Abstract
UNC-49B and UNC-49C are γ-aminobutyric acid (GABA) receptor subunits encoded by the Caenorhabditis elegans unc-49 gene. UNC-49B forms a homomeric GABA receptor, or can co-assemble with UNC-49C to form a heteromeric receptor. The pharmacological properties of UNC-49B homomers and UNC-49B/C heteromers were investigated in Xenopus oocytes.
The UNC-49 subunits are most closely related to the bicuculline- and benzodiazepine-insensitive RDL GABA receptors of insects. Consistent with this classification, bicuculline (10 μM) did not inhibit, nor did diazepam (10 μM) enhance UNC-49B homomeric or UNC-49B/C heteromeric receptors.
The UNC-49C subunit strongly affects the pharmacology of UNC-49B/C heteromeric receptors. UNC-49B homomers were much more picrotoxin sensitive than UNC-49B/C heteromers (IC50=0.9±0.2 μM and 166±42 μM, respectively). Pentobarbitone enhancement was greater for UNC-49B homomers compared to UNC-49B/C heteromers. Propofol (50 μM) slightly enhanced UNC-49B homomers but slightly inhibited UNC-49B/C heteromers. Penicillin G (10 mM) inhibited UNC-49B homomers less strongly than UNC-49B/C heteromers (30% compared to 53% inhibition, respectively).
Several aspects of UNC-49 pharmacology were unusual. Picrotoxin sensitivity strongly correlates with dieldrin sensitivity, yet UNC-49B homomers were highly dieldrin resistant. The enhancing neurosteroid pregnanolone (5β-pregnan-3α-ol-20-one; 10 μM) strongly inhibited both UNC-49 receptors. Alphaxalone (10 μM), another enhancing neurosteroid, did not affect UNC-49B homomers, but slightly inhibited UNC-49B/C heteromers.
UNC-49 subunits and mammalian GABAA receptor α, β, and γ subunit classes all share roughly the same degree of sequence similarity. Thus, although they are most similar to other invertebrate GABA receptors, the UNC-49 receptors share significant structural and pharmacological overlap with mammalian GABAA receptors.
Keywords: UNC-49, GABAA receptors, C. elegans, nematodes
Introduction
Ionotropic GABA receptors are the major inhibitory neurotransmitter receptors in both vertebrate and invertebrate nervous systems. These receptors contain five subunits which are arranged around a central chloride-selective pore. Binding of GABA causes a conformational change that opens the pore and allows chloride ions to flow into the cell, resulting in reduced cellular excitability (MacDonald & Olsen, 1994). GABA receptors have considerable medical and economic importance. In humans, these receptors (the GABAA receptors) are targets of many drugs that reduce neuronal excitability. Such drugs are used to treat anxiety, epilepsy, and insomnia, and to produce anaesthesia (Sieghart, 1995). In invertebrates, these receptors are the targets of pesticides and anti-parasitic compounds used in agriculture and medicine (Ffrench-Constant et al., 1993; Martin, 1993).
To better understand the mechanisms of allosteric regulation of GABA receptors, it is useful to identify receptor residues that mediate the binding of allosteric regulators, and transduce this binding into changes in receptor function. The chimeric structure-function approach has been effective in achieving this goal. In this approach, two homologous receptors with different sensitivities to an allosteric regulator are first identified. Then, segments are swapped between the receptors to identify the domains that are responsible for the functional differences. Finally, individual amino acids that play key roles are identified by site-directed mutagenesis. Because this approach requires receptors with diverse functional properties, it is valuable to compare receptors from widely divergent genomes.
We have recently cloned the unc-49 gene from the nematode Caenorhabditis elegans. unc-49 encodes the GABA receptor that mediates the inhibitory neurotransmission between nerve and muscle. Inhibitory transmission at muscles is required for co-ordinated locomotion in nematodes (McIntire et al., 1993). unc-49 is an alternatively-spliced gene which can potentially encode three distinct GABA receptor subunits, UNC-49A, UNC-49B and UNC-49C. However only two subunits, UNC-49B and UNC-49C, are expressed at high levels in vivo. UNC-49B can assemble as a homomer. Alternatively, UNC-49B can assemble with UNC-49C to produce a distinct heteromultimeric receptor (Bamber et al., 1999). In this study, we survey the sensitivity of both UNC-49B homomeric receptors and UNC-49B/C heteromeric receptors to a variety of positive and negative allosteric regulators of GABA receptor function.
Methods
Electrophysiology
All electrophysiological analysis was performed on Xenopus oocytes that had been injected with cRNA encoding the UNC-49 subunits (Bamber et al., 1999). The UNC-49B cRNA was generated using the plasmid pBGR4 as a template, which contained a full-length UNC-49B.1 cDNA (NCBI protein database accession number AAD42383). The UNC-49C cRNA was generated using the plasmid pBGR9 as a template, which contained a full-length UNC-49C cDNA (NCBI protein database accession number AAD42386). Both plasmids were linearized using the restriction enzyme Asp 718-1 (Roche Molecular Biochemicals, Indianapolis, IN, U.S.A.), and cRNA was synthesized using the mMessage mMachine T3 kit (Ambion, Austin, TX, U.S.A.). To express UNC-49B homomers, oocytes were injected with 0.5 μg μl−1 UNC-49B cRNA, and to express UNC-49B/C heteromers, oocytes were injected with a mixture of UNC-49B and UNC-49C cRNA, each at 0.5 μg μl−1 (27.6 nl total). Oocyte injections, and two-electrode voltage clamp electrophysiology were performed as previously described (Donevan et al., 1998). Injected oocytes were incubated for 2 days at 18°C, and then placed at 4°C. Recordings were performed 2–7 days after injection. Recordings were performed using electrodes with 1–2 MΩ ðresistance, and oocytes were voltage-clamped at −60 mV. Drugs were delivered using a multiple-reservoir perfusion system. By activating the appropriate valves, the perfusion solution was switched from saline alone to saline plus GABA, with or without allosteric regulators.
The effects of most allosteric regulators were determined by comparing the peak currents evoked by GABA alone (at EC50) to those evoked with simultaneous co-application of GABA plus the allosteric regulator. Co-application of GABA and modulatory drugs is the method we routinely use unless there is evidence for slow equilibration of drug binding to receptor. Slow equilibration would produce slow rising or falling of plateau currents in the presence of GABA plus the drug, distinct from the desensitization caused by GABA alone. Although this pattern was not consistently observed for any of the allosteric regulators tested, it is still possible that the simultaneous co-application method used here could result in an underestimate of drug efficacy. The effect of modulators presented in Table 1 were calculated by pooling data from three to 10 oocytes. Each oocyte was exposed to GABA alone, followed by GABA plus drug, and then usually re-tested with GABA alone to ensure complete wash-out. A normalized current value for a single such application regimen was calculated for each oocyte. Average normalized values and standard error of the mean (s.e.mean) values were then calculated for the pool of oocytes. P values were calculated from raw data before normalization, using the two-tailed paired Student's t-test or the two-tailed Wilcoxon signed rank test.
