Abstract
Glucagon and glucagon-like peptide-1 (GLP-1) are homologous peptide hormones with important functions in glucose metabolism. The receptors for glucagon and GLP-1 are homologous family B G-protein coupled receptors. The GLP-1 receptor amino-terminal extracellular domain is a major determinant of glucagon/GLP-1 selectivity of the GLP-1 receptor. However, the divergent residues in glucagon and GLP-1 that determine specificity for the GLP-1 receptor amino-terminal extracellular domain are not known. Less is known about how the glucagon receptor distinguishes between glucagon and GLP-1.
We analysed chimeric glucagon/GLP-1 peptides for their ability to bind and activate the glucagon receptor, the GLP-1 receptor and chimeric glucagon/GLP-1 receptors. The chimeric peptide GLP-1(7–20)/glucagon(15–29) was unable to bind and activate the glucagon receptor. Substituting the glucagon receptor core domain with the GLP-1 receptor core domain (chimera A) completely rescued the affinity and potency of GLP-1(7–20)/glucagon(15–29) without compromising the affinity and potency of glucagon. Substituting transmembrane segment 1 (TM1), TM6, TM7, the third extracellular loop and the intracellular carboxy-terminus of chimera A with the corresponding glucagon receptor segments re-established the ability to distinguish GLP-1(7–20)/glucagon(15–29) from glucagon. Corroborant results were obtained with the opposite chimeric peptide glucagon(1–14)/GLP-1(21–37).
The results suggest that the glucagon and GLP-1 receptor amino-terminal extracellular domains determine specificity for the divergent residues in the glucagon and GLP-1 carboxy-terminals respectively. The GLP-1 receptor core domain is not a critical determinant of glucagon/GLP-1 selectivity. Conversely, the glucagon receptor core domain contains two or more sub-segments which strongly determine specificity for divergent residues in the glucagon amino-terminus.
Keywords: Chimeric glucagon/GLP-1 ligands, chimeric glucagon/GLP-1 receptors
Introduction
Glucagon and glucagon-like peptide-1 (GLP-1) are regulatory peptides in glucose metabolism and they originate from a common precursor, preproglucagon, by tissue-specific processing (Mojsov et al., 1986). In mammals, glucagon is a product of the pancreatic α-cells and its major function is to stimulate glycogenolysis and gluconeogenesis in the liver. GLP-1 is produced by the intestinal L-cells and potentiates glucose-induced insulin secretion from the pancreatic β-cells. In addition, GLP-1 inhibits glucagon secretion from the pancreatic α-cells, inhibits gastric emptying, lowers food intake, stimulates neogenesis and proliferation of β-cells and inhibits apoptosis of β-cells (Drucker, 2001; Holst, 2000). At the target cells, glucagon and GLP-1 interact with specific G-protein coupled receptors (GPCRs), which couple to intracellular signalling pathways including adenylate cyclase and phospholipase C (Gromada et al., 1998). Both receptors are interesting drug targets for the treatment of type 2 diabetes (Knudsen et al., 2001; Madsen et al., 1999).
The glucagon and GLP-1 receptors belong to family B of the seven transmembrane (7TM) G-protein coupled receptors. Family B includes receptors for related peptide hormones, such as glucagon-like peptide-2 (GLP-2), glucose-dependent insulinotropic polypeptide (GIP), vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase activating polypeptide (PACAP), growth hormone-releasing hormone (GHRH) and secretin. Family B also includes receptors for other peptide hormones such as calcitonin, corticotropin releasing factor (CRF) and parathyroid hormone (PTH). The N-terminal extracellular domain of family B receptors is important for selective ligand interaction however, the extracellular loops and the extracellular end of the transmembrane segments can provide additional determinants of ligand selectivity (Bergwitz et al., 1996; Couvineau et al., 1996; Gelling et al., 1997; Holtmann et al., 1995; 1996; Lutz et al., 1999). Therefore multiple discontinuous segments can play a role in selective ligand recognition of family B receptors. The primary structure of the N-terminal extracellular domain is highly divergent among different family B receptors, although a conserved pattern of six cysteines suggests a common structural fold (Asmann et al., 2000; Bazarsuren et al., 2002). In addition, a disulfide bond between cysteines conserved in the entire GPCR super family is believed to connect the extracellular end of the third transmembrane segment and the second extracellular loop (Knudsen et al., 1997; Palczewski et al., 2000).
Structure-activity studies of family B receptor peptide ligands suggest that the N-terminals constitute the activation domain and the C-terminals constitute the binding domain. Exendin-4 (1–39) and the N-terminally truncated exendin-4 (9–39) are GLP-1 receptor ligands with similar binding affinities, however exendin-4 (1–39) is a full agonist whereas exendin-4 (9–39) is a potent antagonist (Thorens et al., 1993). The N-terminally modified glucagon analogue desHis1Glu9-glucagon is a potent glucagon receptor antagonist, which illustrates the importance of His1 and Asp9 of native glucagon in receptor activation (Unson et al., 1991). The corresponding His7 and Asp15 of GLP-1(7–36)amide/GLP-1(7–37), are important for the biological activity of GLP-1, but a clear segregation of residues important for receptor binding versus activation is not obvious. Alanine scanning of GLP-1 showed that the N-terminal residues His7, Phe12, Thr13 and Asp15 were important for receptor binding and activation (Adelhorst et al., 1994). Substitutions in the GLP-1 C-terminus gave only subtle effects, except for Phe28-Ala and Ile29-Ala. In addition, several residues in the C-terminus can be derivatized with long fatty acids without loss of potency (Knudsen et al., 2000). However, N-terminal truncation of two residues and C-terminal truncation of four residues both dramatically decrease the affinity and potency and therefore it appears that the entire length of GLP-1 is required for optimal biological activity (Montrose-Rafizadeh et al., 1997; Xiao et al., 2001). Interestingly, a C-terminally truncated PTH-analogue, conformationally constrained by alpha-aminoisobutyric acid is able to mediate a full agonist response through the juxtamembrane region of the PTH-1 receptor (Shimizu et al., 2001). Furthermore, constitutively active receptors were generated by substitution of the N-terminal extracellular domain of the PTH-1 and CRF receptors with N-terminal peptide-fragments of PTH-1 and CRF, respectively (Nielsen et al., 2000; Shimizu et al., 2000). Therefore it appears that the CRF and PTH-1 N-terminals are sufficient to activate their respective receptors.
