Abstract
There appears to be a controversy in the study of myocardial ischaemia-reperfusion injury and preconditioning whether nitric oxide (NO) plays a protective or detrimental role. A number of findings and the interpretation of the results to date do not support such a controversy. An understanding of the latest developments in NO, superoxide (O2−·) and peroxynitrite (ONOO−) biology, as well as the various ischaemic animal models utilized is necessary to resolve the apparent controversy. NO is an important cardioprotective molecule via its vasodilator, antioxidant, antiplatelet, and antineutrophil actions and it is essential for normal heart function. However, NO is detrimental if it combines with O2−· to form ONOO− which rapidly decomposes to highly reactive oxidant species. There is a critical balance between cellular concentrations of NO, O2−·, and superoxide dismutase which physiologically favour NO production but in pathological conditions such as ischaemia and reperfusion result in ONOO− formation. In contrast, exposure of the heart to brief episode(s) of ischaemia markedly enhances its ability to withstand a subsequent ischaemic injury. The triggering of this endogenous cardioprotective mechanism known as preconditioning requires both NO and O2−· synthesis. However, preconditioning in turn attenuates the overproduction of NO, O2−· and ONOO− during a subsequent episode of ischaemia and reperfusion, thereby protecting the heart. Here we review the roles of NO, O2−·, and ONOO− in both ischaemia-reperfusion injury and preconditioning.
Keywords: Ischaemia, reperfusion, cardioprotection, ischaemic preconditioning, nitric oxide, superoxide, peroxynitrite, matrix metalloproteinase
Myocardial ischaemia-reperfusion andpreconditioning
Ischaemic heart disease, a major cause of mortality in industrialized societies, is characterized by insufficient blood supply to regions of the myocardium which leads to tissue necrosis (infarction). Ischaemic heart disease develops as a consequence of many diseases, including hypertension, atherosclerosis, hyperlipidaemia, and diabetes. The treatment of this condition has entered a new era where mortality can be approximately halved by procedures which allow the rapid return of blood flow, i.e. reperfusion, to the ischaemic zone of the myocardium. Reperfusion, however, may lead to further complications such as diminished cardiac contractile function and arrhythmias (see for review: Braunwald, 1985). Therefore, development of cardioprotective agents which improve myocardial function, decrease the incidence of arrhythmias, lessen the necrotic tissue mass, and delay the onset of necrosis during ischaemia/reperfusion is of great clinical importance. Previous attempts to attenuate the consequences of ischaemia/reperfusion injury with pharmacological tools have been largely unsuccessful. However, the heart was found to be able to adapt to ischaemic stress (Murry et al., 1986). Ischaemic preconditioning is a well-described adaptive response in which brief exposure to ischaemia markedly enhances the ability of the heart to withstand a subsequent ischaemic injury (see for review: Przyklenk & Kloner, 1998). The discovery of this endogenous cardioprotective mechanism encouraged the exploration of new ways to protect the ischaemic/reperfused myocardium. The exact cellular mechanism of preconditioning is still a question of debate, however, among several other mediators, NO, superoxide (O2−·), and peroxynitrite (ONOO−) certainly play a role in both ischaemia/reperfusion injury and in the development of the cardioprotective effect of preconditioning (see for reviews: Baxter & Ferdinandy, 2001; Bolli, 2001; Bolli et al., 1998; Schulz et al., 2001).
Nitric oxide: cardioprotective or detrimental?
There appears to be a controversy in the study of myocardial ischaemia-reperfusion injury and preconditioning whether NO plays a protective or detrimental role. Findings from our laboratories and others, and interpretation of the results to date, do not support such a controversy. An understanding of the latest development in NO, O2−· and ONOO− biology as well as the various ischaemic animal models utilized is necessary to resolve the apparent controversy.
Coronary endothelium, endocardial endothelium, cardiac nerves, and cardiomyocytes of the normal heart are all sources of the basal production of NO by Ca2+-dependent NO synthases (NOS) (Curtis & Pabla, 1997; Pabla & Curtis, 1996). This serves a number of important physiological roles in the regulation of cardiac function including coronary vasodilation, inhibiting platelet and neutrophil actions, antioxidant effects, modulation of cardiac contractile function, and inhibiting cardiac oxygen consumption (Hare & Comerford, 1995; Xie & Wolin, 1996).
NO plays protective roles in the ischaemic heart by several mechanisms (Figure 1) such as stimulating soluble guanylate cyclase and thus reducing [Ca2+]i partly through activation of cGMP-dependent protein kinase, terminating chain propagating lipid radical reactions caused by oxidative stress (Rubbo et al., 1994), and by inhibiting the activity of platelets and neutrophils and their adhesion to the endothelial surface (Kubes et al., 1991; Radomski et al., 1987). NO (either via NOS acitivity or through NO donors) protects against the toxic effects of exogenously supplied ONOO− in the coronary circulation (Villa et al., 1994), on platelets (Moro et al., 1994), or in liposomes (Rubbo et al., 1994). Whether NO-induced inhibition of mithochondrial respiration and modulation of apoptosis is protective or detrimental in ischaemia/reperfusion is not clear in the literature. NO through its interaction with components of the mitochondrial respiratory chain might function not only as a physiological regulator of cell respiration, but also as a modulator of the generation of reactive oxygen species by mitochondria, thereby affecting mechanisms of cell survival or death (Moncada & Erusalimsky, 2002). It should be noted that the exact role of NO in apoptosis is not known as NO has been shown to exert both proapoptotic and antiapoptotic effects in the myocardium (Gao et al., 2002a; Iwai-Kanai et al., 2002; Sam et al., 2001; Taimor et al., 2000; Weiland et al., 2000). The controversy is possibly due to the lack of ONOO− measurements in these studies.
Figure 1.
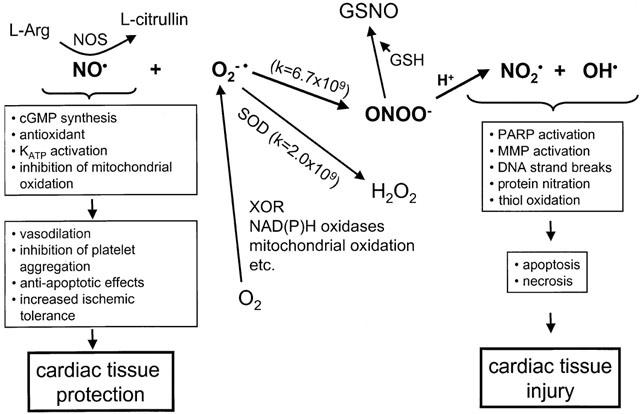
Cellular mechanisms of nitric oxide (NO), superoxide (O2−·), and peroxynitrite (ONOO−) actions. NO is an important cardioprotective molecule via its vasodilator, antioxidant, antiplatelet, and antineutrophil actions and it is essential for normal heart function. However, NO is detrimental if it combines with O2−· to form ONOO− which rapidly decomposes to highly reactive oxidant species leading to tissue injury. There is a critical balance between cellular concentrations of NO, O2−·, and superoxide dismutase (SOD) which physiologically favor NO production but in pathological conditions such as ischaemia and reperfusion result in ONOO− formation. ONOO− is detoxified if it combines with reduced glutathione (GSH) or other thiols to form S-nitrosoglutathione (GSNO) or other nitrosothials, an NO donor molecule. MMP – matrix metalloproteinase; NOS – NO synthase; PARP – poly-ADP ribose synthase; XOR – xanthine oxidoreductase.
Toxicity of NO via reaction with O2−· toform ONOO−
NO is necessary for normal cardiac physiology, but it is potentially toxic in excess concentrations. It is now understood that many of the toxic actions of NO are not directly due to NO itself but are mediated via production of ONOO−, the reaction product of NO with O2−· (Figure 1; Beckman & Koppenol, 1996; Rubbo et al., 1996). Although formation of ONOO− has been shown to contribute to several cardiovascular pathologies including ischaemia/reperfusion injury, the possible physiological role of ONOO− is not known.
Possible sources of O2−· in the heart include NAD(P)H oxidoreductases (Mohazzab et al., 1997), xanthine oxidoreductases (Hille & Nishino, 1995), mitochondrial electron transport chain activity, activated neutrophils, arachidonic acid metabolism, and auto-oxidation of certain tissue metabolites. The highly oxidative heart muscle is subjected to its own basal production of O2−· (Boveris & Chance, 1973) and NO (Hare & Comerford, 1995). Mitochondrial Mn-superoxide dismutase (SOD), cytosolic Cu-Zn SOD, extracellular Cu-Zn SOD, glutathione (GSH), uric acid, and catalase are the more important endogenous antioxidants.
NO and O2−· combine at a reaction rate which is only limited by diffusion to form ONOO−. ONOO− at pH<8 is protonated to form the unstable intermediate, peroxynitrous acid, which spontaneously decomposes to yield highly reactive oxidant species (Figure 1). Understanding the balance between local concentrations of NO, O2−·, and SOD is critical in understanding NO biology and its potential toxicity in the form of ONOO− (Beckman & Koppenol, 1996; Rubbo et al., 1996). One must consider the competition between NO and SOD for O2−·. Under normal physiological conditions in vascular endothelium, [NO] is ∼10 nM, [SOD] is ∼1 μM. Therefore, the reaction rate of O2−· to form ONOO− is only a fraction of the dismutation rate of O2−· by SOD, and, as a result, very little ONOO− is formed. However, at maximal vascular rates of NO production (i.e. which may occur during acute reperfusion of ischaemic tissue or during inducible NOS expression), [NO] is⩾1 μM. Therefore, the formation of ONOO− will predominate over the dismutation of O2−·. Under a variety of cellular stress conditions where NO production is upregulated in the endothelium and cardiomyocytes, either by acute increase in Ca2+-dependent NOS activity via changes in shear stress or high [Ca2+]i which occurs during acute reperfusion and ischaemia, or via de novo expression of inducible NOS in cells or tissues as a result of the action of pro-inflammatory cytokines, one predicts the formation of ONOO− (Csonka et al., 1999; Depre & Hue, 1994; Ferdinandy et al., 2000).
