Abstract
Photorelaxation is the reversible relaxation of vascular smooth muscle (VSM) when irradiated with ultraviolet (UV) light resulting from the release of nitric oxide (NO). In this study we characterize the involvement of endothelial nitric oxide synthase (eNOS) in the photorelaxation response of thoracic aorta from endothelial NOS deficient (−/−) and control (C57BL/6j) mice.
Cirazoline contracted aortae were repeatedly exposed to 30 s of UV light every 3–4 min. Equal levels of photorelaxation (45±2%; n=34) was observed in both strains.
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), K+, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO), 4-aminopyridine (4-AP) and ethacrynic acid significantly reduced the photorelaxation response. In C57BL/6j mice diethyldithiocarbamate (DETCA) also reduced photorelaxation.
Control endothelium-intact and -denuded aorta and L-NAME (100 μM) treated and untreated eNOS (−/−) aortae were repeatedly exposed to UV light for 5 min every 10 min until no photorelaxation response was observed. After 1 h of rest in the dark the vessels showed between 30–70% recovery of the photorelaxation response indicating regeneration of the store in the absence of the endothelium and eNOS.
The results of this study suggest that photorelaxation in mouse aorta VSM results from the release of NO from a stable store of RSNOs, which activates soluble guanylate cyclase (sGC), leading to cGMP-dependent relaxation that is partially mediated by an increase in KV channel activation and hyperpolarization. In addition, the eNOS isoform is not essential for the formation of the photorelaxation store and a non-NOS source of NO may be involved in the maintenance of this store.
Keywords: Photorelaxation, UV light, mouse aorta, S-nitrosothiols, eNOS deficient mice
Introduction
First described by Furchgott et al. (1955), photorelaxation is the reversible relaxation of smooth muscle in response to an exposure to ultraviolet (UV) light. Subsequent studies have shown that photorelaxation is correlated with an increase in guanosine 3′5′ cyclic monophosphate (cGMP) levels (Karlsson et al., 1984; Furchgott et al., 1985) and reduced by scavengers of NO such as haemoglobin (HbO) and methylene blue (Matsunaga & Furchgott, 1989). Given this evidence it was not surprising that in response to UV light NO release from rat aortic rings has been detected with a porphorynic sensor (Kubaszewski & Malinski, 1994). However, studies have shown that photorelaxation is not inhibited by nitric oxide synthase (NOS) inhibitors such as NG-monomethyl-L-arginine (L-NMMA) (Matsunaga & Furchgott, 1989) but in some cases is enhanced (Chen & Gillis, 1992; Chang et al., 1993) suggesting that photorelaxation is not directly dependent upon activation of NOS.
It was initially thought that photorelaxation was endothelium-independent (Matsunaga & Furchgott, 1989). However, subsequent studies revealed that photorelaxation, in the absence of endothelium, could be progressively reduced upon repeated exposures to light (Venturini et al., 1993). In addition, endothelium-intact rat tail artery, depleted of its photorelaxation stores, recovered slowly in the dark whereas those treated with L-NNA did not, leading to the postulation that eNOS was essential for repriming the NO store utilized by photorelaxation (Megson et al., 1995; 2000). Conversely, it has been reported that the endothelium can attenuate photorelaxation through an as yet undetermined mechanism (Chang et al., 1997). These findings present a confusing picture on the role of the endothelium and NOS in the photorelaxation response and demonstrate the need for further studies.
It has also been suggested that S-nitrosothiols (RSNOs) are the tissue source of NO utilised in photorelaxation (Kubaszewski et al., 1994; Megson et al., 1995), which was later confirmed by the studies of Lovren & Triggle (1998) and Megson et al. (2000). RSNOs, under suitable conditions can either decompose to liberate NO or be involved in transnitrosation and S-thiolation reactions where they can mediate signal transduction and enzyme activity (for review see Hogg, 2002). Typically, they are biologically stable molecules and the sulphur nitrogen covalent bond is not usually sensitive to homolysis. However, when RSNOs are irradiated by strong direct light, the sulphur nitrogen bond undergoes a homolytic cleavage to yield a disulphide and NO (Williams, 1985; Sexton et al., 1994). The identification of the smooth muscle source of RSNOs and the physiological modulators that regulate its release may provide a novel target for pharmacological manipulation in conditions where NO bioavailability is reduced. Although it is unlikely that UV light is the physiological mediator for the release of NO from the RSNO ‘store', it provides a convenient tool for the study into the mechanisms underlying its formation. Therefore, in this study we examine photorelaxation in aortic rings from eNOS deficient mice to determine the contribution of eNOS to the formation of the ‘store' of RSNOs in vascular smooth muscle.
Methods
Animal sources
Wild type (WT) genetic background strain (C57BL/6j) mice were used as controls and matched for size for endothelial NOS deficient (eNOS (−/−)) mice. All mice were purchased from The Jackson Laboratory (ME, U.S.A.). Protocols for the animal used were conducted in accordance with the University of Calgary Animal Care Committee guidelines.
Isolated mouse aorta preparation
Male mice (25–35 g) were killed via cervical dislocation. The thoracic aorta was excised into ice-cold modified Krebs' solution (composition (mM): NaCl 118, KCl 4.7, MgSO4 1.2, NaHCO3 25, KH2PO4 0.8, CaCl2 2.5 and glucose 11; pH=7.4) and gassed with carbogen (95% O2, 5% CO2). Aortae were cleaned of connective tissue and fat and four rings of 3 mm were cut and mounted on two wires in a small vessel myograph (J.P. Trading, Denmark), containing Krebs buffer and warmed to 37°C. The rings were placed under a 5 mN resting tension for a 1 h equilibration period. Isometric measurements of vessel force were captured online via the use of Myodaq 2.01 data acquisition system and later analysed with Myodata 2.02.
