Abstract
On the basis of previous findings that cannabinoids inhibit the function of human and rat 5-HT3 receptors in vitro, we investigated whether cannabinoid receptor agonists also modulate the activity of the rat peripheral 5-HT3 receptors on the terminals of cardiopulmonary afferent C-fibres in vivo.
In urethane-anaesthetized rats, pre-treated intravenously (i.v.) with the CB1 receptor antagonist SR 141716A (3 μmol kg−1) and with the β1/β2 adrenoceptor antagonist propranolol (0.3–0.4 μmol kg−1), bolus injection of the serotonin 5-HT3 receptor agonist phenylbiguanide (3–10 μg kg−1, i.v.) or the vanilloid VR1 receptor agonist capsaicin (3–10 μg kg−1, i.v.) caused an immediate decrease in heart rate and mean arterial blood pressure (the von Bezold–Jarisch reflex).
The phenylbiguanide-induced bradycardia was dose-dependently attenuated by the cannabinoid receptor agonists CP 55,940 (0.1–1 μmol kg−1, i.v.) and WIN 55,212-2 (0.1–3 μmol kg−1, i.v.) 20 min after injection, but not by the inactive S-(−)enantiomer of the latter, WIN 55,212-3 (1 μmol kg−1, i.v.). The inhibition was reversible within 30 min. The extent of inhibition by the highest doses of cannabinoid receptor agonists amounted to about 50%. Both cannabinoid receptor agonists failed to affect the capsaicin-evoked bradycardia.
In conclusion, our results demonstrate that cannabinoid receptor agonists modulate the von Bezold–Jarisch reflex by inhibiting peripheral serotonin 5-HT3 receptors in rats in vivo. An analogous mechanism of cannabinoid receptor agonists may be assumed to be involved in other serotonin 5-HT3 receptor-mediated responses.
Keywords: Anaesthetized rats; von Bezold–Jarisch reflex; cannabinoid receptors; CP 55,940; peripheral serotonin 5-HT3 receptors; SR 141716A; WIN 55,212-2
Introduction
Two specific cannabinoid receptor subtypes termed CB1 and CB2 have been identified and characterized; they belong to the superfamily of G protein-coupled receptors (for reviews, see e.g. Ameri, 1999; Pertwee, 2000; 2001). In the nervous system, cannabinoid receptor agonists induce their effects by activating CB1 receptors whereas the CB2 receptors are expressed by immune cells. However, cannabinoid receptor-independent effects of cannabinoids have also been reported for ligand- and voltage-gated ion channels, such as vanilloid VR1 receptors (Zygmunt et al., 1999; Malinowska et al., 2001a), TASK-1 K+ channels (Maingret et al., 2001), Shaker-related Kv 1.2 K+ channels (Poling et al., 1996) and T-type Ca2+ channels (Chemin et al., 2001).
In this context and in view of the involvement of both cannabinoid (for references, see above) and 5-HT3 receptors (Boess & Martin, 1994; Costall & Naylor, 1997; Karim et al., 1996; Voog et al., 2000; Simpson et al., 2000) in pain and emesis, it is of particular interest to note that in electrophysiological experiments on rat nodose ganglion neurones in the whole cell configuration, cannabinoid receptor agonists stereoselectively inhibited the 5-HT3 receptor-mediated currents (Fan, 1995). This inhibitory effect could be due to a direct action of the cannabinoid receptor agonists at 5-HT3 receptor channels themselves or, alternatively, occurs via inhibitory cannabinoid CB1 receptors, which may be present on these cells in addition to 5-HT3 receptors. The latter possibility could not be ruled out, when considering the lack of experiments with cannabinoid receptor antagonists in the study of Fan (1995). However, recently it was shown in a combined patch-clamp and radioligand binding study on outside-out patches and membrane preparations, respectively, of HEK 293 cells expressing recombinant human 5-HT3A (h5-HT3A) receptors that cannabinoid receptor ligands directly act at the h5-HT3A receptor, probably by binding to an allosteric modulatory site (Barann et al., 2002; see also Commentary by Townsend IV et al., 2002).
On the basis of these considerations and findings, it was the aim of the present study to examine whether a cannabinoid receptor-independent, 5-HT3 receptor-mediated action of cannabinoid receptor agonists can also be detected in vivo. The von Bezold–Jarisch reflex, i.e. vagal nerve mediated reflex bradycardia and hypotension induced by activation of peripheral 5-HT3 receptors on cardiac afferent nerves in anaesthetized rats treated with a CB1 receptor antagonist, is a suitable model to investigate cannabinoid receptor-independent effects of cannabinoids at the 5-HT3 receptor.
Preliminary results of the present study have been published in abstract form (Godlewski et al., 2002).
Methods
General procedure
Male Wistar rats (weighing 150–260 g) were anaesthetized by intraperitoneal (i.p.) injection of urethane (1.25 g kg−1; this injection was sufficient to maintain anaesthesia until the end of experiments, since we did not observe any pain reflexes elicited by paw-pinch) and tracheotomized. The left carotid artery was carefully separated from the vagus nerve and cannulated to measure the mean arterial pressure via a pressure transducer (Gould P23ID). Heart rate was recorded from the ECG by means of subcutaneous electrodes. Body temperature was monitored by a rectal probe transducer and was maintained constant at 37±1°C using a tungsten lamp. The transducers were connected to the monitor Trendscope 8031 (AxMediTec, Białystok, Poland). The left femoral vein was cannulated for intravenous (i.v.) administration of drugs in a volume of 0.5 ml kg−1. After 15–30 min of equilibration period, during which the cardiovascular parameters were allowed to stabilize, the experiments were performed. This protocol proved to be suitable to study the von Bezold–Jarisch reflex responses in our former studies (e.g. Malinowska et al., 1996; 2001a).
