Abstract
Prostaglandin E1 (PGE1, alprostadil) is used as a vasodilator for the treatment of peripheral vascular diseases.
Previous reports suggested a pro-angiogenic effect for PGE1.
We studied the in vitro and in vivo effect of PGE1, complexed with α-cyclodextrin, on the angiogenic process. Contrary to what was expected, we found that, in human umbilical vein endothelial cells (HUVECs), PGE1 inhibited proliferation, migration and capillary-like structure formation in Matrigel.
By RT–PCR studies, the expression of the EP2 and EP3 subtypes of the PG receptor was detected in HUVECs.
PGE1 alone stimulated adenylate cyclase activity at micromolar concentrations, while at nanomolar concentrations potentiated the forskolin-induced cAMP accumulation.
8-Bromoadenosine-3′:5′-cyclic monophosphate (Br-cAMP) mimicked the inhibitory effect of PGE1 on endothelial cell growth, motility and tube formation.
Sulprostone, an agonist at the EP3 subtype of PG receptors, mimicked the in vitro anti-angiogenic effects of PGE1, while butaprost, an EP2 receptor agonist, had no effect.
Finally, in the plug assay model of angiogenesis in mice, PGE1 showed a strong inhibitory effect on Matrigel neovascularization.
Thus, PGE1 possesses strong anti-angiogenic activity in vitro and in vivo.
Keywords: Prostaglandins, angiogenesis, human endothelial cells, cell signalling, in vivo neovascularization, Matrigel
Introduction
Prostaglandin E1 (PGE1, alprostadil) has been shown to induce vasodilation and to inhibit platelet aggregation. Based on these properties, PGE1, alone or complexed with α-cyclodextrin to improve solubility and stability in water (Wiese et al., 1991), has been administered for the treatment of peripheral vascular diseases (ICAI Study, 1999). The availability of a drug capable of inducing the formation of new vessels, operating a sort of endogenous bypass, would be of great value for treating diseases like critical leg ischemia. For this reason, recently, angiogenic factors like vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) have been administered to animals, and in few cases to humans, as a recombinant protein (Schumacher et al., 1998) or by gene transfer (Baumgartner et al., 1998). Although promising, potential toxicity, bioavailability problems and other complex factors have delayed the use of these technologies.
Since PGE1 has been suggested to induce angiogenesis in vivo (Ziche et al., 1982; Diaz-Flores et al., 1994), we studied the effect of PGE1/α-cyclodextrin, which is a well tolerated drug, easily administered and relatively inexpensive, upon human endothelial cell functions related to the angiogenic process: proliferation, migration, and formation of capillary-like structures on Matrigel, in vitro. We also studied the effect of PGE1/α-cyclodextrin in vivo, using the Matrigel plug assay in mice. Contrary to expectations, the drug showed strong anti-angiogenic effects in vitro and in vivo.
Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from freshly derived umbilical cords by digestion with collagenase essentially as described by Jaffe et al. (1973). Cells were routinely grown in 199 medium, supplemented with 20% heat-inactivated foetal bovine serum (FBS), 25 μg ml−1 endothelial cell growth factor and 50 μg ml−1 heparin, and used at passages 2–6.
Proliferation assays
HUVECs, plated at a density of 2×104 cells well−1 in 96-well plates, were pre-treated for 30 min with PGE1/α-cyclodextrin, and then stimulated for 48 h with 20 ng ml−1 VEGF or 20 ng ml−1 bFGF in the presence of the drug. [3H]-Thymidine (1 μCi well−1; specific activity 2 Ci mmol−1) was added during the last 6 h of incubation. The radioactivity associated to the TCA-insoluble fraction was measured after 10% TCA extraction and NaOH solubilization.
Chemotaxis assays
Migration assays were performed in a 48-well modified Boyden chamber. Nucleopore polyvinylpyrrolidone (PVP)-free polycarbonate filters (8 μm) were coated with 10 μg ml−1 of human fibronectin, and placed over a bottom chamber containing 50 ng ml−1 VEGF. The cells, suspended in 199 medium containing 0.1% fatty acid free bovine serum albumin (BSA), were added to the upper chamber at a density of 5×104 cells well−1 in the presence of the drug. After 6 h of incubation at 37°C, non-migrated cells on the upper surface of the filter were removed by scraping. The cells migrated to the lower side of the filter were stained with Diff-quick stain, and 5 to 8 unit fields per filter were counted at 160× magnification with a microscope (Zeiss). The assays were run in triplicate.
In vitro angiogenesis assays
The formation of vascular-like structures was assessed on a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma (Matrigel), frequently used for the evaluation of in vitro angiogenesis (for reviews see Baatout, 1997; Benelli & Albini, 1999). Twenty-four well plates were coated with Matrigel and the cells were seeded on the polymerized matrix at a density of 5×104 cells well−1. VEGF (10 ng ml−1) and bFGF (10 ng ml−1) were used as angiogenic stimuli. PGE1/α-cyclodextrin was present in the medium during the incubation. After 12–18 h at 37°C in 5% CO2, cells were fixed in 4% paraformaldehyde, and images were acquired using an Axiovert microscope (Zeiss) with a PCO SuperVGA SensiCam (Axon Instruments, U.S.A.). The degree of cord formation was quantified by measuring the area occupied by the tubes in five random fields from each well using the National Institute of Health (NIH) Image Program.
Reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was isolated using a Rneasy total RNA isolation kit (Qiagen, GmbH, Germany) following the supplier's protocol. One μg of total RNA was reverse-transcribed by using oligo-dT and amplified with 35 PCR cycles. A set of oligonucleotide primers specific for human EP receptors were used, as described in Sheng et al. (2001). The amplified products were visualized on 1.5% agarose gels.
Determination of intracellular cAMP
HUVECs, plated at a density of 1–1.5×105 cells well−1 in 24-well plates, were preincubated for 10 min with 1 mM isobutylmethylxanthine (IBMX) in 199 medium before stimulation for 20 min at 37°C with PGE1/α-cyclodextrin. The reaction was terminated by aspiration of the medium followed by the addition of 0.5 ml of cold absolute ethanol. After overnight freezing at −20°C, the ethanol supernatants were dried, and the intracellular cAMP levels were evaluated with a commercial kit.