Light source
The radiation source for photorelaxation was a halogen dissecting lamp generating polychromatic light (0.17 W/cm2). Light was beamed through a bifurcated fibre optic light guide at the tissues from a distance of 5 cm. Light intensity was measured with a radiometer (IL 1700, Ealing Scientific).
Experimental protocol
Tissues were contracted with a potassium depolarizing solution (KPSS: composition (mM): KCl 123, MgSO4 1.2, NaHCO3 25, KH2PO4 0.8, CaCl2 2.5 and glucose 11) to determine their viability. Concentration-response curves cirazoline (0.01–50 μM) were constructed to determine the concentration needed to contract the tissue to 50% of its maximum (30–300 nM). Then tissues were submaximally contracted with cirazoline and a single dose of acetylcholine (10 μM) added to assess the responsiveness of the endothelium (no response was expected in eNOS (−/−) mice (Huang et al., 1995)). Tissues were submaximally contracted with cirazoline and the photorelaxation response was determined. Three different protocols were used. (1) Five 30 s exposures every 3–4 min or (2) three 5 min exposures every 10 min. In both these protocols tissues were then washed and allowed to recover for 1 h in the dark and the same protocol was repeated in the absence or presence of inhibitors. (3) Three 5 min exposures every 10 min until the photorelaxation response was no longer seen followed by 1 h recovery in the dark and then three 5 min exposures every 10 min to assess the effect of the rundown of the photorelaxation store. In protocol (1) when inhibitors were used they were added 20 min prior to submaximally constricting the tissue with cirazoline while in protocols (2) and (3) inhibitors were added immediately after the last of the control exposures to UV light. In each experiment one tissue served as a control and was not exposed to any inhibitors.
Data analysis and statistics
Photorelaxation responses are expressed as a percentage reversal of the cirazoline precontraction. Since the nature of the photorelaxation response was altered during the 5 min exposures to UV light, in protocol 2 & 3, two measurements were taken. An initial photorelaxation response was measured immediately after the beginning of the photorelaxation response (initial) and another measurement was taken 5 min after the commencement of the photorelaxation response (5 min). Results in the text are given as mean±s.e.mean. Paired or unpaired Student's t-tests were used to test several values that were dependent. All statistical calculations were performed using SigmaStat (Jandal Scientific, U.S.A.). Values were considered statistically significant if P<0.05.
Drugs
Acetylcholine; 4-aminopyridine (4-AP); 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO); cirazoline; diethyldithiocarbamate (DETCA); ethacrynic acid; L-cysteine; 7-nitroindazole (7-NI); NG-nitro-L-arginine methyl ester (L-NAME); 1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ); sodium nitroprusside (SNP); polyethyleneglycol-superoxide dismutase (PEG-SOD) and tetraethylammonium chloride (TEA) were purchased from Sigma (MA, U.S.A.).
Results
Photorelaxation response
In response to repeated exposure to 30 s irradiation's of UV light every 3–4 min, segments of mouse aorta from both strains relaxed (45±3%; n=18) in a reversible manner which was not significantly reduced after 10 exposures (n=5–12; P>0.05; Student's t-test; Figure 1a, b). In addition, there was no significant difference between the level of photorelaxation of the eNOS (−/−) mice and their WT control (n=6–12; P>0.05; Student's t-test; Figure 1b).
Figure 1.
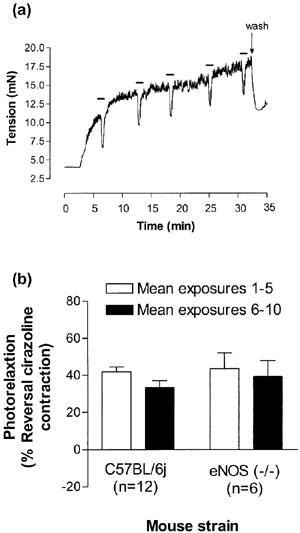
(a) An original recording illustrating changes in tension (mN) in cirazoline contracted aorta from C57BL/6j mice in response to 30 s exposures to UV light (Protocol 1). Bars represent periods of illumination with UV light. (b) Photorelaxation responses in thoracic aortae from eNOS (−/−) and their WT control (C57BL/6j) represented as percentage of reversal of the cirazoline contraction. Open bars represent the mean of 30 s UV light exposures 1–5 and the closed bars the mean of exposures 6–10 (Protocol 1). There were no significant differences in photorelaxation responses between strains (n=6–12; P>0.05; Student's t-test).
In a separate series of experiments the effect of three exposures of UV light for 5 min every 10 min was examined in aorta from endothelium-intact and -denuded C57BL/6j and endothelium-intact eNOS (−/−) mice (Protocol 2). The photorelaxation response in endothelium-intact control aorta was comprised of an initial transient vasorelaxation (42±3%) followed by a rebound contraction by the end of the 5 min irradiation period (−5+6%; n=4; P<0.05; Student's t-test; Figures 2 and 3). Conversely, the photorelaxation was maintained for the duration of the UV irradiation in endothelium-denuded control (51±3% initial vs 47±4% at 5 min; n=6; Figure 3) and eNOS (−/−) vessels (52±9% initial vs 60±9% at 5 min; n=6; P>0.05; Student's t-test; Figure 2b and 3).
Figure 2.
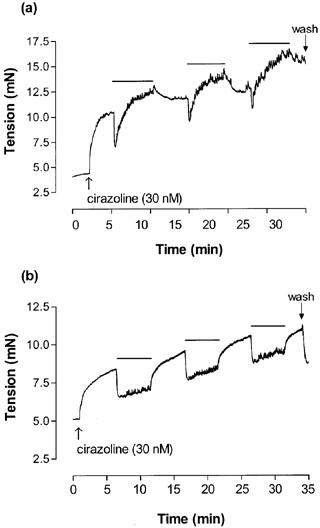
An original recording illustrating changes in tension (mN) in cirazoline contracted thoracic aortae from (a) C57BL/6j mice and (b) eNOS (−/−) mice in response to 5 min exposures to UV light (Protocol 2). Bars represent periods of illumination with UV light.