Pathophysiology of ONOO− in myocardialischaemia-reperfusion
Acute injury
We have shown first that ONOO− is produced during the acute reperfusion of ischaemic hearts and that drugs which inhibit ONOO− formation or antagonize its toxicity protect the heart from reperfusion injury (Yasmin et al., 1997; Cheung et al., 2000b). We have also developed a novel, simple and effective methodology to detect cardiac ONOO− based on the reaction of ONOO− with tyrosine to form dityrosine (Ferdinandy & Schulz, 2001a; Yasmin et al., 1997). Rapid generation of ONOO− during reperfusion of the ischaemic heart has also been detected using luminol chemiluminescence in the perfusate and anti-nitrotyrosine labelling of myocardial proteins (Wang & Zweier, 1996). We showed that low concentrations of the NOS inhibitor NG-monomethyl-L-arginine (L-NMMA), or a cell permeable SOD mimetic, MnTBAP protected the hearts from ischaemia-reperfusion injury. The beneficial effect of L-NMMA fell within a narrow range of concentrations and was lost at higher concentrations which further reduced coronary flow (Yasmin et al., 1997). Our data also showed that a NO donor, at subvasodilatory concentration, protected hearts from endogenous ONOO−-mediated injury. This study provided the first mechanistic evidence of how either NO donors by detoxifying ONOO− or NOS inhibitors by inhibiting ONOO− formation are able to reduce ischaemia-reperfusion injury (Yasmin et al., 1997).
Myocardial infarction (late injury, neutrophil dependent)
What is the importance of the neutrophil-independent free radical release during acute reperfusion, as opposed to the neutrophil-dependent reperfusion events which occur several hours later? It should be noted that the late influx of neutrophils does not substantially contribute to the development of infarction, however, the literature is somewhat controversial in this regard (Williams et al., 1994; Baxter, 2002; Gao et al., 2002b; Jordan et al., 1999). One very important defect in the heart resulting from acute reperfusion following ischaemia is endothelial injury, which is manifested by reduced endothelium-dependent vasodilator response within the first minute of reperfusion (Siegfried et al., 1992). We speculate that this damage is self-inflicted, resulting from the burst of endogenous ONOO− from the vascular endothelium immediately at reperfusion. This would cause enhanced susceptibility of the endothelial surface to neutrophil and platelet adhesion, platelet aggregation and neutrophil activation, events which are normally inhibited by the physiological production of endothelium-derived NO (Kubes et al., 1991; Radomski et al., 1987). Villa et al. (1994) showed that bolus injection of ONOO− into isolated hearts acutely inhibited endothelium-dependent coronary vasodilation. Myocardial ONOO− generation has also been shown to occur during the neutrophil-dependent phase of ischaemia-reperfusion injury, seen 5 h after reperfusion, using an in vivo model of regional ischaemia in rats (Liu et al., 1997). An understanding of the oxidative damage which occurs during acute reperfusion following ischaemia is thus crucial to help devise strategies to reduce the possible subsequent neutrophil-mediated damage in the later stages of reperfusion injury.
Cellular targets of ONOO−
The possible downstream targets of ONOO− which mediate its toxicity are several (Figure 1). Its highly reactive decomposition products at physiological or acidic pH can attack protein (oxidation of sulfhydryls, nitration of tyrosine residues), lipids (formation of lipid peroxides), and DNA (strand breakage). This results in the depletion of low molecular weight antioxidants such as glutathione and most often the inhibition of several enzyme activities including superoxide dismutase, aconitase and other enzymes of the mitochondrial respiratory chain, creatine kinase, Ca2+-ATPase, Na+-K+-ATPase, glutathione peroxidase, prostacyclin synthase, α-antiproteinase and many others, just to name a few (see for reviews: Beckman & Koppenol, 1996; Ronson et al., 1999; Rubbo et al., 1996; Szabó, 1996). As a result of DNA strand breakage activation of the NAD+ consuming DNA repair enzyme poly-ADP ribose synthase contributes further to the depletion of cellular energy stores (Szabó, 1996). ONOO− has been also shown to cause irreversible inhibition of the mitochondrial respiratory chain (Brown, 2001; Xie & Wolin, 1996) and to trigger apoptosis of cardiac myocytes (Arstall et al., 1999; Beckman, 1999). An important question is which of these multiple effects represents an early event in ONOO−-mediated toxicity, at a time point prior to the onset of irreversible energy depletion, cellular damage and death.
Interestingly, ONOO− may also activate some proteins, again due to its ability to react with critical thiol residues. At low micromolar concentrations Maeda's group have shown that ONOO− activates the zymogen form of matrix metalloproteinases (MMPs) (Okamoto et al., 1997; 2001). This family of proteolytic enzymes, best known for their ability to degrade and remodel the extracellular matrix, are now recognized to have a variety of novel actions as proteases, both physiological and pathological, on substrates apart from the extracellular matrix. As we showed that ONOO− biosynthesis peaks in isolated rat hearts in the first minute of reperfusion following ischaemia and contributes to contractile dysfunction (Yasmin et al., 1997) we addressed whether MMP activation could play a role in the detrimental actions of ONOO− in the heart. We found that MMP-2 is activated and released from the reperfused heart, peaking in the first minute of reperfusion, and that inhibition of MMP-2 activity functionally protected the heart (Cheung et al., 2000a). Moreover, infusion of ONOO− into isolated rat hearts resulted in MMP-2 activation which preceded the onset of mechanical dysfunction, an effect which could be abolished either with glutathione or an inhibitor of MMP activity (Wang et al., 2002a). MMP-2 was found to colocalize with the contractile protein regulatory element troponin I in cardiac myocytes and upon ischaemia-reperfusion injury was found to be responsible for the proteolytic cleavage of troponin I (Wang et al., 2002b), one of the contributory factors to altered contractility in myocardial ischaemia/reperfusion injury (Bolli & Marban, 1999). It is thus apparent that an important target of the early phase of ONOO−-induced oxidative stress injury in the heart is activation of MMP-2 and its resultant proteolysis of novel intracellular targets, including troponin I.
Can ONOO− protect the heart againstmyocardial ischaemia-reperfusion?
The literature is somewhat controversial as to whether ONOO− is either cytotoxic or cytoprotective (Ferdinandy & Schulz, 2001b; Vinten-Johansen, 2000). It seems that deleterious effects of ONOO− in the heart are predominantly observed in in vitro or ex vivo crystalloid buffer perfused systems, however, ONOO− is cardioprotective if applied in vivo. However, this controversy derives solely from the rapid reactions of exogenously administered ONOO− with a variety of biomolecules. Simply, exogenous administration of ONOO− does not accurately reflect pathological conditions in which intracellular generation of ONOO− is enhanced.
Due to the very short half life of ONOO− at physiological pH, it has very little chance to reach its cellular targets when it is applied via the blood, as it rapidly reacts with plasma proteins and thiols such as GSH. Thus, ONOO− is likely to be detoxified before it has a chance to reach tissues downstream of the injection site, let alone the intracellular compartment (Ishida et al., 1999). ONOO− oxidizes thiols (Cheung et al., 1998), e.g. it reacts with GSH to form the nitrosothiol, nitrosoglutathione, a NO donor (Figure 1; Mayer et al., 1995; Prendergast et al., 1997; Villa et al., 1994). By this reaction ONOO− is a vasodilator (Villa et al., 1994; Wu et al., 1994) and inhibitor of platelet aggregation (Moro et al., 1994). Thus nature has a built-in mechanism to transform toxic ONOO− into a NO donor. Indeed, hearts with enhanced endogenous GSH levels are less susceptible to ischaemia-reperfusion injury (Kirshenbaum & Singal, 1993) and micromolar concentrations of GSH added to the perfusate protects isolated hearts from post-ischaemic contractile dysfunction through the reduced formation of ONOO− at reperfusion (Cheung et al., 2000b). Exogenously administered ONOO− was also shown to inhibit leukocyte-endothelial cell interactions and to protect against ischaemia-reperfusion injury in rats in vivo (Lefer et al., 1997). Intraventricular infusion of ONOO−reduced myocardial infarct size and preserved coronary endothelium after ischaemia and reperfusion in cats (Nossuli et al., 1997), which effects were mediated by the intermediate formation of S-nitrosothiols (Nossuli et al., 1998).
Exogenously applied ONOO−, however, has been shown to be detrimental to cellular functions when it was applied in crystalloid buffer systems, in which the concentrations of extracellular antioxidants and both free and protein bound thiols are limited. In this case, exogenous ONOO− and its toxic metabolites have a greater chance to reach their cellular targets and cause injury, this of course being dependent upon concentration and the duration of exposure. We have shown that continuous infusion of 40 but not 4 μM ONOO− into isolated working rat hearts perfused with Krebs-Henseleit buffer impaired cardiac contractile function within 45 min (Schulz et al., 1997). Authentic ONOO− inhibited contractile function in cardiac myocytes (Ishida et al., 1996) and in isolated rat papillary muscle (Digerness et al., 1999). Administration of the ONOO− generator SIN-1 exerted either cardiodepression in crystalloid buffer-perfused or cardioprotection in blood-perfused rat hearts (Ma et al., 2000).
