Abstract
The role of nigral tachykinin NK1, NK2 and NK3 receptors in central cardiovascular regulation was studied by measuring the effects of selective agonists and antagonists on mean arterial pressure (MAP) and heart rate (HR) after bilateral microinjection into the substantia nigra of spontaneously hypertensive rats (SHR). Quantitative in vitro autoradiography was also performed in the midbrain of SHR and Wistar-Kyoto (WKY) with the NK3 receptor ligand [125I]-HPP-Senktide.
Tachycardia was elicited by the NK1 ([Sar9,Met(O2)11]SP) and NK2 ([βAla8]NKA(4-10)) agonists at 25 and 100 pmol while the NK3 agonist (senktide, 50 and 100 pmol) had no significant effect. The three agonists had no effect on behaviour, and increases in MAP were elicited by the NK1 agonist only.
Whereas antagonists at NK1 (RP 67580, 500 pmol) and NK2 (SR 48968, 500 pmol) receptors had no significant effect on MAP and HR, the NK3 antagonist (R-820, 500 pmol) reduced MAP for over 3 h in SHR. That anti-hypertensive effect did not occur after intracerebroventricular or intravenous injection of R-820. Also, R-820 had no cardiovascular effect in WKY.
The affinity (KD: 0.7 nM) and densities of specific NK3 receptor binding sites measured in the substantia nigra, ventral tegmental area, hippocampus and amygdala were not significantly different in SHR and WKY.
It is concluded that endogenous tachykinins exert a tonic activity on NK3 receptors in the substantia nigra of SHR to maintain high blood pressure. Hence, nigral tachykinin NK3 receptors may represent a promising therapeutic target in the treatment of arterial hypertension.
Keywords: Tachykinins; NK1, NK2 and NK3 receptors; substantia nigra; central autonomic regulation; hypertension
Introduction
Compelling evidence suggests a role for tachykinin receptors in central autonomic cardiovascular regulation (Unger et al., 1988; Culman & Unger, 1995; Culman et al., 1997; Cellier et al., 1997; 1999) and in the modulation of the nigro-striatal dopaminergic system (Reid et al., 1990; Humpel et al., 1991; Humpel & Saria, 1993; Bannon & Whitty, 1995; Nalivaiko et al., 1997; Marco et al., 1998). Tachykinins are known to modulate nigro-striatal dopaminergic neurons by stimulating the release, turnover and metabolism of striatal dopamine via the activation of neurokinin-1 (NK1) and neurokinin-3 (NK3) receptors in the substantia nigra (SN) (Reid et al., 1990; Humpel et al., 1991; Humpel & Saria, 1993; Bannon & Whitty, 1995; Marco et al., 1998). Autoradiographic, immunohistochemical, in situ and/or solution hybridization and single cell reverse transcription-polymerase chain reaction techniques have shown the presence of substance P (SP) and neurokinin A (NKA) containing nerve terminals and tachykinin NK1 and NK3 receptors and/or their mRNA in the SN (Helke et al., 1990; Stoessl & Hill, 1990; Stoessl, 1994; Bannon & Whitty, 1995; Whitty et al., 1995; Shughrue et al., 1996; Futami et al., 1998; Chen et al., 1998; Ribeiro-Da-silva et al., 2000). Also, SP and NKA are released in vivo (Lindefors et al., 1989) and in vitro (Jessel, 1978; Humpel & Saria, 1989) in the SN from striato-nigral projecting neurons.
Lesion of the nigro-striatal dopaminergic pathway attenuates the development of hypertension in young spontaneously hypertensive rats (SHR) that points to a putative role for this neuronal pathway in the development of hypertension (Van den Buuse et al., 1991; Linthorst et al., 1994).
Previous studies have shown that central activation of tachykinin NK3 receptors by i.c.v. injection of selective agonists (senktide or [MePhe7]NKB) leads to increases of mean arterial pressure, heart rate and behavioural activity in conscious rats (Itoi et al., 1992; Picard et al., 1994; Cellier et al., 1997). Intravenous pre-treatment with the dopamine D2 receptor antagonist haloperidol blocked those cardiovascular and behavioural changes and unmasked a vasopressin-dependent bradycardia (Cellier & Couture, 1997). Although anatomical and physiological studies suggest an interaction between tachykinins and the nigro-striatal dopamine pathway in normotensive rats, there is no information regarding the implication of tachykinins in hypertension at the level of the SN.
With the purpose to follow up the recent pharmacological evidence suggesting that the SN is a potential site of modulation of cardiac autonomic activity by tachykinins in the normotensive rat (Lessard & Couture, 2001), the present study was undertaken to assess the cardiovascular effects of selective tachykinin agonists and antagonists bilaterally injected into the SN of SHR. The respective agonists and antagonists were: [Sar9,Met(O2)11]SP (Regoli et al., 1988) and RP 67580 (Garret et al., 1991) for NK1 receptors; [β-Ala8]NKA(4-10) (Rovero et al., 1989) and SR 48968 (Advenier et al., 1992; Emonds-Alt et al., 1992) for NK2 receptors; senktide (Wörmser et al., 1986) and R-820 (Regoli et al., 1994) or SR 142801 (Emonds-Alt et al., 1995) for NK3 receptors. To avoid the spurious effects of anaesthesia and the stress induced by immobilization, these studies were carried out in awake, unrestrained rats. Moreover, in vitro autoradiography was performed to evaluate the density and dissociation constant (KD) of NK3 receptor binding sites in the SN and other midbrain areas of SHR and normotensive control Wistar-Kyoto rats (WKY).
Methods
Animal source and care
Male SHR (15 weeks, n=88) and age-matched WKY (n=17) were purchased 3–5 days prior to experiments from Charles River, St-Constant, Québec, Canada and housed four to five per cage under a 12 h light–dark cycle in a room with controlled temperature (20°C), humidity (53%) with food (Charles River Rodent) and tap water available ad libitum. The care of animals and research protocols conformed to the guiding principles for animal experimentation as enunciated by the Canadian Council on Animal Care and approved by the Animal Care Committee of our University.
Animal preparation
Rats were anaesthetized with an intraperitoneal (i.p.) injection of 65 mg kg−1 sodium pentobarbitone (Somnotol; M.T.C. Pharmaceuticals, Cambridge, Ontario, Canada) and then positioned in a stereotaxic frame (David Kopf Instrumentation, Tujunga, CA, U.S.A.) with the incisor bar set at 3.3 mm below the interaural line. The skull was exposed, cleaned and a hole was drilled above each SN (coordinates: 5.3 mm posterior to the bregma, 2.0 mm lateral to the midline, 6.3 mm ventral from the skull surface for SHR and WKY; Paxinos & Watson, 1998). Two 23-gauge stainless steel guide cannulae targeted 2 mm dorsal to each SN were implanted and fixed with two screws and dental cement to the skull. An additional group of SHR was implanted with one 23-gauge stainless steel guide cannulae into the right lateral ventricle (coordinates: 1 mm posterior to the bregma, 1.4 mm lateral to the midline, 3.0 mm ventral from the skull surface, Paxinos & Watson, 1998). Stylets (31-gauge stainless steel) were inserted into the guide cannulae to avoid their obstruction and the loss of cerebrospinal fluid. Then, the skin was replaced and sutured. Animals were housed in individual plastic cages (40×23×20 cm) in the same controlled conditions and allowed to recover for 7 days. Then, the rats were re-anaesthetized with sodium pentobarbitone (65 mg kg−1, i.p.) and an intravascular siliconized (Sigmacote, Sigma-Aldrich Canada) polyethylene tubing PE-60 catheter (Intramedics, Clay Adams, NJ, U.S.A.), filled with physiological saline containing 100 i.u. ml−1 heparin sodium salt (Sigma-Aldrich Canada), was inserted into the abdominal aorta via the right femoral artery for direct blood pressure recording. The catheter was tunnelled subcutaneously to emerge at the back of the neck. Another group of SHR was implanted with an intravenous (i.v.) siliconized polyethylene tubing PE-10 catheter, filled with physiological saline containing 100 i.u. ml−1 heparin sodium salt, into the right jugular vein for i.v. injection. The catheter was tunnelled subcutaneously to emerge with the femoral catheter at the back of the neck. Before surgery, the animals received Ethacilin (5 mg kg−1, i.m., rogar/S.T.B. Inc., London, Ontario, Canada) and Ketoprophen (anafen, 10 mg kg−1, i.m., Merial Canada Inc., Baie d'Urfé, Québec, Canada). Recovery from anaesthesia was monitored closely under a warming lamp to maintain the body temperature of animals. Thereafter, rats were housed individually in polyethylene cages with a top grid and returned to their resident room. Rats with apparent abnormal behaviour (loss of >25% of body weight, anorexia, weaknesses) were immediately humanely killed with an overdose of pentobarbitone. Experimental protocols were initiated 48 h after the final intervention, in conscious and unrestrained rats.