Table 1.
Allosteric regulation of UNC-49B and UNC-49B/C. Values represent the per cent of current observed in the presence of GABA plus the allosteric regulator, compared to the same concentration of GABA alone. For all drugs except pentobarbitone, GABA concentrations corresponded to the respective EC50 values for the UNC-49B and UNC-49B/C receptors. For pentobarbitone, GABA concentrations corresponded to EC10. Error values are s.e.mean
GABA EC50 values were determined for each batch of oocytes by performing a GABA dose-response curve, and fitting the data with the Hill equation:
where I is current at a given GABA concentration, Imax is current at saturation, EC50 is the GABA concentration required to produce half-maximal current, and n is the Hill coefficient.
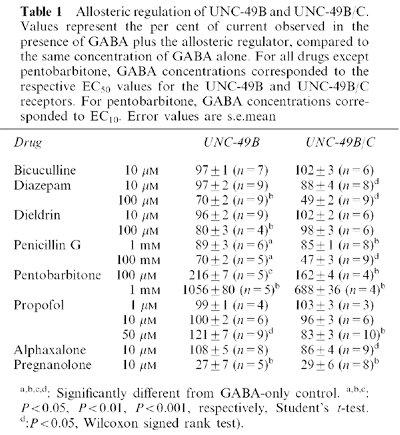
Pentobarbitone (PB) dose-response curves (Figure 2A) were generated by co-applying EC10 GABA with varying concentrations of pentobarbitone. First, GABA dose-response curves were generated, and the EC10 was determined using the Hill equation above (n=3). For subsequent oocytes, the responses to EC10 and saturating GABA (3000 μM) were measured, to verify that the EC10 GABA produced 10% of the maximal current for that particular oocyte. If so, then we proceeded to co-apply EC10 GABA and varying concentrations of pentobarbitone. The pentobarbitone dose-response curves were fitted with the Hill equation above, to determine the pentobarbitone EC50 and Hill coefficient.
Figure 2.
Pentobarbitone responses of UNC-49B and UNC-49B/C. (A) Pentobarbitone dose-response curves for enhancement of EC10 GABA currents. Closed circles represent UNC-49B homomers (n=5), open circles represent UNC-49B/C heteromers (n=4). Error bars are s.e.mean. (B) Direct activation by 1 mM pentobarbitone was observed in Xenopus oocytes expressing UNC-49B homomers (upper trace), but not UNC-49B/C heteromers (lower trace). (C) 10 mM pentobarbitone inhibits both the UNC- 49B (upper traces) and UNC-49B/C receptors (lower traces) when co-applied with GABA (GABA concentrations correspond to the EC40 and EC10 for the UNC-49B and UNC-49B/C receptors, respectively). Bars above traces in (B) and (C) represent duration of drug exposure.
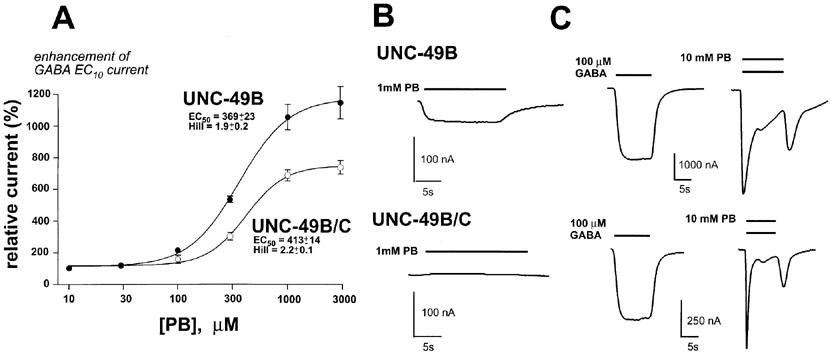
The picrotoxin (PTX) dose-response curves (Figure 6C) were generated by co-applying EC50 GABA and picrotoxin at varying concentrations. Picrotoxin dose-response curves were fitted using the equation:
whereIPTX+/IPTX− is the current in the presence of picrotoxin relative to GABA alone, IC50 is the concentration of picrotoxin required to block 50% of the GABA evoked current, and n is the Hill coefficient. The program NFIT (Island Products, Galveston, TX, U.S.A.) was used for non-linear curve-fitting. Error values presented for dose-response curves are s.e.mean, and P values (two-tailed) were calculated using the unpaired Student's t-test.
Figure 6.
UNC-49C confers picrotoxin resistance. (A) A Xenopus oocyte expressing the UNC-49B homomer shows strong inhibition by 100 μM picrotoxin. (B) An oocyte expressing the UNC-49B/C heteromer shows little inhibition by 100 μM picrotoxin. Bars in (A) and (B) represent the duration of drug exposure. (C) Picrotoxin dose-response curves for UNC-49B homomers (closed circles) and UNC-49B/C heteromers (open circles). Error bars represent s.e.mean, n=4 oocytes for each data point. GABA concentrations corresponded to the respective GABA EC50 values for the UNC-49B and UNC-49B/C receptors.
All drugs were obtained from Sigma (St. Louis, MO, U.S.A.). Stock solutions of alphaxalone (10 mM), pregnanolone (10 mM), pentobarbitone (300 mM), propofol (100 mM), picrotoxin (100 mM), and dieldrin (1 M) were prepared in dimethyl sulphoxide. GABA (1 M), and penicillin G (penG; 1 M) were prepared in water, Diazepam (DZ; 10 mM) was prepared in ethanol, and (−) bicuculline methiodide (BIC; 100 mM) was prepared in Ringer's saline. All stocks were stored at −20°C except diazepam, which was prepared fresh each day.
Phylogenetic analysis
Alignment of M2 sequences was performed using the DNAstar analysis software (Madison, WI, U.S.A.). Per cent similarity values were also determined using DNAstar software (Clustal alignment method, PAM250 residue weight table). The dendrogram and bootstrap analysis was performed using ClustalW software (Thompson et al., 1994). The M3–M4 intracellular loop sequences of each subunit were not included in these analyses, because they are not conserved. Accession numbers (NCBI protein database) for the aligned subunits are as follows: UNC-49A (AAD42382), UNC-49B (AAD42383), UNC-49C (AAD42386); Rat GABAA receptor subunits α1 (P18504), α2 (P23576), α3 (P20236), α4 (P28471), β1 (P15431), β2 (P15432), β3 (P15433), γ1 (P23574), γ2 (P18508), γ3 (P28473), δ(P18506), ε(AAG17631); Rat GABAC receptor subunits ρ1 (P50572), ρ2 (P47742), ρ3 (P50573); Rat glycine receptor subunits α1 (P07727), α2 (P22771), α3 (P24524), β(P20781); Human GABAA receptors α5 (CAA01920), α6 (NP000802), Drosophila melanogaster Rdl gene product (P25123), Drosophila GABA receptor β-subunit (Q08832); lymnaea stagnalis GABA receptor β-subunit (P26714); C. elegans β-subunit (NP499661); and avermectin-sensitive glutamate-gated chloride channel subunits α1 (S50864), α2B (CAA04170), β(S50865); mouse nicotinic acetylcholine receptor α1 subunit (P04756). Potassium channel sequence comparisons were performed with the human HERG channel (PIR I38465) and the Streptomyces lividans KcsA channel (PIR S60172).