One can conclude that exogenous ONOO− is toxic when applied in crystalloid perfused hearts, however, it can be protective under experimental conditions in which ONOO− first reacts with thiol groups, thereby forming NO donors. It is questionable whether this mechanism of ONOO− detoxification is always of the capacity to eliminate a sufficient portion of ONOO− at the site of its endogenous formation, especially under conditions of ischaemia/reperfusion. To our knowledge, there is no literature showing any tissue protective effect of endogenously formed ONOO−. In contrast, many studies show that enhanced formation of ONOO− in the myocardium is cytotoxic to the heart and contributes to ischaemia/reperfusion injury in isolated rat hearts (Wang & Zweier, 1996; Yasmin et al., 1997) and anaesthetized rats (Liu et al., 1997), and in other pathological models of myocardial dysfunction in several species in vivo or ex vivo (Ferdinandy et al., 1999; 2000; Ishiyama et al., 1997; Oyama et al., 1998; Sakurai et al., 1999; Weinstein et al., 2000) including myocardial inflammation in humans (Kooy et al., 1997). Many of these studies showed a correlation between endogenous ONOO− formation and deterioration of cardiac function.
Taken together, there is a consensus in the literature that endogenously formed ONOO− contributes to the injury to the heart as a result of ischaemia/reperfusion and other cardiac pathologies.
NO is cardioprotective in ischaemia-reperfusion injury
A number of ex vivo studies using isolated crystalloid-buffer perfused hearts have shown that enhancing NO levels, either by applying NO donors, NO-dependent vasodilators, L-arginine supplementation, angiotensin converting enzyme inhibitors, or pretreating the animal with endotoxin analogues which among several other effects enhance the activities of myocardial inducible NOS, cyclo-oxygenase, and antioxidant enzymes, functionally protect the heart from acute ischaemia/reperfusion injury and/or infarct size development (Masini et al., 1991; Massoudy et al., 1995; Schoelkens & Linz, 1992; Xi et al., 1999; Yasmin et al., 1997). Whether this protective effect is due to the antioxidant properties of NO, lowering [Ca2+]i via activation of guanylate cyclase, modulating mitochondrial respiration, influencing apoptosis, or other mechanisms is not completely understood at the moment.
In vivo studies show that NO donors improve the recovery of mechanical function and/or reduce infarct size following ischaemia-reperfusion. Subvasodilatory doses of NO donors (Siegfried et al., 1992), L-arginine (Weyrich et al., 1992) or agonists of endothelium-derived NO (Richard et al., 1995) were shown to be protective (see for review: Grisham et al., 1998). In vivo studies using endothelial NOS knockout mice enhance the notion that the basal release of NO in the heart is an important endogeneous cardiac protectant, particularly in regards to the prevention of neutrophil sticking and platelet activation. Infarct size in endothelial NOS knockout mice was larger than in the wildtype controls, with higher P-selectin expression and significantly more neutrophils in hearts from endothelial NOS knockout mice (Jones et al., 1999a). Yang et al. (1999) showed that the protective effect of an angiotensin converting enzyme inhibitor in ischaemia-reperfusion injury in wildtype mice was lost in the corresponding endothelial NOS knockout mice. In contrast, an ex vivo study showed that the functional recovery of hearts from endothelial NOS knockout mice was improved in comparison to wildtype controls (Flogel et al., 1999), suggesting that NO and thus ONOO− generated from endothelial NOS contributes to ischaemia/reperfusion injury seen in early reperfusion.
Inhibition of NOS: can it also be protective in ischaemia-reperfusion injury?
Ex vivo studies
Despite evidence that supplementation of NO can protect hearts from ischaemia-reperfusion injury, reports that inhibition of NOS can also prevent such injury began to appear. Most importantly, all studies using NOS inhibitors must be evaluated in regard to the concentration, potency and efficacy of the NOS inhibitor used, as well as to experimental details (i.e. constant flow versus constant pressure perfusion, global versus low-flow ischaemia, etc.). Low to moderate inhibitory concentrations of L-NAME (3 μM) or L-NMMA (30 μM) protected isolated working rabbit hearts from ischaemia/reperfusion injury when given prior to the onset of global, no-flow ischaemia (Schulz & Wambolt, 1995). We later showed that the mechanism of cardioprotection by L-NMMA in constant pressure-perfused rat hearts subjected to ischaemia-reperfusion was by reducing the formation of ONOO− at reperfusion (Yasmin et al., 1997). Depre et al. (1995) showed that L-NMMA at concentrations between 0.001–10 μM significantly protected isolated rabbit hearts perfused at constant flow and subjected to low-flow ischaemia. They suggested that the protective effect of L-NMMA was by the enhancement of glycolysis during ischaemia, however, they could not exclude a protective effect during reperfusion itself. Another study using constant flow-perfused hearts suggested additional protective effects of L-NAME (30 μM), including the stimulation of adenosine release which occurs only at such high levels of NOS inhibition (Woolfson et al., 1995). A study in isolated rat hearts which used 1 mM L-NAME (Wang & Zweier, 1996), supramaximal in terms of blocking NO biosynthesis, may have included protective effects unrelated to NOS inhibition, as L-NAME in particular has been shown to be a muscarinic receptor antagonist at concentrations greater than 100 μM (Buxton et al., 1993).
In vivo studies
Essentially there is no study to date which carefully assesses the dose-dependent actions of NOS inhibitors in myocardial ischaemia-reperfusion injury in vivo. This is an obvious gap in the literature. Studies using high doses of NOS inhibitors (i.e.>IC50), which significantly raise mean arterial blood pressure, uniformly provide evidence of a worsened outcome in terms of infarct size or function post ischaemia reperfusion (Hoshida et al., 1995; Williams et al., 1995). Interestingly, a study in open-chested rats subjected to only 4 min of regional ischaemia and 4 min of reperfusion showed that low doses of L-NAME caused a marked reduction in mortality due to ventricular fibrillation. This protective effect was abolished by co-administration of L-arginine and potentiated by co-administration of SOD, suggesting that reperfusion arrythmias may be due to the generation of ONOO− (Ohoi & Takeo, 1996).
Myocardial adaptation to ischaemia:preconditioning
Preconditioning confers a remarkable cardioprotection in a variety of species including humans (see for review: Przyklenk & Kloner, 1998), although the cardioprotective effectiveness of ischaemic preconditioning might be attenuated in the heart during aging and some disease states such as hyperlipidaemia and diabetes (see for review: Ferdinandy et al., 1998). Preconditioning can be elicited by different sublethal stress signals, such as brief periods of ischaemia, hypoxia, rapid electrical pacing, heat stress, or administration of bacterial endotoxin, etc. The cardioprotective effect of preconditioning shows two distinct phases. The early phase is manifested within minutes after the preconditioning stimulus and has a duration of less than 3 h. The late phase is characterized by a slower onset (⩾20 h) and a duration of up to 72 h. Both phases of preconditioning involve reduction of necrotic tissue mass (infarct size), improvement of cardiac performance and reduction of arrhythmias following ischaemia and reperfusion (see for reviews: Baxter & Ferdinandy, 2001; Ferdinandy et al., 1998; Przyklenk & Kloner, 1998).
Among several other mediators, NO, oxygen free radicals, and antioxidant enzymes have been suggested to be, and also refuted as, key triggers and mediators of preconditioning. NO has been also suggested to play a role in the mechanism of other well known triggers of preconditioning, such as adenosine, bradykinin, and opioids (see for review: Bolli, 2001). There is still a considerable debate regarding the exact cellular mechanism of ischaemic preconditioning (see for review: Schulz et al., 2001). The mechanisms of early and late preconditioning seem to be different. Further discrepancies are generally attributed to species differences, different stimuli to induce preconditioning, and different study end-points, i.e. myocardial function, arrhythmias, or infarct size. Understanding the cellular pathways involved in the ischaemic adaptation of the myocardium may lead to the development of ‘preconditioning mimetic' drugs for patients suffering from ischaemic heart disease.
NO, O2−·, and ONOO− in earlypreconditioning
The role of NO in early preconditioning was suggested first by Vegh et al. (1992). They demonstrated that 10 mg kg−1 L-NAME administered both before and after preconditioning abolished the antiarrhythmic effect of preconditioning in a coronary occlusion model of anaesthetized, open chest dogs (Vegh et al., 1992). Bilinska et al. (1996) reported that infusion of the NO donors nitroglycerin (500 μM) or SIN-1 (10 μM) for 5 min before coronary occlusion mimicked the antiarrhythmic effect of ischaemic preconditioning in isolated rat hearts. Using the electron spin resonance technique to directly measure cardiac NO content we have shown that a decrease in basal cardiac NO content before preconditioning, due to either in vivo pretreatment with 1 mg kg−1 L-nitroarginine (Ferdinandy et al., 1996), experimental hypercholesterolaemia (Ferdinandy et al., 1997b), or selective depletion of neurotransmitters including NO from cardiac sensory neurons (Ferdinandy et al., 1997a) leads to the loss of pacing-induced preconditioning in isolated working rat hearts. However, preconditioning, in turn, markedly decreased the accumulation of NO in the heart tissue during subsequent ischaemia and reperfusion in isolated working rat hearts (Csonka et al., 1999). In the presence of 4.6 μM L-nitroarginine, a nonvasoactive concentration in this model which reduced basal NO synthesis, preconditioning failed to protect against ischaemia-reperfusion and failed to attenuate NO accumulation produced by ischaemia-reperfusion. When L-nitroarginine was applied after the preconditioning protocol, the effect of preconditioning on test ischaemia-reperfusion and ischaemic NO accumulation was not affected (Csonka et al., 1999; Ferdinandy et al., 1997b). These results prove that intact NO biosynthesis is required for the triggering mechanism of preconditioning and further show that the cardioprotection provided by preconditioning involves a mechanism which decreases the accumulation of NO in the myocardium during ischaemia and reperfusion. In accordance with this, Woolfson et al. (1995) reported that the limitation of infarct size after inhibition of NO synthesis by L-NAME shares a common mechanism with ischaemic preconditioning in rabbit hearts. Furthermore, preliminary studies by Wang & Zweier (1997) showed that preconditioning decreases NO synthesis during subsequent ischaemia and this is associated with the protective effect of preconditioning in isolated rat hearts.