Measurement of cardiovascular parameters
During all experiments, continuous direct recordings of blood pressure and heart rate were made respectively with a Statham pressure Transducer (P23ID) and a cardiac tachometer (model 7P4) (triggered by the arterial blood pressure pulse) coupled to a Grass polygraph (model 79; Grass Instruments Co., Quincy, MA, U.S.A.). Cardiovascular responses were measured 1 h after the rats were transported to an isolated and quiet testing-room, where only the experimenter had access. Rats remained in their resident cage but the top grid was removed and they had no more access to the food and water for the duration of the experiment, which lasted for a period of 3–6 h. When resting blood pressure and heart rate were stable, microinjections were made simultaneously into each SN of undisturbed, freely moving rats using two hand-held Hamilton microsyringes (5 μl, Fisher Scientific Ltd, Montréal, Québec, Canada) connected to 60 cm length polyethylene tubing PE-10. Five to 10 min prior to injection, two 31-gauge stainless steel injectors, pre-connected to the PE-10 tubing, were inserted into the guide cannulae without handling the rats. All solutions for microinjections were freshly prepared and injected (volume of 0.1 μl in each SN, 1 μl for i.c.v. and 1 ml kg−1 for i.v.) over a period of 1 min.
Measurement of behavioural parameters
Behavioural activity was measured as previously reported (Picard et al., 1994). Briefly, during every consecutive period of 15 s, a score of 1 or 0 was given systematically depending on whether the animal showed the specific type of behaviour or not, whatever its frequency, intensity or duration during that period. Summation of scores for the first 30 min period following SN injection gave the behavioural scores for face washing, grooming, sniffing and rearing. The maximal theoretical score was 120 (15 s intervals ×30 min). Wet dog shakes and locomotion (number of complete exploratory circles within the cage) behaviours were measured according to the number of episodes or frequency during the first 30 min period.
Histology
At the end of the experiments, the rats received 0.1 μl of Evans Blue dye (Sigma-Aldrich Canada) in each SN and they were immediately sacrificed with an overdose of sodium pentobarbitone. The brains were removed and fixed with 10% (v v−1) formol and 20% (w v−1) sucrose. Coronal sections (40 μm, cut on a freezing microtome) were mounted on glass slides and stained with cresyl violet for histological examination of the microinjection sites. Twenty-three out of 69 SHR (33%) implanted in the SN were rejected, either for evidence of cerebral haemorrhage (17 rats) or because the microinjection site was outside the accepted area (6 rats) (Figure 1). The latter six rats displayed no cardiovascular effect to agonists (n=2) or R-820 (n=4). Thus, numbers indicated in results represent only rats which were included in the study.
Figure 1.
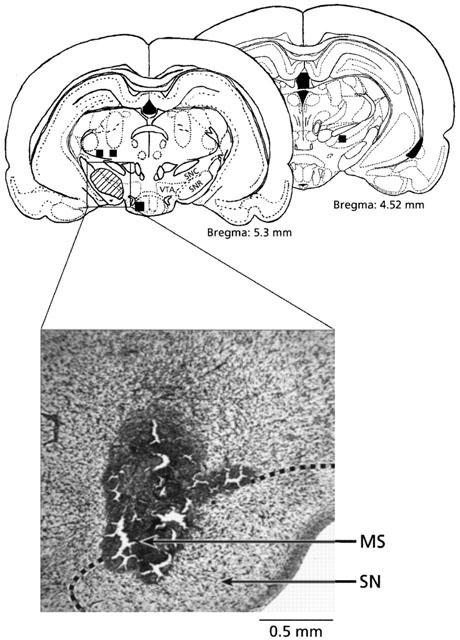
Identification of the microinjection sites in the substantia nigra (SN) following post-mortem histological examination of microinjected Evans Blue dye (0.1 μl bilaterally). The hatched zone represents accepted microinjection sites and the squares represent microinjection sites of R-820 (n=4) excluded from the results and kept as negative control for spread. A rat was considered successfully injected when both cannula tips were shown to be slightly above SN or within a distance of 0.5 mm of the SN (AP=5.3 mm posterior to the bregma) (Paxinos & Watson, 1998). Abbreviations: MS, microinjection site; SN, substantia nigra; SNC, SN pars compacta; SNR, SN pars reticulata; VTA, ventral tegmental area. Scale: 0.5 mm.
Experimental protocols
Experiment 1: cardiovascular effects of SN microinjection of selective tachykinin agonists
SHR (n=8) and WKY (n=3) received initially a microinjection of artificial cerebrospinal fluid (aCSF) into the SN followed 60 min later by the injection of 25 or 50 pmol of the selective NK1 agonist [Sar9,Met(O2)11]SP, the selective NK2 agonist [β-Ala8]NKA(4-10) and the selective NK3 agonist senktide at random. Additional SHR received only the NK2 and NK3 agonists (n=2) or only the NK3 agonist (n=3). The dose of 100 pmol of each agonist was given to four SHR at random and two additional rats received only NK2 and NK3 agonists. Only one dose of the same agonist was given to any animal. Agonists were injected at a minimum of 60 min intervals to enable blood pressure and heart rate to return to baseline values. Cardiovascular effects induced by agonists were not significantly different whatever their order of injection or if given alone or together to the same animal, suggesting the absence of cross desensitization between the three agonists. Each dose refers to the summation of doses given on each side of the SN. Doses of agonists were chosen on the basis of our previous study in Wistar rats (Lessard & Couture, 2001).
Experiment 2: cardiovascular effects of SN microinjection of selective tachykinin antagonists
In order to assess the contribution of endogenous tachykinins and their receptors, SHR were microinjected into the SN with one of the three antagonists (anti-NK1, RP 67580, 500 pmol, n=5; anti-NK2, SR 48968, 500 pmol, n=6; anti-NK3, R-820, 500 pmol, n=12 or SR 142801, 500 pmol, n=4). Each rat received only one antagonist, and doses were chosen on the basis of their ability to block in a selective and reversible manner the cardiovascular effects induced by the over-mentioned tachykinin agonists following their SN injection in Wistar rats (Lessard & Couture, 2001).
Since intranigral microinjection of RP 67580 and SR 48968 had no cardiovascular effect in SHR (present study) and Wistar rats (Lessard & Couture, 2001), while R-820 reduced blood pressure in SHR, WKY received an intranigral microinjection of R-820 (500 pmol, n=10) only. To control for the site of the anti-hypertensive action of R-820, two groups of SHR received R-820 into the right lateral ventricle (500 pmol, n=8) and the right jugular vein (500 pmol, n=6).
