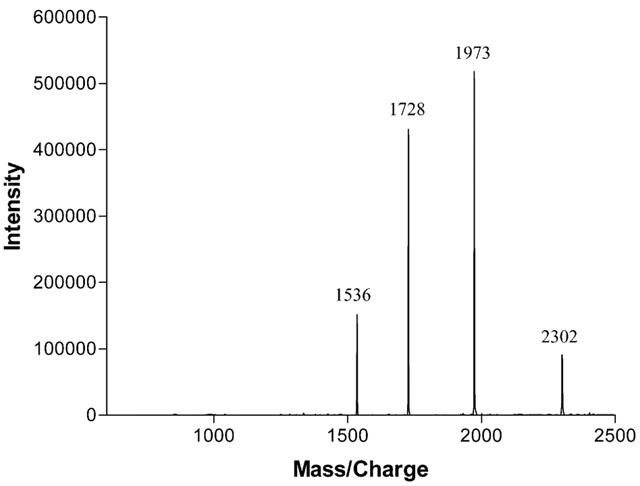
Electrospray mass spectrometry of acanmyotoxin-1. The spectrum shows a series of multiple-charged ions, related to molecules bearing 6–9 protons.
N-terminal amino acid sequence
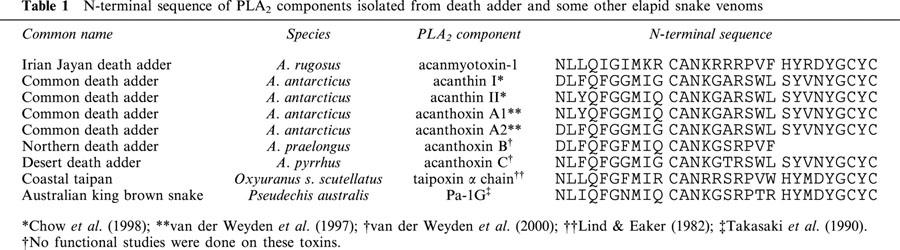
The N-terminal amino acid sequence of acanmyotoxin-1 was determined (Table 1). The location of half-cystines was typical of elapid PLA2 enzymes. The N-terminal sequence of acanmyotoxin-1 was compared with other protein sequences at the National Center for Biotechnology Information (NCBI) database using the BLAST service. Acanmyotoxin-1 shared highest identity with taipoxin α chain (75%) from the coastal taipan (Oxyuranus s. scutellatus) and Pa-1G (65%) from the Australian king brown snake (Pseudechis australis). Acanmyotoxin-1 shared lower identity with other previously isolated death adder PLA2 components such as acanthin II (55%) and acanthin I (51%).
Table 1.
N-terminal sequence of PLA2 components isolated from death adder and some other elapid snake venoms
Phospholipase A2 activity
High PLA2 activity was detected in both A. rugosus venom and the isolated component acanmyotoxin-1. A. rugosus venom had a specific activity of 140.2±10.4 μmol min−1 mg−1 (n=6) while acanmyotoxin-1 had a specific activity of 153.4±11 μmol min−1 mg−1 (n=6). The positive control, bee venom PLA2 had a specific activity of 287.5±17.5 μmol min−1 mg−1 (n=4). 4-BPB modified acanmyotoxin-1 had no PLA2 activity (n=6).
Chick isolated biventer cervicis nerve-muscle preparation
Neurotoxic studies
The positive control and presynaptic neurotoxin, paradoxin (0.07 μM) caused time-dependent inhibition of indirect twitches, whereas acanmyotoxin-1 (0.1 μM) had no significant inhibitory effect on twitch height compared to the vehicle, 0.1% bovine serum albumin in 0.9% saline (n=4–8; one-way ANOVA, P<0.05; Figure 3a). Paradoxin (0.07 μM), acanmyotoxin-1 (0.1 μM) and vehicle had no significant effect on the contractile responses to exogenous ACh (1 mM), CCh (20 μM) and KCl (40 mM) (n=4–8; Student's paired t-test, P<0.05; Figure 3b). However, acanmyotoxin-1 (0.1 μM) caused a significant increase in baseline tension compared to the vehicle (n=4–8; one-way ANOVA, P<0.05; data not shown).
Figure 3.