All experiments were approved by the Local Animal Ethics Committee in Białystok (Poland).
Experimental protocols
In order to determine the influence of the cannabinoid receptor antagonist SR 141716A on the dose-response curve for phenylbiguanide, rats injected with propranolol (0.3 μmol kg−1, i.v.) were given three or four increasing doses of phenylbiguanide (1, 3, 10 and 30 μg kg−1, i.v.) with sufficient time between the injections for full recovery to preinjection parameters. This procedure was repeated 10 min after the injection of SR 141716A (3 μmol kg−1, i.v.). In one experimental group both propranolol and SR 141716A were omitted.
In order to examine the effects of cannabinoid receptor agonists on the von Bezold–Jarisch reflex, animals were injected i.v. with the cannabinoid CB1 receptor antagonist SR 141716A (3 μmol kg−1) at the onset of the experiment and 5 min later with the β1/β2-adrenoceptor antagonist propranolol (0.3–0.4 μmol kg−1). The von Bezold–Jarisch reflex (bradycardia and hypotension) was evoked 10 min after the administration of SR 141716A by rapid i.v. bolus injection of only one dose (between 3–10 μg kg−1) of phenylbiguanide or capsaicin which produced a decrease in heart rate by about 15–25% of the basal value. Injections of an appropriate dose of phenylbiguanide/capsaicin were repeated every 10 min until bradycardic responses were reproducible three times (S1A; S1B, S1C). Subsequently, a single dose of the cannabinoid receptor agonist under study, WIN 55,212-2 (0.1, 1 or 3 μmol kg−1), WIN 55,212-3 (1 μmol kg−1), CP 55940 (0.1, 0.3 or 1 μmol kg−1) or their vehicle was given i.v. The effects of the cannabinoid receptor agonist under study or its vehicle on phenylbiguanide/capsaicin-induced reflex bradycardia were studied 10, 20 and 30 min following the injection of the cannabinoid or its vehicle (S2, S3 and S4).
Calculation and statistics
Results are given as means±s.e.means (n=number of animals). The decrease in heart rate was calculated as % of the basal heart rate immediately before the injection of phenylbiguanide or capsaicin. The effects of the cannabinoid receptor agonists on the phenylbiguanide/capsaicin-induced bradycardia obtained during S2, S3 or S4 were calculated as a ratio over S1, where S1 was the mean of three subsequently reproduced responses (S1A, S1B and S1C). For comparison of mean values, Student's t-test for paired or unpaired data was used. When two or more treatment groups were compared to the same control, the one-way analysis of variance (ANOVA) followed by the Dunnett test was used. Differences were considered as significant when P<0.05.
Drugs
R(+) - [2,3 - dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-yl]-(1-naphthalenyl)-methanone mesylate (benzoxazin-yl]-(1-naphthalenyl)-methanone mesylate (WIN 22,212-2), S(−)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de] -1,4-benzoxazin-yl] - (1-naphthalenyl)-methanone mesylate (benzoxazin-yl]-(1-naphthalenyl)-methanone mesylate (WIN 22,212-3), 2-hydroxypropyl-β-cyclodextrin (cyclodextrin) (RBI, Natick, U.S.A.); N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carbox-amide hydrochloride (SR 141716A) (Sanofi Recherche, Montpellier, France); (−)-cis-3-[2-hydroxy-4-(1,1- dimethylheptyl)phenyl]-trans - 4 - (3-hydroxypropyl) cyclohexanol (CP 55,940) (Tocris Cookson, Bristol, U.K.); 1-phenylbiguanide (phenylbiguanide) (ICN, Costa Mesa, U.S.A.); 8-methyl-N-vanillyl-6-nonenamide (capsaicin), urethane, (S)-1-(isopropylamino)-3-(1-naphthyloxy)-2-propanolol hydrochloride (propranolol), derivative of castor oil and ethylene oxide (Cremophor El), dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Steinheim, Germany); ethyl alcohol (ethanol) (PPOCh, Gliwice, Poland).
Propranolol, urethane and cyclodextrin were dissolved in saline, WIN 55,212-2, WIN 55,212-3 and CP 55,940 in a 19% w v−1 solution of cyclodextrin, capsaicin in a mixture of saline and ethanol (15 : 1). Stock solution of SR 141716A was prepared in a mixture of DMSO and Cremophor El, which was further diluted in cyclodextrin immediately before the experiment (1 : 1 : 10). Cyclodextrin solution and the solvent for SR 141716A caused a short-lasting decrease in heart rate and blood pressure followed by an increase in blood pressure by about 10 mmHg which turned to basal values within 1–2 min. Saline and the vehicle for capsaicin did not affect the basal blood pressure and the heart rate.
