Abstract
Gamma radiation impairs vascular function, leading to the depression of endothelium-dependent vasodilatation. Loss of the nitric oxide (NO) pathway has been implicated, but little is known about radiation effects on other endothelial mediators.
This study investigated the mechanisms of endothelial dysfunction in rabbits subjected to whole-body irradiation from a cobalt60 source.
The endothelium-dependent relaxation of rabbit aorta evoked by acetylcholine (ACh) or A23187 was impaired in a dose-dependent manner by irradiation at 2 Gy or above. Inhibition was evident 9 days post-irradiation and persisted over the 30 day experimental period.
Endothelium-independent responses to glyceryl trinitrate (GTN), sodium nitroprusside (SNP) and 3-morpholino-sydnonimine (SIN-1) were suppressed over a similar dose range at 7–9 days post-irradiation, but recovered fully by 30 days post-irradiation.
In healthy vessels, ACh-induced relaxation was inhibited by L-Nω-nitroarginine (L-NA; 3×10−4 M) and charybdotoxin (10−8 M) plus apamin (10−6 M) but resistant to indomethacin, indicating the involvement of NO and endothelium-derived hyperpolarizing factor (EDHF). Supporting this, ACh caused smooth muscle hyperpolarization that was reduced by L-NA and charybdotoxin plus apamin.
In irradiated vessels, responses to ACh were insensitive to L-NA but abolished by charybdotoxin plus apamin, indicating selective loss of NO-mediated relaxation.
In animals treated shortly after irradiation with the antioxidant, α-tocopherol acetate, the NO-dependent relaxation was restored without effect on the EDHF-dependent component.
The results imply that radiation selectively impairs the NO pathway as a consequence of oxidative stress, while EDHF is able to maintain endothelium-dependent relaxation at a reduced level.
Keywords: Rabbit aorta, endothelium, radiation, NO, EDHF, oxidative stress
Introduction
The most common health problems in the surviving Ukrainian population that was exposed to radiation, as a result of the 1986 Chernobyl nuclear accident, are diseases of the cardiovascular system (Khomazjuk, 1998; Leliuk & Guskova, 1998; Klimov & Adziarkha, 2001). Cardiovascular disease is also prevalent among survivors of the Hiroshima and Nagasaki atomic bombs (Wong et al., 1993). The increased incidence of cardiovascular disease may be due in part to the deleterious effects of ionizing radiation on blood vessels that have been widely reported. One year before the Chernobyl accident it was acknowledged that even mild irradiation (0.2–0.5 Gy) can change the contractile tone of blood vessels and their sensitivity to constrictor and dilator mediators (Vorobyov & Stepanov, 1985). A growing mass of data now strongly indicates that damage to the blood vessel endothelium occurs following exposure to ionizing radiation, leading to a loss of endothelium-dependent vasodilation (Maynard et al., 1992; Taranenko et al., 1992; Menendez et al., 1998; Qi et al., 1998; Sugihara et al., 1999; Beckman et al., 2001). Clarification of the mechanisms underlying the chronic effects of ionizing radiation on blood vessels may therefore help to identify therapeutic interventions that can improve the treatment of cardiovascular disease associated with radiation exposure.
The impairment of locally irradiated human and rabbit arteries is associated with loss of nitric oxide-dependent relaxation and endothelial nitric oxide synthase (eNOS) activity (Qi et al., 1998; Sugihara et al., 1999), suggesting that the main vascular effect of irradiation may be to inhibit the nitric oxide (NO) component of endothelium-dependent vasodilatation. Reduced production in irradiated vascular rings of the endothelium-derived vasodilator, prostacyclin (Allen et al., 1981), suggests however, that other endothelium-derived mediators could also contribute. In addition, there is evidence that vascular smooth muscle can be affected directly by radiation, resulting in altered sensitivity to endothelium-independent vasoactive substances, including NO (Warfield et al., 1989; Taranenko et al., 1992; Major et al., 1996; Qi et al., 1998; Tishkin, 1998).
The goal of this study was to determine the mechanisms underlying the depression of endothelium-dependent vasodilatation that develops in rabbit aorta several days after whole-body irradiation with a non-lethal dose from a cobalt60 source. We were particularly interested to determine whether or not irradiation selectively inhibits the NO-mediated component of endothelium-dependent vasodilatation.