In vivo angiogenesis
We used the Matrigel sponge model of angiogenesis introduced by Passaniti et al. (1992) and Albini et al. (1994). This widely used method for in vivo angiogenesis provides the opportunity for quantifying the effect of angiogenic stimulators and inhibitors more easily than other methods (CAM and rabbit corneal assay) (Jain et al., 1997). Briefly, C57/bl6 female mice (6–8 weeks of age, Charles River, Calco, LC, Italy) were injected subcutaneously with 0.5 ml of Matrigel supplemented with 150 ng ml−1 aFGF and 60 units heparin ml−1 near the abdominal midline. In vivo, the gel quickly polymerized to form a solid gel. Although the commercially available Matrigel is naturally enriched with growth factors, variability among different batches can occur. So additional factors are normally added in a wide range of concentrations, from 1 ng to 1 μg ml−1 of FGF (Passaniti et al., 1992; Maeshima et al., 2000; Bakre et al., 2002). In our hands, the most reproducible results were obtained with the concentrations of FGF and heparin indicated above.
PGE1/α-cyclodextrin (20 ng day−1) was systemically administered by means of osmotic pumps (Alzet, Charles River) implanted subcutaneously in the back of the animals, posterior to the scapulae. The pumps continuously delivered the drug at controlled rates, with a pumping rate of 0.5 μl h−1. The control animals were implanted with the same pumps, filled with saline. After 4 days, mice were killed, the Matrigel pellets were collected and their haemoglobin content was evaluated using a Drabkin reagent kit. Animal care was in accordance with the Italian State regulation governing the care and the treatment of laboratory animals (permission n° 14/2001).
Materials
Prostaglandin E1 (PGE1, alprostadil), in the form of inclusion complex with α-cyclodextrin (Wiese et al., 1991), and heparin were supplied by Schwarz Pharma (Monheim, Germany). Butaprost and sulprostone were from Cayman Chemical (Ann Arbor, MI, U.S.A.). Matrigel and human fibronectin were purchased from Becton Dickinson (Bedford, MA, U.S.A.), VEGF165 from PeproTech Inc. (Rocky Hill, NJ, U.S.A.), FGFs, IBMX, forskolin and Br-cAMP from Sigma Chemicals (St. Louis, MO, U.S.A.). All tissue culture reagents (199 medium, FBS, and endothelial cell growth factor) were purchased from Sigma Chemicals, St. Louis, MO, U.S.A. [3H]-Thymidine and kits for the radioimmunological detection of cAMP were from Amersham Pharmacia Biotech, U.K. The RNA isolation kit was supplied by Qiagen, GmbH, Germany. The Drabkin reagent kit and Diff-quick were from Sigma Chemicals, St. Louis, MO, U.S.A. and VWR Scientific Products, Bridgeport, NJ, U.S.A., respectively.
Statistical analysis
All data obtained in vitro are presented as mean±s.e.mean, where n is the number of individual experiments, each performed in triplicate. For in vivo studies, results are expressed as mean±s.d. of two independent experiments, which were performed using 5–6 animals for each treatment. Statistical analysis was carried out by Student's t-test or by one-way analysis of variance (ANOVA) followed by Bonferroni's test for multiple comparison, using a statistics package (PRISM, GraphPad Software, San Diego, CA, U.S.A.). Differences were considered significant when P<0.05.
Results
The inclusion complex PGE1/α-cyclodextrin was used in all the experiments. To simplify the text and the figures, the complex PGE1/α-cyclodextrin has been referred to as PGE1.
Effect of PGE1 on endothelial cell proliferation
The effect of PGE1 on HUVECs proliferation was assessed by [3H]-thymidine incorporation as described under the Methods section. Figure 1A shows that PGE1 reduced endothelial cell proliferation (up to 100% inhibition) in a concentration dependent manner, displaying an IC50 of 400 nM. In the absence of any growth factor, the drug had no effect on cell proliferation (data not shown). A concentration of α-cyclodextrin alone, equivalent to that present in 5 μM PGE1/α-cyclodextrin complex, did not modify the 3H-thymidine incorporation (Figure 1B), suggesting that the antiproliferative effect of PGE1/α-cyclodextrin is due to its PGE1 component.
Figure 1.
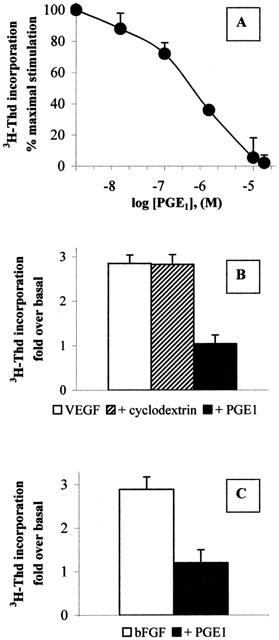
PGE1 inhibits endothelial cell proliferation (A) HUVECs were treated for 48 h with the indicated concentrations of PGE1/α-cyclodextrin in the presence of 20 ng ml−1 VEGF. [3H]-Thymidine ([3H]-Thd) incorporated by the cells was evaluated as described in the Methods section. Results are expressed as per cent of maximal stimulation induced by the growth factor and are the mean±s.e.m. of 3–5 independent experiments. (B) HUVECs were treated for 48 h with 20 ng ml−1 VEGF in the absence (open bar) or in the presence (solid bar) of 5 μM PGE1/α-cyclodextrin. α-cyclodextrin alone (diagonal bar) was added at a concentration equivalent to the one present in 5 μM PGE1. Results are expressed as fold increase in [3H]-Thd incorporated by the cells under basal conditions (199 medium alone, 1042±102 c.p.m./well, n=3) and are the mean±s.e.m. of three independent experiments. (C) HUVECs were treated for 48 h with 20 ng ml−1 of bFGF in the absence (open bar) or in the presence (solid bar) of 5 μM PGE1/α-cyclodextrin. Results are expressed as in Figure 1B.
PGE1 exerted a similar inhibitory effect on HUVECs stimulated by basic FGF (bFGF), another growth inducer of endothelial cells (Presta et al., 1986) (Figure 1C).