Results
General
In urethane-anaesthetized rats, basal heart rate and mean arterial pressure were 365±6 beats min−1 and 73±2 mmHg, respectively (n=66). Intravenous administration of the cannabinoid CB1 receptor antagonist SR 141716A (3 μmol kg−1) did not modify the baseline cardiovascular parameters (5 min after its administration the respective values amounted to 355±6 beats min−1 and 80±2 mmHg). Propranolol (as a rule, 0.3 μmol kg−1; 0.4 μmol kg−1 when the initial heart rate exceeded 400 beats min−1) given 5 min after SR 141716A decreased the basal heart rate and increased the mean arterial pressure. Thus, immediately before S1 (first injection of phenylbiguanide, capsaicin or their vehicle) basal heart rate and blood pressure were 336±4 beats min−1 and 87±2 mmHg (n=66), respectively. These values did not differ among the treatment groups and remained stable throughout the experiments (Table 1).
Table 1.
Baseline cardiovascular parameters in various experimental groups of urethane-anaesthetized rats pre-treated with SR 141716A (3 μmol kg−1) and propranolol (0.3–0.4 μmol kg−1)
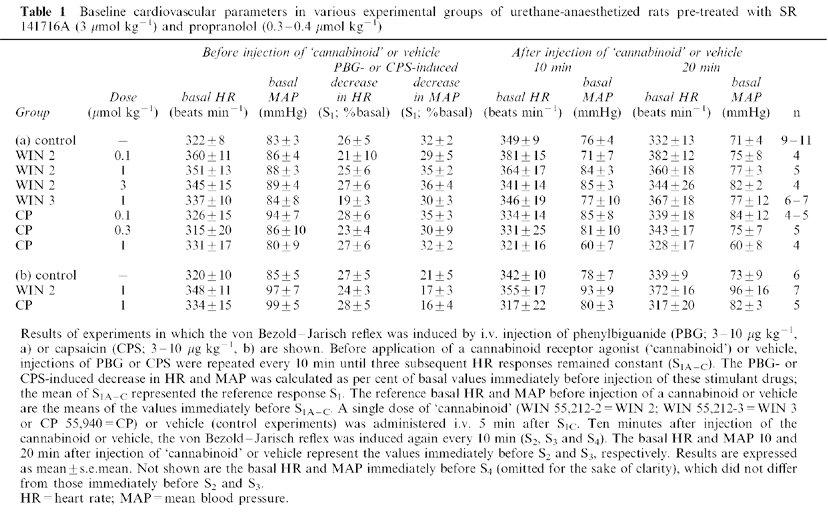
Intravenous administration of the cannabinoid receptor agonists WIN 55,212-2 (0.1, 1, 3 μmol kg−1), WIN 55,212-3 (1 μmol kg−1), CP 55,940 (0.1, 0.3 μmol kg−1) in the presence of SR 141716A and propranolol did not affect the basal cardiovascular parameters 10, 20 (Figure 1; Table 1) and 30 min (Figure 1) after treatment. In the experiments in which the von Bezold–Jarisch reflex was elicited by phenylbiguanide (Figure 1a and Table 1a; but not in those in which it was evoked by capsaicin: Figure 1b and Table 1b), the highest dose of CP 55,940 (1 μmol kg−1) tended to decrease mean arterial pressure (by about 10 mmHg); however, this effect was not significant (see Table 1a for means±s.e.means).
Figure 1.
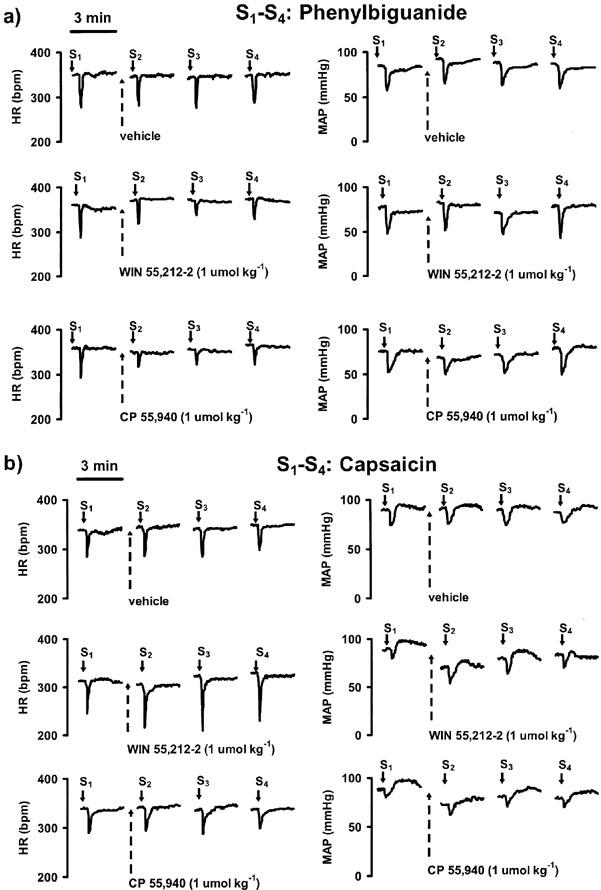
Traces from representative experiments showing the effects of cannabinoid receptor agonists on the phenylbiguanide (a)- and capsaicin (b)- induced reflex decrease in heart rate (HR; left panel) and mean blood pressure (MAP; right panel) in urethane-anaesthetized rats pre-treated with SR 141716A (3 μmol kg−1) and propranolol (0.3–0.4 μmol kg−1). Heart rate is expressed as beats min−1 (b.p.m.). Phenylbiguanide or capsaicin (each 3–10 μg kg−1, solid arrows) was injected i.v. every 10 min until three subsequent heart rate responses remained constant (S1A, S1B, S1C); only one representative tracing of these control responses is given (S1). Five min after S1C a single dose of cannabinoid receptor agonist or its vehicle (hatched arrows) was administered i.v. Reflex decrease in HR and MAP was induced again by injection of phenylbiguanide or capsaicin 10, 20 and 30 min after application of cannabinoid receptor agonist or its vehicle (S2, S3, and S4, respectively). The horizontal bars above the upper left tracings of a and b represent the 3-min periods of recording.
