Abstract
The human dopamine D2long (D2L) receptor was expressed with four different G proteins in Sf9 cells using the baculovirus expression system. When co-expressed with Gi/Go G proteins (Gi1α, Gi2α, Gi3α, or Goα, plus Gβ1 and Gγ2), the receptor displayed a high-affinity binding site for the agonists (dopamine and NPA), which was sensitive to GTP (100 μM), demonstrating interaction between the receptor and the different G proteins.
The receptor to G protein ratio (R : G ratio) was evaluated using [3H]-spiperone saturation binding (R) and [35S]-GTPγS saturation binding (G). R : G ratios of 1 : 12, 1 : 3, 1 : 14 and 1 : 5 were found for Gi1, Gi2, Gi3, and Go preparations, respectively. However, when R : G ratios of 1 : 2 and 1 : 12 were compared for Gi2 and Go, no difference was found for the stimulation of [35S]-GTPγS binding.
Several agonists were tested for their ability to stimulate [35S]-GTPγS binding to membranes co-expressing the receptor and various G proteins. All the compounds tested showed agonist activity in preparations expressing Gi3 and Go. However, for Gi2 and Gi1 preparations, compounds such as S-(−)-3-PPP and p-tyramine were unable to stimulate [35S]-GTPγS binding.
Most of the compounds showed higher relative efficacies (compared to dopamine) and higher potencies in the preparation expressing Go. Comparison of the effects of different agonists in the different preparations showed that each agonist differentially activates the four G proteins.
We conclude that the degree of selectivity of G protein activation by the D2L receptor can depend on the conformation of the receptor stabilised by an agonist.
Keywords: dopamine D2 receptor, G proteins, baculovirus expression system, [3H]-spiperone binding, [35S]-GTPγS binding
Introduction
The mechanisms of activation of G protein-coupled receptors (GPCRs) by agonists are still poorly understood, despite extensive work in the field. Several models have been developed to explain the mechanisms of GPCR activation, including the ternary complex model and its extensions (for a historical review, see Kenakin (1997). The extended ternary complex model proposes that the GPCRs exist in an inactive ground state (R), which can isomerise to a partially activated state (R*) that couples more efficiently to the G protein to form the active species (R*G). The formation of R*G may occur spontaneously, but agonists stabilise the active (R*) and (R*G) states. The model also proposes that GPCRs can activate G proteins, even in the absence of ligand (‘constitutive activity') and that inverse agonists suppress this constitutive activity (Lefkowitz et al., 1993; Gether & Kobilka, 1998). There are, however, several experimental results which cannot be explained by the extended ternary complex model. For example, it was shown that the efficacy and potency of β2-adrenergic receptor agonists depend on the specific purine nucleotide present for G protein activation and the specific G protein to which the β2-adrenergic receptor couples (Seifert et al., 1999; Wenzel-Seifert & Seifert, 2000). In addition, some ligands have been shown to act as agonists in one setting and as inverse agonists in another setting (Chidiac et al., 1994). These findings and others (Zuscik et al., 1998; Thomas et al., 2000) suggest a multistate model of GPCR activation, in which ligands stabilise unique and ligand-specific GPCR conformations, which enable GPCRs to activate cognate G protein(s) in a ligand-specific manner (Gether & Kobilka, 1998; Kenakin, 2002).
The D2 dopamine receptor, a member of the dopamine receptor family (which comprises five receptors, D1–D5), represents a good model for the analysis of the mechanisms of GPCR activation. Indeed, it has been shown to activate a large diversity of second messenger pathways, including inhibition of adenylyl cyclase (Hall & Strange, 1999), stimulation of phospholipase C (Vallar et al., 1990), potentiation of arachidonic acid release (Kanterman et al., 1991), regulation of K+- and Ca2+-channels activity (Lledo et al., 1992; Seabrook et al., 1994), as well as modulation of the activity of the Na+/H+ exchanger (Coldwell et al., 1999). Most of these signalling pathways involve interaction of D2 receptors with G proteins of the Gi/o family.
Alternative splicing of the D2 receptor mRNA results in two isoforms of the receptor, termed D2S (short) and D2L (long) (Giros et al., 1989). The long isoform of the receptor differs from the short one by an additional 29 amino acid insert in the putative third intracellular loop. Both D2S and D2L are expressed widely in the brain and exhibit small differences in their pharmacological profile (Castro & Strange, 1993; Malmberg et al., 1993). The D2S and D2L have been shown to couple differently to G proteins (Montmayeur et al., 1993; Senogles, 1994). Interestingly, the D2S receptor couples preferentially to Gi1 over Gi2 when expressed in Sf9 cells (Grünewald et al., 1996) and distinct roles for G protein subunits in the modulation of cAMP accumulation and calcium mobilisation by D2 receptors were described (Liu et al., 1994; Ghahremani et al., 1999). It was also suggested that the 29 amino acid insert of D2L might confer selectivity for interaction with Gi2 (Guiramand et al., 1995). These observations suggest differences in the molecular mechanisms of the interaction of each isoform of the D2 receptor with different G proteins, which may account for different signalling and therefore different functions. The differential coupling of the D2 receptor to different G proteins may have pharmacological consequences. For example the relative efficacies of quinpirole and (+)-3-PPP are reversed when tested on D2 receptors in the striatum and in the pituitary gland (Meller et al., 1992). This could reflect the interaction of the receptor with different G proteins in the two tissues.
We previously reported a selective interaction of the rat D2L receptor with Go over Gi2 G proteins when expressed in Sf21 insect cells (Cordeaux et al., 2001). The interaction of D2L with Go appeared to be stronger than with Gi2. In addition, functional coupling of D2L was more efficient at Go than at Gi2. Moreover, the degree of selectivity depended on the agonist used. These findings prompted us to investigate the interaction of the D2L receptor with other members of the Gi/o family of G proteins using the same system. In order to compare the interaction of the D2L receptor with the different G proteins in the same environment, we have reconstituted the interaction of the human D2L receptor interaction with Gi1, Gi2, Gi3, and Go G proteins by co-expression in baculovirus-infected Sf9 cells.
Materials
Bromocriptine, (+)-butaclamol, clozapine, dopamine, haloperidol, (−)-quinpirole, m-tyramine, p-tyramine, S-(−)-3-PPP, R-(+)-3-PPP, R-(−)-propylnorapomorphine (NPA) were purchased from Sigma (Saint Louis, Missouri, U.S.A.). Antibodies specific for different G protein subunits were from Chemicon International (Harrow, U.K.) and Santa Cruz Biotechnology (Calne, U.K.) as indicated. [3H]-spiperone (15–30 Ci mmol−1) and [35S]-GTPγS (1099–1148 Ci mmol−1) were from Amersham International, Buckinghamshire, U.K. All the other reagents were obtained as indicated.
