Abstract
In order to explore potential therapeutic implications of cannabinoid antagonists, the effects of the prototypical cannabinoid antagonist SR141716A on monoamine efflux from the medial prefrontal cortex and the nucleus accumbens of the rat were investigated by in vivo microdialysis.
SR141716A moderately increased serotonin efflux and concentrations of its metabolite 5-HIAA, both in the medial prefrontal cortex and the nucleus accumbens, and increased norepinephrine, dopamine and their metabolites in the medial prefrontal cortex. In contrast, it had no effect on norepinephrine, dopamine and their metabolites in the nucleus accumbens.
At the same doses, SR141716A increased acetylcholine efflux in the medial prefrontal cortex, in agreement with previous studies; contrary to the effects in cortex, SR141716A had no effect on acetylcholine efflux in the nucleus accumbens.
The efficacy of SR141716A in the psychostimulant-induced hyperlocomotion and the forced swimming paradigms was also explored in mice. SR141716A attenuated phenylcyclidine- and d-amphetamine-induced hyperlocomotion, without affecting locomotor activity when administered alone, and decreased immobility in the forced swimming test.
These results suggest that the cortical selectivity in the release of catecholamines, dopamine in particular, induced by the cannabinoid antagonist SR141716A, its procholinergic properties, together with its mild stimulatory effects on serotonin and norepinephrine efflux make similar compounds unique candidates for the treatment of psychosis, affective and cognitive disorders.
Keywords: SR141716A, DA, NE, 5-HT, ACh, microdialysis, prefrontal cortex, antidepressant, antipsychotic, ADHD
Introduction
The identification of the G-protein coupled type 1 cannabinoid (CB1) receptor that is predominantly localized in the central nervous system (CNS), and the isolation of endogenous ligands, arachidonic acid derivatives, that bind to and activate this receptor, has led to the unravelling of a neuromodulatory endocannabinoid system that is still far from being completely characterized. Presently, a considerable effort is aimed at understanding the role of this emerging system in the pathophysiology of the CNS, exploring the therapeutic possibilities that positive or negative modulators of endocannabinoid activity may offer, developing new molecules with direct or indirect agonistic or antagonistic activity, and determining relevant therapeutic indications for these compounds (Piomelli et al., 2000; Fowler & Jacobsson, 2002). In this respect, the synthesis of the potent and selective prototypical antagonist of the CB1 receptor, SR141716A (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1 H-pyrazole-3-carboxamide-hydrochloride) (Rinaldi-Carmona et al., 1994), has enabled the evaluation of CB1 receptor antagonists as a novel class of pharmacologically active molecules and potentially efficient therapeutic tools.
Studies in animal models have shown that SR141716A reduces the perception of the rewarding/appetitive value of positive reinforcers (reviewed in Chaperon & Thiebot, 1999). This effect may be a key component in the observed anti-addictive properties of SR141716A that reportedly attenuates morphine induced place preference and withdrawal (Mas-Nieto et al., 2001), alcohol intake in alcohol preferring rats (Colombo et al., 1998) and cocaine craving (de vries et al., 2001). Interestingly, and along the same line, SR141716A also suppresses both palatable and non-palatable food intake under operant and non-operant experimental schedules in animals (reviewed in Chaperon & Thiebot, 1999) and reduces body weight of obese patients (communicated by G. Le Fur, 2001, International Cannabinoid Research Society Meeting, El Escurial, Spain). In addition, SR141716A has been shown to increase arousal (Santucci et al., 1996), improve olfactory memory as assessed by the social recognition test (Terranova et al., 1996) and enhance spatial memory in the radial-arm maze task (Lichtman, 2000) in rodents. Furthermore, studies with CB1 knock-out mice have shown a role of endogenous cannabinoids in adaptative processes that control feeding (di marzo et al., 2001), drug dependence and learning and memory (reviewed in Lichtman et al., 2002), supporting the notion that the pharmacological effects of SR141716A result from the disruption of an actively operating endocannabinoid tone.
Little is still known about the neuroanatomical mechanisms by which endocannabinoids modulate behaviour. However, it has been shown that endocannabinoids interact with other neuromodulator/neurotransmitter systems at multiple levels. Interactions between the activity of the endocannabinoid system and opioid, glutamatergic, GABAergic and dopaminergic neurotransmission have been extensively reported (reviewed in Schlicker & Kathmann, 2001). Therefore, pharmacological activity of CB1 receptor antagonists may result from the ability of these compounds to modify major neurotransmitter systems (Chaperon & Thiebot, 1999; Schlicker & Kathmann, 2001). A number of in vitro experiments suggest an excitatory action of SR141716A on regional neurotransmitter release and neuronal activity. Thus, it has been shown that SR141716A increases: (i) glutamate-mediated (Kathmann et al., 1999) norepinephrine (NE) release from hippocampal slices, (ii) electrically evoked acetylcholine (ACh) release from hippocampal slices (Gifford & Ashby, 1996) as well as (iii) glutamatergic EPSCs in prefrontal cortex slices (Auclair et al., 2000). It is interesting to note that these stimulatory in vitro effects of SR141716A are dependent on the neurotransmitter system and the region studied. Indeed, SR141716A when applied alone failed to affect glutamatergic EPSCs in the striatum (Gerdeman & Lovinger, 2001), electrically evoked DA release from striatal and nucleus accumbens (n.Acc.) slices (Cadogan et al., 1997; Szabo et al., 1999) or striatal ACh release (Gifford & Ashby, 1996) at doses effective in blocking the effects of exogenously applied cannabinoid agonists.
In vivo, SR141716A increases ACh efflux in hippocampal microdialysates (Gessa et al., 1998) and NE efflux in the anterior hypothalamus (Tzavara et al., 2001) at behaviourally active doses. Here, by using in vivo microdialysis, we studied the effects of SR141716A on the extracellular concentrations of monoamines (dopamine (DA), NE, setotonin (5-HT), ACh) and monoamine metabolites (DOPAC, HVA, 5-HIAA) from the medial prefrontal cortex and the n.Acc. of awake, freely moving rats in view of the involvement of these areas in the pathophysiology and pharmacotherapy of neuropsychiatric diseases, such as schizophrenia and depression (e.g. Meyer-Lindenberg et al., 2002; Baxter et al., 1989). In addition, we evaluated the ability of SR141716A to produce antipsychotic-like effects through reversal of hyperlocomotion induced by psychostimulants (phenylcyclidine (PCP) and d-amphetamine), and to induce antidepressant-like effects in a behavioural despair paradigm, the forced swimming test in mice. Pharmacological effects in these animal models of antipsychotic- and antidepressant-like activities are invariably associated with changes in the activity of monoaminergic pathways (see e.g. Carlsson et al., 2000; Lucki, 1997).
