Abstract
To clarify the involvement of Th2 responses in the development of allergen-induced airway remodelling, we investigated the effect of anti-CD4 monoclonal antibody (mAb) and anti-CD8 mAb, and the responses of IL-4 gene-knockout (KO) mice in a murine model of allergic asthma.
Mice were immunized twice by intraperitoneal injections of ovalbumin (OA), and exposed to aeroallergen (OA, 1% w v−1) for 3 weeks. Twenty-four hours after the final challenge, airway responsiveness to acetylcholine was measured, and bronchoalveolar lavage (BAL) and histological examinations were carried out.
Anti-CD4 mAb (1 mg kg−1) clearly inhibited allergen-induced increases in airway responsiveness to acetylcholine, the number of eosinophils in BAL fluid, serum OA-specific IgE levels, IL-13 and transforming growth factor-β1 levels in BAL fluid, and amount of hydroxyproline in the lung by 100, 99, 100, 100, 84, and 60%, respectively. Furthermore, the antibody (1 mg kg−1) also attenuated allergen-induced goblet cell hyperplasia in the epithelium and subepithelial fibrosis by 72 and 83%, respectively. In contrast, anti-CD8 mAb (1 mg kg−1) showed no effect on each parameter. Furthermore, all these parameters were attenuated in IL-4KO mice by 57, 93, 100, 45, 84 and 60%, and also 72 and 83%, respectively.
These findings suggest that Th2 responses play a critical role for the development of allergen-induced airway remodelling, and that the inhibition of Th2 responses, e.g. using anti-CD4 mAb, is a therapeutic approach for the treatment of airway remodelling in asthma.
Keywords: Airway, bronchial asthma, goblet cell, inflammation, remodelling, subepithelial fibrosis, T helper type 2 cells, transforming growth factor-β1
Introduction
Bronchial asthma has been suggested to be a reversible disorder, characterized by variable airflow limitation, airway eosinophilic inflammation and airway hyperresponsiveness (AHR) (Djukanovic et al., 1990), however, some patients with asthma have been shown to have irreversible changes in lung function (Brown et al., 1984; Jeffery et al., 1992; Wilson, 1998), despite appropriate and aggressive anti-inflammatory therapy. This was suggested to be the result of structural changes in the airways that occur as a result of airway remodelling, which are characterized by goblet cell hyperplasia/hypertrophy, subepithelial fibrosis and smooth muscle hyperplasia/hypertrophy (Aikawa et al., 1992; Heard & Hossain, 1973; Roche et al., 1989). Although it was suggested that airway remodelling is an injury-repair response driven by variable mediators derived from inflammatory cells in the airways, the precise mechanisms of the development of airway remodelling are unknown.
To date, several clinical investigations have demonstrated that CD4+ T lymphocytes were observed in the asthmatic airways, and T helper type 2 (Th2) cytokines produced by CD4+ T lymphocytes were suggested to play a central role in initiating and sustaining asthma responses (Robinson, 2000). For, example, IL-4 is critical for the commitment of naïve CD4+ T cells into the Th2 phenotype, and IL-13, which shares several biological responses with IL-4, may also be important to the development of allergic airway eosinophilia and AHR (de vries, 1998; Kopf et al., 1993). IL-4 and IL-13 are also required for IgE isotype switching in B cells (Finkelman et al., 1988; McKenzie et al., 1993). Moreover, IL-4 and IL-13 also increase airway mucus secretion, which can exacerbate airway responsiveness (Temann et al., 1997; Zuhdi Alimam et al., 2000). IL-5 is known to affect eosinophil differentiation, maturation and prolongation of survival, and promote eosinophilic inflammation in the airways (Foster et al., 1996; Lopez et al., 1988; Yamaguchi et al., 1988), which may be involved in asthmatic responses. These responses induced by CD4+ T lymphocytes via the production of Th2 cytokines were suggested to be important in the pathophysiology in asthma. Recent studies in animal models of allergic asthma support the involvement of CD4+ T lymphocytes. Shortly, depletion of CD4+ T lymphocytes by administration of antibody clearly inhibited allergen-induced airway eosinophilia and AHR (Nakajima et al., 1992; Gavett et al., 1994). More recently, Foster et al. (2002) demonstrated that CD4+ T lymphocytes are critical for the development of airway epithelial changes and subepithelial fibrosis as well as AHR in a murine model of chronic asthma. However, the role of Th2 responses in the development of allergen-induced airway remodelling has not been fully investigated in vivo.
In the present study, to clarify the role of Th2 responses in the development of airway remodelling, we investigated the effect of depletion of CD4+ or CD8+ T lymphocytes using each monoclonal antibody in a murine model of allergic asthma. Furthermore, we examined the deficiency of IL-4 using the gene-knockout (KO) mouse.
Methods
Animals
Seven-week-old female BALB/c mice (Japan SLC, Shizuoka, Japan), 7-week-old female IL-4 KO (Noben-Trauth et al., 1996; BALB/cJ background, Jackson Laboratory, Bar Harbor, ME, U.S.A.) and age matched female wild-type animals (Jackson Laboratory) were used. Experiments were undertaken following the guidelines for the care and use of experimental animals of the Japanese Association for Laboratory Animals Science in 1987.
Agents
The following drugs and chemicals were purchased commercially and used: ovalbumin (OA, Seikagaku Kogyo, Tokyo, Japan), acetylcholine chloride (ACh, Nacalai Tesque, Inc., Kyoto, Japan), bovine serum albumin (BSA, Seikagaku Kogyo), Türk solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan), pancuronium bromide (Sigma, St. Louis, MO, U.S.A.), sodium pentobarbitone (Abbott Lab., Chicago, IL, U.S.A.), disodium ethylenediaminetetraacetic acid (EDTA-2Na, Nacalai Tesque), Diff-Quick solution (International Reagent Corp., Ltd., Kobe, Japan), monoclonal rat anti-mouse IgE antibody (LO-ME-3, Serotec Co., Ltd., Oxford, U.K.), polyclonal goat anti-mouse IgG1 antibody (ST-AR81, Serotec Co. Ltd.), monoclonal rat anti-mouse IgG2a antibody (LO-MG2a-7, Serotec Co. Ltd.), peroxidase-conjugated streptavidin (Dakopatts a/s, Glostrup, Denmark) and hydroxy-L-proline (Nacalai Tesque).
