Abstract
Intravenous injection of ioxaglate (4 g iodine kg−1), an iodinated radiographic contrast medium, caused a marked protein extravasation, pulmonary oedema and a decrease in the arterial partial oxygen pressure in rats.
All of these reactions to ioxaglate were reversed by the pretreatment with gabexate mesilate (10 and 50 mg kg−1, 5 min prior to injection) or nafamostat mesilate (3 and 10 mg kg−1), in which the inhibition was complete after injection of nafamostat mesilate (10 mg kg−1).
Both gabexate mesilate and nafamostat mesilate inhibited the activity of purified human lung tryptase, although the latter compound was far more potent than the former.
Ioxaglate enhanced the nafamostat-sensitive protease activity in the extracellular fluid of rat peritoneal mast cell suspensions.
Tryptase enhanced the permeability of protein through the monolayer of cultured human pulmonary arterial endothelial cells. Ioxaglate, when applied in combination with rat peritoneal mast cells, also produced the endothelial barrier dysfunction. These effects of tryptase and ioxaglate were reversed by nafamostat mesilate.
Consistent with these findings, immunofluorescence morphological analysis revealed that tryptase or ioxaglate in combination with mast cells increased actin stress fibre formation while decreasing VE-cadherin immunoreactivity. Both of these actions of tryptase and ioxaglate were reversed by nafamostat mesilate.
These findings suggest that tryptase liberated from mast cells plays a crucial role in the ioxaglate-induced pulmonary dysfunction. In this respect, nafamostat mesilate may become a useful agent for the cure or prevention of severe adverse reactions to radiographic contrast media.
Keywords: Radiographic contrast media, vascular hyperactivity, pulmonary edema, endothelial barrier function, tryptase, actin stress fibre, VE-cadherin
Introduction
In spite of increasing use of radiographic contrast media (RCM), no effective prevention or therapy for the anaphylactoid reactions, including bronchospasm, dyspnea, laryngeal oedema and pulmonary oedema accompanying respiratory distress, induced by the intravascular injection of RCM has yet been established. The pulmonary oedema is a serious life-threatening adverse event, although the incidence is rare. The RCM-induced pulmonary oedema is a non-cardiogenic type and appears to be related to the increase in pulmonary vascular permeability subsequent to the activation of inflammatory cascade or the release of a variety of chemical mediators (Bouachour et al., 1991). Although the precise mechanisms underlying the RCM-induced vascular hyperpermeability or pulmonary dysfunction remain to be clarified, several lines of evidence have suggested that histamine is implicated in the pathogenesis of the RCM-related adverse events. It has been reported that the concentration of histamine in human plasma increases after injection of RCM (Brasch et al., 1970; Cogen et al., 1979; Robertson et al., 1985), in which the extent of histamine release is closely related to the severity of the adverse events (Laroche et al., 1998). A variety of RCM stimulate histamine release from human basophils (Assem et al., 1983) as well as mast cells (Stellato et al., 1996). Moreover, several anti-histaminic agents have been used to prevent or alleviate the anaphylactic reactions to RCM (Lieberman, 1990). However, the effectiveness of anti-histaminic therapy is incomplete, thereby indicating the contribution of other mechanisms than histamine release.
It has been demonstrated that plasma level of tryptase increases in patients who show severe symptoms after intravascular RCM injection (Laroche et al., 1998; Mita et al., 1998). In isolated human lung mast cells, several RCM induce a concentration-dependent tryptase release (Stellato et al., 1996). Taken together, it is probable that tryptase contributes significantly to the immediate anaphylactoid reactions to RCM.
Tryptase is a serine protease and abundant in mast cell granules (Schwartz et al., 1987; Schwartz, 1990; Hogan & Schwartz, 1997). Increased secretion of mast cell tryptase is observed in nasal lavage fluid of grass pollen-allergic patients (Jacobi et al., 1998), and in serum or bronchoalveolar lavage fluid of asthmatic patients after allergen challenge (Wenzel et al., 1988; Swystun et al., 2000). On the other hand, mast cell tryptase is reported to be a potent stimulator of microvascular leakage and augments the process in allergic inflammation (He & Walls, 1997).
In spite of the presence of the data showing that RCM cause the release of mast cell tryptase both in vivo and in vitro, there have hitherto been few studies on the role of tryptase in the vascular-related adverse reactions to the intravascular injection of RCM because of the lack of available potent tryptase inhibitors. Recently, gabexate mesilate has been reported to be a potent inhibitor of human tryptase (Erba et al., 2001). Gabexate mesilate and its structurally related compound nafamostat mesilate are synthetic low molecular-weight inhibitors of trypsin-like serine proteases (Menegatti et al., 1986; Aramoto et al., 1993; Ramjee et al., 2000), although the latter compound is about 100 times more potent than the former in suppressing trypsin activity. Both compounds inhibit reversibly the activities of a number of enzymes, including trypsin, thrombin, kallikrein and plasmin (Aoyama et al., 1984), and used for the therapy of disseminated intravascular coagulation and acute pancreatitis. Moreover, nafamostat mesilate but not gabexate mesilate has been reported to inhibit, though less potently, the complement activation, such as generation of C3a, C4a and C5a induced by heat aggregated IgG, zymosan and Cobra venom factor (Issekutz et al., 1990).
Therefore, in the present study, we examined whether the RCM-induced pulmonary dysfunction is reversed by gabexate mesylate or its structurally related analogue nafamostat mesylate. The effects of these compounds on the activity of purified human lung tryptase were also compared.
Methods
The present experimental procedures were all approved by the Committee for the Care and Use of Laboratory Animals at the Faculty of Medicine, Kyushu University, and the law (No. 105) and notification (No. 6) of the Japanese government.