Methods
Construction of recombinant baculoviruses
cDNA encoding for human D2L dopamine receptor was subcloned into the vector TOPO® (Invitrogen) between a NdeI site at the 5′ end of the insert and an EcoRI site at the 3′ end of the insert, to produce the recombinant plasmid TOPOD2L. In order to add an epitope tag to the receptor in its amino terminus, complementary synthetic oligonucleotides encoding an HIV epitope tag sequence (Mckeating et al., 1993) were designed as follows: 5′-AGTACTAGTATCAGAGGCAAGGTACAACATATG-3′ and 5′-CATATGTTGTACCTTGCCTCTGATACTAGTACT-3′. This introduces a 3′ NdeI site to the tag sequence. These oligonucleotides were annealed, and digested subsequently with NdeI. TOPOD2L was digested with EcoRI and NdeI. The DNA fragment and the HIV tag were then ligated. The ligation mixture was subjected to PCR to selectively amplify tagged receptor whilst at the same time adding an XhoI site and a start codon to the 5′ end of the tag. For this purpose the following primers were used: 5′-TTGAATTCTCAGCAGTGGAGGATC-3′ and 5′-TTCTCGAGGATGGATAGTACTAGTATCAGAGGC-3′. Both PCR products were digested with XhoI and EcoRI and ligated into the plasmid pBlueBac4.5 (Invitrogen), to produce the recombinant plasmid pBBHD2L. The plasmid was then co-transfected with Bac-N-Blue™ DNA (Invitrogen) in Sf9 cells and underwent recombination, to produce recombinant baculovirus. All the constructs were verified by DNA sequencing. The baculoviruses expressing the human G protein subunits αi1–3, αo, β1 and γ2 were generously donated by T. Kozasa (University of Illinois, Chicago, U.S.A.). These baculoviruses were subjected to PCR to verify their purity. All the viruses were purified using plaque purification and amplified by serial infection of Sf9 insect cells.
Cell culture
Sf9 cells were grown in suspension in TC-100 medium supplemented with 10% foetal calf serum (FCS) and 0.1% pluronic F-68®. The cells were maintained at a density of 0.5 to 2.5×106 cells ml−1 and were split every two to three days. For infections, cells were seeded at a density of 0.3 to 0.6×106 cells ml−1 and infected when they reached a density of about 1×106 cells ml−1. Infections were carried out with various multiplicities of infection (m.o.i.) of baculoviruses. When the receptor was expressed alone, an m.o.i. of 5 was used. When the receptor was co-expressed with G proteins the m.o.i. used were 6 : 10 : 10 : 10 for D2L : Gαi/o : Gβ1 : Gγ2, respectively. Preliminary experiments showed these combinations to be optimal for G protein activation. In the preparations where only D2L, Gβ1 and Gγ2 were used, m.o.i. of 6, 10 and 10 were used for each baculovirus, respectively. Cells were harvested 48 h after infection.
Membrane preparation
Cells were collected by centrifugation (1700×g, 10 min, 4°C) and resuspended in 15 ml of buffer (mM) (HEPES 20, MgCl2 6, EDTA 1, EGTA 1, pH 7.4). Cell suspensions were then homogenised using an Ultra Turrax at setting 4–5 for 20 s. The homogenate was centrifuged at 1700×g for 10 min and the supernatant was collected and centrifuged at 48,000×g for 1 h at 4°C. The resulting pellet was resuspended in buffer and stored at −80°C in aliquots of 500 μl. The protein concentration was determined by the method of Lowry et al. (1951), using bovine serum albumin as standard.
Immunoblot analysis
Membrane protein (20–40 μg) was incubated in 10 μl electrophoresis loading buffer (30 mM Tris-HCl, pH 6.8, 5% glycerol, 10% β-mercaptoethanol, 0.4% SDS, 0.04% bromophenol blue) and denatured by heating at 90°C for 5 min. Denatured membrane proteins were then separated by SDS polyacrylamide gel electrophoresis on 10% acrylamide gels. Samples were transferred to nitrocellulose membranes using the Biorad semi dry transfer system. Nitrocellulose membranes were incubated for 1 h with 5% dried milk (w/v) in buffer (mM) (NaCl 137, KCl 3, 0.1% Tween 20, Tris-HCl 25, pH 7.5). Membranes were then incubated overnight at 4°C with single primary antibodies (mAb3073 anti-Gαo, 1 μg ml−1 (Chemicon); C-10 anti-Gαi-3 (a rabbit polyclonal antibody able to recognise Gαi1, Gαi2 and Gαi3 subunits), 1 μg ml−1 (Santa Cruz, specificity verified by Marston & Strange, unpublished); mAb3077 anti-Gαi2, 1 μg ml−1 (Chemicon); C-16 anti-Gβ1, 0.4 μg ml−1 (Santa Cruz); A-16 anti-Gγ2, 0.4 μg ml−1 (Santa Cruz, specificity verified in Cordeaux et al., 2001) in buffer containing 5% dried milk (w/v). Membranes were washed four times with buffer (15 min each) before incubation with secondary antibody (anti-mouse (Gαo, Gαi2) or anti-rabbit (Gαi-3, Gβ1, Gγ2)) immunoglobulin horseradish peroxidase conjugate (Sigma, 1 : 5000) for 1 h. After three washes with buffer (10 min each), membranes were exposed to equal volumes of Enhanced Chemiluminescence (ECL) detection reagents 1 and 2 (Amersham) and bands were visualised after exposure of the membranes to Hybond-ECL X-ray film (Amersham) for between 30 s to 3 min.
[35S]-GTPγS binding measurements
Stimulation of [35S]-GTPγS (1099-1148 Ci mmol−1; Amersham International, Buckinghamshire, U.K.) binding was measured on membranes co-expressing human dopamine D2L and different G protein subunits. Membrane protein (5 μg) was incubated with 1 μM GDP and various concentrations of dopamine D2 receptor ligands in buffer (mM) (HEPES 20, NaCl 100, MgCl2 6, pH 7.4) in a final volume of 80 μl. Reactions were performed in triplicate and were initiated by the addition of membrane proteins. After 30 min of pre-incubation at 30°C, 20 μl of [35S]-GTPγS (0.1 nM final concentration) was added and the incubation continued for a further 30 min. Reactions were terminated by rapid filtration through Whatman GF/C glass fibre filters using a 96-well plate Brandel cell harvester, followed with four washes of 1.5 ml of ice-cold PBS. Radioactivity was determined as described above.
For [35S]-GTPγS saturation binding, the final reaction volume was 1 ml. A final concentration (0.1 nM) of [35S]-GTPγS was incubated in triplicate with a range of concentrations (0–60 nM) of non-radioactive GTPγS. The reactions were initiated by the addition of membranes. After 30 min incubation at 30°C, the reactions were terminated and radioactivity determined as described above. For each [35S]-GTPγS saturation binding experiment, membranes prepared from cells expressing only receptor and Gβ1 and Gγ2 subunits were used as control. [35S]-GTPγS binding in the preparations containing receptor and G protein α, β, γ subunits was then corrected for this background binding. Saturation binding curves were derived to estimate the total number of G proteins expressed. The amount of ligand bound to G protein (BOUNDTOT) was calculated by equation 1: BOUNDTOT=[35S] - GTPγSBOUND×GTPγSTOT/[35S] - GTPγSCONC, where [35S]-GTPγSBOUND is the corrected ligand binding, [35S]-GTPγSCONC is the [35S]-GTPγS concentration in the tubes (0.1 nM) and GTPγSTOT is [35S]-GTPγSCONC plus GTPγS concentration.