Sensitization and antigen challenge
Experiments were performed as reported previously (Tanaka et al., 2001). Mice were actively sensitized by intraperitoneal injections of 50 μg OA with 1 mg alum on day 0 and 12. Starting on day 22, they were exposed to OA (1% w v−1 diluted in sterile physiological saline) for 30 min every day for 3 consecutive weeks. Negative control animals were injected with saline or OA plus alum and exposed to saline in a similar manner. Airway responsiveness to Ach, bronchoalveolar lavage (BAL) and the measurement of hydroxyproline content in right lungs were performed 24 h after the final antigen challenge.
Treatment of anti-CD4 monoclonal antibody (mAb) and anti-CD8 mAb
Monoclonal antibodies against mouse CD4 (GK1.5, American Tissue Culture Collection, Manassas, VA, U.S.A.) and mouse CD8 (2.43, American Tissue Culture Collection) were purified from ascites by sequential (NH4)2SO4 precipitation and protein A affinity chromatography, dialyzed against phosphate-buffered saline (PBS), and kept at −80°C until use. Either anti-CD4 or -CD8 mAb (0.5 or 1 mg per animal) was treated by intraperitoneal injection 6 h after every exposure every fourth day from the day before first antigen inhalation. Control antibody (rat IgG) was treated in a similar manner.
Flow cytometric analyses of the depleted CD4+ or CD8+ cells in vivo
Mouse splenocytes were suspended in PBS containing 0.1% BSA. The cells were incubated with anti-FcγII/III receptor (Fc BlockTM; rat IgG2b clone 2.4G2, PharMingen, San Diego, CA, U.S.A.) for 5 min, and then stained with either phycoerythrin (PE)-conjugated anti-CD4 antibody (L3T4; rat IgG2a clone RM4-5, PharMingen) or fluorescein isothiocyanate-conjugated anti-CD8 antibody (Ly-2; rat IgG2a clone 53-6.7, PharMingen) for 30 min at 4°C in the dark. After washing with PBS containing 0.1% BSA and 0.1% NaN3, the cells were analysed using FACS scan and LYSIS II software (Becton-Dickinson, San Jose, CA, U.S.A). The lymphocyte-gated region was determined based on forward and side scatter signals of control splenocytes stained with PE-conjugated anti-CD45 (Ly-5/T-200; rat IgG2b clone 30-F11, PharMingen). Dead cells were excluded using propidium iodide. For each sample, at least 10,000 cells in the lymphocytes gated region were analysed.
Measurement of antigen-specific IgE, IgG1 and IgG2a
At week 3 (day 43), immediately prior to the final antigen challenge, blood was collected and sera were obtained by centrifugation and stored at −80°C. Antigen-specific IgE in serum was measured using the enzyme-linked immunosorbent assay (ELISA) as previously described (Nagai et al., 1997, Tanaka et al., 2001). Briefly, serum OA-specific IgE was measured by coating flat-bottom 96-well microtiter plates (Nunc Immuno-Plate I 96-F, Roskilde, Denmark) with monoclonal rat anti-mouse IgE antibody (LO-ME-3) at a concentration of 5 μg ml−1. After blocking with 1% BSA, serum dilutions were incubated for 1 h followed by biotinylated-OA and peroxidase-conjugated streptavidin. Serum OA-specific IgG1 was measured by coating the microtiter plates with polyclonal goat anti-mouse IgG1 antibody (ST-AR81). After blocking, serum dilutions were incubated for 1 h followed by biotinylated-OA and peroxidase-conjugated streptavidin. Serum OA-specific IgG2a was measured by coating the microtiter plates with OA solution (20 μg ml−1). After blocking, serum dilutions were incubated for 1 h followed by peroxidase-conjugated monoclonal rat anti-mouse IgG2a antibody (LO-MG2a-7). Sequentially diluted monoclonal anti-OA IgE, IgG1 and IgG2a (donated by Dr M. Kiniwa, Taiho Pharmaceutical Co. Ltd., Saitama, Japan) were used as a standard. Optical densities of the enzymatic reactions were read using an automatic ELISA plate reader (Multiscan MS, ver 8, Labsystems Oy, Helsinki, Finland) at 450 nm (reference 690 nm) and analysed using Deltasoft 3 (Biometallics Inc., Princeton, NJ, U.S.A.). Each detection limit was 1 ng ml−1, 10 μg ml−1 and 3 ng ml−1, respectively.
BAL
To evaluate airway inflammation, we examined the accumulation of inflammatory cells in BAL fluid (BALF). Experiments were performed according to previously described methods (Tanaka et al., 2001). Animals were killed with an intraperitoneal injection of sodium pentobarbitone (100 mg kg−1). The trachea was cannulated and the left bronchi were tied for histological examination. Then, the right air lumen was washed four times with 0.5-ml calcium- and magnesium-free PBS containing 0.1% BSA and 0.05 mM EDTA-2Na. This procedure was repeated three times (total volume; 1.3 ml, recovery >85%). BALF from each animal was pooled in a plastic tube, cooled on ice and centrifuged (150×g) at 4°C for 10 min. Cell pellets were resuspended in the same buffer (0.5 ml). BALF was stained with Türk solution and the number of nucleated cells was counted in a Burker chamber. A differential count was made on a smear prepared with a cytocentrifuge (Cytospin II, Shandon, Cheshire, U.K.) and stained with Diff-Quick solution (based on standard morphologic criteria) of at least 300 cells (magnification×500). The supernatant of BALF was stored at −30°C for determination of cytokine production.