Methods
Drugs
SR141716A was synthesized in Eli Lilly and Company. PCP and d-amphetamine were purchased from Sigma.
Animals
All studies were performed according to the guidelines set forth by the National Institutes of Health and implemented by the Animal Care and Use Committee of Eli Lilly and Company. Male Wistar rats (250–300 g) were used for microdialysis experiments. Male C57/BL6 (25–30 g) mice were used for psychostimulant-induced hyperlocomotion and male NIH Swiss mice (25–30 g) for forced swimming test experiments. Animals (purchased from Harlan Sprague–Dawley, Indianapolis, IN, U.S.A.) were housed in a vivarium for at least 1 week prior to use; water and food were available ad libitum during this acclimatization period.
In vivo microdialysis studies
Two weeks prior to the microdialysis experiments, rats were anaesthetized with a mixture of chloral hydrate and pentobarbital (170 mg kg−1 and 36 mg kg−1 in 30% propylene glycol and 14% ethanol), placed in a stereotaxic apparatus and implanted with a guide cannula (BAS). Twenty-four hours before testing, a 4 mm or 2 mm concentric microdialysis probe was implanted in the medial prefrontal cortex (BAS, BR-4) or in the n.Acc. (BAS, BR-2). Coordinates for the medial prefrontal cortex were AP: +3.2 mm ML: +0.6 mm DV: −2.2 mm and for the n.Acc. AP: +1.6 mm ML: +1.2 mm DV: −6.3 mm, according to Paxinos & Watson (1986). The correct location of the probe was verified histologically at the end of the experiment.
Measurements of DA, NE, 5-HT and their metabolites
On the day of the experiment, a modified Ringer's solution in mM (NaCl 150, KCl 3, CaCl2 1.7 and MgCl2 0.9, pH=6.0) was perfused at a rate of 1 μl min−1. Samples were collected every 30 min into a refrigerated fraction collector and analysed the same day of the experiment with HPLC coupled to electrochemical detection as previously described (Perry & Fuller, 1997).
ACh measurements
On the day of the experiment, a modified Ringer's solution supplemented with 0.1 μM neostigmine in mM (NaCl 147.0, KCl 3.0, CaCl2 1.3, MgCl2 1.0, Na2HPO4×7H2O 1.0, NaH2PO4×H2O 0.2 pH=7.25) was perfused at a rate of 2.4 μl min−1. Samples were collected every 15 min and analysed immediately, on-line, with HPLC coupled to electrochemical detection, with a 150×3 mm ACH-3 column (ESA, Inc.) maintained at 35°C. The mobile phase was comprised of 100 mM di-Sodium hydrogen phosphate, 2 mM 1-octanesulfonic acid and 50 μl l−1 of a microbicide (reagent MB, ESA, Inc.) (pH 8.0, adjusted with phosphoric acid) and was delivered by an HPLC pump (ESA, Inc.) at 0.4 ml min−1. A coulometric detector was used for electrochemical detection (ESA Coulochem II) connected with a solid phase reactor for ACh (ESA; ACH-SPR) and with an analytical cell with platinum target (ESA 5041).
For all microdialysis experiments, SR141716A was dissolved in saline containing 2% DMSO, 2% cremophor EL, and injected i.p. at a volume of 3 ml kg−1. Data (n=5–7 rats per group) are expressed as multifold change from baseline, which is the average of the five basal values immediately before SR141716A administration and were analysed with two-way ANOVA (treatment: independent variable×time: dependent variable-within subjects factor) followed by Duncan's test.
Psychostimulant-induced hyperlocomotion
To assess the effects of SR141716A on psychostimulant (PCP- and d-amphetamine-) induced hyperlocomotion, locomotor activity was measured with a 20 station Photobeam Activity System (San Diego Instruments, San Diego, CA, U.S.A.) with seven photocells per station. Mice were placed in the locomotor activity boxes (40.6×20.3×15.2 cm) for a habituation period of 20 min. Immediately after habituation, the psychostimulants, d-amphetamine 2.5 mg kg−1 or PCP 4 mg kg−1 (dissolved in saline) were administered i.p. at a volume of 10 ml kg−1, simultaneously with SR141716A (3 mg kg−1 or 10 mg kg−1 i.p. dissolved in 2% DMSO, 2% cremophor EL, saline) or vehicle. Locomotion was assessed for a 60 min period after the injection. Data (n=8–12 mice per group) are expressed as total ambulations (where ambulation was defined as the sequential breaking of adjacent photobeams) for the entire 60 min period following the injection and were analysed with two-way ANOVA (treatment with the stimulant×treatment with SR141716A) followed by Duncan's test.
Forced swimming test
To assess the effects of SR141716A on behavioural despair paradigms, mice (7–8 per experimental group) were tested in the forced swimming test 30 min after a single administration of SR141716A (0.3 mg kg−1 −3 mg kg−1 dissolved in 2% DMSO, 2% cremophor EL, saline) or vehicle injected i.p. at a volume of 10 ml kg−1. The forced swimming test was conducted in clear plastic cylinders (diameter 10 cm; height 25 cm) filled with 6 cm of water (22–25°C) for 6 min. The duration of immobility was recorded using the programme PORSOLT (Infallible Software, Rockville, MD, U.S.A.), during the last 4 min of the 6-min trial. A mouse was regarded as immobile when floating motionless or making only those movements necessary to keep its head above the water. Statistical analysis was performed with one-way ANOVA and Duncan's post-hoc test.
Results
In vivo microdialysis studies
The effects of a single i.p. administration of SR141716A (1, 3 and 10 mg kg−1) on the monoamines 5-HT, DA, NE, ACh and on the monoamine metabolites DOPAC, 5-HIAA, and HVA were assessed by in vivo microdialysis in the prefrontal cortex and the n.Acc. of the rat. There were no statistically significant differences in the basal values of parent amines (DA, NE, 5-HT, ACh) or of metabolites (DOPAC, HVA, 5-HIAA) among the groups receiving vehicle or SR141716A. Therefore the basal values were pooled and presented together. For each of the parameters examined the effects of SR141716A are presented both over a course of time, every 30 min after the injection of the drug, as well as overall effects during the four-hour observation period after the injection of the drug (area under curve). When data are graphed over a course of time, statistical significance for each time point as compared to vehicle is indicated with an asterisk only for the 10 mg kg−1 dose for reasons of simplicity and clarity of presentation. For the same reason all levels of statistical significance mentioned in the Results section, are indicated in the Figures only with one asterisk signifying at least P<0.05.