Methods
The study was performed on male New Zealand White rabbits weighing 2.5–3.0 kg, according to the recommendations of the declaration of Helsinki and internationally accepted principles for the care and use of experimental animals. Groups of six animals were allocated to one of the following treatments: whole body irradiation at a dose of 6 Gy, with sacrifice on the 9th day post irradiation; whole body irradiation at a dose of 6 Gy with sacrifice on the 30th day post irradiation; irradiation at 6 Gy followed 1 h later with a single, oral dose of α-tocopherol acetate (50 mg kg−1) and sacrifice at 9 days post-irradiation; irradiation at 6 Gy followed 1 h later with a single, oral dose of vehicle (olive oil) and sacrifice at 9 days post-irradiation; no irradiation (control). The level of radiation used is low compared with the dose of 10–12 Gy that was previously found to be lethal in 50% of animals at 30 days post-irradiation (LD50/30; see for example Gratwohl et al., 1998), and all animals survived throughout the experimental period (up to 30 days). The radiation dose used is also below the threshold dose (8.3–8.8 Gy) for producing noticeable morphological changes in endothelial cells (Vorobyov & Stepanov, 1985), while being sufficient to produce distinct changes in endothelium-dependent relaxation of rabbit aorta. Study times post-irradiation were chosen on the basis of previous reports on the time course of functional (Qi et al., 1998) and morphological (Vorobyov & Stepanov, 1985) changes in the endothelium. Although tested at higher radiation doses, these studies found that the maximal loss of endothelium-dependent relaxation and the largest changes in endothelial cell morphology occurred at 1 week after irradiation and, although endothelial function remained depressed for several weeks, the morphological effects disappeared by 30 days post-irradiation.
To estimate the dose-dependence of the effect of radiation, a further 30 rabbits were exposed to a dose of 1, 2 or 4 Gy and sacrificed on the 7th or 9th day of the post-irradiation period, with a similar number of untreated animals acting as the controls. Whole-body irradiation was performed with gamma rays delivered at a rate of 0.307 Gy min−1 from a cobalt60 source (TGT ROCUS M, Russia) positioned 50 cm distant from the animal. During irradiation, rabbits were restrained in a plastic box specifically designed for this study, and the radiation beam was focussed on the animal's chest. There was no change in housing, standard food or drinking water following irradiation. The animals were closely observed for unwanted effects and there was no visible sign of discomfort or illness.
Experimental preparation
Experiments were performed on ring preparations (2–3 mm wide) of rabbit thoracic aorta, prepared with care in order to keep the endothelium intact. Aortic rings were mounted isometrically under a resting tension of 3 g in a flowing tissue bath, between a stationary stainless steel hook and an isometric force transducer (AE 801, SensoNor A/S, Norten, Norway) coupled to a chart recorder (model 202, Cole-Parmer Instrument Company, U.S.A.). Vessels were maintained at 37°C in an organ bath containing 0.5 ml of modified Krebs-bicarbonate buffer solution of the following composition (in mM): NaCl 133; KCl 4.7; NaHCO3 16.3; NaH2PO4 1.38; CaCl2 2.5; MgCl2 1.2; glucose, 7.8; pH, 7.4. The solution was continuously gassed with 16% O2, 5% CO2 and 79% N2. Rings were allowed to equilibrate for 1 h under resting tension before experiments commenced. Following the equilibration period, they were exposed several times to high K+ (60 mM) solution until reproducible contractile responses were obtained. High K+ solution was prepared by replacement of NaCl with equimolar KCl in order to avoid a change in ionic strength of the solution.
The relaxant responses to ACh, A23187, GTN, SNP and SIN-1 were examined during contractions induced by 10 μM phenylephrine. The magnitude of the contraction produced by phenylephrine at this concentration was near 90% of the maximal response to phenylephrine in both control and irradiated vessels. Both at 9 and 30 days after being irradiated, vessels showed no significant difference in the maximal contraction or sensitivity to phenylephrine when compared with the control vessels (Taranenko et al., 1992; Qi et al., 1998). Thus, relaxation responses were studied at equal levels of pre-contraction in healthy and irradiated tissues. Except where indicated, experiments were carried out in the presence of indomethacin (5×10−6 M) to exclude any involvement of prostanoids in the endothelium-dependent relaxation response to ACh. Tissues were incubated with indomethacin, L-NA or charybdotoxin and apamin for at least 30 min before testing their effects on ACh-induced relaxation.
Measurement of membrane potential
Aortic rings were inverted and suspended with the endothelial surface upward between two horizontal stainless steel hooks in an organ bath and were superfused continuously with modified Krebs solution at 37°C. Glass microelectrodes filled with 3M KCl (tip resistance 60–80 MΩ) were inserted into smooth muscle cells from the intimal side and transmembrane potentials recorded with a microelectrode amplifier MZ-4 (Nihon Kohden, Japan) connected to a chart recorder (model 202, Cole-Parmer Instrument Company, U.S.A.) and computer, via a Digidata 1200 interface (Axon Instruments, U.S.A.). The following criteria were used to assess the validity of a successful impalement into a smooth muscle cell: (i) a sudden negative shift in voltage at a depth of more than 15 μM below the intimal surface of the vascular wall, (ii) a stable negative voltage for more than 2 min, and (iii) an instantaneous return to the previous voltage level on removal of the microelectrode. The first stable negative voltage shift recorded just after contact with the tissue was attributed to penetration into endothelial cells. Records were collected and analysed using AxoScope 7 software (Axon Instruments, U.S.A.). Stable membrane potentials were measured for 2–3 min before applying ACh (10−5 M). The ACh was then washed out and inhibitors of endothelium-dependent vasodilation added to the superfusing solution for 30–35 min before impaling cells and testing ACh again.