Cytokine levels in BALF
The amount of cytokine in the supernatant of BALF was measured using ELISA (Endogen Inc., Woburn, MA, U.S.A. for interferon-γ (IFN-γ); R&D Systems Inc., Minneapolis, MN, U.S.A. for IL-13). The transforming growth factor (TGF)-β1 content in BALF was also measured using ELISA (Genzyme Tecne, Minneapolis), which can detect mouse TGF-β1 protein, because of the high homology of TGF-β1 across species. The assay detects only the active form of TGF-β1. Each sample was activated before measuring according to the manufacturer's recommendations. The detection limit of each kit was 10 pg ml−1 for IFN-γ, 1.5 pg ml−1 for IL-13, or 7 pg ml−1 for TGF-β1, respectively.
Measurement of hydroxyproline content in right lungs
Whole collagen content of the right lung was evaluated by determining hydroxyproline contain as described previously (Tanaka et al., 2001). Briefly, after recovery of BALF, the right lung lobes were removed and cut into sections (1 mm thick). The chopped lungs were dried with acetone. Then, the dried lung samples were hydrolyzed with 2 ml of 6 N HCl at 120°C for 24 h in sealed glass tubes. The amount of hydroxyproline in the hydrolysate was measured according to Kivirikko et al. (1967). Authentic hydroxyproline (hydroxy-L-proline) was used to establish a standard curve.
Measurement of airway function
Measurement of bronchial responsiveness to intravenous ACh was performed as previously described (Nagai et al., 1997; Tanaka et al., 2001). Briefly, to measure airway responsiveness to ACh, mice were anaesthetized with sodium pentobarbitone (60 mg kg−1, i.p.) and the jugular vein was cannulated for intravenous injection of acetylcholine. Mice were injected with pancuronium bromide (0.1 mg kg−1, i.v.) to stop spontaneous respiration, and animals were ventilated with a rodent ventilator (New England Medical Instruments Inc., Medway, MA, U.S.A). Bronchoconstriction was measured according to the overflow method, using a bronchospasm transducer (Ugo Basil 7020, Milan, Italy) connected to the tracheal cannula. To measure airway responsiveness to ACh, changes in respiratory overflow volume were measured using increasing doses of ACh. The increase in respiratory overflow volume induced by ACh was represented as a percentage of the maximal overflow volume (100%) obtained by clamping the tracheal cannula. The area under the curve (AUC) calculated from dose-response curves for ACh are expressed as a magnitude of AHR. Briefly, each dose was converted logarithmically, and then AUC was calculated and represented as arbitrary units (Tanaka et al., 2001).
Histological study
The left lungs were distended with 10% buffered formalin via the trachea (10 cm H2O) for 30 min, and then excised and immersed in the fresh fixative for 24 h. Tissues were sliced and embedded in paraffin, and 6 μm sections were stained with periodic acid-Schiff (PAS) and Masson-Trichrome for light microscopy examination.
Examination of goblet cell hyperplasia was carried out using a method previously described by Padrid et al. (1995) with a slight modification (Tanaka et al., 2001) using a Leica image analysis system (Leica, Cambridge, U.K.). Briefly, two to four specimens of the PAS-stained histological preparations of the left lobe, in which the total length of the epithelial basement membrane of the bronchioles were 1.0–2.5 mm, were selected and the pathological changes were evaluated according to the modified 5-point scoring system (Tanaka et al., 2001) using the Leica microscope (×20 objectives). The preparations, in which the maximum internal diameter of the bronchioles were 2 fold or greater as large as the minimum internal diameter, were not used for analysis. The hyperplasia of the goblet cells in the epithelial lining was expressed by a score according to the percentage of the goblet cells in the epithelial cells. To minimize sampling errors, the 5-point scoring system (grade 0–4) (Tanaka et al., 2001) was adopted: grade 0 (no goblet cells); grade 1, <25%; grade 2, 25–50%; grade 3, 50–75%; grade 4, ⩾75%. The mean score of the total epithelial cells in two to four preparations of one mouse were counted. The mean scores of hyperplasia of the goblet cells were calculated in five to six animals.
Masson-trichrome stained sections were used for assessment of subepithelial fibrosis using a Leica image analysis system (Leica). As described above, two to four specimens of the Masson-trichrome -stained histological preparations of the left lobe, in which the total length of the epithelial basement membrane of the bronchioles was 1.0–2.5 mm, were selected and the fibrotic area (stained in blue) beneath the basement membrane was measured. The mean score of the fibrotic area divided by basement membrane length in two to four preparations of one mouse were calculated, then, the mean scores of subepithelial fibrosis were calculated in five to six animals.
Statistical analysis
Values are presented as the mean with standard error. Statistical significance between two groups was estimated using the two-tailed Student's t-test or the Mann–Whitney U-test after the variances of the data were evaluated with F-test. To define statistically significant differences among control animals and mAb treated animals, the data was subjected to Bartlett's analysis followed by a parametric or a non-parametric Dunnett's multiple range test. Spearman's correlation test was used to analyse for correlation between the cytokine productions in BALF, goblet cells hyperplasia and subepithelial fibrosis. P values less than 0.05 were considered to be significant.
Results
Expression of CD4 and CD8 on splenocytes after their Abs treatment in vivo
To confirm the depletion of CD4+ or CD8+ T lymphocytes in vivo, we examined their expression in splenocytes prepared from the mice 1 to 4 days after their Abs treatment using flow cytometric analyses.
Splenocytes of normal mice showed 31.5±2.1% and 13.2±1.8% of CD4 and CD8 positive cells, respectively (n=4). In contrast, the percentages of each population were clearly decreased 24 h after the treatment of each mAb (1.3± 0.5% for CD4+ and 0.4±0.2% for CD8+, respectively). The effects persisted for at least 4 days after their treatment (data not shown). Control antibody (rat IgG) had no effect on the expression of each population (data not shown). Therefore, mice were treated with their mAbs every fourth day from the day before first antigen inhalation.