Results
We have characterized the responses of the UNC-49B homomers and UNC-49B/C heteromers to a variety of allosteric regulators. We expressed both homomeric and heteromeric UNC-49 receptor in Xenopus oocytes, and tested compounds that typically enhance GABA receptor function (diazepam, pentobarbitone, propofol, and the neurosteroids alphaxalone and pregnanolone), and compounds that typically inhibit GABA receptor function (bicuculline, picrotoxin, dieldrin, and penicillin G). Allosteric regulators were tested at the GABA EC50 for both UNC-49B homomers and UNC-49B/C heteromers, with the exception of pentobarbitone, which was tested at the GABA EC10. As previously reported, the GABA-responsiveness of both UNC-49 receptors was variable (Bamber et al., 1999), but UNC-49B homomers were always more GABA-sensitive, and had larger Hill coefficients than UNC-49B/C heteromers. To obtain reliable GABA dose-response data, we characterized the GABA responsiveness of each new batch of oocytes expressing UNC-49 receptors. We always observed the expected differences between the two receptor types, with EC50 values ranging from 78 to 267 μM for UNC-49B homomers, and from 139 to 660 μM for UNC-49B/C heteromers, and Hill coefficients ranging from 2 to 3.1 for UNC-49B homomers, and from 1 to 1.5 for UNC-49B/C heteromers. We also observed that UNC-49B/C heteromers tended to desensitize faster than UNC-49B homomers, although we did not attempt to quantify this effect. Both receptors typically produced currents in the 1 to 3 μA range, although there was a large degree of cell-to-cell variability in the absolute current magnitude, with some cells producing maximal currents in the hundred nA range. We did not observe any differences in drug efficacy or potency that correlated with current magnitude or extent of apparent desensitization, so these properties were not used as a basis for excluding cells from the data set.
Positive allosteric regulators: diazepam, pentobarbitone, propofol, neurosteroids
Diazepam
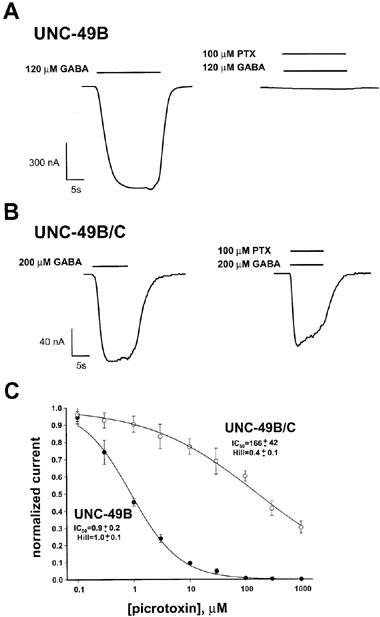
We tested whether the UNC-49 receptors showed benzodiazepine modulation using the full benzodiazepine agonist diazepam. Diazepam acts specifically on mammalian GABAA receptors containing α, β, and γ ðsubunits (Burt & Kamatchi, 1991). Diazepam enhances GABA-evoked currents of these receptors by shifting the GABA dose-response curve to the left. Typical diazepam EC50 values range from 50 nM to 4 μM; maximal enhancement for a variety of recombinant and native mammalian GABAA receptors has been observed at 10 μM (Puia et al., 1991). We found that 10 μM diazepam did not enhance either UNC-49B homomers or UNC-49B/C heteromers, consistent with the known requirement for the γ subunit for diazepam action. Instead, diazepam produced a novel effect on the UNC-49 receptors. We observed a slight dose-dependent inhibition, which was potentiated by the UNC-49C subunit (Figure 1, Table 1). This effect was fully reversible within 50 s.
Figure 1.
Responses of UNC-49B and UNC-49B/C to diazepam. Xenopus oocytes expressing UNC-49B homomers (upper traces) or UNC-49B/C heteromers (lower traces) were exposed to GABA (left), or GABA plus diazepam (right). Bars above traces indicate the duration of drug application. GABA concentrations correspond to the respective EC50 values for UNC-49B homomers and UNC-49B/C heteromers.
Pentobarbitone
Barbiturates such as pentobarbitone can enhance GABA-evoked currents, directly activate GABA receptors, and at high concentrations, block GABA receptors. Barbiturates show little specificity among ligand-gated chloride channels. They affect mammalian GABAA receptors (Sieghart, 1995), insect GABA receptors (Hosie & Sattelle, 1996), and mammalian glycine receptors (Belelli et al., 1999). EC50 values for pentobarbitone enhancement are in the range of 20–35 μM for several recombinant mammalian GABAA receptors (Thompson et al., 1996), and 300–800 μM for the Drosophila RDL receptor and its splice variant (Belelli et al., 1996; Chen et al., 1994). Both forms of UNC-49 receptors were sensitive to allosteric regulation by pentobarbitone (Figure 2). At concentrations of 3000 μM or less, pentobarbitone caused simple potentiation of the GABA-evoked currents. The EC50 values this enhancement (using EC10 GABA) were not significantly different between UNC-49B homomers and UNC-49B/C heteromers. However, the efficacy of pentobarbitone was significantly greater at the UNC-49B homomer than at the UNC-49B/C heteromer (Figure 2A, Table 1). UNC-49B homomers were maximally enhanced by 11.5±1.0 fold (n=5) while UNC-49B/C heteromers were maximally enhanced by 7.4±0.4 fold (n=4; P<0.05). This enhancement was 100% reversible within 30 s. Higher concentrations of pentobarbitone (1 mM), in the absence of GABA, evoked inward currents from oocytes expressing the UNC-49B homomer (n=4), but not from oocytes expressing the UNC-49B/C heteromer (n=5; Figure 2B). This pentobarbitone direct activation produced currents that were 0.5 to 1% of the amplitude of the EC50 GABA-evoked currents. This effect is relatively small: pentobarbitone direct activation of mammalian GABAA receptors can produce currents which range from 33 to 168% of the maximal GABA-evoked currents (Thompson et al., 1996). However, the subunit specificity of this pentobarbitone direct activation suggests that it is not an artefact of the Xenopus oocyte expression system. Finally, pentobarbitone had a complex action at very high concentrations (10 mM). As shown in Figure 2C, co-application of GABA and 10 mM pentobarbitone caused a rapid current spike that was larger than the current produced by the same concentration of GABA alone (not shown), suggesting the initial action of pentobarbitone was to enhance the GABA-evoked current. Following this spike, the current decreased to a much lower steady-state value. Finally a large tail spike occurred upon removal of both drugs. This behaviour is characteristic of an allosteric enhancer that also has open channel blocking activity, and is thought to be caused by faster dissociation from the channel blocking site than the enhancing site (Akaike et al., 1987; Peters et al., 1988; Robertson, 1989; Thompson et al., 1996). This effect was observed regardless of whether UNC-49C was present in the receptor.