Chemicals
Phenylephrine, acetylcholine chloride (ACh), sodium nitroprusside (SNP), 3-morpholino-sydnonimine (SIN-1), L-Nω-nitroarginine (L-NA), α-Tocopherol acetate and indomethacin were obtained from Sigma (St. Louis, U.S.A.). Glyceryl trinitrate (GTN) was from Bio-Therabel (Brussels, Belgium), A23187 was from Calbiochem-Behring (La Jolla, U.S.A.) and Na2ATP was from Serva (Heidelberg, Germany). All drugs were dissolved in distilled water except for indomethacin and A23187 which were dissolved in deionized water with an equimolar concentration of Na2CO3 and 95% ethanol, respectively. The final bath concentration of ethanol never exceeded 0.01% which had no effect on its own. α-Tocopherol acetate was dissolved in olive oil.
Statistical analysis
The data are shown as means±s.e.mean; n indicates the number of preparations tested. Concentration–response curves were fitted to the Hill equation and EC50 and maximal response values were estimated using the Origin 6.1 Program (OriginLab Corporation, U.S.A.). Pairs of data were compared using the Student's t-test. Multiple comparisons were made using one-way analysis of variance (ANOVA), with repeated measures when data were collected from the same preparations, and if any significant difference was found the Scheffe's multiple comparison test was applied. Differences were considered to be statistically significant when P was less than 0.05.
Results
The effect of radiation on sensitivity and responsiveness of the vascular wall to ACh and A23187
Acetylcholine evoked concentration-dependent relaxation of rabbit thoracic aortae precontracted with phenylephrine (10−5 M), provided the endothelium remained intact. Figure 1 compares the effects of ACh on intact aortae from healthy rabbits with aortae removed from rabbits 9 days after exposure to 6 Gy radiation. In healthy tissue, ACh produced a maximum relaxation (Rm) of 54±3% (n=6) at concentrations above 5 μM and 50% of the maximum response at around 230 nM (pD2=6.6±0.1). When the same experiment was repeated on aortae from irradiated animals, ACh induced a smaller maximum relaxation of 40±3% (n=6, P<0.05 vs control value). Interestingly, although the amplitudes of relaxations produced by high ACh concentrations (>1.3×10−7 M) were significantly decreased after irradiation, the relaxant responses to low concentrations (<1.3×10−7 M) were significantly (P<0.05) increased. Thus, the sensitivity of the smooth muscle to ACh was increased, resulting in a lower EC50 (pD2=7.1±0.2, P<0.05 vs control value).
Figure 1.
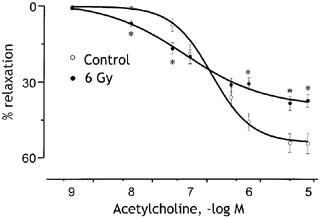
Concentration–relaxation curves to ACh obtained on the thoracic aortae from healthy (open circles) rabbits and irradiated (closed circles) rabbits. The radiation dose was 6 Gy and vessels were studied 9 days later. Experiments were carried out on 24 rings from six control animals and 24 rings from six irradiated animals. Relaxations are expressed as per cent decrease in the tension evoked by 10−5 M phenylephrine. *P<0.05 compared with control responses to the same ACh concentration.
The reduction in maximum response to ACh depended on the radiation dose as illustrated in Figure 2. Vessels from rabbits exposed to 2 Gy showed a significantly reduced response to 10 μM ACh (P<0.05) and at 4 Gy vessel relaxation was reduced to around 30%. Since 4 Gy (Figure 2) and 6 Gy (Figure 1) reduced the responses to 10 μM ACh by similar amounts, these doses may be sufficient to produce the maximal effect. To determine whether or not the loss of responsiveness to ACh reflected a change in the activity of muscarinic receptors or downstream events, we tested the effects of radiation on endothelium-dependent relaxation responses to the receptor-independent Ca2+ ionophore, A23187. As shown in Figure 2, in healthy rabbit aorta 0.1 μM A23187 produced relaxation with a similar amplitude to 1 μM ACh. Moreover, the responsiveness to A23187 was reduced following whole-body irradiation over the same dose levels that reduced responses to ACh (Figure 2). The loss of endothelium-dependent vasodilatation therefore reflects altered activity downstream of endothelial receptors.
Figure 2.
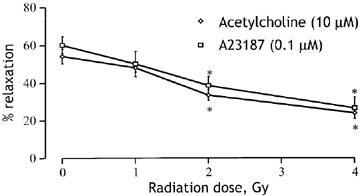
Radiation dose–response curves compared for endothelium-dependent relaxation induced by 10 μM ACh (diamonds) and 0.1 μM A23187 (squares). Responses to each agent were obtained in 22 vessel rings from six control animals and sets of 15 rings from five animals receiving 1, 2 or 4 Gy radiation from a cobalt60 source. Relaxations are expressed as per cent decrease in the tension evoked by 10−5 M phenylephrine. *P<0.05 compared with the appropriate response in control rings.