Tissue preparation for autoradiography
SHR (n=5) and WKY (n=4) used for autoradiography had previously received i.c.v. injection (500 pmol) of the NK1 (RP 67580) and the NK2 (SR 48968) antagonists which caused no cardiovascular effect in a parallel study. At least 2 days after the last i.c.v. injection, rats were sacrificed by an intra-arterial injection of an overdose of sodium pentobarbitone. Brains were immediately removed and frozen in 2-methyl butane cooled at −45–55°C with liquid nitrogen, and then stored at −80°C until use. Coronal sections (20 μm) were cut on a cryostat and fixed at a temperature between −11 and −13°C. Slices were thaw-mounted on 0.2% gelatine/0.033% chromium potassium sulphate coated glass slides and stored at −80°C.
Peptide iodination
Iodination of HPP-Senktide was performed according to the chloramine T method (Hunter & Greenwood, 1962). Briefly, 5 μg of peptide were incubated in 0.05 M phosphate buffer for 30 s in the presence of 0.5 mCi (18.5 MBq) of Na125I and 220 nmol of chloramine T in a total volume of 85 μl. The mono-iodinated peptide was then immediately purified by high pressure liquid chromatography on a C4 Vydac column (0.4×250 mm) (The Separations Group, Hesperia, CA, U.S.A.) with 0.1% trifluoroacetic acid and acetonitrile as mobile phases. The specific activity of the iodinated peptide was calculated as 2000 c.p.m. fmol−1 or 1212 Ci mmol−1.
In vitro receptor autoradiography
Sections were thawed at room temperature, pre-incubated for 30 s in 25 mM PIPES buffer (pH 7.4; 4°C) and incubated at room temperature for 90 min in 25 mM PIPES buffer (pH 7.4; 4°C) containing: 1,10-phenanthroline 1 mM, dithiothreitol 1 mM, bacitracin 0.014%, captopril 0.1 mM, BSA 0.2% (protease free) and magnesium chloride 7.5 mM in the presence of 0.7–1.7 nM of [125I]-HPP-Senktide in order to construct a saturation curve. The non-specific binding was determined in the presence of 1 μM of unlabelled HPP-Senktide or senktide. At the end of the incubation period, slides were transferred sequentially through four rinses of 4 min each in 25 mM PIPES (pH 7.4, 4°C) and dipped for 15 s in distilled water (4°C) to remove the excess of salts and air-dried. [3H]-Hyperfilm was juxtaposed onto the slides in the presence of [125I]-microscales and exposed at room temperature for 24 h. The films were developed in D-19 (Kodak developer) and fixed in Kodak Ektaflo. Autoradiograms were quantified by densitometry using an image analysis system (MCIDTM, Imaging Research Inc., Ontario, Canada). Standard curves from [125I]-microscales were used to convert density levels into fentomoles per milligram of tissue (fmol mg−1 tissue). Specific binding was determined by subtracting non-specific labelling from total binding taken from adjacent sections. Quantification of total and non-specific binding was made on 200 and 160 tissue sections in 5 SHR and 4 WKY, respectively that correspond to eight tissue sections per rat for each of the five concentrations of the radioligand. Anatomical parameters and nomenclature were determined according to Paxinos & Watson (1998).
[125I]-HPP-Senktide was chosen instead of [3H]-Senktide used in previous autoradiographic studies (Dam et al., 1990; Stoessl & Hill, 1990; Stoessl, 1994; Langlois et al., 2001) because the iodinated ligand has the advantage to display greater specific activity (1212 Ci mmol−1) over the tritiated ligand (50–63.5 Ci mmol−1). Hence, the time of tissue exposure to the [3H]-Hyperfilm can be reduced from 8–10 weeks with [3H]-Senktide to 1 day with [125I]-HPP-Senktide without affecting the selectivity of the agonist for the NK3 receptor.
Chemicals and materials
The composition of aCSF was, in mM: NaCl 128.6, KCl 2.6, MgCl2 2.0 and CaCl2 1.4; pH adjusted to 7.2. [Sar9,Met(O2)11]SP was obtained from Peninsula Lab. Inc. (San Carlos, CA, U.S.A.), while [β-Ala8]NKA (4-10) and senktide were purchased from Bachem Bioscience Inc. (King of Prussia, PA, U.S.A.). The non-peptide antagonists RP 67580 (racemic form of 7,7-diphenyl-2[1-imino-2(2-methoxy-phenyl)-ethyl]perhydroisoindol-4-one (3aR, 7aR), SR 48968 ((S)-N-methyl -N- [4- (4 - acetylamino - 4-phenylpiperidino) -2- (3,4- dichlorophenyl)-butyl]benzamide) and SR 142801 ((S)-(N)-(1-(3- (1-benzoyl-3- (3,4-dichlorophenyl)piperidin-3-yl)propyl) - 4-phenylpiperidin-4-yl)-N-methylacetamide) were provided by Dr C. Garret (Rhone Poulenc, Paris, France) and X. Emonds-Alt (Sanofi Recherche, Montpellier, France), respectively. The antagonist R-820 (3-Indolyl-carbonyl-Hyp-Phg-N(Me)-Bzl) was generously provided by Dr J.L. Fauchère (Servier, Paris, France). [Sar9,Met(O2)11]SP was solubilized in aCSF while senktide, [β-Ala8]NKA (4-10) and all antagonists were solubilized in 1–15% (v v−1) DMSO (Fisher Scientific, Montréal, Québec, Canada). The solution was then completed with aCSF (i.c.v. and SN) or saline (i.v. injection) which contained 20% of 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich Canada). Stock solutions (10 mg ml−1) of agonists and antagonists were stored in aliquots of 100 μl at −20°C until use. In all experiments, vehicle (aCSF containing 10% DMSO) was injected as control and had no significant effect on any parameters when compared to baseline values. HPP-Senktide (3-4 hydroxyphenyl-propionyl-Asp-Asp-Phe-N-MePhe-Gly-Leu-Met-NH2 (MW:1006.5) is derived from the selective NK3 receptor agonist senktide (Wörmser et al., 1986). It was synthesized by W. Neugebauer (Department of Pharmacology, Université de Sherbrooke, Canada). Autoradiographic [125I]-microscales (20 μm) and [3H]-Hyperfilm (single-coated, 24×30 cm) were purchased from Amersham Pharmacia Biotech Canada. Piperazine-N,N′-bis[2-ethanesulphonic-acid] (PIPES), 1,10-phenanthroline, dithiothreitol, bacitracin, captopril and bovine serum albumin (BSA) (protease free) were purchased from Sigma-Aldrich Canada.
Statistical analysis of data
Results are expressed as means±s.e.mean of (n) rats. Results were analysed for statistical significance using a two-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni confidence intervals. Statistical analysis for specific binding sites was performed with a Student's t-test for unpaired samples. Only probability values (P) less than 0.05 were considered to be statistically significant.
Results
Cardiovascular response induced by the NK1 agonist [Sar9,Met(O2)11]SP
The effects on MAP and HR of two doses of [Sar9,Met(O2)11]SP in SHR are shown in Figures 2A, 3A and 4B. [Sar9,Met(O2)11]SP (25 pmol and 100 pmol) evoked increases in HR and MAP which were significant (P<0.05) when compared to vehicle (aCSF) values (n=8). Thus, the tachycardia was significant at 25 pmol (5–45 min, n=8) and 100 pmol (5–15 min, n=4). The maximal rise in HR was 71±24 b.p.m. (25 pmol, 30 min) and 43±21 b.p.m. (100 pmol, 15 min). [Sar9,Met(O2)11]SP produced an increase in MAP at 25 pmol (7–45 min, n=8) and 100 pmol (5–45 min, n=4) in SHR. The maximal rise in MAP was 13±6 mmHg (25 pmol, 30 min) and 11±7 mmHg (100 pmol, 15 min). There was no significant effect on behaviour. Baseline HR and MAP values were 293±11 b.p.m. and 160±7 mmHg, respectively.
