Abstract
We tested the hypothesis, whether G Protein-coupled receptors (GPCRs) may differentially regulate specific signalling pathways by constitutive and agonist-induced activation using the human sphingosine 1-phosphate receptor S1P5 as a model.
S1P5 receptor-expressing HEK293 cells exhibited a high degree of basal activity for both inhibition of adenylyl cyclase and extracellular signal regulated kinase (ERK) when cultured in serum, which contains high levels of sphingosine 1-phosphate (S1P). However, basal activity was independent of the presence of S1P: (i) constitutive activity remained when cells were cultured in delipidated serum, (ii) addition of S1P to delipidated serum did not increase basal S1P5 receptor signalling.
Conversely, constitutive inhibition of forskolin-stimulated adenylyl cyclase was further enhanced by S1P in S1P5-HEK293 cells.
Transfection of several mammalian cell lines (CHO-K1, HEK293, NIH-3T3, RH7777) with the S1P5 receptor induced cell rounding, which was more pronounced in the presence of S1P-containing serum. Rounded cell morphology did not correlate with apoptotic cell death, but led to detachment of cells.
Cell surface ELISA assays showed that a fraction of plasma membrane S1P5 receptors were dose-dependently internalized with S1P.
These data reveal that intrinsic inhibition of unstimulated adenylyl cyclase or ERK activity by the S1P5 receptor is insensitive to ligand modulation. Conversely, effects on forskolin-stimulated adenylyl cyclase, cell morphology and internalization can be further augmented with S1P. Our results suggest that different signal transduction pathways are not equally activated through constitutively active GPCRs with promiscuous signalling characteristics.
Keywords: Human S1P5, cell rounding, constitutive activity, sphingosine 1-phosphate, cAMP assay, internalization
Introduction
G protein-coupled receptors (GPCRs) constitute the largest protein family of integral membrane receptors. Despite the wide variety of activating ligands, they share a common seven transmembrane topology and common activation mechanisms (for review see Bockaert & Pin, 1999; Meng & Bourne, 2001). Information provided by extracellular stimuli is transduced into intracellular second messengers by their coupling to heterotrimeric G proteins that subsequently regulate diverse effector systems within the cells (Iiri et al., 1998; Marinissen & Gutkind, 2001).
According to the two-state model, GPCRs can spontaneously isomerize between an inactive (R) and an active state (R*) (Samama et al., 1993; Weiss et al., 1996). The active state R* triggers exchange of GTP for GDP on the α subunit of heterotrimeric G proteins, resulting in a measurable cellular or pharmacological response (Samama et al., 1993). Several GPCRs show constitutive activity when overexpressed in recombinant systems (Samama et al., 1993; van sande et al., 1995; Kenakin, 1996; Chen et al., 2000) and this feature can be used in screenings of GPCRs. Ligands with affinity are applied to redistribute the receptor species: inverse agonist ligands preferentially stabilize the inactive conformation (R), agonist ligands preferentially stabilize the active conformation (R*) (Milligan et al., 1995), and neutral antagonists do not display any preference for a given receptor conformation. Interestingly, several established antagonist drugs targeting GPCRs have been recognized to be inverse agonists (McCune et al., 2000; Stevens et al., 2000; Miserey-Lenkei et al., 2002). Besides constitutively active receptors (CARs), many constitutively active mutants (CAMs) have been generated that mimic the active state and signal in the absence of a ligand (e.g. Pei et al., 1994; Jensen et al., 2000; Stevens et al., 2000; Scheer et al., 2000; Ramsay et al., 2001; Greasley et al., 2001; Miserey-Lenkei et al., 2002). Naturally occurring activating mutations are associated with human disease states like male-limited precocious puberty (luteinizing hormone receptor, Shenker et al., 1993), hyperfunctioning thyroid adenoma (thyrotropin receptor, Parma et al., 1993), retinitis pigmentosa (rhodopsin, Rao et al., 1994), and autosomal dominant hypocalcaemia (Ca2+ sensing receptor, Brown, 1999), for review see Coughlin, 1994). CAMs have been extensively used in recent years to provide insight into conformational changes which occur in GPCRs upon ligand-binding (Lefkowitz et al., 1993; Scheer & Cotecchia, 1997; Leurs et al., 1998; Pauwels & Wurch, 1998). In contrast, little information is available whether CAMs can differentially alter separate signalling cascades. A rare example for which this issue has been investigated is the α1b-adrenoceptor: a Cys128Phe mutant displays constitutive activity when phospholipase Cβ activation is monitored but not when phospholipase A2 activity is measured, whereas an Ala293Glu mutant of the same receptor shows constitutive activity in both pathways (Perez et al., 1996). Likewise, it is not known so far whether CARs with the ability to signal through more than one pathway differentially alter separate signalling cascades.
Lipid receptors, in particular the family of sphingosine 1-phosphate (S1P) receptors S1P1-5 (formerly known as edg receptors; Chun et al., 2002) have received increasing attention in recent years, and many reports show that expression of various S1P receptor subtypes in mammalian (primary) cells causes phenotypical changes such as morphological alterations, stress fibre formation, cytoskeletal reorganization and cell proliferation (Lee et al., 1998; 1999; Moolenaar, 1999; van brocklyn et al., 1999; Robert et al., 2001). In addition, it has been suggested that the plethora of effects mediated by S1P receptors is in part due to their ability to signal through more than one pathway. In all cases, however, phenotypical changes were dependent on the presence of S1P (Lee et al., 1998; van brocklyn et al., 1999) and no reports are available about ligand-independent activities of S1P receptor subtypes.
To test whether lipid receptors exhibit constitutive signalling characteristics and differentially alter various effector systems, signalling and cellular responses have to be determined in delipidated serum, that does not contain S1P. In this study, we have analysed several S1P receptors and chose the recently cloned human S1P5 receptor as an example to demonstrate that it constitutively activates some signalling pathways while others still remain sensitive to agonist stimulation.
Methods
Molecular cloning of various human S1P5 receptor constructs
As the human S1P5 receptor (AC073749) sequence is intronless, we cloned the receptor from human genomic DNA via PCR. PCR conditions, established to amplify the S1P5 receptor sequence, were 94°C for 10 min followed by 35 cycles of 94°C, 1 min, 60°C, 1 min, 72°C, 2 min using the GC-melt kit. Primers designed to amplify the S1P5 receptor sequence contained a HindIII site in the forward, and an EcoRI-site in the reverse primer, respectively. The 1197-bp PCR product was cloned into the pcDNA3.1(+) mammalian expression vector and sequenced in both directions.
To clone the construct used for generation of stable cell lines the S1P5 receptor coding region was excised from pcDNA3.1(+) with HindIII(5′) and NotI(3′) and inserted into the peak 8 vector (Edge Biosystems) using T4-DNA ligase.
To clone the S1P5–GFP construct the stop codon was deleted by PCR. Primers were designed to introduce an EcoRI site upstream of the initiating methionine codon and a BamHI site 5′ of the stop codon. The PCR product was cloned into the EcoRI–BamHI site of the pEGFP-N3 vector using T4-DNA ligase.
To monitor cell surface expression of the S1P5 receptor, the HA epitop tag (YPYDVPDYA) was introduced immediately after the initiating methionine codon of the S1P5 receptor sequence into the pcDNA3.1(+) expression vector.
Cell culture and transfection
HEK293 cells were grown in Dulbecco's modified Eagles Medium (DMEM) (with Glutamax-1, w/o sodiumpyruvate, with 4500 mg l−1 glucose, with pyridoxine) supplemented with 10% foetal bovine serum (FBS), penicillin-streptomycin (10,000 IU ml/10,000 μg ml−1) and 25 mM HEPES pH 7.0. Peak stable cells were cultivated in DMEM (with Glutamax-1, w/o sodiumpyruvate, with 4500 mg l−1 glucose, with pyridoxine) supplemented with 10% FBS, penicillin-streptomycin (10,000 IU ml per 10,000 μg ml−1), 25 mM HEPES pH 7.0 and G418 (400 μg per ml). CHO-K1 cells were cultured in basal Iscove medium supplemented with 10% FBS, penicillin-streptomycin (10,000 IU ml per 10,000 μg ml−1), 50 μg ml−1 gentamicin, 2 mM L-Glutamin. All cells were cultivated at 37°C in a humidified 5% CO2 incubator.
For transient transfections 4×105 HEK293 or 2×105 CHO-K1 cells were seeded onto 35-mm dishes. About 24 h later cells were transiently transfected at 50–80% confluency with the indicated receptor (1 μg of plasmid DNA) using the FuGENE 6 transfection reagent for HEK293 or the LipofectAMINE reagent for CHO cells according to the manufacturer's instructions. For visualization of transfected cells, receptors were cotransfected with the cDNA encoding the green fluorescent protein (GFP) at a 4 : 1 ratio. Transfection efficiencies typically amounted to 30–40% as determined by counting green, GFP-transfected cells.
