Abstract
The involvement of Rho-kinase (ROCK) in the contractile mechanisms mediating smooth muscle contraction of the rat urinary bladder was investigated using expression studies and the ROCK inhibitor Y-27632.
Both isoforms of ROCK (ROCK I and ROCK II) were detected in high levels in rat urinary bladder.
Y-27632 (10 μM) significantly attenuated contractions of rat urinary bladder strips evoked by the G-protein coupled receptor agonists carbachol (58.1±10.5% at 0.3 μM) and neurokinin A (68.6±12.7% at 1 μM) without affecting contractions to potassium chloride (10–100 mM). In addition, basal tone was reduced by 47.8±2.0% by 10 μM Y-27632 in the absence of stimulation.
Contractions of urinary bladder strips evoked by the P2X receptor agonist α,β-methylene ATP (α,β-mATP; 10 μM) were also attenuated by Y-27632 (30.0±7.2% at 10 μM).
Y-27632 (10 μM) significantly attenuated contractions evoked by electrical field stimulation (2–16 Hz). The effect of Y-27632 on the tonic portion of the neurogenic response (4–16 Hz) was not significantly different from the effect of atropine (1 μM) alone.
While the mechanism underlying the ability of Y-27632 to inhibit α,β-mATP-evoked contractions remains undetermined, the results of the present study clearly demonstrate a role for ROCK in the regulation of rat urinary bladder smooth muscle contraction and tone.
Keywords: Urinary bladder, Rho-kinase, G-protein coupled receptors, smooth muscle, Ca2+-sensitization, signal transduction
Introduction
It is widely accepted that the key signal to activate the contractile apparatus in smooth muscle is an increase in the intracellular calcium concentration ([Ca2+]i). The rise in intracellular Ca2+ promotes binding to calmodulin (CaM), which in turn activates the phosphorylation of myosin light chain (MLC) by myosin light chain kinase (MLCK), resulting in smooth muscle contraction (for review see Horowitz et al., 1996). Recently, however secondary mechanisms have been identified that can modulate smooth muscle contractility independently of Ca2+. Activation of excitatory G-protein coupled receptors can cause contraction of smooth muscle without necessarily changing [Ca2+]i, a process termed ‘Ca2+-sensitization'. The small GTPase Rho and one of its downstream effectors, Rho-associated kinase (Rho-kinase; ROCK) have been shown to play important roles in this process. Activated ROCK phosphorylates, and thus inactivates, smooth muscle myosin phosphatase, preventing the dephosphorylation of MLC, which leads to Ca2+-sensitization of the smooth muscle (for review see Somlyo & Somlyo, 2000).
Y-27632, a specific inhibitor of ROCK (Uehata et al., 1997; Davies et al., 2000; Ishizaki et al., 2000) has been used to demonstrate a role for this enzyme in Ca2+-independent regulation of contraction in a number of tissues including vascular (Uehata et al., 1997), airway (Iizuka et al., 2000; Yamagata et al., 2000) and genital (Chitaley et al., 2001; Rees et al., 2001) smooth muscles. Recently, Jezior et al. (2001) reported that bethanechol-evoked contractions of rabbit isolated urinary bladder smooth muscle are inhibited in the presence of Y-27632. The study described in this report was designed to expand upon these findings by investigating the effects of Y-27632 on rat urinary bladder contractions evoked through both G-protein coupled and non-G-protein coupled mechanisms. In addition, expression studies confirmed the presence of ROCK in the rat urinary bladder.
Methods
Expression studies
Male Sprague–Dawley rats (350–600 g; Charles River, U.S.A.) were anaesthetized with isoflurane (4% in 100% oxygen) and sacrificed by cervical dislocation. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23). The Institutional Animal Care and Use Committee of GlaxoSmithKline approved procedures using laboratory animals. The following tissues were exposed and removed: urinary bladder, aorta, heart, liver, kidney, brain and skeletal muscle (diaphragm).
Western blotting
Western blotting was performed as described previously (Sambrook et al., 1989). Briefly, 1–2 g of frozen tissue was homogenized in 15 ml lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP40) containing protease inhibitors. The homogenate was centrifuged at 10,000×g for 15 min and the supernatant taken. The total protein concentration of each supernatant was measured using Bio-Rad Dc protein assay reagents (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Each sample containing 100 ug of total protein was taken and mixed with 5× sodium dodecyl sulphate (SDS) sample buffer and phosphate buffered saline. After boiling for 10 min, samples were loaded onto Invitrogen NuPAGE 4–12% Bis-Tris Gel (Invitrogen, Carlsbad, CA, U.S.A.). The protein was separated by electrophoresis in a Novex gel box and blotted onto Invitrogen PVDF membranes. Membranes were blocked for 2 h with 5% dry milk in TBST buffer (20 mM Tris pH 7.5, 0.5 M NaCl, 0.1% Tween 20) and then incubated with a primary monoclonal antibody against ROCK I (1 : 50 dilution) or ROCKII (1 : 200 dilution; R54520, BD Transduction Laboratories, San Diego, CA, U.S.A.) at 4°C overnight. For normalization of the protein loading, a monoclonal antibody against GAPDH (RD1-TRK5G4-6C5, Research Diagonostics Inc., Flanders, NJ, U.S.A.) was used in 1 : 5000 dilution in the same manner. After washing the membranes three times with TBST, the membranes were incubated for 1 h with 1 : 5000 HRP linked anti-mouse Ig in TBST containing 5% dry milk. Following three times washes with TBST, the blots were developed with Western Lightning-Chemiluminescence Reagents Plus (NEL104, NEL105, PerkinElmer Life Sciences, Boston, MA, U.S.A.) and exposed on Kodak X-OMAT AR imaging films.