Animals
Male Sprague-Dawley rats weighing 180–230 g (Kyudo Co., Saga, Japan) were used throughout the present experiments. Animals were housed in a room maintained on a 12-h light/dark schedule (lights on at 08:00 h) at a temperature of 23±2°C, and had free access to food and water.
Chemicals and reagents
Ioxaglate was obtained from a commercially available source (Hexabrix, 320 mg iodine ml−1; Mallinckrodt Medical, St. Louis, MO, U.S.A). Evans blue dye (Sigma Chemical, St. Louis) was mixed with RCM and they were simultaneously injected. Nafamostat mesilate and gabexate mesilate were gifts from Torii Pharmaceutical Co. Ltd. (Tokyo, Japan). Human tryptase (5.2 units mg protein−1) was purchased from Wako Pure Chemicals Inc. (Osaka, Japan). The peptide composition and purity (>98%) were ascertained by HPLC analysis, mass analysis and amino acid analysis. t-Butyloxycarbonyl - L-phenylalanyl - L-seryl-L-arginine-4-methylcoumaryl-7-amide (t-Boc-Phe-Ser-Arg-MCA) was purchased from peptide institute, Inc. (Osaka). Anti-VE-cadherin antibody labelled with fluorescein isothiocyanate was obtained from Alexis Biochemicals (San Diego, CA, U.S.A.). Rhodamine-conjugated phalloidin was obtained from Molecular Probes (Eugene, OR, U.S.A.). All other chemicals were of reagent grade.
Assessment of vascular permeability
The vascular permeability in lung tissues was evaluated by the Evans blue extravasation method described previously (Sendo et al., 2000). In brief, under anaesthesia with sodium pentobarbitone (50 mg kg−1, i.p.), rats were injected with saline or ioxaglate (4 g iodine kg−1) in combination with Evans blue (20 mg kg−1) through the femoral vein. The total injection volume, including ioxaglate, Evans blue and test compounds was 20 ml kg−1 and the mixture was injected at a rate of 1.5 ml min−1 using a syringe pump. At 10 min after the start of ioxaglate injection, the thorax was opened and the lung was perfused with physiological saline via a cannula inserted into the pulmonary artery to remove the intravascular Evans blue dye, then the lung parenchyma was dissected and weighed. Half of the dissected lung was immersed into formamide (4 ml g−1 wet weight of tissue) for 24 h to extract Evans blue. The remainder was dried in an oven at 60°C for 24 h. The concentration of Evans blue was determined at 620 nm using spectrophotometric microplate reader (Immuno-mini, NJ-2300, Intermedical, Tokyo). The amount of the dye was calculated from the standard curve for Evans blue (0.5–40 μg ml−1) and expressed as μg dye g dry tissue weight−1.
Measurement of blood gasses
Rats were anaesthetized with sodium pentobarbitone, and a cannula (Angiocath, 24G, Deseret Medical Inc., UT, U.S.A.) was inserted into the femoral artery for blood sampling. Saline (20 ml kg−1, i.v.) or nafamostat mesilate (10 mg kg−1, i.v.) was injected 5 min before ioxaglate injection (4 g iodine kg−1, i.v.). Aliquots (100 μl) of blood specimens were taken before and 5, 10, 20, 40 and 60 min after ioxaglate injection, and various arterial parameters including PaO2, PaCO2 and pH were immediately analysed using an automatic gas analyser (i-STAT Co., East Windor, NJ, U.S.A).
Determination of water, sodium and potassium contents in lung
To determine the water content and electrolytes concentrations in pulmonary tissues, rats were injected with ioxaglate (4 g iodine kg−1, i.v.) and killed by cervical dislocation under light anaesthesia 10 min later. The chest was opened and the lung was removed rapidly. Tissues were placed on absorbent paper and adherent non-pulmonary tissues were carefully removed. Lungs were separated into lobes and the extra-lobar vessels were removed. During this procedure, care was taken to prevent passive bleeding and the discharge of tissue fluid from the lung. The wet tissue weight was recorded and tissues were placed in an oven for 24 h at 100°C to measure the dry tissue weight. The water content (%, w w−1) was calculated as (wet weight – dry weight)×100 wet weight −1. The dried lobes were suspended in a mixture of nitric acid and perchloric acid (2 : 1, v v−1), homogenized, and then centrifuged at 1000×g for 10 min. The resultant supernatant was diluted with distilled water. The concentrations of sodium and potassium were measured by an atomic absorption spectrophotometry (Model AA-6400F, Shimadzu, Kyoto, Japan) using diluted standard solutions (Wako Pure Chemicals Inc.). Data obtained from triplicate assays were expressed as mEq g dry tissue weight−1.
Assay for human tryptase activity
The effect of nafamostat mesilate and gabexate mesilate on tryptase activity was determined by incubating purified human lung tryptase (7×10−4 units ml−1 final concentration) with its peptide substrate t-Boc-Phe-Ser-Arg-MCA (3.75 μM final concentration) in 100 mM Tris-HCl buffer (pH 7.8) in the absence or presence of inhibitors. To minimize the loss of tryptase activity, 20 μM heparin and 10% glycerol were included in the reaction buffer. The reaction was carried at 37°C for 30 min, then terminated by immersing into boiling water for 30–60 s. The resultant degradation product MCA was measured fluorimetrically at the excitation wavelength of 370 nm and the emission wavelength of 460 nm.