SR141716A increases 5-HT, DA, NE and their metabolites in the prefrontal cortex
Basal values (in pmoles ml−1) in the prefrontal cortex were: 0.19 ±0.02 for 5-HT, 0.97±0.07 for NE, 0.47±0.05 for DA, 60±5 for DOPAC, 235±13 for 5-HIAA, and 82±6 for HVA.
Two-way ANOVA on the 5-HT time course data showed significant effects of treatment (F(2,12)=7.77, P<0.01) time (F(9, 108)=11.6, P<0.001) and interaction between these two factors (F(18,108)=2.86, P<0.001). SR141716A at 3 mg kg−1 (F(9, 72)=2.54, P<0.05) and 10 mg kg−1 (F(9, 63)=5.15, P<0.01) increased 5-HT efflux in the prefrontal cortex. 5-HT was increased (up to a peak value of 200%) 1 h after the injection of 10 mg kg−1 SR141716A and returned to baseline levels 3 h later (Figure 1a). One-way ANOVA that was used to analyse the overall effect of SR141716A (at 1, 3 and 10 mg kg−1) also showed a significant effect of the drug (F(3, 18)=15.7, P<0.001), at the 3 and 10 mg kg−1 doses on 5-HT efflux (Figure 1d).
Figure 1.
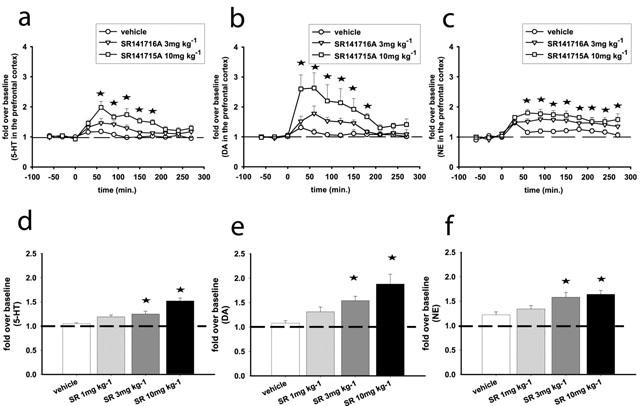
SR141716A increases 5-HT (a,d), DA (b,e) and NE (c,f) efflux in the prefrontal cortex of the rat. (a,b, and c) Represent time course effects after i.p. administration of SR141716A at 3 and 10 mg kg−1. (d,e and f) Represent the average effects of SR141716A (1, 3, 10 mg kg−1; i.p.) over the course of the experiment (overall effects). Data represent mean±s.e.mean of n=5–8 rats per experimental group and are expressed as multifold change from baseline, which is the average of the five basal values immediately before SR141716A administration. Asterisks indicate P<0.05 as compared to the vehicle-receiving group. (a,b,c) Statistical significance for each time point is indicated with an asterisk only for the 10 mg kg−1 dose.
For the time course of DA efflux, two-way ANOVA showed significant effects of treatment (F(2,14)=5.97, P<0.05) time (F(9, 126)=14.7, P<0.001) and interaction between these two factors (F(18,126)=3.73, P<0.001). SR141716A at 3 mg kg−1 (F(9, 90)=4.4, P<0.01) and 10 mg kg−1 (F(9, 81)=5.9, P<0.01) significantly increased DA efflux in the prefrontal cortex. For the 10 mg kg−1 dose this increase (up to a peak value of 263%) reached statistical significance 30 min after the injection of SR141716A and declined to baseline levels by the fourth hour after the injection (Figure 1b). One-way ANOVA performed on the overall effects of SR141716A (at 1, 3 and 10 mg kg−1) also showed a significant effect of the drug (F(3, 18)=6.06, P<0.01), at the 3 and 10 mg kg−1 doses on DA efflux (Figure 1e).
For the time course of NE efflux, significant effects of treatment (F(2,12)=5.97, P<0.05) and time (F(9, 126)=9, P<0.001) were observed. SR141716A dose-dependently increased NE efflux in the prefrontal cortex; at 10 mg kg−1 the increase of NE (up to a peak value of 180%) reached statistical significance 1 h after the injection of SR141716A and persisted throughout the observation period (Figure 1c). One-way ANOVA on the overall effect of SR141716A (at 1, 3 and 10 mg kg−1) also showed a significant effect of the drug (F(3, 19)=6.05, P<0.001), at the 3 and 10 mg kg−1 doses on NE efflux (Figure 1f).
For the time courses of all metabolites examined (DOPAC, 5-HIAA, HVA) two-way ANOVA demonstrated significant interactions between treatment (SR141716A, 3 or 10 mg kg−1) and time (F(18, 135)=3.7, P<0.001 for DOPAC; F(18, 126)=4.9, P<0.001 for 5-HIAA and F(18, 126)=4.37, P<0.001 for HVA). SR141716A dose-dependently increased all metabolites at 3 mg kg−1 (F(9, 99)=4.36, P<0.001 for DOPAC; F(9, 99)=4.87, P<0.001 for 5-HIAA and F(9, 99)=5.52, P<0.001 for HVA) and 10 mg kg−1 (F(9, 99)=5.81, P<0.001 for DOPAC; F(9, 72)=6.61, P<0.001 for 5-HIAA and F(9, 72)=7.81, P<0.001 for HVA). At the 10 mg kg−1 dose the increases (up to peak values of 152%, 140% and 150% for DOPAC, 5-HIAA and HVA respectively) of metabolite levels reached statistical significance 1–2 h after the injection of SR141716A and persisted for at least 3 h (Figure 2 a,b,c). The same pattern of responses was observed when the overall effects of SR141716A (at 1, 3 and 10 mg kg−1) were examined. One-way ANOVA showed a significant effect of SR141716A that increases average metabolite values, at the 3 and 10 mg kg−1 doses (F(3, 18)=5.58, P<0.01 for DOPAC; F(3, 16)=4.08, P<0.05 for 5-HIAA and F(3, 18)=6.13, P<0.01 for HVA) (Figure 2d,e,f).
Figure 2.