RT–PCR
Isolation of total RNA from rat tissues was performed as described previously (Sambrook et al., 1989). Real-time quantitative polymerase chain reaction (RT–PCR) analysis (Heid et al., 1996) was used to determine the relative levels of ROCK I and ROCK II mRNA in rat tissues. 1–2 μg of total RNA from each sample was treated with RQ Dnase 1 (Promega Biotechnology, Madison, U.S.A.) prior to reverse transcription. The resulting cDNA was diluted to 100 μl and 5 μl was loaded to each PCR reaction. Reverse transcription and PCR reactions were performed according to the manufacturer's instructions (Applied Biosystems, Foster City, U.S.A.). ROCK I or ROCK II sequence-specific amplification was detected with an increasing fluorescent signal of FAM reporter dye during the amplification cycle. A rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer and probe set obtained from Applied Biosystems was used to amplify the mRNA level of GAPDH. This amplification was included in the same reaction on all samples tested as an internal control of variations in RNA amounts. Each sequence specific amplification was quadruplicated. Levels of the different mRNAs were subsequently normalized to GAPDH mRNA levels, and the relative mRNA level of each tissue was then compared to that of heart tissue (whilst arbitrarily setting the mRNA heart level as 1.0). Oligonucleotide primers and Taqman probes were designed using Primer Express software (Applied Biosystems) and were synthesized by Applied Biosystems. Sequences of forward primers, reverse primers, and probes were:
ROCK I
forward primer–AGGCCTGTGCCAAACCTTT
reverse primer–TGGTCCCTGTGGGACTTAACA
Taqman probe–CCGCCTGCCCTAGAGTGTCGAAGA
ROCK II
forward primer–CCCGATCATCCCCTAGAACC
reverse primer–TTGGAGCAAGCTGTCGACTG
Taqman probe–CAACAAAACCAGTCCATTCGGCGGC
Contraction studies
Rat urinary bladder was obtained as described above and bisected longitudinally. Bladder strips were mounted in 15 ml vertical tissue baths, aerated with 95% O2 and 5% CO2, and bathed in a physiological salt solution of the following composition (mM): NaCl 118; KCl 4.7; NAHCO3 25; KH2PO4 1.2; MgSO4 0.58; CaCl2 2.5 and glucose 11. The tissues were equilibrated for 1 h under 1 g resting tension and maintained at 37°C. In experiments using carbachol, neurokinin A (NKA) or phorbol 12,13 dibutyrate (PDBu) as agonists, control responses to 100 mM potassium chloride (KCl) were obtained and the tissues washed every 15 min for 1 h. Antagonist or vehicle was then incubated for a period of 45 min.
Cumulative concentration-response curves to carbachol, NKA or PDBu were then constructed. In experiments using KCl as the contractile test substance, tissues were first incubated with Y-27632 or vehicle for 45 min before cumulative doses of KCl were added. In experiments investigating the effects of Y-27632 on α,β-methylene ATP (α,β-mATP)-evoked responses, an initial response was obtained to α,β-mATP, followed by a 60 min washout period (to avoid desensitization of responses). Tissues were then incubated with Y-27632, HA-1077 or vehicle for 45 min and re-challenged with α,β-mATP. In experiments evaluating the effect of various compounds on electrical field stimulation (EFS), tissues were stimulated through ring electrodes using a S88 Square Pulse Stimulator connected to a SIU5 RF Isolation Unit (both from Astro-Med Inc., U.S.A.). Frequency-response curves were carried out using the following parameters: 150 volts, 0.5 ms pulse width for 30 s, at frequencies of 2, 4, 8 and 16 Hz. Tissues were stimulated at 15 min intervals and washed between each stimulation. Test compounds or vehicle was added, equilibrated for 45 min and second frequency-response curves were constructed. Test compounds or vehicles were re-added following washing between each stimulation during construction of second frequency-response curves. In experiments using α,β-mATP as a desensitizing agonist of P2X receptors, following construction of control frequency-response curves, 10 μM α,β-mATP was added to the bath and incubated for 10 min. To confirm desensitization, the tissues were then re-challenged with 10 μM α,β-mATP (which was left in the bath throughout the subsequent 45 min incubation with Y-27632 or vehicle). Second frequency-response curves were then performed as described above.
Data capture and analysis
Changes in bladder tension were recorded using TSD125C 50 g isometric force transducers connected to DA 100B General Purpose Transducer Amplifiers connected to a PC based MP100 System and analysed off-line using AcqKnowledge version 3.5.7 software (all from Biopac Systems Inc., U.S.A.). The amplitude (g) of bladder contractions evoked by application of agonists were measured. For concentration-response curves this measurement was made after plateau to each concentration of agonist had been reached. Changes in bladder tensions evoked by agonists were expressed as percentages of the contractile response to 100 mM KCl in each tissue (where the contractile test substance used was KCl, 100 mM was also the maximal response and concentration used in concentration-response curves) and were compared with vehicle controls by the Mann-Whitney Test followed by the Bonferroni test. Changes in bladder tension evoked by α,β-methylene ATP were expressed as percentage changes before and after the administration of test substances and compared with vehicle controls by Student's unpaired t-test. Both amplitude and area under the curve (AUC) of responses to EFS were measured. Baseline bladder tensions were measured 2 min before administration of test substances, and changes in resting bladder tensions from baseline caused by test substances were measured at 15 min intervals over the 45 min incubation period. Changes in baseline bladder tensions and bladder contractile responses to EFS were expressed as percentage changes before and after the administration of test compounds, and compared with vehicle controls by the Mann–Whitney Test followed by the Bonferroni test. P values <0.05 were considered significant. All values are mean±s.e.mean and n numbers refer to the number of bladder strips examined.
Drugs and solutions
Drugs and chemicals were obtained from the following sources: Y-27632 ((+)-(R)-trans-4-(1-amino-ethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride monohydrate), fasudil (hydrochloride; HA-1077) and GF 109203X (bisindolylmaleimide) from Tocris Cookson Ltd, U.S.A.; carbamylcholine chloride (carbachol), Neurokinin A, potassium chloride, α,β-methylene ATP, atropine (sulphate), tetrodotoxin, MEN 10,367, phorbol 12,13-dibutyrate (PDBu) and dimethyl sulphoxide (DMSO) from Sigma Aldrich Chemicals, U.S.A. All drugs were dissolved in distilled water, except GF 109203X, PDBu and MEN 10,367 that were dissolved in DMSO. Test substances were added to baths in a maximum of 150 μl volume and the concentrations reported are final bath concentrations. The final concentration of DMSO in the bath was always ⩽0.01%. Administration of vehicles had no effect on the responses under study.