Phenylbiguanide and capsaicin-induced reflex bradycardia and hypotension and influence of propranolol and SR 141716A on the dose-response curve of phenylbiguanide
In rats not pre-treated with any drug, i.v. bolus injection of the 5-HT3 receptor agonist phenylbiguanide (1, 3, 10, 30 μg kg−1) produced a transient (5–10 s) fall in heart rate by 6±1, 11±2, 24±4, 33±4%, respectively, and in mean arterial blood pressure by 4±2, 15±5, 27±5 and 28±4%, respectively (expressed as percentage of basal values; n=3–6). In animals treated with propranolol (0.3 μmol kg−1), responses to the same doses of phenylbiguanide were almost identical to those obtained in the absence of the β1/β2-adrenoceptor blockade (for reflex bradycardia in the presence of propranolol, see values represented by open circles in Figure 2; reflex decrease in mean arterial blood pressure amounted to 2±1, 16±3, 28±3 and 41±6% of basal values, respectively; n=3–6). Intravenous application of SR 141716A (3 μmol kg−1) to rats pre-treated with propranolol (0.3 μmol kg−1) also did not alter the phenylbiguanide-induced bradycardia (Figure 2) and hypotension (decrease by 5±2, 18±5, 33±4 and 40±10% of basal blood pressure, respectively; n=3–6).
Figure 2.
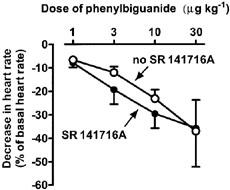
Influence of SR 141716A on the phenylbiguanide-induced reflex bradycardia in urethane-anaesthetized rats pre-treated with propranolol (0.3 μmol kg−1). Animals were given three or four increasing doses of phenylbiguanide (1, 3, 10 and 30 μg kg−1) with sufficient time between injections for full recovery to pre-injection parameters. Determination of the phenylbiguanide dose-response curve was repeated 10 min after the exposure to SR 141716A (3 μmol kg−1). Means±s.e.mean of n=3–6 rats. Bradycardia was expressed as a percentage of the basal heart rate immediately before the injection of phenylbiguanide.
As shown previously (Fozard, 1984; Malinowska et al., 2001a), intravenous bolus injection of capsaicin (3–10 μg kg−1) also induced reflex bradycardia and hypotension. The decrease in heart rate and mean arterial pressure was of similar short duration (5–15 s) and returned to the basal parameters within 1–3 min (see traces in Figure 1).
Influence of cannabinoid receptor agonists on the reflex bradycardia induced by phenylbiguanide and capsaicin
In order to examine the influence of the cannabinoid receptor agonists on the von Bezold–Jarish reflex in rats pre-treated with SR 141716A and propranolol, a single dose of phenylbiguanide (3–10 μg kg−1) or capsaicin (3–10 μg kg−1) was injected.
At the dose applied, these drugs decreased heart rate by about 15–25% of the basal value. We did not observe any significant differences in S1 values among the experimental groups (Table 1). In control animals, the phenylbiguanide- and capsaicin-stimulated decreases in heart rate expressed as ratios S2/S1, S3/S1 and S4/S1 were 1.10±0.07, 1.23±0.09, 1.10±0.11 (n=9–11) and 1.14±0.14, 1.27±0.12, 1.05±0.13 (n=5–6), respectively.
The cannabinoid receptor agonists WIN 55,212-2 (1 and 3 μmol kg−1) and CP 55,940 (1 μmol kg−1) attenuated the phenylbiguanide-induced reflex bradycardia (see Figure 1 for original traces). Figure 3 shows the time-dependence of the effect of cannabinoids at a dose of 1 μmol kg−1. Ten min after injection, both drugs did not yet affect the phenylbiguanide-induced decrease in heart rate, but 20 min after injection the bradycardic response was inhibited by about 40–50% by either cannabinoid receptor agonist; the inhibition was reversible within further 10 min (Figure 3). In the case of the highest dose of WIN 55,212-2 (3 μmol kg−1), a significant inhibition of phenylbiguanide-induced bradycardia occurred already 10 min after injection (55±10% of the control, n=4; P<0.05) and persisted for further 10 min (see Figure 4). Again, the response declined to a value of 85±17% of the control (n=4) within 30 min.
Figure 3.
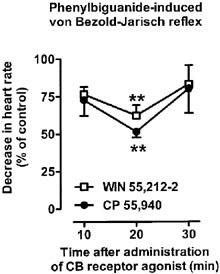
Influence of WIN 55,212-2 (1 μmol kg−1) and CP 55940 (1 μmol kg−1) on the phenylbiguanide-induced von Bezold–Jarisch reflex in urethane-anaesthetized rats pre-treated with SR 141716A (3 μmol kg−1) and propranolol (0.3–0.4 μmol kg−1). Phenylbiguanide (3–10 μg kg−1) was injected i.v. every 10 min until three subsequent heart rate responses remained constant (S1A–C). Five minutes later a single dose of the cannabinoid receptor agonist under study was administered i.v. The reflex decrease in heart rate was induced again 10, 20 and 30 min following exposure to the respective cannabinoid receptor agonist (S2, S3 and S4). The S1A–C and S2–4 values were calculated as percentages of the basal heart rate immediately before the injection of phenylbiguanide. S2, S3 and S4 were then calculated as ratios over S1, where S1 is the mean of three subsequent comparable responses (S1A, S1B and S1C). Results are expressed as the percentage of the control receiving the respective vehicle. Mean±s.e.mean of n=4–11 rats. **P<0.01 compared to the corresponding control.