Figure 3.
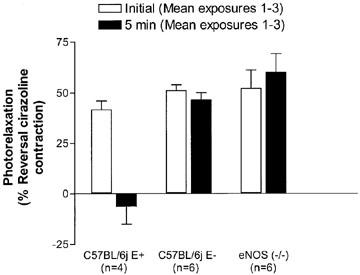
Photorelaxation responses in endothelium-denuded (E−) and -intact (E+) thoracic aortae from C57BL/6j mice and eNOS(−/−) mice represented as percentage of reversal of the cirazoline contraction (Protocol 2). After three consecutive 5 min exposures to UV light, responses at the beginning (open bars, initial) and end (closed bars, 5 min) of each illumination period were averaged (*P<0.05; Student's t-test; n=4–6).
Characterization of the photorelaxation response
The effects of the soluble guanylate inhibitor, ODQ (10 μM), high extracellular K+ (30 mM), the NO scavenger, c-PTIO (50 μM), the voltage-dependent K+ (KV) channel inhibitor, 4-AP (0.5 mM), and the thiol alkylating agent ethacrynic acid (50 μM) were assessed in control and eNOS (−/−) mice using Protocol 1. All inhibitors significantly reduced photorelaxation in aortae from both eNOS (−/−) and C57BL/6j mice (n=3–12; P<0.05; Student's t-test; Figure 4). In addition the effect of the nitroxyl anion scavenger L-cysteine (3 mM), the nNOS inhibitor 7-NI (0.1 mM), the large Ca2+ activated K+ (BKCa) channel inhibitor TEA (1 mM), the SOD inhibitor DETCA (6 mM) and denuding the vessels (E−) on photorelaxation were examined in aortae from control C57BL/6j mice (n=4–5; Figure 4). DETCA significantly reduced photorelaxation (43±8 control vs 8±1% treated; n=4; P<0.05; Student's t-test) whereas L-cysteine (42±4% control vs 44±4% treated; n=5), 7-NI (47±3% control vs 36±2% treated; n=4), TEA (35±2% control vs 29±2% treated; n=4) and denuding the vessels (48±6%; n=5) had no significant effect (n=4–5; P>0.05; Student's t-test).
Figure 4.
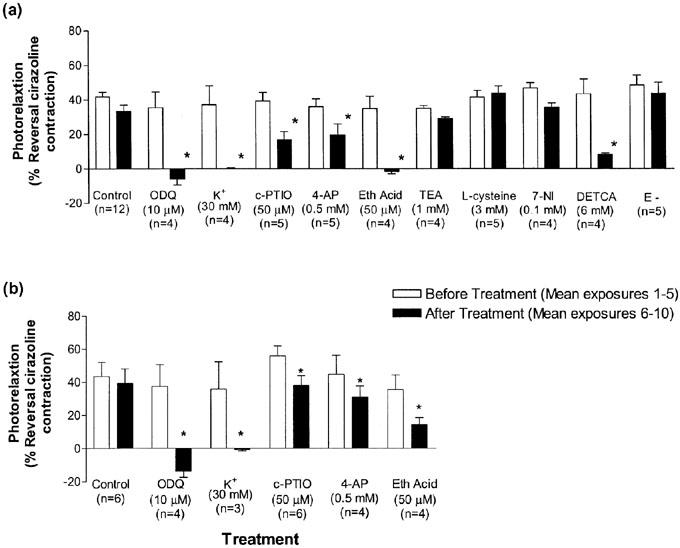
Effects of inhibitors on the photorelaxation response (30 s exposures to UV light represented as percentage of reversal of the cirazoline contraction) in (a) thoracic aortae of C57BL/6j mice and (b) eNOS (−/−) mice (Protocol 1). Open bars represent the mean of photorelaxations 1–5 and the closed bars represent the mean of photorelaxations 6–10 after treatment with inhibitors. (*P<0.05 vs before treatment response; Student's paired t-test; n=4–12).
The differences in the appearance of the photorelaxation response were investigated using Protocol 2 in endothelium-intact WT aortae. There was no significant difference between the initial level of photorelaxation in control, endothelium-denuded, L-NAME (100 μM), and PEG-SOD treated aorta (n=4–6; P>0.05; Student's t-test; Figure 5). However, as similarly reported for Protocol 1, DETCA (6 mM) and c-PTIO (50 μM) significantly reduced the initial photorelaxation response and in this protocol, also increased the rebound contraction response at 5 min (n=4; P<0.05; Student's t-test; Figure 5). Conversely, L-NAME (100 μM) significantly reversed the rebound contraction seen in control vessels as the photorelaxation response was then maintained over the period of irradiation (n=6; P<0.05; Student's t-test; Figure 5).
Figure 5.
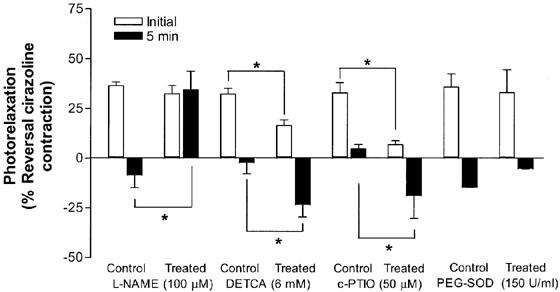
Effects of inhibitors on the photorelaxation responses (5 min exposures to UV light; Protocol 2) in thoracic aortae from C57BL/6j mice represented as percentage of reversal of the cirazoline contraction. After three consecutive 5 min exposures to UV light, responses at the beginning (open bars, initial) and end (closed bars, 5 min) of each illumination period were averaged (*P<0.05; Student's t-test; n=4–6).