The identification of interactions between specific residues of the peptide ligands with specific residues/elements of the corresponding receptors is beginning to emerge. Results obtained by mutagenesis of the VPAC1 receptor and the ligand VIP demonstrated that interactions between Asp3 of VIP and positively charged residues at the extracellular end of the second transmembrane segment (TM2) are important for ligand binding and receptor activation (Solano et al., 2001). Likewise, positively charged residues in the secretin receptor TM2 have been implicated in recognition of Asp3 of secretin (di paolo et al., 1998). Labelling of the receptors for secretin and PTHrP by photoactive peptide-analogues has shown proximity between residues in the C-terminal part of the peptides and residues/segments of the extracellular N-terminal domain of the receptors (Dong et al., 1999; Gensure et al., 2001). These results help position and orientate the ligands with respect to the structural elements of their receptors.
The N-terminal extracellular domain and the first extracellular loop of the human glucagon and GLP-1 receptors are important for ligand binding (Buggy et al., 1995; Unson et al., 1995; 1996; 2002; Xiao et al., 2000). In addition, the GLP-1 receptor N-terminal extracellular domain is an important determinant of glucagon/GLP-1-selectivity (Graziano et al., 1996). Results obtained with chimeric glucagon and GLP-1 peptides have shown that residues in opposite ends of glucagon and GLP-1 determine the selective recognition by their respective receptors (Hjorth et al., 1994). Residues in the upper half of TM2 of the rat glucagon receptor are believed to interact with Gln3 of glucagon and could function as a selectivity determinant of the glucagon receptor (Perret et al., 2002). We have analysed the interaction and function of chimeric glucagon/GLP-1 peptides with chimeric glucagon/GLP-1 receptors, with special emphasis on ligand selectivity. The results provide novel information about how the homologous glucagon and GLP-1 receptors distinguish between their homologous ligands glucagon and GLP-1.
Methods
Receptor constructs
The cDNAs encoding the human GLP-1 receptor and the human glucagon receptor were originally obtained from Dr B. Thorens and Zymogenetics Inc., respectively, and sub-cloned into the mammalian expression vector pcDNA3.1/V5-His-TOPO® (Invitrogen) (Lok et al., 1994; Thorens et al., 1993). In addition, stop-codons were inserted after the coding region of the receptors by QuickchangeTM site-directed mutagenesis (Stratagene). This was done to analyse the effect of the C-terminal V5/His-tag. Chimeric glucagon and GLP-1 receptors were generated by overlap extension PCR, as previously described (Horton et al., 1990). Chimera A was composed of amino-acid residues 1–144 of the human glucagon receptor and residues 148–463 of the human GLP-1 receptor. Chimera B was composed of residues 1–169 and 346–477 of the human glucagon receptor and residues 173–347 of the human GLP-1 receptor. Chimera C was composed of amino-acid residues 1–147 of the human GLP-1 receptor and residues 145–477 of the human glucagon receptor. Plasmid DNA was generated using the Plasmid Maxi Kit (QIAGEN) and sequenced using the DYEnamic ET Dye Terminator Kit and the MegaBACE™ 1000 DNA Analysis System (Amersham Bioscience).
Peptide synthesis and radiolabelling
Glucagon, GLP-1 (7–37) and the chimeric peptides GLP-1(7–20)-glucagon(15–29) and glucagon(1–14)-GLP-1(21–37) were synthesized according to the Fmoc strategy on an Applied Biosystems 431A peptide synthesizer. The peptide was cleaved from 200 mg of the protected peptidyl resin by stirring for 60 min at room temperature in a mixture of 2 ml trifluoro acetic acid (TFA), 50 μl triisopropylsilane and 50 μl water. The cleavage mixture was filtered, concentrated to approximately 0.5 ml by a stream of nitrogen, precipitated with 49.5 ml diethyl ether and washed three times with 50 ml diethyl ether and dried as a white powder. The crude peptide was dissolved in 50% acetic acid, diluted to 10% acetic acid with water, purified by semi-preparative HPLC and lyophilized. The final product was characterized by RP-HPLC/Ion spray mass spectrometry (LC-MS) (retention time and molecular mass) and analytical RP-HPLC (retention time and peptide amount). The RP-HPLC analysis was performed using UV detection at 214 nm and a Vydac 218TP54 4.6×250 mm 5 μm C-18 silica column (The Separations Group, Hesperia). The LC-MS analysis was performed on a PE-Sciex API 100 mass spectrometer. The molecular mass was found to be in agreement with the expected structure within the experimental error of the method (±1 amu) and the purity of the peptides was above 95%.
The radioligand 125I-Glucagon (2.2 Ci μmol−1) was prepared by the chloramine-T method and 125I-GLP-1 (2.2 Ci μmol−1) was prepared by the lactoperoxidase method (Thorell & Johansson, 1971). Both radioligands were purified by reverse-phase HPLC.
Cell culture and transient receptor expression
HEK293-cells were routinely maintained in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum (LifeTechnologies) and penicillin/streptomycin (90 u ml−1 and 90 μg ml−1, respectively). Cells were seeded in T75-flasks, transfected with 9 μg of DNA using the FuGeneTM transfection reagent (Roche), harvested 24 h after transfection and applied directly to functional experiments or plasma membrane preparations.
Functional assay
HEK293 cells transiently expressing the glucagon receptor, the GLP-1 receptor or a chimeric receptor were harvested and resuspended in assay buffer (Flashplate®, NEN) to a cell density of 1.8×106 cells ml−1. Peptides were diluted in PBS with 0.02% Tween 20. Cells (50 μl) and peptides (50 μl) were mixed in 96-well Flashplates® (NEN), gently agitated for 5 min and incubated for 25 min at room temperature. The resulting intracellular level of cAMP was measured according to supplier's manual and data were analysed by non-linear regression using Prism®, (GraphPad Software, Inc.).