Figure 4.
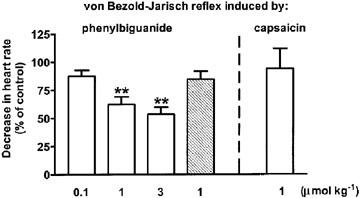
Influence of WIN 55,212-2 (open bars) and WIN 55,212-3 (hatched bar) on the von Bezold–Jarisch reflex bradycardia induced by phenylbiguanide/capsaicin in urethane-anaesthetized rats pre-treated with SR 141716A (3 μmol kg−1) and propranolol (0.3–0.4 μmol kg−1). Phenylbiguanide (3–10 μg kg−1) or capsaicin (3–10 μg kg−1) was injected i.v. every 10 min until three subsequent heart rate responses remained constant (S1A–C). Five minutes later a single dose of the cannabinoid agonist under study was administered i.v. The decrease in heart rate was induced again 10 (not shown) and 20 min following exposure to the respective cannabinoid receptor agonist (S3). The S1A–C and S3 values were calculated as percentages of the basal heart rate immediately before the injection of phenylbiguanide. S3 was then calculated as a ratio over S1, where S1 is the mean of three subsequent comparable responses (S1A, S1B and S1C). Results are expressed as the percentage of the control receiving the respective vehicle. Mean±s.e.mean of n=4–5 rats. **P<0.01 compared to the corresponding control.
Figures 4 and 5 show that the inhibitory effects of WIN 55,212-2 and CP 55,940, measured 20 min after their injection, on the phenylbiguanide-evoked bradycardia were dose-dependent; an inhibition by about 50% represented the maximum effect obtainable at the doses of 3 and 1 μmol kg−1, respectively. The S(−)enantiomer of WIN 55,212-2, WIN 55,212-3, at the dose of 1 μmol kg−1 was inactive 10, 20 and 30 min after injection (the respective values amounted to 93±7, 85±7 and 101±14% of the control, n=7; see also Figure 4 for the responses obtained 20 min after injection).
Figure 5.
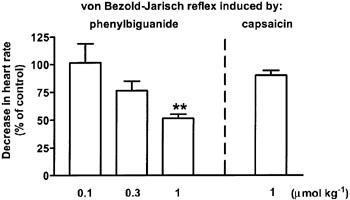
Influence of CP 55,940 on the von Bezold–Jarisch reflex bradycardia induced by phenylbiguanide/capsaicin in urethane-anaesthetized rats pre-treated with SR 141716A (3 μmol kg−1) and propranolol (0.3–0.4 μmol kg−1). Phenylbiguanide (3–10 μg kg−1) or capsaicin (3–10 μg kg−1) was injected i.v. every 10 min until three subsequent heart rate responses remained constant (S1A–C). Five minutes later a single dose of CP 55,940 was administered i.v. The reflex decrease in heart rate was induced again 10 (not shown) and 20 min following exposure to the cannabinoid receptor agonist (S3). The S1A–C and S3 values were calculated as percentages of the basal heart rate immediately before the injection of phenylbiguanide. S3 was then calculated as a ratio over S1, where S1 is the mean of three subsequent comparable responses (S1A, S1B and S1C). Final results are expressed as the percentage of the control receiving the respective vehicle. Mean±s.e.mean of n=4–5 rats. **P<0.01 compared to the corresponding control.
WIN 55,212-2 and CP 55,940 (1 μmol kg−1 each) did not affect the capsaicin-induced reflex bradycardia evoked by S2, S3 and S4 (see Figures 4 and 5, respectively, for the lack of effect on S3; similarly, also the responses evoked by S2 and S4 remained unchanged after injection of WIN 55,212-2 and CP 55,940; results not shown).
Influence of cannabinoid receptor agonists on the reflex hypotension induced by phenylbiguanide and capsaicin
In control animals pre-treated with SR 141716A and propranolol, the ratios S2/S1, S3/S1 and S4/S1 for the phenylbiguanide- and capsaicin-induced decreases in blood pressure were 0.87±0.07, 0.82±0.09, 0.85±0.11 (n=9–11) and 0.97±0.09, 0.94±0.16 and 0.91±0.14 (n=5–6), respectively. Both WIN 55,212-2 and CP 55,940 tended to decrease the hypotensive response to phenylbiguanide and capsaicin at S2 and S3, but this effect was not significant (Figure 6). No such tendency was visible 30 min after injection of the cannabinoids (S4; data not shown).
Figure 6.