Measurement of nafamostat mesilate-sensitive tryptase-like serine protease release from rat peritoneal mast cells
Peritoneal fluids containing mast cells were collected, as described previously (Sueyasu et al., 1997). Briefly, 20 ml of Hank's balanced salt solution (HBSS mM: NaCl 137, KCl 5.36, MgSO4 0.2, Na2HPO4 0.34, KH2PO4 0.44, NaHCO3 4.17, CaCl2 1.26 and glucose 5.6) was injected into the rat peritoneal cavity. After gentle massage of the abdomen for 90 s, the intraperitoneal fluids were collected with a plastic pipette. The cell suspensions were centrifuged at 100×g for 6 min at 25°C, and washed four times with HBSS containing 0.1% BSA. The number of mast cells in cell suspensions was counted after staining with o-toluidine blue, and diluted with HBSS containing 0.1% BSA to the concentration of 2×105 mast cells ml−1. Cell suspensions were mixed with an equal volume of HBSS containing various concentrations of ioxaglate and incubated at 37°C for 10 min. Then, aliquots of the incubation medium was transferred and incubated with 4 μM t-Boc-Phe-Ser-Arg-MCA solution in the absence or presence of nafamostat mesilate for 5 min. The reaction was terminated by immersing the reaction mixture in boiling water. The methylcoumarine produced was determined by a reversed-phase high-performance liquid chromatography with fluorescence detection. The fluorescence intensity determined at the excitation wavelength of 370 nm and the emission wavelength of 460 nm was monitored.
Measurement of histamine release from rat peritoneal mast cells
Cell suspensions containing peritoneal mast cells (2×105 mast cells ml−1), prepared as described above, were incubated for 10 min at 37°C with 100 mg iodine ml−1 of ioxaglate in the absence or presence of nafamostat mesilate. The reaction was stopped by centrifugation at 800×g for 5 min at 4°C. The supernatant was transferred to another tube and perchloric acid was added to the final concentration of 0.4 M. The pellets were homogenized with 0.4 M perchloric acid. The histamine concentrations in both the supernatant and the cell pellet were determined by ion-pair high performance liquid chromatography coupled with post-column fluorescent derivatization, as described previously (Itoh et al., 1992). The histamine release was expressed as the percentage of histamine concentration in the supernatant to the total.
Determination of endothelial barrier function
Human pulmonary arterial endothelial cells (HPAECs) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with 10% foetal bovine serum, 100 u ml−1 penicillin, 100 mg ml−1 streptomycin and 250 mg ml−1 amphotericin B. HPAECs of two to four passages were used for experiments. Cells were seeded at 4.0×104 cells [cm2]−1 onto fibronectin-coated polycarbonate membrane (1.1 cm2, 3.0 μm pore size) of the Transwell insert (12-well type, Corning Costar). The barrier function was determined by the permeability of Evans blue conjugated with bovine serum albumin through the monolayer of the endothelial cells, as described previously (Furuta et al., 2002). In brief, Evans blue (0.67 mg ml−1) was mixed with 4% bovine serum albumin (BSA) and diluted with Krebs–Ringer buffer (KRB mM: NaCl 118.0, KCl 4.7, CaCl2 1.3, MgSO4 1.2, NaH2PO4 1.0, NaHCO3 25, glucose 11, pH 7.4). After washing the cells with KRB three times, the insert was immersed in a well containing 1.5 ml KRB, then BSA-conjugated Evans blue was included in the presence of test compounds. In a set of experiments where the effect of ioxaglate was examined, freshly prepared rat peritoneal lavage fluid containing mast cells was included in the inner chamber at a concentration of 3×105 cells well−1. Aliquots (300 μl) of medium in the outer chamber were taken and replaced by the same volume of freshly prepared KRB at 10, 20 and 30 min to measure the clearance of BSA-conjugated Evans blue. The concentration of Evans blue was measured from the absorbance at 620 nm using a spectrophotometric microplate reader. The clearance (μl min−1) was calculated according to the following equation, and expressed as the percentage of the control values.
Clearance (μl min−1)=[C]O×VO [C]I−1, where [C]O and [C]I are the tracer concentrations (μg μl−1 min−1) in the outer chamber and the initial concentration (μg μl−1) in the inner chamber, respectively, and VO is the volume (μl) of the outer chamber.
Immunofluorescence analysis for actin and VE-cadherin
HPAECs were grown to confluence on gelatin-coated glass coverslips. HPAECs were stimulated with ioxaglate (100 mg iodine ml−1) in the absence or presence of rat peritoneal mast cells prepared as described above, or 7×10−3 units ml−1 tryptase for 10 min, then washed twice with phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS at 25°C for 15 min. Cells were washed twice with PBS and permeabilized with 0.1% Triton X-100 at 25°C for 3–5 min, washed twice with PBS and incubated with blocking buffer (PBS containing 5% foetal bovine serum, 0.2% BSA and 0.1% Triton X-100). The blocking buffer was removed and cells were subsequently stained with rhodamine-conjugated phalloidin for actin staining or fluorescein isothiocyanate-labelled anti-VE-cadherin antibody for VE-cadherin staining in blocking buffer (PBS containing 5% foetal bovine serum, 0.2% BSA and 0.1% Triton X-100) at 25°C for 20 min, followed by washing twice with PBS. A coverslip was mounted onto the slide containing PBS and glycerol (1 : 1) and images were visualized with fluorescence microscopy (BX51, Olympus, Tokyo).
Statistical analyses
Data are expressed as the mean±s.e.mean and statistically analysed by one-way analysis of variance followed by the Bonferroni/Dunnett's test for multiple comparisons, or by Student's t-test for comparison between two groups (StatView; Abacus Concepts, CA, U.S.A). Statistical significance was defined as P<0.05.