Radioligand binding assay
[3H]-spiperone (15–30 Ci mmol−1, Amersham International, Buckinghamshire, U.K.) saturation binding experiments were performed in a final volume of 1 ml buffer (mM) (HEPES 20, MgCl2 6, EDTA 1, EGTA 1, pH 7.4) and 25 μg of membrane protein per tube. Eight different concentrations of radioligand were used, ranging from approximately 10 pM to 2 nM. The reaction was initiated by the addition of membrane proteins and incubated for 3 h at 25°C. Reactions were terminated by rapid filtration through Whatman GF/C glass fibre filters using a Brandel cell harvester followed with four washes of 3 ml of ice-cold PBS (mM) (NaCl 140, KCl 10, KH2PO4 1.5, Na2HPO4 8). Filter discs were soaked in 2 ml of Optiphase Hi-Safe 3 (Wallac) for at least 6 h before the radioactivity was determined by liquid scintillation spectrometry. Non-specific binding was defined in the presence of 3 μM (+)-butaclamol. Assays were performed in triplicate. For [3H]-spiperone competition binding experiments, a range of concentrations of competing ligand was incubated with a fixed concentration of radioligand (typically 0.45 nM) and the reactions were started and terminated as described above.
Analysis of data
Data were analysed using the computer program GraphPad Prism (GraphPad Software Inc.). [3H]-spiperone saturation binding experiments were fitted to a one-site model to define the Bmax (receptor expression level) and KD (dissociation constant for [3H]-spiperone). Competition experiments were fitted to a two-site binding and a one-site binding models and the best fit was determined using an F-test. IC50 values of competitors were derived from this analysis and the Ki (inhibition constants) values were derived using the Cheng & Prusoff (1973) equation. For [35S]-GTPγS binding, concentration-response curves for agonists were analysed by non linear least squares regression fit and EC50 and Emax (maximum effect) values were derived from this analysis. Results are given as mean±s.e.mean of the indicated number of experiments.
Statistical comparisons were performed using Analysis of Variance (ANOVA), followed by Tukey post-hoc test, where appropriate. A value of P<0.05 was considered significant.
Results
Expression of dopamine D2L receptor and G protein subunits
In saturation binding experiments, [3H]-spiperone was found to label a homogeneous and saturable population of specific binding sites in Sf9 cells expressing the human dopamine D2L receptor with or without G protein subunits. The Bmax and the KD values were analysed using one-way ANOVA, and were not significantly different between the five preparations (P>0.05). The Bmax and the KD values for [3H]-spiperone are summarised in Table 1. The expression of G protein subunits was analysed by immunoblot, using antibodies directed against the different subunits. Figure 1 shows the results of immunoblots performed on membranes co-expressing the D2L receptor and different combinations of G protein subunits. Bands corresponding to the size of each G protein subunit were identified. No band was detected with any of the antibodies when the receptor was expressed in the absence of exogenous G protein (lane 1 on Figure 1).
Table 1.
Expression levels of human dopamine D2L receptor (R) and G protein (G) in Sf9 cells
Figure 1.
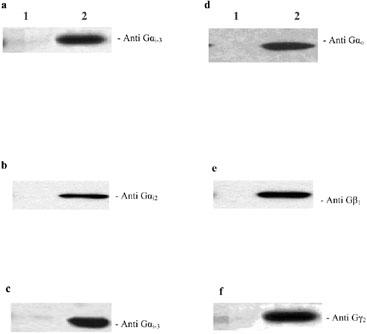
Expression of G protein subunits in Sf9 cells. Sf9 membranes expressing the D2L receptor alone (lane 1) or co-expressing the D2L receptor with different combinations of G protein subunits (lane 2) were separated by SDS–PAGE, transferred to nitrocellulose filters, and probed with the indicated antibodies as described in the Methods section. (a) D2LGi1β1γ2; (b) D2LGi2β1γ2; (c) D2LGi3β1γ2; (d) D2LGoβ1γ2; (e) and (f) D2LGi/oβ1γ2. Representative experiments performed on each membrane preparation are shown.
Analysis of receptor : G protein ratio
In order to assess the G protein expression levels in our system, we used a method which takes into account the relatively high level of guanine nucleotide binding sites in Sf9 cells (Grünewald et al., 1996). Hence, we infected Sf9 cells with baculoviruses for D2L and Gβ1 and Gγ2 subunits only (to switch off expression of endogenous proteins). G protein levels were determined using [35S]-GTPγS saturation binding and the level of [35S]-GTPγS binding in the receptor/Gβγ preparation was subtracted from that in the preparations containing the heterotrimers (Figure 2a, c, e and g). Data were then transformed into saturation curves as described in the Methods section and the apparent KD for GTPγS as well as the relative G protein levels (Bmax) were derived for the different preparations (Figure 2b, d, f and h) and are summarised in Table 1. The apparent KD for GTPγS with different preparations was not significantly different between the preparations containing the four G proteins (one-way ANOVA, P>0.05). Based on these data and the Bmax values for [3H]-spiperone binding, the R : G ratios in the different preparations were calculated and data are given in Tables 1 and 2.
Figure 2.
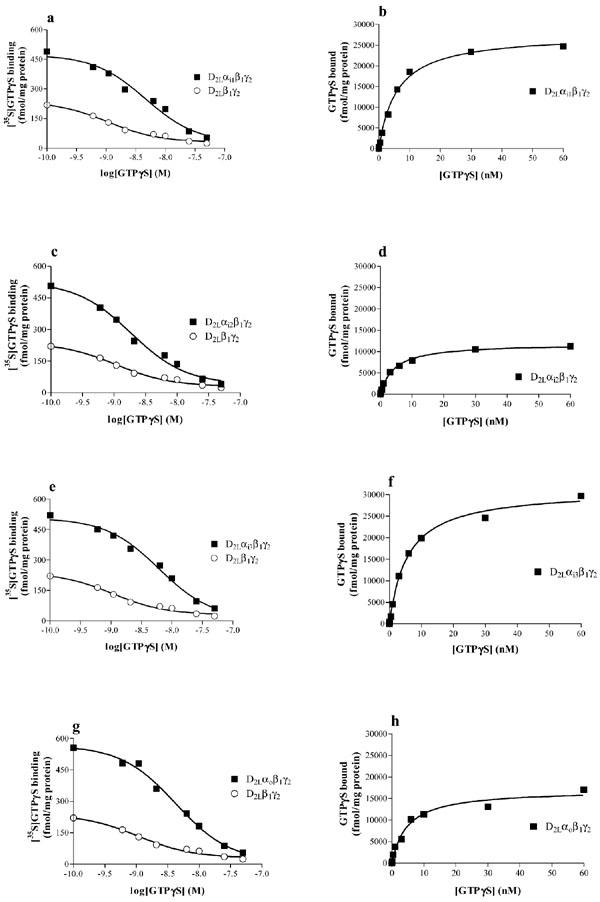
G protein levels analysed by [35S]-GTPγS saturation binding. [35S]-GTPγS saturation binding experiments were performed on Sf9 membranes expressing D2L receptor and Gi1 (a, b), Gi2 (c, d), Gi3 (e, f), and Go (g, h), as described in the Methods section. Data are from representative experiments repeated as in Table 1.