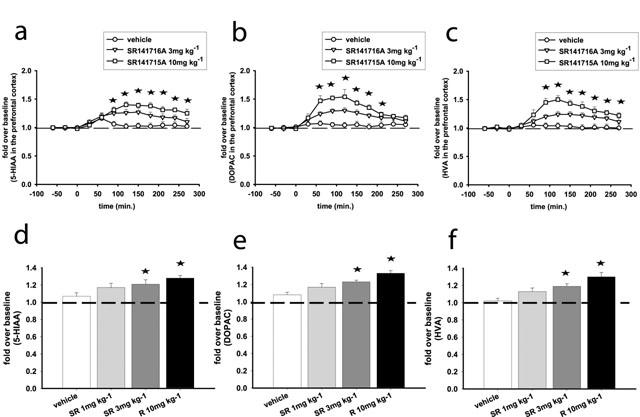
SR141716A increases 5-HIAA (a, d), DOPAC (b, e) and HVA (c, f) efflux in the prefrontal cortex of the rat. (a, b, and c) Represent time course effects after i.p. administration of SR141716A at 3 and 10 mg kg−1. (d, e and f) Represent the average effects of SR141716A (1, 3, 10 mg kg−1; i.p.) over the course of the experiment (overall effects). Data represent mean±s.e.mean of n=5–8 rats per experimental group and are expressed as multifold change from baseline, which is the average of the five basal values immediately before SR141716A administration. Asterisks indicate P<0.05 as compared to the vehicle-receiving group. (a, b, c) Statistical significance for each time point is indicated with an asterisk only for the 10 mg kg−1 dose.
SR141716A does not affect DA, DOPAC and HVA in the n.Acc., while it slightly increases 5-HT and its metabolite 5-HIAA in this region
Basal values (in pmoles ml−1) in the n.Acc. were: 0.09±0.01 for serotonin, 3.4±0.3 for DA, 1281±92 for DOPAC, 227±12 for 5-HIAA, 449±29 for HVA. We could not measure NE in a reliable and reproducible manner in this region.
Among all parameters examined, two-way ANOVA showed significant effects of treatment (F(1, 5)=13.4, P<0.05) and time (F(9, 45)=2.77, P<0.05) for the 5-HT efflux time course at the 10 mg kg−1 dose only. Interestingly, for the same dose, two-way ANOVA also showed significant effects of treatment (F(1,8)=6.96, P<0.05) and time (F(9, 72)=2.8, P<0.01) for the time course of the 5-HT metabolite 5-HIAA. SR141716A at 10 mg kg−1 moderately increased 5-HT (up to a peak value of 139% and an average value of 127%) and its metabolite 5-HIAA (up to a peak value of 120% and an average value of 116%) in the n.Acc. (Figure 3). There was no significant effect of SR141716A at any of the doses tested on DA, DOPAC or HVA (not shown).
Figure 3.
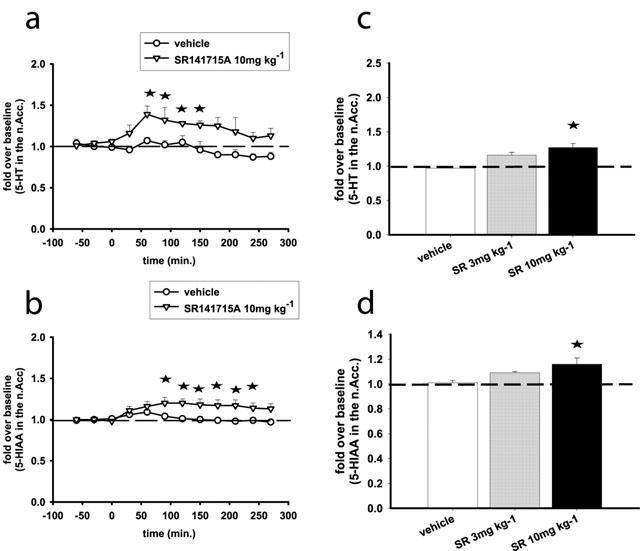
SR141716A increases 5-HT (a, c) and 5-HIAA (b, d) efflux in the n.Acc. of the rat. (a and b) Represent time course effects after i.p. administration of SR141716A at 10 mg kg−1. (c and d) Represent the average effects of SR141716A (3, 10 mg kg−1; i.p.) over the course of the experiment (overall effects). Data represent mean±s.e.mean of n=5–8 rats per experimental group and are expressed as multifold change from baseline, which is the average of the five basal values immediately before SR141716A administration. Asterisks indicate P<0.05 as compared to the vehicle-receiving group.
SR141716A increases ACh efflux in the prefrontal cortex
Basal acetylcholine levels in the prefrontal cortex were 1.2±0.1 pmoles 50 μl−1. For the time course of ACh efflux two-way ANOVA showed significant effects of treatment (F(3,7)=5.54, P<0.05) time (F(12, 84)=9.53, P<0.001) and interaction between these two factors (F(36,84)=1.7, P<0.05). SR141716A at 10 mg kg−1 (F(1, 6)=6.86, P<0.05), 3 mg kg−1 (F(1, 5)=10.48, P<0.05) but not 1 mg kg−1 increased ACh efflux in the prefrontal cortex. At the 10 mg kg−1 dose ACh was increased in drug-treated animals as compared to vehicle-treated animals 30 min after the injection of SR141716A and returned to baseline levels 2 h later (Figure 4a,c).
Figure 4.
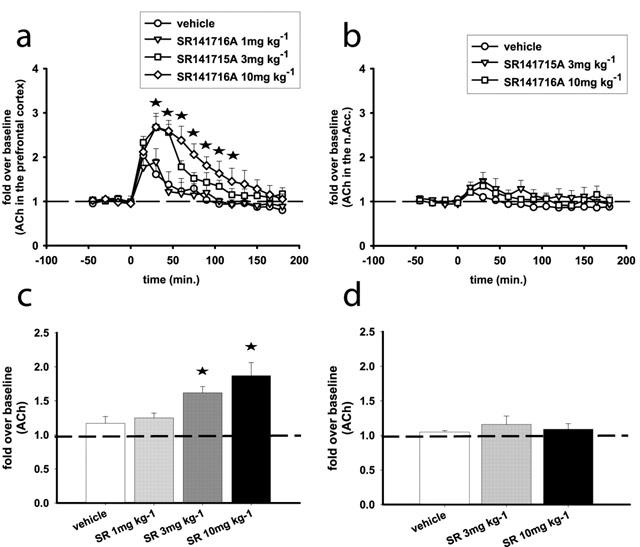
SR141716A increases ACh efflux in the prefrontal cortex (a, c) but does not affect ACh efflux in the n.Acc. (b, d) of the rat. (a and b) Represent time course effects after i.p. administration of SR141716A. (c and d) Represent the average effects of SR141716A over the course of the experiment (overall effects). Data represent mean±s.e.mean of n=5–8 rats per experimental group and are expressed as multifold change from baseline, which is the average of the five basal values immediately before SR141716A administration. (a) Statistical significance for each time point is indicated with an asterisk only for the 10 mg kg−1 dose. Asterisks indicate P<0.05 as compared to the vehicle-receiving group.