Cell cycle distribution, assessed by flow cytometry using propidium iodide, revealed that PGE1 treatment induced accumulation of HUVECs in G0/G1 phase, with a concurrent decrease in the proportion of events in S phase (data not shown).
Effect of PGE1 on endothelial cell migration
The effect of PGE1 on cell migration, using VEGF as chemoattractant, is depicted in Figure 2. The angiogenic factor induced migration of HUVECs with increases of 2.16±0.16-fold above the basal (i.e., in the absence of chemotactic agent), value of 20.9±2.3 cells field−1 (n=13). Addition of increasing concentrations of PGE1 to the cell suspension in the upper part of the Boyden's chamber induced a concentration dependent inhibition of cell migration, with an IC50 of 500 nM, and a maximal inhibition of the chemotactic response (up to 50%) at a concentration between 5 and 10 μM (Figure 2). In the absence of chemoattractant, PGE1 had no effect on endothelial cell migration (data not shown). In the inset (Figure 2), we show that PGE1, when added to the lower part of the chamber, still inhibited migration of HUVECs.
Figure 2.
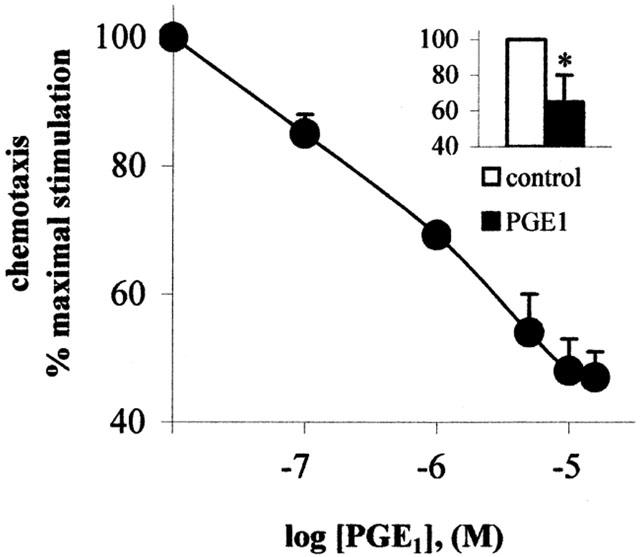
PGE1 inhibits endothelial cell migration. PGE1/α-cyclodextrin was added to the cells in the upper part of a Boyden's chamber at the indicated concentrations, and the chemotaxis assay was performed as described in the Methods section, using 50 ng ml−1 VEGF as attractant. Results are expressed as percent of maximal migration induced by VEGF and are the mean±s.e.m. of 3–5 independent experiments. In the inset, 5 μM PGE1/α-cyclodextrin was added to the lower chamber. *P<0.05, two tailed unpaired Student's t-test.
Effect of PGE1 on the in vitro angiogenic process in endothelial cells
In vitro, endothelial cells plated on Matrigel form cord-like capillary structures, after a few hours, thus mimicking the in vivo angiogenic process (Benelli & Albini, 1999) (Figure 3A).
Figure 3.
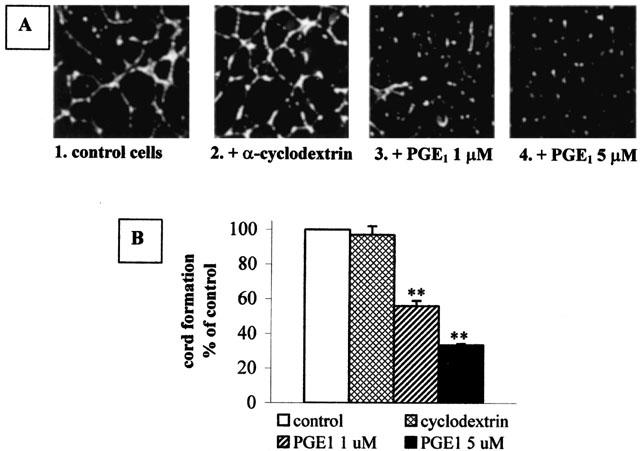
PGE1 inhibits in vitro angiogenesis. (A) HUVECs were seeded on Matrigel and treated as described in the Methods section. Panel 1: control cells stimulated with 10 ng ml−1 VEGF and 10 ng ml−1 bFGF in the absence of any drug. Panel 2: cells treated with these angiogenic factors and α-cyclodextrin alone at a concentration equivalent to the one present in 5 μM PGE1. Panels 3 and 4: cells treated with these angiogenic factors and 1 μM or 5 μM PGE1/α-cyclodextrin, respectively. (B) Quantification of the cord formation shown in (A) by NIH image program. Open bar: control cells. Cross-hitched bar: α-cyclodextrin-treated cells. Diagonal bar: 1 μM PGE1/α-cyclodextrin-treated cells. Solid bar: 5 μM PGE1/α-cyclodextrintreated cells. Each bar is the mean±s.e.m. of 3–4 independent experiments. **P<0.001, One Way ANOVA followed by Bonferroni test.
HUVECs, pre-incubated where indicated for 30 min with 1 and 5 μM PGE1, were then plated on a layer of polymerized Matrigel in the presence of angiogenic factors. PGE1 was present during the entire period of the assay (12–18 h). This treatment resulted in an inhibition of endothelial cell alignment and cord formation (Figure 3A, panels 3 and 4) in comparison with the control (Figure 3A, panel 1). α-Cyclodextrin per se did not modify cord formation (Figure 3A, panel 2). Quantification by optical imaging of the area occupied by the capillary network (Figure 3B) shows that maximal cord formation, observed in the control cells and set at 100%, was reduced by PGE1 to 56±3.0% and 33±0.7% (n=4) at the concentrations of 1 and 5 μM, respectively.
Prostaglandin receptor subtypes expressed in HUVECs
At least eight types of prostaglandin (PG) receptors have been recently cloned, all belonging to the family of seven transmembrane domain receptors coupled to G proteins (Breyer et al., 2001). To our knowledge, it is not known which kind of PG receptors are expressed in HUVECs. The expression of PG receptor subtypes in HUVECs was determined by RT–PCR using specific oligonucleotide primers (Sheng et al., 2001). As can be seen in Figure 4, we observed the expression of the EP2 and EP3 receptor subtypes, while the EP1 and EP4 subtypes were not detected.