Results
Expression of ROCK
Figure 1 demonstrates by Western blotting with antibodies to ROCK I and ROCK II, that both isoenzymes are expressed in rat urinary bladder smooth muscle. The calculated molecular weights were as expected, approximately 160 kDa (Ishizaki et al., 1996). ROCK I and II were also detected in rat aorta, brain and heart. Expression of both ROCK I and ROCK II was low in rat liver, kidney and skeletal muscle. The results of RT–PCR experiments were consistent with Western blotting studies and confirmed the presence of high levels of both ROCK I and ROCK II mRNA in rat urinary bladder (Figure 2). ROCK I expression was highest in rat aorta, followed by significant expression in the urinary bladder. Conversely, the expression level of ROCK II detected in the urinary bladder was approximately twice as high as that detected in the aorta. Expression of both ROCK I and ROCK II was comparatively much lower in rat brain, liver, kidney and skeletal muscle than in urinary bladder and aorta.
Figure 1.
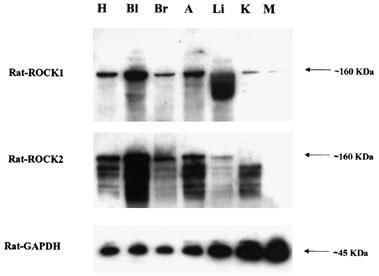
Expression of ROCK I and ROCK II in rat tissues from Western blotting experiments. Tissue homogenates were subjected to SDS–PAGE and immunoblotted with anti-ROCK I, II or GADPH. Each lane represents results from different rat tissues: heart (H), bladder (Bl), brain (Br), aorta (A), liver (Li), kidney (K) and skeletal muscle (diaphragm; M). Results are representative of tissue samples from five animals.
Figure 2.
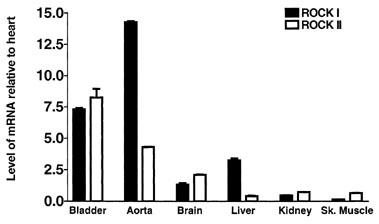
Expression of ROCK I and ROCK II in rat tissues from RT–PCR experiments. ROCK I or ROCK II specific message was normalized to GAPDH specific message and the relative amount of mRNA of each sample was compared to that of heart while arbitrarily setting heart mRNA level as 1.0 unit. Each bar represents the mean value of four tissue samples from separate animals and vertical bars show s.e.mean.
Effect of test substances on baseline bladder tension
Y-27632 (10 μM) caused a significant decrease (47.8±2.0%) in baseline bladder tension over a 45 min incubation period (Figure 3; n=28). HA-1077 (10 μM) evoked a similar decrease in baseline bladder tension, causing a 37.54±1.0% decrease after 45 min (Figure 3; n=9). The effects of Y-27632 and HA-1077 on baseline bladder tension were not significantly different. In addition, 10 μM GF 109203X evoked a decrease in bladder tension of 38.6±5.9% following 45 min incubation (Figure 3; n=5). This was not significantly different from the effects of Y-27632 and HA-1077 on baseline bladder tension. MEN 10,367 (10 μM) evoked an increase in baseline bladder tension of 176.2±52.3%, that returned to pre-drug levels within approximately 15 min (n=4). All other test substances used in the present study had no effect on baseline bladder tensions (not shown).
Figure 3.
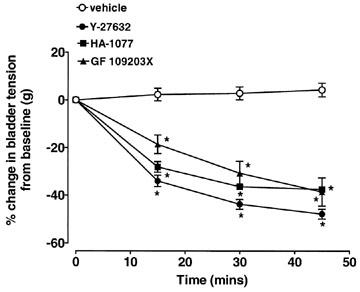
Effects of 10 μM Y-27632, 10 μM HA-1077 and 10 mM GF 109203X on baseline bladder tension in rat urinary bladder strips. Data is expressed as a percentage of pre-treatment tension. Each point represents the mean value and vertical bars show s.e.mean of five experiments. *P<0.05 compared to vehicle.
Effect of Y-27632 on carbachol- and NKA-evoked bladder contractions
Cumulative additions of carbachol (1 nM–10 μM) evoked sustained concentration-dependent contractions of rat urinary bladder strips (Figure 4). The maximal response to carbachol in vehicle experiments was 5.3±0.9 g at 10 μM, which was 105.0±28.2% of the response to 100 mM KCl (Figure 4; n=6). The carbachol EC50 value was 183.5±68.9 nM. Pretreatment with 10 μM Y-27632 significantly attenuated carbachol-evoked contractions at concentrations of carbachol between 1–300 nM, with an inhibition of 58.1±10.5% at 300 nM (Figure 4; n=4). At higher carbachol concentrations (1–100 μM), contractions were attenuated by Y-27632, however the inhibition was not statistically significant. The carbachol EC50 value in the presence of 10 μM Y-27632 was 1034.3±336.1 nM, with a calculated agonist dose ratio (DR) of 5.63. (1 and 3 μM Y-27632 had no effect on carbachol-evoked contractions and therefore 10 μM Y-27632 was used in subsequent experiments).
Figure 4.
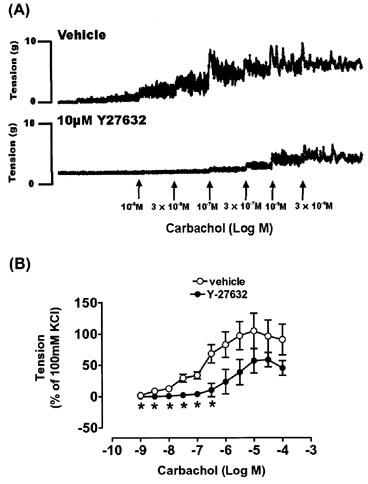
Effect of 10 μM Y-27632 on carbachol-evoked contractions of rat urinary bladder strips. Typical traces are shown in (A), while summary data (n=4–6 for each point) is shown in (B). Responses are expressed as a percentage of the response evoked by 100 m MCl. Vertical bars indicate s.e.mean. *P<0.05 compared to vehicle.