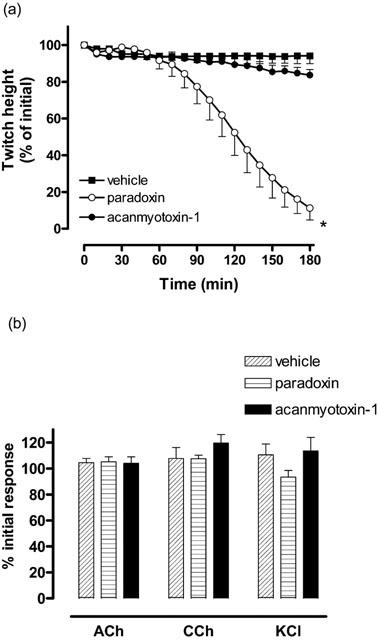
The effect of acanmyotoxin-1 (0.1 μM; n=4), paradoxin (positive control; 0.07 μM; n=4) or vehicle (n=8) on (a) indirect twitches or (b) contractile responses to exogenous ACh, CCh and KCl in the CBCNM preparation. *P<0.05, significantly different from vehicle, one-way ANOVA.
Myotoxic studies
A. rugosus venom (30–50 μg ml−1) caused a significant inhibition of direct twitches compared to the vehicle (n=4–8; one-way ANOVA, P<0.05; Figure 4a). This effect was dose-dependent with A. rugosus venom (50 μg ml−1) causing a significant inhibition of direct twitches compared to A. rugosus venom at 10 μg ml−1 (n=4–5; one-way ANOVA, P<0.05; Figure 4a). In addition, A. rugosus venom (10–50 μg ml−1) induced a significant increase in baseline tension compared to the vehicle control (n=4–8; one-way ANOVA, P<0.05; Figure 4b). Again this effect was dose-dependent with A. rugosus venom (50 μg ml−1) causing a significantly greater contraction compared to A. rugosus venom at 10 μg ml−1 (n=4–5; one-way ANOVA, P<0.05; Figure 4b).
Figure 4.
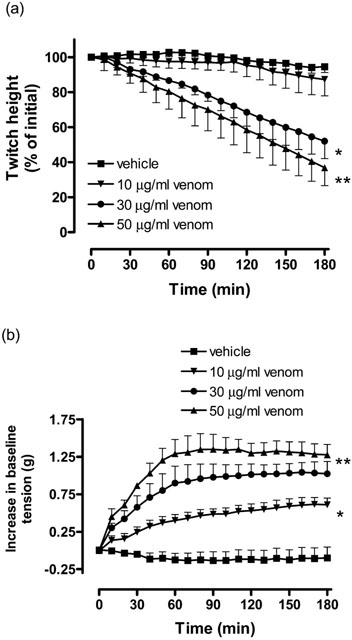
The effect of A. rugosus venom (10–50 μg ml−1; n=4) or vehicle (n=8) on (a) direct twitches or (b) baseline tension of the CBCNM preparation. *P<0.05, significantly different from vehicle, one-way ANOVA. **P<0.05, significantly different from A. rugosus venom (10 μg ml−1), one-way ANOVA.
Acanmyotoxin-1 (1 μM) caused a significant inhibition of direct twitches compared to the vehicle, whereas acanmyotoxin-1 (0.1 μM) had no significant effect on the twitch height (n=4–6; one-way ANOVA, P<0.05; Figure 5a). In addition, acanmyotoxin-1 (0.1–1 μM) induced a significant increase in baseline tension compared to the vehicle (n=4–6; one-way ANOVA, P<0.05; Figure 5b). This response was dose-dependent with acanmyotoxin-1 (1 μM) causing a significantly greater contraction than acanmyotoxin-1 at 0.1 μM (n=4; one-way ANOVA, P<0.05; Figure 5b).
Figure 5.
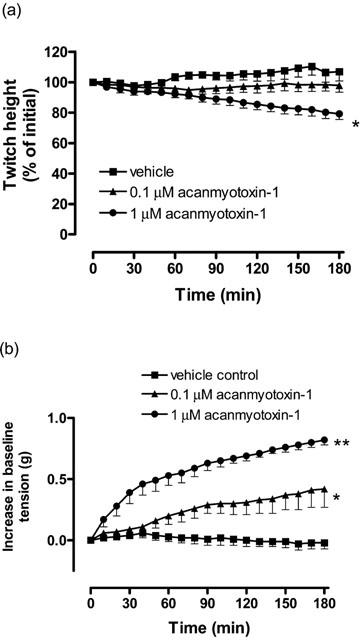
The effect of acanmyotoxin-1 (0.1–1 μM; n=4) or vehicle (n=6) on (a) direct twitches or (b) baseline tension of the CBCNM preparation. *P<0.05, significantly different from vehicle, one-way ANOVA.