The nature of preconditioning-induced inhibition of NO synthesis is not known. Preconditioning may decrease the rate of enzymatic and/or nonenzymatic (Zweier et al., 1995) NO production during ischaemia-reperfusion by altering cellular pH and the availability of cofactors and/or arginine for NO synthesis, or may possibly stimulate the formation of endogenous NOS inhibitors (Vallance et al., 1992).
In contrast to the aforementioned studies, Lu et al. (1995) reported that 10 mg kg−1 L-NMMA or L-NAME did not affect the antiarrhythmic effect of preconditioning in anaesthetized rats with coronary occlusion/reperfusion. Weselcouch et al. (1995) showed that in rat hearts, 30 μM L-NAME did not interfere with the effect of preconditioning on post-ischaemic myocardial function. In an isolated rabbit heart study, 100 μM L-NAME failed to block the infarct size limiting effect of preconditioning, nor had it any effect on infarct size without preconditioning. However, enhancement of exogenous NO levels by pretreatment with a NO donor induced preconditioning via an antioxidant pathway (Nakano et al., 2000). In these studies, surprisingly, the different NOS inhibitors neither interfered with preconditioning nor the outcome of ischaemia-reperfusion without the preceding preconditioning. Since neither NO generation nor NOS activities were determined in these studies and only a single dose of NOS inhibitors were used, it is difficult to interpret these negative results.
The role of NO in the mechanism of other well established triggers of early preconditioning such as adenosine and bradykinin has not been well established. Little is known whether NO plays a significant role in adenosine-induced early cardioprotection (Cargnoni et al., 1999; Gao et al., 2000; Gattullo et al., 1999; Mubagwa & Flameng, 2001). It seems that bradykinin, a well known endothelium-dependent vasodilator, triggers cardioprotection via an NO-independent pathway in rats and rabbits (Bugge & Ytrehus, 1996; Goto et al., 1995).
The possible role for oxygen free radicals in preconditioning was suggested by Tanaka et al. (1994) who showed that the antioxidants SOD or mercaptopropionyl glycine were both able to inhibit the protective effect of preconditioning on infarct size in rabbits. Osada et al. (1991) reported that the antiarrhythmic effect of preconditioning was lost when the preconditioning ischaemia was applied in the presence of both SOD and catalase in isolated rat hearts. Tritto et al. (1997) showed that a 5 min infusion of O2−· generated by purine/xanthine oxidase prior to ischaemia-reperfusion resulted in a reduction of infarct size in rabbit hearts, similar to the effect of preconditioning. Activation of mitochondrial KATP channels has been shown in several studies to play a role in preconditioning (Liu et al., 1998; Schulz et al., 2001), however, it was recently suggested that opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals (Pain et al., 2000). These studies strongly suggest that the generation of free radicals during preconditioning stimuli is necessary to trigger the protective machinery of preconditioning. Preconditioning, in turn, attenuates the increased free radical synthesis during subsequent ischaemia-reperfusion (Tosaki et al., 1994). Others, however, showed that preconditioning was not affected by SOD in rabbit hearts (Iwamoto et al., 1991; Omar et al., 1991), however, it can be explained by the very low cell permeability of SOD.
As the majority of studies show that NO and oxygen free radicals are both required to elicit preconditioning, it was plausible to speculate that formation of ONOO− is an important oxidative stimulus to trigger cellular adaptive mechanisms. Altug et al., (2000; 2001) have found that brief exposure of isolated rat hearts to 1 μM exogenous ONOO− was capable of mimicking the beneficial effects of ischaemic preconditioning, and this was abolished by the administration of the antioxidant mercaptopropionylglycine. Others have shown that administration of a ONOO− generating system triggered early preconditioning in rabbits in vivo. As exogenous ONOO− may not properly reflect the effect of endogenous ONOO− formation, we have recently measured endogenous ONOO− formation during preconditioning induced by three brief cycles of ischaemia and reperfusion and also during subsequent test ischaemia/reperfusion in isolated working rat hearts to clarify the possible contribution of endogenous ONOO− to ischaemic preconditioning. When test ischaemia and reperfusion were preceded by preconditioning, ONOO− formation was markedly attenuated upon reperfusion (Csonka et al., 2001). We have also found that the first brief period of ischaemia/reperfusion significantly enhanced endogenous ONOO− formation, which was reduced after subsequent cycles of brief ischaemia/reperfusion (Csonka et al., 2001). These findings are in accordance with that of Liaudet et al. (2001) showing that in mice and rats with coronary occlusion, myocardial preconditioning attenuates reperfusion-induced activation of poly-ADP ribose synthase. This is possibly due to inhibition of ONOO− formation, as ONOO− is a well-known activator of poly-ADP ribose synthase (Szabó et al., 1996).
Taken together, these results show that ONOO− formation during ischaemia/reperfusion might act as a trigger for preconditioning, but preconditioning in turn decreases formation of ONOO− upon subsequent cycle(s) of ischaemia/reperfusion (Figure 2).
Figure 2.
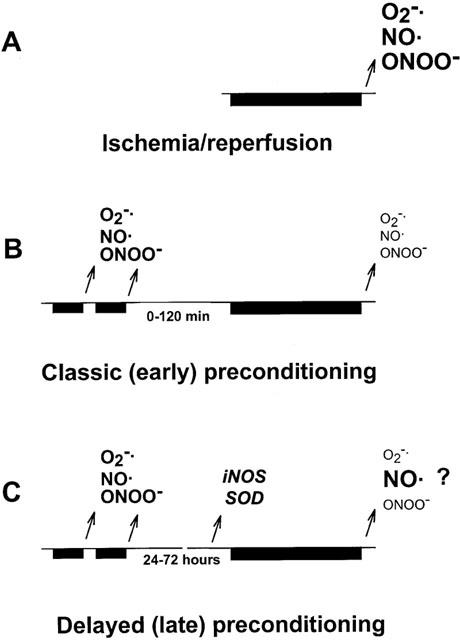
Increased synthesis of nitric oxide (NO) and superoxide (O2−·) leads to a pronounced formation of the toxic metabolite peroxynitrite (ONOO−) upon reperfusion thereby leading to myocardial injury (A). Preceding short periods of ischaemia/reperfusion during preconditioning, however, results in a moderate formation of reactive oxygen species which triggers a cardioprotective mechanism leading to subsequent inhibition of ONOO− formation (B). A similar mechanism is suspected during late preconditioning, however, expression of iNOS and antioxidant enzymes such as superoxide dismutase (SOD) also occurs. Changes in cardiac NO, O2−·, and ONOO− and the exact role of increased iNOS are questions of debate (C). Bars denote periods of ischaemia, arrows indicate formation of reactive oxygen species upon reperfusion or expression of enzymes, font size shows relative amount of NO, O2−·, and ONOO− formation.
NO, O2−·, and ONOO− in late preconditioning
In a conscious rabbit model of preconditioning with repetitive coronary occlusion/reperfusion, Bolli et al. (1997) reported that L-nitroarginine, given either during preconditioning or 24 h later, abrogated the protective effect of preconditioning and that the selective inducible NOS inhibitors aminoguanidine or S-methylisothiourea abolished preconditioning only when applied 24 h after preconditioning. They also showed that preconditioning induces an increase in inducible NOS mRNA levels in the ischaemic regions of the rabbit heart, and that this induction is triggered by increased generation of NO during the preconditioning stimulus (Jones et al., 1999b). Targeted disruption of the inducible NOS gene in mice led to a complete blockade of late preconditioning (Guo et al., 1999). Dexamethasone or selective inducible NOS inhibitors inhibited the late effect of preconditioning on infarct size in anaesthetized rabbits (Imagawa et al., 1999) and on arrhythmias in anaesthetized dogs (Kis et al., 1999). These results suggest a dual role of NO in late preconditioning, as intact NO synthesis by endothelial NOS is necessary to trigger late preconditioning and NO derived from inducible NOS is a mediator of late protection (Figure 2, see for review: Bolli et al., 1998). However, the exact role of inducible NOS in late preconditioning is not known as neither NO levels, nor NOS activities were determined in the aforementioned studies.
The role of NO in the mechanism of other well-known triggers of late preconditioning, such as adenosine and bradykinin has been investigated by several laboratories. The late infarct size limiting effect of bradykinin has been shown to be NO-dependent in a rat coronary occlusion/reperfusion model (Ebrahim et al., 2001). The role of NO in adenosine-induced late cardioprotection is somewhat controversial. Adenosine A(1) receptor stimulation has been shown to induce late preconditioning via an iNOS-dependent pathway in mice (Kudo et al., 2002). The lack of protective effect of adenosine A(1) receptor activation in iNOS gene-knockout mice suggests a direct cause-and-effect relationship of iNOS in adenosine-induced late cardioprotection (Zhao et al., 2000). However, another study showed that delayed protection against myocardial infarction observed 24 h after pharmacologic preconditioning with an adenosine A1 agonist occurred independently of either early generation of NO or subacute induction of inducible nitric oxide synthase in rabbits (Dana et al., 2001). An interesting study showed that in iNOS knock-out mice, eNOS plays a role in delayed A(1) receptor triggered preconditioning (Bell et al., 2002). Others have demonstrated that A(1) receptor-induced late preconditioning uses an NOS-dependent pathway whereas A(3) receptor-induced late preconditioning is mediated by an NOS-independent pathway in rabbits (Takano et al., 2001). In contrast, the inhibition of iNOS with S-methylisothiourea and targeted disruption of the iNOS gene also abolished the protective effect of adenosine A(3) receptor stimulation in mice hearts which shows an essential role of iNOS in A(3) adenosine receptor-induced late preconditioning (Zhao & Kukreja, 2002). The controversial findings can be related to the use of single concentration of inhibitors of adenosine receptor and NOS in the aforementioned studies.