Cumulative additions of NKA (1 nM–1 μM) evoked sustained concentration-dependent contractions of rat urinary bladder strips (Figure 5). The maximal response to NKA in vehicle experiments was 4.2±0.4 g at 300 nM NKA, which was 136.2±24.8% of the response to 100 mM KCl (Figure 5; n=4). The EC50 value for NKA was 6.5±3.9 nM. Pretreatment with 10 μM Y-27632 significantly attenuated NKA-induced contractions at all NKA concentrations with an inhibition of 68.6±12.7% at 1 μM NKA (Figure 5; n=5). The EC50 value for NKA in the presence of Y-27632 was 32.4±9.4 nM, with a calculated agonist dose ratio (DR) of 4.98.
Figure 5.

Effect of 10 μM Y-27632 on NKA-evoked contractions of rat urinary bladder strips. Responses are expressed as a percentage of the response evoked by 100 mM KCl (n=4–5 for each point). Vertical bars indicate s.e.mean. *P<0.05 compared to vehicle.
Effect of Y-27632 on KCl-evoked bladder contractions
Cumulative additions of KCl (1–100 mM) induced sustained concentration-dependent contractions of rat urinary bladder strips at concentrations of 10 mM and above (Figure 6). The maximal response to KCl in vehicle experiments was 8.7±0.5 g at 100 mM KCl (n=5), which was unchanged in the presence of 10 μM Y-27632 (8.3±0.4; n=5). The EC50 value for KCl was 31.0±4.3 mM, which was unchanged in the presence of 10 μM Y-27632 (32.4±9.4 mM; Figure 6; n=5).
Figure 6.

Effect of 10 μM Y-27632 on KCl-evoked contractions of rat urinary bladder strips. Responses are expressed as a percentage of the response evoked by 100 mM KCl (n=5 for each point). Vertical bars indicate s.e.mean.
Effects of Y-27632 and HA-1077 on α,β-mATP-evoked bladder contractions
Addition of 10 μM α,β-mATP-evoked contractile responses of rat urinary bladder strips with a mean amplitude of 3.9±0.5 g (Figure 7A; n=14). Re-challenge of tissues with α,β-mATP following incubation with vehicle evoked a similar contraction with an amplitude of 3.7±0.7 g (n=5). Y-27632 (10 μM) significantly attenuated the amplitude of α,β-mATP-evoked bladder contractions by 30.0±7.2% (Figure 7A,B; n=4). In an effort to confirm that this unexpected effect was due to ROCK inhibition, another ROCK inhibitor, HA-1077 (fasudil hydrochloride; Davies et al., 2000), was tested. HA-1077 (10 μM) also significantly attenuated α,β-mATP-evoked bladder contractions by 22.1±5.7% (Figure 7A,B; n=5). The degree of inhibition produced by HA-1077 was similar in magnitude to that of Y-27632.
Figure 7.

Effects of Y-27632 and HA-1077 on α,β-mATP evoked contractions of rat urinary bladder strips. (A) Typical traces of responses to 10 μM α,β-mATP in the absence and presence of 10 μM Y-27632 or 10 μM HA-1077. Shaded circles indicate addition of α,β-mATP to the tissue strips. Summary data (n=4–6 for each) is displayed in (B). Vertical bars indicate s.e.mean. *P<0.05 compared to vehicle.
Effect of Y-27632, HA-1077 and GF 109203X on PDBu-evoked bladder contractions
Cumulative additions of PDBu, a protein kinase C (PKC) activator (3 nM–10 μM; for review see Nambi et al., 1996), evoked sustained concentration-dependent contractions of rat urinary bladder strips (Figure 8). The maximal response to PDBu in vehicle experiments was 1.7±0.6 g at 300 nM, which was 37.1±7.3% of the response to 100 mM KCl (Figure 8; n=6). The PDBu EC50 value was 180±25 nM, which was unchanged following pretreatment with 10 μM Y-27632 (151±80 nM; Figure 8; n=5) or 10 μM HA-1077 (175±70 nM; Figure 8; n=4). The selective PKC antagonist, GF109203X (Toullec et al., 1991; 10 μM), significantly attenuated PDBu-evoked contractions at concentrations of PDBu between 0.3 and 10 μM, with an inhibition of 33.4±7% at 10 μM (Figure 8; n=4). The PDBu EC50 value in the presence of 10 μM GF-109203X was 1189±82 nM, with a calculated agonist DR of 6.61.
Figure 8.
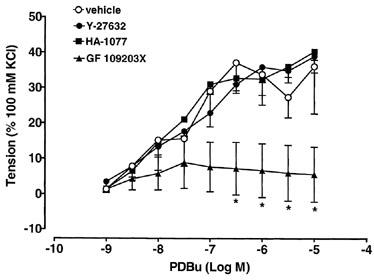
Effects of 10 μM Y-27632, 10 μM HA-1077 and 10 μM GF 109203X on PDBu-evoked contractions of rat urinary bladder strips. Responses are expressed as a percentage of the response evoked by 100 mM KCl (n=4–6 for each point). Vertical bars indicate s.e.mean. *P<0.05 compared to vehicle.
Effect of TTX, Y-27632, atropine, α,β-mATP desensitization and MEN 10,376 on electrically-evoked bladder contractions
Contractions of rat urinary bladder strips induced by EFS at 2, 4, 8 and 16 Hz were frequency-dependent and consisted of an initial phasic contraction (measured by amplitude) followed by a more sustained tonic contraction (measured by AUC). The mean amplitude values of bladder contractions evoked by 2, 4, 8 and 16 Hz were 1.7±0.3 g, 2.5±0.4 g, 3.3±0.5 g and 4.0±0.6 g respectively. The mean AUC values for these contractions were 16.6±5.7, 22.1±8.1, 27.3±10.8 and 29.9±12.8 at 2, 4, 8 and 16 Hz respectively (n=25). TTX (1 μM) significantly attenuated both the amplitude and AUC of EFS-evoked bladder contractions at all frequencies of stimulation (73.2±4.1% and 73.4±1.2%, respectively, averaged across the four frequencies; Figure 9; n=4).
Figure 9.