SR141716A does not affect ACh efflux in the n.Acc
Basal ACh levels in this region were 0.22±0.03 pmoles 50 μl−1. There was no significant effect of SR141716A at any of the doses tested (3 and 10 mg kg−1) on ACh efflux in this region (Figure 4b,d). In the relevant microdialysis literature it is generally accepted that the effects of compounds affecting directly cholinergic neurotransmission (e.g. muscarinic receptor antagonists and cholinesterase inhibitors) on ACh efflux may vary quantitatively depending on the concentration of acetylcholinesterase inhibitor in the perfusion solution. On the other hand, the effects of physiological or other pharmacological manipulations appear less dependent on changes in this parameter (see e.g. Moor et al., 1998). Importantly, ACh measurements in the medial prefrontal cortex and the n.Acc. were conducted under identical conditions revealing an exclusive effect of SR141716A on ACh efflux in the medial prefrontal cortex, i.e. no increase was found in the n.Acc.
SR141716A attenuates psychostimulant-induced hyperlocomotion
For PCP induced hyperlocomotion, two-way ANOVA (treatment with SR14176A×treatment with PCP) showed a significant effect of PCP (F(1, 72)=15.65, P<0.001) as well as a significant level of interaction between the two factors (F(2, 72)=3.35, P<0.05). Post-hoc analysis revealed that SR141716A (3 and the 10 mg kg−1) reversed PCP-induced hyperlocomotion, without having any effect on locomotion in the absence of PCP (Figure 5a). Two-way ANOVA (treatment with SR14176A×treatment with d-amphetamine) showed a significant effect of d-amphetamine (F(1, 60)=34.73, P<0.001) as well as a significant level of interaction between the two factors (F(2, 60)=11.36, P<0.001). Post-hoc analysis revealed that 3 and 10 mg kg−1 SR141716A reversed d-amphetamine-induced hyperlocomotion, without having any effect on locomotion in the absence of d-amphetamine (Figure 5b).
Figure 5.
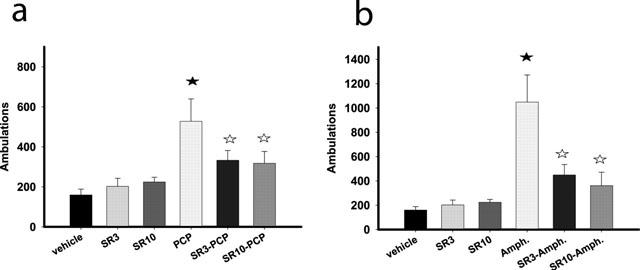
SR141716A attenuates d-amphetamine (a) and PCP (b) induced hyperlocomotion without affecting basal locomotor activity in the mouse. Data (ambulations over a 60 min period after concurrent i.p. administration of each of the drug combinations) represent mean±s.e.mean of n=8–12 mice per experimental group. Closed asterisks indicate P<0.05 as compared to the vehicle-receiving group and open asterisks P<0.05 as compared to the d-amphetamine- (a) or PCP- (b) receiving group.
SR141716A reduces immobility in the forced swimming test
One way ANOVA revealed a significant effect of treatment (F(3, 27)=3.8, P<0.05). SR141716A dose dependently decreased immobility during the test session, this effect reaching statistical significance at the 3 mg kg−1 dose (Figure 6).
Figure 6.
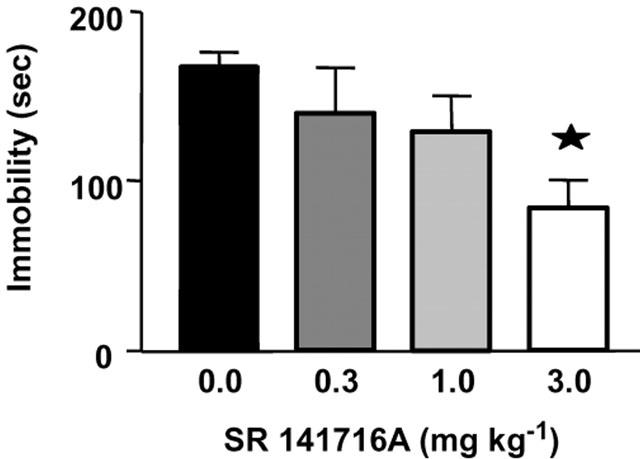
SR141716A reduces immobility in the forced swimming test in the mouse. Data (immobility for the last 4 min of a 6 min trial) represent mean±s.e.mean of n=7–8 mice per experimental group. Asterisks indicate P<0.05 as compared to the vehicle-receiving group.
Discussion
In this study we show an in vivo stimulatory effect of SR141716A on monoaminergic neurotransmitter systems in the prefrontal cortex of the rat. Acute administration of SR14116A selectively increased DA efflux in the prefrontal cortex versus the n.Acc. The same selectivity was observed for ACh efflux. These results suggest a role for CB1 receptors in regulating monoamine efflux in the cortex. It is widely established that endocannabinoids act as retrograde signalling molecules that regulate presynaptic activity and neurotransmitter release (reviewed in Schlicker & Kathmann, 2001). Thus, the neurochemical effects of SR141716A that we observed can be attributed to the CB1 receptor antagonist activity of the compound resulting in the disruption of an endogenous cannabinoid tone and would reflect regional/circuit differences in endocannabinoid mediated modulation of monoaminergic neurotransmission.
The prefrontal cortex receives extensive serotonergic, dopaminergic, cholinergic and noradrenergic afferents that interact widely at the terminal level. Although a direct action of SR141716A on presynaptic CB1 receptors localized on the terminals of monoaminergic neurons that project to the prefrontal cortex cannot be excluded at this point, the effects of this compound could also be indirect, involving interposed neurons that supply monosynaptic or trans-synaptic input to monoaminergic neurons. It should be noted that cannabinoid agonists administered at low doses also increase DA (Chen et al., 1990) and ACh (Acquas et al., 2000) in the prefrontal cortex. Interestingly, the effects of low doses of exogenously applied natural (Δ9-tetrahydrocannabinol (THC)) or synthetic (WIN-55,212-2) cannabinoid agonists in a number of biochemical or behavioural readouts are opposite of those elicited by higher doses of these compounds (Chaperon & Thiebot, 1999). In this respect, Gessa et al. (1998) have shown that higher doses of cannabimimetics decrease cortical ACh release.