Antivenom studies
Prior incubation (10 min) of CSL death adder antivenom (5 units ml−1) prevented the inhibition of direct twitches and the increase in baseline tension caused by acanmyotoxin-1 (1 μM; n=4; Figures 6a,b).
Figure 6.
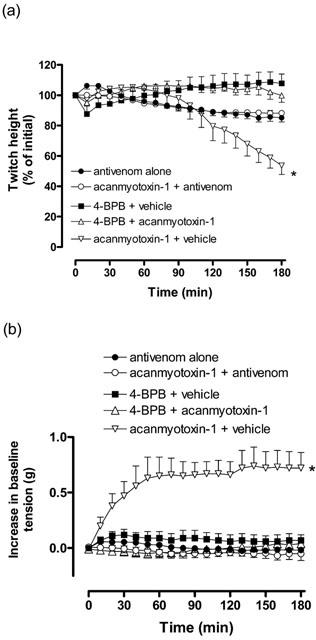
The effect of acanmyotoxin-1 (1 μM; n=4) or vehicle (BSA; n=4) in the presence of antivenom (5 units ml−1), and effect of acanmyotoxin-1 (1 μM; n=4) or vehicle (sodium cacodylate; n=6) incubated in 4-BPB (1.8 mM) on (a) direct twitches or (b) baseline tension of the CBCNM preparation. Positive control was acanmyotoxin-1 (1 μM; n=4) incubated in vehicle (acetone). *P<0.05, significantly different from 4-BPB plus vehicle, one-way ANOVA.
4-BPB modified acanmyotoxin-1 studies
Acanmyotoxin-1 (1 μM) plus vehicle (acetone) significantly inhibited direct twitches compared to 4-BPB plus vehicle (sodium cacodylate; n=4–6; one-way ANOVA, P<0.05; Figure 6a). However, acanmyotoxin-1 (1 μM) plus 4-BPB had no significant inhibitory effect on direct twitches compared to 4-BPB plus vehicle (n=4–6; one-way ANOVA, P<0.05; Figure 6a). In addition, acanmyotoxin-1 (1 μM) plus vehicle induced a significant increase in baseline tension compared to 4-BPB plus vehicle, while acanmyotoxin-1 (1 μM) plus 4-BPB had no significant effect on the baseline tension (n=4–6; one-way ANOVA, P<0.05; Figure 6b).
Morphological studies
Light microscopy studies of tissues exposed to A. rugosus venom (10–50 μg ml−1) and acanmyotoxin-1 (0.1–1 μM) showed dose-dependent morphological changes in skeletal muscle compared to the vehicle control tissues (Figures 7a,b,c,d; data not shown for 10 and 50 μg ml−1 A. rugosus venom). These changes included muscle fibre damage and vacuolation of the muscle cells. Prior incubation of CSL death adder antivenom (5 units ml−1) prevented morphological changes from occurring due to acanmyotoxin-1 (1 μM; Figure 7e). No detectable morphological changes were seen in tissues equilibrated with antivenom alone (data not shown). While acanmyotoxin-1 (1 μM) plus vehicle (i.e. acetone) induced morphological changes similar to acanmyotoxin-1 (1 μM) alone, no detectable morphological changes were seen in acanmyotoxin-1 (1 μM) plus 4-BPB or vehicle plus 4-BPB treated tissues (data not shown).
Figure 7.
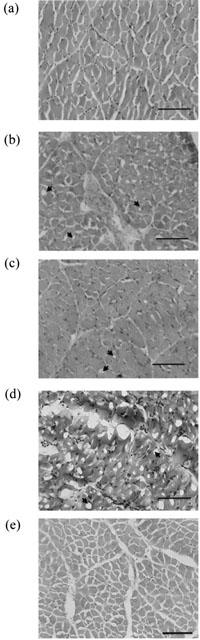
Transverse sections of CBCNM preparations exposed to (a) vehicle (BSA); (b) A. rugosus venom (30 μg ml−1); (c) acanmyotoxin-1 (0.1 μM); (d) acanmyotoxin-1 (1 μM); (e) acanmyotoxin-1 (1 μM) in the presence of antivenom (5 units ml−1). Scale bars, 100 μm in all micrographs. Arrowheads indicate prominent vacuoles.