Effects of 10 μM Y-27632, 1 μM TTX, 10 μM atropine, desensitization of P2X receptors with 10 μM α,β-mATP and 10 μM MEN 10,367 on electrically-evoked contractions of rat urinary bladder strips. Data are expressed as percentage changes from control responses in the amplitude (A) and area under the curve (B) of EFS-evoked contractions at 2 (a), 4 (b), 8 (c) and 16 (d) Hz stimulation, following incubation with vehicle or antagonists. Each bar represents the mean value of 4–5 experiments and vertical bars show s.e.mean. *P<0.05 compared to vehicle.
To characterize the effects of Y-27632 on electrically-evoked bladder contractions, experiments were carried out to compare the effects of Y-27632 with muscarinic acetylcholine receptor blockade with atropine, NK2 receptor blockade with MEN 10,376 (Maggi et al., 1991a, b; 1992) or P2X receptor desensitization with α,β-mATP.
Y-27632 (10 μM) significantly attenuated the amplitude of EFS-evoked bladder contractions at all frequencies of stimulation (41.0±3.4% averaged across the four frequencies; Figure 9A) and significantly attenuated the tonic portion of the response at 4, 8 and 16 Hz stimulation (Figure 9B; n=4). Atropine (1 μM) also significantly attenuated the tonic portion of EFS-evoked bladder contractions at 4, 8 and 16 Hz stimulation (Figure 9B), but had no effect on the amplitude of these responses (Figure 9A; n=5). The attenuating effects of Y-27632 and atropine on the tonic portion of the bladder contractile response to EFS at 4, 8 and 16 Hz stimulation were not significantly different. Y-27632 and atropine also attenuated the tonic portion of the response at 2 Hz, although this was not statistically significant (Figure 9B).
Desensitization of P2X receptors with α,β-mATP (see Methods) did not significantly affect the AUC of EFS-evoked bladder contractions at all frequencies of stimulation (Figure 9B; n=4). However, phasic responses to EFS at 8 and 16 Hz stimulation were significantly attenuated by 24.7±10.0% and 21.1±13.1%, respectively (Figure 9A; n= 4). The attenuating effects of Y-27632 and α,β-mATP on the phasic portion of the bladder contractile response to electrical stimulation at 8 and 16 Hz were not significantly different (Figure 9A).
MEN 10,367 (10 μM) did not have a significant effect on either the phasic or tonic components of EFS-evoked contractile responses of the rat urinary bladder (Figure 9A,B; n=4).
Discussion
The results of the present study indicate that ROCK plays an important role in the regulation of rat urinary bladder smooth muscle contraction and tone. ROCK I and ROCK II are highly expressed and the selective ROCK inhibitor Y-27632 attenuates contractions evoked by G-protein coupled receptor agonists in rat urinary bladder smooth muscle. Contractions induced by carbachol and NKA have previously been shown to be mediated by the G-protein coupled muscarinic M3 (Longhurst et al., 1995; Tong et al., 1997) and tachykinin NK2 receptors (Hall et al., 1992) respectively (for reviews see Eglen et al., 1994; Khawaja & Rogers, 1996). The current results demonstrate that responses of urinary bladder strips to both agonists are inhibited by approximately 50% in the presence of 10 μM Y-27632. In addition to affecting contractions induced by exogenous agonists, the ROCK inhibitors Y-27632 and HA-1077 cause a significant decrease in bladder tension in the absence of stimulation, which could indicate a role for ROCK in the maintenance of urinary bladder tone.
ROCK is a serine/threonine protein kinase (Matsui et al., 1996) with two isoforms, namely ROCK I (p160ROCK/ROKβ; Ishizaki et al., 1996) and ROCK II (ROKα; Leung et al., 1995). ROCK I has 64% sequence identity with ROCK II and the two isoforms share 90% identity in the kinase domain (for review see Amano et al., 2000). In the present study, expression of both ROCK I and ROCK II were detected in rat urinary bladder using both Western blotting and RT–PCR techniques. Furthermore, RT–PCR experiments demonstrated that the level of both isoenzymes was higher than those detected in all other rat tissues tested (aside from ROCK I in aorta), including brain, liver, kidney and skeletal muscle. This is the first time expression of ROCK has been demonstrated in the urinary bladder and provides support of a role for these isoenzymes in the contractile mechanisms of this tissue.
Y-27632 is a highly selective inhibitor of both isoforms of ROCK with reported Ki values of 0.14 μM (Uehata et al., 1997) and 0.22 μM (Ishizaki et al., 2000) for ROCK I and 0.30 μM for ROCK II (Ishizaki et al., 2000). Furthermore, Y-27632 is reportedly 200–300 (Uehata et al., 1997; Ishizaki et al., 2000) and 1250 fold (Uehata et al., 1997) more selective for ROCK isoforms than protein kinase C (PKC) and MLCK respectively. Davies et al. (2000) have shown that Y-27632 has a minimal effect on a large number of other serine/threonine protein kinases. The concentration of Y-27632 that was used in the present study (10 μM) should inhibit both ROCK I and ROCK II on the basis of previously published literature (Uehata et al., 1997; Ishizaki et al., 2000), whilst having no effect on other kinases relevant to smooth muscle contractility, including MLCK and PKC. However, a recent study by Eto et al. (2001) reported that 10 μM Y-27632 inhibited contractions of isolated permeabilized rabbit femoral artery induced by stimulation with the PCK activator PDBu (for review see Nambi et al., 1996). Therefore in an attempt to confirm the selectivity of Y-27632 for ROCK versus PKC in the present study, the effects of this compound were examined on PDBu-evoked contractile responses of isolated rat urinary bladder. Ten μM Y-27632 had no effect on PDBu-evoked contractile responses, which were inhibited by the selective PKC antagonist GF109203X (Toullec et al., 1991). Therefore, the attenuation of carbachol- and NKA-evoked bladder contractions following Y-27632 treatment is most likely due to an inhibition of ROCK rather than an effect on PKC in the urinary bladder. Y-27632 has also been shown to attenuate G-protein mediated contractile responses in other tissues including airway, gastrointestinal and bronchial smooth muscles (Janssen et al., 2001; Sward et al., 2000; Yamagata et al., 2000). The reason for an apparent lack of effect of Y-27632 on PKC in the rat urinary bladder compared with an effect of this compound in the rabbit femoral artery remains unclear. However, differences in the types and levels of PKC isoenzymes or in the regulation of Ca2+-sensitization mechanisms between these smooth muscle types and/or species cannot be ruled out. Interestingly, GF 109203X evoked a decrease in rat urinary bladder smooth muscle tension alone, in similarity to the decrease evoked by Y-27632 and HA-1077, suggesting roles for ROCK and PKC in the maintenance of bladder tone. Previous studies using GF 109203X in mouse bladder do not discuss an effect of this inhibitor on baseline tone (Liu & Lin-Shiau, 2000), however a role for PKC in tracheal (de diego et al., 1995) and vascular (Henrion & Laher, 1993) smooth muscle tone has previously been suggested.