Figure 2.
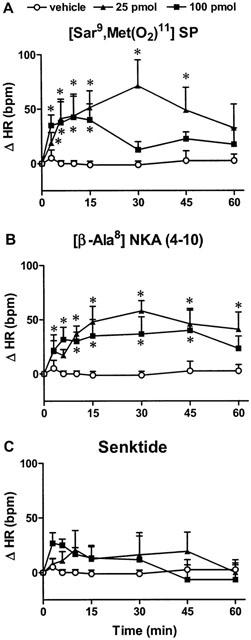
Changes in heart rate (Δ HR) induced by SN microinjection of the vehicle (aCSF, n=8–13) or (A) the NK1 agonist [Sar9,Met(O2)11]SP (25 pmol (n=8), 100 pmol (n=4)); (B) the NK2 agonist [β-Ala8]NKA (4-10) (25 pmol (n=10), 100 pmol (n=6)) and (C) the NK3 agonist senktide (50 pmol (n=13), 100 pmol (n=6)) in SHR. Each point represents the mean±s.e.mean of (n) rats. Comparison to vehicle values is indicated by *P<0.05.
Figure 3.
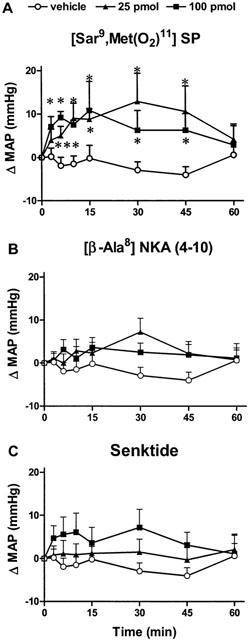
Legend as Figure 2 for changes in mean arterial pressure (Δ MAP) in the same animals.
Figure 4.
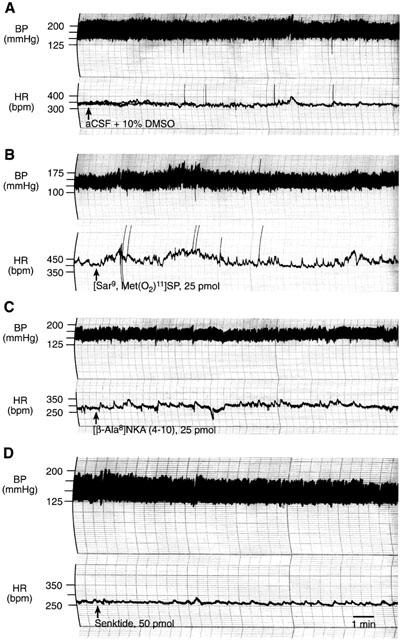
Original traces showing changes in heart rate (HR) and blood pressure (BP) induced by SN microinjection of (A) vehicle (aCSF with 10% DMSO), (B) the NK1 agonist [Sar9,Met(O2)11]SP (25 pmol), (C) the NK2 agonist [β-Ala8]NKA (4-10) (25 pmol) and (D) the NK3 agonist senktide (50 pmol) in SHR.
Cardiovascular response induced by the NK2 agonist[β-Ala8]NKA (4-10)
The effects on MAP and HR of two doses of [β-Ala8]NKA (4-10) in SHR are shown in Figures 2B, 3B and 4C. [β-Ala8]NKA (4-10) evoked a significant tachycardia (P<0.05) which was similar at 25 pmol (10–60 min, n=10) and 100 pmol (7–45 min, n=6) when compared to vehicle (aCSF with 7% DMSO) values (n=10). The maximal rise in HR was 58±10 b.p.m. (25 pmol, 30 min) and 40±19 b.p.m. (100 pmol, 45 min). [β-Ala8]NKA (4-10) (25 and 100 pmol) had no significant effect on MAP when compared to vehicle values. There was no significant effect on behaviour. Baseline HR and MAP values were 323±31 b.p.m. and 161±9 mmHg, respectively.
Cardiovascular response induced by the NK3 agonist senktide
The effects on MAP and HR of two doses of senktide in SHR are shown in Figures 2C, 3C and 4D. Senktide at the dose of 50 pmol (n=13) and 100 pmol (n=6) had no significant effect on MAP and HR when compared to vehicle values (n=13). There was no significant effect on behaviour. Baseline HR and MAP values were 338±25 b.p.m. and 160±10 mmHg, respectively.
Effect of tachykinin agonists in WKY
In WKY (n=3), the NK1 [Sar9,Met(O2)11]SP, 25 pmol), NK2 ([β-Ala8]NKA (4-10), 25 pmol) and NK3 (senktide, 50 pmol) receptor agonists evoked increases in HR which were similar in amplitude and duration to the tachycardia measured in Wistar rats (Lessard & Couture, 2001). As observed in Wistar rats, 25 pmol of these agonists failed to alter MAP and behaviour in WKY (data not shown).
Effects of tachykinin antagonists in SHR and WKY
The effects on MAP of the NK1 receptor antagonist RP 67580 (500 pmol, n=5) and the NK2 receptor antagonist SR 48968 (500 pmol, n=6) in SHR are shown in Figure 5A. Neither of the two antagonists caused significant changes in MAP compared to vehicle values (aCSF with 10% DMSO, n=6). Both antagonists also failed to alter HR (data not shown). Moreover, SN microinjection of the NK3 antagonist SR 142801 (500 pmol, n=4) was devoid of cardiovascular effect in this study (data not shown).
Figure 5.
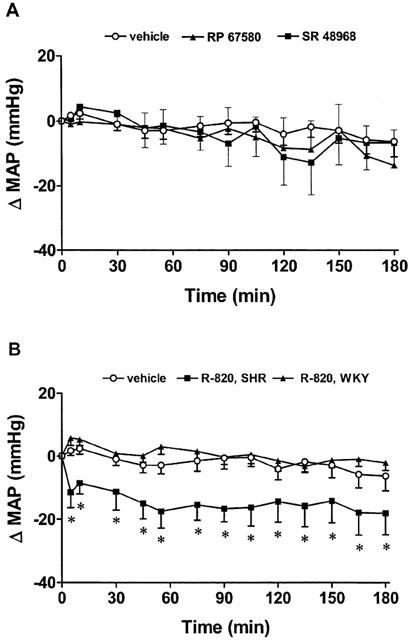
Time-course effects on changes in mean arterial pressure (Δ MAP) induced by SN microinjection of (A) vehicle (aCSF with 10% DMSO, n=6), RP 67580 (500 pmol, n=5) and SR 48968 (500 pmol, n=6) in SHR, and (B) vehicle (aCSF with 10% DMSO, n=12) and R-820 (500 pmol) in SHR (n=12) and WKY (n=10). Each point represents the mean±s.e.mean of (n) rats. Comparison to vehicle values is indicated by *P<0.05.
The effects on MAP of the NK3 receptor antagonist R-820 (500 pmol) microinjected into the SN of SHR (n=12) and WKY (n=10) are shown in Figure 5B. Whereas R-820 failed to alter MAP when compared to vehicle values (aCSF with 10% DMSO, n=12) in WKY, it reduced significantly (P<0.05, 10 min–3 h) MAP in SHR for a period that lasted over 3 h post-injection when compared to vehicle values (n=12). The maximal fall in MAP was −18±5 mmHg at 55 min. However, R-280 did not modify HR in both WKY and SHR (data not shown). Contrarily to SN microinjection, i.c.v. (500 pmol, n=8) or intravenous (500 pmol, n=6) injection of R-820 failed to elicit significant changes in MAP from time 0 to 3 h post-injection when compared to vehicle values (data not shown).
