Abstract
Optimal dipeptide and peptidomimetic drug transport across the intestinal mucosal surface is dependent upon the co-operative functional activity of the di/tripeptide transporter hPepT1 and the Na+/H+ exchanger NHE3. The ability of the anti-absorptive enteric neuropeptide VIP (vasoactive intestinal peptide) to modulate dipeptide uptake was determined using human intestinal (Caco-2) epithelial cell monolayers.
Uptake of glycylsarcosine (Gly-Sar) across the apical membrane of Caco-2 cell monolayers is inhibited by basolateral exposure to either VIP, pituitary adenylate cyclase-activating polypeptide (PACAP), or the VPAC1 receptor agonist [11,22,28Ala]-VIP. Inhibition of Gly-Sar uptake is observed only in the presence of extracellular Na+. Reverse-transcription polymerase chain reaction (RT–PCR) demonstrates that VPAC1 mRNA is expressed in Caco-2 cells whereas VPAC2 mRNA is not detected.
The VIP-induced inhibition of Gly-Sar uptake is abolished in the presence of the protein kinase A (PKA) inhibitor H-89 (N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide.2HCl).
22Na+ uptake across the apical membrane is inhibited by the selective NHE3 inhibitor S1611. Experiments with BCECF [2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein]-loaded Caco-2 cells demonstrate that VIP reduces the NHE3-dependent recovery of intracellular pH (pHi) after dipeptide-induced acidification. Western blot of Caco-2 cell protein demonstrates expression of the NHE regulatory factor NHERF1 (expression of which is thought to be required for PKA-mediated inhibition of NHE3).
VIP has no effect on Gly-Sar uptake in the presence of S1611 suggesting that VIP and S1611 both modulate dipeptide uptake via the same mechanism.
These observations demonstrate that VIP (and PACAP) modulate activity of the H+/dipeptide transporter hPepT1 in a Na+-dependent manner consistent with the modulation being indirect through inhibition of NHE3.
Keywords: Dipeptide transport, hPepT1, Caco-2 cell, human intestine, epithelial transport, VIP, PACAP, NHE3, VPAC1, NHERF
Introduction
For orally-delivered drugs, the initial barrier to effective oral bioavailability is the mucosal surface of the small intestinal wall. Many orally-active drugs cross this absorption barrier by movement through endogenous nutrient transporters such as the intestinal di/tripeptide transporter PepT1 (Yang et al., 1999). The human intestinal H+-coupled di/tripeptide transporter hPepT1 (Liang et al., 1995) is located at the apical membrane of human intestinal enterocytes (Walker et al., 1998) and transports a range of drugs across the epithelial brush-border including β-lactam antibiotics (e.g. cefadroxil, cephalexin and loracarbef), ACE inhibitors (enalapril maleate and captopril), bestatin and pro-drugs such as L-DOPA-L- Phe (Dantzig & Bergin, 1990; Saito & Inui, 1993; Thwaites et al., 1994; 1995; Wenzel et al., 1995; Tamai et al., 1998; Yang et al., 1999). The wide substrate specificity of hPepT1 highlights this transport protein as a potential route for absorption of other peptidomimetic drugs.
When studied in isolation (e.g. following expression in Xenopus laevis oocytes or HeLa cells), hPepT1 functions as a H+-coupled, Na+-independent transporter (Liang et al., 1995). However, studies in intact intestinal tissues or cultured intestinal cell lines demonstrate that di/tripeptide transport is dependent upon the presence of extracellular Na+ (Ganapathy & Leibach, 1985; Thwaites et al., 2002). Ganapathy & Leibach (1985) proposed a model to account for this apparent Na+-dependence, which suggested a close functional relationship between the apical H+-coupled di/tripeptide transporter hPepT1 and an apically-localized Na+/H+ exchanger (now known as NHE3) (Orlowski, 1993; Brant et al., 1995). The model predicted that removal of extracellular Na+ would prevent the Na+/H+ exchanger from extruding protons, transported into the cell by H+/dipeptide symport, which would in turn lead to a reduction in the driving force for hPepT1 activity (the trans-apical membrane H+ electrochemical gradient) and thus further transport. Studies using the human intestinal epithelial cell line Caco-2 demonstrate that hPepT1 selectively activates the apical NHE isoform NHE3 (which is quiescent at normal mucosal pH) but not the basolateral NHE1 (Thwaites et al., 1999). Conversely, pharmacological inhibition of NHE3 (by forskolin or the selective NHE3 inhibitor S1611) reduces dipeptide uptake in a pH and Na+-dependent manner consistent with the effect being indirect by a reduction in the driving force for transport (Thwaites et al., 2002). The acid microclimate (an area of low pH adjacent to the mucosal surface) provides the physiological driving force for hPepT1 activity and has been measured between pH 6.2–6.8 at the jejunal surface in vivo and in vitro (McEwan et al., 1988; Daniel et al., 1989). This acid microclimate is alkalinized by a number of factors (e.g. forskolin, cholera toxin or E. coli STa enterotoxin) that increase [cAMP]i or [cGMP]i (McEwan et al., 1988). NHE3 is highly regulated and is inhibited by factors that increase [cAMP]i and [cGMP]i (McSwine et al., 1998). There are relatively few studies of the short-term regulation of hPepT1 function, although dipeptide transport is modulated directly by some endogenous factors e.g. leptin (Buyse et al., 2001) and α2-adrenergic receptor stimulation (Berlioz et al., 2000). Clearly, optimal nutrient absorption or drug transport via the oral route are dependent upon the functional co-operation of a number of transport proteins present in the epithelial cells lining the small intestinal wall. Since epithelial transport is under the control of a variety of chemicals released by endocrine and neural pathways within the walls of the gastrointestinal tract, any physiological (pathophysiological or pharmacological) modulation of either hPepT1 or NHE3 will also effect the oral absorption of any drugs transported by the peptide transporter.
Vasoactive intestinal peptide (VIP) is a neuropeptide contained in the enteric nervous system within the walls of the gastrointestinal tract and is believed to have secretory and anti-absorptive actions (Brown & Miller, 1991). In the human jejunum and ileum, VIP-containing neurones extend into the mucosa where they form a dense network in contact with the luminal epithelium (Ferri et al., 1982). Although the secretory action of VIP is well characterized the VIP-containing nerves are more abundant in the villus (an area where absorption is more pronounced) than crypt region (Ferri et al., 1982). Consistent with the distribution of VIP-containing nerves, the VIP receptor on the intestinal enterocyte is found solely on the basolateral membrane (Dharmsathaphorn et al., 1983) and in human jejunal membranes is more abundant (7–8×) in villus than crypt cells (Salomon et al., 1993). In rat jejunum, the expression of VIP receptors along the crypt-villus axis is homogeneous (Voisin et al., 1990). The receptor has been cloned from a human jejunal cDNA library (Couvineau et al., 1994) and has been named the VPAC1 receptor (Harmar et al., 1998). VPAC1 has equal affinity for VIP and the related neuropeptide pituitary adenylate-cyclase activating peptide (PACAP) which exists in two forms, PACAP-27 and PACAP-38 (Vaudry et al., 2000). In human small intestinal tissues in vitro, VIP activates adenylate cyclase, increases cAMP and Isc (Schwartz et al., 1974; Salomon et al., 1993). In vivo, infusion of VIP into human volunteers leads to a decrease in water and Na+ absorption and an increase in Cl− secretion (Krejs et al., 1980).
Thus, although much is known about the control of water and electrolyte transport across the intestinal wall (and in particular the role of VIP), relatively little is known about regulation of nutrient absorption. The main purpose of this investigation, therefore, was to study the regulatory effects of the secretory/anti-absorptive neuropeptides VIP and PACAP on the two transporters (hPepT1 and NHE3) involved in optimising peptide (and peptide-like drug) transport across the mucosal surface of the human intestinal epithelium.