In further support of a role for a Ca2+-sensitization mechanism in urinary bladder smooth muscle contraction through a ROCK mediated pathway, responses of rat urinary bladder strips evoked by KCl were not affected by Y-27632. Contractions of the urinary bladder evoked by KCl have previously been shown to be dependent on extracellular Ca2+ and are inhibited by Ca2+ channel antagonists, indicating an involvement of Ca2+ influx via voltage sensitive Ca2+ channels (Lowe & Noronha-Blob, 1991; Visser & Van Mastrigt, 2000). Thus, the mechanism by which [Ca2+]i is increased following exposure to KCl in the urinary bladder is independent of G-protein coupled pathways. Thus the lack of effect of Y-27632 on KCl induced responses provides further evidence that a ROCK-mediated Ca2+-sensitization mechanism is specifically involved in G-protein coupled contractile responses of this tissue.
α,β-mATP induces contraction of urinary bladder smooth muscle via P2X1 receptors (Vial & Evans, 2000), which mediate both the depolarization of the membrane causing the influx of Ca2+ ions through voltage-dependent Ca2+ channels and the influx of Ca2+ ions directly through the receptor (Schneider et al., 1991). Therefore, in addition to experiments using KCl stimulation, the effect of Y-27632 on α,β-mATP-evoked responses was investigated to confirm that Y-27632 inhibits responses through a ROCK-mediated pathway that is specifically coupled to G-protein mediated receptors. Unexpectedly, Y-27632 significantly attenuated α,β-mATP-evoked contractions by approximately 30%. In an effort to confirm that this effect was due to ROCK inhibition, the experiment was repeated using HA-1077 (10 μM) and similar results were obtained. (PDBu-evoked bladder contractile responses were unaffected by this concentration of HA-1077 demonstrating selectivity of this inhibitor for ROCK versus PKC). It is unclear why these compounds affect the response to α,β-mATP. However a possible explanation is that Y-27632 and HA-1077 cause inhibition by acting directly at the P2X1 receptor either by interfering with the extracellular ATP binding site or perhaps interfering with the intracellular phosphorylation site on the P2X1 receptor (Ennion & Evans, 2002). Indeed, Y-27632 has been shown to inhibit ROCK by competing with ATP for binding at the ROCK catalytic site (Ishizaki et al., 2000). Regardless of the explanation, Y-27632 does exhibit some degree of selectivity in the attenuation of G-protein mediated responses (carbachol and NKA) versus α,β-mATP-evoked responses (c.f. approximately 50% versus 30%).
The response to EFS of the rodent urinary bladder has been shown to be mediated by a co-release of ATP and acetylcholine, resulting in a biphasic contraction with ATP contributing mainly to the phasic and acetylcholine to the tonic portion of the neurogenic response (Maggi et al., 1985; Brading & Williams, 1990). In addition, it has been proposed that each of these neurotransmitters is responsible for a different component of the micturition response, ATP being largely responsible for the initiation of voiding, while cholinergic transmission responsible for the maintenance of voiding (Theobald, 1995). In order to investigate the role of ROCK in the electrically-evoked response of urinary bladder smooth muscle, studies were conducted to compare the effects of Y-27632 with muscarinic acetylcholine receptor blockade with atropine, NK2 receptor blockade with MEN 10,376 (Maggi et al., 1991a,b; 1992) or P2X receptor desensitization with α,β-mATP. Similar to published results, the bladder contractile response to EFS in the present study was biphasic, comprising an initial phasic contraction followed by a secondary sustained tonic contractile response (Brading & Williams, 1990). EFS-evoked bladder contractions were inhibited by TTX at all frequencies of stimulation, indicating their neural origin. The secondary tonic component of the biphasic response to EFS was attenuated by atropine at frequencies of stimulation of 4 Hz and above, while P2X receptor desensitization had no effect. Y-27632 was found to have a similar effect to atropine, consistent with the view that the contractile effects of cholinergic stimulation in the bladder, represented by a tonic response to electrical stimulation in vitro, involve a ROCK-mediated pathway. The amplitude of the contractile response to EFS was significantly attenuated by P2X receptor desensitization at high frequencies of stimulation (8 and 16 Hz), but atropine had no effect at all frequencies tested. Y-27632 also significantly attenuated the amplitude of EFS-evoked responses at these frequencies of stimulation (8 and 16 Hz) and to a similar degree as α,β-mATP. As discussed above, Y-27632 also attenuated the response to exogenous α,β-mATP by approximately 30%, suggesting that effects on purinergic transmission may mediate the effects of Y-27632 on the phasic response to high frequencies of electrical stimulation. However, Y-27632 also significantly attenuated the phasic response to electrical stimulation at lower frequencies of stimulation (2 and 4 Hz), whereas neither atropine nor P2X receptor desensitization had an effect. However, it is possible that a combined effect on both these transmitter pathways by Y-27632 may offer an explanation for the present results. Indeed, Patra & Westfall (1994) found that responses of the guinea-pig bladder to EFS were inhibited 20% by desensitization with α,β-mATP and 35% by atropine, but when used together the neurogenic response was inhibited by 85%. Furthermore, a role for other transmitters and modulatory pathways in the bladder contractile response to EFS cannot be ruled out. In this respect, a role for tachykinins in the contractile response to EFS has previously been reported (Meini & Maggi, 1994) and Y-27632 attenuates bladder contractions evoked by exogenous NKA, suggesting the effects of Y-27632 on neurogenic bladder contractions may also be mediated by an effect on tachykinergic transmission. However, in the present study, the selective NK2 receptor antagonist, MEN 10,367, had no effect on either the phasic or tonic contractile response to electrical stimulation. Previous studies have suggested that tachykinins released from the peripheral endings of primary afferent neurons mediate the atropine-resistant, capsaicin-sensitive tonic contractile response to electrical stimulation (Meini & Maggi, 1994). Thus, as the effects of MEN 10,367 were examined on the response to electrical stimulation per se, the effects of NK2 receptor blockade may be masked by the presence of other neurotransmitters. In this respect, the ability of Y-27632 to attenuate EFS-evoked bladder contractions may be complex and involve various contributions and interactions of the different neurotransmitters (acetylcholine, ATP and tachykinins) and components (phasic and tonic) of the contractile response to electrical stimulation. These possibilities require more detailed investigation. Interestingly, MEN 10,367 alone evoked a contractile response of the isolated rat bladder, which was not observed in previous in vitro studies with this inhibitor at the same concentration (Maggi et al., 1991b) or from in vivo studies (Maggi et al., 1991a). Similar agonist effects of other selective NK2 receptor antagonists, for example MEN 10,207 have previously been observed (Maggi et al., 1991b), although the mechanism of action of the agonist effect of MEN 10,367 in the present study remains unclear.