Generation of a stable S1P5 receptor HEK293 cell line
4×105 peak stable cells (PSC, HEK293 cells resistant to G418) were seeded onto 35-mm dishes. About 24 h later cells were transfected at 50% confluency with 1 μg receptor (S1P5 receptor in peak 8) or peak 8 (empty vector) cDNA using the FuGENE 6 transfection reagent. Forty-eight hours later cell selection with puromycin was initiated until a final concentration of 1 μg ml−1 puromycin was reached. The stable cell lines were grown in DMEM (with Glutamax-1, w/o sodiumpyruvate, with 4500 mg l−1 glucose, with pyridoxine) supplemented with 10% FBS, penicillin–streptomycin (10,000 IU ml per 10,000 μg ml−1), 25 mM HEPES pH 7.0 and 1 μg ml−1 puromycin at 37°C in a humidified 5% CO2 incubator.
Measurement of cyclic AMP (cAMP) accumulation
1×104 HEK293 cells stably expressing the indicated receptor or vector DNA were cultivated in medium supplemented with 10% FBS or charcoal-stripped FBS (csFBS) (±S1P) for 24 h prior to the assay. Accumulation of cAMP was determined in the presence of 500 μM isobutylmethylxanthine (IBMX) with the alpha-screen® cAMP Detection Kit (Packard BioScience) according to the manufacturer's instructions. Luminescence was measured with the fusion α-luminometer (Canberra-Packard GmbH). Data are means±s.e.mean of 5–10 independent experiments, each performed in quintuplets.
Cell rounding assay
CHO-K1 cells were plated at a density of 1×105 cells/well on 35-mm dishes and transfected after 24 h with the indicated receptor expression plasmids as described above at a 4 : 1 ratio with the pEGFP N1 plasmid, which encodes a green fluorescence protein (GFP). Sixteen hours after transfection cells were washed once with PBS and grown for 32 h in Iscove supplemented with 10% FBS or 10% csFBS. Nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI). Transfected cells were visualized by phase contrast and fluorescence microscopy; apoptotic cells were identified by the blue colour of their nuclei representing fragmented DNA.
To determine pertussis toxin (PTX) sensitivity, PTX (100 ng ml−1) was present in the medium during the last 24 h before analysis.
Caspase activity assay
8×105 CHO-K1 cells were seeded onto 80-cm2 dishes and transfected about 24 h later with cDNAs encoding for the S1P5 receptor, rS1P3 receptor or empty pcDNA3.1(+) vector cDNA as a control. Transfection medium was replaced after 16 h with basal Iscove medium supplemented with 10% FBS and cells were cultivated for an additional 8 h. 4×104 cells were then seeded into 96-well plates and grown for 16 h in Iscove medium supplemented with 10% csFBS. Cells were serum starved for an additional 6 h in Iscove prior to addition of S1P or staurosporin (5 μM). Caspase activity was then measured using the fluorometric homogeneous caspase assay® as per manufacturer's instructions. Fluorescence was recorded with the FluoStar Galaxy (BMG LabTechnologies GmbH) using an excitation filter at 485 nm and an emission filter at 520 nm. Data represent means±s.e.mean of six independent experiments.
ERK in vitro kinase reactions
Cell culture and transfections: CHO-K1 cells were grown on 6-well plates in Ham's F12 medium containing 10% FBS to about 50–70% confluency. Transfections were performed in medium with 10% csFBS using 0.75 μg of receptor and 0.75 μg of HA-ERK2 cDNA/well and the FuGENE 6 transfection method according to the supplier's manual. About 24 h after transfection cells were serum-starved in Ham's F12 medium containing 0.1% (m/V) FAF BSA for another 24 h until used for the experiments.
ERK activity was analysed as previously described (Blaukat et al., 2000). Briefly, serum-starved cells on 6-well plates were stimulated with the indicated ligands at 37°C followed by lysis in 0.5 ml of ice-cold buffer containing (mM) HEPES 50, pH 7.2, NaCl 150, EDTA 1, NaF 20, sodium orthovanadate 2, 1% (w v−1) Triton X-100, 10% (w v−1) glycerol and protease inhibitors (1 mM Pefabloc, 10 μg ml−1 leupeptin and 1% Trasylol®). Equal amounts of soluble fractions were subjected to immunoprecipitation with 5 μl of a polyclonal antiserum against the HA-tag (Santa Cruz Biotechnology) and 35 μl protein A-agarose slurry for 3 h at 4°C. Resultant precipitates were washed three times with lysis buffer followed by two washes with kinase buffer (mM) (Tris 10, pH 7.5, MgCl2 10). Kinase reactions were started by addition of 25 μl kinase buffer including 200 μM ATP, 1 μCi γ[32P]-ATP and 10 μg myelin basic protein (MBP). After 20 min at 30°C reactions were stopped by addition of 20 μl SDS sample buffer and boiling for 2 min. Proteins were separated by 12% SDS polyacrylamide gel electrophoresis (SDS–PAGE) and gels were cut at the 30-kDa marker band. Upper parts were transferred onto nitrocellulose membranes and probed with 0.5 μg ml−1 anti-ERK2 antibodies in 5% BSA in TBS (Tris 50 mM, pH 7.6, NaCl 150 mM) to check equal immunoprecipitation of HA–ERK2. Lower parts were stained with Coomassie brilliant blue R250 to monitor amounts of MBP. Substrate phosphorylation was analysed using a PhosphoImager (BioRad, Molecular Imager FX). Western blotting of total cell lysates with monoclonal HA antibodies was performed to prove equal HA–ERK2 levels.
ELISA studies
To estimate cell surface expression of receptors carrying an N-terminal HA-tag, we used an indirect cellular ELISA (Schöneberg et al., 1997), further referred to as ‘surface ELISA'. Briefly, COS-7 cells were seeded into 48-well plates and transfected (0.25 μg of DNA and 0.6 μl of LipofectAMINE/well). Seventy-two hours after transfection cells were formaldehyde-fixed for 10 min without disrupting the cell membrane, blocked with DMEM plus 10% FBS for 1 h and incubated with a biotin-labelled anti-HA monoclonal antibody (12CA5, Boehringer Mannheim, Mannheim, Germany; 1 μg antibody ml−1 DMEM plus 10% FBS) for 2 h. Incubation with S1P as indicated was performed in DMEM for 30 min at 37°C prior fixation. After three times washing with PBS bound anti-HA antibody was then detected with the help of a peroxidase-labelled streptavidin conjugate (Sigma; 1 : 5000 in DMEM plus 10% FBS). After removal of excess unbound conjugate, H2O2 and o-phenylenediamine (2.5 mM each in 0.1 M phosphate-citrate buffer, pH 5.0) were added to serve as substrate and chromogen, respectively. After 15 min the enzyme reaction was stopped by the addition of 1 M HCl containing 0.05 M Na2SO3, and colour development was measured bichromatically at 492 and 620 nm using an ELISA reader (Titertek Multiskan MCC/340, Flow Laboratories, McLean, VA, U.S.A.).
Data analysis
Results are expressed as means±standard deviation or standard error, shown as error bars in the figures. Statistical comparisons were made by the Student's t-test, with significance considered when P<0.05. One-way ANOVA with Bonferroni testing was employed in case of multiple comparisons (data of Figure 8).
Figure 8.
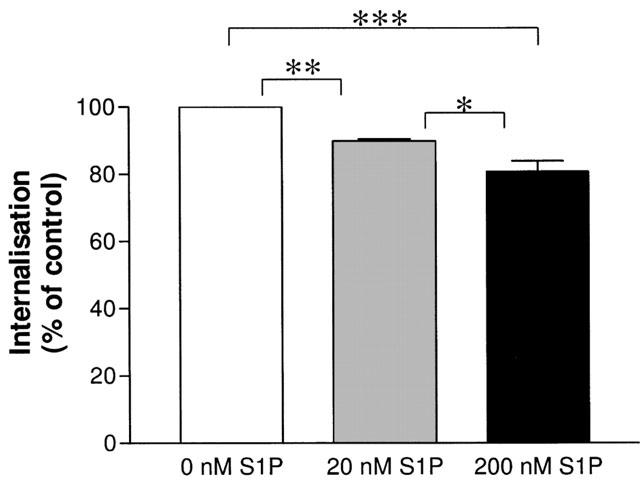
S1P-mediated internalization of a HA-tagged wild-type S1P5 receptor. COS-7 cells transiently expressing the HA-tagged S1P5 receptor were grown and transfected in 48-well plates. Seventy-two hours after transfection cells were fixed and surface expressed receptors detected with a surface ELISA as described in detail in the Method section. S1P concentration-dependently internalizes a certain percentage of S1P5 receptors. Data are shown as means±s.e.mean of three independent experiments performed in quadruplicates. *P<0.05, **P<0.01, ***P<0.001 indicate significant differences of the respective multiple comparisons.