Effect of anti-CD4 mAb or anti-CD8 mAb on allergen-induced AHR, airway eosinophilia, IgE production in serum, cytokine production in BALF
To investigate the role of CD4+ or CD8+ T lymphocytes in the onset of allergen-induced AHR, inflammatory infiltrates in the airway, IgE production and Th1/Th2 cytokine balance, we examined the effect of anti-CD4 or anti-CD8 mAb treatment in this model. As shown in Figure 1, repeated antigen challenge induced increases in airway responsiveness to ACh, the number of eosinophils in BALF, serum antigen-specific IgE production, IL-13 production in BALF and the decreases in IFN-γ production in BALF compared with those in saline-inhaled mice. In contrast, the systemic treatment of anti-CD4 mAb during antigen inhalation clearly inhibited all these responses in a dose-dependent manner, whereas the administration of anti-CD8 mAb showed no effect on these parameters.
Figure 1.
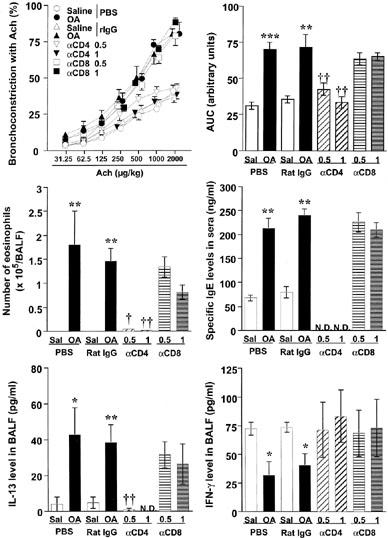
Effect of anti-CD4 monoclonal antibody (mAb) (GK1.5) or anti-CD8 mAb (2.43) on allergen-induced airway hyperresponsiveness to acetylcholine, the increases in the numbers of eosinophils in bronchoalveolar lavage fluid (BALF), serum antigen-specific IgE level, IL-13 production in BALF and the decrease in interferon-γ ðproduction in BALF in sensitized BALB/c mice. Values represent the mean with standard error of five to six mice in each group. Sal, saline-exposed; OA, ovalbumin-exposed; αCD4, anti-CD4 mAb-treated (0.5 mg or 1 mg per animal, i.p.); αCD8, anti-CD8 mAb-treated; PBS, phosphate-buffered saline-injected; Rat IgG, control Ab-injected (1 mg per animal, i.p.); AUC, area under the curve for the dose-response to acetylcholine (range: 31.25–2000 μg kg−1); N.D., not detected. *P<0.05, **P<0.01, ***P<0.001 (vs saline-inhaled group); †P<0.05, ††P<0.01 (vs ovalbumin-inhaled, rat IgG-treated group).
The effect of depletion of CD4+ or CD8+ T lymphocytes on allergen-induced airway remodelling
To investigate the role of CD4+ or CD8+ T lymphocytes in the development of allergen-induced airway remodelling, we examined the histological changes in the airways, TGF-β1 production in BALF and the amount of hydroxyproline content, which is an amino acid specifically consisting of collagen, in the right lung tissue 24 h after the final antigen challenge. Figure 2 shows the representative sections of each group stained with PAS for detection of goblet cells (Figure 2 A–D) and stained with Masson-Trichrome for detection of connective tissue (Figure 2E–H). The quantitative findings of the histological examination, TGF-β1 production and the amount of hydroxyproline in each group are shown in Table 1. As shown in Figure 2 and Table 1, OA-exposed mice showed severe goblet cell hyperplasia compared with saline-exposed animals (Figure 2A vs B). In contrast, the depletion of CD4+ T lymphocytes clearly prevented the goblet cell hyperplasia, while the depletion of CD8+ T lymphocytes showed no effect on the epithelial changes (Figure 2C,D, and Table 1).
Figure 2.
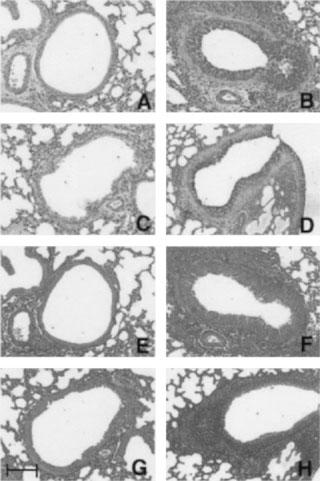
Histological analysis of lung sections stained with periodic acid-Schiff (A–D) and with Masson-trichrome (E–H) 24 h after the final antigen challenge (×50) in sensitized BALB/c mice. (A and E) Rat IgG-injected saline-exposed animal; (B and F) rat IgG-injected ovalbumin-exposed animal; (C and G) anti-CD4 monoclonal antibody (mAb) (GK1.5)-treated ovalbumin-exposed animal; (D and H) anti-CD8 mAb (2.43)-treated ovalbumin-exposed animal. Scale bar 100 μm.
Table 1.