All tachykinin receptor antagonists tested in this study were devoid of behavioural activity in SHR and WKY. Four rats that had injection sites beside the SN after histological post-mortem examination failed to evoke any significant changes in MAP with 500 pmol R-820 (Figure 1).
Midbrain NK3 receptor binding sites in SHR and WKY
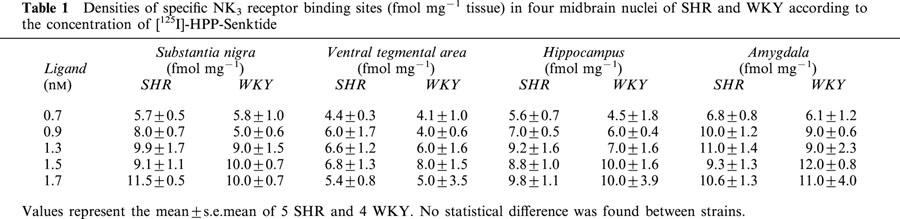
Densities of total, non-specific and specific NK3 receptor binding sites in the SN of SHR according to the concentration of [125I]-HPP-Senktide are shown in Figure 6. Non-specific binding defined in the presence of 1 μM unlabelled senktide accounted for approximately 30% of total binding at ligand concentration of 1.3 nM. In all midbrain areas, only small amounts of specific NK3 receptor binding sites were detected (Figure 7, Table 1). Specific binding with [125I]-HPP-Senktide in SN displayed a Bmax which was not significantly different in SHR (11.5 fmol mg−1 of tissue) and WKY (10 fmol mg−1 tissue). Likewise, no difference was seen between SHR and WKY regarding densities of specific NK3 receptor binding sites in the ventral tegmental area, the hippocampus and the amygdala whatever the concentration of the radioligand (Table 1). Furthermore, the affinity of the binding as defined by the dissociation constant (KD) was identical in all these regions (0.7 nM) from both SHR and WKY.
Figure 6.
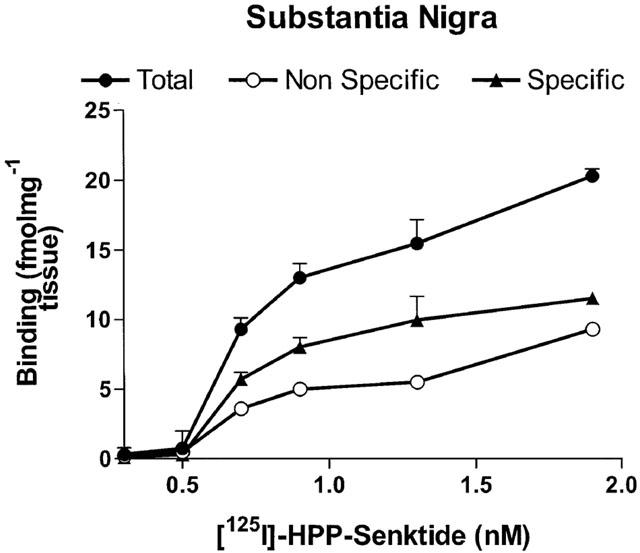
Total, non-specific and specific binding (fmol mg−1 tissue) of [125I]-HPP-Senktide as a function of its concentration in the substantia nigra of SHR. Non-specific binding was measured with the addition of 1 μM HPP-Senktide. Quantification was performed on 40 sections (4 sections per animal×5 SHR) for each concentration (×5 concentrations) corresponding to 200 sections for total and non-specific binding.
Figure 7.
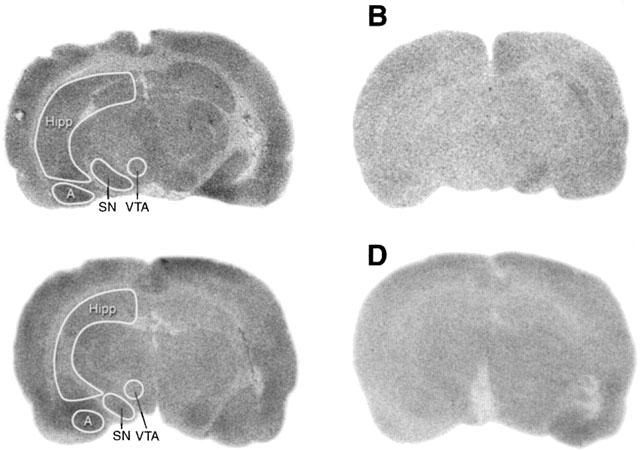
Autoradiographic distribution of [125I]-HPP-Senktide binding sites in the midbrain of SHR (A,B) and WKY (C,D). Total binding measured with 0.7 nM [125I]-HPP-Senktide is shown in panels A and C while the non-specific binding in the presence of 1 μM HPP-Senktide is shown in panels B and D. The areas under study are delimited by a white line. Abbreviations: SN, substantia nigra; VTA, ventral tegmental area; Hipp, hippocampus; A, amygdala.
Table 1.
Densities of specific NK3 receptor binding sites (fmol mg−1 tissue) in four midbrain nuclei of SHR and WKY according to the concentration of [125I]-HPP-Senktide
Discussion
The profile of the cardiovascular responses elicited by bilateral microinjection of low doses (pmol range) of selective tachykinin NK1 and NK3 receptor agonists into the SN of SHR is quite different from that obtained in normotensive rats using the same approach (Lessard & Couture, 2001). While a tachycardia occurred without changes in MAP with the NK1 agonist in normotensive Wistar rats (Lessard & Couture, 2001) and WKY, same doses of agonist increased both heart rate and MAP in SHR. These results are consistent with a previous report showing a 3 fold increase in pressor effect and a marked heart rate increase following i.c.v. injection of substance P in SHR when compared with age-matched WKY (Unger et al., 1980). While the NK3 agonist evoked a marked heart rate increase in the absence of blood pressure changes in Wistar rats (Lessard & Couture, 2001) and WKY, this agonist was found inactive on both cardiovascular parameters in SHR. However, the NK2 agonist elicited a similar tachycardia in Wistar, WKY and SHR with no effect on blood pressure.
In previous experiments, the tachycardiac responses to NK2 and NK3 agonists were abolished in the presence of atenolol whereas a treatment with atenolol and atropine was required to block the tachycardiac response of the NK1 agonist (Lessard & Couture, 2001). It was concluded that the three tachykinin receptors increase the sympatho/adrenal drive to the heart and that the NK1 agonist can additionally inhibit cardiovagal activity. Therefore, it is likely that the pressor effect of the NK1 agonist in SHR derived from the well documented enhanced activity and reactivity of the sympathetic nervous system in SHR (de champlain, 1998). Additionally, the inhibition of vagal activity by the NK1 agonist might be altered in SHR. Indeed, the greater sympathetic drive in SHR is associated with a reduced vagal activity and baroreceptor reflex (Julius, 1988; Korner, 1989; de champlain, 1998; Grassi et al., 1998).