Discussion
Until recently, research on death adders has been largely focused on the venom from the Australian A. antarcticus (common death adder). Both in vivo and in vitro studies have shown that this venom has no significant myotoxic activity (Mebs & Samejima, 1980; Sutherland et al., 1981; Wickramaratna & Hodgson, 2001). Therefore, it has been thought that death adder venoms are devoid of myotoxic activity. However, a recent clinical study reported evidence of rhabdomyolysis in patients following death adder envenomations, in Papua New Guinea, by a species not closely aligned with A. antarcticus (Lalloo et al., 1996). Consequently, the present study examined A. rugosus venom for myotoxic activity, and isolated the first myotoxin from a death adder venom.
Acanmyotoxin-1 was isolated as a single peak from A. rugosus venom by successive RP-HPLC separations. As seen from the RP-HPLC chromatogram of the A. rugosus venom, acanmyotoxin-1 is also the last major peak to elute at the given conditions. Using electrospray mass spectrometry the molecular mass of acanmyotoxin-1 was determined to be 13811 daltons. It is well documented that elapid snake venom PLA2 components usually have molecular mass in the range of 12–14 kDa (Dawson & Hemington, 1967; Sim, 1998). While the molecular mass of acanmyotoxin-1 is consistent with other snake venom PLA2 components it is about 1 kDa bigger than PLA2 components previously isolated from A. antarcticus and A. praelongus venoms. Comparison of the N-terminal sequences showed that acanmyotoxin-1 shared highest identity with taipoxin α chain (75%) and Pa-1G (65%). The taipoxin α chain is the subunit of a potent presynaptic neurotoxin possessing myotoxic and PLA2 activity (Harris & Maltin, 1982; Lind & Eaker, 1982), and Pa-1G is a myotoxic PLA2 component (Geh et al., 1992). Interestingly, lower sequence identity was seen with the antiplatelet active death adder PLA2 components.
Due to the sequence homology and molecular mass resemblance of acanmyotoxin-1 to other elapid venom PLA2 components, the specific activity of acanmyotoxin-1 was determined. High PLA2 activity was detected in both A. rugosus venom and the isolated component. Given the high PLA2 activity of A. rugosus whole venom it is possible that the whole venom may have other components with high PLA2 activity.
A. rugosus venom and acanmyotoxin-1 were examined for in vitro myotoxicity using the directly stimulated CBCNM preparation. Both A. rugosus venom and acanmyotoxin-1 caused dose-dependent inhibition of direct twitches. Furthermore, both A. rugosus venom and acanmyotoxin-1 induced a dose-dependent increase in baseline tension. An inhibition of direct twitches and a rise in baseline tension is indicative of myotoxic activity (Harvey et al., 1994). In addition to these results, light microscopy studies of tissues exposed to A. rugosus venom and acanmyotoxin-1 showed obvious morphological changes in skeletal muscle compared to tissues exposed to the vehicle. Together, these results suggest that both A. rugosus venom and acanmyotoxin-1 cause myotoxicity.
Several studies have shown that some elapid venom PLA2 components, such as notexin and notechis II-5, are myotoxic as well as presynaptically neurotoxic (Harris & Johnson, 1978; Harris, 1991; Dixon & Harris, 1996). Therefore, acanmyotoxin-1 was examined for in vitro neurotoxicity using the indirectly stimulated CBCNM preparation. Acanmyotoxin-1 (0.1 μM) had no significant inhibitory effect on the indirect twitch height compared to the vehicle control, thus, suggesting it to be lacking in any detectable neurotoxic activity at this concentration. However, acanmyotoxin-1 (0.1 μM) caused a significant increase in baseline tension compared to vehicle. Given the myotoxic activity, it was not possible to further examine the neurotoxic activity of acanmyotoxin-1 at a higher concentration. In contrast to acanmyotoxin-1 (0.1 μM), paradoxin (0.07 μM) caused almost full inhibition of the indirect twitches over 3 h. Electrophysiological studies are required to further examine the neurotoxic activity of acanmyotoxin-1.
Due to habitat destruction and consequential decrease in species population levels, death adder envenomations are a rare occurrence in Australia, although these are still a significant health problem in Papua New Guinea (Currie et al., 1991; Sutherland, 1992; Lalloo et al., 1995; 1996). CSL death adder antivenom is indicated for use in envenomation by any death adder species (AMH, 1998; White, 1998). Since A. antarcticus venom lacks myotoxic activity, and given that death adder antivenom has been raised against A. antarcticus venom, it was of clinical relevance to examine the efficacy of death adder antivenom against the in vitro myotoxicity of acanmyotoxin-1. Previously, we have studied the efficacy of CSL death adder antivenom against the in vitro neurotoxicity of A. rugosus venom (Fry et al., 2001). Prior incubation of antivenom (5 units ml−1) prevented the inhibition of direct twitches and the increase in baseline tension caused by acanmyotoxin-1 (1 μM). Furthermore, antivenom prevented morphological changes from occurring due to acanmyotoxin-1 (1 μM). Thus, CSL death adder antivenom is effective in neutralizing the in vitro myotoxic activity of acanmyotoxin-1.