The effects of radiation on responsiveness and sensitivity of the vascular wall to NO donors
The loss of response to ACh and A23187 may partly reflect reduced responsiveness of the smooth muscle cell layer to endothelium-derived NO, a major mediator of the relaxations. The evidence for this is presented in Figure 3, which shows the effects of radiation on relaxation responses to the endothelium-independent vasodilators GTN, SNP. The main effect on the concentration-response curves to GTN and SNP was a radiation-dose dependent reduction of the maximal relaxation. The maximal relaxation to GTN was significantly (P<0.05) reduced from 61±6 % (n=5) in healthy vessels, to 22±2% (n=5) at 7 days after irradiation (Figure 3a). The maximal relaxation to SNP was also significantly (P<0.05) reduced from 71±7% (n=5) in healthy vessels to 33±3% (n=5) at 7 days after irradiation (Figure 3b). In both cases, the loss of maximal response was accompanied by a significant increase in sensitivity. Thus the pD2 for GTN increased from 5.6±0.1 to 6.2±0.2 (P<0.05), while the pD2 for SNP increased from 5.4±0.2 to 6.6±0.3 (P<0.05) at 7 days following irradiation. Responses to near maximal concentrations of GTN (10 μM) and SNP (10 μM) remained significantly depressed at 9 days following irradiation at 2 or 4 Gy (Figure 3c).
Figure 3.
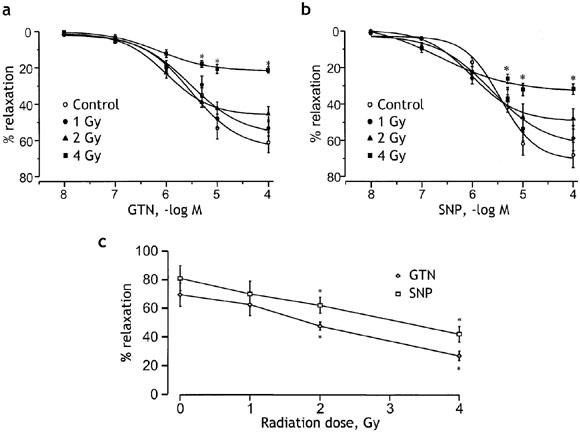
Effects of different doses of radiation on the concentration–response curves for GTN (a) and SNP (b). The relaxant responses obtained in control preparations are compared with those in preparations at the 7th day after irradiation with doses of 1, 2 or 4 Gy. The responses are expressed as per cent relaxation of phenylephrine-induced contraction. Points are means and vertical lines s.e.mean of 17 preparations from five animals in each case. (c) Radiation dose response curves showing a dose-dependent reduction in the maximum relaxation response to GTN (10 μM, diamonds) and SNP (10 μM, squares) measured at 9 days after irradiation. Experiments were performed on 18 aortic rings from six control animals and 15 rings from five animals in each of the other groups receiving 1, 2 or 4 Gy radiation. *P<0.05 vs the respective control values.
Difference in maximal effects of radiation on endothelium-dependent and endothelium-independent relaxation
As shown in Figure 4, the endothelium-dependent relaxation response to 10 μM ACh remained depressed at 30 days following irradiation at 6 Gy. There was no significant difference in amplitude between the responses measured at 9 or 30 days after irradiation, and at both time points the responses were significantly less than control. In contrast, although responses to the endothelium-independent vasodilators GTN (10 μM) and SIN-1 (10 μM) were significantly (P<0.05) depressed at 9 days following 6 Gy irradiation, by 30 days the responses had fully recovered to the control level. The impaired response to ACh at this later period must therefore be due entirely to an effect of radiation on endothelial cells.
Figure 4.
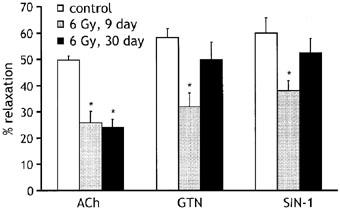
Histogram comparing maximum relaxation responses to 10 μM ACh, 10 μM GTN and 10 μM SIN-1 in vessels from control animals (open bars) and animals exposed to 6 Gy radiation either 9 (grey bars) or 30 days (black bars) earlier. The number of animals employed was six for each drug, with 14 rings tested in each condition. Relaxations are expressed as per cent decrease in the tension evoked by 10−5 M phenylephrine. *P<0.05 compared with the appropriate response in control rings.
The effects of radiation on the EDRF/NO but not the EDHF-dependent component of ACh-induced vasorelaxation
To test if altered release or action of endothelium-derived relaxing factors might contribute to the loss of responsiveness to ACh at 9 days post-irradiation, we tested the effects of agents that interfere with endothelium-dependent pathways on responses of vessels from healthy and irradiated animals (Figure 5). Indomethacin (5×10−6 M) was used to inhibit the synthesis and release of prostanoids. When added to the bath solution, indomethacin had no significant effect on the relaxation induced by ACh (10 μM) in either healthy or irradiated vessels. When in addition to indomethacin, L-Nω-nitroarginine (L-NA; 300 μM) was added to inhibit the synthesis of NO, the responses of healthy vessels to ACh were reduced to 25±4 % (P<0.05, n=14), indicating the involvement of NO. In contrast, responses of vessels from irradiated animals, which were already significantly depressed, were unaffected by 300 μM L-NA (25±2% relaxation before and 24±3% after adding L-NA, n=14), suggesting that irradiation had seriously impaired NO-dependent vasodilatation. To assess the potential contribution of endothelium-derived hyperpolarising factor (EDHF) to the ACh-induced relaxation and its modulation by radiation, we replaced L-NA with the K channel inhibitors, charybdotoxin (10−8 M) and apamin (10−6 M), which when applied together block EDHF-mediated responses in several vessels (Mombouli & Vanhoutte, 1997). In the presence of these drugs, the relaxation induced by ACh was reduced by around 50% in control vessels, but was abolished in the vessels from irradiated animals. The addition of 300 μM L-NA to the solution containing charybdotoxin and apamin led to complete inhibition of the ACh-induced relaxation in healthy and irradiated vessels.