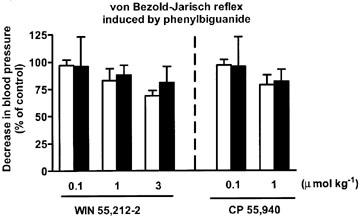
Influence of WIN 55,212-2 and CP 55,940 on the von Bezold–Jarisch reflex hypotension induced by phenylbiguanide in urethane-anaesthetized rats pre-treated with SR 141716A (3 μmol kg−1) and propranolol (0.3–0.4 μmol kg−1). Phenylbiguanide (3–10 μg kg−1) or capsaicin (3–10 μg kg−1) was injected i.v. every 10 min until three subsequent heart rate responses remained constant (S1A–C); blood pressure responses also were constant at S1A–C. Five min later a single dose of the cannabinoid agonist under study was administered i.v. The reflex decrease in heart rate and blood pressure was induced again 10 min (S2; open bars) and 20 min (S3; filled bars) following exposure to the respective cannabinoid receptor agonist. The S1A–C, S2 and S3 values were calculated as percentages of the basal blood pressure immediately before the injection of phenylbiguanide. S2 and S3 was then calculated as a ratio over S1, where S1 is the mean of three subsequent comparable responses (S1A, S1B and S1C). Final results are expressed as the percentage of the control receiving the respective vehicle. Mean±s.e.mean of n=3–5 rats.
The hypotensive responses to capsaicin were not at all depressed by CP 55,940 1 μmol kg−1 (n=4–5) nor by WIN 55,212-2 1 μmol kg−1 (n=7; data not shown).
Discussion
The present study in urethane-anaesthetized rats was designed to investigate whether cannabinoid receptor agonists modulate the function of peripheral 5-HT3 receptors in vivo in a cannabinoid receptor-independent manner. The reflex bradycardia induced by bolus injection of the 5-HT3 receptor agonist phenylbiguanide or the vanilloid VR1 receptor agonist capsaicin into systemic circulation is a suitable model for this purpose. It has previously been shown that the bradycardia in response to phenylbiguanide is abolished by the 5-HT3 receptor antagonist ondansetron (Malinowska et al., 1996), but is insensitive to the vanilloid VR1 receptor antagonist capsazepine (Lee & Lundberg, 1994), whereas the response to capsaicin is abolished by capsazepine (Lee & Lundberg, 1994; Malinowska et al., 2001a), but is insensitive to ondansetron (Malinowska et al., 1996). This clearly indicates that phenylbiguanide and capsaicin activate distinct 5-HT3 and VR1 receptors located on vagal afferent C-fibres in the heart. In our study, capsaicin was used to distinguish whether the modulatory effects of cannabinoids occur exclusively at the level of cardiac 5-HT3 receptors or whether central 5-HT3 receptors (Sévoz et al., 1996) or VR1 receptors or other components of the von Bezold–Jarisch reflex common for 5-HT3 and VR1 receptors might also be affected.
In order to exclude the involvement of neuronal CB1 receptors at any conceivable level of the von Bezold–Jarisch reflex in the effects of the cannabinoid receptor agonists and to counteract the pronounced hypotensive effects elicited by the cannabinoid receptor agonists (Lake et al., 1997; Malinowska et al., 1997), all experiments were performed in the presence of the CB1 receptor antagonist SR 141716A (Rinaldi-Carmona et al., 1995). In our experiments, SR 141716A by itself did not affect the phenylbiguanide-induced reflex-bradycardia, but it prevented the CB1 receptor-mediated decrease in blood pressure. The effectiveness of the blockade of the CB1 receptor-mediated responses by SR 141716A at a dose of 3 μmol kg−1 can also be derived from the following observations. (1) In urethane-anaesthetized rats, the antagonistic effect of SR 141716A against the hypotensive response to cannabinoid receptor agonist was achieved within 10 min of its intravenous administration and persisted unchanged for at least 1 h (Varga et al., 1995). (2) In pithed rats, the i.v dose of SR 141716A required to block the inhibitory effects of cannabinoid agonists on the neurogenic tachycardia (Malinowska et al., 2001b) or vasopressor response (Malinowska et al., 1997) was 30 fold or even 100 fold lower, respectively, than in the presence study; thus even if in our experiments a part of SR 141716A should have been eliminated until the third injection of phenylbiguanide after application of cannabinoid receptor agonist, a sufficient amount of the antagonist should have been left in the organism to block the CB1 receptors. Propranolol was routinely applied, since according to our experience the bradycardic responses were better reproducible. Under the present conditions, the extent of the bradycardic responses was virtually identical to that in propranolol-untreated rats.
In rats pre-treated in this manner, a cannabinoid receptor agonist was injected, and 10, 20 and 30 min later the von Bezold–Jarisch reflex was elicited by phenylbiguanide or capsaicin. Twenty min after injection, WIN 55,212-2 and CP 55,940 inhibited the phenylbiguanide-induced bradycardia in a dose-dependent manner. In the experiments with WIN 55,212-2 3 μmol kg−1, the inhibition already occurred after 10 min and persisted during the subsequent 10 min. The inhibition was reversible within the period from the 20th to 30th minute after injection of the cannabinoid, and it was stereoselective, since WIN 55,212-3, an enantiomer of WIN 55,212-2, was inactive. Simultaneously with the reduction of reflex bradycardia, both WIN 55,212-2 and CP 55,940 tended to attenuate the hypotensive component of the von Bezold–Jarisch reflex in a dose-dependent manner. This effect was probably less pronounced because the hypotensive component occurs partly secondary to the intense bradycardic response (Sévoz et al., 1996; Malinowska et al., 2001a).