Propofol
Propofol is a general anaesthetic which is similar to pentobarbitone in its specificity and action at ligand-gated chloride channels. The binding sites and transduction mechanisms appear to be similar for these two compounds (Belelli et al., 1999; Davies et al., 1998; Pistis et al., 1997; 1999). The EC50 for propofol enhancement has been reported to be in the range of 5–25 μM for mammalian and insect GABA receptors (Belelli et al., 1996; Chen et al., 1994; Lam & Reynolds, 1998; Pistis et al., 1999). At 50 μM, propofol can directly activate mammalian GABAA receptors to produce currents that are 10–20% of the maximal GABA-evoked currents (Lam & Reynolds, 1998). We tested whether propofol could enhance or directly activate the UNC-49B homomeric and UNC-49B/C heteromeric GABA receptors at 1, 10, and 50 μM. Propofol, at 1 or 10 μM, showed no enhancement or direct activation of either form of the UNC-49 GABA receptor (Table 1, not shown). However at 50 μM (Figure 3), propofol slightly enhanced the GABA EC50 current of UNC-49B homomers (Table 1). The same propofol concentration enhanced the GABA EC10 current of UNC-49B homomers by 70% (P<0.05, n=4 oocytes; not shown). These data indicate that propofol has relatively low efficacy at the UNC-49B homomer, since propofol can potentiate mammalian GABAA receptors by 5 to 34 fold when activated over a broad range of GABA concentrations (Davies et al., 1997; Lam & Reynolds, 1998). Propofol enhancement was antagonized by UNC-49C: Instead of enhancing, 50 μM propofol slightly but significantly inhibited currents from UNC-49B/C heteromers (P<0.01; Table 1). Both the enhancing and inhibiting effects of propofol were 100% reversible within 90 s. We did not observe direct activation of either UNC-49B homomers (n=5) or UNC-49B/C heteromers (n=3).
Figure 3.
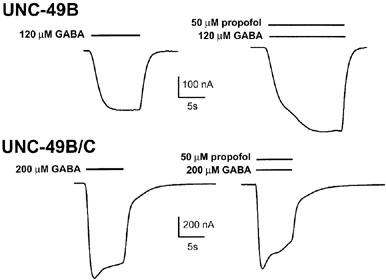
Propofol enhances UNC-49B but inhibits UNC-49B/C. GABA-evoked currents in Xenopus oocytes expressing UNC-49B homomers are potentiated by 50 μM propofol (upper traces). GABA-evoked currents in oocytes expressing UNC-49B/C heteromers are slightly inhibited by 50 μM propofol (lower traces). GABA concentrations corresponded to EC50 values, bars represent duration of drug exposure.
Neurosteroids
GABA-evoked currents of mammalianGABAA receptors are enhanced by endogenous pregnane neurosteroids such as pregnanolone, and the synthetic neurosteroid anaesthetic alphaxalone. EC50 values for neurosteroid enhancement of several subtypes of recombinant GABAA receptors were reported to be in the 100–500 nanomolar range for pregnane steroids, and in the low micromolar range for alphaxalone (Maitra & Reynolds, 1998; Park-Chung et al., 1999). We found that alphaxalone and pregnanolone were largely inhibitory at the C. elegans GABA receptor (Figure 4). Pregnanolone caused strong inhibition of the UNC-49 receptors, regardless of whether the UNC-49C subunit was present or absent (Figure 4, Table 1). By contrast, alphaxalone had no effect on UNC-49B homomers, while it modestly inhibited UNC-49B/C heteromers (Figure 4, Table 1). Alphaxalone inhibition was fully reversible within 1 min. Pregnanolone inhibition was also fully reversible, but required over 15 min to fully wash out. Thus, neurosteroid response of UNC-49 differs from that of mammalian GABAA receptors because both of these steroids at this concentration strongly enhance the mammalian receptors (Maitra & Reynolds, 1998; Park-Chung et al., 1999). It also differs from the response of Drosophila RDL, which is weakly potentiated by 10 μM alphaxalone, and by 10 μM 5α-pregnan-3α-ol-20-one (a closely-related pregnane steroid; Chen et al., 1994; Hosie & Sattelle, 1996).
Figure 4.
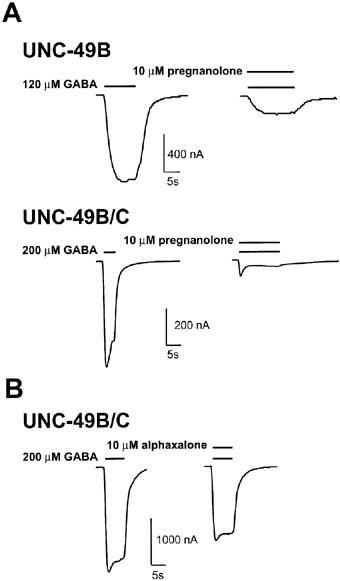
Neurosteroid responses of UNC-49B and UNC-49B/C. (A) Pregnanolone inhibited both the UNC-49B homomer (upper traces) and the UNC-49B/C heteromer (lower traces). (B) Alphaxalone inhibited GABA-evoked currents in oocytes expressing UNC-49B/C heteromers. GABA concentrations corresponded to the EC50 value for each receptor, bars above traces represent duration of drug exposure.
Negative allosteric regulators: bicuculline, picrotoxin, dieldrin, penicillin G
Bicuculline
Bicuculline is generally regarded as a GABAA receptor competitive antagonist, although there is evidence that it may also act allosterically (Ueno et al., 1997). It is highly specific for vertebrate GABAA receptors, and is effective in the low micromolar range. We tested the UNC-49 receptors for bicuculline sensitivity, and found that 10 μM bicuculline had no effect on either UNC-49B homomers or UNC-49B/C heteromers (Figure 5, Table 1). This result confirms that the UNC-49 receptors are pharmacologically distinct from the mammalian GABAA receptors.
Figure 5.
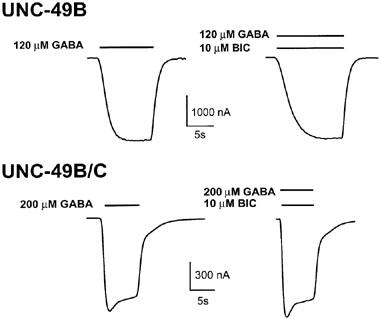
Bicuculline does not affect UNC-49 receptors. UNC-49B homomers (upper traces) and UNC-49B/C heteromers (lower traces) were exposed to GABA (left), or GABA plus 10 μM bicuculline (right). The increased current rise time in the UNC-49B homomer trace shown is not consistently observed. Bars above traces indicate the duration of drug application. GABA concentrations corresponded to the respective EC50 values. GABA-only traces are the same as shown in Figure 1.