Figure 5.
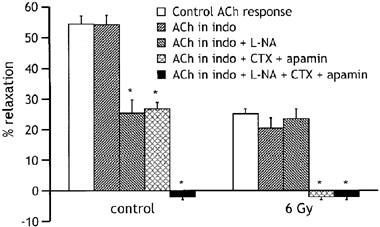
Histogram comparing relaxations to 10 μM ACh in aortic rings from control animals (n=5) and animals exposed 9 days earlier to 6 Gy radiation (n=5), in the absence of any drug (open bars) and in the presence of 5 μM indomethacin (indo), indomethacin plus 300 μM L-NA, indomethacin plus 10−8 M charybdotoxin (CTX) and 10−6 M apamin and indomethacin plus 300 μM L-NA plus 10−8 M CTX and 10−6 M apamin. Relaxations are expressed as per cent decrease in the tension evoked by 10−5 M phenylephrine. *P<0.05, compared with the response to ACh in the absence of drugs.
Electrophysiological experiments were carried out to determine whether or not the relaxant response to ACh involved smooth muscle hyperpolarization. As shown in Figure 6a, ACh induced smooth muscle hyperpolarization in healthy vessels, and the response was reduced in amplitude by pre-exposure to L-NA (10 μM), indicating that at least part of the response was due to endothelium-derived NO. Figure 6b compares the effects of 300 μM L-NA and the combined application of charybdotoxin (10−8 M) and apamin (10−6 M) on the amplitude of the ACh-induced hyperpolarization recorded from healthy vessels and vessels from rabbits that were exposed 9 days earlier to 6 Gy radiation. In healthy vessels both treatments reduced the response to ACh, suggesting that both NO and EDHF were involved in mediating the response. In contrast, the hyperpolarization evoked in vessels from irradiated animals was not significantly affected by 300 μM L-NA, but it was essentially abolished by charybdotoxin plus apamin. Moreover, the hyperpolarization evoked by ACh in irradiated vessels was of a similar amplitude to that evoked in healthy vessels exposed to 300 μM L-NA. Irradiation therefore appeared to eliminate the NO-mediated component, without preventing the EDHF-mediated component of the ACh-induced hyperpolarization.
Figure 6.
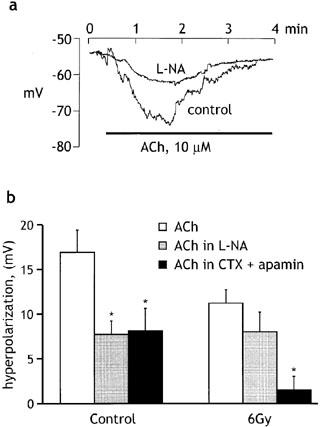
Influence of radiation on ACh-induced hyperpolarization. (a) Original records of membrane potential changes induced by ACh (10−5 M) before and during exposure to L-NA (10−5 M). (b) Histogram comparing the peak amplitude of the ACh-induced (10−5 M) hyperpolarization recorded under control conditions and in the presence of 300 μM L-NA or apamin (10−6 M) plus charybdotoxin (10−8 M), in vessels from non-irradiated (control) and irradiated (6 Gy) animals. All experiments were performed in the presence of indomethacin (5×10−6 M). Data shown as means±s.e.mean of 6–8 experiments (5 animals) for each group. *P<0.05 compared with the response to ACh in the absence of drugs.
The effects of α-tocopherol on radiation-induced changes in endothelium-dependent vascular responses
α-Tocopherol is the main lipid-soluble antioxidant in human plasma and lipoproteins. Oral administration of this vitamin to cholesterol fed rabbits was found to prevent the loss of endothelial vasodilator function that develops early in the course of atherosclerosis, a problem that may result from oxidative modification of low density lipoproteins (Keaney et al., 1993). To shed light on the mechanisms underlying the radiation-induced loss of NO-mediated relaxation, we tested the effects of oral administration of a single dose of 50 mg kg−1 α-tocopherol acetate, given 1 h after the exposure to irradiation. As shown in Figure 7, this treatment restored the response to 10 μM ACh in irradiated vessels, such that the relaxation produced by ACh 9 days after irradiation was not significantly different from that measured in control vessels. Furthermore, neither irradiation nor α-tocopherol had any significant effect on the relaxation induced by ACh in the presence of 300 μM L-NA. Vehicle (olive oil) administered at same dose had no protection effect. Thus α-tocopherol was able to protect against the radiation-induced loss of the NO-mediated relaxation.