A quantitative in vitro autoradiography was performed to address possible changes in affinity or number of NK3 receptors into the midbrain of SHR. This was tested as a putative mechanism to account for the loss of senktide-induced tachycardia in SHR. Data reveal that modest densities of NK3 receptor binding sites are similarly distributed in SN, amygdala, hippocampus and ventral tegmental area in SHR and WKY. This is consistent with autoradiographic and in situ hybridization studies regarding the distribution of NK3 receptor binding sites and mRNA in the midbrain of normotensive rats (Shughrue et al., 1996; Langlois et al., 2001). These studies revealed moderate density of NK3 receptor binding sites in the rat midbrain. Bmax values measured in the SN with [125I]-HPP-Senktide (11.5 and 10 fmol mg−1 tissue in SHR and WKY, respectively) are in the same range than the value previously reported with [3H]-Senktide (16±3 fmol mg−1 tissue) (Langlois et al., 2001). This suggests that both radioligands yield similar results, yet [125I]-HPP-Senktide has the advantage to require only 24 h of exposure to the film contrary to 8–10 weeks needed for [3H]-Senktide which has a much lower specific activity. Moreover, [125I]-HPP-Senktide bound with higher affinity (KD=0.7 nM) to midbrain areas than [3H]-Senktide (KD=2.3–2.8 nM) to rat brain cortex and striatum (Dam et al., 1990; Stoessl & Hill, 1990). Because both the dissociation constant (KD values) and densities of receptors in the four analysed midbrain areas were not significantly different between SHR and WKY, it is concluded that the reduced cardiovascular effect following microinjection of the NK3 agonist in SHR is unlikely to be attributable to the loss of NK3 receptors or to changes in affinity.
In SHR, the NK3 antagonist R-820 reduced MAP for over 3 h when microinjected into the SN while, at the same dose, NK1 (RP 67580) and NK2 (SR 48968) antagonists were devoid of any cardiovascular effect. The latter receptor antagonists blocked in a selective and reversible manner the cardiovascular responses induced by i.c.v. or SN injection of the selective tachykinin agonists mentioned above in Wistar rats (Picard et al., 1994; Cellier et al., 1999; Lessard & Couture, 2001). Likewise, R-820 inhibited the cardiovascular, antidiuretic and antinatriuretic effects induced by i.c.v. and/or nigral injection of senktide or [MePhe7]NKB (Cellier et al., 1997; Yuan & Couture, 1997; Lessard & Couture, 2001), and the thermo-hypoalgesia induced by the intrathecal injection of the two NK3 agonists in the rat tail-flick test (Couture et al., 2000).
In previous studies, we showed that the high affinity human NK3 receptor antagonist SR 142801 behaves as a tachykinin NK3 receptor agonist (blocked by R-820) when injected intracerebroventricularly or intrathecally in conscious rats (Cellier et al., 1997; Couture et al., 2000). When injected into the SN, SR 142801 did not reproduce the fall in MAP observed with R-820 but was inactive as the agonist senktide. This was expected since this SR compound displays a low affinity at rat NK3 receptors (Emonds-Alt et al., 1995).
In the present study, rats which showed injection sites beside the SN failed to evoke any significant changes in MAP to agonists or R-820. Therefore, the described cardiovascular effects are unlikely to be due to the diffusion of the injection outside the SN. This possibility is also unlikely in this study because the volume (0.1 μl) of injection is 5–10 fold smaller than the volume (0.5 and 1 μl) generally employed in this nucleus (Humpel et al., 1991; Humpel & Saria, 1993; Stoessl et al., 1995). Furthermore, the cardiovascular responses to agonists are unrelated to changes of behavioural activity which occurred at doses higher than 1 nmol (Stoessl et al., 1995; Lessard & Couture, 2001).
A role for nigral tachykinin NK3 receptors in the tonic control of blood pressure in SHR
Since the three antagonists did not affect resting blood pressure and heart rate upon their central administration (i.c.v. and SN) in Wistar or WKY, it appears that endogenous tachykinins do not play a primary role in the tonic control of blood pressure and heart rate in the brain of normotensive animals. However, data highlight a potential role for tachykinins in the maintenance of hypertension in SHR by activating tonically NK3 receptors in the SN. Thus, the occupancy of NK3 receptors by an over-production of endogenous tachykinins may account for the lack of effect with senktide.
The distinctive effects of NK1 and NK3 receptor agonists could be related to the activation of different neuronal pathways from the SN. Indeed, in situ and/or solution hybridization, autoradiographic and immunocytochemical studies have shown that NK3 receptors are located mainly on dopaminergic neurons in the substantia nigra pars compacta (Stoessl & Hill, 1990; Stoessl, 1994; Bannon & Whitty, 1995; Whitty et al., 1995; Shughrue et al., 1996; Chen et al., 1998) while NK1 receptors are mostly located on GABAergic neurons of the substantia nigra pars reticulata (Sivam & Krause, 1992; Stoessl, 1994; Bannon & Whitty, 1995). Electrophysiological and microdialysis studies reveal that senktide activates mostly dopaminergic neurons of the SN pars compacta that leads to an increase of extracellular dopamine concentration in the striatum (Nalivaiko et al., 1997; Marco et al., 1998). The activation of that dopaminergic pathway by senktide was blocked by selective NK3 antagonists. Interestingly, an autoradiographic study suggests an up-regulation of dopamine D1 and D2 receptors in the caudate-putamen of SHR (Kirouac & Ganguly, 1993). It is tempting to suggest that the tonic activation of nigral NK3 receptors by endogenous tachykinins is facilitated by a hyperactive dopaminergic nigro-striatal system in SHR. This neuronal pathway could be involved in the maintenance of hypertension in SHR.
Although the SN has been traditionally associated with the central control of motor activity, evidence suggests that this midbrain nucleus exerts a role in central cardiovascular regulation (Barbeau et al., 1969; Micieli et al., 1987; Van den Buuse et al., 1991; Lin & Yang, 1994; Linthorst et al., 1994). Electrical or chemical stimulation of the SN leads to increases in blood pressure, heart rate and striatal dopamine levels in anaesthetized rats (Lin & Yang, 1994). On the other hand, orthostatic and postprandial hypotension have been reported in patients with Parkinson's disease who are known to suffer from a degeneration of the nigro-striatal dopaminergic pathway (Barbeau et al., 1969; Micieli et al., 1987). Also, lesion of the nigro-striatal dopaminergic pathway attenuates the development of hypertension in young SHR (Van den Buuse et al., 1991; Linthorst et al., 1994). In the present study, an attenuation of the arterial hypertension has been observed in SHR under blockade of nigral tachykinin NK3 receptors.
Conclusion
With the use of highly selective tachykinin receptor agonists, it was evidenced that the modulation of cardiac activity by NK3 receptor activation in the SN is markedly altered in SHR which is not the case for NK2 receptors. This is unlikely to be the consequence of changes in receptor density and affinity. Although significant pressor responses were evoked by activation of NK1 receptors, the latter do not appear to be tonically active in SHR contrary to NK3 receptors. Hence, this study using selective antagonists provides the first pharmacological evidence suggesting a pathophysiological role for tachykinin NK3 receptors in the maintenance of hypertension at the level of the substantia nigra.
Acknowledgments
The authors acknowledge Dr C. Garet (Rhone Poulenc, France), Dr X. Emonds-Alt (Sanofi Recherche, France) and Dr J.L. Fauchère (Institut de recherches Servier, France) for the donation of RP 67580, SR 48968/SR 142801 and R-820, respectively. The authors are also grateful to Dr Jean-Guy Chabot (Douglas Hospital Research Center, Montreal) for his invaluable help and advices in autoradiography, and to Mr Claude Gauthier for the Artwork. A. Lessard holds Studentships from the Heart and Stroke Foundation of Canada and the FRSQ-FCAR program. M.M. Campos was awarded a post-doctoral Fellowship from the GRSNA at Université de Montréal. This work was supported by a grant from the Canadian Institutes of Health Research (MT-14379).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- HR
heart rate
- i.c.v.
intracerebroventricular
- i.v.
