Abstract
Our previously published data indicate that an endogenously produced 5-lipoxygenase metabolite can strongly contract isolated endothelium-preserved rat aortic strips when cyclo-oxygenase isoenzymes are inhibited. Therefore, we decided to investigate if cysteinyl-containing leukotrienes (Cys Lts) are involved in this endothelium-dependent contraction.
The isometric contraction of endothelium-preserved rat aortic strips was recorded in preparations preincubated with 5 μM indomethacin and precontracted with phenylephrine, adjusting resting tension at 0.7 g. Acetylcholine (ACh) contracted control strips. Montelukast and MK-571, selective type 1 Cys Lts receptor (Cys Lt1) antagonists and the Cys Lt1/Cys Lt2 (type 2 Cys Lts receptor) antagonist BAYu9773 dose-dependently prevented ACh-induced contraction, their IC50s being 2.2, 3.1 and 7.9 nM respectively. The leukotriene B4 receptor antagonist U75302 was far less potent (IC50 1.5 μM).
In rat aorta smooth muscle cells (RASMs), Western blot analysis showed the presence of Cys Lt1 and Cys Lt2 receptors, the Cys Lt1 receptor being predominantly expressed.
In fura-2 loaded RASMs, LTD4 (0.01–100 nM) and LTC4 (200–800 nM) dose-dependently increased intracellular calcium concentration ([Ca2+]i). Montelukast (1–100 nM) reduced LTD4-induced [Ca2+]i increase, its IC50 being approximately 10 nM. BAY u9773 exhibited significantly low effectiveness.
LTD4 (10 nM) induced a redistribution of smooth muscle actin fibres throughout the cytoplasm as visualized by confocal microscopy.
In conclusion, Cys Lt1 activation by endogenously produced Cys Lts, can contract rat aortas, while Cys Lt2 only marginally influences aortic tone. Intracellularly, this effect is mediated by an increase in [Ca2+]i. Therefore, Cys Lts, by inducing vascular contraction, can contribute to systemic hypertension.
Keywords: Rat aortic strips, rat aortic smooth muscle cells, lipoxygenase metabolites, 5-lipoxygenase, cysteinyl-containing leukotriene receptors, cysteinyl-containing leukotriene receptor antagonists, intracellular calcium
Introduction
Cysteinyl-containing leukotrienes (Cys Lts) are arachidonic acid metabolites (for review see: Samuelsson et al., 1987; Samuelsson, 2000). Two enzymatic steps are required for Cys Lts biosynthesis: 5-lipoxygenase (5-LOX) forms leukotriene A4 (LTA4) from arachidonic acid, then cysteinyl-leukotriene synthase adds the peptide glutathione, yielding cysteinyl-leukotriene C4 (LTC4). Cleavage reactions yield cysteinyl-leukotriene D4 (LTD4) and the less potent, more stable cysteinyl-leukotriene E4 (LTE4). Cys Lts are potent contracting factors, acting on smooth muscles from the bronchi, vasculature and intestine (Lewis et al., 1990). They are also known to induce proliferation of smooth muscle and endothelial cells (Palmberg et al., 1991; Modat et al., 1987) and collagen synthesis in rat lung fibroblasts (Phan et al., 1988).
Two different human receptor types for Cys Lts have been cloned. The cloned human CysLT type 1 receptor (hCys Lt1) is a seven-loop Gq-associated membrane receptor (Lynch et al., 1999; Sarau et al., 1999). The receptor is widely expressed, the spleen, placenta and peripheral blood leukocytes being particularly rich in this receptor. LTD4 can induce an increase in intracellular calcium concentration ([Ca2+]i) after transient transfection of this receptor in COS-7 (Lynch et al., 1999) and HEK293T cells (Sarau et al., 1999). MK-571 (Lynch et al., 1999) and pranlukast, zafirlukast, montelukast and pobilukast (Sarau et al., 1999) can specifically inhibit the LTD4-induced increase in [Ca2+]i, their IC50s being in the low nanomolar range. Binding studies performed by both groups have determined a relative potency of agonists, i.e. LTD4>>LTC4>LTE4. Zafirlukast, pranlukast, and montelukast, tested by both groups, can competitively displace LTD4 binding with high potency (Lynch et al., 1999; Sarau et al., 1999). More recently, a murine LT receptor, mCys Lt1, has also been cloned and characterized (Martin et al., 2001). Two forms of this receptor can be predicted on the basis of cDNA analysis of start codons, encoding two proteins, a ‘short' (339 amino acids) and a ‘long' form (352 amino acids). Both isoforms show an 87% amino acid identity with the hCys Lt1. The rank order affinity for the mCys Lt1 is LTD4>>LTE4=LTC4>>LTB4. Pranlukast, MK571 and zafirlukast can displace LTD4 specific binding (Ki 0.25±0.02 nM, 0.69±0.12 nM and 0.72±0.09 nM respectively), while the Cys Lt1/Cys Lt2 non-selective antagonist BAY u9773 (Tudhope et al., 1994) shows a Ki from 500 to 1000 fold higher than Cys Lt1 specific antagonists (451±196 nM). Similarly to the human receptor, the mCys Lt1 is associated with a Gα-protein linked to calcium mobilization.
The type 2 human receptor (hCys Lt2) is also a seven-loop G-protein associated transmembrane receptor (Heise et al., 2000; Takasaki et al., 2000; Nothacker et al., 2000). Expression of hCys Lt2 is particularly high in the heart and spleen, where it exceeds hCys Lt1 (Takasaki et al., 2000). Activation of this receptor can also induce an increase in [Ca2+]i, LTD4 and LTC4 acting on this receptor with a similar potency. Montelukast and zafirlukast are unable to prevent receptor activation, while BAY u9773 prevented it. Moreover, according to Nothacker et al. (2000), BAY u9773 acts as partial agonist in HEK 293T cells transiently expressing hCys Lt2 (HPN321), since it can either induce an increase in [Ca2+]i with an EC50 of 92±15 nM or antagonize the increase in [Ca2+]i induced by 10 nM LTD4 with an IC50 of 300±92 nM. However, in HEK 293T cells transiently transfected with hCys Lt1, BAY u9773 antagonizes the LTD4-induced [Ca2+]i increase with an IC50 of 440±182 mM (Nothacker et al., 2000). Cys Lts may play a role in the pathogenesis of hypertension since they may contribute to the constrictor response to angiotensin II (ATII) in in vitro preparations of aortas from spontaneously hypertensive rats (Stanke-Labesque et al., 2001). In ATII-induced hypertensive rats, another experimental model of hypertension, a strong relationship between ATII and the lipoxygenase pathway is also suggested by the up-regulation in LTA4 hydrolase, an enzyme able to convert LTA4 to LTB4 in the heart (Ishizaka et al., 1999). In addition, Cys Lts can induce a concentration-dependent contraction in human atherosclerotic coronary artery preparations, whereas non-atherosclerotic coronary artery segments are unresponsive (Allen et al., 1998).