Figure 7.
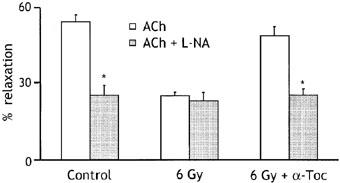
Effects of irradiation (6 Gy) on relaxations induced by acetylcholine (10 μM) in the absence and presence of 300 μM L-NA, compared with and without the administration of the antioxidant α-tocopherol acetate (α-Toc), given orally at 50 mg kg−1 1 h after exposure to radiation. Relaxations are expressed as per cent decrease in the tension evoked by 10−5 M phenylephrine. All experiments were performed in the presence of indomethacin (5×10−6 M). Data shown as means±s.e.mean of 15 experiments (five animals) for each group. *P<0.05.
Discussion
The loss of endothelial function is one of the most commonly observed vascular effects of ionized radiation. As has been widely reported, irradiation was found to cause a loss of endothelium-dependent vasodilatation in response to ACh, although it was observed only at ACh concentrations above 100 nM. Loss of response to ACh began at a radiation dose as low as 2 Gy and increased in magnitude with increasing dose. Thus, the changes in vascular activity reported here were observed at lower radiation doses than previously investigated (Qi et al., 1998; Sugihara et al., 1999). The loss of activity was clear at 9 days post-irradiation and remained at a similar level for the remainder of the 30-day experimental period. This agrees with a previous report that responses of rabbit ear artery to ACh were equally impaired between 1 and 10 weeks after local irradiation (Qi et al., 1998). Impairment of the endothelium-dependent relaxations evoked by ACh and A23187 showed a similar dependence on radiation dose. This indicates that the loss of relaxant response to ACh was not due to altered muscarinic receptors on the endothelium, but resulted either from the altered production or release of relaxant factors from the endothelium or altered smooth muscle sensitivity to the released factors.
Relaxation responses to a range of NO donor drugs, including GTN, SNP and SIN-1, were also depressed at 9 days post irradiation. Since these agents act directly on vascular smooth muscle, altered smooth muscle sensitivity to NO is likely to contribute to the impairment of endothelium-dependent relaxation evident a few days after irradiation. However, unlike the impaired response to ACh, at 30 days post-irradiation the relaxant responses to NO donor drugs recovered to the level seen in control animals. It appears therefore that the smooth muscle damage caused by radiation is relatively short-lived, and that the persistent impairment of responses to endothelium-dependent vasodilators in the longer term is due entirely to dysfunctional endothelial cells.
Endothelium-dependent relaxation of thoracic aorta, taken from either control or irradiated rabbits, was insensitive to indomethacin, implying that it did not involve the release of prostanoids. In healthy vessels, the relaxation evoked by ACh was probably mediated by a combination of NO and EDHF. This is suggested by the finding that both the relaxation and smooth muscle hyperpolarization induced by ACh were only partially inhibited by L-NA. Other studies in rabbit aorta also found that high concentrations of L-NAME (10−4 M) were only able to decrease the maximal relaxation induced by ACh to 56% (Ruiz & Tejerina, 1999). The involvement of EDHF is further suggested by the finding that the combined application of the K-channel inhibitors, charybdotoxin and apamin, reduced both the relaxation and hyperpolarization induced by ACh and abolished the L-NA resistant component.
In contrast to its effect in healthy vessels, L-NA had no effect on either the relaxation or hyperpolarization response to ACh in vessels from irradiated animals, implying that the NO-mediated component of the response was impaired. The remaining response appeared to be mediated by EDHF, because both the hyperpolarization and relaxation evoked by ACh were abolished in the presence of charybdotoxin and apamin. This implies that the EDHF pathway is relatively resistant to irradiation. In fact it can be seen from Figure 6 that the charybdotoxin- and apamin-sensitive components of the ACh-induced hyperpolarization were of similar amplitudes in control and irradiated vessels, suggesting that this component was completely unaffected by irradiation. This stability of the EDHF pathway appears to argue against the hypothesis that EDHF is a metabolite of arachidonic acid, formed by the cytochrome P450 mono-oxygenase pathway (Mombouli & Vanhoutte, 1997). This is because lipid oxidation, a widely recognized consequence of radiation-induced oxidative stress, might be expected to affect this pathway.