The fact that SR141716A administration increases monoamine efflux selectively in the prefrontal cortex suggests that the stimulation of monoaminergic neurotransmission is not due to non-specific stress mediated responses, which would also augment monoamines in the n.Acc. (Imperato et al., 1991). The enhancement of cortical monoaminergic neurotransmission by SR141716A could be relevant to the anti-addictive and cognitive enhancing properties of this compound that are described in the literature. Thus, the cortical selectivity in the effects of SR141716A on DA efflux, could explain, partially at least, some of its anti-addictive properties. Cortical DA circuits control mesolimbic DA activation, and increases in DA in the prefrontal cortex such as these produced by SR141716A are expected to reduce DA activity in the n.Acc. (Deutch et al., 1991). Increased mesolimbic DA neurotransmission has been associated with the perception of appetitive properties of positive reinforcers and with the reinstatement of drug taking behaviour (di chiara, 1999), processes that are blocked by SR141716A (see Introduction). On the other hand, it is believed that the levels of cholinergic activity in the cortex and the hippocampus could be linked to an increase in arousal and could enhance attention, trace formation and ultimately, by increasing signal-to-noise ratio, quality of acquisition (see e.g. Mcgaughy et al., 2002). Therefore, the increase in ACh efflux that we observed with SR141716A could be related to the effects of this compound in arousal, learning and memory (see Introduction). Indeed, Lichtman (2000) has shown that SR141716A, administered at the doses that increase ACh in the cortex and hippocampus (Gessa et al., 1998; our observations), increases performance in a T-maze by facilitating attention and/or consolidation.
More importantly, the stimulation of cortical monoaminergic neurotransmission by SR141716A suggests a possible therapeutic potential of this compound in affective and cognitive disorders, since cholinergic and catecholaminergic cortical circuits have an important regulatory role in integrating affect, attention, memory and reward processes into coordinated patterns of behavioural responses. Hypoactivity of the prefrontal cortex has been shown in schizophrenic patients (e.g. Meyer-Lindenberg et al., 2002) as well as in depressed subjects (Baxter et al., 1989). It has been proposed that dysregulated monoaminergic neurotransmission could be involved in the pathogenesis of schizophrenia, depression, and attention deficit/hyperactivity disorder (ADHD) (Deutch et al., 1991; Tanda et al., 1994; Biederman & Spencer, 1999). Agents that enhance monoaminergic activity in the prefrontal cortex are effective medications for these disorders (see below).
Alonso et al. (1999) by assessing c-fos expression in the rat have shown that SR141716A increases neuronal activity in the mesocorticolimbic system in a pattern similar to that produced by known atypical antipsychotics (Robertson et al., 1994) and have suggested a possible antipsychotic potential for this compound. In this respect, Poncelet et al. (1999) have shown that SR141716 antagonizes d-amphetamine-induced reinstatement of exploratory behaviour in gerbils, a paradigm used commonly to reveal antipsychotic-like pharmacological activity. On the contrary, Masserano et al. (1999) have reported that SR141716A potentiates the stimulant locomotor effects of d-amphetamine. In our study, SR141716A attenuated the hyperlocomotion produced by both PCP and d-amphetamine without affecting basal locomotor activity, in line with the findings of Poncelet et al. (1999). Interestingly, Masserano et al. (1999) have used animals that were not familiarized to the experimental environment, while Poncelet et al. as well as we, have assessed the effects of SR141716A in animals that were habituated to the locomotor activity test cages.
A key-component in the mechanism of action of prototypical and putative, clinically effective, atypical antipsychotics is their ability to preferentially increase levels of DA and NE in the prefrontal cortex, an effect related to their beneficial therapeutic profile in alleviating negative symptoms of schizophrenia (Westerink et al., 2001; Zhang et al., 2000; Nomikos et al., 1994). The magnitude and time-course of the increase in DA and NE neurotransmission induced by SR141716A in the prefrontal cortex (10 mg kg−1 SR141716A elevated DA and NE values up to a peak value of 263 and 180% of the baseline and an average value of 188 and 163% of the baseline respectively) are identical to those elicited in the same brain region by the atypical antipsychotics olanzapine, clozapine or risperidone (Zhang et al., 2000). Furthermore, similarly to SR141716A, olanzapine and clozapine increase ACh efflux in the cortex but not in the n.Acc. (Ichikawa et al., 2002), although the stimulatory action of olanzapine was clearly more pronounced than that of either clozapine or SR141716A (Shirazi-Southall et al., 2002; present study). The fact that SR141716A shares key neurochemical actions of atypical antipsychotics suggests that cannabinoid antagonists should be further studied in pre-clinical animal models predictive for efficacy in the treatment of negative and cognitive aspects of psychosis.
Putative antidepressants, such as 5-HT1A agonists (Arborelius et al., 1993), and established antidepressants such as the 5-HT uptake inhibitor fluoxetine (Bymaster et al., 2002b), and the NE uptake inhibitors desipramine and reboxetine (Tanda et al., 1994; Linner et al., 2001) increase DA in the prefrontal cortex but not in the n.Acc., an effect that has been proposed to play a role in their antidepressant effects (Tanda et al., 1994). The magnitude and time-course of DA elevation in the prefrontal cortex elicited by SR141716A are similar to those induced by the aforementioned antidepressants. In addition, a known effect of antidepressants is to increase NE, and in the case of fluoxetine also 5-HT, in the prefrontal cortex and other brain regions. We have previously shown that like desipramine and fluoxetine (Perry & Fuller, 1997) SR141716A markedly increased NE efflux in the hypothalamus (Tzavara et al., 2001). We have therefore assessed the actions of SR141716A in the forced swimming test, a behavioural despair model that is predictive of antidepressant efficacy (Porsolt et al., 1977). Known antidepressants that belong to different structural and pharmacological classes increase escape attempts in this test. SR141716A dose-dependently increased escape attempts (reduced immobility) at doses that did not affect basal locomotor activity. In the microdialysis experiments, in addition to DA and NE efflux, SR141716A elevated 5-HT efflux (up to a peak value of 198% of the baseline and an average value of 152% of the baseline) in the prefrontal cortex. SR141716A also increased, although only slightly, 5-HT efflux in the n.Acc. Compared to the changes induced by 5-HT uptake inhibitor antidepressants such as fluoxetine the changes in 5-HT neurotransmission elicited by SR141716A are relatively small, especially in the n.Acc. In this respect the physiological relevance of cannabinoid-5-HT interactions as well as the pharmacological significance of the reduction in immobility in the forced swimming test induced by SR141716A warrant further investigation. In particular alternative animal models of potential antidepressant efficacy should be employed to counteract the limitations of the forced swimming test, especially the possibility of ‘false positive' results.