In order to determine whether the PLA2 activity of acanmyotoxin-1 is necessary for the myotoxic action, acanmyotoxin-1 was subjected to 4-BPB modification. Many studies have shown that PLA2 activity can be inhibited by selective acylation of His-48 using 4-BPB (Volwerk et al., 1974; Abe et al., 1977). When acanmyotoxin-1 was incubated with 4-BPB, the enzymatic activity as well as myotoxic activity was abolished. Thus, suggesting that PLA2 activity is essential for the myotoxic activity of acanmyotoxin-1. In contrast to acanmyotoxin- 1, some studies have shown that Lys-49 PLA2 components lack catalytic activity on artificial substrates (Soares et al., 2000; 2001). However, 4-BPB modification of these Lys-49 PLA2 components prevented some of their pharmacological effects (Soares et al., 2000; 2001). Thus, it was suggested that inhibition of the pharmacological effects by 4-BPB modification were not due to the inhibition of enzymatic activity (Soares et al., 2000). Recently, it has been shown that some Lys-49 PLA2 components are catalytically active on biological substrates (Soares et al., 2002). Therefore, the observed reduction in pharmacological effects after 4-BPB modification of Lys-49 PLA2 components may still be the result of inhibition of the catalytic activity. However, it is possible that His-48 may be important in the pharmacological site of PLA2 components.
In conclusion, A. rugosus venom caused dose-dependent in vitro myotoxicity in the CBCNM preparation. Acanmyotoxin-1 is the first myotoxic component to be isolated from any death adder venom. Although CSL death adder antivenom has been raised against A. antarcticus venom it is effective in neutralizing the myotoxic activity of acanmyotoxin-1. Furthermore, studies with 4-BPB suggest that PLA2 activity is essential for the myotoxic activity of acanmyotoxin-1. Given the results of this study clinicians may need to be mindful of possible myotoxicity following death adder envenomation in Irian Jaya. In light of this finding, other death adder venoms should be examined for myotoxic activity.
Acknowledgments
We thank Dr Susan Luff (Microscopy and Imaging Research Facility, Monash University) and Dr Jane Black (Dept. of Anatomy, Monash University) for their input into the histology studies. We would also like to thank Dr Geoff Isbister (Clinical Toxicologist and Emergency Physician, Newcastle Mater Misericordiae Hospital, Australia) for his useful comments. We are grateful to CSL Ltd. for the generous gift of death adder antivenom. This research was funded by a Monash Small Grant and the Defence Science and Technology Agency, Singapore.
Abbreviations
- 4-BPB
4-bromophenacyl bromide
- CBCNM
chick biventer cervicis nerve-muscle
- CCh
carbachol
- PLA2
Phospholipase A2
- TFA
trifluoroacetic acid
References
- ABE T., ALEMA S., MILEDI R. Isolation and characterization of presynaptically acting neurotoxins from the venom of Bungarus snakes. Eur. J. Biochem, 1977;80:1–12. doi: 10.1111/j.1432-1033.1977.tb11849.x. [DOI] [PubMed] [Google Scholar]
- AMH . Australian Medicines Handbook. Adelaide. Adelaide: Australian Medicines Handbook Pty Ltd; 1998. [Google Scholar]
- BARFARAZ A., HARVEY A.L. The use of the chick biventer cervicis preparation to assess the protective activity of six international reference antivenoms on the neuromuscular effects of snake venoms in vitro. Toxicon. 1994;32:267–272. doi: 10.1016/0041-0101(94)90079-5. [DOI] [PubMed] [Google Scholar]
- BELL K.L., SUTHERLAND S.K., HODGSON W.C. Some pharmacological studies of venom from the inland taipan (Oxyuranus microlepidotus) Toxicon. 1998;36:63–74. doi: 10.1016/s0041-0101(97)00060-3. [DOI] [PubMed] [Google Scholar]