Irradiated vascular cells are likely to suffer from oxidative stress (On et al., 2001). Following irradiation, the content of oxygen-derived free radicals (superoxide anion, hydroxyl anions and others) and hydrogen peroxide is known to increase significantly in living cells and biological liquids (Sheng-Tanner et al., 1998; Serkiz, 1997). These radicals are among the main agents responsible for radiation-induced lipid peroxidation (Sydoryk et al., 1997) and can activate a death pathway in mammalian cells (Baraboy, 1997; Sheng-Tanner et al., 1998). Although multiple processes may lead to endothelial damage, the generation of oxygen-derived free radicals and subsequent lipid peroxidation may be one of the key components in the cascade of events. Additionally, a fast interaction between NO and superoxide anions could result in the formation of peroxynitrite (Rubanyi & Vanhoutte, 1986), which is a less potent vasodilator and possesses the ability to initiate lipid peroxidation (Radi et al., 1991). The accelerated degradation of NO and formation of peroxynitrite would reduce the availability of endothelium-derived NO following irradiation, explaining the selective effect of radiation on the NO pathway. A role for oxidative stress in radiation-induced endothelial dysfunction is supported by the protective effects of the well-known antioxidant, vitamin C (ascorbic acid), in rat aorta (On et al., 2001). In that study, chronic treatment of rats with vitamin C for 7 days prior to and after irradiation with a 137Cs source was found to prevent the impairment of ACh-mediated vasodilation. In the present study, another antioxidant, α-tocopherol, was found to prevent the loss of endothelium-dependent relaxation when administered as a single dose shortly after radiation exposure. Since this effect was seen without any change in the EDHF-mediated component of relaxation, the data further support the idea that oxidative stress is responsible for the selective loss of NO-mediated vasodilatation following exposure to ionising radiation.
In conclusion, the data indicate that endothelial function is impaired in blood vessels from γ-irradiated rabbits as a consequence of the selective loss of NO-mediated relaxation. When studied 9 days post-irradiation at 6 Gy, the NO-mediated component of the endothelium-dependent response was abolished, while responses to NO donors were only partially inhibited. It is therefore likely that the impaired release and action of NO both contribute to the loss of endothelium-dependent relaxation. Following the loss of the NO pathway, endothelium-dependent vasodilatation is maintained at a reduced level due to the persistence of an EDHF pathway that is resistant to γ-radiation. Contrary to endothelium-dependent responses, full recovery of the endothelium-independent responses to NO donors were observed 30 days after irradiation. Since this effect was transient, the prolonged loss of endothelial cell function and NO-mediated relaxation is likely to be more important in the development of the cardiovascular disorders seen in the populations exposed to irradiation as a consequence of the Chernobyl accident.
Acknowledgments
This study was supported by a Collaborative Research Initiative Grant (055168/Z/98/Z) from the Wellcome Trust to AI Soloviev and AM Gurney.
Abbreviations
- ACh
acetylcholine
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- GTN
glyceryl trinitrate
- L-NA
L-Nω- nitroarginine
- NO
nitric oxide
- SIN-1
3-morpholino-sydnonimine
- SNP
sodium nitroprusside
References
- ALLEN J.B., SAGERMAN R.H., STUART M.J. Irradiation decreases vascular prostacyclin formation with no concomitant effect on platelet thromboxane production. Lancet. 1981;2:1193–1196. doi: 10.1016/s0140-6736(81)91437-9. [DOI] [PubMed] [Google Scholar]
- BARABOY V.A.Biological effects in animals. Changes in biochemical indices of the organism vitally important systems Chernobyl catastrophe 1997Ukraine, Kiev: Editorial House of Annual Issue ‘Export of Ukraine'; 285–288.Baryakhtar, V. (ed) [Google Scholar]
- BECKMAN J.A., THAKORE A., KALINOWSKI B.H., HARRIS J.R., CREAGER M.A. Radiation therapy impairs endothelium-dependent vasodilation in humans. J. Am. Coll. Cardiol. 2001;37:761–765. doi: 10.1016/s0735-1097(00)01190-6. [DOI] [PubMed] [Google Scholar]
- GRATWOHL A., JOHN L., BALDOMERO H., ROTH J., TICHELLI A., NISSEN C., LYMAN S.D., WODNAR-FILIPOWICZ A. FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood. 1998;92:765–769. [PubMed] [Google Scholar]
- KEANEY J.F., GAZIANO J.M., XU A., FREI B., CURRAN-CELANTANO J., SHWAERY G.T., LOSCALZO J., VITA J.A. Dietary antioxidants preserve endothelium-dependent vessel relaxation in cholesterol-fed rabbits. Proc. Natl. Acad. Sci. USA. 1993;90:11880–11884. doi: 10.1073/pnas.90.24.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHOMAZJUK I.N.The problem of essential hypertension in survivors after Chernobyl catastrophe. Proceedings of the 2nd International Conference. Long-term health consequences of the Chernobyl disaster 1998World Health Organization – Chernobylinterinfom, Kiev; 398–399.p [Google Scholar]