Repriming of the photorelaxation store
This series of experiments was performed to assess the ability of the aorta to reprime the photorelaxation store and recover a response and assess the contribution of eNOS to repriming. C57BL/6j E+ and E− and eNOS (−/−) vessels were repeatedly exposed to UV light for 5 min every 10 min until no photorelaxation response was observed (Protocol 3). After 1 h of rest in the dark all the aortae were then challenged with three 5 min exposures to UV light to determine the effect of rundown of the photorelaxation store. Although after 1 h of rest the initial level of photorelaxation was significantly different from control levels, all vessels showed partial recovery of the photorelaxation response (Control E+, 38%; Control E−, 71% and eNOS (−/−), 28%; n=4–6; P<0.05; Student's t-test; Figure 6). In addition, aorta from eNOS (−/−) mice, treated with the non-selective NOS inhibitor, L-NAME (100 μM) immediately after rundown of the photorelaxation store also showed partial recovery of the photorelaxation store (40%; n=4; P<0.05; Student's t-test; Figure 6). In some instances, after the photorelaxation store was ‘rundown' the effect of the NO donor SNP (10 μM) was examined. Previous studies with aortae from eNOS (−/−) mice have profiled the concentration-response to SNP (Waldron et al., 1999). There was no significant difference in the effect between the level of relaxation before or after rundown (data not shown).
Figure 6.
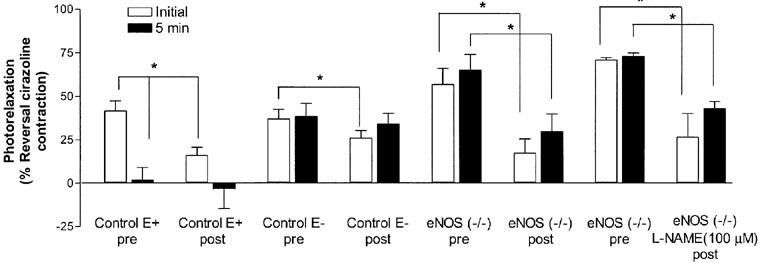
Regeneration of the photorelaxation store following continuous exposures to UV light (5 min every 5 min) in thoracic aortae of control (C57BL/6j) endothelium-intact (E+) and -denuded (E−), eNOS (−/−) and L-NAME (100 μM)-treated eNOS (−/−) mice (Protocol 3). Aortae were exposed to UV light until a photorelaxation response (represented as percentage of reversal of the cirazoline contraction) was no longer observed and then left to recover in the dark for 1 h. All aortae were then exposed to three 5 min exposures to UV light to determine the effect of rundown on the photorelaxation store. After three consecutive 5 min exposures to UV light, responses at the beginning (open bars, initial) and end (closed bars, 5 min) of each illumination period were averaged either before rundown (pre) or 1 h after rundown (post) (*P<0.05; Student's t-test; n=4–6).
Discussion
This study is the first to characterize photorelaxation in mouse aorta and as such there were three main objectives: (1) to characterize the underlying mechanisms of photorelaxation, (2) to investigate the differences in photorelaxation in endothelium-intact and -denuded aorta and (3) to investigate the involvement of eNOS in the formation and repriming of the photorelaxation. The main finding from this study is that photorelaxation was observed in aortae from both strains of mice examined and no significant difference in the level of photorelaxation were observed. These findings indicate that in contrast to findings in rat tail arteries where eNOS was considered essential for the formation of a photorelaxation ‘store' (Megson et al., 1995), neither eNOS nor, based on the failure of both L-NAME and 7-NI to inhibit photorelaxation, any one NOS isoform is essential for the formation and maintenance of the store in mouse aorta. We have also previously reported that photorelaxation is preserved in aortae from nNOS (−/−), the control strain for nNOS (−/−), B6;129SF2, and iNOS (−/−) mice thus providing additional evidence that no single isoform of NOS is essential for providing the NO source for photorelaxation (Andrews et al., 2002).
In mouse aorta, NO, derived from a source other than eNOS, is the predominant, possibly sole mediator of the photorelaxation response since the NO scavenger, c-PTIO, which selectively inactivates the free radical form of NO (Akaike et al., 1993), significantly inhibited the photorelaxation response in both strains of mice examined. Because c-PTIO did not completely inhibit photorelaxation it is possible that other redox forms of NO may be involved. Alternatively, the nitrite and nitrate formed as end products while using c- PTIO might produce NO in the presence of UV light. However, it does not appear likely that other redox forms of NO are involved in photorelaxation because L-cysteine (3 mM), a scavenger of the nitroxyl anion (Pino & Feelisch, 1994) did not significantly reduce photorelaxation.
Once released, NO targets the haem moiety of soluble guanylate cyclase (sGC) in vascular smooth muscle cells, and thus, activates sGC, leading to an increase in cGMP which acts as a second messenger in a cascade of phosphorylation events resulting in vasodilation. It has been reported that ODQ and methylene blue, inhibitors of sGC, reduce photorelaxation (Charpie et al., 1994; Furchgott et al., 1985; Lovren & Triggle, 1998), have no effect (Goud et al., 1996) or even potentiate photorelaxation (Chen & Gillis, 1993). Furthermore, it has been shown that photorelaxation increases cGMP levels in rabbit and rat aortae through the activation of sGC (Karlsson et al., 1984; Furchgott et al., 1985; Chen & Gillis, 1992). In keeping with the majority of these reports, ODQ (at a concentration specific for sGC (Feelisch et al., 1999)) completely abolished photorelaxation in both strains of mice indicating that activation of sGC is a prerequisite to photorelaxation.
The inhibition of photorelaxation of aortae from both strains of mice by elevated concentrations extracellular K+ (30 mM) suggests a membrane hyperpolarization-dependent response. This finding is consistent with those of other investigators who have also reported the inhibition of photorelaxation following contractions by various concentrations of K+ (Furchgott et al., 1961; Charpie et al., 1994; Chang et al., 1997; Lovren & Triggle, 1998). Furthermore, 4-AP (0.5 mM), at a concentration which is selective for KV channels (Beech & Bolton, 1989) reduced the photorelaxation response whereas TEA (1 mM) at a concentration selective for BKCa channels (Langton et al., 1991) had no significant effect. These results support a mechanism in mouse aorta whereby NO activates sGC, leading to cGMP-dependent increase in KV channel activation, which would be expected to result in membrane hyperpolarization and relaxation of vascular smooth muscle. However, 4-AP alone did not completely inhibit photorelaxation and thus cellular events other than hyperpolarization mediated by the cGMP-dependent opening of KV channels may also be involved in mediating photorelaxation.