Interestingly, NE uptake inhibitors like desipramine or the newly developed atomoxetine, have been proposed as alternatives to psychostimulants for the pharmacotherapy of ADHD, a pathology characterized by attentional deficits with or no hyperactivity, co morbid with depression and drug abuse. A common feature between psychostimulants and NE uptake inhibitors is the increase in DA and NE efflux in the prefrontal cortex, a mechanism thought to play a pivotal role in the therapy of ADHD by modulating attention (Linner et al., 2001; Bymaster et al., 2002a). As discussed before the exclusive increases of DA in the prefrontal cortex that we observed with SR141716A reach similar levels, occur at the same dose range and follow a similar time-course as with reboxetine and atomoxetine (Bymaster et al., 2002a). Despite the fact that NE increases elicited by SR141716A in this region are only moderate when compared to those induced by NE inhibitors it is tempting to propose to further investigate the possible role of CB1 antagonists in the treatment of ADHD. This suggestion is strengthened by the fact that, as discussed above, SR141716A has been shown to enhance attention and to modulate reward mechanisms also thought to play a role in the pathogenesis of ADHD. It should be noted, however, that SR141716A and atomoxetine affect monoamine efflux possibly via different mechanisms, given that SR141716A, but not atomoxetine, also increases concentrations of the monoaminergic metabolites. In this respect, the precise neuroanatomical substrates targeted by SR141716A and the molecular mechanism of CB1 receptor antagonist-induced increases of monoamines in the prefrontal cortex need further investigation.
In conclusion, the in vivo stimulatory effects of SR141716A on major neurotransmitter systems in the prefrontal cortex suggests a role for CB1 receptors in modulating affective and cognitive functions that are strongly associated with monoaminergic neurotransmission in this region. The ability to exclusively enhance dopaminergic neurotransmission in the prefrontal cortex, and the procholinergic (a purported mechanism of action of drugs that improve cognition) properties of cannabinoid antagonists together with their stimulatory effects on serotonin and NE efflux uniquely portray this class of compounds and warrants their evaluation as candidates in the treatment of psychosis, affective and cognitive disorders, particularly when overlapping, treatment-resistant symptomatology is targeted.
Abbreviations
- 5-HT
serotonin
- ACh
acetylcholine
- ADHD
attention deficit-hyperactivity disorder
- CB1
type 1 cannabinoid receptor
- CNS
central nervous system
- DA
dopamine
- n.Acc.
nucleus accumbens
- NE
norepinephrine
- PCP
phenylcyclidine
- THC
Δ9-tetrahydrocannabinol
References
- ACQUAS E., PISANU A., MARROCU P., DI CHIARA G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur. J. Pharmacol. 2000;401:179–185. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- ALONSO R., VOUTSINOS B., FOURNIER M., LABIE C., STEINBERG R., SOUILHAC J., LE FUR G., SOUBRIE P. Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience. 1999;91:607–620. doi: 10.1016/s0306-4522(98)00675-7. [DOI] [PubMed] [Google Scholar]
- ARBORELIUS L., NOMIKOS G.G., HACKSELL U., SVENSSON T.H. (R)-8-OH-DPAT preferentially increases dopamine release in rat medial prefrontal cortex. Acta Physiol. Scand. 1993;148:465–466. doi: 10.1111/j.1748-1716.1993.tb09584.x. [DOI] [PubMed] [Google Scholar]
- AUCLAIR N., OTANI S., SOUBRIE P., CREPEL F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J. Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- BAXTER L.R., JR, SCHWARTZ J.M., PHELPS M.E., MAZZIOTTA J.C., GUZE B.H., SELIN C.E., GERNER R.H., SUMIDA R.M. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- BIEDERMAN J., SPENCER T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol. Psychiatry. 1999;46:1234–1242. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- BYMASTER F.P., KATNER J.S., NELSON D.L., HEMRICK-LUECKE S.K., THRELKELD P.G., HEILIGENSTEIN J.H., MORIN S.M., GEHLERT D.R., PERRY K.W. Atomoxetine increases extracellular levels of norepinephrine and dopamine in the prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002a;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- BYMASTER F.P., ZHANG W., CARTER P.A., SHAW J., CHERNET E., PHEBUS L., WONG D.T., PERRY K.W. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology. 2002b;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- CADOGAN A.K., ALEXANDER S.P., BOYD E.A., KENDALL D.A. Influence of cannabinoids on electrically evoked dopamine release and cyclic AMP generation in the rat striatum. J. Neurochem. 1997;69:1131–1137. doi: 10.1046/j.1471-4159.1997.69031131.x. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., WATERS N., WATERS S., CARLSSON M.L. Network interactions in schizophrenia–therapeutic implications. Brain Res. Brain Res. Rev. 2000;31:342–349. doi: 10.1016/s0165-0173(99)00050-8. [DOI] [PubMed] [Google Scholar]
- CHAPERON F., THIEBOT M.H. Behavioral effects of cannabinoid agents in animals. Crit. Rev. Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- CHEN J.P., PAREDES W., LI J., SMITH D., LOWINSON J., GARDNER E.L. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- COLOMBO G., AGABIO R., FA M., GUANO L., LOBINA C., LOCHE A., REALI R., GESSA G.L. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- DE VRIES T.J., SHAHAM Y., HOMBERG J.R., CROMBAG H., SCHUURMAN K., DIEBEN J., VANDERSCHUREN L.J., SCHOFFELMEER A.N. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- DEUTCH A.Y., MOGHADDAM B., INNIS R.B., KRYSTAL J.H., AGHAJANIAN G.K., BUNNEY B.S., CHARNEY D.S. Mechanisms of action of atypical antipsychotic drugs. Implications for novel therapeutic strategies for schizophrenia. Schizophr. Res. 1991;4:121–156. doi: 10.1016/0920-9964(91)90030-u. [DOI] [PubMed] [Google Scholar]
- DI CHIARA G. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., GOPARAJU S.K., WANG L., LIU J., BATKAI S., JARAI Z., FEZZA F., MIURA G.I., PALMITER R.D., SUGIURA T., KUNOS G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., JACOBSSON S.O. Cellular transport of anandamide, 2- arachidonoylglycerol and palmitoylethanolamide-targets for drug development. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:193–200. doi: 10.1054/plef.2001.0357. [DOI] [PubMed] [Google Scholar]