Preparation of plasmamembranes
Approximately 5×107 cells were harvested, resuspended in 5 ml cold 10 mM Tris-HCl (pH 7.4) and 1 mM EDTA, subjected to homogenization by a 10 s burst using a polytron homogenizer and subsequent centrifugation for 15 min at 10,000 r.p.m. (11,951×g) and 4°C. The resulting pellet was resuspended in 5 ml cold buffer (10 mM Tris-HCl (pH 7.4), 1 mM EDTA) and centrifuged as above. The final pellet was resuspended in 1–2 ml 25 mM Tris-HCl (pH 7.4), 20% glycerol and a mixture of protease inhibitors (CompleteTM EDTA-free, Roche). The membrane suspension was pulled through a 25-gauge needle three times and aliquots were frozen at −80°C.
Binding assay
Freshly thawed membrane preparation was pulled through a 25-gauge needle three times and diluted in assay buffer (50 mM HEPES-NaOH, 5 mM MgCl2, 5 mM EGTA, 0.005% Tween20). Peptides and radioligands were also diluted in assay buffer. Membrane-preparation (20 μg of protein), peptide (1 pM–10 μM) and tracer (50 pM) were mixed in 96-well 0.65-μm filter microtiter plates (Millipore) and incubated for 2 h at 30°C. Subsequently, bound and unbound peptide/radioligand were separated using a vacuum manifold (Millipore), plates were washed twice in 100 μl cold incubation buffer and left to dry. Finally, filters were retrieved using the Millipore Punch System, the amount of bound radioligand was determined using a Packard γ-counter and data were analysed by non-linear regression using Prism®, (GraphPad Software, Inc.).
Results
We synthesized two chimeric peptides composed of elements of glucagon and GLP-1; the chimeric peptide GLP-1(7–20)-glucagon(15–29), referred to as GLP-1/Glu and the opposite chimeric peptide glucagon(1–14)-GLP-1(21–37), referred to as Glu/GLP-1. Glucagon, GLP-1 and the two chimeric peptides were analysed for their ability to bind and activate the glucagon receptor, the GLP-1 receptor and chimeric glucagon/GLP-1 receptors. All receptors were expressed with a C-terminal V5/His-tag (Invitrogen). The presence of the C-terminal tag had no influence on the functional response of the receptors (data not shown).
The glucagon receptor
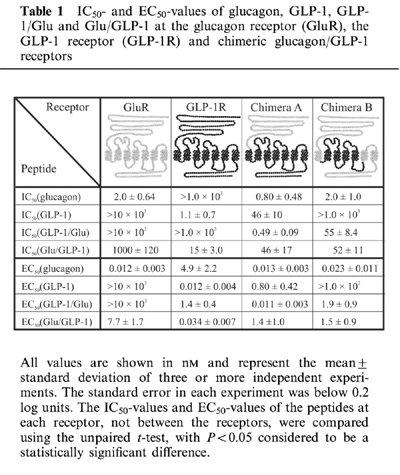
In competition binding experiments, glucagon efficiently displaced 125I-glucagon from the glucagon receptor (IC50 of 2.0 nM) and high concentrations of the chimeric peptide Glu/GLP-1 also displaced 125I-glucagon (IC50 of 1.0 μM), whereas 10 μM of GLP-1 and GLP-1/Glu were unable to displace 125I-glucagon (Figure 1a and Table 1). Whole cells transiently expressing the human glucagon receptor, responded functionally with half-maximal stimulation at 11 pM of glucagon and 7.7 nM of Glu/GLP-1 whereas 1.0 μM of GLP-1 and GLP-1/Glu were unable to activate the glucagon receptor (Figure 1e and Table 1). The results showed that substitution of the glucagon N-terminus with the GLP-1 N-terminus completely eliminated binding to and activation of the glucagon receptor, within the ligand concentrations of the experiments (blue arrows in Figure 1a, e). Substitution of the glucagon C-terminus with the GLP-1 C-terminus decreased the affinity and potency at the glucagon receptor by approximately 1000 fold, relative to glucagon (green arrows in Figure 1a, e).
Figure 1.
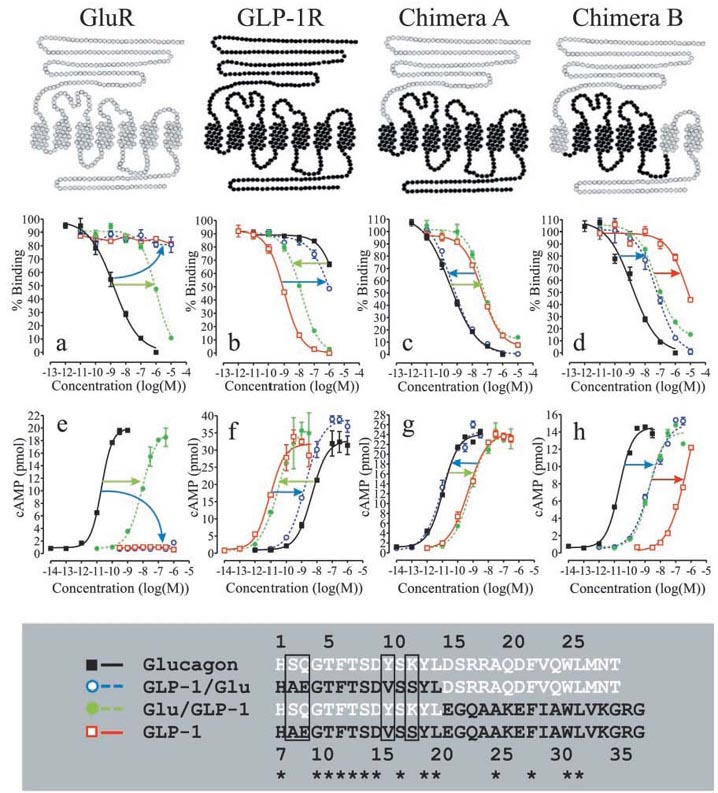
Competition binding and functional analyses of chimeric glucagon/GLP-1 peptides and chimeric glucagon/GLP-1 receptors. The upper panel illustrates the glucagon receptor (GluR) in grey, the GLP-1 receptor (GLP-1R) in black and two chimeric receptors composed of elements of the glucagon and GLP-1 receptor. Below each receptor are the corresponding binding and functional analyses. Each figure is representative of three or more independent experiments performed in triplicates (binding analyses) or duplicates (functional analyses). Competition binding analyses of the glucagon receptor and chimera A and B were performed using 125I-glucagon as the tracer. Competition binding analysis of the GLP-1 receptor was performed using 125I-GLP-1 as the tracer. (a) and (e): Competition-binding analysis (a) and functional analysis (e) of the glucagon receptor. (b) and (f): Competition-binding analysis (b) and functional analysis (f) of the GLP-1 receptor. (c) and (g): Competition-binding analysis (c) and functional analysis (g) of chimera A. (d) and (h): Competition-binding analysis (d) and functional analysis (h) of chimera B. The lower panel shows a sequence alignment of glucagon, GLP-1 and the two chimeric peptides GLP-1/Glu and Glu/GLP-1. The glucagon sequence is shown in white, the GLP-1 sequence is shown in black and a star illustrates amino acid identity. Glucagon is numbered 1–29 and GLP-1 is numbered 7–37.