Sources of materials
Sphingosine 1-phosphate (S1P), dihydrosphingosine 1-phosphate (dhS1P), forskolin, isobutylmethylxanthine (IBMX), staurosporine, ATP, myelin basic protein (MBP), pertussis toxin, and the RT–PCR kit were from Sigma (St. Louis, MO, U.S.A.), HEK293 and CHO-K1 cells were from the American Type culture collection (ATCC, Manassas, VA, U.S.A.), competent E. coli DH5 and cell culture media and sera from GIBCO BRL (Gaithersburg, MD, U.S.A.), the enhanced green fluorescence protein (pEGFP) vector and the GC-melt PCR kit from Clontech (Palo Alto, CA, U.S.A.), FBS and basal Iscove medium was from Biochrom (Berlin, Germany), oligonucleotides from MWG-Biotech AG (Ebersberg, Germany), the expression plasmid pcDNA3.1(+), LipofectAMINE, and E. coli MC 1061 from Invitrogen (Carlsbad, CA, U.S.A.), restriction enzymes and T4-ligase from New England Biolabs (Schwalbach, Germany), charcoal-stripped FBS (csFBS) from PAN Biotech (Aidenbach, Germany), the homogenous Caspases Assay, L-Glutamine, Gentamicin, FuGENE and G418 solution from Roche Diagnostics (Mannheim, Germany), the alphascreen™ cAMP assay kit from Packard BioScience (Meriden, U.S.A.), 4′, 6-diamino-2-phenylindole (DAPI) from Molecular Probes (Eugene, OR, U.S.A.). Polyclonal anti-HA antibodies were a gift from L. Rönnstrand (Ludwig Institute for Cancer Research, Uppsala, Sweden) polyclonal ERK2 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.), protein A-HRP and protein A-sepharose was from Zymed Laboratories (San Francisco, CA, U.S.A.), γ-32P-ATP (6000 Ci mmol−1) was from Amersham (Braunschweig, Germany).
Results
S1P5 receptor expression reduces basal cAMP accumulation
Im et al. (2001) have recently shown that the S1P5 receptor inhibits forskolin-induced stimulation of adenylyl cyclase with nanomolar EC50 values when stimulated with S1P or its reduced form, dhS1P. However, ligand-independent effects of the S1P5 receptor were not addressed in this study nor reported elsewhere thus far.
We observed that stable expression of the S1P5 receptor in HEK293 cells resulted in a 20% reduction of basal cAMP levels as compared with vector expressing cells (Figure 1A) when cultivated in medium containing 10% FBS, which is known to contain high levels (∼400 nM) S1P (Yatomi et al., 1997). To determine whether the observed effects on basal cAMP accumulation were dependent on the presence of S1P in the culture medium, cAMP assays were performed with cells grown in delipidated serum (charcoal-stripped FBS, csFBS). Intrinsic inhibition of cAMP generation did not change significantly when cells were cultured in csFBS containing medium (17%, Figure 1B vs 20%, Figure 1A). To test whether the agonist-independent effect of the S1P5 receptor on adenylyl cyclase signalling is sensitive to ligand modulation, measurement of cAMP accumulation was performed in the presence of csFBS and S1P. As depicted in Figure 1C, 1 μM S1P did not further augment S1P5 receptor-mediated inhibition of basal cAMP production. Furthermore, generation of cAMP in stable S1P5 receptor transfectants via the direct activator of adenylyl cyclase forskolin was significantly impaired in relation to vector transfected control cells (Figure 1D). To rule out non-specific effects of GPCR expression on cAMP levels, GPR63 was included as a control as it does not show intrinsic effects on the adenylyl cyclase pathway (A. Niedernberg, manuscript in preparation). As can be seen in Figure 1A,B and D, effects on intracellular second messenger levels were specifically mediated by the S1P5 receptor.
Figure 1.
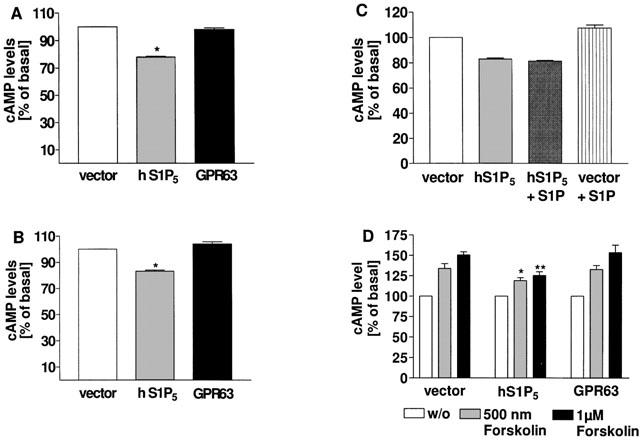
cAMP accumulation in HEK 293 cells stably expressing the human S1P5 receptor, GPR63 or vector. Cells stably expressing the indicated receptors or pcDNA3.1(+) vector DNA as a control were grown in medium containing 10% FBS (A) or charcoal-stripped FBS (csFBS) (B) for 24 h and cAMP levels quantitated in the presence of 500 μM IBMX with the alpha-screen® kit as described in Methods. (C) Transfected cells were cultivated in csFBS for 24 h and stimulated with 1 μM S1P for 30 min prior to quantification of cAMP levels. (D) Transfected cells were stimulated with forskolin for 30 min in the presence of 500 μM IBMX and cAMP levels determined as described above. In cells expressing the human S1P5 receptor basal cAMP levels were significantly lower than in vector or GPR63 expressing cells, whether they were cultivated in the presence (A) or absence (B) of lipids: *P<0.05 indicates significant difference versus cAMP levels in vector transfected cells. Addition of S1P did not significantly affect intrinsic S1P5 receptor-mediated cAMP inhibition (C). Forskolin stimulation of S1P5 receptor transfected cells resulted in significant lower cAMP levels as compared with vector or GPR63 transfectants (D): *P<0.05 and **P<0.01 indicate significant differences versus cAMP levels in vector transfected cells under corresponding conditions. Data are means±s.e.mean (n=4–10).
To investigate whether S1P may enhance S1P5 receptor-mediated inhibition of forskolin-stimulated adenylyl cyclase, i.e. inhibit the enzyme when cAMP levels are elevated as opposed to the conditions in Figure 1, various concentrations of S1P were tested for agonism in S1P5–HEK293 cells, exposed to 1000 nM of forskolin during 30 min (Figure 2). Unlike in non-forskolin treated S1P5–HEK293 cells (Figure 1C), S1P now dose dependently inhibits forskolin-stimulated adenylyl cyclase (EC50=173 nM, log EC50=−6.76+0.15; n=4, Figure 2). Collectively, our adenylyl cyclase data show that S1P acting through the S1P5 receptor further enhances basal inhibition of forskolin-stimulated but not of non-stimulated adenylyl cyclase.
Figure 2.
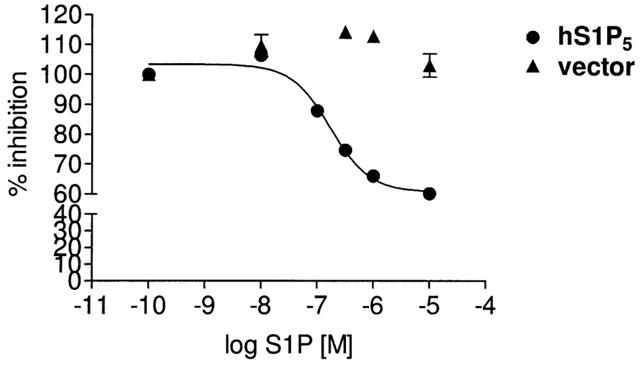
S1P-mediated inhibition of forskolin-stimulated adenylyl cyclase in S1P5-HEK293 cells. Cells stably expressing the S1P5 receptor were grown in medium containing 10% charcoal-stripped FBS (csFBS) for 24 h and cAMP levels were quantitated upon challenge with increasing concentrations of S1P with the alpha-screen® kit as described in Methods. S1P dose dependently inhibits forskolin-stimulated adenylyl cyclase activity in S1P5 receptor but not in vector transfected cells. Data are means±s.e.mean of 3–4 independent experiments, performed in quintuplets. Where not shown error bars lie within the dimensions of the symbols.