In the isolated rabbit heart, blockade of leukocyte-endothelial cell adhesion by specific antibodies prevents Cys Lts transcellular formation and the related increase in coronary resistance and myocardial stiffness (Sala et al., 2000).
Recent data obtained in our laboratory on isolated rat aortas (Franchi-Micheli et al., 2000) show that, in particular experimental conditions, i.e. low resting tension and blockade of cyclo-oxygenase isoenzymes with indomethacin or ibuprofen, ACh can increase the contraction of phenylephrine-precontracted preparations. This ACh paradoxical effect is observed only in the presence of a functional, intact endothelium, whereas after its rubbing ACh is ineffective at doses up to 100 μM. Therefore, it seems likely that the endothelium is necessary for the biosynthesis of contractile factors. Since ACh-induced contraction is totally prevented in the presence of 5-LOX inhibitors, we argue that these contractile factors may be Cys Lts.
The present study was designed to investigate if Cys Lts are involved in the contraction of isolated rat aorta and to elucidate their effect on [Ca2+]i in vascular smooth muscle cells isolated from rat aortas (RASMs). Since ACh administration was unable to induce a contraction in isolated rat aortic strips in the absence of endothelium (Franchi-Micheli et al., 2000) and preliminary data showed an undetectable level of Cys LTs in resting and ACh-stimulated RASMs, we used LTD4 and LTC4 as agonists. Our aim was to provide further insight into the role of Cys Lts in vascular function.
Methods
This investigation conforms to European Community rules for the care and use of laboratory animals.
Functional studies on rat thoracic aortas
Male Wistar rats (300–350 g) were anaesthetized with diethyl ether and killed by cervical dislocation. Thoracic aortas were isolated and mounted in an organ bath of 2 ml volume. The strips were perfused with pre-warmed (37°C) Krebs-Henseleit solution (K-H) of the following composition (mM): NaCl 110, NaHCO3 25, KCl 4.8, KH2PO4 1.2, MgSO4 1.2, D(+)glucose 11, CaCl2 2.5, gassed with 5% CO2 in O2. The resting tension was set at 0.7 g and the tissue was equilibrated for 60 min as described (Franchi-Micheli et al., 2000). After equilibration, the presence of an intact, functional endothelial lining was tested by the administration of an ACh bolus (2 nmol) that induced a 99±3.5% relaxation of 100 nM phenylephrine precontracted strips. After washing, strips were preincubated for 30 min with 5 μM indomethacin and the maximal contraction obtained with 100 nM phenylephrine was expressed as 100% of contractile response. Indomethacin was maintained throughout the experiment. A bolus of 2 nmol ACh was then added to the 2 ml organ bath. The contraction induced by ACh was expressed as the increase in the contractile force from the maximal phenylephrine-induced contraction. Cumulative dose-response curves of Cys Lts antagonists were performed. Cys Lts antagonists were preincubated 15 min at every concentration tested. Of the antagonists tested, MK-571 was dissolved in buffer, montelukast in DMSO/buffer 1 : 10,000 and BAY u9773 and U75302 in ethanol/buffer 1 : 100 at the maximal concentration used. At these concentrations solvents did not influence the contractile response of agonists tested. An 80% relaxation was utilized as the cut-off limit. Experiments were mostly performed on endothelium-intact preparations. In several cases, the endothelium was removed by rubbing the cell layer with a cotton gauze, and the absence of endothelium was determined as described before, where ACh was unable to induce relaxation.
Isolation and culture of smooth muscle cells from aorta (RASMs)
Smooth muscle cells were isolated from thoracic aortas of male Wistar rats (120–200 g) according to Fallier-Becker et al. (1990) with minor modifications. Briefly, thoracic aortas were dissected, transferred into a Petri dish containing cold Dulbecco's modified essential medium (DMEM) and discharged from fatty tissues. Aortas were longitudinally cut and endothelial cells were removed by rubbing. Intima, media and adventitia layers were cut into small pieces and maintained under stirring for 2 h at 37°C in DMEM containing 0.1% elastase and 0.1% collagenase. Disaggregated cells, obtained by centrifugation (250× g for 10 min), were then suspended in 5 ml of culture medium (see below) and plated in 25 cm2 flasks. After 24 h, cells were washed and grown in 5 ml culture medium until confluence (8–10 days). A total of 11 cell lines between the 1st and 3rd culture passage were used for these experiments. Ham's F12 medium (Sigma Chemical Co., St. Louis, MO, U.S.A.), supplemented with 20% foetal calf serum (Gibco BRL, Paisley, U.K.), 250 IU ml−1 penicillin G and 250 μg ml−1 streptomycin, was used as culture medium. Immunocytochemical characterization of cells obtained as described below showed a 97% positive staining with mouse monoclonal anti-α smooth muscle actin antibodies (Sigma, working dilution, 1 : 300).
Western blot analysis of Cys Lt1 and Cys Lt2 receptors
Confluent RASMs were lysed in lysis buffer composed of (mM): Tris/HCl 20, pH 7.4; NaCl 10; MgCl2 1.5; Na2EDTA 1.3; dithiotreitol (DTT) 1; phenylmethylsulphonyl fluoride (PMSF) 1; Triton X-100 0.25%; leupeptin 20 μg ml−1; epstein 1 μg ml−1; Pefabloc SC 1 mg ml−1; and aprotinin 2.5 μg ml−1. With centrifugation at 17,000×g at 4°C, we collected the supernatants and measured the total protein content spectrophotometrically by using the Bradford reagent (Bradford, 1976). 75 μg of proteins were separated by 12% SDS–PAGE according to Laemmli (1970) and electroblotted on a nitrocellulose membrane. The membranes were blocked for 1 h at room temperature with 5% BSA in 0.1% PBS-Tween, and then incubated overnight at 4°C with primary polyclonal antibodies for Cys Lt1 and, after stripping, for Cys Lt2 receptors (Cayman Chemical, Ann Arbor, MI, U.S.A.; working dilution: 1 : 10,000). Immune reaction was revealed by peroxidase-conjugated secondary antibodies (Vector, Burlingame, CA, U.S.A.; working dilution: 1 : 10,000). Detection of the immune reaction was carried out using the chemiluminescent substrate ECL (Amersham, Arlington Heights, IL, U.S.A.).