Table 2.
Dopamine stimulation of [35S]-GTPγS binding to membranes expressing D2L and Gi2 or Go
Effects of dopamine and dopamine receptor agonists on [35S]-GTPγS binding
When the receptor and G protein subunits were expressed using m.o.i. of 6/10/10/10 (R/α/β1/γ2) the R : G ratios in the different preparations were not equivalent (Table 1). Indeed the R : G ratios for the Gi2 and Go preparations were found to be lower than that for the Gi1 and Gi3 preparations. We therefore sought to analyse the effect of varying the R : G ratio on agonist activity at Gi2 and Go. Thus, by varying the m.o.i. of the baculoviruses used, two preparations (with R : G ratios of ∼1 : 2 and ∼1 : 12) were generated for each R/G combination. The effect of dopamine in preparations expressing Gi2 and Go with varying R : G ratios is summarised in Table 2. Thus, the maximal effect and the potency of dopamine were similar (one-way ANOVA, P>0.05) for the two R : G ratios, for Gi2 and Go preparations (Table 2). We also tested the effect of other dopamine D2 receptor agonists at the two R : G ratios. As shown in Figure 3, the relative efficacies, as well as the potencies of bromocriptine, NPA, and quinpirole were similar (one-way ANOVA, P>0.05) at the two R : G ratios. In addition, for the Gi2 preparation, the effect of S-(−)-3PPP and p-tyramine were assessed at two R : G ratios. In one experiment out of five, S-(−)-3PPP showed a slight agonist effect (Emax 11% over basal level, equivalent to 14% the efficacy of dopamine). Thus, the two compounds were virtually inactive in the Gi2 preparation, even when the R : G ratio was increased (Figure 3).
Figure 3.
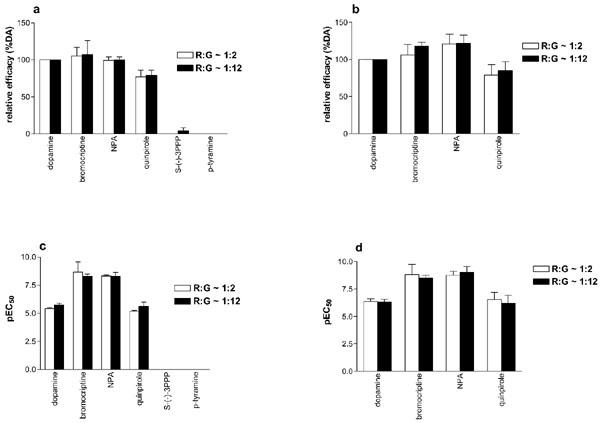
Stimulation of [35S]-GTPγS binding by agonists in membranes of Sf9 cells expressing D2L receptor and Gi2 or Go at various R : G ratios. The stimulation of [35S]-GTPγS binding was determined as described in the Methods section on membranes expressing D2L receptor and Gi2 (a, c), or Go (b, d). The R : G ratios were calculated as in Table 1. Data shown are mean±s.e.mean of 3–17 experiments.
As the stimulation of [35S]-GTPγS binding in these preparations seemed to be independent of the R : G ratio, we pooled data from the different preparations. The average maximal effect of dopamine for stimulation of [35S]-GTPγS binding in the different preparations was 44±4%, 94±14%, 80±9% and 47±3% over basal levels for preparations expressing D2L and Gi1, Gi2, Gi3, and Go, respectively. No agonist effect was found in Sf9 cells expressing the D2L receptor in the absence of exogenous G protein (data not shown).
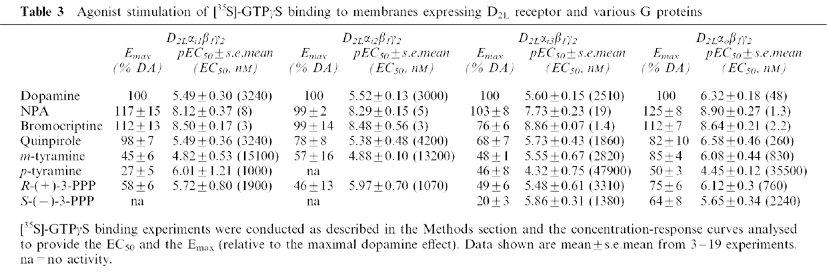
A series of dopamine agonists were then tested for their ability to stimulate [35S]-GTPγS binding in the different preparations (Figure 4, Table 3). All of the agonists tested showed intrinsic activity at Gi3 and Go G proteins (Figure 4c and d). Compounds such as bromocriptine and NPA were potent agonists in all the four preparations. They also behaved as full agonists (relative to dopamine) in all preparations, with the exception of the preparation expressing Gi3, where bromocriptine was a partial agonist (Figure 4c, Table 3). All the other compounds tested showed partial agonist activities (relative to dopamine) in the preparations expressing Gi3 and Go. However, S-(−)-3-PPP was unable to stimulate [35S]-GTPγS binding (Figure 4a and b) in the Gi1 and Gi2 preparations, while p-tyramine was unable to stimulate [35S]-GTPγS binding in the Gi2 preparation (Figure 4b). Compounds such as dopamine, NPA, quinpirole, and m-tyramine showed their highest potencies in the Go preparation (Table 3). Bromocriptine had similar potency in each of the preparations.
Figure 4.
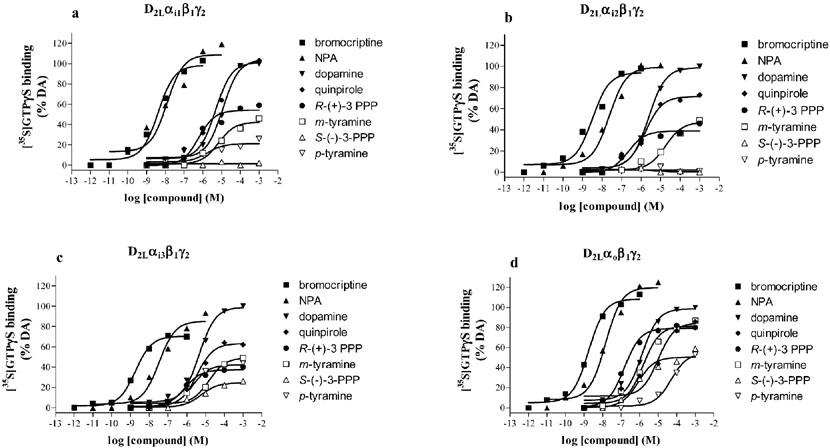
Stimulation of [35S]-GTPγS binding by agonists in membranes of Sf9 cells expressing the D2L receptor and various G proteins. The stimulation of [35S]-GTPγS binding by agonists was determined as described in the Methods section on membranes expressing D2L receptor and Gi1 (a), Gi2 (b), Gi3 (c) or Go (d). Data are representative stimulation curves replicated as in Table 3.