Table 1.
IC50- and EC50-values of glucagon, GLP-1, GLP-1/Glu and Glu/GLP-1 at the glucagon receptor (GluR), the GLP-1 receptor (GLP-1R) and chimeric glucagon/GLP-1 receptors
The GLP-1 receptor
In competition binding, GLP-1 displaced 125I-GLP-1 from the GLP-1 receptor with an IC50 of 1.1 nM. Glu/GLP-1 displaced 125I-GLP-1 with an IC50 of 15 nM whereas glucagon and the reverse chimeric peptide GLP-1/Glu displaced 125I-GLP-1 only at higher concentrations (IC50 >1.0 μM) (Figure 1b and Table 1). Whole cells transiently expressing the human GLP-1 receptor, gave a functional response with half-maximal stimulation at 12 pM of GLP-1 and 34 pM of Glu/GLP-1 (Figure 1f and Table 1). Glucagon and the opposite chimeric peptide GLP-1/Glu were less potent and induced a functional response with half-maximal stimulation at 4.9 nM and 1.4 nM, respectively (Figure 1f and Table 1). The results showed that primarily substitution of the GLP-1 C-terminal with the glucagon C-terminal decreased the affinity and potency at the GLP-1 receptor (blue arrows in Figure 1b, f).
Chimeric glucagon/GLP-1 receptors
The above experiments confirmed the major conclusion from other studies, that primarily residues located at opposite ends of the homologous peptide ligands determine the selective recognition of the glucagon and GLP-1 receptors (Hjorth et al., 1994). In an attempt to locate the receptor domains that interact with the N-terminal and C-terminal parts of glucagon and GLP-1, we substituted the N-terminal extracellular domain of the GLP-1 receptor with the corresponding domain of the glucagon receptor. This chimeric receptor, chimera A, was analysed in competition binding using 125I-glucagon as the tracer and the results showed that chimera A bound glucagon with higher affinity than GLP-1 (Figure 1c and Table 1). In functional experiments with chimera A, half-maximal stimulation occurred at 13 pM of glucagon and at 0.80 nM of GLP-1 and hence the relative binding affinity of glucagon and GLP-1 predicted their relative potency (Figure 1g and Table 1). Therefore, substitution of the GLP-1 receptor extracellular N-terminal domain with the corresponding human glucagon receptor domain shifted the glucagon/GLP-1-selectivity in favour of glucagon. The results agreed with previous analyses, which showed that the extracellular N-terminal domain of the GLP-1 receptor was an important determinant of ligand selectivity (Graziano et al., 1996). In addition, the affinity and potency of the chimeric peptide GLP-1/Glu were nearly identical with that of glucagon, whereas the affinity and potency of the opposite chimeric peptide Glu/GLP-1 were nearly identical with that of GLP-1 (Figure 1c, g and Table 1). Therefore, substitution of the glucagon C-terminus with the GLP-1 C-terminus decreased the affinity and potency at chimera A to that of GLP-1 (green arrows in Figure 1c, g). Substitution of the GLP-1 C-terminus with the glucagon C-terminus increased the affinity and potency at chimera A to that of glucagon (blue arrows in Figure 1c, g). In contrast, substitution of the N-terminals had not apparent effect on either affinity or potency at chimera A.
We also generated the opposite chimeric receptor, composed of the GLP-1 receptor N-terminal extracellular domain and the glucagon receptor core domain. However, we were unable to measure any ligand binding or functional response with that receptor (data not shown).
The glucagon receptor was able to distinguish between glucagon and GLP-1/Glu whereas chimera A was unable to distinguish between glucagon and GLP-1/Glu despite the presence of the glucagon receptor N-terminal extracellular domain. Therefore it appeared that the glucagon receptor core domain distinguished between glucagon and GLP-1/Glu. In an attempt to locate the regions in the glucagon receptor core domain that were responsible for the discrimination between the glucagon and GLP-1 N-terminals, chimera A was modified to become more glucagon receptor-like. TM1, TM6, TM7, the third extracellular loop (ECL3) and the C-terminus of chimera A were substituted with the corresponding glucagon receptor segments and the remaining GLP-1 receptor segments were TM2-TM5 and connecting loops. The resulting chimeric receptor, chimera B, was analysed by competition binding and functional experiments as described for chimera A. Compared with chimera A, the relative affinity and potency of the peptide-ligands had changed. Chimera B bound glucagon with the highest affinity (IC50 of 2.0 nM), GLP-1/Glu and Glu/GLP-1 with lower affinity (IC50 of 52 nM and 55 nM, respectively), and GLP-1 with the lowest affinity (IC50>1.0 μM) (Figure 1d and Table 1). The relative binding affinity of the ligands predicted their relative potency in functional experiments. Glucagon was the most potent peptide (EC50 of 23 pM), GLP-1/Glu and Glu/GLP-1 were less potent (EC50 of 1.9 nM and 1.5 nM, respectively) and GLP-1 was the least potent peptide (EC50>100 nM) (Figure 1h and Table 1). The results showed that substitution of TM1, TM6, TM7, ECL3 and the intracellular C-terminus of chimera A with the corresponding glucagon receptor segments decreased the affinity and potency of GLP-1/Glu relative to glucagon (blue arrows in Figure 1d, h) and GLP-1 relative to Glu/GLP-1 (red arrows in Figure 1d, h). However, the affinity and potency of Glu/GLP-1 relative glucagon and GLP-1/Glu relative to GLP-1 was unaffected by the substitution.