Image analysis of cytosolic intracellular calcium
RASMs were grown until confluence on round coverslips (diameter 25 mm), harvested in serum-free medium for 24 h, loaded with 4 μM fura-2AM, 0.02% Pluronic F for 45 min at room temperature and washed in HEPES/NaHCO3 medium composed of (mM): NaCl 140, KCl 2.9, MgCl2 0.9, NaH2PO4 0.5, NaHCO3 12, glucose 10, HEPES 10, CaCl2 1.5, adjusted to pH 7.4 with 1 N NaOH. Intracellular calcium concentration was measured as previously described (Failli et al., 1995).
Fura-2-loaded RASMs were placed on the platform of an inverted fluorescence microscope (Nikon Diaphot, Japan) equipped with a 75 W Xenon lamp. Fluorescence images were collected with an intensified charge-coupled device (CCD) video camera (ISIS-M, Photonic Science, U.K.) at a videorate of 40 ms. At least eight cells found in an optical field (using 40× magnification objective) were analysed. Similar experimental protocols were performed on at least three different cell preparations.
For quantification of [Ca2+]i, following-in-time images obtained at 340 and 380 nm excitation, emission 510 nm (time interval between two following-in-time images: 800 ms) were digitalized by an analogical-to-digital converter (256×256 pixels×8 bits) and dynamically analysed on line using an image analysis, Microsoft Windows based software (Autolab®, RCS, Florence, Italy). Ratio images were obtained every 1.2 s. A dissociation constant of 224 nM was used for fura-2 and calibration curves were performed using ionomycin and ethylenebis(oxyethylenenitrilo)-tetraacetic acid as described previously (Failli et al., 1995). After measuring [Ca2+]i in resting condition, LTD4 or LTC4 at various concentrations were administered and maintained for the rest of the experiment. Intracellular calcium dynamics were followed for at least 10 min after agonist administration.
All data were exported in ASCII file format and elaborated for graphic presentation using MicroCal Origin® (2.8 version). A noise/signal ratio of 0.5 was considered as the low detectable limit for calcium transient. In order to verify cell responsiveness in several experiments, 1 μM ATII was also administered.
Immunocytochemistry of RASMs for α-smooth muscle actin
RASMs were grown on glass coverslips placed into 24-well plates until subconfluence. Several specimens were treated with LTD4 (10 nM) and after 10 min extensively washed with phosphate-buffered saline (PBS). Control and LTD4-treated cells were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature, washed in PBS, and immunolabelled with mouse monoclonal anti-α smooth muscle actin antibodies (Sigma; working dilution, 1 : 300). The immune reaction was revealed by Alexa 488 nm-labelled goat anti-mouse antibodies (Molecular Probes, Eugene, OR, U.S.A.; working dilution, 1 : 200). The immunostained cells were observed at a Bio-Rad 1024 ES confocal laser-scanning microscope (Bio-Rad, Hempsted, Herts, U.K.) with laser beam excitation at 488 nm wavelength. Three-dimensional images were built using a Confocal Assistant (Bio-Rad) software.
Chemicals
Acetylcholine (ACh), phenylephrine, indomethacin, ATII, collagenase type I, elastase, antibiotic solutions and ethylene diaminetetraacetic acid (EDTA) disodium salt were obtained from Sigma. LTC4, LTD4, MK-571 sodium salt, and the BLT selective antagonist U-75302 (Lawson et al., 1989) were purchased from Cayman Chemical. Fura-2AM and BAYu9773 were obtained from Molecular Probes and Biomol Research Laboratories Inc. (Plymouth, PA, U.S.A.) respectively; all other reagents were of analytical grade. Cell culture plastic supports were purchased from Costar (Corning Costar Co., Costar Italia, Milan, Italy).
The specific Cys Lt1 receptor antagonist montelukast (MK-476, Jones et al., 1995) was the kind gift of Merck Sharp & Dohme, Italy, Rome.
Mathematical and statistical methods
Values are presented as means±s.e.mean. For experiments quantifying [Ca2+]i, 8 to 14 RASMs isolated from at least three different cell lines of the 11 used for these experiments were analysed. The number of cells analysed is reported as c and the number of different preparations used as n. Dose-response curves were analysed with linear regression and compared with repeated measures ANOVA. Sigmoidal dose-response curves were analysed for the statistical comparison of curves with ALLFIT (Delean et al., 1978) and one-way ANOVA followed by Bonferroni's t-test. EC50 and IC50 were calculated using the Implot (version4) program (GraphPad Software, San Diego, CA, U.S.A.) and analysed for the statistical comparison with one-way ANOVA followed by Bonferroni's t-test. A P value of ⩽0.05 was considered significant.
Results
Functional studies on rat aortas
Phenylephrine (100 nM) contracted rat aortic strips, developing a tension of 20±1.2 mg mg−1 of wet tissue. This value was not modified by incubation with 5 μM indomethacin. In these experimental conditions (i.e. resting tension set at 0.7 g and in the presence of 5 μM indomethacin), ACh induced a strong, long-lasting dose-dependent contraction. Adding 2 nmol ACh as a bolus, the increase in contractile tone was up to twice that induced by phenylephrine (40.5±3.5 mg mg−1 of wet tissue). The ACh-induced contraction was dose-dependently blunted by Cys Lt1 antagonists montelukast and MK-571 and by the non-selective Cys Lt1/Lt2 antagonist BAY u9773 (Figure 1), whereas they did not affect the phenylephrine-induced one either in the presence or absence of endothelium. Also in basal condition, antagonists were unable to modify the aortic tone. The dose-inhibitory curve slopes were not significantly different (0.72, 0.71 and 0.76 respectively, ALLFIT). The BLT receptor antagonist U-75302 also reduced the ACh-induced contraction, although its dose-inhibitory curve had a different slope (0.38, F=6.87, P<0.01, ALLFIT) and IC50 (1.5 μM, pIC50=−5.82±0.361, F=276.15, P<0.01, one-way ANOVA) in comparison with the other antagonists used. Of the other antagonists tested, montelukast and MK-571 were the most effective molecules, their IC50 being 2.2 nM (pIC50=−8.66±0.092) and 3.1 nM (pIC50=−8.51±0.161) respectively, followed by BAY u9773 (IC50=7.9 nM, pIC50=−8.10±0.078).