It is believed that RSNOs stored in vascular smooth muscle may be the tissue source of the NO released during photorelaxation. In support of this hypothesis is the finding that after photorelaxation stores were exhausted, pretreatment of rat aorta with the thiol alkylating agent ethacrynic acid inhibited the regeneration of the store (Megson et al., 1995). In addition, studies conducted with rat aorta in our laboratory examined the effect of a variety of different RSNO depleting agents, including ethacrynic acid and altering conditions such as pH, temperature and oxygen levels all of which are known to affect RSNO stability (Lovren & Triggle, 1998). All of these conditions reduced photorelaxation, suggesting that RSNOs are the tissue source of NO utilized in photorelaxation. Finally, when rats were treated with agents that affect biosynthesis of glutathione, a precursor for the synthesis of the RSNO S-nitrosoglutathione, it was reported that the photorelaxation response in the aorta was enhanced or reduced accordingly (Megson et al., 2000). In the present study, ethacrynic acid also reduced photorelaxation in all strains of mice examined indicating that in mouse aorta RSNOs also play a role, and no particular NOS isoform is essential for the formation of a RSNO store in vascular smooth muscle.
The photorelaxation response in aortae with endothelium comprises a transient relaxation followed by a rebound contraction. Surprisingly, the rebound contractile phase that followed photorelaxation during the 5 min period of irradiation in aortae with endothelium was reduced in the (a) L-NAME-treated aorta with endothelium, (b) aorta without endothelium and (c) aorta from eNOS (−/−) mice. Therefore, this contraction appears to be dependent upon the activity of eNOS. This finding is in agreement with studies conducted in the rat aorta where UV light induced contractions in endothelium-intact but not -denuded tissues and as in the present study the contraction was inhibited by L-NAME or L-NMMA (Chang et al., 1997). It may be argued that the NOS inhibitors used in these studies may themselves release NO. It is well documented that NO2 moiety found in L-NNA can decompose upon irradiation with UV light to release NO and thus, enhance photorelaxation in a variety of smooth muscle preparations (Chen & Gillis, 1992). However, it has been reported that L-NAME (100 μM), when exposed to UV light produces only a negligible amount of NO when compared to the same concentration of L-NA (Bauer & Fung, 1994). In the present study, L-NAME (100 μM) was used and since no potentiation of photorelaxation was observed, it appears that, under the conditions that photorelaxation was examined in this study, L-NAME did not release a physiologically significant amount of NO. The mediator of the contraction is not known nor is it clear as to the role of NO synthase in this phenomenon.
One could speculate that superoxide (O2−) or peroxynitrite (ONOO−) production by a UV light-induced uncoupling of NO synthase from regular catalysis of substrates might be responsible i.e. through lipid and protein peroxidations. SOD catalyzes the reaction of O2− to form hydrogen peroxide (H2O2). To date there have been mixed results reported with respect to the effects of SOD which include both a failure to affect photorelaxation (Chen & Gillis, 1992) and the enhancement of photorelaxation (Matsunaga & Furchgott, 1991; Chang et al., 1993). In this study, membrane permeable PEG-SOD (150 U/ml) had no significant effect on the initial photorelaxation response or the rebound contraction in endothelium-intact aortae. However, DETCA (6 mM), a cell permeable inhibitor of SOD, reduced the initial photorelaxation and enhanced the contraction indicating that O2− or ONOO− generation may be involved in the rebound contraction. c-PTIO (50 μM) also enhanced the rebound contraction, however, this finding is almost certainly due to the decrease in NO availability, rather than an effect on the mediator of the contractile effect.
When the photorelaxation store was depleted in control endothelium-denuded and eNOS (−/−) control and L-NAME (100 μM) treated eNOS (−/−) aorta after 1 h of rest in the dark the photorelaxation response was partially restored, indicating regeneration of the store in the absence of the endothelium, eNOS and NOS, respectively. These findings are in contrast to studies in rat aorta where treatment with L-NMMA (100 μM) or the NO scavenger HbO (5 μM) after the photorelaxation store had been depleted prevented the repriming of the store (Megson et al., 1995). It is possible that in the present study the RSNO ‘store' was not totally exhausted, environmental nitrogen oxides are contributing to the regeneration of the ‘store' or that the vascular smooth muscle has become increasingly insensitive to NO. However, the latter would be unlikely since the sensitivity of photorelaxation-depleted vessels to the NO donor, SNP, remained unaltered. The current study has demonstrated that photorelaxation in mouse aortae is independent of the endothelium and also independent of any one isoform of NOS. The question thus arises as to what is the origin of the NO released by photoactivation? Is it from a non-NOS enzymatic source, an enzyme-independent chemical reaction or is the cellular store of NO derived from multiple sources of NO such that the loss of any one NOS isoform does not affect the viability of the store?
Potential non-NOS enzymatic sources include cytochrome P450 (CYP) and xanthine oxidase. CYP, presumably due to its structural similarity to NOS, has been reported to be able to generate NO in smooth muscle in a NADPH and oxygen-dependent manner (Jia et al., 1998; Keseru et al., 2000; Taylor et al., 2001). Xanthine oxidase is thought to catalyze the reduction of nitrite to generate NO in the presence of NADH (Zhang et al., 1997; 1998). There are also several reports of the generation of NO via non-enzymatic pathways, including reactions between L or D-arginine and H2O2 (Nagase et al., 1997) and the reduction of nitrite to NO (Zweier et al., 1995). In addition, the basal release of NO from rat mesenteric smooth muscle cells has been detected via chemiluminescence suggesting that vascular smooth muscle can generate NO in the absence of the endothelium (Mendizabal et al., 2000).