Methods
Materials
[14C]Gly-Sar [glycyl[1-14C]sarcosine (specific activity 56.7 mCi.mmol−1)] was obtained from Cambridge Research Biochemicals (Stockton-on-Tees, U.K.). 22NaCl (specific activity 722 mCi mg−1) was obtained from NEN Life Science Products (Zaventem, Belgium). VIP and PACAP-27 were from Bachem (Saffron Walden, Essex, U.K.). Ouabain was from Sigma-Aldrich Ltd (Poole, Dorset, U.K.). [11,22,28Ala]VIP was from Tocris (Bristol, U.K.). H-89 (N-[2-(p-bromocinnamylamino)ethyl]-5- isoquinolinesulfonamide.2HCl) was from Biomol Research Laboratories (Exeter, U.K.). 2′,7′-Bis(2- carboxyethyl-5(6)-carboxyfluorescein) (BCECF)-AM (acetoxymethyl ester) was from Molecular Probes (Leiden, The Netherlands). S1611 was a gift from H.J. Lang (Aventis Pharma Deutschland GmbH, Frankfurt/Main, Germany). All other chemicals were from Sigma or BDH (Poole, Dorset, U.K.) and were of the highest quality available.
Cell culture
Caco-2 cells (passage number 98-122) were cultured and prepared as monolayers as described previously (Thwaites et al., 1993a; 1999). Cell monolayers were prepared by seeding at high density (4.0–5.0×105 cells cm−2) on 12 mm Transwell polycarbonate filters (Corning-Costar Ltd, High Wycombe, U.K.). Cell monolayers were maintained in culture at 37°C in a humidified atmosphere of 5% CO2 in air. The confluence and integrity of the epithelial monolayers were confirmed by measurement of transepithelial electrical resistance (RT) at 37°C using EVOM chopstick electrode STX-2 and a WPI evometer (World Precision Instruments, Herefordshire, U.K.). Experiments were performed 14–18 days after seeding and 18–24 h after feeding. Confluent polarized monolayers of the human intestinal epithelial cell line Caco-2 are used extensively to characterize intestinal epithelial function. The advantages and limitations of the use of Caco-2 cell monolayers as a model for oral absorption studies are discussed elsewhere (Delie & Rubas, 1997).
Uptake across the apical membrane of Caco-2 cell monolayers
Uptake experiments using either [14C]-Gly-Sar or 22Na+ were performed essentially as described previously (Thwaites et al., 1993a; 1999). Briefly, the cell monolayers were washed in 4×500 ml volumes of modified Krebs' solution (pH 7.4) of composition (mM): NaCl, 137; KCl, 5.4; MgSO4, 0.99; KH2PO4, 0.34; NaH2PO4, 0.3; CaCl2, 2.8; Glucose, 10; (Na+-free solutions were prepared as above except choline chloride replaced NaCl and NaH2PO4 was omitted). For 22Na+ uptake measurements, glucose-free solutions were prepared as above with mannitol replacing glucose. Solution pH was adjusted at 37°C by addition of 10 mM HEPES (pH 6.5 and 7.4) or 10 mM MES (pH 5.5) with addition of Tris base until the required pH was achieved. The apical solution was pH 6.5 unless stated otherwise. In all experiments the basolateral solution was pH 7.4. [14C]-Gly-Sar (0.5 μCi ml−1, 100 μM) was added to the apical chamber and uptake allowed to proceed at 37°C for 15 min. Various compounds (S1611, VIP, PACAP and [11,22,28Ala]-VIP) were added selectively to either the apical or basolateral solution for the duration of uptake (see Figure Legends for details). H-89 required preincubation for 60 min on both sides of the monolayer and was also present throughout the uptake measurements. For 22Na+ uptake experiments, the monolayers were preincubated in an NH4Cl containing solution (Na+- and glucose-free, pH 7.4, 30 mM NH4Cl replacing 30 mM choline chloride) for 15 min. The monolayers were then washed briefly in a Na+-, glucose- and NH4Cl-free Krebs' pH 7.4 solution before apical 22Na+ (1 μCi ml−1, 100 nM) uptake (pH 7.4) was measured for 5 min in the absence or presence of S1611 (0–10 μM). The basolateral solution contained 1 mM ouabain. At the end of the incubation period the cell monolayers were washed in 3×500 ml volumes of ice-cold modified Krebs' (pH 7.4) and removed from the insert. Cell-monolayer associated radioactivity was determined by scintillation counting.
pHi measurements
Intracellular pH (pHi) was measured in Caco-2 cell monolayers loaded with the pH-sensitive dye 2′,7′- bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF)-AM by microspectrofluorimetry, essentially as described previously (Thwaites et al., 1993a; 1999). pHi recovery from a substrate-induced acid load (apical 20 mM Gly-Sar, Na+-free, pH 5.5) was measured first in the absence and then the presence of 5 nM VIP in the basolateral solution. Overall VIP was present in the basolateral solution for 15 min (10 min with the apical solution held at pH 7.4 and then throughout the 5 min acidification period so that as in the uptake experiments VIP was present for 15 min) before pHi recovery was measured. H+ efflux rates (μM.s−1) were calculated by linear regression of the initial 30 s of pHi recovery (using Photon Counter System 4.82 software, Newcastle Photometric Systems, Newcastle upon Tyne, U.K.) using estimates of buffering capacity (Watson et al., 1991; Thwaites et al., 1999).
Reverse-transcriptase polymerase chain reaction(RT–PCR)
Total mRNA was prepared from Caco-2 cells grown in standard conditions. Total mRNA was isolated using an RNeasy MidiKit (Qiagen, U.K.) and a DNase treatment step (RNase-free DNase Set, Qiagen). Total mRNA was reverse transcribed using Omniscript reverse transcriptase (Qiagen). Human brain (whole) cDNA was obtained as part of a Human MTC Panel 1 kit (BD Biosciences Clontech, U.S.A.) and was used as a positive control [as both VPAC1 and VPAC2 receptors are expressed abundantly in the CNS (Harmar et al., 1998)]. Primers were designed based on the published human sequences to amplify: VPAC1 (accession number: NM_004624) from nucleotides 305–707 (giving an expected product size of 403 bp) and were as follows: Forward: 305GATGTGGGACAACCTCACCT324; Reverse: 707GAGGGCCAAGTCTTTGATGA688.
VPAC2 (accession number: XM_004641) from nucleotides 230–624 (giving an expected product size of 395 bp) and were as follows: Forward: 230CCAGAATGCCGATTTCATCT249 Reverse: 624AGCTTCCTGAAGAGGCACAG605.
The control primers were CLONTECH's Human G3PDH (glyceraldehyde-3-phosphate dehydrogenase) Control Amplimer Set (#5406-1) and were obtained as part of the Human MTC Panel 1 kit (BD Biosciences Clontech, U.S.A.) (giving an expected product size of 1000 bp) and were as follows: Forward: 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′; Reverse: 5′-CATGTGGGCCATGAGGTCCACCAC-3′.
Polymerase chain reactions (PCRs) were performed in the manufacturers' buffers over 35 cycles in 1.5 mM MgCl2 using the HotStarTaq DNA Polymerase system (Qiagen). Thermal cycling parameters for VPAC1 were as follows: 95°C for 15 min as an initial step, then 3-step cycling (94°C for 1 min, 57°C for 1 min, 72°C for 1 min) for 35 cycles and finally 72°C for 10 min. The 403 bp product was subcloned (TOPO TA cloning) into the vector pCR2.1 (Invitrogen, San Diego, CA, U.S.A.) and sequenced on an ABI model 377 automated sequencer (Perkin-Elmer, Applied Biosystems, Warrington, U.K.). Thermal cycling parameters for VPAC2 and G3PDH were as follows: 95°C for 15 min as an initial step, then 3-step cycling (94°C for 1 min, 55.5°C for 1 min, 72°C for 1 min) for 35 cycles and finally 72°C for 10 min. Negative control reactions were performed, and no bands observed, in the absence of template DNA (see Figure 3) and in the absence of either Reverse Transcriptase, Caco-2 RNA or both Reverse Transcriptase and Caco-2 RNA (results not shown).
Figure 3.