Figure 4.
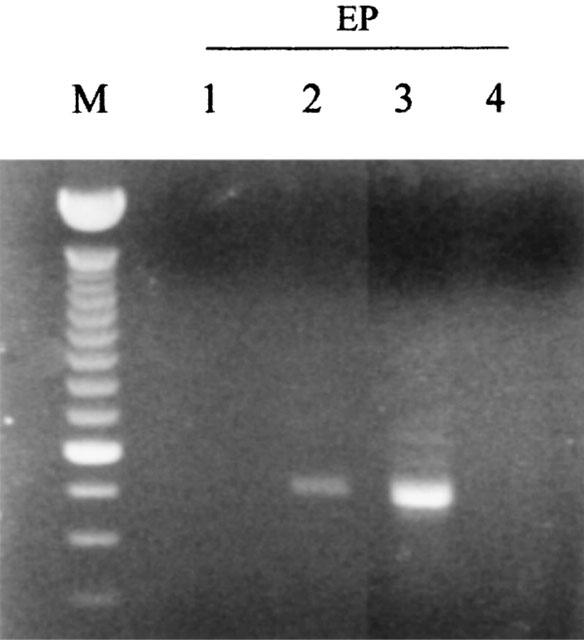
Expression of EP receptor subtypes in HUVECs. One μg of total RNA extracted from HUVECs was reverse-transcribed and amplified for 35 PCR cycles by using specific primers for EP1, EP2, EP3 and EP4. The amplified products were visualized on 1.5% agarose gels. M, molecular weight marker.
Effect of PGE1 on cAMP accumulation in endothelial cells
To define the signal transduction pathway(s) coupled to PGE1-stimulated receptors, we studied the intracellular cAMP accumulation in HUVECs following PGE1 treatment, since both EP2 and EP3 receptors are known to be coupled to adenylate cyclase in a positive and negative manner, respectively. Previously, an association between EP2 receptor subtype and cAMP generation has been found in corneal endothelial cells (Jumblatt & Peterson, 1991).
In HUVECs, PGE1 stimulated, in a concentration-dependent manner, an increase in intracellular cAMP levels (Figure 5A), displaying an EC50 of 1.5 μM and a maximal stimulation of 13.1±5.75 (n=3) fold over basal levels (53.0±13.9 fmol cAMP well−1, n=3) at 5 μM concentration. α-Cyclodextrin per se had no stimulatory effect on the intracellular cAMP content (data not shown).
Figure 5.
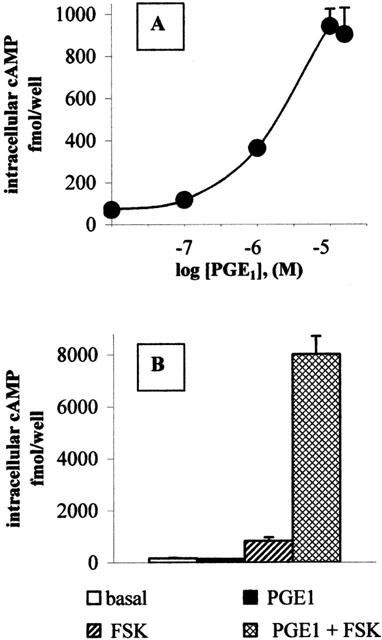
PGE1 induces cAMP accumulation in endothelial cells. (A) Intracellular cAMP was assayed by RIA after ethanol extraction of intact cells stimulated for 20 min with the indicated concentrations of PGE1/α-cyclodextrin. Results are expressed as fmol of intracellular cAMP/well and are the mean±s.d. of duplicate samples from a representative experiment, which was performed three times with similar results. (B) HUVECs were treated with 100 nM PGE1/α-cyclodextrin in the absence (solid bar) or in the presence (cross-hitched bar) of 100 μM forskolin (FSK). Open bar: untreated cells. Diagonal bar: cells treated with FSK 100 μM alone. Results are expressed as in (A).
At nanomolar concentrations, PGE1 per se did not change cAMP levels, but greatly enhanced the forskolin-induced cAMP accumulation (Figure 5B). The augmentation by the EP3 receptor of stimulated cAMP formation has been already reported in COS cells (Hatae et al., 2002). This phenomenon, called superactivation, has been described also for other inhibitory receptors (Thomas & Hoffman, 1996; Avidor-Reiss et al., 1996).
Effect of raised intracellular cAMP on angiogenesis in vitro
Since the experiments described above suggest that the increase in intracellular cAMP is the transduction mechanism coupled to PGE1-stimulated receptors in HUVECs, we tested the effect of Br-cAMP on proliferation, migration and cord formation in cultures of HUVECs. As shown in Figure 6, an increase in intracellular cAMP levels obtained by administering Br-cAMP (500 μM) mimics the effect of PGE1 on the parameters studied, i.e. Br-cAMP inhibited cell proliferation (Figure 6A), cell migration (Figure 6B) and capillary-like structure formation (Figure 6C).
Figure 6.
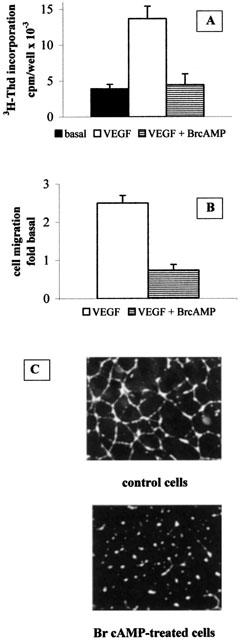
The antiangiogenic effect of PGE1 is mimicked by increasing intracellular cAMP levels. (A) HUVECs were treated as described with 20 ng ml−1 VEGF in the absence (open bar) or in the presence (horizontal bar) of 500 μM Br-cAMP. Results are expressed as c.p.m. of [3H]-thymidine incorporated by the cells and are the mean±s.e.m of three independent experiments. Solid bar: unstimulated cells. (B) 500 μM Br-cAMP (horizontal bar) was added to the cells and chemotaxis assay was performed as described in the Methods section. Results are expressed as fold increase in the number of migrated cells under basal conditions (i.e. in the absence of chemotactic factors) and are the mean±s.e.m. of three independent experiments. Open bar: control cells migrated in the absence of cAMP elevating agents. (C) Cells were plated on Matrigel as described in the Methods section in the absence (upper panel) or in the presence (lower panel) of 500 μM Br-cAMP.