Figure 1.
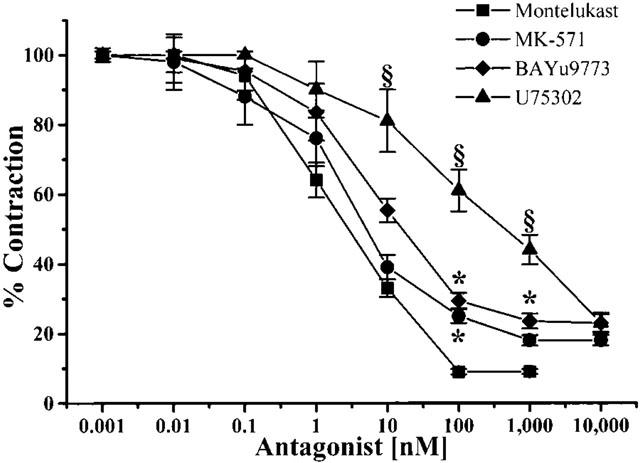
Effect of montelukast, MK-571, BAY u9773 and U-75302 on ACh-induced contraction in phenylephrine-precontracted rat thoracic aortic strips. Cumulative dose-response curves of antagonists were performed on rat thoracic aortic strips stretched to 0.7 g resting tension and preincubated with 5 μM indomethacin. Curves were statistically analysed using ALLFIT (Delean et al., 1978) and one-way ANOVA followed by Bonferroni's t-test. Values are the means±s.e.mean of at least four experiments. *P<0.05 vs montelukast, one-way ANOVA; § P<0.05 vs montelukast, MK-571 and BAY u9773, one- way ANOVA. For more details, see Results.
Western blot analysis of Cys Lt1 and Cys Lt2 receptors
Western blot analysis performed on RASMs revealed the presence of both receptors as immunoreactive bands with a molecular mass between 49 and 34.6 KDa in the cellular extract. As shown in Figure 2, the Cys Lt1 receptor was strongly expressed, while the Cys Lt2 type appeared less abundant.
Figure 2.
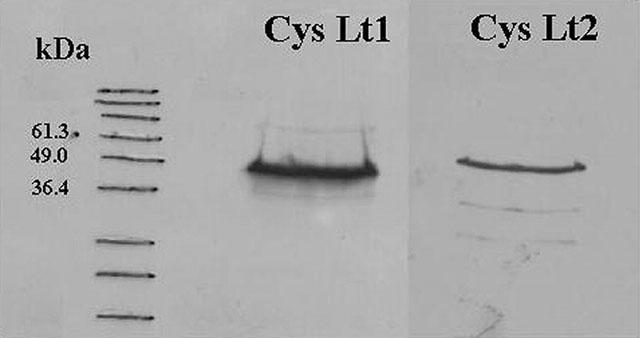
Western blot analysis of Cys Lt1 and Cys Lt2 receptors in isolated rat aortic smooth muscle cells. Representative results from one of three separate rat aortic smooth muscle cell line preparations.
Effect of Cys Lts on RASM intracellular calcium concentration
In resting condition, RASMs showed an [Ca2+]i of 173±4.0 nM (c=189, n=15). The administration of CysLTD4 (0.01–100 nM) to RASMs induced a dose-dependent increase in [Ca2+]i (r2=0.992, P<0.01). The EC50 of LTD4 in these experiments was 0.54 nM (pEC50=−9.27±0.114). LTC4 also increased intracellular calcium concentration. However, effective doses of LTC4 ranged from 200 to 800 nM (not shown).
The maximal effect of LTD4-induced [Ca2+]i increase was obtained using 10 nM LTD4. The non-cumulative dose-response curve of the LTD4-induced [Ca2+]i increase is shown in Figure 3. The maximal dose (10 nM) was used to better define the effect of LTD4. Figure 4 shows two representative time-courses of the [Ca2+]i increase representative of all cell preparations (n=14) analysed after treatment with 10 nM LTD4.
Figure 3.
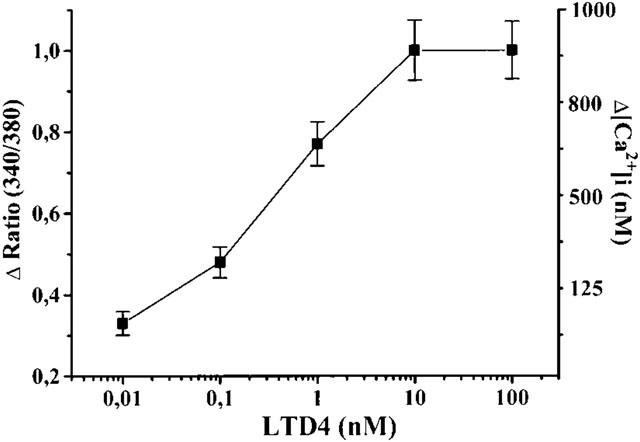
Non-cumulative dose-response curve of LTD4 in isolated rat aortic smooth muscle cells. Each point is the means (±s.e.mean) of at least 60 cells obtained in five separate experiments. Left axis: fluorescent ratio (340/380); Right axis: [Ca2+]i (nM).
Figure 4.
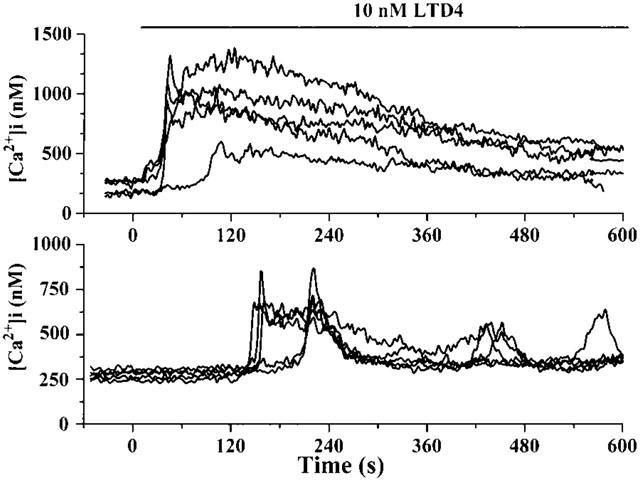
Typical time-course of LTD4-induced calcium transient in isolated rat aortic smooth muscle cells. LTD4 (10 nM) was administered at time=0 and maintained in the perfusion chamber until the end of the experiment, as indicated. The upper panel shows a sustained plateau phase of all cells; in the bottom one calcium oscillations are superimposed on the plateau phase. Each curve represents the time-course of a single fura-2-loaded cell. Intracellular calcium concentration ([Ca2+]i) is measured by image analysis. Representative results from two of 11 separate rat aortic smooth muscle cell line preparations.