Human S1P5 receptor inhibits basal ERK activity in in vitro kinase assays
Little is known about the different signalling cascades initiated by the human S1P5 receptor in a ligand-dependent or -independent manner. So far, the only measured endpoints have been Gαi-mediated responses such as inhibition of cAMP production and stimulation of GTP-γ-S binding (Im et al., 2001). For the rat S1P5 receptor orthologue, dose-dependent inhibition of adenylyl cyclase and ERK activity by S1P have been shown (Malek et al., 2001) but ligand-independent effects on these pathways have not been described so far. Therefore, we tested the hypothesis whether the human S1P5 receptor also modulates MAP-kinase signalling and whether the effect is ligand-dependent or not. ERK activity was determined via phosphorylation of the ERK substrate myelin basic protein (MBP) such that the phosphorylation status of MBP directly reflects ERK activity. As shown in Figure 3A expression of S1P5 receptor in CHO-K1 cells grown in medium supplemented with 10% FBS significantly reduced the kinase activity of ERK in comparison to mock transfected cells. Serum-induced ERK1/2 phosphorylation was not inhibited by S1P in mock transfected cells (Figure 3A, left panel). Moreover, 1 μM S1P did not modify the intrinsic effects of the S1P5 receptor on ERK activity (Figure 3A, right panel). Notably, the inhibition of ERK activity in cells expressing the S1P5 receptor cultivated in medium containing csFBS (Figure 3B, right panel) was as prominent as in cells grown with FBS (Figure 3A, right panel) and again, addition of S1P did not modify the inhibitory effects of the S1P5 receptor on ERK activation (Figure 3B, right panel). Collectively, our data suggest that inhibition of ERK activity through the S1P5 receptor is constitutive and insensitive to the ligand S1P.
Figure 3.
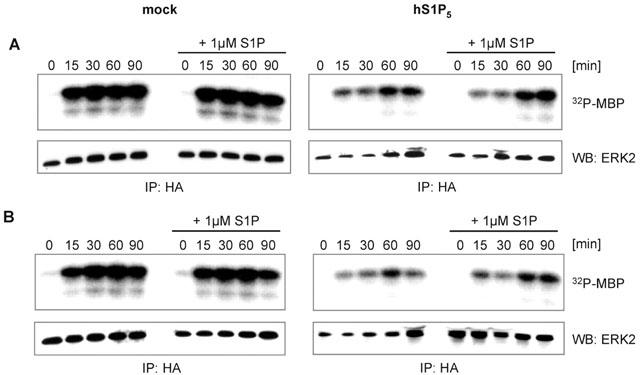
The S1P5 receptor displays ligand-independent inhibition of ERK activity. (A) CHO cells were cotransfected with human S1P5 receptor cDNA or vector (mock) and HA-ERK2 cDNA. After incubation in serum-free medium for 24 h, cells were stimulated with medium containing 10% FBS with or without addition of 1 μM S1P. After cell lysis, immunoprecipitation, in vitro kinase reactions and separation by SDS page (see Methods), myelin basic protein (MBP) phosphorylation was quantified for measurement of ERK activity. HA-ERK2 was quantified via Western blot for confirmation of equal expression levels. (B) The assay was essentially carried out as described above but stimulation was performed in medium containing 10% csFBS with or without S1P. Data shown are representative for 2–4 independent experiments.
Human S1P5 receptor overexpression induces S1P-dependent cell rounding
To test whether the S1P5 receptor-mediated basal activity is correlated with intrinsic effects on cell morphology, the S1P5 receptor in combination with GFP at a 4 : 1 ratio was transiently transfected into mammalian CHO-K1 cells. The rat S1P2 (rS1P2, formerly referred to as H218) receptor was included as a positive control as a recent study by van brocklyn et al. (1999) showed that transfection of rS1P2 receptor into HEK293 cells leads to cell rounding followed by apoptotic cell death.
CHO-K1 cells transfected with the S1P5 receptor, rS1P2 receptor (not shown) or pcDNA3.1(+) vector cDNA and GFP were cultured for 34 h in medium containing FBS (Figure 4). Under these conditions most of the cells expressing the S1P5 receptor displayed a rounded morphology. Expression of the rS1P2 receptor caused cell shape changes that were less pronounced than those seen with the S1P5 receptor (not shown). It is evident that morphological changes are correlated with receptor expression levels, as cells with intense GFP fluorescence, and thus presumably also high S1P receptor levels, exhibit most prominent cell shape changes. Vector-transfected cells displayed the normal CHO cell morphology. As shown in Figure 4, S1P5 receptor-transfected cells exposed to csFBS had ∼30–40% round morphology compared to ∼90% in FBS. S1P5 receptor expressing cells exposed to csFBS containing 1 μM S1P showed a completely round phenotype, comparable to that observed in FBS containing medium. These data suggest that S1P5 receptor-mediated cell rounding does not require, but is enhanced by, S1P stimulation. It should be noted that cell rounding responses were not only observed in CHO cells but also in HEK293, NIH-3T3, COS7, and RH7777 cells (not shown).
Figure 4.
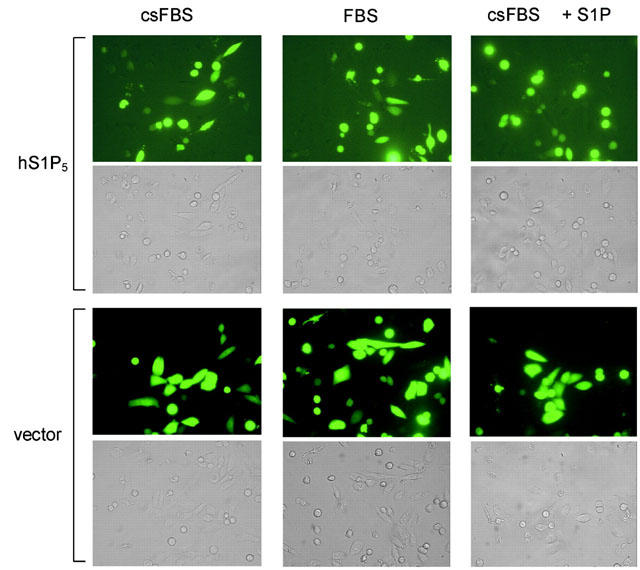
S1P5 receptor expression in CHO-K1 cells causes cell rounding. CHO cells transiently transfected with S1P5 or pcDNA3.1(+) vector and GFP (4 : 1) cDNA were grown for 34 h in Iscove supplemented with FBS, csFBS, or csFBS+1 μM S1P. Plates were then analysed by fluorescence microscopy. The human S1P5 receptor induced cell rounding that was more pronounced in transfected cells cultivated in medium containing 10% FBS as compared with medium containing csFBS. Addition of 1 μM S1P to csFBS containing medium led to a complete round morphology of S1P5 transfected cells. Vector transfected cells displayed the normal CHO cell morphology.
As regulation of the actin cytoskeleton and cell rounding responses are frequently mediated via Gα12/13 and the small G protein Rho, we tested whether S1P5 expressing cells activate Rho signalling in HEK293 cells in a reporter gene assay, that translates receptor-mediated Rho activation into stimulation of a serum responsive element, which in turn promotes transcription of luciferase. The neurotensin receptor served as a positive control in this assay and caused an 8 fold increase of luciferase activity upon 4 h of ligand stimulation. In contrast, the S1P5 receptor did neither display agonist-independent nor S1P-induced modulation of the Rho signalling pathway in the respective reporter gene assay (data not shown).
Human S1P5 receptor does not lead to apoptosis in transfected CHO-K1 cells
To determine whether cell rounding is correlated with apoptotic cell death, nuclei were stained with DAPI. As seen in Figure 5, none of the rounded cells contained fragmented DNA in their nuclei, which would indicate apoptosis. To determine whether Gαi proteins are involved in the cell rounding response, experiments were performed in the presence of PTX which specifically inactivates Gαi proteins. However, PTX did not affect S1P5 receptor-mediated cell rounding (data not shown), indicating a role of either Gαq/11 or Gα12/13. Very similar phenotypical observations were made when the S1P5 receptor was transfected into HEK293, COS-7, RH7777 or NIH-3T3 cells. In all cases cell rounding was induced in the presence as well as in the absence of S1P in the medium (data not shown).
Figure 5.
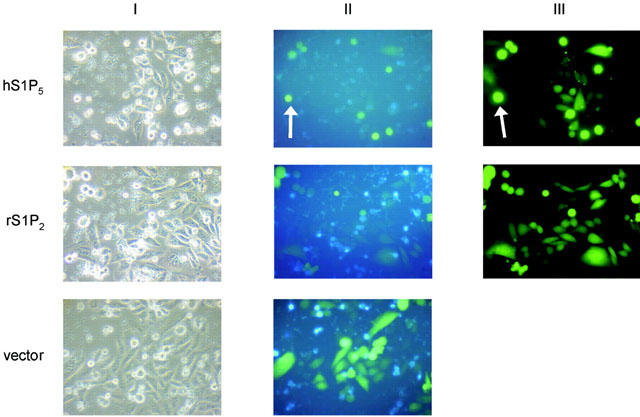
The S1P5 receptor causes cell rounding but not cell death in transiently transfected CHO-K1 cells. CHO cells transiently transfected with the cDNAs of human S1P5 receptor (A), rS1P2 receptor (B), pcDNA3.1(+) (C) and GFP (4 : 1) were grown for 34 h in Iscove supplemented with FBS after transfection and images recorded after 4′, 6-diamino-2-phenylidole (DAPI) nuclear staining. No signs of apoptosis could be seen in S1P5 or rS1P2 receptor transfected cells with rounded morphology (II+III). White arrows highlight selected cells that are round but not apoptotic.