Results
Effects of nafamostat mesilate and gabexate mesilate on ioxaglate-induced pulmonary dysfunction
Intravenous injection of ioxaglate at 4 g iodine kg−1 caused a marked extravasation of serum protein, as assessed by the leakage of Evans blue dye into pulmonary tissues. Pretreatment of nafamostat mesilate (3 or 10 mg kg−1) or gabexate mesilate (50 mg kg−1) significantly inhibited the ioxaglate-induced vascular hyperpermeability. It was noteworthy that the inhibition was almost complete, when pretreated with 10 mg kg−1 nafamostat mesilate (Figure 1). Moreover, the lung developed a marked oedema with a concomitant increase in tissue Na+ content after ioxaglate injection (Figure 2). Pretreatment with nafamostat mesilate (10 mg kg−1) completely blocked the ioxaglate-induced pulmonary oedema and the rise in tissue Na+ content (Figure 2).
Figure 1.
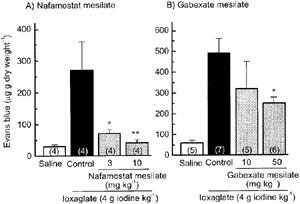
Pretreatment with nafamostat mesilate and gabexate mesilate reverses the ioxaglate-induced pulmonary vascular hyperpermeability in rats. Nafamostat mesilate and gabexate mesilate were injected i.v. 5 min before intravenous injection of ioxaglate (4 g iodine kg−1). Vascular permeability was assessed by the extravasation of Evans blue dye to lung tissues 10 min after ioxaglate injection. Each column represents the mean±s.e.mean. Number of animals was shown in each parenthesis. *P<0.05, **P<0.01 versus control.
Figure 2.
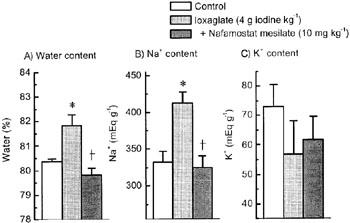
Pretreatment with nafamostat mesilate abolishes the ioxaglate-induced pulmonary oedema and increase in tissue Na+ content in the rat lung. Nafamostat mesilate (10 mg kg−1) was injected i.v. 5 min before intravenous injection of ioxaglate (4 g iodine kg−1). Lungs were dissected 10 min after ioxaglate injection. Pulmonary oedema was assessed by tissue water content. Each column represents the mean±s.e.mean for n=4–6 experiments. *P<0.05 versus control; †P<0.05 versus ioxaglate alone.
In rats with intravenous ioxaglate injection, arterial partial oxygen pressure was remarkably but transiently reduced (from 110.3±3.7 mmHg to 77.0±5.0 mmHg, mean ±s.e.mean, n=6), while arterial partial carbon dioxide pressure was slightly and not significantly elevated (Figure 3). On the other hand, arterial pH was not significantly changed by ioxaglate injection. Pretreatment with nafamostat mesilate (10 mg kg−1) completely reversed the decrease in arterial partial oxygen pressure induced by ioxaglate injection.
Figure 3.
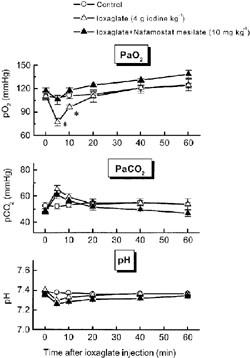
Pretreatment with nafamostat mesilate abolishes the ioxaglate-induced decrease in arterial PaO2 in rats. Rats were injected with nafamostat mesilate (10 mg kg−1) 5 min before ioxaglate treatment. Arterial gasses were monitored by an automatic gas analyser (i-STAT) for 60 min after ioxaglate injection. Control animals were injected with saline. Each point represents the mean±s.e.mean for n=4–6 experiments. *P<0.05 versus control.
Inhibition of tryptase activity by nafamostat mesilate and gabexate mesilate
To determine whether nafamostat mesilate inhibits the tryptase activity similarly to gabexate mesilate, the effects of these compounds on purified human lung tryptase activity were compared. Generally consistent with the previous data (Erba et al., 2001), gabexate mesilate inhibited human tryptase activity with an IC50 of 1.9×10−7 M, as determined by the degradation of synthetic tryptase substrate peptide t-Boc-Phe-Ser-Arg-MCA (Figure 4). It was noteworthy that nafamostat mesilate was far more potent than gabexate mesilate in blocking human tryptase activity, the IC50 of which was 1.6×10−11 M. Thus, the potency in tryptase inhibition was approximately 12,000 fold higher in nafamostat mesilate than in gabexate mesilate.
Figure 4.
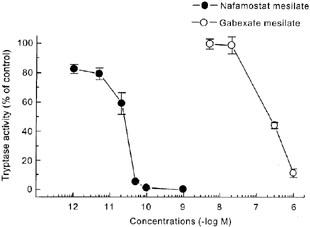
Nafamostat mesilate and gabexate mesilate inhibit the activity of purified human lung tryptase. Tryptase activity was assessed by the degradation of a synthetic tryptase substrate t-Boc-Phe-Ser-Arg-MCA to methylcoumarine. Each point represents the mean±s.e.mean for n=4–5 experiments.
Enhancement by ioxaglate of the release of tryptase-like protease from rat peritoneal mast cells
To confirm that ioxaglate stimulates the release of mast cell tryptase, the effect of this contrast medium on the tryptase-like protease activity in the extracellular fluid of the rat peritoneal mast cell suspensions. As shown in Figure 5, ioxaglate produced a marked and concentration-dependent increase in the tryptase-like protease activity in the extracellular fluid. This action of ioxaglate was almost totally inhibited by nafamostat mesilate (10 nM).
Figure 5.
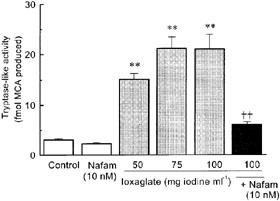
Ioxaglate produces a concentration-dependent increase in the release of nafamostat mesilate-sensitive tryptase-like protease from rat peritoneal mast cells. Tryptase activity was assessed by the degradation of a synthetic tryptase substrate t-Boc-Phe-Ser-Arg-MCA to methylcoumarine, and its release was expressed as the percentage of activity in the incubation medium to the total. The tryptase-like activity was determined in the presence of 10 nM nafamostat mesilate (Nafam). Each column represents the mean±s.e.mean for n=4–6 experiments. **P<0.01 versus control, ††P<0.01 versus ioxaglate (100 mg iodine ml−1) alone.