Clearly further studies are required, however, it is of interest that ‘rundown' of the store combined with treatment with a NOS inhibitor failed to prevent the photosensitive store from being restored. This finding suggests that the NO store is most likely derived, at least in part, from a non-NOS source. Support for this hypothesis is provided by our data that photorelaxation in aortae from eNOS (−/−) mice is unaltered and yet aortae from these mice fail to relax to acetylcholine indicating that eNOS is the sole source of the NO released by endothelium-dependent vasorelaxants (Huang et al., 1995).
Physiological implications
Several questions still remain concerning the nature and physiological relevance of the photosensitive NO store. Thus, what is the functional relevance of a RSNO in vascular smooth muscle and can this store of NO be manipulated pharmacologically to be presented as a potential novel treatment for many different pathophysiological conditions, particularly where increased NO release would be beneficial? It has been proposed that there could be beneficial effects from the photochemotherapeutic application of UV light in disease states such as leukaemia (Sexton et al., 1994). Although the use of UV light appears to be a convenient tool for the assay of RSNOs in vascular smooth muscle, it is unlikely that UV light is the physiological stimulus in vivo that induces the release of NO from this store. Which leads to the question of what is the physiological stimulus? Further studies are required to address this question.
To date, there are several theories as to how NO may be released from RSNOs in vivo; however, it is important to note that these findings are largely from studies carried out in vitro. The enzyme γ-glutamyl transpeptidase has been implicated in some tissues (Askew et al., 1995; Lipton et al., 2001), but not others (Gordge et al., 1995; Hogg et al., 1997), indicating possible tissue-dependent differences for the expression of this enzyme. In addition, CuZn-SOD (Jourd'heuil et al., 1999; Singh et al., 1999), the thioredoxin system (Nikitovic & Holmgren, 1996), the putative enzyme ‘GSNO lysase' (Gordge et al., 1998), and xanthine oxidase (Trujillo et al., 1998) have all been suggested as catalysts for the release of NO. However, perhaps the most promising finding to date is the identification of glutathione-dependent formaldehyde dehydrogenase as a highly specific modulator of GSNO, controlling intracellular levels of both GSNO and other RSNOs (Jensen et al., 1998; Keseru et al., 2000). Moreover, when the gene for this enzyme is deleted in mice, increased levels of GSNO and RSNOs were detected together with decreased release of NO from GSNO (Keseru et al., 2000).
Another important finding is that the photorelaxation responses in rat aorta of stroke-prone spontaneously hypertensive rats were significantly enhanced when compared to control (Charpie et al., 1994; Kubaszewski & Malinski, 1994). These studies indicate that the photosensitive NO store may not be reduced in disease states in parallel with the reduction in endothelium-derived NO that is typically observed. Therefore, the RSNO ‘store' in vascular smooth muscle may be a new target for the release of NO in many pathophysiological conditions where NO production is impaired.
Conclusions
The sensitivity of photorelaxation in vascular smooth muscle of mouse aorta to inhibitors such as c-PTIO, ethacrynic acid, ODQ, high extracellular K+ and 4-AP suggests that photorelaxation involves the release of NO, derived from RSNOs, evidently from a stable form of NO, which mediates a guanylate cyclase-dependent relaxation mediated, in part, by the activation of K+ channels, notably KV channels, and reversible vasorelaxation. In addition it does not appear that the eNOS isoform, or any one specific NOS isoform, is essential for the formation of the photorelaxation store and that a non-NOS source of NO may be involved in the maintenance of this store.
Acknowledgments
These studies were supported by the Canadian Hypertension Society/Canadian Institutes of Health Research/Merck Frosst Postdoctoral Fellowship (to K.L. Andrews), the Astra/Zeneca/Heart and Stroke Foundation/Canadian Institutes of Health Research Postdoctoral Fellowship (to J.J. McGuire) and the research funding (to C.R. Triggle) from the Heart and Stroke Foundation of Canada.
Abbreviations
- 4-AP
4-aminopyridine
- 7-NI
7-nitroindazole
- BKCa
large conductance Ca2+ activated K+ channels
- c-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- CYP
cytochrome P450
- DETCA
diethyldithiocarbamate
- E−
endothelium-denuded
- E+
endothelium-intact
- eNOS
endothelial nitric oxide synthase
- HbO
haemoglobin
- iNOS
inducible nitric oxide synthase
- KV
voltage-dependent K+ channels
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- L-NNA
NG-nitro-L-arginine
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- O2−
superoxide
- ONOO−
peroxynitrite
- ODQ
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one
- PEG-SOD
polyethyleneglycol-superoxide dismutase
- RSNO
S-nitrosothiol
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- UV
ultraviolet
- WT
wild type
References