The results of the present study are particularly interesting with regard to the pharmacological treatment of lower urinary tract dysfunction. Overactive bladder is treated primarily with anti-muscarinics, however their use is often limited by adverse side-effects, including dry mouth and constipation (for reviews see Chapple, 2000; Wyndaele, 2001). Thus, ROCK inhibitors could potentially provide a benefit over the anti-muscarinics by working downstream of the muscarinic receptor.
In conclusion, the results of the present study demonstrate that ROCK I and ROCK II are expressed in the rat urinary bladder and that ROCK plays a role in the regulation of rat urinary bladder smooth muscle contraction and tone. Therefore, ROCK inhibitors may prove to be therapeutically useful in the treatment of lower urinary tract disorders associated with changes in the physiology of bladder smooth muscle contractility.
Acknowledgments
The authors greatly appreciate the assistance of Mark Burgert with statistical analysis.
Abbreviations
- α,β-mATP
α,β-methylene ATP
- NKA
neurokinin A
- PDBu
phorbol 12,13-dibutyrate
- ROCK
Rho-kinase
References
- AMANO M., FUKATA Y., KAIBUCHI K. Regulation and functions of Rho-associated kinase. Exper. Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- BRADING A.F., WILLIAMS J.H. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and α,β-methylene ATP. Br. J. Pharmacol. 1990;99:493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPLE C.R. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology. 2000;55:33–46. doi: 10.1016/s0090-4295(99)00492-6. [DOI] [PubMed] [Google Scholar]
- CHITALEY K., WINGARD C.J., WEBB R.C., BRANAM H., STOPPER V.S., LEWIS R.W., MILLS T.M. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nature Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DIEGO A., CORTIJO J., VILLAGRASA V., PERPINA M., MORCILLO E.J. H-7, a protein kinase C inhibitor, inhibits spontaneous tone and spasmogenic responses in normal and sensitized guinea pig trachea. Gen. Pharmacol. 1995;26:1747–1755. doi: 10.1016/0306-3623(95)00075-5. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., REDDY H., WATSON N., CHALLISS R.A.J. Muscarinic acetylcholine receptor subtypes in smooth muscle. Trends Pharmacol. Sci. 1994;15:114–119. doi: 10.1016/0165-6147(94)90047-7. [DOI] [PubMed] [Google Scholar]
- ENNION S.J., EVANS R.J. P2X1 receptor subunit contributing to gating revealed by a dominant negative PKC mutant. Biochem. Biophys. Res. Comm. 2002;291:611–616. doi: 10.1006/bbrc.2002.6488. [DOI] [PubMed] [Google Scholar]
- ETO M., KITAZAWA T., YAZAWA M., MUKAI H., ONO Y., BRAUTIGAN D.L. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C α and δ isoforms. J. Biol. Chem. 2001;276:29072–29078. doi: 10.1074/jbc.M103206200. [DOI] [PubMed] [Google Scholar]
- HALL J.M., FLOWERS J.M., MORTON I.K. A pharmacological study of NK1 and NK2 tachykinin receptor characteristics in the rat isolated urinary bladder. Br. J. Pharmacol. 1992;107:777–784. doi: 10.1111/j.1476-5381.1992.tb14523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEID C.A., STEVENS J., LIVAK K.J., WILLIAMS P.M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- HENRION D., LAHER I. Effects of staurosporine and calphostin C, two structurally unrelated inhibitors of protein kinase C, on vascular tone. Can. J. Physiol. Pharmacol. 1993;71:521–524. doi: 10.1139/y93-076. [DOI] [PubMed] [Google Scholar]
- HOROWITZ A., MENICE C.B., LAPORTE R., MORGAN K.G. Mechanisms of smooth muscle contraction. Physiol. Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- IIZUKA K., SHIMIZU Y., TSUKAGOSHI H., YOSHII A., HARADA T., DOBASHI K., MUROOZONO T., NAKAZAWA T., MORI M. Evaluation of Y-27632, a Rho-kinase inhibitor, as a bronchodilator in guinea-pigs. Eur. J. Pharmacol. 2000;406:273–279. doi: 10.1016/s0014-2999(00)00504-5. [DOI] [PubMed] [Google Scholar]
- ISHIZAKI T., MAEKAWA M., FUJISAWA K., OKAWA K., IWAMATSU A., FUJITA A., WATANABE N., SAITO Y., KAKIZUKA A., MORII N., NARUMIYA S. The small GTP-binding protein Rho binds and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- ISHIZAKI T., UEHATA M., TAMECHIKA I., KEEL J., NONOMURA K., MAEKAWA M., NARUMIYA S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- JANSSEN L.J., WATTIE J., LU-CHAO H., TAZZEO T. Muscarinic excitation-contraction coupling mechanisms in tracheal and bronchial smooth muscles. J. Applied Physiol. 2001;91:1142–1151. doi: 10.1152/jappl.2001.91.3.1142. [DOI] [PubMed] [Google Scholar]
- JEZIOR J.R., BRADY J.D., ROSENSTEIN D.I., MCCAMMON K.A., MINER A.S., RATZ P.H. Dependency of detrusor contractions on calcium sensitization and calcium entry through LOE-908-sensitive channels. Br. J. Pharmacol. 2001;134:78–87. doi: 10.1038/sj.bjp.0704241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAWAJA A.M., ROGERS D.F. Tachykinins: receptor to effector. Int. J. Biochem. Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- LEUNG T., MANSER E., TAN L., LIM L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J. Biol. Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- LIU S.H., LIN-SHIAU S.Y. Protein kinase C regulates purinergic component of neurogenic contractions in mouse bladder. J. Urol. 2000;164:1764–1767. [PubMed] [Google Scholar]