- KLIMOV D.V., ADZIARKHA K.N. Risk factors for the development of hypertension in the liquidators of 1986-1987. (Abstract of the 3rd International Confererence. Health effects of the Chernobyl accident: results of the 15-year follow-up studies. June 4-8, 2001, Kiev, Ukraine) Intern. J. of Radiation Med. 2001;3:59. [Google Scholar]
- LELIUK V.G., GUSKOVA A.K.Evaluation of the relationship between the cerebrovascular diseases and ionizing radiation exposure at the late period after irradiation. Proceedings of the 2nd International Conference: Long-term health consequences of the Chernobyl disaster 1998World Health Organization – Chernobylinterinfom, Kiev; 274–275.p [Google Scholar]
- MAYNARD K.I., STEWART-LEE A.L., MILNER P., BURNSTOCK G. X-irradiation attenuates relaxant responses in the rabbit ear artery. Br. J. Pharmacol. 1992;105:126–128. doi: 10.1111/j.1476-5381.1992.tb14222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJOR O., KEMENY A.A., FORSTER D.M., JAKUBOWSKI J., MORICE A.H. In vitro contractility studies of the rat middle cerebral artery after stereotactic Gamma Knife radiosurgery. Stereotact. Funct. Neurosurg. 1996;66 Suppl 1:17–28. doi: 10.1159/000099697. [DOI] [PubMed] [Google Scholar]
- MENENDEZ J.C., CASANOVA D., AMADO J.A., SALAS E., GARSIA-UNZUETA M.T., FERNANDEZ F., PEREZ DE LA LASTRA M., BERRAZUETA J.R. Effects of radiation on endothelial function. I. J. Radiation Oncology Biol. Phys. 1998;41:905–913. doi: 10.1016/s0360-3016(98)00112-6. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Endothelium-derived hyperpolarizing factor(s): updating the unknown. Trends Pharmacol. Sci. 1997;18:252–256. [PubMed] [Google Scholar]
- ON Y.K., KIM H.S., KIM S.Y., CHAE I.H., OH B.H., LEE M.M., PARK Y.B., CHOI Y.S., CHUNG M.H. Vitamin C prevents radiation-induced endothelium-dependent vasomotor dysfunction and de-endothelialization by inhibiting oxidative damage in the rat. Clin. Exp. Pharmacol. Physiol. 2001;28:816–821. doi: 10.1046/j.1440-1681.2001.03528.x. [DOI] [PubMed] [Google Scholar]
- QI F., SUGIHARA T., HATTORI Y., YAMAMOTO Y., KANNO M., ABE K. Functional and morphological damage of endothelium in rabbit ear artery following irradiation with cobalt60. Br. J. Pharmacol. 1998;123:653–660. doi: 10.1038/sj.bjp.0701654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADI R., BECKMAN J., BUSH K., FREEMAN B. Peroxynitrite-induced membrane lipid peroxydation: the cytotoxic potential of superoxide and nitric oxide. Arch. of Biophys. and Biochem. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Superoxide anions and hyperoxia inactivate EDRR. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- RUIZ E., TEJERINA T.Is L-citrulline an EDHF in the rabbit aorta Endothelim-dependent hyperpolarizations 1999The Netherlands: Harwood Academic Publishers; 399–403.ed. Vanhoutte, P [Google Scholar]
- SERKIZ Y.I.Peculiarities of the low efficiency radiation biological effects Chernobyl catastrophe 1997Ukraine, Kiev, Editorial House of Annual Issue ‘Export of Ukraine'; 279–281.Baryakhtar, V. (ed.) [Google Scholar]
- SHENG-TANNER X., BUMP E.A., HEDLEY D.W. An oxidative stress-mediated death pathway in irradiated human leukemia cells mapped using multilaser flow cytometry. Radiat. Res. 1998;150:636–647. [PubMed] [Google Scholar]
- SUGIHARA T., HATTORI Y., YAMAMOTO Y., QI F.Z., ICHIKAWA R., SATO A., LIU M.Y., ABE K., CANNO M. Preferential impairment of nitric oxide-dependent relaxation in human cervical arteries after irradiation. Circulation. 1999;100:635–641. doi: 10.1161/01.cir.100.6.635. [DOI] [PubMed] [Google Scholar]
- SYDORYK Y.P., DRUZHINA M.O., BURLAKA A.P., PUKNOVA G.G.Biological effects in animals. Changes of biophysical characteristics in the organs and in the blood Chernobyl catastrophe 1997Ukraine, Kiev, Editorial House of Annual Issue ‘Export of Ukraine'; 282–284.Baryakhtar, V. (ed) [Google Scholar]
- TARANENKO V.M., TISHKIN S.M., RUDNEV M.I. The ionised radiation-induced changes of vascular wall reactivity. Proc. Acad. Sci. U.S.S.R. (Moscow) 1992;322:600–603. [PubMed] [Google Scholar]
- TISHKIN S.M. The influence of ionising radiation on some antianginal drugs action. Drugs (Ukraine) 1998;2:62–65. [Google Scholar]
- VOROBYOV V.I., STEPANOV R.P.Ionizing radiation and blood vessels 1985Moscow, Energoatomizdat; 296p [Google Scholar]
- WARFIELD M.E., SCHNEIDKRAUT M.J., CUNARD C.M., RAMWELL P.W., KOT P.A. Reactivity of rat abdominal aorta to U46619 following whole-body gamma irradiation. Radiat. Res. 1989;117:459–468. [PubMed] [Google Scholar]
- WONG F.L., YAMADA M., SASAKI H., KODAMA K., AKIBA S., SHIMAOKA K., HOSODA Y. Noncancer disease incidence in the atomic bomb survivors: 1958-1986. Radiat. Res. 1993;135:418–430. [PubMed] [Google Scholar]