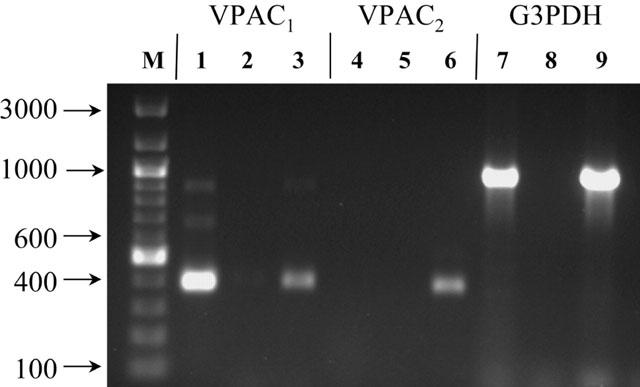
Products of PCR on Caco-2 cDNA (lanes 1, 4 and 7) using primers specific for VPAC1 (lane 1), VPAC2 (lane 4) and G3PDH (control, lane 7). Negative control reactions in the absence of template DNA are shown in lane 2 (VPAC1), lane 5 (VPAC2) and lane 8 (G3PDH). As a positive control for primer competence, products of PCR on human brain (whole) cDNA using primers specific for VPAC1 (lane 3), VPAC2 (lane 6) and G3PDH (lane 9) are shown. Lane M shows DNA markers with sizes indicated. Products were separated on a 1% agarose gel and stained with ethidium bromide. Primer sequences and thermal cycling parameters are given in Methods.
Generation and affinity purification of an anti-hNHERF1 antibody
An anti-hNHERF1 antibody was raised in rabbit to a synthetic peptide (GLLAGDRLVEVNGEN) corresponding to residues 52–67 of human NHERF1 (accession number: AAC04572; Reczek et al., 1997; Murthy et al., 1998) following a standard 77 day immunization (Sigma Genosys, Cambridge, U.K.). For affinity purification of the polyclonal serum, 5 mg of the synthetic peptide was coupled to a HiTRAP NHS activated column (Amersham Pharmacia Biotech, U.K.) following the manufacturer's instructions. The column was equilibrated with starting buffer, (0.5 M Tris, 0.5 M EDTA, 1 mg ml−1 NaN3, 300 mM NaCl and 0.05% Tween-20, pH 7.4) and 5 ml of crude serum applied. After washing with starting buffer, antibody was eluted with elution buffer (200 mM glycine and 137 mM NaCl, pH 2.8), the pH adjusted to pH 9 and stored at −20°C.
Western blot
Caco-2 cells were washed twice with ice-cold PBS and then lysed for 30 min at 4°C in lysis buffer (2% Nonidet P40, 0.2% SDS, 1 mM DTT, and complete mini protease inhibitor cocktail (Roche) in PBS). The lysate was centrifuged at 10,000×g for 10 min at 4°C, and the protein concentration quantified in the supernatant. Thirty μg of protein were separated on a 12% polyacrylamide gel in NuPage MOPS SDS Running Buffer (Invitrogen, U.K.). Proteins were transferred to a polyvinylidene difluoride membrane (PVDF) (Hybond-P, Pharmacia Biotech, U.K.) and the membrane was blocked for 1 h in TBS-T buffer (consisting of 20 mM Tris, 137 mM NaCl, 0.1% Tween-20, pH 7.6) containing 5% non-fat milk. The membrane was incubated for 2 h at room temperature with either the primary anti-hNHERF1 antibody (purified and non-purified) or pre-immune serum (all diluted 1 : 1000 in TBS-T buffer) and then rinsed five times with TBS-T buffer. The membrane was then incubated with the secondary antibody (goat anti-rabbit IgG, horseradish peroxidase-linked whole antibody, 1 : 40,000, room temperature, 1 h) (Amersham Biosciences, U.K.). The membrane was washed with TBS-T buffer and protein detected by the enhanced chemiluminescence method using an ECL Plus Western blotting detection system (Amersham Biosciences, U.K.). To determine the specificity of the anti-hNHERF1 antibody binding, purified or nonpurified serum was pre-incubated with 100 μg of the antigenic peptide in PBS for 1 h at 37°C and then overnight at 4°C. Western blotting was then performed as described above. Band sizes are estimated by comparison with High Range (M.W. 29–205K) Colour Markers (Sigma).
Statistical analysis
Data are expressed as mean±s.e.mean (n). Statistical comparisons of mean values were made using paired two-tailed Student's t-test or one-way analysis of variance (ANOVA) (using the Tukey-Kramer or Bonferroni's multiple comparisons post-test) as appropriate. Sigmoidal dose response curves were fitted using GraphPad Prism version 3.00.
Results
pH- and Na+-dependent inhibition of dipeptide uptake by VIP and PACAP
Gly-Sar (100 μM) uptake (15 min) across the apical membrane of Caco-2 cell monolayers was measured in the presence and absence of extracellular Na+. Unless stated otherwise, dipeptide uptake measurements were at apical pH 6.5 which is within the range of ‘physiological' pH values (pH 6.2–6.8) measured at the small intestinal mucosal surface (McEwan et al., 1988; Daniel et al., 1989). At apical pH 6.5 in the presence of Na+, basolateral addition of either VIP (5 nM) or PACAP (100 nM) caused a 45 or 47% reduction (P<0.001 vs control) in Gly-Sar uptake (Figure 1). No further inhibition of Gly-Sar uptake (below that observed when the compounds were used individually) was observed when VIP and PACAP were used in combination (P<0.001; Figure 1). Gly-Sar uptake was significantly reduced when extracellular Na+ was removed (P<0.001; Figure 1). No effect of VIP or PACAP was detected in Na+-free conditions (P>0.05; Figure 1). The effects of VIP and PACAP on the Na+-dependent component of Gly-Sar uptake were concentration dependent (Figure 2a), half-maximal inhibition (IC50) being measured at 0.3 nM and 0.8 nM for PACAP and VIP, respectively. Maximal inhibition by either peptide was observed at 3 nM, no further inhibition being observed up to 100 nM (Figure 2a) confirming that the effects of VIP and PACAP in Figure 1 represented maximal inhibition (>3 nM concentrations of VIP and PACAP were used throughout this investigation). Similar observations were made at apical pH 7.4 where VIP (50 nM) caused a 42% reduction (P<0.001) in apical Gly-Sar uptake from 170±8 to 98±4 (n=5) pmol cm−2(15 min)−1. In Na+-free conditions VIP had no effect (P>0.05) on Gly-Sar uptake at pH 7.4 [92±4 and 86±3 (n=5) pmol cm−2(15 min)−1, in the absence and presence of VIP, respectively].
Figure 1.
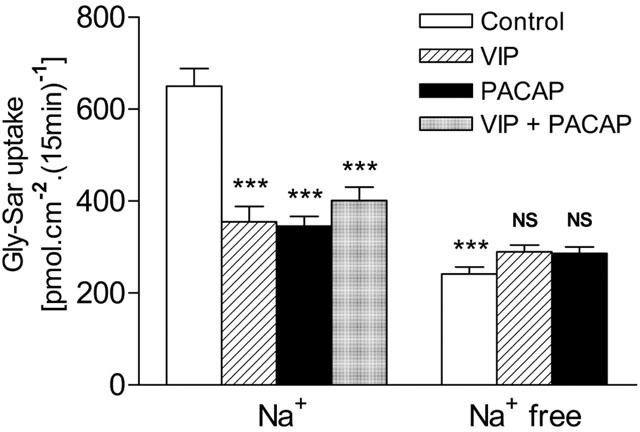
Inhibition of Gly-Sar uptake (100 μM) across the apical membrane of Caco-2 cell monolayers by vasoactive intestinal peptide (VIP, 5 nM) and pituitary adenylate cyclase activating polypeptide 1-27 (PACAP, 100 nM) in the presence (Na+) but not in the absence (Na+ free) of extracellular Na+. Apical uptake was measured for 15 min at apical pH 6.5 (basolateral pH 7.4) in the presence of VIP and PACAP in the basolateral solution only. Data are mean±s.e.mean (n=6). ***, P<0.001 vs control in the presence of Na+. NS, P>0.05 vs control in the absence of Na+.
Figure 2.