Picrotoxin
Picrotoxin is a broadly-active inhibitor of ligand-gated chloride channels. Picrotoxin inhibition has been reported for mammalian and insect GABA receptors, glycine receptors, and glutamate-gated chloride channels (Etter et al., 1999; Ffrench-Constant et al., 1993; Rajendra et al., 1997; Sieghart, 1995). Picrotoxin IC50 values are reported to be in the 500–1000 nanomolar range for recombinant mammalian GABAA receptors (Gurley et al., 1995; Krishek et al., 1996a), and around 50 nanomolar for the Drosophila RDL splice variant (Chen et al., 1994). We observed that UNC-49B homomers are picrotoxin sensitive, while UNC-49B/C heteromers are strongly picrotoxin resistant (Figure 6). Picrotoxin inhibition was fully reversible within 30 s. The site of picrotoxin action is likely to be near the pore of the receptor. First, residues important for picrotoxin sensitivity have been identified in the M2 pore-forming domain (Gurley et al., 1995; Pribilla et al., 1992; Wang et al., 1995). Second, substituted-cysteine-accessibility experiments implicate pore residues in picrotoxin action (Xu et al., 1995). Thus, our results extend an earlier observation (Bamber et al., 1999) that the UNC-49C subunit makes an important contribution to the pore properties of the UNC-49B/C heteromer.
Dieldrin
Dieldrin is a pesticide that strongly inhibits invertebrate GABA receptors. Dieldrin also affects mammalian GABAA receptors, but has a complex effect consisting of a fast enhancement followed by a slowly developing inhibition (Nagata et al., 1994a, b). The dieldrin inhibitory binding site and transduction mechanism are thought to be similar, if not identical, to those of picrotoxin (Ffrench-Constant et al., 1993). Therefore since UNC-49B was highly picrotoxin sensitive, we were surprised to find that UNC-49B homomers are strongly dieldrin resistant (Figure 7). UNC-49B homomers are unaffected by 10 μM dieldrin, whereas the wild-type Drosophila RDL GABA receptor is completely inhibited by this concentration of dieldrin (Ffrench-Constant et al., 1993). However UNC-49B homomers were not completely dieldrin resistant; modest inhibition was observed at 100 μM dieldrin. As with picrotoxin inhibition, the UNC-49C subunit reversed this sensitivity to high concentrations of dieldrin. UNC-49B/C heteromers were not affected by either 10 or 100 μM dieldrin (Table 1). Dieldrin inhibition was fully reversible within 90 s.
Figure 7.
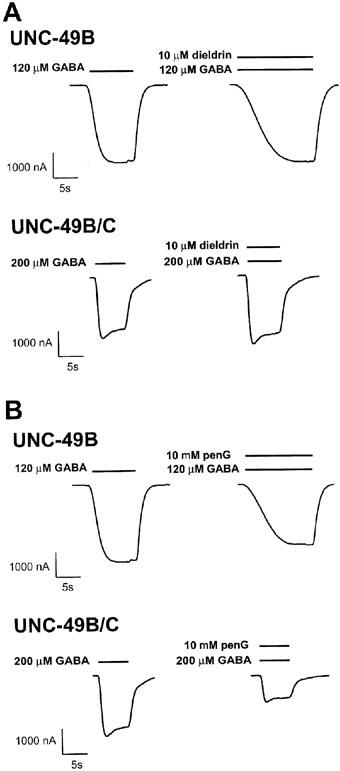
Responses of UNC-49B and UNC-49B/C to other channel blockers. (A) GABA alone (left traces), and GABA plus dieldrin (right traces) were applied to Xenopus oocytes expressing UNC-49B homomers (upper traces) or UNC-49B/C heteromers (lower traces). (B) GABA alone (left traces) or GABA plus penicillin G (right traces) were applied to Xenopus oocytes expressing UNC-49B homomers (upper traces) or UNC-49B/C heteromers (lower traces). The increased current rise times of the UNC-49B homomer traces shown, in the presence of dieldrin and penicillin G, are not consistently observed. The EC50 GABA concentration was used for each receptor, bars represent duration of drug exposure.
Penicillin G
β-lactam antibiotics such as penicillin G are pro-convulsive, and are known to act as open-channel blockers of GABA receptors (Fujimoto et al., 1995). We tested whether UNC-49B homomers and UNC-49B/C heteromers were sensitive to inhibition by penicillin G. At 1 mM, penicillin G slightly inhibited the GABA-evoked currents from both receptors. However, at 10 mM, penicillin G inhibited UNC-49B/C heteromer currents about twice as much as UNC-49B homomer currents (Figure 7, Table 1). Penicillin G inhibition was fully reversible within 40 s.
Discussion
We have characterized the actions of several positive and negative allosteric regulators of the vertebrate GABAA receptor on the UNC-49B homomeric and UNC-49B/C heteromeric GABA receptors of C. elegans. We draw four major conclusions from our study: First, UNC-49C modulates the sensitivity of the UNC-49B/C heteromer to several allosteric regulators. Second, the properties of muscle GABA receptors in parasitic nematodes are more similar to the UNC-49B/C heteromer than the UNC-49B homomer, suggesting that a conserved UNC-49C homologue may be modulating the pharmacology of these receptors as well. Third, considering both pharmacological and phylogenetic criteria, the UNC-49 receptors can be placed with the Drosophila RDL receptor into a group which is distinct from mammalian GABAA receptors, but is nonetheless a conserved part of the GABA receptor family. Fourth, the binding sites and transduction mechanisms for certain classes of allosteric regulators may be more diverse than has been previously appreciated. Channel blockers and anaesthetics thought to act through a common site have different effects on the UNC-49 receptors. The conservation among ionotropic GABA receptors predicts that this diversity may also exist in the mammalian GABAA receptors.
The actions of most allosteric regulators on the UNC-49B receptor were greatly altered by the addition of the UNC-49C subunit to the receptor. These effects are interesting from a structure-function point of view, because in some cases, potential effector residues in the pore-lining M2 domain have been identified from structure-function studies of other GABA receptors, and these residues differ in a consistent manner in the UNC-49B and UNC-49C subunits. In the first example, sequence differences between UNC-49B and UNC-49C subunits can account for differential picrotoxin sensitivity. The conserved threonine at the 6′ position in the M2 domain (Figure 8A) is an important determinant of picrotoxin sensitivity. Replacement of this threonine with a methionine in the rat ρ1 GABAC receptor subunit, or with a phenylalanine in the rat β2 GABAA receptor subunit has been demonstrated to cause strong picrotoxin resistance (Gurley et al., 1995; Zhang et al., 1995). The UNC-49B subunit contains a threonine at this position (Figure 8A) and the UNC-49B homomer is picrotoxin sensitive. UNC-49C contains a methionine at this position (Figure 8A), and the UNC-49B/C receptor is relatively picrotoxin resistant. In the second example, previous data indicated that pentobarbitone direct activation is greatly reduced when a methionine residue is inserted at the 15′ position of the M2 domain, in place of the wild-type asparagine residue in the human β3 subunit (Pistis et al., 1999). Conversely, pentobarbitone direct activation is gained when the 15′ methionine of the Drosophila RDL receptor is mutated to serine (Pistis et al., 1999). UNC-49C contains a methinonine at the 15′ position, and pentobarbitone direct activation is lost when the UNC-49C subunit is present in the receptor. In the third example, this same methionine residue causes reduced sensitivity to propofol enhancement (Pistis et al., 1999); we observe that the modest propofol enhancement of the UNC-49B homomer is lost in UNC-49B/C heteromer. It is more difficult to predict the structural basis for the effects of UNC-49C on diazepam, penicillin G, and alphaxalone actions, and the efficacy of pentobarbitone enhancement, however UNC-49C may be useful for further investigation of these mechanisms.