- LONGHURST P.A., LEGGETT R.E., BRISCOE J.A.K. Characterization of the functional muscarinic receptors in the rat urinary bladder. Br. J. Pharmacol. 1995;116:2279–2285. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE V.C., NORONHA-BLOB L. Effect of extracellular Ca2+ on cholinergic, KCl and phorbol ester-mediated phosphoinositide turnover and guinea-pig urinary bladder contraction. Eur. J. Pharmacol. 1991;195:273–279. doi: 10.1016/0014-2999(91)90546-3. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., BALLATI L., LECCI A., MANZINI S., PATACCHINI R., RENZETTI A.R., ROVERO P., QUARTARA L., GIACHETTI A. In vivo evidence for tachykininergic transmission using a new NK-2 receptor selective antagonist, MEN 10,367. J. Pharmacol. Exp. Ther. 1991a;257:1172–1178. [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., EGLEZOS A., QUARTARA L., GIULIANI S., GIACHETTI A. Tachykinin receptors in the guinea-pig renal pelvis: activation by exogenous and endogenous tachykinins. Br. J. Pharmacol. 1992;107:27–33. doi: 10.1111/j.1476-5381.1992.tb14459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., SANTICIOLI P., GIULIANI S. Tachykinin antagonists and capsaicin-induced contraction of the rat isolated urinary bladder: evidence for tachykinin-mediated cotransmission. Br. J. Pharmacol. 1991b;103:1535–1541. doi: 10.1111/j.1476-5381.1991.tb09823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., MELI A. Pharmacological evidence for the existence of two components in the twitch response to field stimulation of detrusor strips from the rat urinary bladder. J. Auto. Pharmacol. 1985;5:221–230. doi: 10.1111/j.1474-8673.1985.tb00123.x. [DOI] [PubMed] [Google Scholar]
- MATSUI T., AMANO M., YAMAMOTO T., CHIHARA K., NAKAFUKU M., ITO M., NAKANO T., OKAWA K., IWAMATSU A., KAIBUCHI K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:29051–29054. [PMC free article] [PubMed] [Google Scholar]
- MEINI S., MAGGI C.A. Evidence for a capsaicin-sensitive, tachykinin-mediated, component in the NANC contraction of the rat urinary bladder to nerve stimulation. Br. J. Pharmacol. 1994;112:1123–1131. doi: 10.1111/j.1476-5381.1994.tb13200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAMBI P., AMEGADZIE B., MATTERN M. Protein kinase C, a therapeutic target. Pharmacol. Rev. Comm. 1996;8:29–38. [Google Scholar]
- PATRA P.B., WESTFALL D.P. Potentiation of purinergic neurotransmission in guinea-pig urinary bladder by histamine. J. Urol. 1994;151:787–790. doi: 10.1016/s0022-5347(17)35088-7. [DOI] [PubMed] [Google Scholar]
- REES R.W., RALPH D.J., ROYLE M., MONCADA S., CELLEK S. Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br. J. Pharmacol. 2001;133:455–458. doi: 10.1038/sj.bjp.0704124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMBROOK J., FRITSCH E.F., MANIATIS T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- SCHNEIDER P., HOPP H.H., ISENBERG G. Ca2+ influx through ATP-gated channels increments [Ca2+]i and inactivates ICa in myocytes from guinea-pig urinary bladder. J. Physiol. 1991;440:479–496. doi: 10.1113/jphysiol.1991.sp018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMYLO A.P., SOMYLO A.V. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARD K., DREJA K., SUSNJAR M., HELLSTRAND P., HARTSHORNE D.J., WALSH M.P. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J. Physiol. 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEOBALD R.J. Purinergic and cholinergic components of bladder contractility and flow. Life Sci. 1995;56:445–454. doi: 10.1016/0024-3205(94)00909-0. [DOI] [PubMed] [Google Scholar]
- TONG Y.C., HUNG Y.C., LIN S.N., CHENG J.T. Pharmacological characterization of the muscarinic receptor subtypes responsible for the contractile response in the rat urinary bladder. J. Auto. Pharmacol. 1997;17:21–25. doi: 10.1046/j.1365-2680.1997.00436.x. [DOI] [PubMed] [Google Scholar]
- TOULLEC D., PIANETTI P., COSTE H., BELLENESQUE P., GRAND-PERRET T., AJAKANE M., BAUDET M., BOISSIN P., BOURSIER E., LORIOLLE F., DUHAMEL L., CHARON D., KIRILOVSKY J. The bisindolylmaleimide GF109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISSER A.J., VAN MASTRIGT R. The role of intracellular and extracellular calcium in mechanical and intracellular electrical activity of human urinary bladder smooth muscle. Urol. Res. 2000;28:260–268. doi: 10.1007/s002400000109. [DOI] [PubMed] [Google Scholar]
- WYNDAELE J.J. The overactive bladder. Br. J. Urol. 2001;88:135–140. doi: 10.1046/j.1464-410x.2001.02296.x. [DOI] [PubMed] [Google Scholar]
- YAMAGATA S., ICHINOSE M., SUGIURA H., KOARAI A., KOIKE K., SHIRATO K. Effect of a calcium sensitization modulator, Y-27632, on isolated human bronchus and pulmonary artery. Pulm. Pharmacol. Ther. 2000;13:25–29. doi: 10.1006/pupt.1999.0227. [DOI] [PubMed] [Google Scholar]