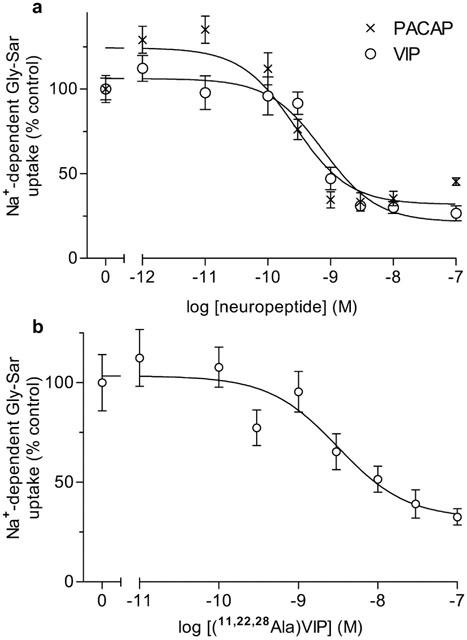
Concentration-response curves for the effect of the neuropeptides PACAP and VIP, and the VPAC1-receptor selective agonist [11,22,28Ala]-VIP on Na+-dependent Gly-Sar uptake. (a) Top panel: Gly-Sar (100 μM) uptake (15 min) was measured across the apical membrane of Caco-2 cell monolayers in the presence or absence of basolateral VIP (open circles) or PACAP (X) (both 10−7–10−12 M) in the presence of extracellular Na+. The results are expressed as the percentage uptake under control (in the absence of either VIP or PACAP) conditions (after subtraction of the uptake in Na+ free conditions). Best-fit concentration response curves were fitted using GraphPad Prism version 3.00 (r2= 0.996 for VIP; r2=0.994 for PACAP). Data are mean±s.e.mean (n=6–18). (b) Bottom panel: Gly-Sar (100 μM) uptake (15 min) was measured across the apical membrane of Caco-2 cell monolayers in the presence or absence of basolateral [11,22,28Ala]-VIP (10−7–10−11 M) in the presence of extracellular Na+. The results are expressed as the percentage uptake under control (in the absence of [11,22,28Ala]-VIP) conditions (after subtraction of the uptake in Na+ free conditions). The best-fit concentration response curve was fitted using GraphPad Prism version 3.00 (r2=0.912). Data are mean±s.e.mean (n=10–16).
Evidence for a role of the VPAC1 receptor in the VIP/PACAP-induced response
The concentration-dependent effect of the VPAC1 selective agonist [11,22,28Ala]-VIP (Nicole et al., 2000) on Gly-Sar uptake (Figure 2b) was measured to determine which receptor of the VIP/PACAP receptor family (Harmar et al., 1998) was responsible for the inhibitory effect observed in Figure 1. [11,22,28Ala]-VIP inhibited Gly-Sar uptake in a dose-dependent manner (IC50=3.1 nM). Expression of VPAC1 and VPAC2 mRNA in Caco-2 cells was determined by RT–PCR (Figure 3) using primers specific for the published human VPAC1 (Couvineau et al., 1994) and VPAC2 (Svoboda et al., 1994) sequences. Agarose gel electrophoresis of the VPAC1 PCR products revealed a band of the predicted size (403 bp) which was subcloned and sequenced and found to have 100% identity to the published human sequence. In contrast, although a band of the predicted size (395 bp) was obtained for VPAC2 following PCR with human brain (whole) cDNA, no product was obtained from Caco-2 cells.
Role of the protein kinase A pathway in the VIP-induced inhibition
The effect of VIP on Gly-Sar uptake was determined after the Caco-2 cell monolayers had been incubated with the PKA inhibitor H-89 (0–100 μM, 60 min). As demonstrated in Figure 4, preincubation of the cells with H-89 caused a reduction in the inhibitory effect of VIP on Gly-Sar uptake. The ability of H-89 to protect the cells from VIP-induced inhibition was concentration-dependent. H-89 blocked the VIP effect at H-89 concentrations of 25–100 μM (P<0.001 vs VIP alone) but had no effect at 0.5–5 μM (P>0.05 vs VIP alone).
Figure 4.
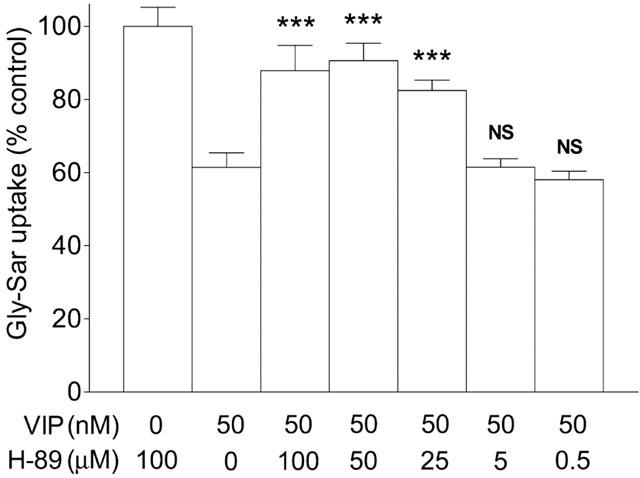
The concentration-dependent protective effect of the PKA inhibitor H-89 on the VIP-induced inhibition of Gly-Sar uptake. Caco-2 cell monolayers were preincubated (apical pH 7.4, basolateral pH 7.4) with H-89 (0–100 μM, in both apical and basolateral solutions) for 60 min prior to the measurement of dipeptide uptake and throughout the 15 min uptake period. Apical Gly-Sar (100 μM) uptake was measured for 15 min (apical pH 7.4, basolateral pH 7.4) in the presence or absence of basolateral VIP (50 nM). Results are expressed as a percentage of the uptake measured in ‘control' cells which are those preincubated in the presence of 100 μM H-89 but where uptake was determined in the absence of VIP. Data are mean±s.e.mean (n=6). ***, P<0.001 vs VIP only (0 μM H-89). NS, P>0.05 vs VIP only (0 μM H-89).
Inhibition of NHE3 and hPepT1 by VIP and S1611
We have shown previously that, after a dipeptide-induced intracellular acidification in BCECF-loaded Caco-2 cell monolayers, the mechanism responsible for recovery to resting pHi levels is the apically-localized Na+/H+ exchanger NHE3 (Thwaites et al., 1993b; 1999; 2002). NHE3 activity is down-regulated by activation of the protein kinase A pathway. The typical response shown in Figure 5a demonstrates that a 15 min exposure to basolateral VIP (5 nM) markedly reduces the ability of the Caco- 2 cells to recover from a dipeptide-induced intracellular acidification even in the presence of Na+ in the apical superfusate. Figure 5b demonstrates that this represents a significant decrease (P<0.01) in the H+ efflux rate (from 88.0±17.0 to 37.1±6.8 (n=8) μM s−1, in the absence and presence of VIP, respectively). To confirm the identity of the apical Na+/H+ exchanger as NHE3, 22Na+ (100 nM) uptake across the apical membrane of Caco-2 cell monolayers was measured (after intracellular acidification using the NH4Cl prepulse-and-release manoeuvre) in the presence of the selective NHE3 inhibitor S1611 (Wiemann et al., 1999) (Figure 6a). S1611 reduced 22Na+ uptake in a dose-dependent manner and inhibition was consistent with inhibition of NHE3 (IC50=0.2 μM). To determine whether inhibition of dipeptide uptake by both VIP and S1611 was ultimately through the same mechanism (inhibition of NHE3) maximally active concentrations of VIP (5 nM, to the basolateral solution) and S1611 (3 μM, to the apical solution) were tested in combination. Figure 6b shows that there was no significant difference (P>0.05) in dipeptide uptake in the presence of both VIP and S1611 compared to uptake in the presence of either VIP or S1611 alone.
Figure 5.