Figure 8.
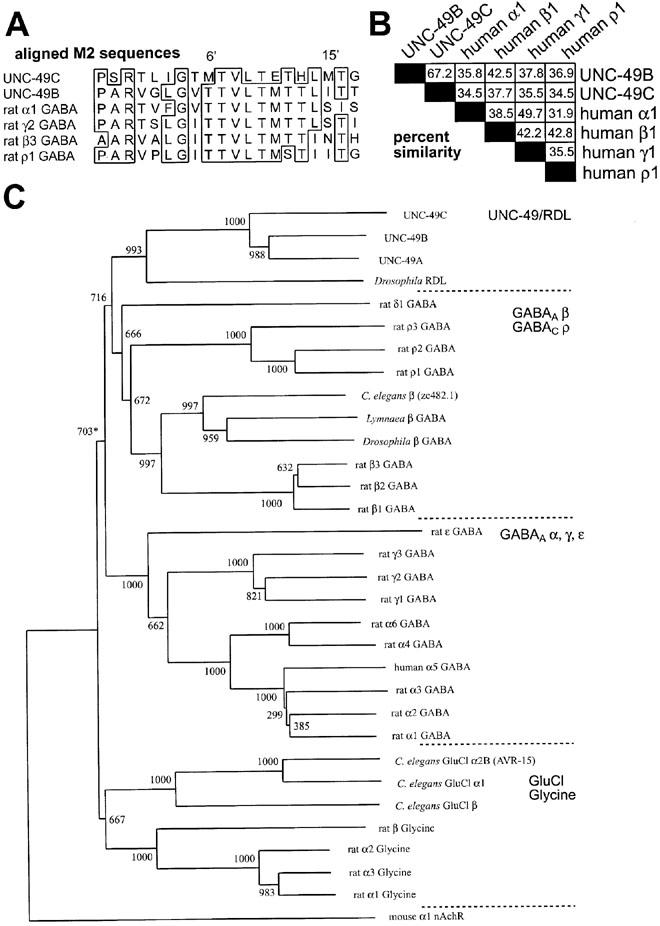
Sequence comparisons and phylogenetic classification of ligand-gated chloride channel subunits. (A) Alignment of the M2 segments of selected subunits. Residues are numbered according to the scheme used by Lester (1992). (B) Matrix of per cent sequence similarity between UNC-49 and mammalian GABA receptor subunits. (C) Phylogenetic tree showing the relationships among GABA receptor subunits and other ligand-gated chloride channel subunits. The mouse nicotinic acetylcholine receptor (nAChR) α1 subunit was included to root the tree. Bootstrap values are shown above each branch; the bootstrap value for the grouping of the UNC-49 subunits with mammalian GABAA receptor subunits is marked with an asterisk. This dendrogram was constructed using ClustalW sequence analysis software. The intracellular loops of the subunits were excluded from the analysis in (B) and (C) because they are non-conserved.
Modulation of receptor pharmacology by an UNC-49C-like subunit may be a conserved feature of nematode GABA receptors. Native GABA receptors in muscle cells of the parasitic nematode Ascaris suum show properties quite similar to the UNC-49B/C heteromer. They are neither inhibited by bicuculline nor enhanced by benzodiazepines, a feature shared with other invertebrate GABA receptors. They are dieldrin resistant, suggesting that they are more like UNC-49 than like RDL receptors. Finally they are picrotoxin resistant, and thus resemble the UNC-49B/C heteromer rather than the UNC-49B homomer (Holden-Dye et al., 1989; Martin, 1993; Walker et al., 1992). A simple explanation for this unique pharmacology would be that nematode muscle GABA receptors share a conserved subunit structure; namely a heteromultimer composed of subunits homologous to UNC-49B and UNC-49C. If so, then screening for compounds that specifically target the C. elegans UNC-49B/C heteromer might be an effective strategy for developing novel nematocidal agents.
Since the UNC-49 subunits are potentially useful tools for probing the structure-function relationships of GABAA receptors in general, it is important to know how closely the UNC-49 subunits are related to the other ligand-gated chloride channel subunits. A simple comparison of subunit sequences reveals that UNC-49B and UNC-49C are about 35–45% similar to the mammalian α, β, γ, and ρ subunits (Figure 8B). The mammalian subunits show the same degree of similarity to one another, indicating that the UNC-49 subunits are conserved members of the GABA receptor subunit family, rather than a divergent branch of this family. This degree of similarity suggests that the subunits will have conserved overall structures, and conserved binding pockets and transduction mechanisms for allosteric regulators. Molecular modelling studies, based on the crystal structure of the bacterial KcsA potassium channel, suggest that the overall structure, and the mechanisms of channel function and allosteric regulation are conserved between KcsA and the human HERG potassium channels, even though those channels are only 22% similar within the pore-forming region present in the crystal structure (Doyle et al., 1998; Mitcheson et al., 2000).
A more-detailed phylogenetic analysis of the ionotropic GABA receptor subunits reveals three additional relationships among vertebrate and invertebrate receptors (Figure 8C). First, UNC-49 subunits are most closely related to the RDL receptors of Drosophila melanogaster and other insects, as suggested in a previous study (Bamber et al., 1999). Thus, while UNC-49 and the vertebrate GABAA receptors are related, the UNC-49 subunits are not specific homologues of any particular class of mammalian GABAA receptor subunits (i.e. α, β, or γ). This conclusion is consistent with our demonstration that the UNC-49 receptors, like RDL receptors, do not show typical responses to the GABAA receptor-specific modulators bicuculline and diazepam. Second, UNC-49 and the mammalian GABAA receptor subunits fall into a single group, which is distinct from the glycine receptors and glutamate-gated chloride channels (GluCl; Figure 8C). This grouping is likely to be significant because its bootstrap value was large (703), and it has been estimated that bootstrap values of 700 or greater indicate a confidence level of ⩾95% (Hillis & Bull, 1993). Third, the UNC-49/RDL group of receptors are more similar to the mammalian GABAA receptor β subunits than to the α or γ subunits. Our phylogenetic analysis indicates a greater degree of sequence similarity with the β subunits (bootstrap value of 716; Figure 8B). Furthermore, experimental analysis has revealed that members of these groups share an important functional similarity, in that they can assemble to form homomers. This ability is shared among UNC-49 and RDL (Ffrench-Constant et al., 1993), the ρ subunits (Cutting et al., 1991), and the GABAA receptor β subunits (Krishek et al., 1996b; Taylor et al., 1999; Wooltorton et al., 1997). One interpretation of these results is that the UNC-49/RDL group and the GABAA receptor β subunit group may have evolved from a common β-like ancestor which existed prior to the divergence of vertebrate and invertebrate lineages. Consistent with this idea, invertebrate genomes contain other subunits which are direct homologues of the mammalian β subunits (i.e. the predicted subunit zc482.1 from C. elegans, and the β subunits from Lymnaea stagnalis and Drosophila; Figure 8B). These subunits may be direct descendants of a putative ancestral β-like subunit.