Table 3.
Agonist stimulation of [35S]-GTPγS binding to membranes expressing D2L receptor and various G proteins
Dopamine D2L receptor and G protein interaction
Interaction of the dopamine D2L receptor with G proteins was assessed in agonist competition experiments versus [3H]-spiperone binding to membranes prepared from cells co-expressing the receptor and the different G protein subunits. Figure 5 shows competition binding between [3H]-spiperone and dopamine or NPA. In the absence of GTP, competition curves for both compounds were best fitted to a two binding site model (F-test, P<0.002), with higher and lower affinity binding sites defined by dissociation constants Kh, and Kl. The percentage of higher affinity binding sites found with each agonist was comparable (one-way ANOVA, P>0.05) between the four preparations (Table 4). As expected, the affinity of NPA was higher than that of dopamine in each of the preparations. In the presence of a high concentration of GTP (100 μM), the competition curves were best fitted to a one binding site model (F-test, P<0.05). The affinity of dopamine and NPA measured in the presence of GTP (pKGTP) was similar (one-way ANOVA, P>0.05) to their lower affinity (pKl) measured in the absence of GTP (Table 4).
Figure 5.
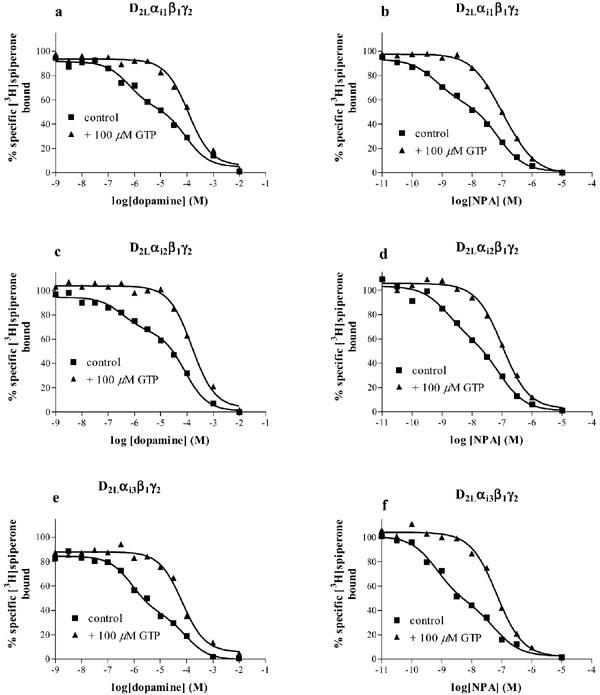
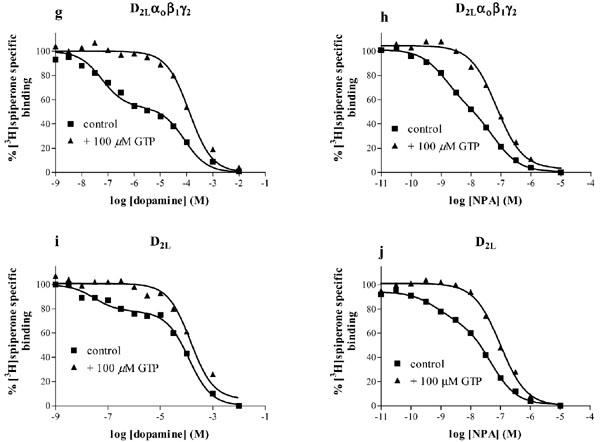
Agonist binding to membranes of Sf9 cells expressing the D2L dopamine receptors and various G proteins. The binding of dopamine or NPA to the D2L receptor co-expressed with Gi1 (a, b), Gi2 (c, d), Gi3 (e, f), Go (g, h) or alone (i, j) was determined in competition vs [3H]-spiperone binding in the absence or presence of 100 μM GTP, as described in the Methods section. Data shown are representative experiments replicated as in Table 4 and the curves are the best fit curves to one site (+GTP) (F-test, P<0.05) or two site models (control) (F-test, P<0.002).
Table 4.
Binding of dopamine and NPA to membranes of Sf9 cells expressing the D2L dopamine receptor and different G proteins
Competition of dopamine or NPA for [3H]-spiperone binding also revealed a higher affinity binding state of the receptor when the D2L receptor was expressed without exogenous G protein subunits (Figure 5i, j). This higher affinity binding site was sensitive to GTP (100 μM), with pKiGTP values comparable (one-way ANOVA, P>0.05) to the pKl values for both compounds (Figure 5, Table 4).
The binding of several antagonists was also tested in competition binding experiments versus [3H]-spiperone. As shown in Figure 6, all the competition curves were best fitted by a one binding site model (F-test, P<0.05) and the rank order of affinity was (+)-butaclamol>haloperidol>clozapine⩾raclopride. The derived Ki values are consistent with those obtained for the receptor expressed in mammalian cell lines and they show that the dopamine D2L receptor expressed in Sf9 cells behaves similarly to a system where the receptor couples exclusively with endogenous mammalian G proteins.
Figure 6.
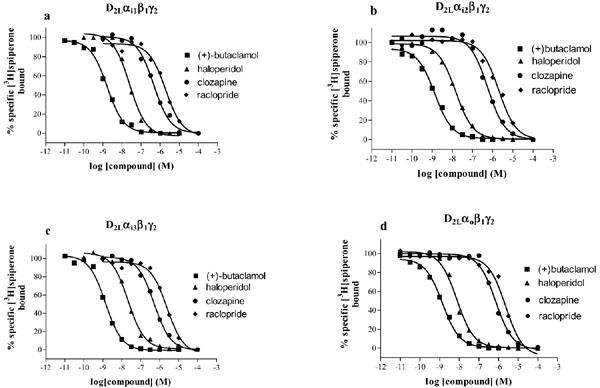
Antagonist binding to membranes of Sf9 cells expressing the D2L receptor and various G proteins. The binding of (+)-butaclamol, haloperidol, clozapine, and raclopride to the D2L receptor co-expressed with Gi1 (a), Gi2 (b), Gi3 (c) or Go (d) was determined in competition vs [3H]-spiperone binding as described in the Methods section. Data shown are representative experiments replicated two to three times and the curves are the best fit curves to a one binding site model. Mean Ki values (nM) for the different compounds are as follows: (+)-butaclamol (0.25, 0.30, 0.33, 0.31); haloperidol (3.9, 2.4, 4.0, 1.8); clozapine (87, 93, 100, 115); raclopride (417, 372, 447, 240) in D2Gi1, D2Gi2, D2Gi3, D2Go, respectively.