Although DAPI staining did not indicate that the S1P5 receptor activates the cell death machinery, we wanted to confirm this finding with a second independent assay. A caspase assay was chosen as caspase activation is one of the cellular processes triggered during apoptosis (Nicholson & Thornberry, 1997; Andersson et al., 2000), and precedes complete fragmentation of the nuclei and cell death.
As shown in Figure 6, CHO-K1 cells transfected with the S1P5 receptor neither showed intrinsic nor ligand-induced induction of caspase activity when incubated for 2 h without serum or in the presence of various concentrations of S1P. In contrast, addition of 10% FBS clearly inhibits starvation-induced caspase activation, whereas staurosporin promotes cell death in all transfectants tested. As recent literature indicated that caspases are not necessarily involved in the very early events of apoptosis (Dumont et al., 2000) we repeated the assay with 7 h incubation. Again, staurosporin induced caspase activation (4–5 fold in comparison to no serum) but no pro-apoptotic effect could be detected in S1P5 transfected cells treated with different concentrations of S1P (data not shown). It appeared however, that S1P5 receptor expression unexpectedly exerted a protective effect, as evidenced by (i) lack of induction of caspases by 300 and 1000 nM S1P in S1P5 transfectants as compared with vector transfected cells and (ii) decreased caspase activity upon staurosporin treatment in S1P5 as compared with vector transfected cells (Figure 6). Our data suggest that the S1P5 receptor does not promote but constitutively protects CHO cells from cell death as determined in caspase assays.
Figure 6.
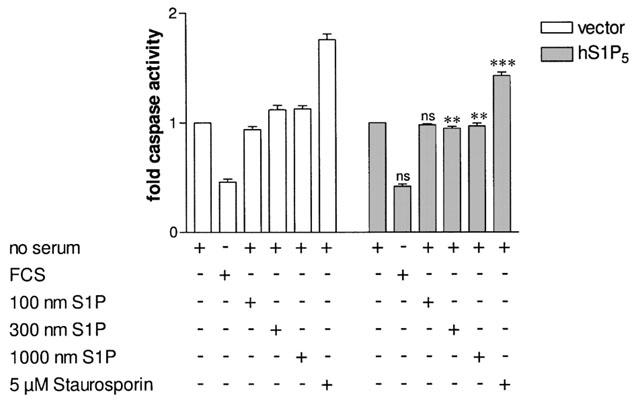
Caspase activity assay in CHO cells transiently transfected with S1P5 receptor or pcDNA3.1(+) cDNAs. Thirty-six hours after transfection, CHO-K1 cells were grown in no serum containing medium for 6 h prior to the assay. Cells were then incubated for 2 h in serum-free medium, 10% FBS containing medium, or the indicated S1P concentration or staurosporin in serum-free medium. Fluorescence was measured using the Homogeneous Caspase Assay® kit according to the manufacturer's instructions. Data are means±s.e.mean of eight independent experiments. *P<0.05, **P<0.01, ***P<0.005 indicate significant differences versus fold caspase activity in vector transfected cells under the corresponding conditions, ns=non-significant.
A S1P5-green fluorescent protein (GFP) fusion protein is mainly located in intracellular compartments
As the S1P5 receptor does not require any ligand to stimulate most cellular responses such as inhibition of adenylyl cyclase and ERK activity, we wondered whether internalization of the S1P5 receptor could be achieved by addition of the activating ligand S1P, or whether the S1P5 receptor population may already be completely internalized. To this end, a S1P5 receptor-GFP fusion protein was transfected into HEK293 cells and images were analysed with confocal microscopy. As seen in Figure 7 (A1–3), most of the transfected cells showed cell rounding, whereas non-transfected cells displayed the characteristic HEK293 cell morphology Figure 7 (A2–3). Frequently, images as in B1–3 were observed with a significant amount of GFP fluorescence in intracellular compartments, some cells (Figure 7, C1–3) displayed GFP fluorescence at the plasma membrane. Cell rounding per se complicates an analysis of ligand-induced internalization, and indeed 10 μM S1P stimulation for 10 min (Figure 7, D1, 2) or 20 min (Figure 7, E1, 2) did not lead to a detectable internalization in this assay. Identical cell rounding responses were obtained upon transfection of S1P5–GFP into COS-7 and CHO-K1 cells (data not shown) and prevented application of this technique for characterization of internalization responses.
Figure 7.
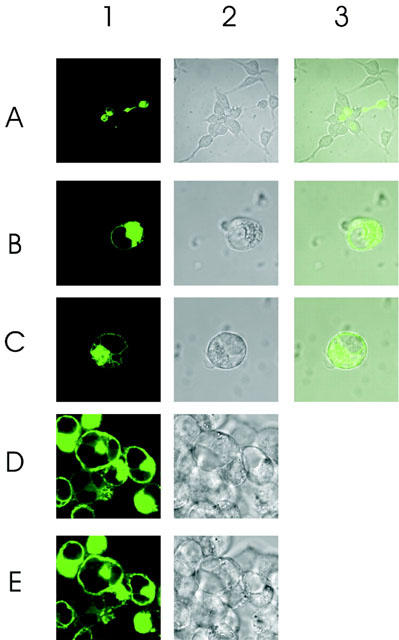
Cellular distribution pattern of GFP-tagged wild type S1P5 receptor. (A) C-terminally GFP-tagged S1P5 receptor was expressed transiently in HEK293 cells and images were analysed confocally. A1–3: the majority of transfected cells are round whereas non transfected cells display the characteristic HEK293 cell morphology (A2,3). B1–3: images frequently observed showing a significant amount of GFP fluorescence in intracellular compartments. Some cells (C1–3) display GFP fluorescence at the plasma membrane. D: cells were stimulated with 10 μM S1P for 10 min. E: cells were stimulated with 10 μM S1P for 20 min. Cell rounding does not allow evaluation of internalization responses.
S1P induces S1P5 receptor internalization in a cell surface ELISA assay
We constructed an N-terminally tagged version of the S1P5 receptor carrying the influenza virus haemagglutinin epitope (HA) sequence immediately after the initiating methionine codon, and applied a cell surface ELISA technique to verify the cell surface expression of the S1P5 receptor and to monitor potential disappearance of cell surface receptors to intracellular compartments upon stimulation with S1P. As depicted in Figure 8, the S1P5 receptor dose-dependently internalized with S1P to a significant extent when cultivated in medium containing FBS: 20 nM S1P internalized 11% and 200 nM S1P internalized 18% of receptors present on the plasma membrane. Interestingly, ligand-induced internalization did not change when cells were kept in csFBS containing medium. It should be noted that our cell surface ELISA was performed in COS7 cells and not with HEK293 or CHO cells used throughout the study, as this was the only cell type that allowed to apply the ELISA protocol without significant detachment of cells.
Discussion
Constitutive activity of GPCRs has been extensively examined in recent years to understand conformational changes imparted on GPCRs upon ligand binding, or distinct features of ligand-induced internalization, trafficking or inverse agonist-induced up-regulation (McCune et al., 2000; Stevens et al., 2000; Miserey-Lenkei et al., 2002; Chen et al., 2002). Some studies are available on the influence of constitutive activity imbued by mutational alteration at different receptor sites on distinct signalling cascades (e.g. Perez et al., 1996). These and related findings lead to the hypothesis that constitutively active mutants and the conformational alterations they produce in a given receptor are unlikely to be equivalent (Stevens et al., 2000). Hence, very little is known whether constitutive activity of wild-type GPCRs that mediate various cellular responses differentially affects those responses.
A family of GPCRs reported to exert intrinsic effects on cells are the members of the sphingosine 1-phosphate family S1P1,2,3,4 (Lee et al., 1998; van brocklyn et al., 1999). However, in most cases their ‘constitutive activity' is a consequence of the high S1P levels in the cell culture medium. Presence of the agonist S1P in the medium facilitated identification of its cognate S1P1 receptor (Lee et al., 1998). HEK293 cells stably expressing the S1P1 receptor exhibited enhanced adherens junction-based cell–cell aggregation, exaggerated cadherin expression and were phenotypically differentiated. Removal of lipids from FBS by charcoal-stripping completely removed the factor responsible for morphogenetic differentiation. Subsequently, S1P was identified as the serum factor responsible for the phenotypic changes. Therefore, in case of the S1P1 receptor, morphogenetic differentiation of cells does not seem to be an intrinsic feature of the receptor due to agonist-independent signalling but a consequence of S1P presence in the medium.