Effect of anti-CD4 mAb and anti-CD8 mAb on allergen-induced airway remodelling in sensitized BALB/c mice
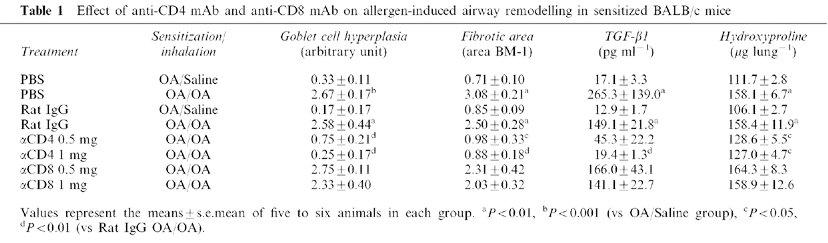
TGF-β1 is known to be a fibrogenic factor. We measured the level in BALF, and evaluated the extent of subepithelial fibrosis using morphological examination and quantitative analyses. Repeated antigen inhalation caused the increases in TGF-β1 production in BALF and marked subepithelial fibrosis, observed beneath the basement membrane of the bronchi and peripheral bronchiole compared with saline-inhaled mice (Figure 2F vs E and Table 1). Treatment of anti-CD4 mAb prevented the increases in TGF-β1 production and the development of subepithelial fibrosis, whereas the depletion of CD8+ T lymphocytes had no effect (Figure 2G vs H). These results were confirmed by the findings in fibrotic area around the airways and the amount of hydroxyproline in the right lung tissue (Table 1).
Effect of IL-4 gene deficiency on allergen-induced AHR, airway eosinophilia, IgE production in serum, cytokine production in BALF
To investigate the role of IL-4 in the onset of allergen-induced AHR, inflammatory infiltrates in the airway, IgE production and Th1/Th2 cytokine balance, we examined the effect of IL-4 gene deficiency using IL-4 KO mice compared with wild-type (BALB/cJ) mice. As shown in Figure 3, repeated antigen challenge in sensitized wild-type mice induced increases in airway responsiveness to ACh, the number of eosinophils in BALF, serum antigen-specific IgE production, IL-13 production in BALF and the decreases in IFN-γ production in BALF compared with those in saline-inhaled mice. In contrast to antigen-inhaled wild-type mice, all these responses were significantly attenuated in IL-4 KO mice. Furthermore, antigen challenge induced a marked increase in serum antigen-specific IgG1 (Th2 responses in mice) in wild-type mice (3.00±0.32 mg ml−1), however, the production was also significantly reduced in IL-4 KO mice (0.50±0.04 mg ml−1), whereas the level of serum antigen-specific IgG2a (Th1 response in mice) in IL-4 KO mice (33.8±4.9 μg ml−1) was markedly increased compared with those in wild-type mice (0.07±0.02 μg ml−1).
Figure 3.
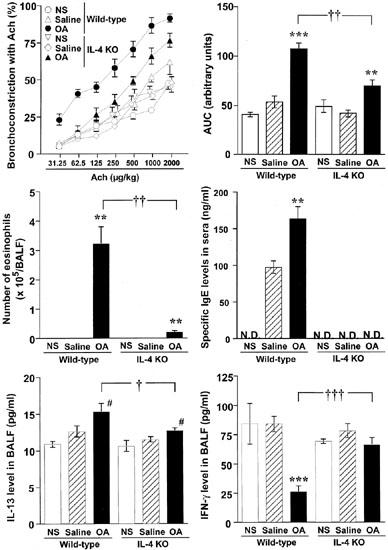
Effect of IL-4 gene deficiency on allergen-induced airway hyperresponsiveness to acetylcholine, the increases in the numbers of eosinophils in bronchoalveolar lavage fluid (BALF), serum antigen-specific IgE level, IL-13 production in BALF and the decrease in interferon-γ production in BALF in BALB/cJ mice. Values represent the mean with standard error of five to six mice in each group. NS, non-sensitized; Saline, saline-exposed; OA, ovalbumin-exposed; AUC, area under the curve for the dose-response to acetylcholine (range: 31.25–2000 μg kg−1); N.D., not detected. **P<0.01, ***P<0.001 (vs saline-inhaled group); #P<0.05 (vs non-sensitized group); ††P<0.01, †††P<0.001 (vs ovalbumin-inhaled wild-type animals).
The effect of IL-4 gene deficiency on allergen-induced airway remodelling
Figure 4 shows the representative sections of each group stained with PAS for detection of goblet cells (Figure 4A–D) and stained with Masson-Trichrome for detection of connective tissue (Figure 4E–H). The quantitative findings of the histological examination, TGF-β1 production and the amount of hydroxyproline in each group are shown in Table 2.
Figure 4.
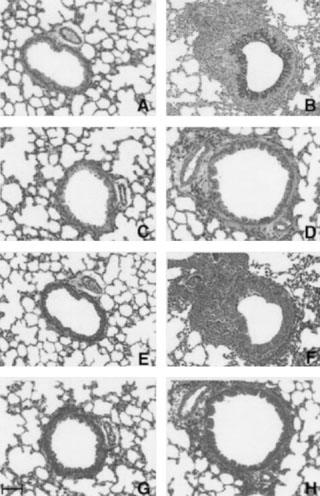
Histological analysis of lung sections stained with periodic acid-Schiff (A–D) and with Masson-trichrome (E–H) 24 h after the final antigen challenge (×50) in IL-4 gene knockout (KO) mice or wild-type (BALB/cJ) mice (×50). (A and E) Saline-exposed wild-type mice; (B and F) ovalbumin-exposed wild-type mice; (C and G) saline-exposed IL-4 KO mice; (D and H) ovalbumin-exposed IL-4 KO mice. Scale bar 100 μm.
Table 2.
Effect of IL-4 gene deficiency on allergen-induced airway remodelling in BALB/cJ mice
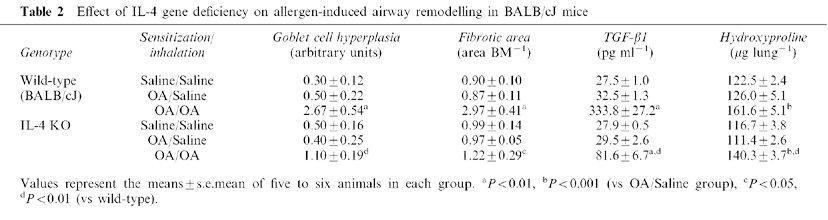
As shown in Figure 4 and Table 2, OA-exposed wild-type showed severe goblet cell hyperplasia compared with saline-exposed wild-type animals (Figure 4A vs B). In contrast, marked decreases in the number of goblet cells lining the epithelium was observed in OA-exposed IL-4 KO mice compared with those of wild-type mice (Figure 4B vs D, and Table 2).