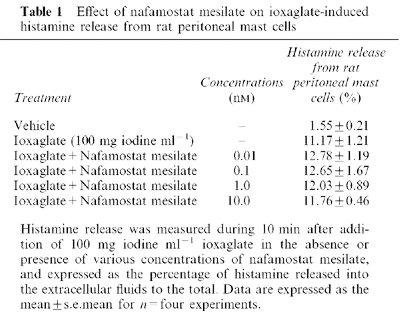
On the other hand, nafamostat mesilate (0.01–10 nM) did not affect the ioxaglate induced histamine release from rat peritoneal mast cells (Table 1).
Table 1.
Effect of nafamostat mesilate on ioxaglate-induced histamine release from rat peritoneal mast cells
Endothelial barrier dysfunction induced by tryptase and ioxaglate in combination with rat peritoneal mast cells, and their reversal by nafamostat mesilate in cultured HPAECs
Tryptase (1.4×10−3 units ml−1) enhanced the permeability of BSA-conjugated Evans blue through the monolayer of cultured HPAECs (Figure 6A). Ioxaglate (100 mg iodine ml−1), when added in combination with rat peritoneal mast cells (3×105 cells well−1), also significantly enhanced the protein permeability through the endothelial monolayer, although ioxaglate alone caused a slight and not significant change in the protein permeability (Figure 6B). Both the effects of tryptase and ioxaglate in combination with mast cells were markedly reversed by 0.1 nM nafamostat mesilate.
Figure 6.
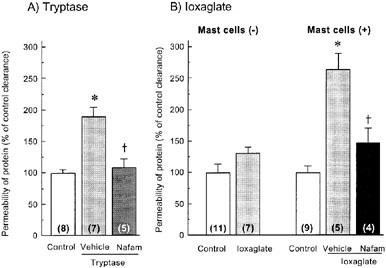
Endothelial barrier dysfunction induced by tryptase (A) and ioxaglate in combination with rat peritoneal mast cells (B) in cultured human pulmonary arterial endothelial cells (HPAECs), and their reversal by nafamostat mesilate. Barrier function was estimated from the permeability of albumin-conjugated Evans blue through the monolayer of HPAECs, as described in Methods. In (A), cells were exposed to 1.4×10−3 units ml−1 tryptase for 30 min. In (B), ioxaglate (100 mg iodine ml−1) was included in the absence or presence of rat peritoneal mast cells (3×105 cells/well−1). Nafamostat mesilate (Nafam: 0.1 nM) was added together with 1.4×10−3 units ml−1 tryptase (A) or 100 mg iodine ml−1 of ioxaglate (B). The clearance of BSA-conjugated Evans blue in control groups were 0.21±0.04 μl min−1 (mean±s.e.mean; (A) 0.32±0.02 μl min−1 (B) in the absence of mast cells) and 0.41±0.03 μl min−1 (B) in the presence of mast cells). Each column represents the mean±s.e.mean. KRB was used for vehicle. Number of experiments is shown in each parenthesis. *P<0.05 versus control. †<0.05 versus vehicle.
Changes in immunoreactivities for actin and VE-cadherin after exposure of HPAECs to ioxaglate
Immunohistochemical staining for actin and VE-cadherin was conducted in cultured HPAECs using rhodamine-conjugated phalloidin and anti-serum against VE-cadherin. As shown in Figure 7, non-stimulated HPAECs revealed a definite and extensively tight staining of VE-cadherin along the intercellular junction, while showing a faint staining with actin stress fibres. In contrast, the VE-cadherin staining was disrupted and partially disappeared in cells exposed to 100 mg iodine ml−1 ioxaglate in the presence of rat peritoneal mast cells. Moreover, a marked enhancement of actin stress fibre staining was observed in these cells. Similar rearrangement of VE-cadherin and the enhanced actin stress fibre staining was observed in cells treated with 7×10−3 units ml−1. The ioxaglate-induced decrease in VE-cadherin staining was dramatically reversed by nafamostat mesilate (1 nM).
Figure 7.
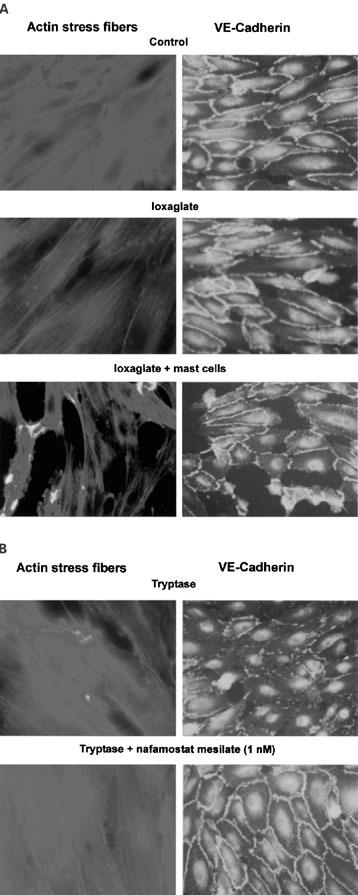
Actin stress fibres increase, while VE-cadherin immunoreactivity decreases after exposure of cultured HPAECs to ioxaglate (100 mg iodine ml−1), ioxaglate+mast cells, and tryptase (7×10−3 units ml−1). The increase in actin stress fibres and the decrease in VE-cadherin induced by ioxaglate+mast cells or tryptase are blocked by nafamostat mesilate (1 nM).