As shown, the increase in [Ca2+]i induced by 10 nM LTD4 was characterized by an initial peak phase, the maximal [Ca2+]i value being obtained 190.2±18.22 s after LTD4 administration (range 2.4–424.29 s, c=148, n=14). Before the peak began, a lag time of about 60 s occurred (mean value 57.4±4.29 s; range 1.2±171.96 s, c=147, n=14). After reaching the maximal value, the [Ca2+]i slowly decreased. A second phase of long-lasting, sustained increase in [Ca2+]i was typically observed (see upper panel of Figure 4, typical example). This second phase lasted until the end of our experimental time (10 min). At this time, [Ca2+]i was up to 2–2.5 times higher than the resting value (mean value 353.9±21.02 nM, P<0.001 vs resting value, c=159, n=14). The lag time (r2=0.992, P<0.001) and time to the maximal [Ca2+]i value (r2=0.993, P<0.001) of the LTD4-induced calcium transient were linearly related to the LTD4 dose (Table 1). In several experiments, calcium oscillations were superimposed on the plateau phase (see bottom panel of Figure 4, typical example). Figure 5 shows the effect of 10 mM LTD4 as observed by video-imaging analysis in a representative experiment. A lag time of about 30 s after 10 nM LTD4 administration occurred before [Ca2+]i began to increase, reaching the highest level after 100 s. The peak phase was found not to be synchronized in the analysed cells. Moreover, in several cells, the increase was mainly localized at the cell periphery.
Table 1.
Dose-response curve of LTD4 on lag time and time to the maximal [Ca2+]i value in RASMs
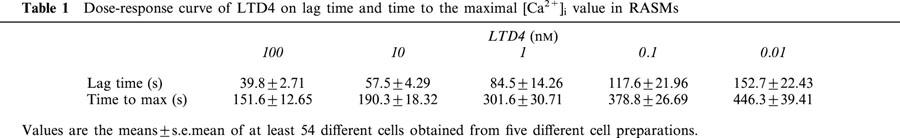
Figure 5.
Time sequence of [Ca2+]i in individual fura-2-loaded rat aortic smooth muscle cells in response to 10 nM LTD4 (representative experiment). Frames are described left to right and up to down. Frame 1 shows resting [Ca2+]i before the addition of LTD4. Frames 2, 3, 4 (29, 58 and 105 s after the addition of LTD4 respectively) illustrate the LTD4-induced [Ca2+]i increase. [Ca2+]i (nM) is colour-coded according to the calibration bar.
Preincubation (20 min at 37°C) with 3 nM thapsigargin (which induced a slow and sustained increase in [Ca2+]i) nearly abolished the LTD4-induced [Ca2+]i transient in RASMs, although five out of 77 cells showed a moderate [Ca2+]i increase.
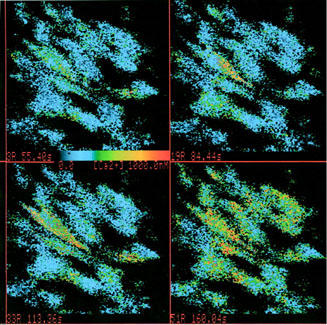
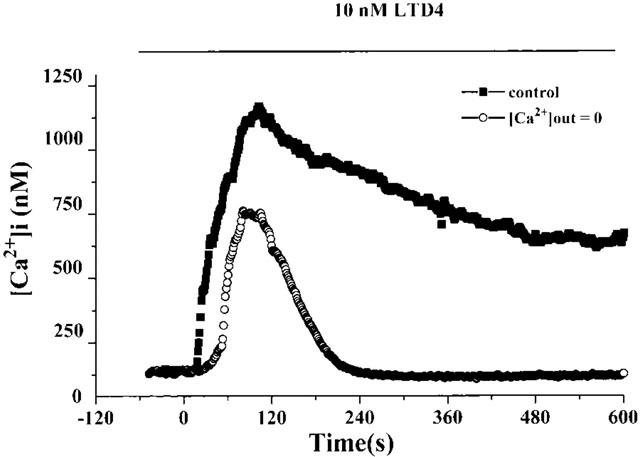
When 10 nM LTD4 was administered in the absence of extracellular calcium (200 μM EDTA in the calcium-free medium), the peak phase of the [Ca2+]i increase was reduced as compared with the experiments performed in the calcium-containing medium and the plateau phase was absent (Figure 6). The L-type calcium channel blocker nifedipine (10 nM) also influenced the LTD4-induced calcium mobilization. The resting [Ca2+]i value was not significantly modified by nifedipine, whereas both the peak and plateau phase of the [Ca2+]i increase induced by 10 nM LTD4 were reduced by about 50% (Figure 7). Similar results were obtained using 1 μM verapamil (not shown).
Figure 6.
Typical time-course of LTD4-induced [Ca2+]i increase in isolated rat aortic smooth muscle cells in the absence of extracellular calcium (200 μM EDTA). LTD4 (10 nM) was administered at time=0 and maintained in the perfusion chamber until the end of experiment as indicated. Curves are the mean of at least 11 single cells. In each point, standard errors (s.e.mean) do not exceed 10% of values.
Figure 7.
Typical time-course of LTD4-induced [Ca2+]i increase in isolated rat aortic smooth muscle cells in control condition or after preincubation (10 min) with 10 nM nifedipine. LTD4 (10 nM) was administered at time=0 and maintained in the perfusion chamber until the end of experiment, as indicated. Curves are the mean of at least 12 cells. In each point, standard errors (s.e.mean) do not exceed 10% of values.