The involvement of oxygen free radicals in late preconditioning was suggested by Sun et al. (1996) who showed that a combination of antioxidants (SOD, catalase, mercaptopropionyl-glycine) infused during the preconditioning stimulus completely abolished the late effect of preconditioning on stunning in conscious pigs. Zhou et al. (1996) reported that isolated rat myocytes preconditioned by anoxia or with the administration of O2−· induced late cytoprotection which was characterized by increased MnSOD activity and decreased O2−· production. Takano et al. (1998) demonstrated that i.v. infusion of the NO donors DETA/NO or SNAP induces cardioprotection 24 h later in conscious rabbits and this effect was lost when they were infused with mercaptopropionyl-glycine. This suggests that the mechanism whereby NO induces preconditioning involves the generation of oxidant species, possibly ONOO−.
Taken together, these results suggest that both NO and oxygen free radicals are necessary to trigger late preconditioning. Xuan et al. (2000) have suggested that endothelial NOS is the source of NO during the trigger phase of delayed preconditioning. Since both free radical scavengers and inhibitors of endothelial NOS abolish delayed preconditioning, it has been proposed that the formation of ONOO− is an important upstream event upon the triggering mechanism of delayed preconditioning (Bolli, 2000). Preliminary studies by Emani et al. (1999) have reported that administration of a ONOO− generating system to rabbits evoked a delayed protective response 24 h later, although interpretation of this study is difficult because under these conditions ONOO− could serve as a donor of NO as discussed in detail above. It should be noted that most of the aforementioned conclusions are based mainly on pharmacological studies. To elucidate the exact role of NO, O2−·, and ONOO− in preconditioning requires further biochemical evidence.
Conclusions/Relevance
Severe myocardial ischaemia and reperfusion results in the deterioration of myocardial function and leads to the development of infarction. However, brief episodes of ischaemia trigger an endogenous cardioprotective mechanism known as preconditioning. The role of NO in cardiac ischemia and preconditioning is complex.
In order to understand NO biology, we need to know its relationship with key oxidants such as O2−·. The study of ONOO− in the heart will lead to a better understanding of basic physiological and pathological mechanisms relevant to cardiac ischaemia and reperfusion injury and give new insight to novel therapeutic targets and strategies for its treatment or prevention. Specifically targeting ONOO− is an exciting new strategy to protect the heart from oxidant stress in ischaemia and reperfusion injury.
Acknowledgments
Both authors acknowledge the support of a NATO Cooperative Linkage Grant (LST.CLG.976650). Peter Ferdinandy acknowledges the support of grants from the Hungarian National Scientific Research Fund (OTKA T029843), Hungarian Ministries of Education (FKFP-0340/2000 and FKFP 0057/2001) and Health (ETT 51/2000), the Hungarian National R&D Program (NFKP-1/040), the Bolyai Fellowship of the Hungarian Academy of Sciences, and the Széchenyi Professors Scholarship. Richard Schulz acknowledges the support of grants from the Canadian Institutes for Health Research (MT-11563 and MT-14741) and the Heart and Stroke Foundation of Alberta, NWT and Nunavut. He is a Senior Scholar of the Alberta Heritage Foundation for Medical Research.
Abbreviations
- GSH
glutathione
- KATP
ATP-sensitive potassium channel
- L-NAME
NG-nitro-L-arginine-methyl-ester
- L-NMMA
NG-monomethyl-L-arginine
- MMP
matrix metalloproteinase
- NO
nitric oxide
- NOS
nitric oxide synthase
- O2−·
superoxide
- ONOO−
peroxynitrite
- SOD
superoxide dismutase
References
- ALTUG S., DEMIRYUREK A.T., AK D., TUNGEL M., KANZIK I. Contribution of peroxynitrite to the beneficial effects of preconditioning on ischaemia-induced arrhythmias in rat isolated hearts. Eur. J. Pharmacol. 2001;415:239–246. doi: 10.1016/s0014-2999(01)00843-3. [DOI] [PubMed] [Google Scholar]
- ALTUG S., DEMIRYUREK A.T., KANE K.A., KANZIK I. Evidence for the involvement of peroxynitrite in ischaemic preconditioning in rat isolated hearts. Br. J. Pharmacol. 2000;130:125–131. doi: 10.1038/sj.bjp.0703280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARSTALL M.A., SAWYER D.B., FUKAZAWA R., KELLY R.A. Cytokine-mediated apoptosis in cardiac myocytes: the role of inducible nitric oxide synthase induction and peroxynitrite generation. Circ. Res. 1999;85:829–840. doi: 10.1161/01.res.85.9.829. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F. The neutrophil as a mediator of myocardial ischaemia-reperfusion injury: time to move on. Basic Res. Cardiol. 2002;97:268–275. doi: 10.1007/s00395-002-0366-7. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F., FERDINANDY P. Delayed preconditioning of myocardium: current perspectives. Basic Res. Cardiol. 2001;96:329–344. doi: 10.1007/s003950170041. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S. Parsing the effects of nitric oxide, S-nitrosothiols, and peroxynitrite on inducible nitric oxide synthase-dependent cardiac myocyte apoptosis. Circ. Res. 1999;85:870–871. doi: 10.1161/01.res.85.9.870. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BELL R.M., SMITH C.C., YELLON D.M. Nitric oxide as a mediator of delayed pharmacological (A(1) receptor triggered) preconditioning; is eNOS masquerading as iNOS. Cardiovasc. Res. 2002;53:405–413. doi: 10.1016/s0008-6363(01)00472-2. [DOI] [PubMed] [Google Scholar]
- BILINSKA M., MACZEWSKI M., BERESEWICZ A. Donors of nitric oxide mimic effects of ischaemic preconditioning on reperfusion induced arrhythmias in isolated rat heart. Mol. Cell. Biochem. 1996;160–161:265–271. doi: 10.1007/978-1-4613-1279-6_34. [DOI] [PubMed] [Google Scholar]
- BOLLI R. The late phase of preconditioning. Circ. Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- BOLLI R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischaemia and preconditioning: an overview of a decade of research. J. Mol. Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- BOLLI R., DAWN B., TANG X.L., QIU Y., PING P., XUAN Y.T., JONES W.K., TAKANO H., GUO Y., ZHANG J. The nitric oxide hypothesis of late preconditioning. Basic Res. Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLI R., MANCHIKALAPUDI S., TANG X.L., TAKANO H., QIU Y., GUO Y., ZHANG Q., JADOON A.K. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischaemic preconditioning. Circ. Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- BOLLI R., MARBAN E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- BOVERIS A., CHANCE B. The mitochondrial generation of hydrogen peroxyde: general properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUNWALD E.K. Myocardial reperfusion: a double-edged sword. J. Clin. Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G.C. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta. 2001;1504:46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- BUGGE E., YTREHUS K. Bradykinin protects against infarction but does not mediate ischaemic preconditioning in the isolated rat heart. J. Mol. Cell Cardiol. 1996;28:2333–2341. doi: 10.1006/jmcc.1996.0226. [DOI] [PubMed] [Google Scholar]
- BUXTON I.L., CHEEK D.J., ECKMAN D., WESTFALL D.P., SANDERS K.M., KEEF K.D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ. Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- CARGNONI A., CECONI C., BORASO A., BERNOCCHI P., MONOPOLI A., CURELLO S., FERRARI R. Role of A2A receptor in the modulation of myocardial reperfusion damage. J. Cardiovasc. Pharmacol. 1999;33:883–893. doi: 10.1097/00005344-199906000-00008. [DOI] [PubMed] [Google Scholar]
- CHEUNG P.Y., DANIAL H., JONG J., SCHULZ R. Thiols protect the inhibition of myocardial aconitase by peroxynitrite. Arch. Biochem. Biophys. 1998;350:104–108. doi: 10.1006/abbi.1997.0496. [DOI] [PubMed] [Google Scholar]
- CHEUNG P.Y., SAWICKI G., WOZNIAK M., WANG W., RADOMSKI M.W., SCHULZ R. Matrix metalloproteinase-2 contributes to ischaemia-reperfusion injury in the heart. Circulation. 2000a;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- CHEUNG P.Y., WANG W., SCHULZ R. Glutathione protects against myocardial ischaemia-reperfusion injury by detoxifying peroxynitrite. J. Mol. Cell Cardiol. 2000b;32:1669–1678. doi: 10.1006/jmcc.2000.1203. [DOI] [PubMed] [Google Scholar]
- CSONKA C., CSONT T., ONODY A., FERDINANDY P. Preconditioning decreases ischaemia/reperfusion-induced peroxynitrite formation. Biochem. Biophys. Res. Commun. 2001;285:1217–1219. doi: 10.1006/bbrc.2001.5308. [DOI] [PubMed] [Google Scholar]