Discussion
In the glucagon and GLP-1 N-terminals, 10 out of 14 residues are identical and in the C-terminals, four out of 15 residues are identical. Only the divergent residues in glucagon and GLP-1 can determine selective receptor recognition, whereas conserved residues are more likely to serve the same function at the glucagon and GLP-1 receptors, respectively, be it interaction and/or activation. Previous results suggest that residues in the glucagon N-terminus determined the selective interaction with the glucagon receptor, whereas residues in the GLP-1 C-terminus determined the selective interaction with the GLP-1 receptor (Hjorth et al., 1994). The purpose of this study was to identify receptor-domains that are important for the ability of the glucagon and GLP-1 receptors to distinguish between their homologous ligands, glucagon and GLP-1.
The results obtained with the glucagon receptor agreed with previous results and extended the analysis to include functional experiments (Hjorth et al., 1994). The chimeric peptide GLP-1/Glu could neither bind nor activate the glucagon receptor, although only four residues diverge between glucagon and GLP-1/Glu; Ser2/Ala2, Gln3/Glu3, Tyr10/Val10 and Lys12/Ser12 in glucagon and GLP-1/Glu respectively. Previous structure-function analyses of glucagon, addressing Ser2, Gln3, Tyr10 and Lys12 individually, suggest that substitution at each position contributes to the dramatic loss of affinity and potency of GLP-1/Glu relative to glucagon at the glucagon receptor (Andreu & Merrifield, 1987; Azizeh et al., 1996; Unson et al., 1998; Unson & Merrifield, 1994). Therefore, the four divergent residues in the glucagon and GLP-1 N-terminals are important for selective recognition by the glucagon receptor. Substitution of the glucagon C-terminus with the GLP-1 C-terminus also decreased affinity and potency, relative to glucagon at the glucagon receptor. Therefore, divergent residues in the glucagon and GLP-1 C-terminus are also involved in the selective recognition by the glucagon receptor.
The chimeric peptide Glu/GLP-1 was nearly equipotent with GLP-1 at the GLP-1 receptor, and previous results showed that the individual substitutions Ala8-Ser, Val16-Tyr and Ser18-Lys in GLP-1 had only minor effects on binding and activation of the GLP-1 receptor (Adelhorst et al., 1994; Gallwitz et al., 1995). Therefore, the four divergent residues in the glucagon and GLP-1 N-terminals are not critical for the glucagon/GLP-1 selectivity of the GLP-1 receptor. Instead the GLP-1 receptor distinguishes primarily between the divergent residues in the glucagon and GLP-1 C-terminals. Previous results suggest that the GLP-1 receptor N-terminal extracellular domain is a major determinant of glucagon/GLP-1 selectivity of the GLP-1 receptor (Graziano et al., 1996). Therefore we suggest that the GLP-1 receptor relies primarily on the N-terminal extracellular domain to distinguish between divergent residues in the glucagon and GLP-1 C-terminals.
The extracellular N-terminal domain is known to be important for selective ligand recognition of family B receptors. It has been proposed that the N-terminal parts of VIP, secretin, calcitonin, PTH and CRF interact with the core domain of their respective receptor whereas the C-terminal parts interact with the extracellular N-terminal domain of the receptors (Bergwitz et al., 1996; Gourlet et al., 1996; Juarranz et al., 1999; Nielsen et al., 2000). In this study, substitution of the GLP-1 receptor N-terminal extracellular domain with the corresponding glucagon receptor domain shifted the glucagon/GLP-1-selectivity in favour of glucagon, compared to the GLP-1 receptor (Figure 1f, g). The results confirm that the GLP-1 receptor N-terminal extracellular domain is the major glucagon/GLP-1 selectivity determinant of the GLP-1 receptor. In addition, glucagon and GLP-1/Glu were equally potent and GLP-1 and Glu/GLP-1 were equally but less potent at chimera A (Figure 1g). Therefore, the divergent residues in the glucagon and GLP-1 N-terminals are not important for the glucagon/GLP-1 selectivity of chimera A. Instead interactions between the N-terminal extracellular domain of chimera A and divergent residues in the glucagon and GLP-1 C-terminals determined the relative affinity and potency of glucagon, GLP-1, GLP-1/Glu and Glu/GLP-1 at chimera A. These results suggest that the glucagon receptor N-terminal extracellular domain is able to distinguish between divergent residues in the glucagon and GLP-1 C-terminals.
Comparing the relative affinity and potency of glucagon and GLP-1/Glu at the glucagon receptor to their relative affinity and potency at chimera A showed that substitution of the glucagon receptor core domain with the GLP-1 receptor core domain completely rescued the affinity and potency of GLP-1/Glu (Figure 1a, c, e, g). Therefore Ala2, Glu3, Val10 and/or Ser12 of GLP-1/Glu are likely to interact with the GLP-1 receptor core domain of chimera A. The results suggest a binding model in which the N-terminus of GLP-1/Glu interacts primarily with the GLP-1 receptor core domain of chimera A and the C-terminus of GLP-1/Glu interacts primarily with the glucagon receptor N-terminal extracellular domain of chimera A (Figure 2). It is possible that the corresponding Ser2, Gln3, Tyr10 and/or Lys12 of glucagon interact with the glucagon receptor core domain and that the glucagon receptor core domain is able to distinguish between one or more of the divergent residues in the glucagon and GLP-1 N-terminals. This hypothesis was supported by the results obtained with chimera B. Substitution of TM1, TM6, TM7, ECL3 and the intracellular C-terminus of chimera A with the corresponding glucagon receptor segments decreased the affinity and potency of GLP-1/Glu relative to glucagon and GLP-1 relative to Glu/GLP-1 (blue and red arrows in Figure 1d, h). Thus, to some degree the ability to distinguish GLP-1/Glu from glucagon and GLP-1 from Glu/GLP-1 was re-established in chimera B. Therefore, residues in TM1, TM6, TM7 and/or ECL3 are important for the ability of the glucagon receptor to distinguish between divergent residues in the glucagon and GLP-1 N-terminals. However, GLP-1/Glu was able to bind and activate chimera B but not the glucagon receptor. Therefore, residues in the glucagon receptor region TM2-TM5 are also important for the ability of the glucagon receptor core domain to distinguish between the divergent residues in the glucagon and GLP-1 N-terminals. This agrees with recent results which suggest that residues in the upper half of the rat glucagon receptor TM2 interact with Gln3 of glucagon (Perret et al., 2002). In previous studies of secretin and PTH receptor ligand selectivity, residues in the transmembrane segments were proposed to function as selectivity filters that distinguished between different ligands (Turner et al., 1996). We suggest that the glucagon receptor core domain constitutes a selectivity filter with two or more selectivity determinants that distinguish between the divergent residues in the glucagon and GLP-1 N-terminals.