After preincubation (10 min at 37°C) with montelukast in the range of 1–100 nM, the resting [Ca2+]i was not modified (F=1.84, not significant; repeated measures ANOVA). Conversely, the LTD4-induced [Ca2+]i increase was reduced dose-dependently (r2=0.999, P<0.05), 10 nM montelukast being able to reduce the [Ca2+]i increase by about 51% (Figure 8). After removal of montelukast and thorough washing of the RASMs (20 min at a constant flux of 1.5 ml min−1), the effect of 10 nM LTD4 was restored. Using 1 nM LTD4 as agonist, 1, 10 and 100 nM montelukast reduced the [Ca2+]i increase by 52±7.4, 66±5.4 and 88±7.3% respectively. The non-selective Cys Lt1/Cys Lt2 antagonist BAY u9773, in the range 1–100 nM, did not modify the resting [Ca2+]i (F=6.45, not significant; repeated measures ANOVA), whereas it did reduce the 10 nM LTD4-induced [Ca2+]i increase in a dose-dependent way (Figure 8, r2=0.998, P<0.05). The BAY u9773 dose-response curve was significantly different from that of montelukast (F=49.56, P<0.05, repeated measures ANOVA; Figure 8). A higher dose of BAY u9773 (1 μM) induced calcium oscillation in resting condition and was therefore not further analysed. These calcium oscillations were absent in RASMs preincubated with 10 nM montelukast.
Figure 8.
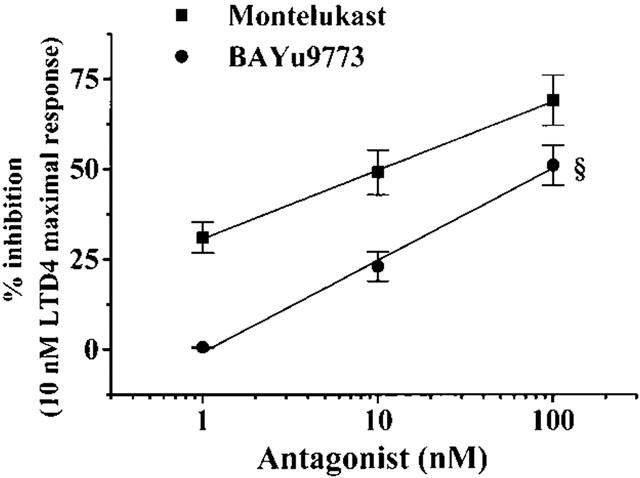
Non cumulative dose-response curves of montelukast and BAY u9773 on LTD4-induced [Ca2+]i increase in isolated rat aortic smooth muscle cells. Antagonists were preincubated 10 min before administration of 10 nM LTD4. Values (means±s.e.mean of at least 46 cells obtained in four separate experiments) were calculated as per cent inhibition of the LTD4-induced [Ca2+]i increase. *P<0.05, F=49.56, repeated measures ANOVA.
Effect of LTD4 on actin microfilaments
Administration of 10 nM LTD4 to RASMs induced a redistribution of actin microfilaments, indicating a contractile effect of this molecule. Moreover, as shown in Figure 9, cells changed their shape from a planar to a globular shape.
Figure 9.
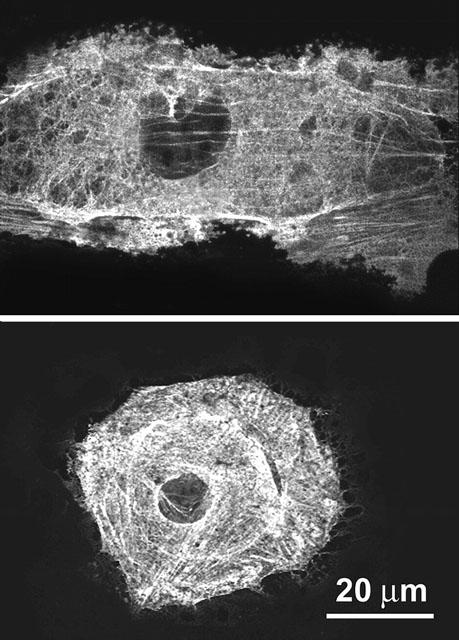
Representative confocal micrographs of cultured rat aortic smooth muscle cells immunostained to reveal α-smooth muscle actin in resting conditions and upon addition of 10 nM LTD4. In the resting cell, cytoplasmic actin is in the form of typical stress fibres, whereas in the LTD4-stimulated cell actin is distributed evenly throughout the cytoplasm and the overall cell shape suggests cell contraction. Cells were fixed 10 min after addition of the stimulus (see Methods). Immune reaction was revealed with Alexa 488 nm-labelled goat anti-mouse antibodies.
Discussion
Our data reveal a new endogenous pathway regulating rat aortic tone due to Cys Lts. When cyclo-oxygenase isoenzymes are inhibited, the arachidonic acid metabolism can produce leukotrienes able to strongly contract the aortic smooth muscle cell coat. As discussed in our previous work, Cys Lts production of rat aortas is undetectable using commercially available methods (Franchi-Micheli et al., 2000), but according to our functional results, this contraction is mainly due to Cys Lts acting through Cys Lt1 stimulation. Indeed, the Cys Lt1 antagonists montelukast and MK-571 can specifically inhibit this contraction, their IC50s being in the low nanomolar range. These IC50s are very similar to those reported by Lynch et al. (1999) and Sarau et al. (1999) who performed radiolabel binding studies in Cos-7 and HEK293 cells transfected with hCys Lt1. Of note, while 100 nM montelukast is the maximal inhibiting dose of ACh-induced contraction, the maximal inhibition is obtained with 1 μM MK-571. Also the non-selective Cys Lt1/Cys Lt2 antagonist BAY u9773 can counteract ACh-induced contraction, reaching the maximal effect at 1 μM.
Due to the similar efficacy in inhibiting the ACh-induced contraction by Cys Lt1 selective and non-selective antagonists, endogenously produced Cys Lts seem to chiefly activate Cys Lt1. However, as the murine type of Cys Lt2 has not yet been cloned, we cannot exclude that montelukast and MK-571, currently thought to be selective Cys Lt1 antagonists, might also influence the Cys Lt2 response. The BLT antagonist U-75302 shows a significantly different dose-response curve and a low inhibitory potency as demonstrated by its curve and high IC50. These data are in agreement with those obtained in binding studies by Martin et al. (2001), which show a high Ki of LTB4 in competition with LTD4 to bind to mCys Lt1-transfected Cos-7 cells.
Western blot analysis performed on RASMs demonstrates the simultaneous presence of Cys Lt1 and Cys Lt2 receptors at the plasma membrane, Cys Lt1 being predominant. Its immunoreactive band ostensibly has a higher molecular mass than hCys Lt1, suggesting that a long form similar to mCys Lt1 is present on RASMs.