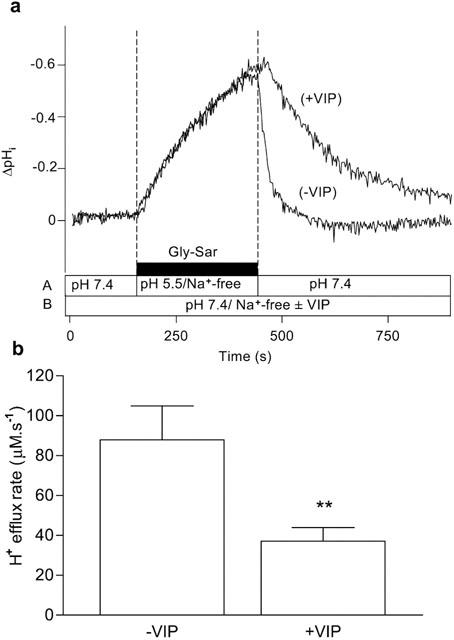
Intracellular pH recovery from a Gly-Sar-induced acidification in the presence and absence of VIP. Caco-2 cell monolayers were exposed to Gly-Sar (20 mM) in the apical superfusate (apical pH 5.5, Na+-free solution) for 5 min (the basolateral membrane was superfused with a Na+-free Krebs' solution (pH 7.4) throughout the experiment). The apical superfusate was then replaced with a pH 7.4, Na+- containing, Gly-Sar-free solution and pHi recovered to basal levels. This ‘control' manoeuvre was performed in the absence of VIP (−VIP). After the ‘control' (−VIP) manoeuvre was completed the cell monolayer was exposed to VIP (5 nM) for 10 min at the basolateral membrane before the manoeuvre (acidification for 5 min with Gly-Sar followed by recovery at pH 7.4/Na+) was repeated in the presence of 5 nM basolateral VIP (+VIP) (total exposure time to VIP before recovery was 15 min). (a) Top panel: The trace represents the composite response seen in a single monolayer to successive recoveries in the absence (−VIP) and presence (+VIP) of VIP. The composition and pH of the apical (A) and basolateral (B) solutions are indicated by the horizontal bars. The results are expressed as a ΔpHi. (b) Bottom panel: The H+-efflux rate was calculated in each individual monolayer from the rate of the initial 30 s of recovery (after removal from the apical chamber of the Gly-Sar/pH 5.5/Na+-free solution) both in the absence (−VIP) and presence (+VIP) of VIP (as described in Methods). Data are mean±s.e.mean (n=8). **, P<0.01.
Figure 6.
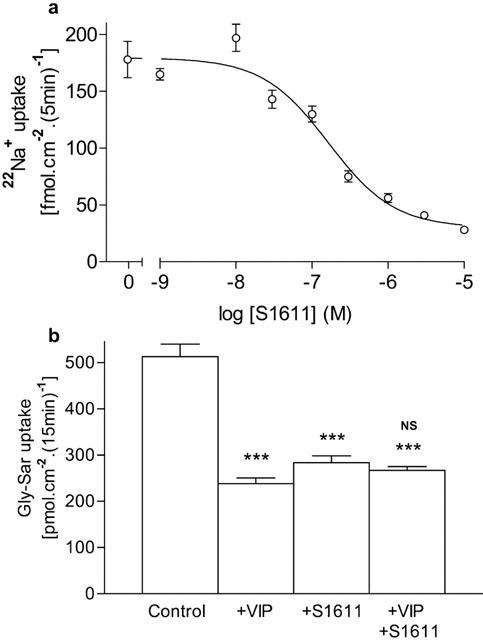
(a) Top panel: Concentration-dependent inhibition of apical 22Na+ uptake (5 min) into Caco-2 cell monolayers by the NHE3 selective inhibitor S1611. Cells were acidified by the NH4Cl prepulseand- release manoeuvre. After acidification, apical 22Na+ influx (1 μCi ml−1, 100 nM, 5 min) was measured at apical pH 7.4 in the absence or presence of S1611 (10−5–10−9 M). The basolateral solution was Na+-free (pH 7.4) and contained 1 mM ouabain. The best-fit concentration response curve was fitted using GraphPad Prism version 3.00 (r2=0.997). Data are mean±s.e.mean (n=11–12). (b) Bottom panel: Gly-Sar uptake across the apical membrane of Caco-2 cell monolayers. Gly-Sar (100 μM) uptake (apical pH 6.5, basolateral pH 7.4) was measured in the presence of extracellular Na+ in the presence of basolateral VIP (5 nM), apical S1611 (3 μM) or both VIP and S1611. Data are mean±s.e.mean (n=11–12). ***, P<0.001 vs Control. NS, P>0.05 VIP+S1611 vs either VIP alone or S1611 alone.
Identification of hNHERF1 protein in Caco-2 cells
Western blotting analysis of hNHERF1 expression in Caco-2 cells demonstrates that a protein of the expected size (approx. 50 kDa) (Reczek et al., 1997) is detected using both the non-purified serum and purified anti-hNHERF1 antibody (Figure 7). In contrast this band was not detected by the preimmune serum. The 50 kDa band appears to be specific as preincubation with the hNHERF1 antigenic peptide selectively reduced the band intensity observed with the non-purified antiserum and eliminated the single band detected by the purified anti-hNHERF1 antibody (Figure 7).
Figure 7.
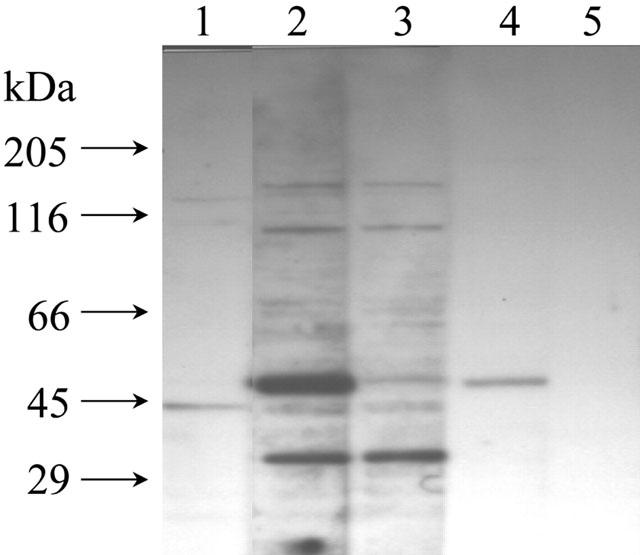
Western blotting analysis of hNHERF1 protein expression in Caco-2 cells. A band of the expected size (approximately 50 kDa) is observed with both the non-purified (lane 2) and purified (lane 4) antihNHERF1 antibody. Lane 1, pre-immune serum (as control). Lane 2, non-purified anti-hNHERF1 serum. Lane 3, non-purified anti-hNHERF1 serum (after conjugation with the antigenic peptide). Lane 4, affinity-purified hNHERF1 antibody. Lane 5, affinity-purified hNHERF1 antibody (after conjugation with the antigenic peptide). Each lane was loaded with 30 μg of Caco-2 cell protein. Size markers are indicated in kDa.
Discussion
The gastrointestinal epithelium represents a major barrier to absorption of many hydrophilic compounds of nutritional and pharmaceutical interest. However, a number of peptide-like drugs have surprisingly high oral bioavailability (Humphrey & Ringrose, 1986). Confluent monolayers of the human intestinal epithelial cell line Caco-2 are used routinely to identify potential peptide-like drug substrates for the endogenous intestinal di/tripeptide transporter hPepT1. The specificity of the di/tripeptide transporter expressed in heterologous systems (e.g. Xenopus laevis oocytes or HeLa cells) is identical to that observed at the apical membrane of Caco-2 cell monolayers (Thwaites et al., 1993a; 1994; 1995; Liang et al., 1995; Wenzel et al., 1995). However, the overall absorptive process is not only a function of membrane transporters involved directly in solute movement (e.g. hPepT1) but also transporters involved in the development and maintenance of transmembrane ionic gradients. For optimal absorption of solutes (absorbed via ion-driven transport proteins) to occur, therefore, it is essential that the driving force (the transepithelial or transmembrane ion gradient) is maintained during absorption. In the case of H+-coupled dipeptide or peptide-like drug transport via hPepT1, dipeptide induced H+-influx into the intestinal enterocyte would quickly run down the transmembrane driving force (the H+ gradient) unless the gradient was maintained by a pHi homeostatic mechanism, e.g. the apically-localized Na+/H+ exchanger NHE3. Since it is the local ionic composition (both intracellular and extracellular) within the villus microdomain that will determine the net direction of ion-driven solute transport, it is possible to conclude that any up- or down-regulation of NHE3 activity will modulate the local microdomain ionic (H+) composition and, therefore, the absorptive capacity of the intestinal epithelium (Thwaites et al., 2002). Our recent studies using Caco-2 cell monolayers support this conclusion, as although hPepT1 has no potential PKA site (Liang et al., 1995) dipeptide uptake is reduced (by a reduction in capacity) by the selective NHE3 inhibitor S1611 (Wiemann et al., 1999) and activators of the protein kinase A pathway [forskolin and 8-Br-cAMP (8-bromoadenosine-3′,5′- cyclic monophosphate)] but only under conditions in which NHE3 will be active (Orlowski, 1993; Thwaites et al., 2002).