- CSONKA C., SZILVASSY Z., PALI T., BLASIG I.E., TOSAKI A., SCHULZ R., FERDINANDY P. Classic preconditioning decreases the harmful accumulation of nitric oxide during ischaemia and reperfusion in rat hearts. Circulation. 1999;100:2260–2266. doi: 10.1161/01.cir.100.22.2260. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., PABLA R. Nitric oxide supplementation or synthesis block – which is the better approach to treatment of heart disease. TIPS. 1997;18:239–244. doi: 10.1016/s0165-6147(97)01080-8. [DOI] [PubMed] [Google Scholar]
- DANA A., BAXTER G.F., YELLON D.M. Delayed or second window preconditioning induced by adenosine A1 receptor activation is independent of early generation of nitric oxide or late induction of inducible nitric oxide synthase. J. Cardiovasc. Pharmacol. 2001;38:278–287. doi: 10.1097/00005344-200108000-00014. [DOI] [PubMed] [Google Scholar]
- DEPRE C., HUE L. Cyclic GMP in the perfused rat heart. Effect of ischaemia, anoxia and nitric oxide synthase inhibitor. FEBS Lett. 1994;345:241–245. doi: 10.1016/0014-5793(94)00459-5. [DOI] [PubMed] [Google Scholar]
- DEPRE C., VANOVERSCHELDE J.L., GOUDEMANT J.F., MOTTET I., HUE Protection against ischaemic injury by nonvasoactive concentrations of nitric oxide synthase inhibitors in the perfused rabbit heart. Circulation. 1995;92:1911–1918. doi: 10.1161/01.cir.92.7.1911. [DOI] [PubMed] [Google Scholar]
- DIGERNESS S.B., HARRIS K.D., KIRKLIN J.W., URTHALER F., VIERA L., BECKMAN J.S., DARLEY-USMAR V. Peroxynitrite irreversibly decreases diastolic and systolic function in cardiac muscle. Free Rad. Biol. Med. 1999;27:1386–1392. doi: 10.1016/s0891-5849(99)00184-7. [DOI] [PubMed] [Google Scholar]
- EBRAHIM Z., YELLON D.M., BAXTER G.F. Bradykinin elicits ‘second window' myocardial protection in rat heart through an NO-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1458–H1464. doi: 10.1152/ajpheart.2001.281.3.H1458. [DOI] [PubMed] [Google Scholar]
- EMANI V.R., OCKAILI R.A., BROWN M.H., KROTTAPALLI K., HESS M.L., KUKREJA R.C.Peroxynitrite directly triggers early and delayed preconditioning in vivo via opening of mitochondrial KATP channels in the rabbit heart Circulation 1999100I–259.Abstract [Google Scholar]
- FERDINANDY P., CSONT T., CSONKA C., TOROK M., DUX M., NEMETH J., HORVATH L.I., DUX L., SZILVASSY Z., JANCSO G. Capsaicin-sensitive local sensory innervation is involved in pacing-induced preconditioning in rat hearts: role of nitric oxide and CGRP. Naunyn Schmiedebergs Arch. Pharmacol. 1997a;356:356–363. doi: 10.1007/pl00005062. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., DANIAL H., AMBRUS I., ROTHERY R.A., SCHULZ R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ. Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., PANAS D., SCHULZ R. Peroxynitrite contributes to spontaneous loss of cardiac efficiency in isolated working rat hearts. Am. J. Physiol. 1999;276:H1861–H1867. doi: 10.1152/ajpheart.1999.276.6.H1861. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Inhibition of peroxynitrite-induced dityrosine formation with oxidized and reduced thiols, nitric oxide donors, and purine derivatives. Antioxid. Redox. Signal. 2001a;3:165–171. doi: 10.1089/152308601750100704. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Peroxynitrite: toxic or protective in the heart. Circ. Res. 2001b;88:e12–e13. doi: 10.1161/01.res.88.2.e12. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SZILVASSY Z., BALOGH N., CSONKA C., CSONT T., KOLTAI M., DUX L. Nitric oxide is involved in active preconditioning in isolated working rat hearts. Ann. NY Acad. Sci. 1996;793:489–493. doi: 10.1111/j.1749-6632.1996.tb33547.x. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SZILVASSY Z., BAXTER G.F. Adaptation to myocardial stress in disease states: is preconditioning a healthy heart phenomenon. Trends Pharmacol. Sci. 1998;19:223–229. doi: 10.1016/s0165-6147(98)01212-7. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SZILVASSY Z., HORVATH L.I., CSONT T., CSONKA C., NAGY E., SZENTGYÖRGYI R., NAGY I., KOLTAI M., DUX L. Loss of pacing-induced preconditioning in rat hearts: role of nitric oxide and cholesterol-enriched diet. J. Mol. Cell. Cardiol. 1997b;29:3321–3333. doi: 10.1006/jmcc.1997.0557. [DOI] [PubMed] [Google Scholar]
- FLOGEL U., DECKING U.K., GODECKE A., SCHRADER J. Contribution of NO to ischaemia-reperfusion injury in the saline-perfused heart: a study in endothelial NO synthase knockout mice. J. Mol. Cell Cardiol. 1999;31:827–836. doi: 10.1006/jmcc.1998.0921. [DOI] [PubMed] [Google Scholar]
- GAO F., CHRISTOPHER T.A., LOPEZ B.L., FRIEDMAN E., CAI G., MA X.L. Mechanism of decreased adenosine protection in reperfusion injury of aging rats. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H329–H338. doi: 10.1152/ajpheart.2000.279.1.H329. [DOI] [PubMed] [Google Scholar]
- GAO F., GAO E., YUE T.L., OHLSTEIN E.H., LOPEZ B.L., CHRISTOPHER T.A., MA X.L. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischaemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002a;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- GAO F., YUE T.L., SHI D.W., CHRISTOPHER T.A., LOPEZ B.L., OHLSTEIN E.H., BARONE F.C., MA X.L. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovasc. Res. 2002b;53:414–422. doi: 10.1016/s0008-6363(01)00488-6. [DOI] [PubMed] [Google Scholar]
- GATTULLO D., LINDEN R.J., LOSANO G., PAGLIARO P., WESTERHOF N. Ischaemic preconditioning changes the pattern of coronary reactive hyperaemia in the goat: role of adenosine and nitric oxide. Cardiovasc. Res. 1999;42:57–64. doi: 10.1016/s0008-6363(98)00319-8. [DOI] [PubMed] [Google Scholar]
- GOTO M., LIU Y.G., YANG X.M., ARDELL J.L., COHEN M.V., DOWNEY J.M. Role of bradykinin in protection of ischaemic preconditioning in rabbit hearts. Circ. Res. 1995;77:611–621. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- GRISHAM M.B., GRANGER D.N., LEFER D.J. Modulation of leukocyteendothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischaemic heart disease. Free Rad. Biol. Med. 1998;25:404–433. doi: 10.1016/s0891-5849(98)00094-x. [DOI] [PubMed] [Google Scholar]
- GUO Y., JONES W.K., XUAN Y.T., TANG X.L., BAO W., WU W.J., HAN H., LAUBACH V.E., PING P., YANG Z., QIU Y., BOLLI R. The late phase of ischaemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc. Natl. Acad. Sci. USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARE J.M., COMERFORD M.L. Role of nitric oxide in the regulation of myocardial function. Prog. Lipid Res. 1995;38:155–166. doi: 10.1016/s0033-0620(05)80004-0. [DOI] [PubMed] [Google Scholar]
- HILLE R., NISHINO T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- HOSHIDA S., YAMASHITA N., IGARASHI J., NISHIDA M., HORI M., KAMADA T., KUZUYA T., TADA M. Nitric oxide synthase protects the heart against ischaemia-reperfusion injury in rabbits. J. Pharmacol. Exp. Ther. 1995;274:413–418. [PubMed] [Google Scholar]
- IMAGAWA J., YELLON D.M., BAXTER G.F. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. Br. J. Pharmacol. 1999;126:701–708. doi: 10.1038/sj.bjp.0702368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIDA H., GENKA C., NAKAZAWA H. Application of authentic peroxynitrite to biological materials. Methods Enzymol. 1999;301:402–409. doi: 10.1016/s0076-6879(99)01104-0. [DOI] [PubMed] [Google Scholar]
- ISHIDA H., ICHIMORI K., HIROTA Y., FUKAHORI M., NAKAZAWA H. Peroxynitrite-induced cardiac myocyte injury. Free Rad. Biol. Med. 1996;20:343–350. doi: 10.1016/0891-5849(96)02060-6. [DOI] [PubMed] [Google Scholar]
- ISHIYAMA S., HIROE M., NISHIKAWA T., ABE S., SHIMOJO T., ITO H., OZASA S., YAMAKAWA K., MATSUZAKI M., MOHAMMED M.U., NAKAZAWA H., KASAJIMA T., MARUMO F. Nitric oxide contributes to the progression of myocardial damage in experimental autoimmune myocarditis in rats. Circulation. 1997;95:489–496. doi: 10.1161/01.cir.95.2.489. [DOI] [PubMed] [Google Scholar]
- IWAI-KANAI E., HASEGAWA K., FUJITA M., ARAKI M., YANAZUME T., ADACHI S., SASAYAMA S. Basic fibroblast growth factor protects cardiac myocytes from iNOS-mediated apoptosis. J. Cell Physiol. 2002;190:54–62. doi: 10.1002/jcp.10036. [DOI] [PubMed] [Google Scholar]
- IWAMOTO T., MIURA T., ADACHI T., NOTO T., OGAWA T., TSUCHIDA A., IIMURA O. Myocardial infarct size-limiting effect of ischaemic preconditioning was not attenuated by oxygen free-radical scavengers in the rabbit. Circulation. 1991;83:1015–1022. doi: 10.1161/01.cir.83.3.1015. [DOI] [PubMed] [Google Scholar]
- JONES S.P., GIROD W.G., PALAZZO A.J., GRANGER D.N., GRISHAM M.B., JOURD'HEUIL D., HUANG P.L., LEFER D.J. Myocardial ischaemia reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am. J. Physiol. 1999a;276:H1567–H1573. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- JONES W.K., FLAHERTY M.P., TANG X.L., TAKANO H., QIU Y., BANERJEE S., SMITH T., BOLLI R. Ischaemic preconditioning increases iNOS transcript levels in conscious rabbits via a nitric oxide-dependent mechanism. J. Mol. Cell. Cardiol. 1999b;31:1469–1481. doi: 10.1006/jmcc.1999.0983. [DOI] [PubMed] [Google Scholar]