In view of the sequence similarities among the ionotropic GABA receptor subunits, two of our observations may offer particular insight into the allosteric regulatory mechanisms of GABA receptors in general. Specifically, the mechanisms for channel blockers and anaesthetics seem to be more diverse than has previously been appreciated. For example, the mechanisms of picrotoxin and dieldrin inhibition have thus far been indistinguishable. Resistance to both compounds are strongly cross-correlated in insects and vertebrates (Kadous et al., 1983; Yarbrough et al., 1986). Moreover, both compounds compete for the same binding site (Matsumura & Ghiasuddin, 1983), and the same mutation in RDL that causes dieldrin resistance also causes picrotoxin resistance (Ffrench-Constant et al., 1993). However, we have found that UNC-49B homomers are highly picrotoxin sensitive but highly dieldrin resistant. Thus, these two compounds cannot have identical mechanisms of inhibition. Similarly, evidence suggests that pentobarbitone and propofol may act through a common site or mechanism of action (Davies et al., 1998; Pistis et al., 1997; 1999). However, we observed that the UNC-49B/C heteromer was enhanced by pentobarbitone but inhibited by propofol, highlighting possible differences in their respective mechanisms. In view of the sequence conservation among ionotropic GABA receptor subunits, this mechanistic diversity may also extend to the mammalian GABAA receptors.
Together, the results of this study suggest that the UNC-49 subunits are a useful addition to the tools available for studying GABAA receptor structure-function relationships. The UNC-49B homomer and UNC-49B/C heteromer both have unique pharmacological profiles, suggesting that they have diverse sequences in functionally-important regions. UNC-49B forms a homomeric receptor, which will simplify the task of identifying functionally important sequences. Finally, the overall structure of the UNC-49 subunits is highly conserved, so the insights gained by studying UNC-49 can be straightforwardly applied to other receptors, including the mammalian GABAA receptors.
Acknowledgments
We wish to thank Dr. M. Sanguinetti for critical reading of the manuscript, and for supplying Xenopus oocytes. This work was supported by NIH grants NS31519 to R.E. Twyman, NS35307 to E. M. Jorgensen, and MH64699 to B.A. Bamber, and the Klingenstein Fund.
Abbreviations
- BIC
bicuculline
- DZ
diazepam
- GABA
γ-aminobutyric acid
- GluCl
glutamate-gated chloride channel
- nicotinic acetylcholine receptor
nAchR
- PB
pentobarbitone
- penG
penicillin G
- pregnanolone
5β-pregnan-3α-ol-20-one
- PTX
picrotoxin
- s.e.mean
standard error of the mean
References
- AKAIKE N., MARUYAMA T., TOKUTOMI N. Kinetic properties of the pentobarbitone-gated chloride current in frog sensory neurones. J. Physiol. 1987;394:85–98. doi: 10.1113/jphysiol.1987.sp016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAMBER B.A., BEG A.A., TWYMAN R.E., JORGENSEN E.M. The Caenorhabditis elegans unc-49 Locus Encodes Multiple Subunits of a Heteromultimeric GABA Receptor. J. Neurosci. 1999;19:5348–5359. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELELLI D., CALLACHAN H., HILL-VENNING C., PETERS J.A., LAMBERT J.J. Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors expressed in Xenopus laevis oocytes. Br. J. Pharmacol. 1996;118:563–576. doi: 10.1111/j.1476-5381.1996.tb15439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELELLI D., PISTIS M., PETERS J.A., LAMBERT J.J. The interaction of general anaesthetics and neurosteroids with GABA(A) and glycine receptors. Neurochem. Int. 1999;34:447–452. doi: 10.1016/s0197-0186(99)00037-6. [DOI] [PubMed] [Google Scholar]
- BURT D.R., KAMATCHI G.L. GABAA receptor subtypes: from pharmacology to molecular biology. Faseb J. 1991;5:2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- CHEN R., BELELLI D., LAMBERT J.J., PETERS J.A., REYES A., LAN N.C. Cloning and functional expression of a Drosophila gamma-aminobutyric acid receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6069–6073. doi: 10.1073/pnas.91.13.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTTING G.R., LU L., O'HARA B.F., KASCH L.M., MONTROSE-RAFIZADEH C., DONOVAN D.M., SHIMADA S., ANTONARAKIS S.E., GUGGINO W.B., UHL G.R., KAZAZIAN H.H., Jr. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES M., THUYNSMA R.P., DUNN S.M. Effects of propofol and pentobarbital on ligand binding to GABAA receptors suggest a similar mechanism of action. Can. J. Physiol. Pharmacol. 1998;76:46–52. [PubMed] [Google Scholar]
- DAVIES P.A., KIRKNESS E.F., HALES T.G. Modulation by general anaesthetics of rat GABAA receptors comprised of alpha 1 beta 3 and beta 3 subunits expressed in human embryonic kidney 293 cells. Br. J. Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONEVAN S.D., BEG A., GUNTHER J.M., TWYMAN R.E. The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J. Pharmacol. Exp. Ther. 1998;285:539–545. [PubMed] [Google Scholar]
- DOYLE D.A., MORAIS CABRAL J., PFUETZNER R.A., KUO A., GULBIS J.M., COHEN S.L., CHAIT B.T., MACKINNON R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- ETTER A., CULLY D.F., LIU K.K., REISS B., VASSILATIS D.K., SCHAEFFER J.M., ARENA J.P. Picrotoxin blockade of invertebrate glutamate-gated chloride channels: subunit dependence and evidence for binding within the pore. J. Neurochem. 1999;72:318–326. [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., ROCHELEAU T.A., STEICHEN J.C., CHALMERS A.E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993;363:449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO M., MUNAKATA M., AKAIKE N. Dual mechanisms of GABAA response inhibition by β-lactam antibiotics in the pyramidal neurones of the rat cerebral cortex. Br. J. Pharmacol. 1995;116:3014–3020. doi: 10.1111/j.1476-5381.1995.tb15957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURLEY D., AMIN J., ROSS P.C., WEISS D.S., WHITE G. Point mutations in the M2 region of the α, β, or γ subunit of the GABAA channel that abolish block by picrotoxin. Receptors Channels. 1995;3:13–20. [PubMed] [Google Scholar]
- HILLIS D.M., BULL J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993;42:182–192. [Google Scholar]