Although intestinal function is highly regulated by a variety of neuroendocrine factors, relatively little is known about control of endogenous nutrient transporters. VIP is expressed abundantly throughout the submucosal plexus and in neurones which extend towards the intestinal epithelium and in particular the villus cells (Ferri et al., 1982). Although VIP is a potent activator of Cl− and fluid secretion in intestinal epithelial cells it also reduces Na+ absorption (Schwartz et al., 1974; Krejs et al., 1980). Figure 1 demonstrates that VIP and the related neuropeptide PACAP-27, which is also found in the submucosal plexus (Sundler et al., 1992; Vaudry et al., 2000), inhibit dipeptide uptake but only in the presence of extracellular Na+. The IC50 values obtained for VIP (0.8 nM) and PACAP-27 (0.3 nM) (Figure 2a) are similar to the half-maximal effective dose of VIP (0.7 nM) required to activate adenylate cyclase in human jejunal membranes (Salomon et al., 1993). Although there are a family of VIP/PACAP receptors (Harmar et al., 1998) only a single receptor is found on human enterocytes and Caco-2 cells (Laburthe et al., 1987; Salomon et al., 1993). Figures 2b-3 provide evidence for VPAC1 (but not VPAC2) expression in Caco-2 cells, the IC50 for [11,22,28Ala]-VIP inhibition of dipeptide uptake (3.1 nM) in Figure 2b being much closer to the EC50 for adenylate cyclase activation through VPAC1 (0.4 nM) than VPAC2 (1.2 μM) (Nicole et al., 2000). The observation given in Figure 2a, that VIP inhibits Gly-Sar uptake with an IC50 of 0.8 nM, clearly discounts an effect through the PAC1 receptor where the VIP IC50 is 1 μM (Harmar et al., 1998). This suggests that the anti-absorptive effects of VIP and PACAP-27 on dipeptide transport are mediated via the VPAC1 receptor sub-type (Couvineau et al., 1994; Harmar et al., 1998; Nicole et al., 2000). VIP is known to increase adenylate cyclase activity and intestinal secretion in human intestine (Schwartz et al., 1974; Krejs et al., 1980). The ability of the PKA inhibitor H-89 to abolish the VIP-induced inhibition of dipeptide uptake suggests that VIP is inhibiting hPepT1 through a PKA-mediated pathway even though hPepT1 has no potential PKA site (Liang et al., 1995) (Figure 4). The concentration-dependent inhibition of 22Na+ uptake by the selective NHE3 selective inhibitor S1611 confirms that, as in normal human intestine (Hoogerwerf et al., 1996), NHE3 is localized and functional at the apical membrane of Caco-2 cell monolayers (Figure 6a). Basolateral VIP caused a similar reduction in NHE3 activity (Figure 5) to that observed in our previous experiments with the Na+/H+ exchange inhibitor EIPA (Thwaites et al., 1999) or the PKA activator forskolin (Thwaites et al., 2002). Clearly both VIP (Figure 5) and S1611 (Figure 6a) inhibit NHE3 activity. In contrast, VIP, PACAP-27 (Figure 1) and S1611 (Thwaites et al., 2002) all fail to inhibit dipeptide uptake in the absence of extracellular Na+. These observations, when considered alongside our previous study (Thwaites et al., 2002) which showed that forskolin, 8-Br-cAMP and VIP only inhibited dipeptide uptake at apical pHo values at which NHE3 is active, suggest that the inhibitory effects are not direct on hPepT1 but rather indirect on NHE3. That VIP is reducing dipeptide uptake by inhibiting NHE3 (and, therefore, the ability of the enterocyte to maintain the driving force for dipeptide transport) is confirmed by the lack of additivity in the inhibitory responses observed when Caco-2 cells are incubated with both apical S1611 and basolateral VIP (Figure 6b). For cAMP-mediated phosphorylation and inhibition of NHE3 to occur an NHE regulatory factor (NHERF) must be present (Weinman et al., 2000). A previous study has suggested that both hNHERF1 (EBP50) and hNHERF2 (E3KARP/TKA1) are absent from Caco-2 cells (Yun et al., 1997). However, we have identified, cloned and sequenced full-length mRNA transcripts of both hNHERF1 and hNHERF2 from Caco-2 cells (Thwaites et al., 2002). Figure 7 demonstrates that hNHERF1 (EBP50) (Reczek et al., 1997; Murthy et al., 1998) protein is expressed in Caco-2 cells.
The abundant expression of VIP (and PACAP) in the submucosal plexus (Ferri et al., 1982; Brown & Miller, 1991), considered alongside the basolateral localization of the receptor on intestinal epithelial cells (Dharmsathaphorn et al., 1983) and the increased level of binding of 125I-VIP in villus cells compared to human crypt cells (Salomon et al., 1993) suggest that the neuropeptides VIP and PACAP have an important role in control of the intestinal villus epithelium. The effects of VIP on epithelial function are most evident under pathophysiological conditions such as Travellers' diarrhoea or those associated with VIP-producing tumours. Patients suffering from watery diarrhoea syndrome (pancreatic cholera) have elevated plasma VIP levels and a high concentration of VIP in the tumour tissue (Bloom et al., 1973). Cholera enterotoxin can increase [cAMP]i in intestinal epithelial cells directly (Muller et al., 1996; Sellin, 1996), an effect which would inhibit NHE3 function. In addition exposure of the mammalian intestine to cholera toxin also causes the release of VIP from the enteric nervous system (Cassuto et al., 1981). Incubation of rat jejunum with a VIP receptor antagonist ([4Cl- D-Phe6, Leu17]VIP) prevents the cholera toxin-induced increase in fluid and electrolyte secretion (Mourad & Nassar, 2000). In diarrhoeal states the direct effects of VIP on (mal)absorption of nutrients (peptide) and (peptide-like) drugs by the intestinal enterocyte may be masked by the increased fluid secretion and increased motility. It is possible that the enterotoxin induced decrease in absorption could directly inhibit drug transport and the effectiveness of oral antibiotics used to treat bacterial enterotoxin induced diarrhoeas. In addition any pharmacological inhibition of NHE3 will indirectly reduce the bioavailability of peptide-like drugs absorbed by the intestinal transporter hPepT1. Studies of bioavailabilty in humans demonstrate that amiloride (a Na+/H+ exchange inhibitor and commonly prescribed K+-sparing diuretic) reduces the absorption rate and absolute bioavailability of the antibiotic amoxicillin (a substrate for hPepT1) (Westphal et al., 1995).
In conclusion, we provide evidence to demonstrate that the coordinated activity of the intestinal di/tripeptide transporter hPepT1 and the Na+/H+ exchanger NHE3 is required to optimise peptide (and peptide-like drug) transport across the luminal membrane of the human intestinal (Caco-2) epithelium. Down-regulation of NHE3 by VIP and PACAP (neuropeptides expressed abundantly in the submucosal plexus) leads to a reduction in hPepT1 activity and therefore the intestinal absorptive capacity for small peptides. These observations suggest that any physiological (e.g. the neuropeptides VIP and PACAP) or pathophysiological (e.g. enterotoxins such as cholera) induced increase in enterocyte [cAMP]i, or any pharmacological (e.g. amiloride) inhibition of NHE3, will lead to a reduction in the absorptive capacity of any H+-coupled solute transporter which in the case of hPepT1 will lead to a decrease in nutrient absorption and peptide-like drug transport.