Meanwhile, many S1P receptor subtypes have been reported to cause phenotypical changes (morphology, proliferation, stress fibre formation, cytoskeletal reorganization, etc) when transfected in various cell types (Lee et al., 1998; 1999; Moolenaar, 1999; van brocklyn et al., 1999; Robert et al., 2001); in all cases, however, changes were dependent on the presence of lipids in the medium, and no reports are available so far about ligand-independent effects of S1P receptor subtypes. We tested several S1P receptors and chose the poorly characterized human S1P5 receptor as a model to study a possible link between activation of various cellular events and their responsiveness to ligand modulation.
Initially, we observed that expression of the S1P5 receptor in HEK293 cells led to a significant reduction of basal cAMP production. Basal cAMP levels decreased when cells were cultured in medium containing FBS as well as in delipidated medium (i.e. medium supplemented with csFBS). This finding indicates that the S1P5 receptor is constitutively active in the absence of the stimulating ligand. In order to test whether its agonist S1P may modulate the S1P5 receptor effects on basal cAMP production, cells were cultivated in csFBS and then stimulated with 1 μM S1P. We did not observe a further decrease in cAMP levels under these conditions (Figure 1C). It cannot unequivocally be stated that constitutive inhibition of adenylyl cyclase via the S1P5 receptor is insensitive to ligand modulation as vector transfected cells displayed a small but significant increase in basal cAMP production. This increase may have counteracted the S1P effects on S1P5 receptor-mediated inhibition of adenylyl cyclase. S1P action on endogenously present S1P2 and S1P3 receptor may have caused this increase, as to the best of our knowledge no other S1P receptor subtype is positively linked to adenylyl cyclase. Alternatively, increase of cAMP levels in vector transfected cells may be due to an as yet unidentified S1P-responsive receptor. On the other hand, basal cAMP levels in FBS and csFBS were about identical, suggesting that S1P present in FBS at a concentration of ∼400 nM does indeed not affect the intrinsic adenylyl cyclase inhibition. Consistent with the hypothesis of constitutive activity is the finding that stimulation with the direct activator of adenylyl cyclase forskolin induced significantly lower cAMP levels in S1P5 receptor expressing cells as compared to vector transfected cells (Figure 1D). Under these conditions, however, i.e. when cAMP levels of forskolin-stimulated cyclase are increased, the agonist ligand S1P further enhances basal inhibition of adenylyl cyclase via the S1P5 receptor (Figure 2). One may speculate that in living cells the S1P5 receptor fine tunes adenylyl cyclase activity: it acts as a ligand-independent brake of adenylyl cyclase when cAMP levels are not elevated but converts to a potent ligand-dependent inhibitor of adenylyl cyclase when the enzyme is activated and/or intracellular cAMP levels are increased. This mechanism even raises the possibility that cAMP itself may be an intracellular allosteric modulator of various active conformations of the S1P5 receptor and merits further investigation.
So far, a subset of GPCRs, including those for lysophosphatidic acid (LPA), endothelin, angiotensin II, bombesin and thrombin, have been reported to induce stress fibres, cytoskeletal re-arrangement and cell rounding (Hecht et al., 1996; Ishida et al., 1999; Sinnett-Smith et al., 2001; Kawanabe et al., 2002). These cellular responses are well recognized to be mediated via the Gα12/Gα13–Rho signalling pathway. Surprisingly, we could not detect Rho activation in a reporter gene assay. Moreover, insensitivity of cell rounding responses to PTX pretreatment clearly rules out involvement of Gαi proteins. Irrespective of the pathway that is responsible for the phenotypic changes, it is intriguing to note that cell rounding is an intrinsic ability of the S1P5 receptor that can be further modulated by the ligand S1P. In contrast, intrinsic effects on the Gαi-mediated events such as inhibition of non-stimulated adenylyl cyclase or ERK activity are insensitive to ligand stimulation. Our findings suggest that different intracellular effector systems triggered by a given constitutively active receptor are not equally activated and support the notion that inverse agonist ligands will be useful tools to dissect the pharmacology of individual GPCR-mediated signal transduction events.
Apoptotic cell death is often preceded by cell rounding. van brocklyn et al. (1999) have reported that HEK293 cells transfected with the rS1P2 receptor, and to a lesser extent the S1P3 receptor, display rounded cell morphology and signs of apoptosis as evidenced by staining of fragmented DNA in the nuclei of the cells. To determine whether the related S1P5 receptor also causes cell death, DAPI staining was performed in S1P5 receptor transfected cells. Our findings clearly show that cell rounding does not correlate with cell death (Figure 5). In addition, caspase assays that are routinely used to determine activation of cell death suggest that the S1P5 receptor does not activate the cell death machinery. It should be noted that Rho activation has also been associated with dynamic membrane blebbing during the final stages of apoptotic cell death (Mills et al., 1998). Moreover, the Rho effector protein kinase N (PKN) is another possible mediator of apoptosis, because it is proteolytically cleaved by caspases to generate a constitutively active kinase fragment (Takahashi et al., 1998). It has to be emphasized, however, that there is as yet no direct evidence implicating specific signalling molecules downstream of Rho in the apoptotic signalling cascade. Nevertheless, the negative outcome of the caspase assay, together with our inability to demonstrate involvement of the Gα12/Gα13–Rho pathway in S1P5 receptor-mediated signalling, and the fact that Rho activation may lead to apoptosis (Mills et al., 1998; Takahashi et al., 1998, for review see Sah et al., 2000) collectively support the idea that the S1P5 receptor may not utilize the Gα12/Gα13 pathway to transmit signals to the cell interior. It should be noted, that in our study the rS1P2 receptor only led to modest cell rounding but not to apoptosis as described by van brocklyn et al. (1999). Different cell types – HEK293 cells used by van brocklyn et al. (1999) versus CHO-K1 cells in our study – may be the reason for the observed discrepancy. As stated by the authors in van brocklyn et al. (1999), HEK293 cells are much easier to detach from the culture dishes than other cell types, and loss of cell attachment may have been a possible cause for the observed cell death in their study. Whereas our data clearly support the concept that the S1P5 receptor does not act pro-apoptotic, it appears that it even exerts a small but significant protective effect. As can be seen in Figure 6, S1P-dependent stimulation of caspase activity in vector transfected cells is abolished in S1P5-transfected cells and the fold caspase activation induced by staurosporin is decreased in S1P5-transfected cells as compared with vector transfected cells. The S1P5 receptor therefore represents a receptor protecting cells in a constitutive manner against noxious stimuli such as staurosporin (Figure 6).
It is of interest to note that receptors with primarily intracellular localization are still signalling-competent. This raises the question whether activation of cellular signalling cascades requires presence of the receptor on the cell surface or whether internalized receptors are still signalling-competent. It is a general view that upon activation of cellular signalling cascades GPCR kinases are recruited to the plasma membrane to phosphorylate the activated GPCR, which in turn promotes arrestin binding (Lohse et al., 1990; Vilardaga et al., 2002).
Arrestin binding is then thought to uncouple the receptor from the G protein and to initiate a process of internalization. Traditionally, internalization has been thought of as a way to terminate cellular signalling. Recently, however, studies using transfected β2-adrenoceptors or endogenous opioid receptors indicate that internalization of certain GPCRs can also activate cellular signalling. Daaka et al. (1998) and Ignatova et al. (1999) showed that receptor internalization was necessary to activate ERK. In these studies, dominant negative mutants of β-arrestin or dynamin prevented receptor internalization and most importantly, receptor-stimulated ERK activity. It is therefore intriguing to ask whether S1P5 receptor-mediated agonist-independent effects require internalization or whether the observed internalization is the consequence of constitutive signalling. Future studies will address this question in more detail. Irrespective of the order of the cellular events, our data further support the concept that wild-type receptors with agonist independent signalling are mainly located in intracellular compartments of the cell as evidenced by the S1P5-GFP fusion construct. This notion is further underscored by our finding that S1P only internalizes a small percentage of S1P5 receptors which may reflect a high basal internalization rate rather than the inability of S1P to internalize the S1P5 receptor. Availability of S1P5 receptor inverse agonists will help to shed more light on the links between constitutive activity and internalization.