Discussion
A number of studies have shown that mast cells are implicated in the pathogenesis of acute allergic-like reactions to iodinated RCM (Robertson et al., 1985; Stellato et al., 1996; Laroche et al., 1998). Since a variety of RCM cause histamine release from mast cell, histamine H1 antagonists have been used for the prevention of the adverse reactions to RCM (Lieberman, 1990). However, the preventive effects of H1 antagonists are incomplete, indicating the involvement of other chemical mediators. It has been reported that ioxaglate and a non-ionic contrast medium ioversol stimulate the release of the pre-formed mediators such as histamine and tryptase but not the de novo synthesized mediators including prostaglandin D2 and leukotriene C4 from human lung mast cells (Genovese et al., 1996; Stellato et al., 1996). On the other hand, Laroche et al. (1998) reported in patients who showed mild to severe reactions to ioxaglate and ioxitalamate that plasma level of tryptase increases in relation to the severity of the reactions. Taken together, mast cell tryptase may become a potential candidate for the mediators of adverse reactions to RCM. Recently, gabexate mesilate is reported to be a potent inhibitor of human tryptase (Erba et al., 2001). Therefore, in the present study, we examined the effects of gabexate mesilate and its structurally related compound nafamostat mesilate on the pulmonary dysfunction induced in rats by an intravenous injection of ioxaglate.
According to the data reported by Erba et al. (2001), the inhibitory action of gabexate mesilate is more potent on human tryptase (Ki=3.4×10−9 M) than on bovine tryptase (Ki=1.8×10−7 M), as determined by the degradation of the synthetic tryptase substrate t-Boc-Phe-Ser-Arg-MCA. We also confirmed using the same tryptase substrate that gabexate mesilate is a potent human tryptase inhibitor. Moreover, it is noteworthy that nafamostat mesilate was found to be an extremely potent inhibitor of human tryptase, in which it blocked approximately 12,000 fold more potently (IC50=1.6×10−11 M) the activity of purified human lung tryptase than gabexate mesilate. Like gabexate mesilate, nafamostat mesilate is a serine protease inhibitor, and inhibits trypsin, plasmin, pancreatic and plasma kallikrein and thrombin with IC50 values ranging from 2.7×10−8 M to 5.0×10−7 M (Fujii & Hitomi, 1981). In this respect, nafamostat mesilate seems to be, thus far, the most potent and selective human tryptase inhibitor. This protease inhibitor is widely used for the therapy of acute pancreatitis and disseminated intravascular coagulation, and for anti-coagulant agent during extracorporeal circulation.
Rats treated with ioxaglate showed an immediate pulmonary dysfunction, including the protein extravasation, oedema, increase in tissue Na+ content, and reduction in arterial PaO2. Pretreatment with gabexate mesilate or nafamostat mesilate significantly reversed the pulmonary protein extravasation, although the inhibitory effect of gabexate mesilate was much less marked than that of nafamostat mesilate. Moreover, nafamostat mesilate at 10 mg kg−1 completely blocked the ioxaglate-induced pulmonary oedema, elevation of the tissue Na+ content and the decrease in arterial PaO2. However, larger doses of this inhibitor than those expected from in vitro study were required to reduce the ioxaglate-induced pulmonary dysfunction. This may be due to the rapid metabolism of nafamostat mesilate in the plasma. Taken together, it is likely that tryptase released from mast cells contributes at least in part to the pathogenesis of RCM-induced pulmonary dysfunction, although we cannot exclude the possibility that other proteases are implicated in the adverse reactions to RCM.
To further confirm that ioxaglate stimulates tryptase release from mast cells, the effect of this contrast medium on the release of tryptase-like protease from rat peritoneal mast cells was investigated. The tryptase-like protease activity determined by the degradation of t-Boc-Phe-Ser-Arg-MCA was markedly enhanced by 50–150 mg iodine ml−1 ioxaglate in a concentration-dependent manner. Although t-Boc-Phe-Ser-Arg-MCA is hydrolyzed by other trypsin-like proteases including trypsin and 73 kDa thiol protease (Molla et al., 1988), the main proteases included in rat peritoneal mast cells are tryptase, a trypsin-like protease, and chymase, a chymotrypsin-like protease (Muramatu et al., 1988). Moreover, the protease activity was almost completely inhibited by nafamostat mesilate (10−8 M). Taken together, it is suggested that mast cell tryptase is liberated by ioxaglate. This idea was further confirmed by the present findings indicating that the endothelial barrier dysfunction was induced by tryptase alone or ioxaglate in combination with rat peritoneal mast cells. Moreover, both of these actions of tryptase and ioxaglate were reversed by nafamostat mesilate. These findings also suggest that nafamostat mesilate inhibits the activity of rat mast cell tryptase to the similar extent as human tryptase.
On the other hand, nafamostat mesilate did not affect the ioxaglate-induced histamine release from rat peritoneal mast cells. Therefore, it is unlikely that the inhibitory effect of nafamostat on the in vivo and in vitro vascular endothelial dysfunction caused by ioxaglate results from the inhibition of the degranulation of mast cells.
The endothelial barrier function is regulated by the intercellular junctional organization. VE-cadherin is the major constituent of cell-to-cell contact, and interacts with actin cytoskeleton via associated proteins such as α- and β-catenins (Lampugnani et al., 1995). The VE-cadherin-catenin complex is flexible and disappears from the extracellular space upon various stimuli that decrease endothelial barrier function such as thrombin (Rabiet et al., 1996). In the present study, the disappearance of immunoreactive VE-cadherin with a marked development of actin stress fibres was observed after exposure of HPAECs to tryptase in nafamostat-sensitive manner. It is also interesting that ioxaglate in combination with peritoneal mast cells caused a disappearance of VE-cadherin immunoreactivity and enhancement of actin stress fibres, although ioxaglate alone induced no marked changes in the staining pattern for either of these molecules. These findings also suggest that ioxaglate liberates mast cell tryptase, which in turn, causes an increase in actin stress fibre formation as well as a decrease in the tight junctional VE-cadherin, leading to the decrease in the endothelial barrier function.