Effect of ethanol on cell migration and cord formation in HUVECs
As can be seen in Figure 7, ethanol, which is used as a solvent for most of the PGs, shows per se a quite noticeable stimulatory effect compared to control and VEGF-treated cells on both chemiotaxis and cord formation, at concentrations as low as 6 mM.
Figure 7.
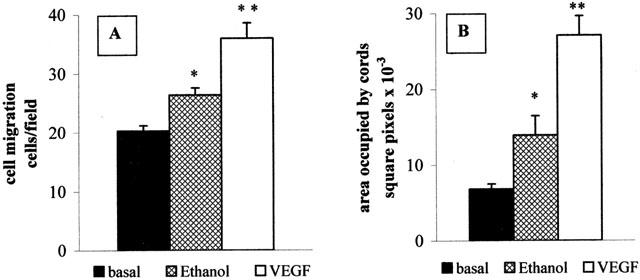
Effects of ethanol on endothelial cell functions. (A) 6 mM ethanol (cross-hatched bar) or 50 ng ml−1 VEGF (open bar) were added in the lower chamber, and chemiotaxis was performed as described. Results are expressed as number of migrated cells in the different experimental conditions and are the mean±s.e.m. of three independent experiments. Solid bar: basal (unstimulated cells). (B) HUVECs were plated on Matrigel as described in the Methods section, in the presence of 6 mM ethanol (cross-hatched bar) or 20 ng ml−1 VEGF (open bar). Results are expressed as area occupied by cords, calculated by means of NIH Image program, and are the mean±s.e.m. of three independent experiments. *P<0.05; **P<0.001 vs basal; One Way ANOVA followed by Bonferroni test.
Selective stimulation of the prostaglandin receptor subtypes
Our RT–PCR studies described above detected EP2 and EP3 receptor subtypes mRNA in HUVECs. Butaprost and sulprostone are considered to be selective agonists of the EP2 and EP3 receptor subtypes, respectively (Breyer et al., 2001). In the experiments showed in Figure 8, butaprost, at a concentration of 1 μM, had no effect either in the proliferation or in the chemiotactic assay. On the contrary, sulprostone, at the concentration of 1 μM, was able to inhibit the 3H-thymidine incorporation by 41.5±5.5% (n=3), and the chemotactic response to VEGF by 36.8±5.9% (n=5), thus mimicking the effect of PGE1.
Figure 8.
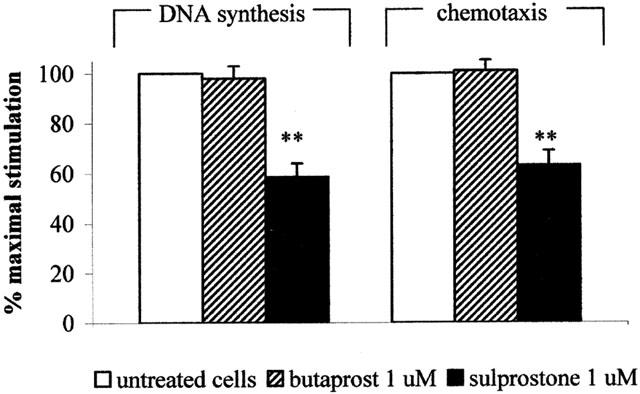
Effects of butaprost and sulprostone on HUVECs growth and motility. HUVECs were treated with 1 μM butaprost (diagonal bar) or 1 μM sulprostone (solid bar), and the effect of the drugs on VEGF-induced DNA synthesis and cell motility was evaluated as described in the Methods section. Results are expressed as per cent of maximal stimulation induced by VEGF, and are the mean±s.e.m. of 3–5 independent experiments. **P<0.001, two tailed unpaired Student's t-test
Effect of PGE1 on the angiogenic process in vivo
The effect of PGE1 on angiogenesis in vivo was tested using the Matrigel plug assay (Passaniti et al., 1992; Albini et al., 1994). Within 4 days of implantation of Matrigel plugs enriched with FGF and heparin, the formation of hemorrhagic lesions in the Matrigel pellets was evident (Figure 9A, left). Treatment with PGE1 administered to mice in a minipump placed subcutaneously and releasing 20 ng animal day−1 visibly reduced the neovascularization process (Figure 9A, right). Quantification of vascularization by determination of haemoglobin content of the Matrigel sponges showed that PGE1 significantly inhibited the FGF-induced angiogenesis (55.2±16.5 mg ml−1 in the control vs 15.4±6.1 mg ml−1 in the treated animals, Figure 9B).
Figure 9.