Repeated antigen inhalation in sensitized wild-type mice also caused the increases in TGF-β1 production in BALF and marked subepithelial fibrosis compared with saline-inhaled mice (Table 2 and Figure 4F vs E). In contrast, these fibrotic changes were clearly attenuated in antigen-inhaled IL-4 KO mice compared with those in wild-type mice (Figure 4H vs F). These results were confirmed in Table 2.
Relation between Th1/Th2 balance and airway remodelling
Finally, we examined the relation between Th1/Th2 balance and airway remodelling in each experiment.
The production of IL-13 in BALF was significantly correlated with goblet cell hyperplasia and subepithelial fibrosis in each experiment (r=0.581/0.594, P<0.001 in the experiment using antibodies; r=0.623/0.710, P<0.001 in the experiment using IL-4 KO mice). In contrast, a significant negative correlation between IFN-γ production and airway remodelling was observed only in the experiment using IL-4 KO mice (r=−0.699/−0.659, P<0.001). Furthermore, in each experiment, there were significant correlations between goblet cell hyperplasia and subepithelial fibrosis (r=0.616, P<0.001 in the experiment using antibodies; r=0.892, P<0.001 in the experiment using IL-4 KO mice).
Discussion
In the present study, we investigated the role of Th2 responses in the development of airway remodelling using anti-CD4 mAb and IL-4 KO mice in a murine model of allergic asthma. First, we investigated the role of the T cell subset in the development of airway remodelling using anti-CD4 or anti-CD8 mAb. As a result, the development of airway remodelling was dependent on CD4+ T cells associated with the increase in Th2 cytokine production and the decrease in Th1 cytokine production. Moreover, the airway remodelling was rarely observed in IL-4 KO mice, suggesting that the airway remodelling was developed dependently on augmented Th2 responses. These findings demonstrated that Th2 responses induced by CD4+ T cells play an important role in the development of allergen-induced epithelial change and subepithelial fibrosis in a murine model of allergic asthma.
The treatment of anti-CD4 mAb during allergen challenge completely prevented the increased level of IL-13 in BALF compared with the control Ab-treated group. In addition, antigen-induced IL-13 production in BALF in IL-4 KO mice was significantly lower than that in wild-type mice. These results suggest that IL-13 production is Th2-dependent, at least, in this model. IL-13 was reported to be critical for the increased mucus production and AHR in experimental asthma models (Wills-Karp et al., 1998; Grünig et al., 1998). Furthermore, the lungs of pulmonary IL-13 transgenic mice have mononuclear and eosinophilic inflammatory infiltrates, epithelial cell hypertrophy, eosinophilic crystals, and greatly increased mucus production (Zhu et al., 1999). In the present study, the production was significantly correlated with epithelial changes in both experiments. These findings suggest that IL-13 produced by CD4+ Th2 cells play an important role in the development of antigen-induced goblet cell hyperplasia.
In IL-4 KO mice, the fibrotic responses were almost abrogated compared with those in wild-type mice. Moreover, the level of IFN-γ in BALF and antigen-specific IgG2a in serum were significantly higher, whereas IL-13 production in BALF and antigen specific IgE/IgG1 production were significantly decreased compared with those in wild-type mice. These findings suggest that Th2 responses are suppressed and Th1 responses are potentiated in IL-4 KO mice. IFN-γ is known to have anti-fibrotic activities (Adelmann-Grill et al., 1987; Elias et al., 1987; Ghosh et al., 2001; Ziesche et al., 1999). For example, it can inhibit fibroblast chemotaxis, proliferation, and production of extracellular matrix macromolecules (Adelmann-Grill et al., 1987; Elias et al., 1987; Ghosh et al., 2001). IFN-γ was also shown to attenuate fibrosis in bleomycin-induced pulmonary fibrosis (Ziesche et al., 1999). Moreover, IFN-γ can also directly antagonize TGF-β effects as described below (Hayashi et al., 1997; Ulloa et al., 1999). TGF-β1 is a multifunctional regulatory cytokine, which has a broad range of activities in cell growth and differentiation (Blobe et al., 2000). In addition to these anti-inflammatory regulatory effects, TGF-β1 also plays a central role in the pathogenesis of various fibrotic disorders including pulmonary fibrosis (Border & Nobel, 1994). Recently, TGF-β1 was suggested to play an important role in asthma (Minshall et al., 1997; Vignola et al., 1997). Increased expression of TGF-β1 in BALF and biopsy or specimens obtained from asthmatics, especially patients with severe asthma, has been reported (Minshall et al., 1997). Furthermore, TGF-β1 expression was found to correlate with the degree of subepithelial fibrosis (Vignola et al., 1997). TGF-β signalling is known to transmit from the membrane to nuclei using Smad protein (Heldin et al., 1997). IFN-γ can up-regulate the expression of SMAD7 (Ulloa et al., 1999). SMAD7 is inhibitory Smad and can bind to TGF-β receptor preventing activation of Smad2 and Smad3, which were phosphorylated by the TGF-β receptor and mediate TGF-β effects (Hayashi et al., 1997). Furthermore, Nakao et al. (1999) showed that gene transfer of Smad7 into T lymphocytes prevented TGF-β-mediated lung fibrosis by the blockade of TGF-β signalling. These basic findings suggest that IFN-γ prevents TGF-β mediated lung fibrosis. In contrast to Th1 cytokine, IL-13 has been shown to be associated with and induce tissue fibrosis. This effect was assumed to be due to the direct effects of IL-13 on fibroblasts since IL-13 stimulates fibroblast proliferation and collagen production in vitro (Chiaramonte et al., 1999; Doucet et al., 1998). It was also reported that IL-13 induces lung tissue fibrosis, to a great extent via the TGF-β induction and activation pathway (Lee et al., 2001). Therefore, the persistent imbalance in expression of Th1/Th2 cytokines may explain the mechanism for progression of subepithelial fibrosis in asthma.