It has been demonstrated that several biological actions of tryptase is considered to be mediated through activation of proteinase-activated receptor-2 (PAR-2) (Molino et al., 1997). PAR-2 is a member of a G protein-coupled receptor family (Nystedt et al., 1994; Böhm et al., 1996), and activated by the tethered ligand after cleavage of N-terminal region by tryptase, trypsin, factor VIIa and Xa (Corvera et al., 1997; Molino et al., 1997; Dery et al., 1998; Coughlin, 2000). The cleavage site by tryptase is assumed to be Arg34-Ser35 in mouse PAR-2 (18) (Nystedt et al., 1994), and Arg36-Ser37 in human PAR-2 (Böhm et al., 1996). The activation of PAR-2 by tryptase is implicated in the pathogenesis of acute inflammation (Saifeddine, 1996; Vergnolle et al., 1999; 2001), increase in airway resistance (Ricciardolo et al., 2000) and vascular hyperpermeability (Vergnolle et al., 1999; Kawabata et al., 1998). Therefore, it is suggested that RCM elicit protein extravasation ultimately via stimulation of endothelial PAR-2.
In conclusion, the pulmonary dysfunction, such as protein extravasation, oedema and decrease in arterial PaO2, induced by an intravenous injection of ioxaglate was dramatically suppressed by gabexate mesilate and nafamostat mesilate. Both compounds inhibited human tryptase activity, in which nafamostat mesilate was by far the strongest inhibitor. In rat peritoneal mast cells, the tryptase-like protease activity in the extracellular fluid was enhanced by ioxaglate. Immunofluorescence examination in cultured HPAECs monolayer revealed that ioxaglate, when treated in combination with mast cells, induced a loss of VE-cadherin and a concomitant increase in actin stress fibres. Therefore, it is likely that ioxaglate induces pulmonary dysfunction by stimulating the release of mast cell tryptase. In addition, our present findings suggest that clinically available nafamostat mesilate is potentially useful for the prevention or cure of anaphylactoid adverse reactions to the intravascular RCM injection.
Acknowledgments
This research was supported in part by Grant-in-Aid for Scientific Research (C :13672390) from the Ministry of Education, Science, Sports and Culture, Japan
Abbreviations
- BSA
bovine serum albumin
- HBSS
Hank's balanced salt solution
- HPAECs
human pulmonary arterial endothelial cells
- PAR-2
proteinase-activated receptor-2
- PBS
phosphate buffered saline
- RCM
radiographic contrast media
- t-Boc-Phe-Ser-Arg-MCA
t-Butyloxycarbonyl-L-phenylalanyl-L-seryl-L-arginine-4-methylcoumaryl-7-amide
References
- AOYAMA T., INO Y., OZEKI M., ODA M., SATO T., KOSHIYAMA Y., SUZUKI S., FUJITA M. Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments. Jpn. J. Pharmacol. 1984;35:203–227. doi: 10.1254/jjp.35.203. [DOI] [PubMed] [Google Scholar]
- ARAMOTO H., SAITO H., SHIGEMATSU H., MUTO T. Synthetic protease inhibitors in the treatment of disseminated intravascular coagulation. Nippon Rinsho. 1993;51:93–98. [PubMed] [Google Scholar]
- ASSEM E.S., BRAY K., DAWSON P. The release of histamine from human basophils by radiological contrast agents. Br. J. Radiol. 1983;56:647–652. doi: 10.1259/0007-1285-56-669-647. [DOI] [PubMed] [Google Scholar]
- BÖHM S.K., KONG W., BROMME D., SMEEKENS S.P., ANDERSON D.C., CONNOLLY A., KAHN M., NELKEN N.A., COUGHLIN S.R., PAYAN D.G., BUNNETT N.W. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUACHOUR G., VARACHE N., SZAPIRO N., L'HOSTE P., HARRY P., ALQUIER P. Noncardiogenic pulmonary edema resulting from intravascular administration of contrast material. Am. J. Roentgenol. 1991;157:255–256. doi: 10.2214/ajr.157.2.1853801. [DOI] [PubMed] [Google Scholar]
- BRASCH R.C., ROCKOFF S.D., KUHN C., CHRAPLYVY M. Contrast media as histamine liberators. II. Histamine release into venous plasma during intravenous urography in man. Invest. Radiol. 1970;5:510–513. [PubMed] [Google Scholar]
- COGEN F.C., NORMAN M.E., DUNSKY E., HIRSHFELD J., ZWEIMAN B. Histamine release and complement changes following injection of contrast media in humans. J. Allergy Clin. Immunol. 1979;64:299–303. doi: 10.1016/0091-6749(79)90148-9. [DOI] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., MCCONALOGUE K., BOHM S.K., KHITIN L.M., CAUGHEY G.H., PAYAN D.G., BUNNETT N.W. Mast cell tryptase regulates colonic myocytes through proteinase-activated receptor-2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUGHLIN S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNET N.W.Proteinase-activated receptors: novel mechanisms of signaling by serine proteases Am. J. Physiol. 1998274C1429–C1452.Pt 1 [DOI] [PubMed] [Google Scholar]
- ERBA F., FIORUCCI L., PASCARELLA S., MENEGATTI E., ASCENZI P., ASCOLI F. Selective inhibition of human mast cell tryptase by gabexate mesilate, an antiproteinase drug. Biochem. Pharmacol. 2001;61:271–276. doi: 10.1016/s0006-2952(00)00550-5. [DOI] [PubMed] [Google Scholar]