These findings are qualitatively consistent with those of the previous in vitro investigations by Fan (1995) and, in particular, Barann et al. (2002). In the former study in rat nodose ganglion cells, stereoselective inhibition of 5-HT3 receptor-mediated currents was shown with CP 55,940 and its less potent enantiomer, CP 56,667, and in the study by Barann et al. (2002) only WIN 55,212-2, but not WIN 55,212-3, reduced the 5-HT-induced currents through recombinant human 5-HT3A receptor in an SR 141716A-resistant manner. However, there are quantitative differences between the present in vivo and the former in vitro investigations: whereas we found CP 55,940 to be roughly equipotent with WIN 55,212-2, the latter drug was more potent than the former one in the in vitro experiments. This difference may be due to pharmacokinetic differences between both cannabinoid receptor agonists, potentially due to differences between them with respect to their hydrophobicity.
A remarkable feature of the WIN 55,212-2- and CP 55,940-induced inhibition of phenylbiguanide-evoked bradycardia is its slow onset. As a rule, this inhibition was not yet visible 10 min after injection (see above), but 20 min had to elapse before the inhibition became manifest. Again, this observation is in line with the slow development of the inhibition in the in vitro experiments. Thus, in rat nodose ganglion, the drugs had to be present in the superfusion fluid for at least 15 min to establish the effect (Fan, 1995). In excised outside-out patches of HEK 293 cells expressing recombinant h5-HT3A receptors, an exposure time with the cannabinoids of 3 min was necessary to obtain the equilibrium effect (Barann et al., 2002). This time period is very long in that preparation when considering that an equilibrium is achieved within seconds with other compounds, e.g. ifenprodil and barbiturates (Barann et al., 1998, 2000).
The long equilibrium time observed with the cannabinoids was interpreted as a hint at a not easily accessible site of action of the cannabinoids at the 5-HT3 receptor (Barann et al., 2002). In particular, a location of this site in the transmembrane or cytosolic domain of the receptor protein was suggested. An action at the ligand recognition site of the 5-HT3 receptor or within its channel pore was excluded by the failure of cannabinoids to inhibit binding of the 5-HT3 receptor radioligand [3H] GR65630 to membranes and, respectively, by the lack of an inhibition of the 5-HT-induced current when the cannabinoids were administered to the patches exclusively during, but not before, stimulation with 5-HT (Barann et al., 2002). These findings in patches of membranes of cells expressing h5-HT3A receptors but no cannabinoid receptors (proven by the lack of binding of CB1 and CB2 receptor radioligands to membranes of these cells) led to the conclusion that the cannabinoids act at an allosteric modulatory site of the 5-HT3 receptor itself.
It has been demonstrated that stimulation of 5-HT3 receptors in the nucleus tractus solitarii inhibits the cardiac von Bezold–Jarisch reflex response in urethane-anaesthetized rats (Sévoz et al., 1996). Thus, by counteracting such an inhibitory effect, cannabinoid receptor agonists might be expected to rather enhance the reflex at the level of the brain, which would counterbalance their inhibitory effects in the periphery. However, the failure of WIN 55,212-2 and CP 55,940 to reduce the capsaicin-evoked, VR1 receptor-mediated reflex bradycardia excludes an action of the cannabinoid receptor agonists on this ligand-gated ion channel and at the central level, strongly supporting the idea that the peripheral 5-HT3 receptor itself on the vagal afferent C-fibres (see above) is the site of action underlying the cannabinoid receptor agonist-induced inhibition of the phenylbiguanide-evoked von Bezold–Jarisch reflex. Irrespective of whether both 5-HT3 and VR1 receptors are located on the same or on different afferent vagal fibres of the heart, all components of the von Bezold–Jarisch reflex subsequent to stimulation of one of these receptors, such as the voltage-gated ion channels involved in propagation of action potentials in the afferent and efferent nerves, the nuclei in the brain (nucleus tractus solitarii, area postrema, dorsal vagal motor nucleus) and the synapse between the vagal nerve and the right atrial pacemaker cells, are identical. In this context, it should be noted that in pithed rats cannabinoid receptor agonists do not influence the bradycardia in response to vagal nerve stimulation (Malinowska et al., 2001b). Thus, by ruling out other possibilities, it may be concluded that it is the peripheral 5-HT3 receptor at which the cannabinoids produce their inhibitory effect.
In conclusion, the present study provides the first piece of evidence that cannabinoid receptor agonists directly inhibit the function of the peripheral 5-HT3 receptors in vivo. An analogous mechanism of cannabinoid receptor agonists may be assumed to be involved in other serotonin 5-HT3 receptor-mediated responses, e.g. analgesia and emesis.
Acknowledgments
This work was supported by the Polish Government (KBN No 3-13431, 4-13901) and by the Deutsche Forschungsgemeinschaft (SFB 400). The authors are also indebted to the Alexander von Humboldt-Stiftung (Bonn, Germany) for generously providing some of the equipment. We wish to thank Mrs I. Malinowska for her skilled technical assistance and the pharmaceutical company SANOFI Recherche for the gift of SR 141716A.