- BROAD A.J., SUTHERLAND S.K., COULTER A.R. The lethality in mice of dangerous Australian and other snake venom. Toxicon. 1979;17:661–664. doi: 10.1016/0041-0101(79)90245-9. [DOI] [PubMed] [Google Scholar]
- CAMPBELL C.H. The death adder (Acanthophis antarcticus): the effect of the bite and its treatment. Med. J. Aust. 1966;2:922–925. doi: 10.5694/j.1326-5377.1966.tb97634.x. [DOI] [PubMed] [Google Scholar]
- CHOW G., SUBBURAJU S., KINI R.M. Purification, characterization, and amino acid sequence determination of acanthins, potent inhibitors of platelet aggregation from Acanthophis antarcticus (common death adder) venom. Arch. Biochem. Biophys. 1998;354:232–238. doi: 10.1006/abbi.1998.0685. [DOI] [PubMed] [Google Scholar]
- COGGER H.G. Reptiles & amphibians of Australia. Sydney: Reed New Holland; 2000. [Google Scholar]
- CRACHI M.T., HAMMER L.W., HODGSON W.C. A pharmacological examination of venom from the Papuan taipan (Oxyuranus scutellatus canni) Toxicon. 1999a;37:1721–1734. doi: 10.1016/s0041-0101(99)00114-2. [DOI] [PubMed] [Google Scholar]
- CRACHI M.T., HAMMER L.W., HODGSON W.C. The effect of antivenom on the in vitro neurotoxicity of venoms from the taipans Oxyuranus scutellatus, Oxyuranus microlepidotus and Oxyuranus scutellatus canni. Toxicon. 1999b;37:1771–1778. doi: 10.1016/s0041-0101(99)00118-x. [DOI] [PubMed] [Google Scholar]
- CURRIE B.J., SUTHERLAND S.K., HUDSON B.J., SMITH A.M.A. An epidemiological study of snake bite envenomation in Papua New Guinea. Med. J. Aust. 1991;154:266–268. doi: 10.5694/j.1326-5377.1991.tb121088.x. [DOI] [PubMed] [Google Scholar]
- DAWSON R.M., HEMINGTON N. Some properties of purified phospholipase D and especially the effect of amphipathic substances. Biochem. J. 1967;102:76–86. doi: 10.1042/bj1020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON R.W., HARRIS J.B. Myotoxic activity of the toxic phospholipase, notexin, from the venom of the Australian tiger snake. J. Neuropathol. Exp. Neurol. 1996;55:1230–1237. doi: 10.1097/00005072-199612000-00006. [DOI] [PubMed] [Google Scholar]
- FRY B.G., WICKRAMARATNA J.C., HODGSON W.C., ALEWOOD P.F., KINI R.M., HO H., WÜSTER W. Electrospray liquid chromatography/mass spectrometry fingerprinting of Acanthophis (death adder) venoms: taxonomic and toxinological implications. Rapid Commun. Mass Spectrom. 2002;16:600–608. doi: 10.1002/rcm.613. [DOI] [PubMed] [Google Scholar]
- FRY B.G., WICKRAMARATNA J.C., JONES A., ALEWOOD P.F., HODGSON W.C. Species and regional variations in the effectiveness of antivenom against the in vitro neurotoxicity of death adder (Acanthophis) venoms. Toxicol. Appl. Pharmacol. 2001;175:140–148. doi: 10.1006/taap.2001.9233. [DOI] [PubMed] [Google Scholar]
- GEH S.L., ROWAN E.G., HARVEY A.L. Neuromuscular effects of four phospholipases A2 from the venom of Pseudechis australis, the Australian king brown snake. Toxicon. 1992;30:1051–1057. doi: 10.1016/0041-0101(92)90050-f. [DOI] [PubMed] [Google Scholar]
- HARRIS J.B.Phospholipases in snake venoms and their effects on nerve and muscle Snake Toxins. 1991New York: Pergamon Press; 91–129.ed. Harvey, A.L.pp [Google Scholar]
- HARRIS J.B., JOHNSON M.A. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. Pharmacol. Physiol. 1978;5:587–600. doi: 10.1111/j.1440-1681.1978.tb00714.x. [DOI] [PubMed] [Google Scholar]
- HARRIS J.B., MALTIN C.A. Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br. J. Pharmacol. 1982;76:61–75. doi: 10.1111/j.1476-5381.1982.tb09191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY A.L., BARFARAZ A., THOMSON E., FAIZ A., PRESTON S., HARRIS J.B. Screening of snake venoms for neurotoxic and myotoxic effects using simple in vitro preparations from rodents and chicks. Toxicon. 1994;32:257–265. doi: 10.1016/0041-0101(94)90078-7. [DOI] [PubMed] [Google Scholar]