intravenous
- MAP
mean arterial blood pressure
- NKA
neurokinin A
- SHR
spontaneously hypertensive rats
- SN
substantia nigra
- SP
substance P
- WKY
Wistar-Kyoto
References
- ADVENIER C., ROUISSI N., NGUYEN Q.T., EMONDS-ALT X., BRELIÈRE J.-C., NELIAT G., NALINE E., REGOLI D. Neurokinin A (NK2) receptor revisited with SR 48968, a potent non peptide antagonist. Biochem. Biophys. Res. Commun. 1992;184:1418–1424. doi: 10.1016/s0006-291x(05)80041-5. [DOI] [PubMed] [Google Scholar]
- BANNON M.J., WHITTY C.J. Neurokinin receptor gene expression in substantia nigra: localization, regulation, and potential physiological significance. Can. J. Physiol. Pharmacol. 1995;73:866–870. doi: 10.1139/y95-119. [DOI] [PubMed] [Google Scholar]
- BARBEAU A., GILLO-JOFFROY L., BOUCHER R., NOWACZYNSKI W., GENEST J. Renin-aldosterone system in Parkinson's disease. Science. 1969;165:291–292. doi: 10.1126/science.165.3890.291. [DOI] [PubMed] [Google Scholar]
- CELLIER E., BARBOT L., IYENGAR S., COUTURE R. Characterization of central and peripheral effects of septide with the use of five tachykinin NK1 receptor antagonists in the rat. Br. J. Pharmacol. 1999;127:717–728. doi: 10.1038/sj.bjp.0702620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELLIER E., BARBOT L., REGOLI D., COUTURE R. Cardiovascular and behavioural effects of intracerebroventricularly administered tachykinin NK3 receptor antagonists in the conscious rat. Br. J. Pharmacol. 1997;122:643–654. doi: 10.1038/sj.bjp.0701435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELLIER E., COUTURE R.Mechanism of cardiovascular and behavioural responses to central NK-3 receptor activation in conscious rat Tachykinins in Health and Disease, International Tachykinin Conference, Cairns (Australia), September 7–11 199740Abstract bookp
- CHEN L.-W., GUAN Z.-L., DING Y.-Q. Mesencephalic dopaminergic neurons expressing neuromedin K receptor (NK3): a double immunocytochemical study in the rat. Brain Res. 1998;780:150–154. [PubMed] [Google Scholar]
- COUTURE R., TOMA N., BARBOT L. SR142801 behaves as a tachykinin NK-3 receptor agonist on a spinal nociceptive reflex in the rat. Life Sci. 2000;66:51–65. doi: 10.1016/s0024-3205(99)00561-5. [DOI] [PubMed] [Google Scholar]
- CULMAN J., KLEE S., OHLENDORF C., UNGER Th. Effect of tachykinin receptor inhibition in the brain on cardiovascular and behavioral responses to stress. J. Pharmacol. Exp. Ther. 1997;280:238–246. [PubMed] [Google Scholar]
- CULMAN J., UNGER Th. Central tachykinins: mediators of defence reaction and stress reactions. Can. J. Physiol. Pharmacol. 1995;73:885–891. doi: 10.1139/y95-122. [DOI] [PubMed] [Google Scholar]
- DAM T.-V., ESCHER E., QUIRION R. Visualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H]senktide. Brain Res. 1990;506:175–179. doi: 10.1016/0006-8993(90)91218-6. [DOI] [PubMed] [Google Scholar]
- DE CHAMPLAIN J. Participation du système nerveux autonome dans l'hypertension artérielle. Médecine Sciences. 1998;14 Suppl. 2:10–22. [Google Scholar]
- EMONDS-ALT X., BICHON D., DUCOUX J.-P., HEAULME M., MILOUX B., PONCELET M., PROIETTO V., VAN BROECK D., VILAIN P., NELIAT G., SOUBRIÉ P., LE FUR G., BRELIÈRE J.-C. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56:PL27–PL32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., VILAIN P., GOULAOUIC P., PROIETTO V., VAN BROECK D., ADVENIER C., NALINE E., NELIAT G., LE FUR G., BRELIÈRE J.-C. A potent and selective non peptide antagonist of the neurokinin A (NK-2) receptor. Life Sci. 1992;50:PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- FUTAMI T., HATANAKA Y., MATSUSHITA K., FURUYA S. Expression of substance P receptor in the substantia nigra. Mol. Brain Res. 1998;54:183–198. doi: 10.1016/s0169-328x(97)00307-0. [DOI] [PubMed] [Google Scholar]
- GARRET C., CARRUETTE A., FARDIN V., MOUSSAOUI S., PEYRONEL J.-F., BLANCHARD J.-C., LADURON P.M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRASSI G., CATTANEO B.M., SERAVALLE G., LANFRANCHI A., MANCIA G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- HELKE C.J., KRAUSE J.E., MANTYH P.W., COUTURE R., BANNON M.J. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990;4:1606–1615. [PubMed] [Google Scholar]
- HUMPEL C., SARIA A. Effects of GABA and L-glutamic acid on the potassium-evoked in vitro release of substance P and neurokinin A-like immunoreactivities are different in the rat striatum and substantia nigra. Neurosci. Lett. 1989;105:159–163. doi: 10.1016/0304-3940(89)90029-3. [DOI] [PubMed] [Google Scholar]
- HUMPEL C., SARIA A. Intranigral injection of selective neurokinin-1 and neurokinin-3 but not neurokinin-2 receptor agonists biphasically modulate striatal dopamine metabolism but not striatal preprotachykinin-A mRNA in the rat. Neurosci. Lett. 1993;157:223–226. doi: 10.1016/0304-3940(93)90742-4. [DOI] [PubMed] [Google Scholar]
- HUMPEL C., SARIA A., REGOLI D. Injection of tachykinins and selective neurokinin receptor ligands into the substantia nigra reticulata increases striatal dopamine and 5-hydroxytryptamine metabolism. Eur. J. Pharmacol. 1991;195:107–114. doi: 10.1016/0014-2999(91)90387-6. [DOI] [PubMed] [Google Scholar]
- HUNTER W.M., GREENWOOD F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- ITOI K., TSCHÖPE C., JOST N., CULMAN J., LEBRUN C., STAUSS B., UNGER T.H.. Identification of the central tachykinin receptor subclass involved in substance P-induced cardiovascular and behavioural responses in conscious rats. Eur. J. Pharmacol. 1992;219:435–444. doi: 10.1016/0014-2999(92)90485-m. [DOI] [PubMed] [Google Scholar]
- JESSEL T.M. Substance P release from the rat substantia nigra. Brain Res. 1978;151:469–478. doi: 10.1016/0006-8993(78)91080-6. [DOI] [PubMed] [Google Scholar]
- JULIUS S. The blood pressure seeking properties of the central nervous system. J. Hypert. 1988;6:177–185. doi: 10.1097/00004872-198803000-00001. [DOI] [PubMed] [Google Scholar]
- KIROUAC G.J., GANGULY P.K. Up-regulation of dopamine receptors in the brain of the spontaneously hypertensive rat: an autoradiographic analysis. Neuroscience. 1993;52:135–141. doi: 10.1016/0306-4522(93)90188-l. [DOI] [PubMed] [Google Scholar]
- KORNER P.I. Baroreceptor resetting and other determinants of baroreflex properties in hypertension. Clin. Exp. Pharmacol. Physiol. 1989;15 Suppl:45–64. doi: 10.1111/j.1440-1681.1989.tb02995.x. [DOI] [PubMed] [Google Scholar]
- LANGLOIS X., WINTMOLDERS C., RIELE P., LEYSEN J.E., JURZAK M. Detailed distribution of neurokinin 3 receptors in the rat, guinea pig and gerbil brain: a comparative autoradiographic study. Neuropharmacol. 2001;40:242–253. doi: 10.1016/s0028-3908(00)00149-0. [DOI] [PubMed] [Google Scholar]