Experiments exploring the effect of Cys Lts on isolated smooth muscle cells, demonstrate that LTD4 in the nanomolar range is able to increase [Ca2+]i in fura-2 loaded RASMs with a 0.54 nM EC50. LTC4 is also able to induce a [Ca2+]i increase, while it is far less active than LTD4. This finding is in keeping with the reported agonist effectiveness rank for Cys Lt1 activation (Lynch et al., 1999; Sarau et al., 1999).
The LTD4-induced [Ca2+]i increase is characterized by a peak phase and a long lasting plateau. Since thapsigargin can abolish it, suppressing both the peak and the plateau phase, a predominant role in the RASM cell response to LTD4 seems to be played by a release of Ca2+ from intracellular stores, that can trigger a calcium influx from extracellular medium as determined in experiments performed in the absence of extracellular calcium in which the plateau phase is suppressed. This calcium influx is also partially prevented by preincubation with nifedipine or verapamil, thus suggesting that voltage-operated calcium channels (VOCCs) of the L-type may be involved in the calcium influx. However, since nifedipine can also reduce the first peak phase, it is likely that the opening of L-type VOCCs may also influence the replenishment of the intracellular stores. These data are in agreement with early work of our group on rat aortic strips (Franchi-Micheli et al., 2000), as well as with the data obtained in canine saphenous veins by Rimile & Vanhoutte (1983) demonstrating that L-type calcium channel blockers are able to prevent leukotriene-induced contraction.
The residual calcium plateau phase observed in nifedipine-treated cells may be dependent on an influx through less defined receptor-operated calcium channels (ROCCs) and/or store-operated calcium channels (SOCCs). This composite interaction between calcium release and calcium influx via different calcium channels after activation of a Gq-coupled receptor is quite characteristic of smooth muscle cells (McFadzean & Gibson, 2002).
Montelukast in the nanomolar range antagonizes the LTD4 effect on [Ca2+]i, supporting the data obtained in aortic strips that the prevalent receptor form involved in calcium mobilization as well as in aortic strip contraction is Cys Lt1. BAY u9773 is far less effective in inhibiting LTD4-induced [Ca2+]i increase. At high doses, BAY u9773 modifies resting [Ca2+]i levels. The weak increase in [Ca2+]i level seems unable to activate contraction as demonstrated in functional experiments performed on rat aortic strips either in the presence or absence of an intact endothelial layer. Further research will clarify this point.
In conclusion, our data demonstrate that when cyclo-oxygenase isoenzymes are inhibited, Cys Lts are endogenously produced in rat aortas; Cys Lts, acting mainly through the activation of Cys Lt1 can actively regulate vascular tone and increase [Ca2+]i and hence may be involved in the control of blood pressure and its derangements in hypertension.
Acknowledgments
This work was supported by grants from the University of Florence and Istituto Gentili S.p.A. (Pisa, Italy). We would like to thank Prof Daniele Bani for reading the manuscript and his kind suggestions and Ms Mary Forrest for editing the manuscript.
Abbreviations
- ACh
acetylcholine
- AT II
angiotensin II
- BAY u-9773
6(R)-(4′-carboxyphenylthio)-5(S)-hydroxy-7(E), 9(E), 11(Z),14(Z)-eicosatetrenoic acid
- BLT
leukotriene B4 receptor
- Cys Lt1
cysteinyl-containing leukotriene type 1 receptor
- Cys Lt2
cysteinyl-containing leukotriene type 2 receptor
- [Ca2+]i
intracellular calcium concentration
- Δ [Ca2+]i
[Ca2+]i at the peak minus [Ca2+]i resting value
- EC50
median effective concentration
- IC50
median inhibitory concentration
- 5-LOX
5-lipoxygenase
- MK-571
(E)-3 [[[3-[2-(7-chloro-2-quinolinyl) ethenyl] phenyl] [[3-(dimethylamino)-3-oxopropyl] thio] methyl] thio] propanoic acid, sodium salt
- Montelukast (MK-476)
[R-(E)]-1 [[[1-[3-[2-(7-chloro-2-quinolinyl) ethenyl] phenyl]-3-[2-(1-hydroxy-1methylethyl) phenyl] propyl] thio] methyl ] cyclopropaneacetic acid, sodium salt
- RASMs
rat aortic smooth muscle cells
- U-75302
6-(6-(3R-hydroxy-1E,5Z-undecadien-1-yl) -2-pyridinyl)-1,5S-exanediol
References
- ALLEN S., DASHWOOD M., MORRISON K., YACOUB M. Differential leukotriene constrictor responses in human atherosclerotic coronary arteries. Circulation. 1998;97:2406–2413. doi: 10.1161/01.cir.97.24.2406. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DELEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families sigmoidal curves: Application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- FALLIER-BECKER P., RUPP J., FINGERLE J., BETZ E.Smooth muscle cells from rabbit aorta Cell Culture techniques in heart and vessel research 1990Berlin: Spring-Verlag; 247–270.ed. Piper, H.M. pp [Google Scholar]
- FAILLI P., RUOCCO C., DE FRANCO R., CALIGIURI A., GENTILINI A., GIOTTI A., GENTILINI P., PINZANI M. The mitogenic effect of platelet-derived growth factor in human hepatic stellate cells requires calcium influx. Am. J. Physiol. 1995;269:C1133–C1139. doi: 10.1152/ajpcell.1995.269.5.C1133. [DOI] [PubMed] [Google Scholar]