Discussion
In the present study, we have analysed the interaction of the D2 dopamine receptor with G proteins by co-expressing human D2L with Gi1, Gi2, Gi3 and Go in Sf9 cells using the baculovirus system. This study extends our previous observations on the rat D2L receptor (Cordeaux et al., 2001). Previous studies have shown that D2 receptors can interact with and signal through pertussis toxin-sensitive G proteins (Gi/o) to couple to different pathways (see for example Montmayeur et al., 1993; Senogles, 1994; Ghahremani et al., 1999). These findings strongly suggested a differential coupling of the D2 receptors with the different subtypes of G proteins. However, most of these interactions have been analysed in a system measuring intracellular events (i.e. cAMP accumulation, calcium mobilisation), where factors such as signal amplification may complicate interpretation. Furthermore, no study has analysed the four subtypes of G proteins in a well-defined system. Our present study is therefore the first to address the question of dopamine D2L interaction with the four G proteins in a well-defined system using a method ([35S]-GTPγS binding), which allows the observation of direct interaction between the receptor and the G proteins.
We have assessed the receptor (R) to G protein (G) ratio using [3H]-spiperone and [35S]-GTPγS saturation binding experiments to measure R and G levels, respectively. Others reported similar methods for determination of G protein levels (Traynor & Nahorski, 1995; Newman-Tancredi et al., 2000). In the present study, we sought to use a method in which no GDP was present and we also take into account the endogenous G proteins or guanine nucleotide-binding proteins by using cells infected with D2L and β1/γ2 only. We assumed that the binding of [35S]-GTPγS to membranes expressing D2Lβ1γ2 corresponds to either endogenous G proteins or other proteins as stated above. These values were, therefore, subtracted from those where receptor was expressed with heterotrimeric G protein (Figure 2). We obtained R : G ratios varying between 1 : 3 and 1 : 14, with the highest ratio being seen with Gi3 preparations (Table 1). These ratios are somewhat lower than those obtained in other systems (see for example Wenzel-Seifert et al., 1998; Neubig, 1994; Ransnäs & Insel, 1988). This variation may reflect differences in the methodology for determination of G protein levels or differences between expression systems.
The receptor to G protein ratio (R : G ratio) has been found to affect both the efficacy and the potency of ligands in several systems (see for example Pauwels et al., 2000; Newman-Tancredi et al., 2000). It was therefore necessary to analyse the effect of the R : G ratio on the signalling event. Thus, for the preparations expressing Gi2 and Go we found that both the efficacies and potencies of dopamine receptor agonists for the stimulation of [35S]-GTPγS binding were similar (one-way ANOVA, P>0.05) for different R : G ratios (Table 2, Figure 3). It seems, therefore, that the overall R : G ratio in the cells may not reflect the functional ratio at a particular receptor. This is not unprecedented as in studies on the reconstitution of receptors and G proteins, even though an R : G ratio much less than one was used, the resulting preparation behaved as though the R : G ratio was greater than one (Jakubik et al., 1998). It may be that receptors and G proteins enter micro domains in the cells with different R : G ratios and that these do not reflect the overall R : G ratio.
A range of agonists was tested for their ability to stimulate [35S]-GTPγS binding to membranes expressing D2L and the different G proteins. All the compounds tested showed agonist activity in the preparations expressing Go and Gi3, whereas in the Gi1 and Gi2 preparations some agonists were unable to stimulate [35S]-GTPγS binding. Most of the compounds showed their highest efficacy (relative to that of dopamine) in the preparation expressing Go (Table 3). This suggests a stronger or a more productive interaction between D2L and Go, and agrees with previous observations on the rat proteins (Cordeaux et al., 2001). Nevertheless, for some compounds such as quinpirole, bromocriptine and NPA, the relative efficacies observed at Gi1 were comparable to those seen at Go. NPA and bromocriptine behaved as potent agonists at all the G proteins with two to three orders of magnitude higher potency compared to dopamine (Figure 4 and Table 3). The potency of several agonists (dopamine, NPA, quinpirole, m-tyramine, R-(+)-3-PPP) was slightly higher in the Go preparation. However, bromocriptine had comparable potency in each preparation. Indeed the potency of bromocriptine in the present study was similar to that reported for the D2 receptor expressed in other recombinant systems (Gardner et al., 1996). It seems, therefore, that the potency of this compound is not dependent on the nature of the G protein and we have speculated that this may be because bromocriptine stabilises the receptor in a conformation close to that in the fully activated R*G state (Cordeaux et al., 2001).
The monohydroxylated compounds S-(−)-3-PPP and p-tyramine were partial agonists in the Go and Gi3 preparations but not in the Gi2 preparation. In the Gi1 preparation, S-(−)-3-PPP exhibited partial agonist activity and p-tyramine was unable to stimulate [35S]-GTPγS binding. These data suggest that the binding of these ligands to the receptor induces a conformation of the receptor which differentially couples to or activates G proteins, with weak interaction occurring between the receptor and Gi1 and Gi2 and more efficient interaction occurring with Gi3 and Go. Interestingly, both compounds appeared ineffective in the Gi2 preparation, even at a higher R : G ratio. This supports the hypothesis of a weak interaction between the Gi2 and the D2L receptor occupied by these agonists. Others have reported increased efficacy of agonists for receptors signalling via Go as compared to signalling via Gi proteins (Yang & Lanier, 1999; Francken et al., 2000). Although the data of the present study show differences in the interaction of one receptor with different G proteins, they do not provide clear evidence for agonist-directed receptor trafficking as proposed by Kenakin (1995) and described in several studies (see for example Berg et al., 1998; Robb et al., 1994; Spengler et al., 1993). In fact no clear reversal in the efficacy or the potency of the ligands was observed. Nevertheless, the present data imply that different agonists select different conformations of the receptor with different affinities for G proteins or abilities to activate them. Thus, the receptor/G protein combination dictates the precise pharmacological profile of the observed response.
The human dopamine D2L receptor expressed in Sf9 cells, with or without exogenous G proteins, exhibited a KD for [3H]-spiperone similar to that of D2 receptors expressed in mammalian cell lines (Gardner et al., 1996) or native receptor (Withy et al., 1981). Competition binding using the agonists dopamine and NPA revealed a high affinity binding component, which was sensitive to GTP, suggesting interaction of the receptor with co-expressed G proteins. The higher affinity agonist binding found in the preparations where D2L was expressed alone may correspond to the interaction of the receptor with either endogenous G proteins or guanyl nucleotide-binding proteins that are not G proteins. In line with this, several studies have reported the presence of endogenous G proteins in Sf9 cells (Graber et al., 1992; Mulheron et al., 1994; Parker et al., 1991; Boundy et al., 1996). It is possible that in our system the D2L receptor interacts with these endogenous proteins in the absence of exogenous G protein. This interaction does not however lead to activation, as we did not observe any agonist effect at the receptor expressed alone. In addition, immunoblot analysis did not reveal any endogenous G protein in our study (Figure 1). We therefore conclude that endogenous G proteins in Sf9 cells may interact with the human dopamine D2L receptor but that this interaction does not lead to activation of G proteins.