Figure 2.
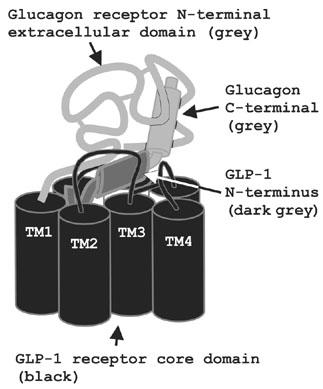
Binding model of the interaction between the chimaeric peptide GLP-1/Glu and chimeric receptor A. The GLP-1 N-terminus of GLP-1/Glu (dark grey) interacts with the GLP-1 receptor core domain of chimera A (black). The glucagon C-terminus (bright grey) interacts with the glucagon receptor N-terminal extracellular domain of chimera A (bright grey).
His1 and Asp9 of glucagon are important for glucagon receptor activation and the residues are conserved in GLP-1 and Exendin-4. N-terminal truncation of exendin-4(1–39) and PACAP(1–38) has generated the GLP-1 receptor antagonist, exendin-4(9–39) and the PACAP receptor antagonist, PACAP(6–38), respectively (Robberecht et al., 1992). These results emphasise the importance of the peptide N-terminus for receptor activation. In this study we show that glucagon and GLP-1/Glu potently activate the GLP-1 receptor core domain of chimera A, with EC50-values of 13 pM and 11 pM, respectively (Figure 1g). We speculate that the glucagon and GLP-1 N-terminals adopt similar conformations, which is required to activate the GLP-1 receptor core domain. The same conformation could also be required to activate the glucagon receptor, but the glucagon receptor core domain has a selectivity filter to distinguish between the divergent residues in the glucagon and GLP-1 N-terminals.
The present knowledge about the three-dimensional structure of receptor-ligand complexes of Class B GPCRs is based on theoretical models refined by constraints obtained by structure-binding/activity analyses, receptor mutagenesis, photo affinity labelling and the presence of conserved cysteines expected to form disulfide bridges. Our results provide novel information about the orientation of glucagon and GLP-1 with respect to the structural elements of their receptors, and show that different domains of the homologous receptors provide the critical determinants of ligand selectivity. On the basis of the results presented here we will attempt to identify specific receptor-ligand interactions that determine ligand selectivity of the glucagon and GLP-1 receptors.
Acknowledgments
We are grateful to Tania P. Rasmussen and Gitte K. Hansen for technical assistance and Carsten E. Stidsen and Sanne M. Knudsen for helpful discussions and critical reading of the manuscript.
Abbreviations
- CRF
corticotropin releasing factor
- ECL3
third extracellular loop
- GLP-1
glucagon-like peptide-1
- GLP-1/Glu
GLP-1(7–20)-glucagon(15–29)
- GPCR
G-protein coupled receptor
- Glu/GLP-1
glucagon(1–14)-GLP-1(21–37)
- PACAP
pituitary adenylate cyclase activating polypeptide
- PTH
parathyroid hormone
- 7TM
seven transmembrane
- TM2
second transmembrane segment
- VIP
vasoactive intestinal polypeptide
References
- ADELHORST K., HEDEGAARD B.B., KNUDSEN L.B., KIRK O. Structure-activity studies of glucagon-like peptide-1. J. Biol. Chem. 1994;269:6275–6278. [PubMed] [Google Scholar]
- ANDREU D., MERRIFIELD R.B. Glucagon antagonists. Synthesis and inhibitory properties of Asp3- containing glucagon analogs. Eur. J. Biochem. 1987;164:585–590. doi: 10.1111/j.1432-1033.1987.tb11167.x. [DOI] [PubMed] [Google Scholar]
- ASMANN Y.W., DONG M., GANGULI S., HADAC E.M., MILLER L.J. Structural insights into the amino-terminus of the secretin receptor: I. Status of cysteine and cystine residues. Mol. Pharmacol. 2000;58:911–919. doi: 10.1124/mol.58.5.911. [DOI] [PubMed] [Google Scholar]
- AZIZEH B.Y., SHENDEROVICH M.D., TRIVEDI D., LI G., STURM N.S., HRUBY V.J. Topographical amino acid substitution in position 10 of glucagon leads to antagonists/partial agonists with greater binding differences. J. Med. Chem. 1996;39:2449–2455. doi: 10.1021/jm960130b. [DOI] [PubMed] [Google Scholar]
- BAZARSUREN A., GRAUSCHOPF U., WOZNY M., REUSCH D., HOFFMANN E., SCHAEFER W., PANZNER S., RUDOLPH R. In vitro folding, functional characterization, and disulfide pattern of the extracellular domain of human GLP-1 receptor. Biophys.Chem. 2002;96:305–318. doi: 10.1016/s0301-4622(02)00023-6. [DOI] [PubMed] [Google Scholar]
- BERGWITZ C., GARDELLA T.J., FLANNERY M.R., POTTS J.T., JR, KRONENBERG H.M., GOLDRING S.R., JUPPNER H. Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin. Evidence for a common pattern of ligand- receptor interaction. J. Biol. Chem. 1996;271:26469–26472. doi: 10.1074/jbc.271.43.26469. [DOI] [PubMed] [Google Scholar]
- BUGGY J.J., LIVINGSTON J.N., RABIN D.U., YOO-WARREN H. Glucagon.glucagon-like peptide I receptor chimeras reveal domains that determine specificity of glucagon binding. J. Biol. Chem. 1995;270:7474–7478. doi: 10.1074/jbc.270.13.7474. [DOI] [PubMed] [Google Scholar]
- COUVINEAU A., ROUYER-FESSARD C., MAORET J.J., GAUDIN P., NICOLE P., LABURTHE M. Vasoactive intestinal peptide (VIP)1 receptor. Three nonadjacent amino acids are responsible for species selectivity with respect to recognition of peptide histidine isoleucineamide. J. Biol. Chem. 1996;271:12795–12800. doi: 10.1074/jbc.271.22.12795. [DOI] [PubMed] [Google Scholar]