S1P acts both as an extracellular ligand via GPCRs, and as an intracellular mediator via as yet unidentified molecular targets (Spiegel & Milstien, 2000; Pyne & Pyne, 2000). Recently, it has been hypothesized that signal transduction cascades are not only initiated at the plasma membrane but also at nuclear membranes (Malviya & Rogue, 1998), as several studies have disclosed that the nuclear envelope plays a major role in signal transduction cascades and a novel nuclear envelope signal transduction (NEST) hypothesis has been put forward (Baldassare et al., 1997; Raben & Baldassare, 2000). Moreover, both heterotrimeric and small G proteins (Baldassare et al., 1997; Saffitz et al., 1994), adenylate cyclase (Yamamoto et al., 1998), phospholipase C (Malviya & Rogue, 1998; Cocco et al., 1999), phospholipase D (Baldassare et al., 1997), and several GPCRs such as the muscarinic M3 receptor (Lind & Cavanagh, 1993), the angiotensin II AT1 receptor (Booz et al., 1992) and prostaglandin EP3 and EP4 receptors (Bhattacharya et al., 1999) have been shown to be localized at the nucleus. It is therefore tempting to speculate that a possible intracellular target for S1P action is represented by a GPCR such as the S1P5 receptor. Clearly, future studies are required to address functionality of GPCRs with intracellular localization.
In summary, our data demonstrate that S1P5 is a constitutively active receptor with the ability to intrinsically stimulate various effector pathways albeit to a different extent. We suggest that different signal transduction pathways are not equally activated through constitutively active GPCRs with promiscuous signalling characteristics and highlight the importance of inverse agonist tools for the understanding of CAR pharmacology.
Abbreviations
- csFBS
charcoal-stripped foetal bovine serum
- dhS1P
dihydro sphingosine 1-phosphate
- ERK
extracellular signal regulated kinase
- FBS
foetal bovine serum
- G protein
guanine nucleotide-binding protein
- GPCR
G protein-coupled receptor
- LPA
lysophosphatidic acid
- PTX
pertussis toxin
- S1P
sphingosine 1-phosphate
References
- ANDERSSON M., SJOSTRAND J., PETERSEN A., HONARVAR A.K., KARLSSON J.O. Caspase and proteasome activity during staurosporin-induced apoptosis in lens endothelial cells. Invest. Ophthalmol. Vis. Sci. 2000;41:2623–2632. [PubMed] [Google Scholar]
- BALDASSARE J.J., JARPE M., ALFERES L., RABEN D.M. Nuclear translocation of RhoA mediates the mitogen-induced activation of phospholipase D involved in nuclear envelope signal transduction. J. Biol. Chem. 1997;272:4911–4914. doi: 10.1074/jbc.272.8.4911. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYA M., PERI K., RIBEIRO-DA-SILVA A., ALMAZAN G., SHICHI H., HOU X., VARMA D.R., CHEMTOB S. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J. Biol. Chem. 1999;274:15719–15724. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
- BLAUKAT A., BARAC A., CROSS M.J., OFFERMANNS S., DIKIC I. G protein-coupled receptor-mediated mitogen-activated protein kinase activation through cooperation of Gαq and Gαl signals. Mol. Cell. Biol. 2000;20:6837–6848. doi: 10.1128/mcb.20.18.6837-6848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCKAERT J., PIN J.P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOZ G.W., CONRAD K.M., HESS A.L., SINGER H.A., BAKER K.M. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology. 1992;130:3641–3649. doi: 10.1210/endo.130.6.1597161. [DOI] [PubMed] [Google Scholar]
- BROWN E.M. Physiology and pathophysiology of the extracellular calcium-sensing receptor. Am. J. Med. 1999;106:238–253. [PubMed] [Google Scholar]
- CHEN G., WAY J., ARMOUR S., WATSON C., QUEEN K., JAYAWICKREME C.K., CHEN W.J., KENAKIN T. Use of constitutive G protein coupled receptor activity for drug discovery. Mol. Pharmacol. 2000;57:125–134. [PubMed] [Google Scholar]
- CHEN S., LIN F., XU M., GRAHAM R.M. Phe(303) in TMVI of the alpha(1B)-adrenergic receptor is a key residue coupling TM helical movements to G-protein activation. Biochemistry. 2002;41:588–596. doi: 10.1021/bi011868k. [DOI] [PubMed] [Google Scholar]
- CHUN J., GOETZL E.J., HLA T., IGARASHI Y., LYNCH K.R., MOOLENAAR W.H., PYNE S., TIGYI G. International Union of Pharmacology. XXXIV. Lysophospholipid Receptor Nomenclature. Pharmacol. Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- COUGHLIN S.R. Expanding horizons for receptors coupled to G proteins: diversity and disease. Curr. Opin. Cell. Biol. 1994;6:191–197. doi: 10.1016/0955-0674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- COCCO L., RUBBINI S., MANZOLI L., BILLI A.M., FAENZA I., PERUZZI D., MATTEUCCI A., ARTICO M., GILMOUR R.S., RHEE S.G. Inositides in the nucleus: presence and characterisation of the isozymes of phospholipase beta family in NIH 3T3 cells. Biochem. Biophys. Acta. 1999;1438:295–299. doi: 10.1016/s1388-1981(99)00061-x. [DOI] [PubMed] [Google Scholar]
- DAAKA Y., LUTTRELL L.M., AHN S., DELLA ROCCA G.J., FERGUSON S.S., CARON M.G., LEFKOWITZ R.J. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- DUMONT C., DURRBACH A., BIDERE N., ROULEAU M., KREOMER G., BERNARD G., HIRSCH F., CHARPENTIER B., SUSIN S.A., SENIK A. Caspase-independent commitment phase to apoptosis in activated blood t lymphocytes: reversibility at low apoptotic insult. Blood. 2000;93:1030–1038. [PubMed] [Google Scholar]
- GREASLEY P.J., FANELLI F., SCHEER A., ABUIN L., NENNIGER-TOSATO M., DEBENEDETTI P.G., COTECCHIA S. Mutational and computational analysis of the alpha(1b)-adrenergic receptor. Involvement of basic and hydrophobic residues in receptor activation and G protein coupling. J. Biol. Chem. 2001;276:46485–46494. doi: 10.1074/jbc.M105791200. [DOI] [PubMed] [Google Scholar]
- HECHT J.H., WEINER J.A., POST S.R., CHUN J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell. Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGNATOVA E.G., BELCHEVA M.M., BOHN L.M., NEUMAN M.C., COSCIA C.J. Requirement of receptor internalisation for opioid stimulation of mitogen-activated protein kinase: biochemical and immunofluorescence confocal microscopic evidence. J. Neurosci. 1999;19:56–63. doi: 10.1523/JNEUROSCI.19-01-00056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IM D.-S., CLEMENS J., MACDONALD T.L., LYNCH K.R. Characterization of the human and mouse sphingosine 1-phosphate receptor, S1P5 (EDG8): Structure-activity relationship of sphingosine 1-phosphate receptors. Biochem. 2001;40:14053–14060. doi: 10.1021/bi011606i. [DOI] [PubMed] [Google Scholar]
- IIRI T., FARFEL Z., BOURNE H.R. G-protein diseases furnish a model for the turn-on switch. Nature. 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- ISHIDA T., ISHIDA M., SUERO J., TAKAHASHI M., BERK B.C. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J. Clin. Invest. 1999;103:789–797. doi: 10.1172/JCI4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN A.A., SPALDING T.A., BURSTEIN E.S., SHEPPARD P.O., O'HARA P.J., BRANN M.R., KROGSGAARD-LARSEN P., BRAUNER-OSBORNE H. Functional importance of the Ala(116)-Pro(136) region in the calcium-sensing receptor. Constitutive activity and inverse agonism in a family C G-protein-coupled receptor. J. Biol. Chem. 2000;275:29547–29555. doi: 10.1074/jbc.M910023199. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., OKAMOTO Y., NOZAK I.K., HASHIMOTO N., MIWA S., MASAKI T. Molecular mechanism for endothelin-1-induced stress-fiber formation: analysis of G proteins using a mutant endothelin(A) receptor. Mol. Pharmacol. 2002;61:277–284. doi: 10.1124/mol.61.2.277. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol. Rev. 1996;48:413–463. [PubMed] [Google Scholar]
- LEE M.J., THANGADA S., CLAFFEY K.P., ANCELLIN N., LIU C.H., KLUK M., VOLPI M., SHA'AFI R.I., HLA T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- LEE M.J., VAN BROCKLYN J.R., THANGADA S., LIU C.H., HAND A.R., MENZELEEV R., SPIEGEL S., HLA T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., COTECCHIA S., SAMAMA P., COSTA T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol. Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- LEURS R., SMIT M.J., ALEWIJNSE A.E., TIMMERMAN H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem. Sci. 1998;23:418–422. doi: 10.1016/s0968-0004(98)01287-0. [DOI] [PubMed] [Google Scholar]