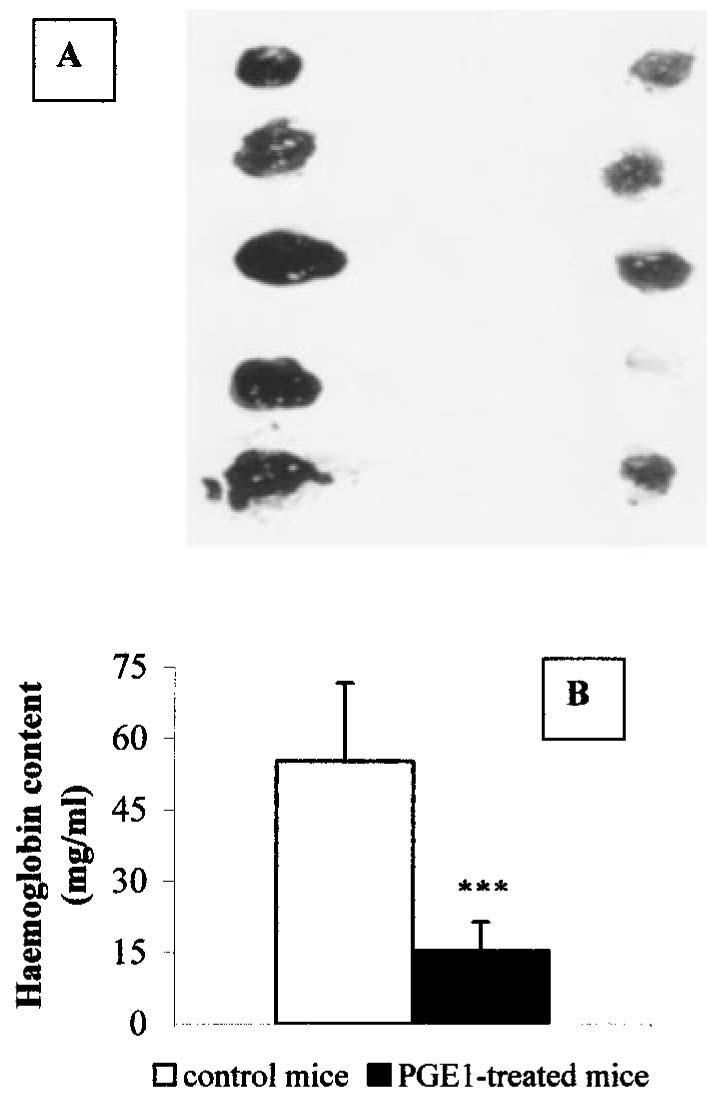
Effects of PGE1 on angiogenesis in vivo. (A) Matrigel pellets containing 150 ng ml−1 aFGF and 60 u ml−1 heparin were removed from mice after 4 days of treatment with pumps filled with isotonic saline (left sponges) or with 20 ng day animal−1 PGE1/α-cyclodextrin (right sponges). For each group, five pellets obtained from different animals were photographed. (B) Haemoglobin content of Matrigel pellets obtained from control animals (open bar) or from PGE1/α-cyclodextrin-treated animals (solid bar). Results are the mean±s.d. of two independent experiments, which were performed using 5–6 animals for each treatment. ***P<0.0001, two tailed unpaired Student's t-test.
Discussion
Angiogenesis is a complex multistep process that includes proliferation and migration of endothelial cells, degradation of extracellular matrix and sprouting of new capillary branches from pre-existing vessels (Yancopoulos et al., 2000). In adult individuals, angiogenesis is a physiological event in the female reproductive cycle and in the repair of tissues, for example during wound healing. Neovascularization is also responsible for or can enhance some pathological conditions such as tumour growth (Carmeliet & Jain, 2000) and retinal pathology (Aiello & Wong, 2000). PGs play a major role in a great number of biological processes, including the regulation of gastrointestinal integrity, kidney and immune function, and reproductive biology. A very limited literature suggests that PGs may stimulate angiogenesis. PGE1 was angiogenic in rabbit corneas (Ziche et al., 1982) and it stimulated formation of new capillaries in rat femoral veins (Diaz-Flores et al., 1994). Indirect evidence involving PGs in angiogenesis has been reported, since inhibitors of cyclooxygenase (COX) have been shown to suppress tumour growth, possibly via an anti-angiogenic action (Tsujii et al., 1998; Masferrer et al., 2000). Our results indicate that the effect of PGE1 is anti-angiogenic, since PGE1 inhibits endothelial cell proliferation, migration and cord formation in vitro, and the vascularization of Matrigel in vivo.
The reasons for the discrepancies between our results and results from other laboratories are not obvious. First, the few data reported in the literature on the pro-angiogenic properties of PGs in vivo were obtained in the rabbit corneal test (Ziche et al., 1982) and in the rat femoral vein sprouting (Diaz-Flores et al., 1994). Our in vivo results were obtained in a murine model of angiogenesis. Moreover, different responses to the same stimulus might be due not only to the different species used, but also to the different vascular beds examined in the same species. In fact, recently Mehrabi et al. (2001) reported an increase in the expression of various markers of angiogenesis in hearts explanted from PGE1-treated patients with idiopathic dilated cardiomyopathy.
Second, it should be pointed out that normally PGE1 is dissolved in ethanol, which has itself recently been shown to induce formation of capillary-like structures (i.e. in vitro angiogenesis) in cultures of immortalized, human endothelial EA-hy926 cells (Jones et al., 1998). Moreover, ethanol has been shown to induce expression of VEGF and stimulate angiogenesis in the chick embryo chorioallantoic membranes (CAM) assay (Gu et al., 2001). Also in our hands, treatment of HUVECs with a concentration of ethanol as low as 6 mM induced a significant stimulation of chemotaxis and cord formation (Figure 7). One might conclude that, in angiogenesis studies, the use of compounds dissolved in ethanol should be avoided. The PGE1 conjugated to α-cyclodextrin we have used in all our experiments is soluble in water.
As mentioned above, the COX isoenzymes seem to be involved in tumour development and progression, since aspirin and other COX inhibitors exert a preventive and protective effect, mostly in colorectal cancer (Giardiello et al., 1993). In these proliferative diseases, a selective role for the COX-2 isoenzyme has been proposed. COX-2 is overexpressed in many other human tumours as well (e.g. breast, prostate and skin) (Williams et al., 1999). Moreover, specific COX-2 inhibitors have been shown to suppress tumour growth in animal models and to prevent development of polyps and colon cancer in humans (for a review, see Gupta & Dubois, 2001). The cellular mechanisms responsible for this anticancer activity are not well understood. However, recent reports suggest that the antitumour effect of COX-2 inhibitors derives from inhibition of angiogenesis (Tsujii et al., 1998; Masferrer et al., 2000; Dormond et al., 2001). In contrast, it has been reported (Trifan et al., 1999) that overexpression of COX-2 causes growth arrest in a variety of cell types, including bovine endothelial and human embryonic cells, via a novel and yet unidentified mechanism of action. Moreover, very recently it has been shown that transgenic mice overexpressing COX-2 developed skin tumours at a much lower frequency than the controls did (Bol et al., 2002). A dramatic 75% reduction of skin tumourigenesis in a multistage mouse skin model was obtained also with homozygous deficiency of COX-1 (Tiano et al., 2002). In the attempt to explain the dissimilar, sometimes conflicting results described above, one should also consider that PGs are not the only metabolic products of the COX enzymes. For example, COX inhibitors certainly decrease the formation of PGs, but also cause an increase in cellular arachidonic acid. Free arachidonic acid can promote apoptosis, again independent from PG formation (Surette et al., 1999; Chan et al., 1998). In conclusion, whether the COX-2 (and as a consequence the COX-2 inhibitors) effects in cancer are directly due to production (or inhibition) of PGs still needs to be fully elucidated.