- DI PAOLO E., DE NEEF P., MOGUILEVSKY N., PETRY H., BOLLEN A., WAELBROECK M., ROBBERECHT P. Contribution of the second transmembrane helix of the secretin receptor to the positioning of secretin. FEBS Lett. 1998;424:207–210. doi: 10.1016/s0014-5793(98)00175-6. [DOI] [PubMed] [Google Scholar]
- DONG M., WANG Y., PINON D.I., HADAC E.M., MILLER L.J. Demonstration of a direct interaction between residue 22 in the carboxyl-terminal half of secretin and the amino-terminal tail of the secretin receptor using photoaffinity labeling. J. Biol. Chem. 1999;274:903–909. doi: 10.1074/jbc.274.2.903. [DOI] [PubMed] [Google Scholar]
- DRUCKER D.J. Minireview: the glucagon-like peptides. Endocrinology. 2001;142:521–527. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- GALLWITZ B., WITT M., PAETZOLD G., MORYS-WORTMANN C., FOLSCH U.R., SCHMIDT W.E. Binding characteristics of N-terminal GIP/GLP-1 hybrid peptides. Endocrinol.Metab. 1995;2:39–46. [Google Scholar]
- GELLING R.W., WHEELER M.B., XUE J., GYOMOREY S., NIAN C., PEDERSON R.A., MCINTOSH C.H. Localization of the domains involved in ligand binding and activation of the glucose-dependent insulinotropic polypeptide receptor. Endocrinology. 1997;138:2640–2643. doi: 10.1210/endo.138.6.9104. [DOI] [PubMed] [Google Scholar]
- GENSURE R.C., GARDELLA T.J., JUPPNER H. Multiple sites of contact between the carboxyl-terminal binding domain of PTHrP-(1-36) analogs and the amino-terminal extracellular domain of the PTH/PTHrP receptor identified by photoaffinity cross-linking. J. Biol. Chem. 2001;276:28650–28658. doi: 10.1074/jbc.M100717200. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VILARDAGA J.P., DE NEEF P., WAELBROECK M., VANDERMEERS A., ROBBERECHT P. The C-terminus ends of secretin and VIP interact with the N-terminal domains of their receptors. Peptides. 1996;17:825–829. doi: 10.1016/0196-9781(96)00107-6. [DOI] [PubMed] [Google Scholar]
- GRAZIANO M.P., HEY P.J., STRADER C.D. The amino terminal domain of the glucagon-like peptide-1 receptor is a critical determinant of subtype specificity. Receptors. Channels. 1996;4:9–17. [PubMed] [Google Scholar]
- GROMADA J., HOLST J.J., RORSMAN P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 1998;435:583–594. doi: 10.1007/s004240050558. [DOI] [PubMed] [Google Scholar]
- HJORTH S.A., ADELHORST K., PEDERSEN B.B., KIRK O., SCHWARTZ T.W. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. J. Biol. Chem. 1994;269:30121–30124. [PubMed] [Google Scholar]
- HOLST J.J. Gut hormones as pharmaceuticals. From enteroglucagon to GLP-1 and GLP-2. Regul. Pept. 2000;93:45–51. doi: 10.1016/s0167-0115(00)00185-3. [DOI] [PubMed] [Google Scholar]
- HOLTMANN M.H., GANGULI S., HADAC E.M., DOLU V., MILLER L.J. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J. Biol. Chem. 1996;271:14944–14949. doi: 10.1074/jbc.271.25.14944. [DOI] [PubMed] [Google Scholar]
- HOLTMANN M.H., HADAC E.M., MILLER L.J. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors. Studies of chimeric receptors. J. Biol. Chem. 1995;270:14394–14398. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]
- HORTON R.M., CAI Z.L., HO S.N., PEASE L.R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- JUARRANZ M.G., VAN RAMPELBERGH J., GOURLET P., DE NEEF P., CNUDDE J., ROBBERECHT P., WAELBROECK M. Different vasoactive intestinal polypeptide receptor domains are involved in the selective recognition of two VPAC(2)-selective ligands. Mol. Pharmacol. 1999;56:1280–1287. doi: 10.1124/mol.56.6.1280. [DOI] [PubMed] [Google Scholar]
- KNUDSEN L.B., AGERSO H., BJENNING C., BREGENHOLT S., CARR R.D., GODTFREDSEN C., HOLST J.J., HUUSFELDT P.O., LARSEN M.O., LARSEN P.J., NIELSEN P.F., RIBEL U., ROLIN B., ROMER J., STURIS J., WILKEN M., KRISTENSEN P. GLP-1 derivatives as novel compounds for the treatment of type 2 diabetes: selection of NN2211 for clinical development. Drug Future. 2001;26:677–685. [Google Scholar]
- KNUDSEN L.B., NIELSEN P.F., HUUSFELDT P.O., JOHANSEN N.L., MADSEN K., PEDERSEN F.Z., THOGERSEN H., WILKEN M., AGERSO H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J. Med. Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- KNUDSEN S.M., TAMS J.W., WULFF B.S., FAHRENKRUG J. A disulfide bond between conserved cysteines in the extracellular loops of the human VIP receptor is required for binding and activation. FEBS Lett. 1997;412:141–143. doi: 10.1016/s0014-5793(97)00714-x. [DOI] [PubMed] [Google Scholar]
- LOK S., KUIJPER J.L., JELINEK L.J., KRAMER J.M., WHITMORE T.E., SPRECHER C.A., MATHEWES S., GRANT F.J., BIGGS S.H., ROSENBERG G.B., SHEPPARD P.O., O'HARA P.J., FOSTER D.C., KINDSVOGEL W. The human glucagon receptor encoding gene: structure, cDNA sequence and chromosomal localization. Gene. 1994;140:203–209. doi: 10.1016/0378-1119(94)90545-2. [DOI] [PubMed] [Google Scholar]
- LUTZ E.M., MACKENZIE C.J., JOHNSON M., WEST K., MORROW J.A., HARMAR A.J., MITCHELL R. Domains determining agonist selectivity in chimaeric VIP2 (VPAC2)/PACAP (PAC1) receptors. Br. J. Pharmacol. 1999;128:934–940. doi: 10.1038/sj.bjp.0702872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADSEN P., BRAND C.L., HOLST J.J., KNUDSEN B. Advances in non-peptide glucagon receptor antagonists. Curr. Pharm. Des. 1999;5:683–691. [PubMed] [Google Scholar]