In conclusion, this study shows a selective interaction of the D2L receptor with G proteins when expressed in Sf9 cells. Our data suggest that agonists can regulate the interaction of dopamine D2L receptor with G proteins, independently of the R : G ratio. These differential interactions of the receptor with different G proteins may account for different functions triggered by D2 dopamine receptors.
Acknowledgments
We thank the BBSRC for the financial support and T. Kozasa for the generous gift of baculoviruses expressing G protein subunits.
Abbreviations
- D2
dopamine D2 receptor
- Gi/o
heterotrimeric GTP binding protein type i or o
- GPCR
G protein-coupled receptor
- GTPγS
guanosine-5′-O-(3-thio)triphosphate
- m.o.i.
multiplicity of infection
- PCR
polymerase chain reaction
- Rh
receptor higher affinity binding site
- 3-PPP
3-(3-hydroxyphenyl)-N-propylpiperidine
References
- BERG K.A., MAAYANI S., GOLDFARB J., SCARAMELLINI C., LEFF P., CLARKE W.P. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- BOUNDY V.A., LU L., MOLINOFF P.B. Differential coupling of rat D2 dopamine receptor isoforms expressed in Spodoptera Frugiperda Insect cells. J. Pharmacol. Exp. Ther. 1996;276:784–794. [PubMed] [Google Scholar]
- CASTRO S.W., STRANGE P.G. Differences in the ligand binding properties of the short and long versions of the D2 dopamine receptor. J. Neurochem. 1993;60:372–375. doi: 10.1111/j.1471-4159.1993.tb05863.x. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of the inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIDIAC P., HEBERT T.E., VALIQUETTE M., DENNIS M., BOUVIER M. Inverse agonist activity of β-adrenergic antagonists. Mol. Pharmacol. 1994;45:490–499. [PubMed] [Google Scholar]
- COLDWELL M.C., BOYFIELD I., BROWN A.M., STEMP G., MIDDLEMISS D.N. Pharmacological characterization of extracellular acidification rate responses in human D2(long), D3 and D4.4 receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1999;127:1135–1144. doi: 10.1038/sj.bjp.0702657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORDEAUX Y., NICKOLLS S.A., FLOOD L.A., GRABER S.G., STRANGE P.G. Agonist regulation of D2 dopamine receptor/G protein interaction: evidence for agonist selection of G protein subtype. J. Biol. Chem. 2001;276:28667–28675. doi: 10.1074/jbc.M008644200. [DOI] [PubMed] [Google Scholar]
- FRANCKEN B.J.B., JOSSON K., LIJNEN P., JURZAK M., LUYTEN W.H.M.L., LEYSEN J.E. Human 5-hydroxytryptamine 5A receptors activate coexpressed Gi and Go proteins in Spodoptera frugiperda 9 cells. Mol. Pharmacol. 2000;57:1034–1044. [PubMed] [Google Scholar]
- GARDNER B., HALL D.A., STRANGE P.G. Pharmacological analysis of dopamine stimulation of [35S]-GTPγS binding via human D2short and D2long dopamine receptors expressed in recombinant cells. Br. J. Pharmacol. 1996;118:1544–1550. doi: 10.1111/j.1476-5381.1996.tb15572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GETHER U., KOBILKA B.K. G protein-coupled receptors. II. Mechanism of agonist activation. J. Biol. Chem. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- GHAHREMANI M.H., CHENG P.H., LEMBO P.M.C., ALBERT P.R. Distinct roles for G alpha(i)2, Galpha(i)3, and G beta gamma in modulation of forskolin- or G(s)- mediated cAMP accumulation and calcium mobilization by dopamine D2S receptors. J. Biol. Chem. 1999;274:9238–9245. doi: 10.1074/jbc.274.14.9238. [DOI] [PubMed] [Google Scholar]
- GIROS B., SOKOLOFF P., MARTRES M-P., RIOU J-F., EMORINE L.J., SCHWARTZ J.-C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;34:923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- GRABER S.G., FIGLER R.A., GARRISON J.C. Expression and purification of functional G protein alpha subunits using a baculovirus expression system. J. Biol. Chem. 1992;267:1271–1278. [PubMed] [Google Scholar]
- GRÜNEWALD S., REILÄNDER H., MICHEL H. In vivo reconstitution of dopamine D2S receptor-mediated G protein activation in baculovirus-infected insect cells: preferred coupling to Gi1 versus Gi2. Biochemistry. 1996;35:15162–15173. doi: 10.1021/bi960757w. [DOI] [PubMed] [Google Scholar]
- GUIRAMAND J., MONTMAYEUR J.-P., CERALINE J., BHATIA M., BORRELI E. Alternative splicing of the dopamine D2 receptor directs specificity of coupling to G-proteins. J. Biol. Chem. 1995;270:7354–7358. doi: 10.1074/jbc.270.13.7354. [DOI] [PubMed] [Google Scholar]
- HALL D.A., STRANGE P.G. Comparison of the ability of dopamine receptor agonists to inhibit forskolin-stimulated adenosine 3′5′-cyclic monophosphate (cAMP) accumulation via D2L (long isoform) and D3 receptor expressed in Chinese hamster ovary (CHO) cells. Biochem. Pharmacol. 1999;58:285–289. doi: 10.1016/s0006-2952(99)00101-x. [DOI] [PubMed] [Google Scholar]
- JAKUBIK J., HAGA T., TUCEK S. Effects of an agonist, allosteric modulator, and antagonist on guanosine-gamma-[35S]thiotriphosphate binding to liposomes with varying muscarinic receptor/Go protein stoichiometry. Mol. Pharmacol. 1998;54:899–906. doi: 10.1124/mol.54.5.899. [DOI] [PubMed] [Google Scholar]
- KANTERMAN R.Y., MAHAN L.C., BRILEY E.M., MONSMA F.J., SIBLEY D.R., AXELROD J., FELDER C.C. Transfected D2 dopamine receptors mediate the potentiation of arachidonic acid release in Chinese hamster ovary cells. Mol. Pharmacol. 1991;39:364–369. [PubMed] [Google Scholar]
- KENAKIN T. Agonist-receptor efficacy II: agonist trafficking of receptor signals. Trends Pharmacol. Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Pharmacological analysis of drug-receptor interaction. Philadelphia, PA, New York, NY, USA: Lippincott-Raven Press; 1997. [Google Scholar]
- KENAKIN T. The ligand paradox between affinity and efficacy: can you be there and not make a difference. Trends Pharmacol. Sci. 2002;23:275–280. doi: 10.1016/s0165-6147(02)02036-9. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., COTECCHIA S., SAMANA P., COSTA T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol. Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- LIU Y.F., JACOBS K.H., RASENICK M.M., ALBERT P.R. G protein specificity in receptor-effector coupling: Analysis of the roles of Go and Gi2 in GH4C1 pituitary cells. J. Biol. Chem. 1994;269:13880–13886. [PubMed] [Google Scholar]