- HOLDEN-DYE L., KROGSGAARD-LARSEN P., NIELSEN L., WALKER R.J. GABA receptors on the somatic muscle cells of the parasitic nematode, Ascaris suum: stereoselectivity indicates similarity to a GABAA-type agonist recognition site. Br. J. Pharmacol. 1989;98:841–850. doi: 10.1111/j.1476-5381.1989.tb14613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSIE A.M., SATTELLE D.B. Allosteric modulation of an expressed homo-oligomeric GABA-gated chloride channel of Drosophila melanogaster. Br. J. Pharmacol. 1996;117:1229–1237. doi: 10.1111/j.1476-5381.1996.tb16720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KADOUS A.A., GHIASUDDIN S.M., MATSUMURA F., SCOTT J.G., TANAKA K. Difference in the Picrotoxinin Receptor between the Cyclodiene-Resistant and Susceptible Strains of the German Cockroach. Pestic. Biochem. Physiol. 1983;19:157–166. [Google Scholar]
- KRISHEK B.J., MOSS S.J., SMART T.G. A functional comparison of the antagonists bicuculline and picrotoxin at recombinant GABAA receptors. Neuropharmacology. 1996a;35:1289–1298. doi: 10.1016/s0028-3908(96)00089-5. [DOI] [PubMed] [Google Scholar]
- KRISHEK B.J., MOSS S.J., SMART T.G. Homomeric beta 1 gamma-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Mol. Pharmacol. 1996b;49:494–504. [PubMed] [Google Scholar]
- LAM D.W., REYNOLDS J.N. Modulatory and direct effects of propofol on recombinant GABAA receptors expressed in xenopus oocytes: influence of alpha- and gamma2- subunits. Brain Res. 1998;784:179–187. doi: 10.1016/s0006-8993(97)01334-6. [DOI] [PubMed] [Google Scholar]
- LESTER H.A. The permeation pathway of neurotransmitter-gated ion channels. Annu. Rev. Biophys. Biomol. Struct. 1992;21:267–292. doi: 10.1146/annurev.bb.21.060192.001411. [DOI] [PubMed] [Google Scholar]
- MACDONALD R.L., OLSEN R.W. GABAA receptor channels. Ann. Rev. Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- MAITRA R., REYNOLDS J.N. Modulation of GABAA receptor function by neuroactive steroids: evidence for heterogeneity of steroid sensitivity of recombinant GABAA receptor isoforms. Can. J. Physiol. Pharmacol. 1998;76:909–920. doi: 10.1139/cjpp-76-9-909. [DOI] [PubMed] [Google Scholar]
- MARTIN R.J. Neuromuscular transmission in nematode parasites and antinematodal drug action. Pharmacol. Ther. 1993;58:13–50. doi: 10.1016/0163-7258(93)90065-l. [DOI] [PubMed] [Google Scholar]
- MATSUMURA F., GHIASUDDIN S.M. Evidence for similarities between cyclodiene type insecticides and picrotoxinin in their action mechanisms. J. Environ. Sci. Health. 1983;18:1–14. doi: 10.1080/03601238309372355. [DOI] [PubMed] [Google Scholar]
- MCINTIRE S.L., JORGENSEN E., KAPLAN J., HORVITZ H.R. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364:337–341. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- MITCHESON J.S., CHEN J., LIN M., CULBERSON C., SANGUINETTI M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA K., HAMILTON B.J., CARTER D.B., NARAHASHI T. Selective effects of dieldrin on the GABAA receptor-channel subunits expressed in human embryonic kidney cells. Brain Res. 1994a;645:19–26. doi: 10.1016/0006-8993(94)91633-0. [DOI] [PubMed] [Google Scholar]
- NAGATA K., NARAHASHI T. Dual action of the cyclodiene insecticide dieldrin on the gamma- aminobutyric acid receptor-chloride channel complex of rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 1994b;269:164–171. [PubMed] [Google Scholar]
- PARK-CHUNG M., MALAYEV A., PURDY R.H., GIBBS T.T., FARB D.H. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- PETERS J.A., KIRKNESS E.F., CALLACHAN H., LAMBERT J.J., TURNER A.J. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br. J. Pharmacol. 1988;94:1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISTIS M., BELELLI D., MCGURK K., PETERS J.A., LAMBERT J.J. Complementary regulation of anaesthetic activation of human (α6β3γ2L) and Drosophila (RDL) GABA receptors by a single amino acid residue. J Physiol (Lond) 1999;515:3–18. doi: 10.1111/j.1469-7793.1999.003ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISTIS M., BELELLI D., PETERS J.A., LAMBERT J.J. The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study. Br. J. Pharmacol. 1997;122:1707–1719. doi: 10.1038/sj.bjp.0701563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIBILLA I., TAKAGI T., LANGOSCH D., BORMANN J., BETZ H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. Embo J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUIA G., VICINI S., SEEBURG P.H., COSTA E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma- aminobutyric acid-gated Cl- currents. Mol. Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- RAJENDRA S., LYNCH J.W., SCHOFIELD P.R. The glycine receptor. Pharmacol. Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br. J. Pharmacol. 1989;98:167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGHART W. Structure and pharmacology of gamma aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- TAYLOR P.M., THOMAS P., GORRIE G.H., CONNOLLY C.N., SMART T.G., MOSS S.J. Identification of amino acid residues within GABA(A) receptor beta subunits that mediate both homomeric and heteromeric receptor expression. J. Neurosci. 1999;19:6360–6371. doi: 10.1523/JNEUROSCI.19-15-06360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON J.D., HIGGINS D.G., GIBSON T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON S.A., WHITING P.J., WAFFORD K.A. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br. J. Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UENO S., BRACAMONTES J., ZORUMSKI C., WEISS D.S., STEINBACH J.H. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J. Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER R.J., COLQUHOUN L., HOLDEN-DYE L. Pharmacological profiles of the GABA and acetylcholine receptors from the nematode, Ascaris suum. Acta Biol Hung. 1992;43:59–68. [PubMed] [Google Scholar]
- WANG T.L., HACKAM A.S., GUGGINO W.B., CUTTING G.R. A single amino acid in gamma-aminobutyric acid rho 1 receptors affects competitive and noncompetitive components of picrotoxin inhibition. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11751–11755. doi: 10.1073/pnas.92.25.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLTORTON J.R., MOSS S.J., SMART T.G. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur. J. Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- XU M., COVEY D.F., AKABAS M.H. Interaction of picrotoxin with GABAA receptor channel-lining residues probed in cysteine mutants. Biophys. J. 1995;69:1858–1867. doi: 10.1016/S0006-3495(95)80056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARBROUGH J.D., ROUSH R.T., BONNER J.C., WISE D.A. Monogenic inheritance of cyclodiene insecticide resistance in mosquitofish, Gambusia affinis. Experientia. 1986;42:851–853. doi: 10.1007/BF01941551. [DOI] [PubMed] [Google Scholar]
- ZHANG D., PAN Z.H., ZHANG X., BRIDEAU A.D., LIPTON S.A. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11756–11760. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]