Acknowledgments
This study was supported by the BBSRC (grants 13/D09145 and 13/D17277). C.M.H. Anderson is supported by a BBSRC Agri-Food Committee Postgraduate Studentship. D.J. Kennedy. is supported by a Postgraduate Studentship from the University of Newcastle upon Tyne Faculty of Medicine. S1611 was obtained from H.J. Lang (Aventis Pharma Deutschland GmbH, Chemical Research, Frankfurt/Main, Germany).
Abbreviations
- BCECF
2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein
- 8-Br-cAMP
8- bromoadenosine-3′,5′-cyclic monophosphate
- EIPA
N-(ethyl-N-isopropyl)-amiloride
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- Gly-Sar
glycylsarcosine
- H-89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide.2HCl
- hPepT1
human intestinal di/tripeptide transporter
- NHE
Na+/H+ exchange
- NHERF
NHE regulatory factor
- PACAP
pituitary adenylate cyclase-activating polypeptide (1-27)
- pHi
intracellular pH
- PKA
protein kinase A
- RT–PCR
reverse transcriptase polymerase chain reaction
- VIP
vasoactive intestinal peptide
- VPAC1
vasoactive intestinal peptide/pituitary adenylate-cyclase activating peptide receptor 1
- VPAC2
vasoactive intestinal peptide/pituitary adenylate-cyclase activating peptide receptor 2
References
- BERLIOZ F., MAORET J.J., PARIS H., LABURTHE M., FARINOTTI R., ROZE C. alpha(2)-adrenergic receptors stimulate oligopeptide transport in a human intestinal cell line. J. Pharmacol. Exp. Ther. 2000;294:466–472. [PubMed] [Google Scholar]
- BLOOM S.R., POLAK J.M., PEARSE A.G.E. Vasoactive intestinal peptide and watery diarrhoea syndrome. Lancet. 1973;2:14–16. doi: 10.1016/s0140-6736(73)91947-8. [DOI] [PubMed] [Google Scholar]
- BRANT S.R., YUN C.H.C., DONOWITZ M., TSE C.M. Cloning, tissue distribution, and functional analysis of the human Na+/H+ exchanger isoform, NHE3. Am. J. Physiol. 1995;269:C198–C206. doi: 10.1152/ajpcell.1995.269.1.C198. [DOI] [PubMed] [Google Scholar]
- BROWN D.R., MILLER R.J.Neurohormonal control of fluid and electrolyte transport in intestinal mucosa Handbook of Physiology 1991Besthesda, MD: American Physiological Society; 527–587.(section 6, Vol 4)eds. Field, M. & Frizzell, R.A. pp [Google Scholar]
- BUYSE M., BERLIOZ F., GUILMEAU S., TSOCAS A., VOISIN T., PERANZI G., MERLIN D., LABURTHE M., LEWIN M.J.M., ROZE C., BADO A. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J. Clin. Invest. 2001;108:1483–1494. doi: 10.1172/JCI13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASSUTO J., FAHRENKRUG J., JODAL M., TUTTLE R., LUNDGREN O. Release of vasoactive intestinal peptide from the cat small intestine exposed to cholera toxin. Gut. 1981;22:958–963. doi: 10.1136/gut.22.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUVINEAU A., ROUYER-FESSARD C., DARMOUL D., MAORET J.J., CARRERO I., OGIER-DENIS E., LABURTHE M. Human intestinal VIP receptor: Cloning and functional expression of two cDNA encoding proteins with different N-terminal domains. Biochem. Biophys. Res. Comm. 1994;200:769–776. doi: 10.1006/bbrc.1994.1517. [DOI] [PubMed] [Google Scholar]
- DANIEL H., FETT C., KRATZ A. Demonstration and modification of intervillous pH profiles in rat small intestine in vitro. Am. J. Physiol. 1989;257:G489–G495. doi: 10.1152/ajpgi.1989.257.4.G489. [DOI] [PubMed] [Google Scholar]
- DANTZIG A.H., BERGIN L. Uptake of the cephalosporin, cephalexin, by a dipeptide transport carrier in the human intestinal cell line, Caco-2. Biochim. Biophys. Acta. 1990;1027:211–217. doi: 10.1016/0005-2736(90)90309-c. [DOI] [PubMed] [Google Scholar]
- DELIE F., RUBAS W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: Advantages and limitations of the Caco-2 model. Crit. Rev. Ther. Drug. 1997;14:221–286. [PubMed] [Google Scholar]
- DHARMSATHAPHORN K., HARMS V., YAMASHIRO D.J., HUGHES R.J., BINDER H.J., WRIGHT E.M. Preferential binding of vasoactive intestinal peptide to the basolateral membrane of rat and rabbit enterocytes. J. Clin. Invest. 1983;71:27–35. doi: 10.1172/JCI110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRI G.L., BOTTI P.L., VEZZADINI P., BILIOTTI G., BLOOM S.R., POLAK J.M. Peptide-containing innervation of the human intestinal mucosa. Histochemistry. 1982;76:413–420. doi: 10.1007/BF00543961. [DOI] [PubMed] [Google Scholar]
- GANAPATHY V., LEIBACH F.H. Is intestinal peptide transport energized by a proton gradient. Am. J. Physiol. 1985;249:G289–G294. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- HARMAR A.J., ARIMURA A., GOZES I., JOURNOT L., LABURTHE M., PISEGNA J.R., RAWLINGS S.R., ROBBERECHT P., SAID S.I., SREEDHARAN S.P., WANK S.A., WASCHEK J.A. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- HOOGERWERF W.A., TSAO S.C., DEVUYST O., LEVINE S.A., YUN C.H.C., YIP J.W., COHEN M.E., WILSON P.D., LAZENBY A.J., TSE C.M., DONOWITZ M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am. J. Physiol. 1996;270:G29–G41. doi: 10.1152/ajpgi.1996.270.1.G29. [DOI] [PubMed] [Google Scholar]
- HUMPHREY M.J., RINGROSE P.S. Peptides and related drugs: a review of their absorption, metabolism and excretion. Drug Metab. Rev. 1986;17:283–310. doi: 10.3109/03602538608998293. [DOI] [PubMed] [Google Scholar]
- KREJS G.J., FORDTRAN J.S., FAHRENKRUG J., SCHAFFALITZKY DE MUCKADELL O.B., FISCHER J.E., HUMPHREY C.S., O'DORISIO T.M., SAID S.I., WALSH J.H., SHULKES A.A. Effect of VIP infusion on water and ion transport in the human jejunum. Gastroenterology. 1980;78:722–727. [PubMed] [Google Scholar]
- LABURTHE M., ROUSSET M., ROUYER-FESSARD C., COUVINEAU A., CHANTRET I., CHEVALIER G., ZWEIBAUM A. Development of vasoactive intestinal peptide-responsive adenylate cyclase during enterocytic differentiation of Caco-2 cells in culture. J. Biol. Chem. 1987;262:10180–10184. [PubMed] [Google Scholar]
- LIANG R., FEI Y.J., PRASAD P.D., RAMAMOORTHY S., HAN H., YANG-FENG T.L., HEDIGER M.A., GANAPATHY V., LEIBACH F.H. Human intestinal H+/peptide cotransporter: cloning, functional expression, and chromosomal localization. J. Biol. Chem. 1995;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- MCEWAN G.T.A., DANIEL H., FETT C., BURGESS M.N., LUCAS M.L. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc. R. Soc. Lond. B. 1988;234:219–237. doi: 10.1098/rspb.1988.0045. [DOI] [PubMed] [Google Scholar]
- MCSWINE R.L., MUSCH M.W., BOOKSTEIN C., XIE Y., RAO M., CHANG E.B. Regulation of apical membrane Na+/H+ exchangers NHE2 and NHE3 in intestinal epithelial cell line C2/bbe. Am. J. Physiol. 1998;275:C693–C701. doi: 10.1152/ajpcell.1998.275.3.C693. [DOI] [PubMed] [Google Scholar]