- LLEDO P.-M., HOMBURGER V., BOCKAERT J., VINCENT J.-D. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat pituitary cells. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MALMBERG A., JACKSON D.M., ERIKSSON A., MOHELL N. Unique binding characteristics of antipsychotic agents interacting with human dopamine D2A, D2B, and D3 receptors. Mol. Pharmacol. 1993;43:749–754. [PubMed] [Google Scholar]
- MCKEATING J.A., SHOTTON C., CORDELL J., GRAHAM S., BALFE P., SULLIVAN N., CHARLES M., PAGE M., BOLMSTEDT A., OLOFSSON S., KAYMAN S.C., WU Z., PINTER A., DEAN C., SADROSKI J., WEISS R.A. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J. Virol. 1993;67:4932–4944. doi: 10.1128/jvi.67.8.4932-4944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLER E., PUZA T., DIAMOND J., LIEU H.D., BOHMAKER K. Comparative effects of receptor inactivation, 17 beta-estradiol and pertussis toxin on dopaminergic inhibition of prolactin secretion in vitro. J. Pharmacol. Exp. Ther. 1992;263:462–469. [PubMed] [Google Scholar]
- MONTMAYEUR J.P., GUIRAMAND J., BORELLI E. Preferential coupling between dopamine D2 receptors and G proteins. Mol. Endocrinol. 1993;7:161–170. doi: 10.1210/mend.7.2.7682286. [DOI] [PubMed] [Google Scholar]
- MULHERON J.G., CASANAS S.J., ARTHUR J.M., GARNOVSKAYA M.N., GETTYS T.W., RAYMOND J.R. Human 5-HT1A receptor expressed in insect cells activates endogenous G(o)-like G protein(s) J. Biol. Chem. 1994;269:12954–12962. [PubMed] [Google Scholar]
- NEUBIG R.R. Membrane organization in G-protein mechanisms. FASEB J. 1994;8:939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., AUDINOT V., MOREIRA C., VERRIELE L., MILLAN M.J. Inverse agonism and constitutive activity as functional correlates of serotonin h5-HT1B receptor/G-protein stoichiometry. Mol. Pharmacol. 2000;58:1042–1049. doi: 10.1124/mol.58.5.1042. [DOI] [PubMed] [Google Scholar]
- PARKER E.M., KAMEYAMA K., HIGASHIJIMA T., ROSS E.M. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. J. Biol. Chem. 1991;266:519–527. [PubMed] [Google Scholar]
- PAUWELS P.J., TARDIF S., WURCH T., COLPAERT F.C. Facilitation of constitutive alpha(2A)-adrenoceptor activity by both single amino acid mutation (Thr(373)Lys) and g(alphao) protein coexpression: evidence for inverse agonism. J. Pharmacol. Exp. Ther. 2000;292:654–663. [PubMed] [Google Scholar]
- RANSNÄS L.A., INSEL P.A. Quantitation of the guanine nucleotide binding regulatory protein Gs in S49 cell membranes using antipeptide antibodies to alpha s. J. Biol. Chem. 1988;263:9482–9485. [PubMed] [Google Scholar]
- ROBB S., CHEEK T.R., HANNAN F.L., HALL L.M., MIDGLEY J.M., EVANS P.D. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 1994;13:1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEABROOK G.R., KNOWLES M.R., BROWN N., MYERS J., SINCLAIR H., PATEL S., FREEDMAN S.B., MCALLISTER G. Pharmacology of high-threshold calcium currents in GH4C1 pituitary cells and their regulation by activation of human D2 and D4 dopamine receptors. Br. J. Pharmacol. 1994;112:728–734. doi: 10.1111/j.1476-5381.1994.tb13138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIFERT R., GETHER U., WENZEL-SEIFERT K., KOBILKA B.K. Effect of guanine, inosine, and xanthine nucleotides on β2-adrenergic receptor/Gs interactions: evidence for multiple receptor conformations. Mol. Pharmacol. 1999;56:348–358. doi: 10.1124/mol.56.2.348. [DOI] [PubMed] [Google Scholar]
- SENOGLES S.E. The D2 dopamine receptor isoforms signal through distinct Gi alpha proteins to inhibit adenylyl cyclase: a study with site-directed mutant Gi alpha proteins. J. Biol. Chem. 1994;269:23120–23127. [PubMed] [Google Scholar]
- SPENGLER D., WAEBER C., PANTALONI C., HOLSBOER F., BOCKAERT J., SEEBURG P.H., JOURNOT L. Differential signal transduction by five splice variant of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- THOMAS W.G., QIAN H., CHANG C.S., KARNIK K. Agonist-induced phosphorylation of the angiotensin II (AT1A) receptor requires generation of a conformation that is distinct from the inositol phosphate signalling state. J. Biol. Chem. 2000;275:2893–2900. doi: 10.1074/jbc.275.4.2893. [DOI] [PubMed] [Google Scholar]
- TRAYNOR J.R., NAHORSKI S. Modulation by μ-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- VALLAR L., MUCA C., MAGNI M., ALBERT P., BUNZOW J., MELDOLESI J., CIVELLI O. Differential coupling of dopaminergic D2 receptors expressed in different cell types. Stimulation of phosphsinositol 4,5-bisphosphate hydrolysis in Ltk− fibroblasts, hyperpolarisation, and cytosolic-free Ca2+ concentration decrease in GH4C1 cells. J. Biol. Chem. 1990;265:10320–10326. [PubMed] [Google Scholar]
- WENZEL-SEIFERT K., HURT C.M., SEIFERT R. High constitutive activity of the human formyl peptide receptor. J. Biol. Chem. 1998;273:24181–24189. doi: 10.1074/jbc.273.37.24181. [DOI] [PubMed] [Google Scholar]
- WENZEL-SEIFERT K., SEIFERT R. Molecular analysis of β2-adrenoceptor coupling to Gs-, Gi-, and Gq-proteins. Mol. Pharmacol. 2000;58:954–966. doi: 10.1124/mol.58.5.954. [DOI] [PubMed] [Google Scholar]
- WITHY R.M., MAYER R.J., STRANGE P.G. Use of [3H]Spiperone for labelling dopaminergic and serotoninergic receptors in bovine caudate nucleus. J. Neurochem. 1981;37:1144–1154. doi: 10.1111/j.1471-4159.1981.tb04664.x. [DOI] [PubMed] [Google Scholar]
- YANG Q., LANIER S.M. Influence of G protein type on agonist efficacy. Mol. Pharmacol. 1999;56:651–656. doi: 10.1124/mol.56.3.651. [DOI] [PubMed] [Google Scholar]
- ZUSCIK M.J., PORTER J.E., GAIVIN R., PEREZ D.M. Identification of a conserved switch residue responsible for selective constitutive activation of β2-adrenergic receptor. J. Biol. Chem. 1998;273:3401–3407. doi: 10.1074/jbc.273.6.3401. [DOI] [PubMed] [Google Scholar]