- JORDAN J.E., ZHAO Z.Q., VINTEN-JOHANSEN J. The role of neutrophils in myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 1999;43:860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- KIRSHENBAUM L.A., SINGAL P.K. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 1993;265:H484–H493. doi: 10.1152/ajpheart.1993.265.2.H484. [DOI] [PubMed] [Google Scholar]
- KIS A., VEGH A., PAPP J.G., PARRATT J.R. Repeated cardiac pacing extends the time during which canine hearts are protected against ischaemia-induced arrhythmias: role of nitric oxide. J. Mol. Cell Cardiol. 1999;31:1229–1241. doi: 10.1006/jmcc.1999.0955. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., LEWIS S.J., ROYALL J.A., YE Y.Z., KELLY D.R., BECKMAN J.S. Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Crit. Care Med. 1997;25:812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUDO M., WANG Y., XU M., AYUB A., ASHRAF M. Adenosine A(1) receptor mediates late preconditioning via activation of PKC-delta signaling pathway. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H296–H301. doi: 10.1152/ajpheart.01087.2001. [DOI] [PubMed] [Google Scholar]
- LEFER D.J., SCALIA R., CAMPBELL B., NOSSULI T.O., HAYWARD R., SALAMON M., GRAYSON J., LEFER A.M. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischaemia-reperfusion injury in rats. J. Clin. Invest. 1997;99:684–691. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAUDET L., YANG Z., AL-AFFAR E.B., SZABO C. Myocardial ischaemic preconditioning in rodents is dependent on poly (ADP-ribose) synthetase. Mol. Med. 2001;7:406–417. [PMC free article] [PubMed] [Google Scholar]
- LIU P.T., HOCK C.E., NAGELE R., WONG P.Y.K. Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury in rats. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H2327–H2336. doi: 10.1152/ajpheart.1997.272.5.H2327. [DOI] [PubMed] [Google Scholar]
- LIU Y., SATO T., O'ROURKE B., MARBAN E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection. Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- LU H.R., REMEYSEN P., DE CLERCK F. Does the antiarrhythmic effect of ischaemic preconditioning in rats involve the L-arginine nitric oxide pathway. J. Cardiovasc. Pharmacol. 1995;25:524–530. doi: 10.1097/00005344-199504000-00003. [DOI] [PubMed] [Google Scholar]
- MA X.L., GAO F., LOPEZ B.L., CHRISTOPHER T.A., VINTEN-JOHANSEN J. Peroxynitrite, a two-edged sword in post-ischaemic myocardial injury-dichotomy of action in crystalloid- versus blood-perfused hearts. J. Pharmacol. Exp. Ther. 2000;292:912–920. [PubMed] [Google Scholar]
- MASINI E., BIANCHI S., MUGNAI L., GAMBASSI F., LUPINI M., PISTELLI A., MANNAIONI P.F. The effect of nitric oxide generators on ischaemia reperfusion injury and histamine release in isolated perfused guinea-pig heart. Agents Actions. 1991;33:53–56. doi: 10.1007/BF01993125. [DOI] [PubMed] [Google Scholar]
- MASSOUDY P., BECKER B.F., GERLACH E. Nitric oxide accounts for postischaemic cardioprotection resulting from angiotensin-converting enzyme inhibition: indirect evidence for a radical scavenger effect in isolated guinea pig heart. J. Cardiovasc. Pharmacol. 1995;25:440–447. doi: 10.1097/00005344-199503000-00014. [DOI] [PubMed] [Google Scholar]
- MAYER B., SCHRAMMEL A., KLATT P., KOESLING D., SCHMIDT K. Peroxynitrite-induced accumulation of cyclic GMP in endothelial cells and stimulation of purified soluble guanylyl cyclase. Dependence on glutathione and possible role of S-nitrosation. J. Biol. Chem. 1995;270:17355–17360. doi: 10.1074/jbc.270.29.17355. [DOI] [PubMed] [Google Scholar]
- MOHAZZAB H., KAMINSKI P.M., WOLIN M.S. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997;96:614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- MONCADA S., ERUSALIMSKY J.D. OPINION: Does nitric oxide modulate mitochondrial energy generation and apoptosis. Nat. Rev. Mol. Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- MORO M.A., DARLEY-USMAR V., GOODWIN D.A., READ N.G., ZAMORA-PINO R., FEELISCH M., RADOMSKI M.W., MONCADA S. Paradoxical fate and biological action of peroxynitrite in human platelets. Proc. Natl. Acad. Sci. USA. 1994;91:6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUBAGWA K., FLAMENG W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc. Res. 2001;52:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- MURRY C.E., JENNINGS R.B., REIMER K.A. Preconditioning with ischaemia: a delay of lethal cell injury in ischaemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- NAKANO A., LIU G.S., HEUSCH G., DOWNEY J.M., COHEN M.V. Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classical ischaemic preconditioning. J. Mol. Cell Cardiol. 2000;32:1159–1167. doi: 10.1006/jmcc.2000.1152. [DOI] [PubMed] [Google Scholar]
- NOSSULI T.O., HAYWARD R., JENSEN D., SCALIA R., LEFER A.M. Mechanisms of cardioprotection by peroxynitrite in myocardial ischaemia and reperfusion injury. Am. J. Physiol. 1998;275:H509–H519. doi: 10.1152/ajpheart.1998.275.2.H509. [DOI] [PubMed] [Google Scholar]
- NOSSULI T.O., HAYWARD R., SCALIA R., LEFER A.M. Peroxynitrite reduces myocardial infarct size and preserves coronary endothelium after ischaemia and reperfusion in cats. Circulation. 1997;96:2317–2324. doi: 10.1161/01.cir.96.7.2317. [DOI] [PubMed] [Google Scholar]
- OHOI I., TAKEO S. Involvement of superoxide and nitric oxide in the genesis of reperfusion arrhythmias in rats. Eur. J. Pharmacol. 1996;306:123–131. doi: 10.1016/0014-2999(96)00231-2. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., AKAIKE T., NAGANO T., MIYAJIMA S., SUGA M., ANDO M., ICHIMORI K., MAEDA H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch. Biochem. Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., AKAIKE T., SAWA T., MIYAMOTO Y., VAN DER VLIET A., MAEDA H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- OMAR B.A., HANSON A.K., BOSE S.K., MCCORD J.M. Ischaemic preconditioning is not mediated by free radicals in the isolated rabbit heart. Free Rad. Biol. Med. 1991;11:517–520. doi: 10.1016/0891-5849(91)90064-a. [DOI] [PubMed] [Google Scholar]
- OSADA M., SATO T., KOMORI S., TAMURA K. Protective effect of preconditioning on reperfusion induced ventricular arrhythmias of isolated rat hearts. Cardiovasc. Res. 1991;25:441–444. doi: 10.1093/cvr/25.6.441. [DOI] [PubMed] [Google Scholar]
- OYAMA J., SHIMOKAWA H., MOMII H., CHENG X., FUKUYAMA N., ARAI Y., EGASHIRA, NAKAZAWA H., TAKESHITA A. Role of nitric oxide and peroxynitrite in the cytokine-induced sustained myocardial dysfunction in dogs in vivo. J. Clin. Invest. 1998;101:2207–2214. doi: 10.1172/JCI986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PABLA R., CURTIS M.J. Effect of endogenous nitric oxide on cardiac systolic and diastolic function during ischaemia and reperfusion in the rat isolated perfused heart. J. Mol. Cell. Cardiol. 1996;28:2111–2121. doi: 10.1006/jmcc.1996.0203. [DOI] [PubMed] [Google Scholar]
- PAIN T., YANG X.M., CRITZ S.D., YUE Y., NAKANO A., LIU G.S., HEUSCH G., COHEN M.V., DOWNEY J.M. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- PRENDERGAST B.D., SAGACH V.F., SHAH A.M. Basal release of nitric oxide augments the Frank-Starling response in the isolated heart. Circulation. 1997;96:1320–1329. doi: 10.1161/01.cir.96.4.1320. [DOI] [PubMed] [Google Scholar]
- PRZYKLENK K., KLONER R.A. Ischaemic preconditioning: exploring the paradox. Prog. Cardiovasc. Dis. 1998;40:517–547. doi: 10.1016/s0033-0620(98)80002-9. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARD V., BLANC T., KAEFFER N., TRON C., THUILLEZ C. Myocardial and coronary endothelial protective effects of acetylcholine after myocardial ischaemia and reperfusion in rats: Role of nitric oxide. Br. J. Pharmacol. 1995;115:1532–1538. doi: 10.1111/j.1476-5381.1995.tb16647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONSON R.S., NAKAMURA M., VINTEN-JOHANSEN J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc. Res. 1999;44:47–59. doi: 10.1016/s0008-6363(99)00184-4. [DOI] [PubMed] [Google Scholar]
- RUBBO H., DARLEY-USMAR V., FREEMAN B.A. Nitric oxide regulation of tissue free radical injury. Chem. Res. Toxicol. 1996;9:809–820. doi: 10.1021/tx960037q. [DOI] [PubMed] [Google Scholar]
- RUBBO H., RADI R., TRUJILLO M., TELLERI R., KALYANARAMAN B., BARNES S., KIRK M., FREEMAN B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- SAKURAI M., FUKUYAMA N., IGUCHI A., AKIMOTO H., OHMI M., YOKOYAMA H., NAKAZAWA H., TABAYASHI K. Quantitative analysis of cardiac 3-L-nitrotyrosine during acute allograft rejection in an experimental heart transplantation. Transplantation. 1999;68:1818–1822. doi: 10.1097/00007890-199912150-00031. [DOI] [PubMed] [Google Scholar]
- SAM F., SAWYER D.B., XIE Z., CHANG D.L., NGOY S., BRENNER D.A., SIWIK D.A., SINGH K., APSTEIN C.S., COLUCCI W.S. Mice lacking inducible nitric oxide synthase have improved left ventricular contractile function and reduced apoptotic cell death late after myocardial infarction. Circ. Res. 2001;89:351–356. doi: 10.1161/hh1601.094993. [DOI] [PubMed] [Google Scholar]