Regarding as the role of IL-4 in the development of allergen-induced airway remodelling, Foster et al. (2000) demonstrated that epithelial hypertrophy and subepithelial fibrosis as well as AHR were potentiated in IL-4 KO mice). They suggested the anti-inflammatory effect of IL-4. The discrepancy between their data and our present data may be due to the differences in the experimental protocol. In their model, mice were immunized with OA with alum, and then exposed OA (2.5% w v−1) 3 days per week for 6 weeks, whereas sensitized mice were exposed OA(1% w v−1) every day for 3 consecutive weeks in the present study. Especially, the frequency and concentration of allergen challenge may influence the mechanisms which cells and/or functional molecules are involved in the development of asthma-like responses as reported (Kobayashi et al., 2000), although similar data were observed with the treatment of anti-CD4 mAb in both experiments.
Recently, a clinical trial of a chimeric antibody to CD4 in severe asthma was carried out (Kon et al., 1998). The antibody is shown to have a potential to improve asthmatic symptoms in patients. Although the present model did not demonstrate the complete features of the asthmatic airway remodelling, our present findings demonstrated that the development of airway remodelling including goblet cell hyperplasia and subepithelial fibrosis was dependent on Th2 responses induced by CD4+ T cells, therefore, the inhibition of the function of CD4+ T cells or the effect of Th2 cytokines may have a therapeutic approach to prevent the airway remodelling in asthma.
Acknowledgments
This work was partly supported by Grants-in-Aid for encouragement of young scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and Takeda Science Foundation. The authors thank Mr Daniel Mrozek for skillful assistance in preparation of this manuscript.
Abbreviations
- AHR
airway hyperresponsiveness
- AUC
area under the curve
- BAL
bronchoalveolar
- BALF
BAL fluid
- ELISA
enzyme-linked immunosorbent assay
- KO
gene-knockout
- OA
ovalbumin
- PBS
phosphate-buffered saline
- TGF
transforming growth factor
- Th2
T helper type 2
References
- ADELMANN-GRILL B.C., HEIN R., WACH F., KRIEG T. Inhibition of fibroblast chemotaxis by recombinant human interferon gamma and interferon alpha. J. Cell Physiol. 1987;130:270–275. doi: 10.1002/jcp.1041300213. [DOI] [PubMed] [Google Scholar]
- AIKAWA T., SHIMURA S., SASAKI H., EBINA M., TAKISHIMA T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- BLOBE G.C., SCHIEMANN W.P., LODISH H.F. Mechanisms of Disease: Role of Transforming Growth Factor β in Human Disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- BORDER W.A., NOBEL N.A. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1291. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- BROWN P.J., GREVILLE H.W., FINUCANE K.E. Asthma and irreversible airflow obstruction. Thorax. 1984;39:131–136. doi: 10.1136/thx.39.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIARAMONTE M.G., DONALDSON D.D., CHEEVER A.W., WYNN T.A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIES J.E. The role of IL-13 and its receptor in allergy and inflammatory responses. J. Allergy Clin. Immunol. 1998;102:165–169. doi: 10.1016/s0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- DJUKANOVIC R., ROCHE W.R., WILSON J.W., BEASLY C.R., TWENTYMAN O., HOLGATE S.T. The role of mucosal inflammation in asthma. Am. Rev. Respir. Dis. 1990;142:434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- DOUCET C., BROUTY-BOYE D., POTTIN-CLEMENCEAU C., JASMIN C., CANONICA G.W., AZZARONE B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J. Clin. Invest. 1998;101:2129–2139. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELIAS J.A., JIMENEZ S.A., FREUNDLICH B. Recombinant gamma, alpha, and beta interferon regulation of human lung fibroblast proliferation. Am. Rev. Respir. Dis. 1987;135:62–65. doi: 10.1164/arrd.1987.135.1.62. [DOI] [PubMed] [Google Scholar]
- FINKELMAN F.D., KATONA I.M., URBAN J., HOLMERS J.F., OHARA J., TUNG A.S., SAMPLE J.V., PAUL W.E. IL-4 is required to generate and sustain in vivo IgE responses. J. Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- FOSTER P.S., HOGAN S.P., RAMSAY A.J., MATTHAEI K.I., YOUNG I.G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER P.S., MING Y., MATTHEI K.I., YOUNG I.G., TEMELKOVSKI J., KUMAR R.K. Dissociation of inflammatory and epithelial responses in a murine model of chronic asthma. Lab. Invest. 2000;80:655–662. doi: 10.1038/labinvest.3780068. [DOI] [PubMed] [Google Scholar]
- FOSTER P.S., YANG M., HERBERT C., KUMAR R.K. CD4+ T-lymphocytes regulate airway remodeling and hyper-reactivity in a mouse model of chronic asthma. Lab. Invest. 2002; 82:455–462. doi: 10.1038/labinvest.3780438. [DOI] [PubMed] [Google Scholar]
- GAVETT S.H., CHEN X., FINKELMAN F., WILLS-KARP M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am. J. Respir. Cell Mol. Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- GHOSH A.K., YUAN W., MORI Y., CHEN S.J., VARGA J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta: integration at the level of p300/CBP transcriptional coactivators. J. Biol. Chem. 2001;276:11041–11048. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- GRÜNIG G., WARNOCK M., WAKIL A.E., VENKAYYA R., BROMBACHER F., RENNICK D.M., SHEPPARD D., MOHRS M., DONALDSON D.D., LOCKSLEY R.M., CORRY D.B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI H., ABDOLLAH S., QIU Y., CAI J., XU Y.Y., GRINNELL B.W., RICHARDSON M.A., TOPPER J.N., GIMBRONE M.A., JR, WRANA J.L., FALB D. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- HEARD B.E., HOSSAIN S. Hyperplasia of bronchial muscle in asthma. J. Pathol. 1973;110:319–331. doi: 10.1002/path.1711010212. [DOI] [PubMed] [Google Scholar]