- MOURAD F.H., NASSAR C.F. Effect of vasoactive intestinal polypeptide (VIP) antagonism on rat jejunal fluid and electrolyte secretion induced by cholera and Escherichia coli enterotoxins. Gut. 2000;47:382–386. doi: 10.1136/gut.47.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER U., BRANDSCH M., PRASAD P.D., FEI Y.J., GANAPATHY V., LEIBACH F.H. Inhibition of the H+/peptide cotransporter in the human intestinal cell line Caco-2 by cyclic AMP. Biochem. Biophys. Res. Comm. 1996;218:461–465. doi: 10.1006/bbrc.1996.0082. [DOI] [PubMed] [Google Scholar]
- MURTHY A., GONZALEZ-AGOSTI C., CORDERO E., PINNEY D., CANDIA C., SOLOMON F., GUSELLA J., RAMESH V. NHE-RF, a regulatory cofactor for Na+/H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J. Biol. Chem. 1998;273:1273–1276. doi: 10.1074/jbc.273.3.1273. [DOI] [PubMed] [Google Scholar]
- NICOLE P., LINS L., ROUYER-FESSARD C., DROUOT C., FULCRAND P., THOMAS A., COUVINEAU A., MARTINEZ J., BRASSEUR R., LABURTHE M. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. J. Biol. Chem. 2000; 275:24003–24012. doi: 10.1074/jbc.M002325200. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI J. Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low affinity (NHE-3) isoforms of the rat Na/H exchanger. J. Biol. Chem. 1993;268:16369–16377. [PubMed] [Google Scholar]
- RECZEK D., BERRYMAN M., BRETSCHER A. Identification of EBP50: a PDZ containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., INUI K.I. Dipeptide transporters in apical and basolateral membranes of the human intestinal cell line Caco-2. Am. J. Physiol. 1993;265:G289–G294. doi: 10.1152/ajpgi.1993.265.2.G289. [DOI] [PubMed] [Google Scholar]
- SALOMON R., COUVINEAU A., ROUYER-FESSARD C., VOISIN T., LAVALLEE D., BLAIS A., DARMOUL D., LABURTHE M. Characterization of a common VIP-PACAP receptor in human small intestinal epithelium. Am. J. Physiol. 1993;264:E294–E300. doi: 10.1152/ajpendo.1993.264.2.E294. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ C.J., KIMBERG D.V., SHEERIN H.E., FIELD M., SAID S.I. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J. Clin. Invest. 1974;54:536–544. doi: 10.1172/JCI107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLIN J.H.The pathophysiology of diarrhea Molecular Biology of Membrane Transport Disorders 1996New York: Plenum Press; 541–563.ed. Schultz, S.G. pp [Google Scholar]
- SUNDLER F., EKBLAD E., ABSOOD A., HAKANSON R., KOVES K., ARIMURA A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46:439–454. doi: 10.1016/0306-4522(92)90064-9. [DOI] [PubMed] [Google Scholar]
- SVOBODA M., TASTENOY M., VAN RAMPELBERGH J., GOOSSENS J.F., DE NEEF P., WAELBROECK M., ROBBERECHT P. Molecular cloning and functional characterization of a human VIP receptor from SUP-T1 lymphoblasts. Biochem. Biophys. Res. Commun. 1994;205:1617–1624. doi: 10.1006/bbrc.1994.2852. [DOI] [PubMed] [Google Scholar]
- TAMAI I., NAKANISHI T., NAKAHARA H., SAI Y., GANAPATHY V., LEIBACH F.H., TSUJI A. Improvement of L-dopa absorption by dipeptidyl derivation, utilizing peptide transporter PepT1. J. Pharm. Sci. 1998;87:1542–1546. doi: 10.1021/js980186o. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., BROWN C.D.A., HIRST B.H., SIMMONS N.L. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H+-coupled carriers at both apical and basal membranes. J. Biol. Chem. 1993a;268:7640–7642. [PubMed] [Google Scholar]
- THWAITES D.T., CAVET M., HIRST B.H., SIMMONS N.L. Angiotensin-converting enzyme (ACE) inhibitor transport in human intestinal epithelial (Caco-2) cells. Br. J. Pharmacol. 1995;114:981–986. doi: 10.1111/j.1476-5381.1995.tb13301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THWAITES D.T., FORD D., GLANVILLE M., SIMMONS N.L. H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J. Clin. Invest. 1999;104:629–635. doi: 10.1172/JCI7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THWAITES D.T., HIRST B.H., SIMMONS N.L. Direct assessment of dipeptide/H+ symport in intact human intestinal (Caco-2) epithelium: a novel method utilising continuous intracellular pH measurement. Biochem. Biophys. Res. Comm. 1993b;194:432–438. doi: 10.1006/bbrc.1993.1838. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., HIRST B.H., SIMMONS N.L. Substrate specificity of the di/tripeptide transporter in human intestinal epithelia (Caco-2): identification of substrates that undergo H+-coupled absorption. Br. J. Pharmacol. 1994;113:1050–1056. doi: 10.1111/j.1476-5381.1994.tb17099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THWAITES D.T., KENNEDY D.J., RALDUA D., ANDERSON C.M.H., MENDOZA M.E., BLADEN C.L., SIMMONS N.L. H+/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na+/H+ exchanger. Gastroenterology. 2002;122:1322–1333. doi: 10.1053/gast.2002.32992. [DOI] [PubMed] [Google Scholar]
- VAUDRY D., GONZALEZ B.J., BASILLE M., YON L., FOURNIER A., VAUDRY H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- VOISIN T., ROUYER-FESSARD C., LABURTHE M. Distribution of common peptide YY-neuropeptide Y receptor along rat intestinal villus-crypt axis. Am. J. Physiol. 1990;258:G753–G759. doi: 10.1152/ajpgi.1990.258.5.G753. [DOI] [PubMed] [Google Scholar]
- WALKER D., THWAITES D.T., SIMMONS N.L., GILBERT H.J., HIRST B.H. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J. Physiol. 1998;507:697–706. doi: 10.1111/j.1469-7793.1998.697bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON A.J.M., LEVINE S., DONOWITZ M., MONTROSE M.H. Kinetics and regulation of a polarized Na+/H+ exchanger from Caco-2 cells, a human intestinal cell line. Am. J. Physiol. 1991;261:G229–G238. doi: 10.1152/ajpgi.1991.261.2.G229. [DOI] [PubMed] [Google Scholar]
- WEINMAN E.J., MINKOFF C., SHENOLIKAR S. Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA. Am. J. Physiol. 2000;279:F393–F399. doi: 10.1152/ajprenal.2000.279.3.F393. [DOI] [PubMed] [Google Scholar]
- WENZEL U., THWAITES D.T., DANIEL H. Stereoselective uptake of β-lactam antibiotics by the intesinal peptide transporter. Br. J. Pharmacol. 1995;116:3021–3027. doi: 10.1111/j.1476-5381.1995.tb15958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTPHAL J.F., JEHL F., BROGARD J.M., CARBON C. Amoxicillin intestinal absorption reduction by amiloride: possible role of the Na+-H+ exchanger. Clin. Pharmacol. Ther. 1995;57:257–264. doi: 10.1016/0009-9236(95)90150-7. [DOI] [PubMed] [Google Scholar]
- WIEMANN M., SCHWARK J.R., BONNET U., JANSEN H.W., GRINSTEIN S., BAKER R.E., LANG H.J., WIRTH K., BINGMANN D. Selective inhibition of the Na+/H+ exchanger type 3 activates CO2/H+-sensitive medullary neurones. Pflügers Arch. 1999;438:255–262. doi: 10.1007/s004240050907. [DOI] [PubMed] [Google Scholar]
- YANG C.Y., DANTZIG A.H., PIDGEON C. Intestinal peptide transport systems and oral drug availability. Pharm. Res. 1999;16:1331–1343. doi: 10.1023/a:1018982505021. [DOI] [PubMed] [Google Scholar]
- YUN C.H.C., OH S., ZIZAK M., STEPLOCK D., TSAO S., TSE C.M., WEINMAN E.J., DONOWITZ M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3010–3015. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]