- LIND G.J., CAVANAGH H.D. Nuclear muscarinic acetylcholine receptors in corneal cells from rabbit. Invest. Ophthalmol. Vis. Sci. 1993;34:2943–2952. [PubMed] [Google Scholar]
- LOHSE M.J., BENOVIC J.L., CODINA J., CARON M.G., LEFKOWITZ R.J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- MALEK R.L., TOMAN R.E., EDSALL L.C., WONG S., CHIU J., LETTERLE C.A., VAN BROCKLYN J.R., MILSTEIN S., SPIEGEL S., LEE N.H. Nrg-1 belongs to the endothelial differentiation gene family of G protein-coupled sphingosine-1-phosphate receptors. J. Biol. Chem. 2001;23:5692–5699. doi: 10.1074/jbc.M003964200. [DOI] [PubMed] [Google Scholar]
- MALVIYA A.N., ROGUE P.J. ‘Tell me where is calcium bred': clarifying the roles of nuclear calcium. Cell. 1998;92:17–23. doi: 10.1016/s0092-8674(00)80895-8. [DOI] [PubMed] [Google Scholar]
- MARINISSEN M.J., GUTKIND J.S. G-protein-coupled receptors and signalling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- MCCUNE D.F., EDELMANN S.E., OLGES J.R., POST G.R., WALDROP B.A., WAUGH D.J., PEREZ D.M., PIASCIK M.T. Regulation of the cellular localization and signalling properties of the alpha(1B)- and alpha(1D)-adrenoceptors by agonists and inverse agonists. Mol. Pharmacol. 2000;57:659–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- MENG E.C., BOURNE H.R. Receptor activation: what does the rhodopsin structure tell us. Trends Pharmacol. Sci. 2001;22:587–593. doi: 10.1016/s0165-6147(00)01825-3. [DOI] [PubMed] [Google Scholar]
- MILLIGAN G., BOND R.A., LEE M. Inverse agonism: pharmacological curiosity or potential therapeutic strategy. Trends Pharmacol. Sci. 1995;16:10–13. doi: 10.1016/s0165-6147(00)88963-4. [DOI] [PubMed] [Google Scholar]
- MILLS J.C., STONE N.L., ERHARDT J., PITTMAN R.N. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISEREY-LENKEI S., PARNOT C., BARDIN S., CORVOL P., CLAUSER E. Constitutive internalisation of constitutively active agiotensin II AT(1A) receptor mutants is blocked by inverse agonists. J. Biol. Chem. 2002;277:5891–5901. doi: 10.1074/jbc.M108398200. [DOI] [PubMed] [Google Scholar]
- MOOLENAAR W.H. Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell. Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- NICHOLSON D.W., THORNBERRY N.A. Caspases: killer proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- PARMA J., DUPREZ L., VAN SANDE J., COCHAUX P., GERVY C., MOCKEL J., DUMONT J., VASSART G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., WURCH T. Review: amino acid domains involved in constitutive activation of G-protein-coupled receptors. Mol. Neurobiol. 1998;17:109–135. doi: 10.1007/BF02802027. [DOI] [PubMed] [Google Scholar]
- PEI G., SAMAMA P., LOHSE M., WANG M., CODINA J., LEFKOWITZ R.J. A constitutively active mutant beta 2-adrenergic receptor is constitutively desensitized and phosphorylated. Proc. Natl. Acad. Sci. 1994;91:2699–2702. doi: 10.1073/pnas.91.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREZ D.M., HWA J., GAIVIN R., MATHUR M., BROWN F., GRAHAM R.M. Constitutive activation of a single effector pathway: evidence for multiple activation states of a G protein-coupled receptor. Mol. Pharmacol. 1996;49:112–122. [PubMed] [Google Scholar]
- PYNE S., PYNE N.J. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABEN D.M., BALDASSARE J.J.Nuclear envelope signalling-role of phospholipid metabolism Eur. J. Histochem. 20004467–80.Review [PubMed] [Google Scholar]
- RAMSAY D., BEVAN N., REES S., MILLIGAN G. Detection of receptor ligands by monitoring selective stabilization of a Renilla luciferase-tagged, constitutively active mutant, G-protein-coupled receptor. Br. J. Pharmacol. 2001;133:315–323. doi: 10.1038/sj.bjp.0704077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO V.R., COHEN G.B., OPRIAN D.D. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994;367:639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- ROBERT P., TSUI P., LAVILLE M.P., LIVI G.P., SARAU H.M., BRIL A., BERREBI-BERTRAND I. EDG1 receptor stimulation leads to cardiac hypertrophy in rat neonatal myocytes. J. Mol. Cell. Cardiol. 2001;33:1589–1606. doi: 10.1006/jmcc.2001.1433. [DOI] [PubMed] [Google Scholar]
- SAFFITZ J.E., NASH J.A., GREEN K.G., LUKE R.A., RANSNAS L.A., INSEL P.A. Immunoelectron microscopic identification of cytoplasmic and nuclear Gs alpha in S49 lymphoma cells. FASEB J. 1994;8:252–258. doi: 10.1096/fasebj.8.2.8119495. [DOI] [PubMed] [Google Scholar]
- SAH V.P., SEASHOLTZ T.M., SAGI S.A., HELLER BROWN J. The role of rho in G protein-coupled receptor signal transduction. Annu. Rev. Pharmacol. Toxicol. 2000;40:459–489. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- SAMAMA P., COTECCHIA S., COSTA T., LEFKOWITZ R.J. A mutation induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- SCHEER A., COSTA T., FANELLI F., DE BENEDETTI P.G., MHAOUTY-KODJA S., ABUIN L., NENNIGER-TOSATO M., COTECCHIA S. Mutational analysis of the highly conserved arginine within the Glu/Asp-Arg-Tyr motif of the alpha(1b)-adrenergic receptor: effects on receptor isomerization and activation. Mol. Pharmacol. 2000;57:219–231. [PubMed] [Google Scholar]
- SCHEER A., COTECCHIA S. Constitutively active G protein-coupled receptors: potential mechanisms of receptor activation. J. Recept. Signal. Transduct. 1997;17:57–73. doi: 10.3109/10799899709036594. [DOI] [PubMed] [Google Scholar]
- SCHÖNEBERG T., SANDIG V., WESS J., GUDERMANN T., SCHULTZ G. Reconstitution of mutant V2 vasopressin receptors by adenovirus-mediated gene transfer. Molecular basis and clinical implication. J. Clin. Invest. 1997;100:1547–1556. doi: 10.1172/JCI119678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHENKER A., LAUE L., KOSUGI S., MERENDINO J.J., JR, MINEGISHI T., CUTLER G.B., JR A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- SINNETT-SMITH J., LUNN J.A., LEOPOLDT D., ROZENGURT E. Y-27632, an inhibitor of Rho-associated kinases, prevents tyrosine phosphorylation of focal adhesion kinase and paxillin induced by bombesin: dissociation from tyrosine phosphorylation of p130(CAS) Exp. Cell. Res. 2001;266:292–302. doi: 10.1006/excr.2001.5219. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., MILSTIEN S. Sphingosine-1-phosphate: signalling inside and out. FEBS Lett. 2000;6:55–57. doi: 10.1016/s0014-5793(00)01670-7. [DOI] [PubMed] [Google Scholar]
- STEVENS P.A., BEVAN N., REES S., MILLIGAN G. Resolution of inverse agonist-induced up-regulation from constitutive activity of mutants of the alpha(1b)-adrenoceptor. Mol. Pharmacol. 2000;58:438–448. doi: 10.1124/mol.58.2.438. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI M., MUKAI H., TOSHIMORI M., MIYAMOTO M., ONO Y. Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc. Nat. Acad. Sci. 1998;95:11566–11571. doi: 10.1073/pnas.95.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BROCKLYN J.R., TU X., EDSALL L.C., SCHMIDT R.R., SPIEGEL S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J. Biol. Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- VAN SANDE J., SWILLENS S., GERARD C., ALLGEIER A., MASSART C., VASSART G., DUMONT J.E. In Chinese hamster ovary K1 cells dog and human thyrotropin receptors activate both the cyclic AMP and phosphatidylinositol 4,5-bisphosphate cascades in the presence of thyrotropin and cyclic AMP cascade in its absence. Eur. J. Biochem. 1995;229:338–343. [PubMed] [Google Scholar]
- VILARDAGA J.P., KRASEL C., CHAUVIN S., BAMBINO T., LOHSE M.J., NISSENSON R.A. Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J. Biol. Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- WEISS J.M., MORGAN P.H., LUTZ M.W., KENAKIN T.P. The cubic ternary complex receptor. Occupancy model. III. Resurrecting efficacy. J. Theor. Biol. 1996;181:381–397. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO S., KAWAMURA K., JAMES T.N. Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry. Microsc. Res. Tech. 1998;40:479–487. doi: 10.1002/(SICI)1097-0029(19980301)40:6<479::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., IGARASHI Y., YANG L., HISANO N., QI R., ASAZUMA N., SATOH K., OZAKI Y., KUME S.P. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]