- HOSER R. Death adders (genus Acanthophis): an overview, including descriptions of five new species and one subspecies. Monitor. 1998; 9:20–41. [Google Scholar]
- KELLAWAY C.H. Observations on the certainly lethal dose of the venom of the death adder (Acanthophis antarcticus) for the common laboratory animals. Med. J. Aust. 1929a;1:764–772. [Google Scholar]
- KELLAWAY C.H. The action of the venoms of the copper-head (Denisonia superba) and of the death adder (Acanthophis antarcticus) on the coagulation of the blood. Med. J. Aust. 1929b;1:772–781. [Google Scholar]
- KIM H.S., TAMIYA N. Isolation, properties and amino acid sequence of a longchain neurotoxin, Acanthophis antarcticus b, from the venom of an Australian snake (the common death adder, Acanthophis antarcticus) Biochem. J. 1981a;193:899–906. doi: 10.1042/bj1930899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM H.S., TAMIYA N. The amino acid sequence and position of the free thiol group of a short-chain neurotoxin from common-death-adder (Acanthophis antarcticus) venom. Biochem. J. 1981b;199:211–218. doi: 10.1042/bj1990211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LALLOO D.G., TREVETT A.J., BLACK J., MAPAO J., SAWERI A., NARAQI S., OWENS D., KAMIGUTI A.S., HUTTON R.A., THEAKSTON R.D.G., WARRELL D.A. Neurotoxicity, anticoagulant activity and evidence of rhabdomyolysis in patients bitten by death adders (Acanthophis sp.) in southern Papua New Guinea. QJM. 1996;89:25–35. doi: 10.1093/oxfordjournals.qjmed.a030134. [DOI] [PubMed] [Google Scholar]
- LALLOO D.G., TREVETT A.J., SAWERI A., NARAQI S., THEAKSTON R.D.G., WARRELL D.A. The epidemiology of snake bite in Central Province and National Capital District, Papua New Guinea. Trans. Roy. Soc. Trop. Med. Hyg. 1995;89:178–182. doi: 10.1016/0035-9203(95)90485-9. [DOI] [PubMed] [Google Scholar]
- LIND P., EAKER D. Amino-acid sequence of the alpha-subunit of taipoxin, an extremely potent presynaptic neurotoxin from the Australian snake taipan (Oxyuranus s. scutellatus) Eur. J. Biochem. 1982;124:441–447. doi: 10.1111/j.1432-1033.1982.tb06612.x. [DOI] [PubMed] [Google Scholar]
- MEBS D., SAMEJIMA Y. Purification, from Australian elapid venoms, and properties of phospholipases A which cause myoglobinuria in mice. Toxicon. 1980;18:443–454. doi: 10.1016/0041-0101(80)90052-5. [DOI] [PubMed] [Google Scholar]
- SHEUMACK D.D., HOWDEN M.E.H., SPENCE I. Isolation and partial characterisation of a lethal neurotoxin from the venom of the Australian death adder (Acanthophis antarcticus) Toxicon. 1979;17:609–616. doi: 10.1016/0041-0101(79)90235-6. [DOI] [PubMed] [Google Scholar]
- SHEUMACK D.D., SPENCE I., TYLER M.I., HOWDEN M.E.H. The complete amino acid sequence of a post-synaptic neurotoxin isolated from the venom of the Australian death adder snake Acanthophis antarcticus. Comp. Biochem. Physiol. 1990;95B:45–50. doi: 10.1016/0305-0491(90)90246-p. [DOI] [PubMed] [Google Scholar]
- SIM K.L. Purification and preliminary characterisation of praelongin phospholipases, antiplatelet agents from the snake venom of Acanthophis praelongus. Biochim. Biophys. Acta. 1998;1379:198–206. doi: 10.1016/s0304-4165(97)00097-4. [DOI] [PubMed] [Google Scholar]
- SOARES A.M., ANDRIAO-ESCARSO S.H., BORTOLETO R.K., RODRIGUES-SIMIONI L., ARNI R.K., WARD R.J., GUTIERREZ J.M., GIGLIO J.R. Dissociation of enzymatic and pharmacological properties of piratoxins- I and -III, two myotoxic phospholipases A2 from Bothrops pirajai snake venom. Arch. Biochem. Biophys. 2001;387:188–196. doi: 10.1006/abbi.2000.2244. [DOI] [PubMed] [Google Scholar]