For the first time, we show here, by RT–PCR studies, that HUVECs express the EP2 and EP3 mRNA receptor subtypes. In fact, PGE1 increases intracellular cAMP and potentiates the forskolin-induced cAMP accumulation, two events which, according to the literature, can be attributed to the EP2 and EP3 receptor stimulation, respectively. Moreover, sulprostone, which is a selective EP3 agonist, mimics the inhibitory effect of PGE1 in HUVECs, while butaprost, a selective EP2 agonist, had no effect. Our data thus suggest an involvement of the EP3 receptor subtype in the anti-angiogenic effect of PGE1.
In the present study, we have also shown that an increase in the levels of intracellular cAMP with Br-cAMP induces the same effect as PGE1 on the parameters studied, i.e. inhibition of HUVECs proliferation, migration and cord formation. That cAMP might be involved in inhibition of angiogenesis is suggested also by recent data showing that treatment of HUVECs with dibutyryl cAMP or forskolin blocked cell migration in vitro and inhibited blood vessel branching in chick embryo CAM assay in vivo (Kim et al., 2000). Moreover, the parathyroid-hormone related peptide has been shown to inhibit angiogenesis by activating endothelial cell protein kinase A (Bakre et al., 2002). However, in our hands, the anti-angiogenic effect of PGE1 occurs in a range of concentrations (nanomolar) where significative increases of cAMP could not be measured. It might very well be that the radioimmunoassay used is not sensitive enough to detect small changes of the second messenger that yet are capable of inhibiting angiogenesis.
Finally, our results obtained in vivo suggest the possibility that cyclodextrin-complexed PGE1 might be used for those diseases that require anti-angiogenic therapy, such as diabetic retinopathy and solid tumours. In vivo experiments are being considered to test the ability of PGE1 to suppress tumour growth in nude mice.
Acknowledgments
We thank P Ferrario, D Mangili, Drs K Ravanelli and L Parigi for their excellent technical assistance. We also thank Drs P Sacerdote for help in chemotaxis experiments, C Verderio for image capturing, and A Flora for technical advice on RT–PCR studies. This work was supported by research grants from MURST-PRIN 2000 and from Schwarz Pharma S.p.A.
Abbreviations
- Br-cAMP
8-Bromoadenosine-3′:5′-cyclic monophosphate
- cAMP
cyclic adenosine monophosphate
- COX
cyclo-oxygenase
- EP
E-prostanoid
- FGF
fibroblast growth factor
- HUVECs
human umbilical vein endothelial cells
- PG
prostaglandin
- PGE1
prostaglandin E1
- VEGF
vascular endothelial growth factor
References
- AIELLO L.P., WONG J.S. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Internat. 2000;58:S113–S119. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- ALBINI A., FONTANINI G., MASIELLO L., TACCHETTI C., BIGINI D., LUZZI P., NOONAN D.M., STETLER-STEVENSON W.G. Angiogenic potential in vivo by Kaposi sarcoma cell-free supernatants and HIV1-tat product: inhibition of KS-like lesions by TIMP-2. AIDS. 1994;8:1237–1244. doi: 10.1097/00002030-199409000-00004. [DOI] [PubMed] [Google Scholar]
- AVIDOR-REISS T., NEVO I., LEVY R., PFEUFFER T., VOGEL Z. Chronic opioid treatment induces adenylyl cyclase V superactivation. Involvement of G-beta gamma. J. Biol. Chem. 1996;271:21309–21315. doi: 10.1074/jbc.271.35.21309. [DOI] [PubMed] [Google Scholar]
- BAATOUT S. Endothelial differentiation using Matrigel. Anticancer Res. 1997;17:451–456. [PubMed] [Google Scholar]
- BAKRE M.M., ZHU Y., YIN H., BURTON D.W., TERKELTAUB R., DEFTOS L.J., VARNER J.A. Parathyroid hormone-related peptide is a naturally occurring, protein kinase A-dependent angiogenesis inhibitor. Nature Med. 2002;8:995–1003. doi: 10.1038/nm753. [DOI] [PubMed] [Google Scholar]
- BAUMGARTNER I., PIECZEK A., MANOR O., BLAIR R., KEARNY M., WALSH K., ISNER J.M. Constitutive expression of phVEGF165 following intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- BENELLI R., ALBINI A. In vitro models of angiogenesis: the use of Matrigel. Int. J. Biol. Marker. 1999;14:243–246. doi: 10.1177/172460089901400408. [DOI] [PubMed] [Google Scholar]
- BOL D.K., ROWLEY R.B., HO C-P., PILZ B., DELL J., SWERDEL M., KIGUCHI K., MUGA S., KLEIN R., FISHER S.M. Cyclo-oxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62:2516–2521. [PubMed] [Google Scholar]
- BREYER R.M., BAGDASSARIAN C.K., MYERS S.A., BREYER M.D. Prostanoid receptors: subtypes and signalling. Ann. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- CARMELIET P., JAIN R.K. Angiogenesis in cancer and other disease. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- CHAN T.A., MORIN P.J., VOGELSTEIN B., KINZLER K.W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAZ-FLORES L., GUTIERREZ R., VALLADARES F., VARELA H., PEREZ M. Intense vascular sprouting from rat femoral vein induced by prostaglandins E1 and E2. Anatomical Record. 1994;238:68–76. doi: 10.1002/ar.1092380109. [DOI] [PubMed] [Google Scholar]
- DORMOND O., FOLETTI A., PAROZ C., RÜEGG C. NSAIDs inhibit αVβ3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nature Med. 2001;7:1041–1047. doi: 10.1038/nm0901-1041. [DOI] [PubMed] [Google Scholar]