- SCHOELKENS B.A., LINZ W. Bradykinin-mediated metabolic effects in isolated perfused rat hearts. Agents Actions Suppl. 1992;38:36–42. [PubMed] [Google Scholar]
- SCHULZ R., COHEN M.V., BEHRENDS M., DOWNEY J.M., HEUSCH G. Signal transduction of ischaemic preconditioning. Cardiovasc. Res. 2001;52:181–198. doi: 10.1016/s0008-6363(01)00384-4. [DOI] [PubMed] [Google Scholar]
- SCHULZ R., DODGE K.L., LOPASCHUK G.D., CLANACHAN A.S. Peroxynitrite impairs cardiac contractile function by decreasing cardiac efficiency. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H1212–H1219. doi: 10.1152/ajpheart.1997.272.3.H1212. [DOI] [PubMed] [Google Scholar]
- SCHULZ R., WAMBOLT R. Inhibition of nitric oxide synthesis protects the isolated working rabbit heart from ischaemia-reperfusion injury. Cardiovasc. Res. 1995;30:432–439. [PubMed] [Google Scholar]
- SIEGFRIED M.R., ERHARDT J., RIDER T., XIN-LIANG M.A., LEFER A.M. Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischaemia-reperfusion. J. Pharmacol. Exp. Ther. 1992;260:668–675. [PubMed] [Google Scholar]
- SUN J.Z., TANG X.L., PARK S.W., QIU Y.M., TURRENS J.F., BOLLI R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J. Clin. Invest. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischaemia-reperfusion injury. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., ZINGARELLI B., SALZMAN A.L. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circ. Res. 1996;78:1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- TAIMOR G., HOFSTAETTER B., PIPER H.M. Apoptosis induction by nitric oxide in adult cardiomyocytes via cGMP-signaling and its impairment after simulated ischaemia. Cardiovasc. Res. 2000;45:588–594. doi: 10.1016/s0008-6363(99)00272-2. [DOI] [PubMed] [Google Scholar]
- TAKANO H., BOLLI R., BLACK R.G.J., KODANI E., TANG X.L., YANG Z., BHATTACHARYA S., AUCHAMPACH J.A. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ. Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- TAKANO H., TANG X.L., QIU Y., GUO Y., FRENCH B.A., BOLLI R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ. Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA M., FUJIWARA H., YAMASAKI K., SASAYAMA S. Superoxide dismutase and N-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischaemic preconditioning in the rabbit. Cardiovasc. Res. 1994;28:980–986. doi: 10.1093/cvr/28.7.980. [DOI] [PubMed] [Google Scholar]
- TOSAKI A., CORDIS G.A., SZERDAHELYI P., ENGELMAN R.M., DAS D.K. Effects of preconditioning on reperfusion arrhythmias, myocardial functions, formation of free radicals, and ion shifts in isolated ischaemic/reperfused rat hearts. J. Cardiovasc. Pharmacol. 1994;23:365–373. [PubMed] [Google Scholar]
- TRITTO I., D'ANDREA D., ERAMO N., SCOGNAMIGLIO A., DE SIMONE C., VIOLANTE A., ESPOSITO A., CHIARIELLO M., AMBROSIO G. Oxygen radicals can induce preconditioning in rabbit hearts. Circ. Res. 1997;80:743–748. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- VALLANCE P., LEONE A., CALVER A., COLLIER J., MONCADA S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- VEGH A., SZEKERES L., PARRATT J.R. Preconditioning of the ischaemic myocardium; involvement of the L-arginine nitric oxide pathway. Br. J. Pharmacol. 1992;107:648–652. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLA L.M., SALAS E., DARLEY-USMAR V.M., RADOMSKI M.W., MONCADA S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc. Natl. Acad. Sci. USA. 1994;91:12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINTEN-JOHANSEN J. Physiological effects of peroxynitrite. Potential products of the environment. Circ. Res. 2000;87:170–172. doi: 10.1161/01.res.87.3.170. [DOI] [PubMed] [Google Scholar]
- WANG P.H., ZWEIER J.L. Measurement of nitric oxide and peroxynitrite generation in the postischaemic heart – evidence for peroxynitrite-mediated reperfusion injury. J. Biol. Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- WANG P.H., ZWEIER J.L.Ischaemic preconditioning decreases nitric oxide (NO) formation and NO mediated injury in the postischaemic heart Circulation 199796Suppl.172Abstract [Google Scholar]
- WANG W., SAWICKI G., SCHULZ R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc. Res. 2002a;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- WANG W., SCHULZE C.J., SUAREZ-PINSON W.L., DYCK J.R.B., SAWICKI G., SCHULZ R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischaemia and reperfusion injury. Circulation. 2002b;106:1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- WEILAND U., HAENDELER J., IHLING C., ALBUS U., SCHOLZ W., RUETTEN H., ZEIHER A.M., DIMMELER S. Inhibition of endogenous nitric oxide synthase potentiates ischaemia-reperfusion-induced myocardial apoptosis via a caspase-3 dependent pathway. Cardiovasc. Res. 2000;45:671–678. doi: 10.1016/s0008-6363(99)00347-8. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN D.M., MIHM M.J., BAUER J.A. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J. Pharmacol. Exp. Ther. 2000;294:396–401. [PubMed] [Google Scholar]
- WESELCOUCH E.O., BAIRD A.J., SLEPH P., GROVER G.J. Inhibition of nitric oxide synthesis does not affect ischaemic preconditioning in isolated perfused rat hearts. Am. J. Physiol. Heart Circ. Physiol. 1995;268:H242–H249. doi: 10.1152/ajpheart.1995.268.1.H242. [DOI] [PubMed] [Google Scholar]
- WEYRICH A.S., MA X., LEFER A.M. The role of L-arginine in ameliorating reperfusion injury after myocardial ischaemia in the cat. Circulation. 1992;86:279–288. doi: 10.1161/01.cir.86.1.279. [DOI] [PubMed] [Google Scholar]
- WILLIAMS F.M., KUS M., TANDA K., WILLIAMS T.J. Effect of duration of ischaemia on reduction of myocardial infarct size by inhibition of neutrophil accumulation using an anti-CD18 monoclonal antibody. Br. J. Pharmacol. 1994;111:1123–1128. doi: 10.1111/j.1476-5381.1994.tb14861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS M.W., TAFT C.S., RAMNAUTH S., ZHAO Z.Q., VINTEN-JOHANSEN J. Endogenous nitric oxide (NO) protects against ischaemia-reperfusion injury in the rabbit. Cardiovasc. Res. 1995;30:79–86. [PubMed] [Google Scholar]
- WOOLFSON R.G., PATEL V.C., NEILD G.H., YELLON D.M. Inhibition of nitric oxide synthesis reduces infarct size by an adenosine-dependent mechanism. Circulation. 1995;91:1545–1551. doi: 10.1161/01.cir.91.5.1545. [DOI] [PubMed] [Google Scholar]
- WU M., PRITCHARD K.A.J., KAMINSKI P.M., FAYNGERSH R.P., HINTZE T.H., WOLIN M.S. Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am. J. Physiol. 1994;266:H2108–H2113. doi: 10.1152/ajpheart.1994.266.5.H2108. [DOI] [PubMed] [Google Scholar]
- XI L., JARRETT N.C., HESS M.L., KUKREJA R.C. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 1999;99:2157–2163. doi: 10.1161/01.cir.99.16.2157. [DOI] [PubMed] [Google Scholar]
- XIE Y.W., WOLIN M.S. Role of nitric oxide and its interaction with superoxide in the suppression of cardiac muscle mitochondrial respiration. Involvement in response to hypoxia/reoxygenation. Circulation. 1996;94:2580–2586. doi: 10.1161/01.cir.94.10.2580. [DOI] [PubMed] [Google Scholar]
- XUAN Y.T., TANG X.L., QIU Y., BANERJEE S., TAKANO H., HAN H., BOLLI R. Biphasic response of cardiac NO synthase isoforms to ischaemic preconditioning in conscious rabbits. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2360–H2371. doi: 10.1152/ajpheart.2000.279.5.H2360. [DOI] [PubMed] [Google Scholar]
- YANG X.P., LIU Y.H., SHESELY E.G., BULAGANNAWAR M., LIU F., CARRETERO O.A. Endothelial nitric oxide gene knockout mice: cardiac phenotypes and the effect of angiotensin-converting enzyme inhibitor on myocardial ischaemia/reperfusion injury. Hypertension. 1999;34:24–30. doi: 10.1161/01.hyp.34.1.24. [DOI] [PubMed] [Google Scholar]
- YASMIN W., STRYNADKA K.D., SCHULZ R. Generation of peroxinitrite contributes to ischaemia-reperfusion injury in isolated rat hearts. Cardiovasc. Res. 1997;33:422–432. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- ZHAO T., XI L., CHELLIAH J., LEVASSEUR J.E., KUKREJA R.C. Inducible nitric oxide synthase mediates delayed myocardial protection induced by activation of adenosine A(1) receptors: evidence from gene-knockout mice. Circulation. 2000;102:902–907. doi: 10.1161/01.cir.102.8.902. [DOI] [PubMed] [Google Scholar]
- ZHAO T.C., KUKREJA R.C. Late preconditioning elicited by activation of adenosine A(3) receptor in heart: role of NF- kappa B, iNOS and mitochondrial K(ATP) channel. J. Mol. Cell Cardiol. 2002;34:263–277. doi: 10.1006/jmcc.2001.1510. [DOI] [PubMed] [Google Scholar]
- ZHOU X.B., ZHAI X., ASHRAF M. Direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocytes. Circulation. 1996;93:1177–1184. doi: 10.1161/01.cir.93.6.1177. [DOI] [PubMed] [Google Scholar]
- ZWEIER J.L., WANG P., SAMUILOV A., KUPPUSAMY P. Enzyme-independent formation of nitric oxide in biological tissues. Nature Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]