- MOJSOV S., HEINRICH G., WILSON I.B., RAVAZZOLA M., ORCI L., HABENER J.F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J. Biol. Chem. 1986;261:11880–11889. [PubMed] [Google Scholar]
- MONTROSE-RAFIZADEH C., YANG H., RODGERS B.D., BEDAY A., PRITCHETTE L.A., ENG J. High potency antagonists of the pancreatic glucagon-like peptide-1 receptor. J. Biol. Chem. 1997;272:21201–21206. doi: 10.1074/jbc.272.34.21201. [DOI] [PubMed] [Google Scholar]
- NIELSEN S.M., NIELSEN L.Z., HJORTH S.A., PERRIN M.H., VALE W.W. Constitutive activation of tethered-peptide/corticotropin-releasing factor receptor chimeras. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10277–10281. doi: 10.1073/pnas.97.18.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALCZEWSKI K., KUMASAKA T., HORI T., BEHNKE C.A., MOTOSHIMA H., FOX B.A., LE T.I., TELLER D.C., OKADA T., STENKAMP R.E., YAMAMOTO M., MIYANO M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- PERRET J., CRAENENBROECK M.V., LANGER I., VERTONGEN P., GREGOIRE F., ROBBERECHT P., WAELBROECK M. Mutational analysis of the glucagon receptor: similarities with the vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase- activating peptide (PACAP)/secretin receptors for recognition of the ligand's third residue. Biochem. J. 2002;362:389–394. doi: 10.1042/0264-6021:3620389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBERECHT P., GOURLET P., DE NEEF P., WOUSSEN-COLLE M.C., VANDERMEERS-PIRET M.C., VANDERMEERS A., CHRISTOPHE J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6-38) as a potent antagonist. Eur. J. Biochem. 1992;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- SHIMIZU M., CARTER P.H., GARDELLA T.J. Autoactivation of type-1 parathyroid hormone receptors containing a tethered ligand. J. Biol. Chem. 2000;275:19456–19460. doi: 10.1074/jbc.M001596200. [DOI] [PubMed] [Google Scholar]
- SHIMIZU N., GUO J., GARDELLA T.J. Parathyroid Hormone (PTH)-(1-14) and -(1-11) Analogs Conformationally Constrained by alpha-Aminoisobutyric Acid Mediate Full Agonist Responses via the Juxtamembrane Region of the PTH-1 Receptor. J. Biol. Chem. 2001;276:49003–49012. doi: 10.1074/jbc.M106827200. [DOI] [PubMed] [Google Scholar]
- SOLANO R.M., LANGER I., PERRET J., VERTONGEN P., JUARRANZ M.G., ROBBERECHT P., WAELBROECK M. Two basic residues of the h-VPAC1 receptor second transmembrane helix are essential for ligand binding and signal transduction. J. Biol. Chem. 2001;276:1084–1088. doi: 10.1074/jbc.M007696200. [DOI] [PubMed] [Google Scholar]
- THORELL J.I., JOHANSSON B.G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim. Biophys. Acta. 1971;251:363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- THORENS B., PORRET A., BUHLER L., DENG S.P., MOREL P., WIDMANN C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- TURNER P.R., BAMBINO T., NISSENSON R.A. A putative selectivity filter in the G-protein-coupled receptors for parathyroid hormone and secretion. J. Biol. Chem. 1996;271:9205–9208. doi: 10.1074/jbc.271.16.9205. [DOI] [PubMed] [Google Scholar]
- UNSON C.G., CYPESS A.M., KIM H.N., GOLDSMITH P.K., CARRUTHERS C.J., MERRIFIELD R.B., SAKMAR T.P. Characterization of deletion and truncation mutants of the rat glucagon receptor. Seven transmembrane segments are necessary for receptor transport to the plasma membrane and glucagon binding. J. Biol. Chem. 1995;270:27720–27727. doi: 10.1074/jbc.270.46.27720. [DOI] [PubMed] [Google Scholar]
- UNSON C.G., CYPESS A.M., WU C.R., GOLDSMITH P.K., MERRIFIELD R.B., SAKMAR T.P. Antibodies against specific extracellular epitopes of the glucagon receptor block glucagon binding. Proc. Natl. Acad. Sci. U.S.A. 1996;93:310–315. doi: 10.1073/pnas.93.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNSON C.G., MACDONALD D., RAY K., DURRAH T.L., MERRIFIELD R.B. Position 9 replacement analogs of glucagon uncouple biological activity and receptor binding. J. Biol. Chem. 1991;266:2763–2766. [PubMed] [Google Scholar]
- UNSON C.G., MERRIFIELD R.B. Identification of an essential serine residue in glucagon: implication for an active site triad. Proc. Natl. Acad. Sci. U.S.A. 1994;91:454–458. doi: 10.1073/pnas.91.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNSON C.G., WU C.R., CHEUNG C.P., MERRIFIELD R.B. Positively charged residues at positions 12, 17, and 18 of glucagon ensure maximum biological potency. J. Biol. Chem. 1998;273:10308–10312. doi: 10.1074/jbc.273.17.10308. [DOI] [PubMed] [Google Scholar]
- UNSON C.G., WU C.R., JIANG Y., YOO B., CHEUNG C., SAKMAR T.P., MERRIFIELD R.B. Roles of specific extracellular domains of the glucagon receptor in ligand binding and signaling. Biochemistry. 2002;41:11795–11803. doi: 10.1021/bi025711j. [DOI] [PubMed] [Google Scholar]
- XIAO Q., GIGUERE J., PARISIEN M., JENG W., ST PIERRE S.A., BRUBAKER P.L., WHEELER M.B. Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry. 2001;40:2860–2869. doi: 10.1021/bi0014498. [DOI] [PubMed] [Google Scholar]
- XIAO Q., JENG W., WHEELER M.B. Characterization of glucagon-like peptide-1 receptor-binding determinants. J. Mol. Endocrinol. 2000;25:321–335. doi: 10.1677/jme.0.0250321. [DOI] [PubMed] [Google Scholar]