- GIARDIELLO F.M., HAMILTON S.R., KRUSH A.J., PIANTADOSI S., HYLIND L.M., CELANO P., BOOKER S.V., ROBINSON C.R., OFFERHAUS G.J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. New Engl. J. Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- GU J.W., ELAM J., SARTIN A., LI W., ROACH R., ADAIR T.H. Moderate levels of ethanol induce expression of vascular endothelial growth factor and stimulate angiogenesis. Am. J. Physiol. 2001;281:R365–R372. doi: 10.1152/ajpregu.2001.281.1.R365. [DOI] [PubMed] [Google Scholar]
- GUPTA R.A., DUBOIS R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature Rev. Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- HATAE N., YAMAOKA K., SUGIMOTO Y., NEGISHI M., ICHIKAWA A. Augmentation of receptor-mediated adenylyl cyclase activity by Gi-coupled prostaglandin receptor subtype EP3 in a Gβγ subunit-independent manner. Biochem. Biophys. Res. Comm. 2002;290:162–168. doi: 10.1006/bbrc.2001.6169. [DOI] [PubMed] [Google Scholar]
- ICAI STUDY Group Prostanoids for chronic critical leg ischemia. A randomised, controlled, open-label trial with prostaglandin E1. Ann. Intern. Med. 1999;130:412–421. [PubMed] [Google Scholar]
- JAFFE E.A., NACHMAN R.L., BECKER C.G., MINICK C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAIN R.K., SCHLENGER K., HÖCKEL M., YUAN F. Quantitative angiogenesis assays: progress and problem. Nature Med. 1997;3:1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- JONES M.K., SARFEH I.J., TARNAWSKI A.S. Induction of in vitro angiogenesis in the endothelial-derived cell line, EA hy926, by ethanol is mediated through PKC and MAPK. Biochem. Biophys. Res. Comm. 1998;249:118–123. doi: 10.1006/bbrc.1998.9095. [DOI] [PubMed] [Google Scholar]
- JUMBLATT M.M., PETERSON C.A. Prostaglandin E2 effects on corneal endothelial cyclic adenosine monophosphate synthesis and cell shape are mediated by a receptor of the ED2 subtype. Invest. Ophthalmol. Vis. Sci. 1991;32:360–365. [PubMed] [Google Scholar]
- KIM S., HARRIS M., VARNER J.A. Regulation of αvβ3-mediated endothelial cell migration and angiogenesis by integrin α5β1 and protein kinase A. J. Biol. Chem. 2000;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- MAESHIMA Y., COLORADO P.C., TORRE A., HOLTHAUS K.A., GRUNKEMEYER J.A., ERICKSEN M.B., HOPFER H., XIAO Y., STILLMAN I.E., KALLURI R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J. Biol. Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- MASFERRER J.L., LEAHY K.M., KOKI A.T., ZWEIFEL B.S., SETTLE S.L., WOERNER B.M., EDWARDS D.A., FLICKINGER A.G., MOORE R.J., SEIBERT K. Antiangiogenic and antitumor activities of cyclooxygenases–2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- MEHRABI M.R., EKMEKCIOGLU C., STANEK B., THALHAMMER T., TAMADDON F., PACHER R., STEINER G.E., WILD T., GRIMM M., SPIECKERMANN P.G., MALL G., GLOGAR H.D. Angiogenesis stimulation in explanted hearts from patients pre-treated with intravenous prostaglandin E1. J. Heart Lung Transpl. 2001;20:465–473. doi: 10.1016/s1053-2498(00)00317-x. [DOI] [PubMed] [Google Scholar]
- PASSANITI A., TAYLOR R.M., PILI R., GUO Y., LONG P.V., HANEY J.A., PAULY R.R., GRANT D.S., MARTIN G.R. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- PRESTA M., MOSCATELLI D., JOSEPH-SILVERSTEIN J., RIFKIN D.B. Purification from a human hepatoma cell line of a basic fibroblast growth factor-like molecule that stimulates capillary endothelial cell plasminogen activator production, DNA synthesis and migration. Mol. Cell. Biol. 1986;6:4060–4066. doi: 10.1128/mcb.6.11.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUMACHER B., PECHER P., VON SPECHT B.U., STEGMANN H. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- SHENG H., SHAO J., WASHINGTON M.K., DUBOIS R.N. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- SURETTE M.E., FONTECH A.N., BERNATCHEZ C., CHILTON F.H. Perturbations in the control of cellular arachidonic acid levels block cell growth and induce apoptosis in HL-60 cells. Carcinogenesis. 1999;20:757–763. doi: 10.1093/carcin/20.5.757. [DOI] [PubMed] [Google Scholar]
- THOMAS J.M., HOFFMAN B.B. Isoform-specific sensitization of adenylyl cyclase activity by prior activation of inhibitory receptors: role of beta gamma subunits in transducing enhanced activity of type VI isoform. Mol. Pharmacol. 1996;49:907–914. [PubMed] [Google Scholar]
- TIANO H.F., LOFTIN C.H., AKUNDA J., LEE C.A., SPALDING J., SESSOMS A., DUNSON D.B., ROGAN E.G., MORHAM S.G., SMART R.C., LANGENBACH R. Deficiency of either cyclo-oxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- TRIFAN O.C., SMITH R.M., THOMPSON B.D., HLA T. Overexpression of cyclooxygenase-2 induced cell cycle arrest–Evidence for a prostaglandin-independent mechanism. J. Biol. Chem. 1999;274:34141–34147. doi: 10.1074/jbc.274.48.34141. [DOI] [PubMed] [Google Scholar]
- TSUJII M., KAWANO S., TSUJII S., SAWAOKA H., HORI M., DUBOIS R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- WIESE M., CORDES H.-P., CHI H., SEYDEL J.K., BACKENSFELD T., MÜLLER B.W. Interaction of Prostaglandin E1 with α-Cyclodextrin in aqueous systems: stability of the inclusion complex. J. Pharmacol. Sci. 1991;80:153–156. doi: 10.1002/jps.2600800213. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.S., MANN M., DUBOIS R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- YANCOPOULOS G.D., DAVIS S., GALE N.W., RUDGE J.S., WIEGAND S.J., HOLASH J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- ZICHE M., JONES J., GULLINO P.M. Role of prostaglandin E1 and copper in angiogenesis. J. Natl. Cancer Inst. 1982;69:475–482. [PubMed] [Google Scholar]