- GERDEMAN G., LOVINGER D.M. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J. Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- GESSA G.L., CASU M.A., CARTA G., MASCIA M.S. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur. J. Pharmacol. 1998;355:119–124. doi: 10.1016/s0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- GIFFORD A.N., ASHBY C.R., JR Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J. Pharmacol. Exp. Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- ICHIKAWA J., DAI J., O'LAUGHLIN I.A., FOWLER W.L., MELTZER H.Y. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacology. 2002;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
- IMPERATO A., PUGLISI-ALLEGRA S., CASOLINI P., ANGELUCCI L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991;538:111–117. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- KATHMANN M., BAUER U., SCHLICKER E., GOTHERT M. Cannabinoid CB1 receptor-mediated inhibition of NMDA- and kainate-stimulated noradrenaline and dopamine release in the brain. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:466–470. doi: 10.1007/pl00005377. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur. J. Pharmacol. 2000;404:175–179. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., VARVEL S.A., MARTIN B.R. Endocannabinoids in cognition and dependence. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:269–285. doi: 10.1054/plef.2001.0351. [DOI] [PubMed] [Google Scholar]
- LINNER L., ENDERSZ H., OHMAN D., BENGTSSON F., SCHALLING M., SVENSSON T.H. Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J. Pharmacol. Exp. Ther. 2001;297:540–546. [PubMed] [Google Scholar]
- LUCKI I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- MAS-NIETO M., POMMIER B., TZAVARA E.T., CANEPARO A., DA NASCIMENTO S., LE FUR G., ROQUES B.P., NOBLE F. Reduction of opioid dependence by the CB(1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br. J. Pharmacol. 2001;132:1809–1816. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSERANO J.M., KAROUM F., WYATT R.J. SR 141716A, a CB1 cannabinoid receptor antagonist, potentiates the locomotor stimulant effects of d-amphetamine and apomorphine. Behav. Pharmacol. 1999;10:429–432. doi: 10.1097/00008877-199907000-00010. [DOI] [PubMed] [Google Scholar]
- MCGAUGHY J., DALLEY J.W., MORRISON C.H., EVERITT B.J., ROBBINS T.W. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J. Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER-LINDENBERG A., MILETICH R.S., KOHN P.D., ESPOSITO G., CARSON R.E., QUARANTELLI M., WEINBERGER D.R., BERMAN K.F. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- MOOR E., SCHIRM E., JACSO J., WESTERINK B.H. Effects of neostigmine and atropine on basal and handling-induced acetylcholine output from ventral hippocampus. Neuroscience. 1998;82:819–825. doi: 10.1016/s0306-4522(97)00331-x. [DOI] [PubMed] [Google Scholar]
- NOMIKOS G.G., IURLO M., ANDERSSON J.L., KIMURA K., SVENSSON T.H. Systemic administration of amperozide, a new atypical antipsychotic drug, preferentially increases dopamine release in the rat medial prefrontal cortex. Psychopharmacology. 1994;115:147–156. doi: 10.1007/BF02244765. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- PERRY K.W., FULLER R.W. Fluoxetine increases norepinephrine release in rat hypothalamus as measured by tissue levels of MHPG-SO4 and microdialysis in conscious rats. J. Neural. Transm. 1997;104:953–966. doi: 10.1007/BF01285563. [DOI] [PubMed] [Google Scholar]
- PIOMELLI D., GIUFFRIDA A., CALIGNANO A., RODRIGUEZ DE FONSECA F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- PONCELET M., BARNOUIN M.C., BRELIERE J.C., LE FUR G., SOUBRIE P. Blockade of cannabinoid (CB1) receptors by 141716 selectively antagonizes drug-induced reinstatement of exploratory behaviour in gerbils. Psychopharmacology. 1999;144:144–150. doi: 10.1007/s002130050987. [DOI] [PubMed] [Google Scholar]
- PORSOLT R.D., BERTIN A., JALFRE M. Behavioral despair in mice: a primary screening test for antidepressants. Archives Internationales de Pharmacodynamie et de Therapie. 1977;229:327–336. [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIERE J.C., LE FUR, G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- ROBERTSON G.S., MATSUMURA H., FIBIGER H.C. Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J. Pharmacol. Exp. Ther. 1994;271:1058–1066. [PubMed] [Google Scholar]
- SANTUCCI V., STORME J.J., SOUBRIE P., LE FUR G. Arousal-enhancing properties of the CB1 cannabinoid receptor antagonist SR 141716A in rats as assessed by electroencephalographic spectral and sleep-waking cycle analysis. Life Sci. 1996;58:PL103–110. doi: 10.1016/0024-3205(95)02319-4. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., KATHMANN M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- SHIRAZI-SOUTHALL S., RODRIGUEZ D.E., NOMIKOS G.G. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- SZABO B., MULLER T., KOCH H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J. Neurochem. 1999;73:1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- TANDA G., CARBONI E., FRAU R., DI CHIARA G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential. Psychopharmacology. 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- TERRANOVA J.P., STORME J.J., LAFON N., PERIO A., RINALDICARMONA M., LE FUR G., SOUBRIE P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology. 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- TZAVARA E.T., PERRY K.W., RODRIGUEZ D.E., BYMASTER F.P., NOMIKOS G.G. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur. J. Pharmacol. 2001;426:R3–4. doi: 10.1016/s0014-2999(01)01228-6. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H., KAWAHARA Y., DE BOER P., GEELS C., DE VRIES J.B., WIKSTROM H.V., VAN KALKEREN A., VAN VLIET B., KRUSE C.G., LONG S.K. Antipsychotic drugs classified by their effects on the release of dopamine and noradrenaline in the prefrontal cortex and striatum. Eur. J. Pharmacol. 2001;412:127–138. doi: 10.1016/s0014-2999(00)00935-3. [DOI] [PubMed] [Google Scholar]
- ZHANG W., PERRY K.W., WONG D.T., POTTS B.D., BAO J., TOLLEFSON G.D., BYMASTER F.P. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology. 2000;23:250–262. doi: 10.1016/S0893-133X(00)00119-6. [DOI] [PubMed] [Google Scholar]