- LESSARD A., COUTURE R. Modulation of cardiac activity by tachykinins in the rat substantia nigra. Br. J. Pharmacol. 2001;134:1749–1759. doi: 10.1038/sj.bjp.0704401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN M.-T., YANG J.-J. Stimulation of the nigrostriatal dopamine system produces hypertension and tachycardia in rats. Am. J. Physiol. (Heart Circ. Physiol. 35) 1994;266:H2489–H2496. doi: 10.1152/ajpheart.1994.266.6.H2489. [DOI] [PubMed] [Google Scholar]
- LINDEFORS N., BRODIN E., TOSSMAN U., SEGOVIA J., UNGERSTEDT U. Tissue levels and in vivo release of tachykinins and GABA in striatum and substantia nigra of rat brain after unilateral striatal dopamine denervation. Expl. Brain Res. 1989;74:527–534. doi: 10.1007/BF00247354. [DOI] [PubMed] [Google Scholar]
- LINTHORST A.C.E., VAN GIERSBERGEN P.L.M., GRAS M., VERSTEEG D.H.G., DE JONG W. The nigrostriatal dopamine system: role in the development of hypertension in spontaneously hypertensive rats. Brain Res. 1994;639:261–268. doi: 10.1016/0006-8993(94)91739-6. [DOI] [PubMed] [Google Scholar]
- MARCO N., THIRION A., MONS G., BOUGAULT I., LE FUR G., SOUBRIÉ P., STEINBERG R. Activation of dopaminergic and cholinergic neurotransmission by tachykinin NK3 receptor stimulation: an in vivo microdialysis approach in guinea pig. Neuropeptides. 1998;32:481–488. doi: 10.1016/s0143-4179(98)90075-0. [DOI] [PubMed] [Google Scholar]
- MICIELI G., MARTIGNONI E., CAVALLINI A., SANDRINI G., NAPPI G. Postprandial and orthostatis hypotension in Parkinson's disease. Neurology. 1987;37:386–393. doi: 10.1212/wnl.37.3.386. [DOI] [PubMed] [Google Scholar]
- NALIVAIKO E., MICHAUD J.-C., SOUBRIÉ P., LE FUR G., FELTZ P. Tachykinin neurokinin-1 and neurokinin-3 receptor-mediated responses in guinea-pig substantia nigra: an in vitro electrophysiological study. Neuroscience. 1997;78:745–757. doi: 10.1016/s0306-4522(96)00625-2. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The Rat Brain in Stereotaxic Coordinates 1998California, USA: Academic Press, San Diego; 4th edn [Google Scholar]
- PICARD P., REGOLI D., COUTURE R. Cardiovascular and behavioural effects of centrally administered tachykinins in the rat: characterization of receptors with selective antagonists. Br. J. Pharmacol. 1994;112:240–249. doi: 10.1111/j.1476-5381.1994.tb13058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGOLI D., DRAPEAU G., DION S., COUTURE R. New selective agonists for neurokinin receptors: pharmacological tools for receptor characterization. Trends Pharmacol. Sci. 1988;9:290–295. doi: 10.1016/0165-6147(88)90013-2. [DOI] [PubMed] [Google Scholar]
- REGOLI D., NGUYEN Q.T., JUKIC D. Neurokinin receptor subtypes characterized by biological assays. Life Sci. 1994;54:2035–2047. doi: 10.1016/0024-3205(94)00712-8. [DOI] [PubMed] [Google Scholar]
- REID M.S., HERRERA-MARSCHITZ M., HÖKFELT T., LINDEFORS N., PERSSON H., UNGERSTEDT U. Striatonigral GABA, dynorphin, substance P and neurokinin A modulation of nigrostriatal dopamine release: evidence for direct regulatory mechanisms. Exp. Brain Res. 1990;82:293–303. doi: 10.1007/BF00231249. [DOI] [PubMed] [Google Scholar]
- RIBEIRO-DA-SILVA A., MCLEOD A.L., KRAUSE J.E.Neurokinin receptors in the CNS Handbook of Chemical Neuroanatomy 2000Vol. 16Amsterdam: Elsevier Science B.V; 195–240.Part I, Peptide Receptorseds. Quirion, R., Björklund, A., Hökfelt, T. pp [Google Scholar]
- ROVERO P., PESTILLINI V., PATACCHINI R., GIULIANI S., SANTICIOLI P., MAGGI C.A., MELI A., GIACHETTI A. A potent and selective agonist for the NK-2 tachykinin receptor. Peptides. 1989;10:593–595. doi: 10.1016/0196-9781(89)90148-4. [DOI] [PubMed] [Google Scholar]
- SHUGHRUE P.J., LANE M.V., MERCHENTHALER I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J. Comp. Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- SIVAM S.P., KRAUSE J.E. Tachykinin systems in the spinal cord and basal ganglia: influence of neonatal capsaicin treatment or dopaminergic intervention on levels of peptides, substance P-encoding mRNAs, and substance P receptor mRNA. J. Neurochem. 1992;59:2278–2284. doi: 10.1111/j.1471-4159.1992.tb10121.x. [DOI] [PubMed] [Google Scholar]
- STOESSL A.J. Localization of striatal and nigral tachykinin receptors in the rat. Brain Res. 1994;646:13–18. doi: 10.1016/0006-8993(94)90052-3. [DOI] [PubMed] [Google Scholar]
- STOESSL A.J., BRACKSTONE M., RAJAKUMAR N., GIBSON C.J. Pharmacological characterization of grooming induced by a selective NK-1 tachykinin receptor agonist. Brain Res. 1995;700:115–120. doi: 10.1016/0006-8993(95)00940-r. [DOI] [PubMed] [Google Scholar]
- STOESSL A.J., HILL D.R. Autoradiographic visualization of NK-3 tachykinin binding sites in the rat brain, utilizing [3H]senktide. Brain Res. 1990;534:1–7. doi: 10.1016/0006-8993(90)90105-k. [DOI] [PubMed] [Google Scholar]
- UNGER Th., CAROLUS S., DEMMERT G., GANTEN D., LANG R.E., MASER-GLUTH C., STEINBERG H., VEELKEN R. Substance P induces a cardiovascular defense reaction in the rat: pharmacological characterization. Circ. Res. 1988;63:812–820. doi: 10.1161/01.res.63.4.812. [DOI] [PubMed] [Google Scholar]
- UNGER Th., ROCKHOLD R.W., YUKIMURA T., RETTIG R., GANTEN D. Blood pressure and heart rate responses to centrally administered substance P are increased in spontaneously hypertensive rats. Clin. Sci. 1980;59:299s–302s. doi: 10.1042/cs059299s. [DOI] [PubMed] [Google Scholar]
- VAN DEN BUUSE M., LINTHORST A.C.E., VERSTEEG D.H.G., DE JONG W. Role of brain dopamine systems in the development of hypertension in the spontaneously hypertensive rat. Clin. Exp. Hyp. 1991;A13:653–659. [Google Scholar]
- WHITTY C.J., WALKER P.D., GOEBEL D.J., POOSCH M.S., BANNON M.J. Quantitation, cellular localization and regulation of neurokinin receptor gene expression within the rat substantia nigra. Neuroscience. 1995;64:419–425. doi: 10.1016/0306-4522(94)00373-d. [DOI] [PubMed] [Google Scholar]
- WÖRMSER U., LAUFER R., HART Y., CHOREV M., GILON C., SELINGER Z. Highly selective agonists for substance P receptor subtypes. EMBO J. 1986;5:2805–2808. doi: 10.1002/j.1460-2075.1986.tb04571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN Y.D., COUTURE R. Renal effects of intracerebroventricularly injected tachykinins in the conscious saline-loaded rat: receptor characterization. Br. J. Pharmacol. 1997;120:785–796. doi: 10.1038/sj.bjp.0700972. [DOI] [PMC free article] [PubMed] [Google Scholar]