- AKAIKE T., YOSHIDA M., MIYAMOTO Y., SATO K., KOHNO M., SASAMOTO K., MIYAZAKI K., UEDA S., MAEDA H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor (NO) through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- ANDREWS K.L., MCGUIRE J.J., TRIGGLE C.R. Characterization of vascular smooth muscle photorelaxation in aorta from NOS knockout mice. The Pharmacologist. 2002;44:A214. [Google Scholar]
- ASKEW S.C., BUTLER A.R., FLITNEY F.W., KEMP G.D., MEGSON I.L. Chemical mechanisms underlying the vasodilator and platelet anti-aggregating properties of S-nitroso-N-acetyl-DL-penicillamine and S-nitrosoglutathione. Bioorg. Med. Chem. 1995;3:1–9. doi: 10.1016/0968-0896(94)00139-t. [DOI] [PubMed] [Google Scholar]
- BAUER J.A., FUNG H.L. Photochemical generation of nitric oxide from nitro-containing compounds: possible relation to vascular photorelaxation phenomena. Life Sci. 1994;54:L1–L4. doi: 10.1016/0024-3205(94)00578-8. [DOI] [PubMed] [Google Scholar]
- BEECH D.J., BOLTON T.B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J. Physiol. 1989;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG K.C., BONGGWAN S., LEE H.Y., KANG Y.J. Characterization of ultraviolet (UV) light-induced relaxation in rat aorta. Pharmacol. Rev. Commun. 1997;9:177–183. [Google Scholar]
- CHANG K.C., CHONG W.S., PARK B.W., SEUNG B.W., CHUN G.W., LEE I.J., PARK P.S. NO- and NO2-carrying molecules potentiate photorelaxation in rat trachea and aorta. Biochem. Biophys. Res. Commun. 1993;191:509–514. doi: 10.1006/bbrc.1993.1247. [DOI] [PubMed] [Google Scholar]
- CHARPIE J.R., PETERS A., WEBB R.C. A photoactivable source of relaxing factor in genetic hypertension. Hypertension. 1994;23:894–898. doi: 10.1161/01.hyp.23.6.894. [DOI] [PubMed] [Google Scholar]
- CHEN X., GILLIS C.N. Enhanced photorelaxation in aorta, pulmonary artery and corpus cavernosum produced by BAY K 8644 or N-nitro-L-arginine. Biochem. Biophys. Res. Commun. 1992;186:1522–1527. doi: 10.1016/s0006-291x(05)81579-7. [DOI] [PubMed] [Google Scholar]
- CHEN X., GILLIS C.N. Methylene blue enhanced photorelaxation in aorta, pulmonary artery and corpus cavernosum. Biochem. Biophys. Res. Commun. 1993;190:559–563. doi: 10.1006/bbrc.1993.1084. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., KOTSONIS P., DIEBE J., CLEMENT B., SCHMIDT H.H.H.W. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo-[4,3,-a]quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide bioactivation. Mol. Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., EHHREICH S.J., GREENBLATT E. The photoactivated relaxation of smooth muscle of rabbit aorta. J. Gen. Physiol. 1961;44:499–519. doi: 10.1085/jgp.44.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., MARTIN W., CHERRY P.D., JOTHIANANDAN D., VILLANI G.M.Endothelium-dependent relaxation, photorelaxation and cyclic GMP 5th International Symposium, Vascular Neuroeffector Mechanisms 1985Amsterdam: Elsevier; 105–114.eds. Bevan, J.A., Godfrain, T., Maxwell, R.A., Stoclet, J.C. & Worcel, M. [Google Scholar]
- FURCHGOTT R.F., SLEATOR W.J., MCCAMAN M.W., ELCHLEPP J. Relaxation of arterial strips by light and the influence of drugs on this photodynamic effect. J. Pharmacol. Exp. Ther. 1955;113:22. [Google Scholar]
- GORDGE M.P., ADDIS P., NORONHA-DUTRA A.A., HOTHERSALL J.S. Cell-mediated biotransformation of S-nitrosoglutathione. Biochem. Pharmacol. 1998;55:657–665. doi: 10.1016/s0006-2952(97)00498-x. [DOI] [PubMed] [Google Scholar]
- GORDGE M.P., MEYER D.J., HOTHERSALL J., NEILD G.H., PAYNE N.N., NORONHA-DUTRA A. Copper chelation-induced reduction of the biological activity of S-nitrosothiols. Br. J. Pharmacol. 1995;114:1083–1089. doi: 10.1111/j.1476-5381.1995.tb13317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUD C., WATTS S.W., WEBB R.C. Photorelaxation is not attenuated by inhibition of the nitric oxide-cGMP pathway. J. Vasc. Res. 1996;33:299–307. doi: 10.1159/000159157. [DOI] [PubMed] [Google Scholar]
- HOGG N. The biochemistry and physiology of s-nitrosothiols. Annu. Rev. Pharmacol. Toxicol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- HOGG N., SINGH R.J., KONOREV E., JOSEPH J., KALYANARAMAN B. S-Nitrosoglutathione as a substrate for gamma-glutamyl transpeptidase. Biochem. J. 1997;323:477–481. doi: 10.1042/bj3230477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG P.L., HUANG Z., MASHIMO H., BLOCH K.D., MOSKOWITZ M.A., BEVAN J.A., FISHMAN M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- JENSEN D.E., BELKA G.K., DU BOIS G.C. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 1998;331:659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIA Y., ZACOUR M., TOLLOCZKO B., MARTIN J.G. Nitric oxide synthesis by tracheal smooth muscle cells by a nitric oxide synthase-independent pathway. Am. J. Physiol. 1998;275:L895–L901. doi: 10.1152/ajplung.1998.275.5.L895. [DOI] [PubMed] [Google Scholar]
- JOURD'HEUIL D., LAROUX F.S., MILES A.M., WINK D.A., GRISHAM M.B. Effect of superoxide dismutase on the stability of S-nitrosothiols. Arch. Biochem. Biophys. 1999;361:323–330. doi: 10.1006/abbi.1998.1010. [DOI] [PubMed] [Google Scholar]
- KARLSSON J.O., AXELSSON K.L., ANDERSSON R.G. Effects of ultraviolet radiation on the tension and the cyclic GMP level of bovine mesenteric arteries. Life Sci. 1984;34:1555–1563. doi: 10.1016/0024-3205(84)90610-6. [DOI] [PubMed] [Google Scholar]