- HELDIN C.H., MIYAZONO K., TEN DIJKE, P. TGF-β signalling from the membrane to the nucleus through SMAD proteins. Nature. 1997;390:465–473. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- JEFFERY P.K., GODFREY R.W., ÄDELROTH E., NELSON F., ROGERS A., JOHANSSON S.A. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma: a quantitative light and electron microscopy study. Am. Rev. Respir. Dis. 1992;145:890–899. doi: 10.1164/ajrccm/145.4_Pt_1.890. [DOI] [PubMed] [Google Scholar]
- KIVIRIKKO K.I., LAITINEN O., PROCKOP D.J. Modifications of a specific assay for hydroxyproline in murine. Annal. Biochem. 1967;19:249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., MIURA T., HABA T., SATO M., SERIZAWA I., NAGAI H., ISHIZAKA K. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J. Immunol. 2000;164:3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- KON O.M., SIHRA B.S., COMPTON C.H., LEONARD T.B., KAY A.B., BARNES N.C. Randomised, dose-ranging, placebo-controlled study of chimeric antibody to CD4 (keliximab) in chronic severe asthma. Lancet. 1998;352:1109–1113. doi: 10.1016/S0140-6736(97)12261-9. [DOI] [PubMed] [Google Scholar]
- KOPF M., LE GROS G., BACHMANN M., LAMERS M.C., BLUETHMANN H., KOHLER G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- LEE C.G., HOMER R.J., ZHU Z., LANONE S., WANG X., KOTELIANSKY V., SHIPLEY J.M., GOTWALS P., NOBLE P., CHEN Q., SENIOR R.M., ELIAS J.A. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J. Exp. Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ A.F., SANDERSON C.J., GAMBLE J.R., CAMPBELL H.D., YOUNG I.G., VADAS M.A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J. Exp. Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKENZIE A.N., CULPEPPER J.A., DE WAAL MALEFYT R., BRIERE F., PUNNONEN J., AVERSA G., SATO A., DANG W., COCKS B.G., MENON S., DE VRIES J.E., BANCHEREAU J., ZURAWSKI G. Interleukin 13, a T-Cell-Derived Cytokine that Regulates Human Monocyte and B-Cell Function. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINSHALL E.M., LEUNG D.Y.M., MARTIN R.J., SONG Y.L., CAMERON L., ERNST P., HAMID Q. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am. J. Respir. Cell Mol. Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- NAGAI H., MAEDA Y., TANAKA H. The effect of anti-IL-4 monoclonal antibody, rapamycin and interferon-γ on airway hyperreactivity to acetylcholine in mice. Clin. Exp. Allergy. 1997;27:218–224. [PubMed] [Google Scholar]
- NAKAJIMA H., IWAMOTO I., TOMOE S., MATSUMURA F., TOMIOKA H., TAKATSU K., YOSHIDA S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am. Rev. Respir. Dis. 1992;146:374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- NAKAO A., FUJII M., MATSUMURA R., KUMANO K., SAITO Y., MIYAZONO K., IWAMOTO I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Invest. 1999;94:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBEN-TRAUTH N., KROPF P., MULLER I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:912–913. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- PADRID P., SNOOK S., FINUCANE T., SHIUE P., COZZI P., SLOWAY J., LEFF A.R. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am. J. Respir. Crit. Care Med. 1995;151:184–193. doi: 10.1164/ajrccm.151.1.7812551. [DOI] [PubMed] [Google Scholar]
- ROBINSON D.S. Th-2 cytokines in allergic disease. Br. Med. Bull. 2000;56:956–968. doi: 10.1258/0007142001903625. [DOI] [PubMed] [Google Scholar]
- ROCHE W.R., WILLIAMS J.H., BEASLY R., HOLGATE S.T. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- TANAKA H., MASUDA T., TOKUOKA S., KOMAI M., NAGAO K., TAKAHASHI Y., NAGAI H. The effect of allergen-induced airway inflammation on airway remodeling in murine model of allergic asthma. Inflamm. Res. 2001;50:616–624. doi: 10.1007/PL00000243. [DOI] [PubMed] [Google Scholar]
- TEMANN U.A., PRASAD B., GALLUP M.W., BASBAUM C., HO S.B., FLAVELL R.A., RANKIN J.A. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 1997;16:471–478. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- ULLOA L., DOODY J., MASSAGUE J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- VIGNOLA A.M., CHANEZ P., CHIAPPARA G., MERENDINO C., PACE E., RIZZO A., ROCCA A.M., BELLIA V., BONSIGNORE G., BOUSQUEST J. Transforming growth factor-β expression in mucosal biopsies in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- WILLS-KARP M., LUYIMBAZI J., XU X., SCHOFIELD B., NEBEN T.Y., KARP C.L., DONALDSON D.D. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- WILSON J.W. What causes airway remodeling in asthma. Clin. Exp. Allergy. 1998;28:534–536. doi: 10.1046/j.1365-2222.1998.00298.x. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI Y., SUDA T., SUDA J., EGUCHI M., MIURA Y., HARADA N., TOMINAGA A., TAKATSU K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J. Exp. Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIESCHE R., HOFBAUER E., WITTMANN K., PETKOV V., BLOCK L.H. A preliminary study of long-term treatment with interferon gamma-1β and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 1999;341:1264–1269. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]
- ZHU Z., HOMER R.J., WANG Z., CHEN Q., GEBA G.P., WANG J., ZHANG Y., ELIAS J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUHDI ALIMAM M., PIAZZA F.M., SELBY D.M., LETWIN N., HUANG L., ROSE M.C. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am. J. Respir. Cell Mol. Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]