- FRANCHI-MICHELI S., FAILLI P., MAZZETTI L., BANI D., CIUFFI M., ZILLETTI L. Mechanical stretch reveals different components of endothelial-mediated vascular tone in rat aortic strips. Br. J. Pharmacol. 2000;131:1355–1362. doi: 10.1038/sj.bjp.0703707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEISE C.E., O'DOWD B.F., FIGUEROA D.J., SAWYER N., NGUYEN T., IM D.S., STOCCO R., BELLEFEUILLE J.N., ABRAMOVITZ M., CHENG R., WILLIAMS D.L., ZENG Z., LIU Q., MA L., CLEMENTS M.K., COULOMBE N., LIU Y., AUSTIN C.P., GERAGE S.R., O'NEIL G.P., METTERS K.M., LYNCH K.R., EVANS J.F. Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- ISHIZAKA N., NAKAO A., OHISHI N., SUZUKI M., AIZAWA T., TAGUCHI J., NAGAI R., SHIMIZU T., OHNO M. Increased leukotriene A(4) hydrolase expression in the heart of angiotensin II-induced hypertensive rat. FEBS Lett. 1999;463:155–159. doi: 10.1016/s0014-5793(99)01607-5. [DOI] [PubMed] [Google Scholar]
- JONES T.R., LABELLE M., BELLEY M., CHAMPION E., CHARETTE L., EVANS J., FORD-HUTCHINSON A.W., GAUTHIER J.Y., LORD A., MASSON P., MCAULIFFE M., MCFARLANE C.S., METTERS K.M., PICKETT C., PIECHUTA H., ROCHETTE C., RODGER I.W., SAWYER N., YOUNG R.N., ZAMBONI R., ABRAHAM W.M. Pharmacology of montelukast sodium (Singulair), a potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1995;73:191–201. doi: 10.1139/y95-028. [DOI] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LAWSON C.F., WISHKA D.G., MORRIS J., FITZPATRICK F.A. Receptor antagonism of leukotriene B4 myotropic activity by the 2,6 disubstituted pyridine analog U-75302: characterization on lung parenchyma strips. J. Lipid Mediat. 1989;1:3–12. [PubMed] [Google Scholar]
- LEWIS R.A., AUSTEN K.F., SOBERMAN R.J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- LYNCH K.R., O'NEILL G.P., LIU Q., IM D.S., SAWYER N., METTERS K.M., COULOMBE N., ABRAMOVITZ M., FIGUEROA D.J., ZENG Z., CONNOLLY B.M., BAI C., AUSTIN C.P., CHATEAUNEUF A., STOCCO R., GREIG G.M., KARGMAN S., HOOK S.B., HOSFIELD E., WILLIAMS D.L., FORD-HUTCHINSON A.W., CASKEY C.T., EVANS J.F. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- MARTIN V., SAWYER N., STOCCO R., UNETT D., LERNER M.R., ABRAMOVITZ M., FUNK C.D. Molecular cloning and functional characterization of murine cysteinyl-leukotriene 1 (CysLT(1)) receptors. Biochem. Pharmacol. 2001;62:1193–1200. doi: 10.1016/s0006-2952(01)00774-2. [DOI] [PubMed] [Google Scholar]
- MCFADZEAN I., GIBSON A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODAT G., MULLER A., MARY A., GREGOIRE C., BONNE C. Differential effects of leukotrienes B4 and C4 on bovine aortic endothelial cell proliferation in vitro. Prostaglandins. 1987;33:531–538. doi: 10.1016/0090-6980(87)90276-0. [DOI] [PubMed] [Google Scholar]
- NOTHACKER H.P., WANG Z., ZHU Y., REINSCHEID R.K., LIN S.H.S., CIVELLI O. Molecular cloning and characterization of a second human cysteinyl-leukotriene receptor: Discovery of a subtype selective agonist. Mol. Pharmacol. 2000;58:1601–1608. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- PALMBERG L., LINDGREN J.A., THYBERG J., CLAESSON H.E. On the mechanism of induction of DNA synthesis in cultured arterial smooth muscle cells by leukotrienes. Possible role of prostaglandin endoperoxide synthase products and platelet-derived growth factor. J. Cell. Sci. 1991;98:141–149. doi: 10.1242/jcs.98.2.141. [DOI] [PubMed] [Google Scholar]
- PHAN S.H., MCGARRY B.M., LOEFFLER K.M., KUNKEL S.L. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry. 1988;27:2846–2853. doi: 10.1021/bi00408a028. [DOI] [PubMed] [Google Scholar]
- RIMELE T.J., VANHOUTTE P.M. Effect of inhibitors of arachidonic acid metabolism and calcium entry on responses to acetylcholine, potassium and norepinephrine in the isolated canine saphenous vein. J. Pharmacol. Exp. Ther. 1983;225:720–728. [PubMed] [Google Scholar]
- SALA A., ROSSONI G., BERTI F., BUCCELLATI C., BONAZZI A., MACLOUF J., FOLCO G. Monoclonal anti-CD18 antibody prevents transcellular biosynthesis of cysteinyl leukotrienes in vitro and in vivo and protects against leukotriene-dependent increase in coronary vascular resistance and myocardial stiffness. Circulation. 2000;101:1436–1440. doi: 10.1161/01.cir.101.12.1436. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B. The discovery of the leukotrienes. Am. J. Respir. Crit. Care Med. 2000;161:S2–S6. doi: 10.1164/ajrccm.161.supplement_1.ltta-1. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B., DAHLEN S.E., LINDGREN J.A., ROUZER C.A., SERHAN C.N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- SARAU H.M., AMES R.S., CHAMBERS J., ELLIS C., ELSHOURBAGY N., FOLEY J.J., SCHMIDT D.B., MUCCITELLI R.M., JENKINS O., MURDOCK P.R., HERRITY N.C., HALSEY W., SATHE G., MUIR A.I., NUTHLAGANTI P., DYTKO G.M., BUCKLEY P.T., WILSON S., BERGSMA D.J., HAY D.W.P. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol. Pharmacol. 1999;56:657–663. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- STANKE-LABESQUE F., DEVILLIER P., VEITL S., CARON F., CRACOWSKI J., BESSARD G. Cysteinyl leukotrienes are involved in angiotensin II-induced contraction of aorta from spontaneously hypertensive rats. Cardiovasc. Res. 2001;49:152–160. doi: 10.1016/s0008-6363(00)00238-8. [DOI] [PubMed] [Google Scholar]
- TAKASAKI J., KAMOHARA M., MATSUMOTO M., SAITO T., SUGIMOTO T., OHISHI T., ISHII H., OTA T., NISHIKAWA T., KAWAI Y., MASUHO Y., ISOGAI T., SUZUKI Y., SUGANO S., FURUICHI K. The molecular characterization and tissue distribution of the human cysteinyl leukotriene CysLT2 receptor. Biochem. Biophys. Res. Commun. 2000;274:316–322. doi: 10.1006/bbrc.2000.3140. [DOI] [PubMed] [Google Scholar]
- TUDHOPE S.R., CUTHBERT N.J., ABRAM T.S., JENNINGS M.A., MAXEY R.J., THOMPSON A.M., NORMAN P., GARDINER P.J. BAY u9773, a novel antagonist of cysteinyl-leukotrienes with activity against two receptor subtypes. Eur. J. Pharmacol. 1994;264:317–323. doi: 10.1016/0014-2999(94)00485-4. [DOI] [PubMed] [Google Scholar]