Abbreviations
- SR 141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carbox-amide hydrochloride
- WIN 22,212-2
R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)-methanone mesylate (benzoxazin-yl]-(1-naphthalenyl)-methanone mesylate
- WIN 22,212-3
S(−)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-yl]-(1-naphthalenyl)-methanone mesylate benzoxazin-yl]-(1-naphthalenyl)-methanone mesylate
References
- AMERI A. The effects of cannabinoids on the brain. Prog. Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- BARANN M., BÖNISCH H., URBAN B.W., GÖTHERT M. Inhibition of 5-HT3 receptor cation channels by ifenprodil in excised patches of N1E-115 cells. Naunyn Schmiedeberg's Arch. Pharmacol. 1998;358:145–152. doi: 10.1007/pl00005236. [DOI] [PubMed] [Google Scholar]
- BARANN M., MEDER W., DORNER Z., BRÜSS M., BÖNISCH H., GÖTHERT M., URBAN B.W. Recombinant human 5-HT3A receptors in outside-out patches of HEK 293 cells: basic properties and barbiturate effects. Naunyn Schmiedeberg's Arch. Pharmacol. 2000;362:255–265. doi: 10.1007/s002100000288. [DOI] [PubMed] [Google Scholar]
- BARANN M., MOLDERINGS G., BRÜSS M., BÖNISCH H., URBAN B.W., GÖTHERT M. Direct inhibition by cannabinoids of human 5-HT3A receptor: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- CHEMIN J., MONTEIL A., DUBEL S., NARGEOT J., LORY P. The alpha1I T-type calcium channel exhibits faster gating properties when overexpressed in neuroblastoma/glioma NG 108-15 cells. Eur. J. Neurosci. 2001;14:1678–1686. doi: 10.1046/j.0953-816x.2001.01796.x. [DOI] [PubMed] [Google Scholar]
- COSTALL B., NAYLOR R.J.Neuropharmacology of 5-HT3 receptor ligands Serotonergic neurons and 5-HT receptors in the CNS 1997Berlin: Springer-Verlag; 409–438.ed. Baumgarten, G.H. & Göthert, M [Google Scholar]
- FAN P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in the rat nodose ganglion neurons. J. Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R. MDL 72222: a potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1984;326:36–44. doi: 10.1007/BF00518776. [DOI] [PubMed] [Google Scholar]
- GODLEWSKI G., KWOLEK G., MALINOWSKA B., BARANN M., MOLDERINGS G.J., URBAN B.W., GÖTHERT M. Inhibitory effects of cannabinoid receptor agonists on the 5-HT3 receptor-mediated Bezold-Jarisch reflex in rats and human 5-HT3A receptors in HEK-293 cells. Naunyn Schmiedebergs Arch. Pharmacol. 2002;365 Suppl.1:R28. [Google Scholar]
- KARIM F., ROERIG S.C., SAPHIER D. Role of 5-hydroxytryptamine3 (5-HT3) antagonists in the prevention of emesis caused by anticancer therapy. Biochem. Pharmacol. 1996;52:685–692. doi: 10.1016/0006-2952(96)00346-2. [DOI] [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- LEE L.Y., LUNDBERG G.M. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anaesthetized rats. J. Appl. Physiol. 1994;76:1848–1855. doi: 10.1152/jappl.1994.76.5.1848. [DOI] [PubMed] [Google Scholar]
- MAINGRET F., PATEL A.J., LAZDUNSKI M., HONORE E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOWSKA B., GODLEWSKI G., BÜCHER B., SCHLICKER E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., GODLEWSKI G., BUCZKO W., GÖTHERT M. Facilitation by substance P and inhibition by (+)-tubocurarine of the 5-HT3 receptor-mediated Bezold-Jarisch reflex in rats. Eur. J. Pharmacol. 1996;315:159–164. doi: 10.1016/s0014-2999(96)00604-8. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., KWOLEK G., GÖTHERT M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001a;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., PISZCZ J., KONECZNY B., HRYNIEWICZ A., SCHLICKER E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic opioid OP4 and cannabinoid CB1 receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 2001b;364:233–241. doi: 10.1007/s002100100450. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Neuropharmacology and therapeutic potential of cannabinoids. Addiction Biol. 2000;5:37–46. doi: 10.1080/13556210071252. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoid receptors and pain. Progress Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- POLING J.S., ROGAWSKI M.A., SALEM N., JR, VICINI S. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology. 1996;35:983–991. doi: 10.1016/0028-3908(96)00130-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HÉAULME M., ALONSO R., SHIRE D., CONGY S., SORUBRIÉ P., BRELIÈRE J.C., LE FUR, G. Biochemical and pharmacological characterisation of SR 141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- SÉVOZ C., NOSJEAN A., CALLERA J.C., MACHADO B., HAMON M., LAGUZZI R. Stimulation of 5-HT3 receptors in the NTS inhibits the cardiac Bezold–Jarisch reflex response. Am. J. Physiol. 1996;271:H80–H87. doi: 10.1152/ajpheart.1996.271.1.H80. [DOI] [PubMed] [Google Scholar]
- SIMPSON K., SPENCER C.M., MCCLELLAN K.J. Tropisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2000;59:1297–1315. doi: 10.2165/00003495-200059060-00008. [DOI] [PubMed] [Google Scholar]
- TOWNSEND DEW., IV, THAYER S.A., BROWN D.R. Commentary. Cannabinoids throw up a conundrum. Br. J. Pharmacol. 2002;137:575–577. doi: 10.1038/sj.bjp.0704913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGA K., LAKE K., MARTIN B.R., KUNOS G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur. J. Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- VOOG O., ALSTERGREN P., LEIBUR E., KALLIKORM R., KOPP S. Immediate effects of the serotonin antagonist granisetron on temporomandibular joint pain in patients with systemic inflammatory disorders. Life Sci. 2000;68:591–602. doi: 10.1016/s0024-3205(00)00965-6. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARCO V., JULIUS D., HÖGSTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