- SOARES A.M., GUERRA S.I.Q.M.R., BORJA-OLIVEIRA C.R., RODRIGUES V.M., RODRIGUES-SIMIONI L., RODRIGUES V., FONTES M.R., LOMONTE B., GUTIERREZ J.M., GIGLIO J.R. Structural and functional characterization of BnSP-7, a Lys49 myotoxic phospholipase A(2) homologue from Bothrops neuwiedi pauloensis venom. Arch. Biochem. Biophys. 2000;378:201–209. doi: 10.1006/abbi.2000.1790. [DOI] [PubMed] [Google Scholar]
- SOARES A.M., OSHIMA-FRANCO Y., VIEIRA C.A., LEITE G.B., FLETCHER J.E., JIANG M.S., CINTRA A.C., GIGLIO J.R., RODRIGUES-SIMIONI L. Mn(2+) ions reduce the enzymatic and pharmacological activities of bothropstoxin-I, a myotoxic Lys49 phospholipase A(2) homologue from Bothrops jararacussu snake venom. Int. J. Biochem. Cell. Biol. 2002;34:668–677. doi: 10.1016/s1357-2725(01)00174-1. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND S.K. Deaths from snake bite in Australia, 1981–1991. Med. J. Aust. 1992;157:740–745. doi: 10.5694/j.1326-5377.1992.tb141272.x. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND S.K., CAMPBELL D.G., STUBBS A.E. A study of the major Australian snake venoms in the monkey (Macaca fascicularis) Pathology. 1981;13:705–715. doi: 10.3109/00313028109086644. [DOI] [PubMed] [Google Scholar]
- TAKASAKI C., YUTANI F., KAJIYASHIKI T. Amino acid sequences of eight phosphlipases A2 from the venom of Australian King Brown snake, Pseudechis australis venom. Toxicon. 1990;28:329–339. doi: 10.1016/0041-0101(90)90068-i. [DOI] [PubMed] [Google Scholar]
- TYLER M.I., RETSON-YIP K.V., GIBSON M.K., BARNETT D., HOWE E., STOCKLIN R., TURNBULL R.K., KUCHEL T., MIRTSCHIN P. Isolation and amino acid sequence of a new long-chain neurotoxin with two chromatographic isoforms (Aa e1 and Aa e2) from the venom of the Australian death adder (Acanthophis antarcticus) Toxicon. 1997;35:555–562. doi: 10.1016/s0041-0101(96)00159-6. [DOI] [PubMed] [Google Scholar]
- VAN DER WEYDEN L., HAINS P.G., BROADY K.W. Characterisation of the biochemical and biological variations from the venom of the death adder species (Acanthophis antarcticus, A. praelongus and A. pyrrhus) Toxicon. 2000;38:1703–1713. doi: 10.1016/s0041-0101(00)00101-x. [DOI] [PubMed] [Google Scholar]
- VAN DER WEYDEN L., HAINS P., BROADY K., SHAW D., MILBURN P. Amino acid sequence of a neurotoxic phospholipase A2 enzyme from common death adder (Acanthophis antracticus) venom. J. Nat. Toxins. 2001;10:33–42. [PubMed] [Google Scholar]
- VAN DER WEYDEN L., HAINS P., MORRIS M., BROADY K. Acanthoxin, a toxic phospholipase A2 from the venom of the common death adder (Acanthophis antarcticus) Toxicon. 1997;35:1315–1325. doi: 10.1016/s0041-0101(97)00004-4. [DOI] [PubMed] [Google Scholar]
- VOLWERK J.J., PIETERSON W.A., DE HAAS G.H. Histidine at the active site of phospholipase A2. Biochemistry. 1974;13:1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- WHITE J. Envenoming and antivenom use in Australia. Toxicon. 1998;36:1483–1492. doi: 10.1016/s0041-0101(98)00138-x. [DOI] [PubMed] [Google Scholar]
- WICKRAMARATNA J.C., HODGSON W.C. A pharmacological examination of venoms from three species of death adder (Acanthophis antarcticus, Acanthophis praelongus and Acanthophis pyrrhus) Toxicon. 2001;39:209–216. doi: 10.1016/s0041-0101(00)00117-3. [DOI] [PubMed] [Google Scholar]
- WUSTER W., GOLAY P., WARRELL D.A. Synopsis of recent developments in venomous snake systematics, No. 3. Toxicon. 1999;37:1123–1129. doi: 10.1016/s0041-0101(98)00248-7. [DOI] [PubMed] [Google Scholar]