- KESERU G.M., VOLK B., BALOGH G.T. Cytochrome P450 catalyzed nitric oxide synthesis: a theoretical study. J. Biomol. Struct. Dyn. 2000;17:759–767. doi: 10.1080/07391102.2000.10506565. [DOI] [PubMed] [Google Scholar]
- KUBASZEWSKI E., PETERS A., MCCLAIN S., BOHR D., MALINSKI T. Light-activated release of nitric oxide from vascular smooth muscle of normotensive and hypertensive rats. Biochem. Biophys. Res. Commun. 1994;200:213–218. doi: 10.1006/bbrc.1994.1436. [DOI] [PubMed] [Google Scholar]
- LANGTON P.D., NELSON M.T., HUANG Y., STANDEN N.B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am. J. Physiol. 1991;260:H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- LIPTON A.J., JOHNSON M.A., MACDONALD T., LIEBERMAN M.W., GOZAL D., GASTON B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- LOVREN F., TRIGGLE C.R. Involvement of nitrosothiols, nitric oxide and voltage-gated K+ channels in photorelaxation of vascular smooth muscle. Eur. J. Pharmacol. 1998;347:215–221. doi: 10.1016/s0014-2999(98)00095-8. [DOI] [PubMed] [Google Scholar]
- MATSUNAGA K., FURCHGOTT R.F. Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J. Pharmacol. Exp. Ther. 1989;248:687–695. [PubMed] [Google Scholar]
- MATSUNAGA K., FURCHGOTT R.F. Responses of rabbit aorta to nitric oxide and superoxide generated by ultraviolet irradiation of solutions containing inorganic nitrite. J. Pharmacol. Exp. Ther. 1991;259:1140–1146. [PubMed] [Google Scholar]
- MEGSON I.L., FLITNEY F.W., BATES J., WEBSTER R. Repriming of vascular smooth muscle photorelaxation is dependent upon endothelium-derived nitric oxide. Endothelium. 1995;3:39–46. [Google Scholar]
- MEGSON I.L., HOLMES S.A., MAGID K.S., PRITCHARD R.J., FLITNEY F.W. Selective modifiers of glutathione biosynthesis and ‘repriming' of vascular smooth muscle photorelaxation. Br. J. Pharmacol. 2000;130:1575–1580. doi: 10.1038/sj.bjp.0703499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDIZABAL V.E., POBLETE I., LOMNICZI A., RETTORI V., HUIDOBRO-TORO J.P., ADLER-GRASCHINSKY E. Nitric oxide synthase-independent release of nitric oxide induced by KCl in the perfused mesenteric bed of the rat. Eur. J. Pharmacol. 2000;409:85–91. doi: 10.1016/s0014-2999(00)00789-5. [DOI] [PubMed] [Google Scholar]
- NAGASE S., TAKEMURA K., UEDA A., HIRAYAMA A., AOYAGI K., KONDOH M., KOYAMA A. A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and D- or L-arginine. Biochem. Biophys. Res. Commun. 1997;233:150–153. doi: 10.1006/bbrc.1997.6428. [DOI] [PubMed] [Google Scholar]
- NIKITOVIC D., HOLMGREN A. S-nitrosoglutathione is cleaved by the thioredoxin system with liberation of glutathione and redox regulating nitric oxide. J Biol. Chem. 1996;271:19180–19185. doi: 10.1074/jbc.271.32.19180. [DOI] [PubMed] [Google Scholar]
- PINO R.Z., FEELISCH M. Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO−) using L-cysteine. Biochem. Biophys. Res. Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- SEXTON D.J., MURUGANANDAM A., MCKENNEY D.J., MUTUS B. Visible light photochemical release of nitric oxide from S-nitrosoglutathione: potential photochemotherapeutic applications. Photochem. Photobiol. 1994;59:463–467. doi: 10.1111/j.1751-1097.1994.tb05065.x. [DOI] [PubMed] [Google Scholar]
- SINGH R.J., HOGG N., GOSS S.P., ANTHOLINE W.E., KALYANARAMAN B. Mechanism of superoxide dismutase/H(2)O(2)-mediated nitric oxide release from S-nitrosoglutathione–role of glutamate. Arch. Biochem. Biophys. 1999;372:8–15. doi: 10.1006/abbi.1999.1447. [DOI] [PubMed] [Google Scholar]
- TAYLOR H.J., CHAYTOR A.T., EDWARDS D.H., GRIFFITH T.M. Gap junction-sependent increases in smooth muscle cAMP underpin the EDHF phenomenon in rabbit arteries. Biochem. Biophys. Res. Commun. 2001;283:583–589. doi: 10.1006/bbrc.2001.4791. [DOI] [PubMed] [Google Scholar]
- TRUJILLO M., ALVAREZ M.N., PELUFFO G., FREEMAN B.A., RADI R. Xanthine oxidase-mediated decomposition of S-nitrosothiols. J. Biol. Chem. 1998;273:7828–7834. doi: 10.1074/jbc.273.14.7828. [DOI] [PubMed] [Google Scholar]
- VENTURINI C.M., PALMER R.M., MONCADA S. Vascular smooth muscle contains a depletable store of a vasodilator which is light-activated and restored by donors of nitric oxide. J. Pharmacol. Exp. Ther. 1993;266:1497–1500. [PubMed] [Google Scholar]
- WALDRON G.J., DING H., LOVREN F., KUBES P., TRIGGLE C.R. Acetylcholine-induced relaxation of arteries isolated from mice lacking endothelial nitric oxide synthase. Br. J. Pharmacol. 1999;128:653–658. doi: 10.1038/sj.bjp.0702858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D.L. S-nitrosation and the reaction of S-nitroso compounds. Chem. Soc. Rev. 1985;14:171–196. [Google Scholar]
- ZHANG Z., NAUGHTON D.P., BLAKE D.R., BENJAMIN N., STEVENS C.R., WINYARD P.G., SYMONS M.C., HARRISON R. Human xanthine oxidase converts nitrite ions into nitric oxide (NO) Biochem. Soc. Trans. 1997;25:524S. doi: 10.1042/bst025524s. [DOI] [PubMed] [Google Scholar]
- ZHANG Z., NAUGHTON D., WINYARD P.G., BENJAMIN N., BLAKE D.R., SYMONS M.C. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem. Biophys. Res. Commun. 1998;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- ZWEIER J.L., WANG P., SAMOUILOV A., KUPPUSAMY P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]