- FUJII S., HITOMI Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim. Biophys. Acta. 1981;661:342–345. doi: 10.1016/0005-2744(81)90023-1. [DOI] [PubMed] [Google Scholar]
- FURUTA W., YAMAUCHI A., DOHGU S., NAKAGAWA S., SENDO T., MAKINO K., OISHI R., KATAOKA Y. Contrast media increase vascular endothelial permeability by inhibiting nitric-oxide production. Invest. Radiol. 2002;37:13–19. doi: 10.1097/00004424-200201000-00004. [DOI] [PubMed] [Google Scholar]
- GENOVESE A., STELLATO C., PATELLA V., LAMPARTER-SCHUMMERT B., DE CRESCENZO G., ADT M., MARONE G. Contrast media are incomplete secretagogues acting on human basophils and mast cells isolated from heart and lung, but not skin tissue. Int. J. Clin. Lab. Res. 1996;26:192–198. doi: 10.1007/BF02592981. [DOI] [PubMed] [Google Scholar]
- HE S., WALLS A.F. Human mast cell tryptase: a stimulus of microvascular leakage and mast cell activation. Eur. J. Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- HOGAN A.D., SCHWARTZ L.B. Markers of mast cell degranulation. Methods. 1997;13:43–52. doi: 10.1006/meth.1997.0494. [DOI] [PubMed] [Google Scholar]
- ISSEKUTZ A.C., ROLAND D.M., PATRICK R.A. The effect of FUT-175 (Nafamstat Mesilate) on C3a, C4a and C5a generation in vitro and inflammatory reactions in vivo. Int. J. Immunopharmacol. 1990;12:1–9. doi: 10.1016/0192-0561(90)90062-r. [DOI] [PubMed] [Google Scholar]
- ITOH Y., OISHI R., ADACHI N., SAEKI K. A highly sensitive assay for histamine using ion-pair HPLC coupled with postcolumn fluorescent derivatization. J. Neurochem. 1992;58:884–889. doi: 10.1111/j.1471-4159.1992.tb09339.x. [DOI] [PubMed] [Google Scholar]
- JACOBI H.H., SKOV P.S., KAMPEN G.T., POULSEN L.K., REIMERT C.M., BINDSLEV-JENSEN C., PRAETORIUS C., MALLING H.J., MYGIND N. Histamine and tryptase in nasal lavage fluid following challenge with methacholine and allergen. Clin. Exp. Allergy. 1998;28:83–91. doi: 10.1046/j.1365-2222.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., MINAMI T., KATAOKA K., TANEDA M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br. J. Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPUGNANI M.G., CORADA M., CAVEDA L., BREVIARIO F., AYALON O., GEIGER B., DEJANA E.R. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin) J. Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAROCHE D., AIMONE-GASTIN I., DUBOIS F., HUET H., GERARD P., VERGNAUD M.C., MOUTON-FAIVRE C., GUEANT J.L., LAXENAIRE M.C., BRICARD H. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology. 1998;209:183–190. doi: 10.1148/radiology.209.1.9769830. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN P. The use of antihistamines in the prevention and treatment of anaphylaxis and anaphylactoid reactions. J. Allergy Clin. Immunol. 1990;86:684–686. doi: 10.1016/s0091-6749(05)80241-6. [DOI] [PubMed] [Google Scholar]
- MENEGATTI E., BOLOGNESI M., SCALIA S., BORTOLOTTI F., GUARNERI M., ASCENZI P. Gabexate mesylate inhibition of serine proteases: thermodynamic and computer-graphics analysis. J. Pharm. Sci. 1986;75:1171–1174. doi: 10.1002/jps.2600751211. [DOI] [PubMed] [Google Scholar]
- MITA H., TADAKORO K., AKIYAMA K. Detection of IgE antibody to a radiocontrast medium. Allergy. 1998;53:1133–1140. doi: 10.1111/j.1398-9995.1998.tb03832.x. [DOI] [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J.A., SCHECHTER J.A., WOOLKALIS M., BRASS L.F. Interaction of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- MOLLA A., YAMAMOTO T., MAEDA H. Characterization of 73 kDa thiol protease from Serratia marcescens and its effect on plasma proteins. J. Biochem. (Tokyo) 1988;104:616–621. doi: 10.1093/oxfordjournals.jbchem.a122521. [DOI] [PubMed] [Google Scholar]
- MURAMATU M., ITOH T., TAKEI M., ENDO K. Tryptase in rat mast cells: properties and inhibition by various inhibitors in comparison with chymase. Biol. Chem. Hoppe. Seyler. 1988;369:617–625. doi: 10.1515/bchm3.1988.369.2.617. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994; 91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABIET M.J., PLANTIER J.L., RIVAL Y., GENOUX Y., LAMPUGNANI M.G., DEJANA E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler. Thromb. Vasc. Biol. 1996;16:488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L., STEINHOFF M., AMADESI S., GUERRINI R., TOGNETTO M., TREVISANI M., CREMINON C., BERTRAND C., BUNNETT N.W., FABBRI L.M., SALVADORI S., GEPPETTI P. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am. J. Respir. Crit. Care Med. 2000;161:1672–1680. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- RAMJEE M.K., HENDERSON I.M., MCLOUGHLIN S.B., PADOVA A. The kinetic and structural characterization of the reaction of nafamostat with bovine pancreatic trypsin. Thromb. Res. 2000;98:559–569. doi: 10.1016/s0049-3848(00)00206-1. [DOI] [PubMed] [Google Scholar]
- ROBERTSON P.W., FREWIN D.B., ROBERTSON A.R., MAHAR L.J., JONSSON J.R. Plasma histamine levels following administration of radiographic contrast media. Br. J. Radiol. 1985;58:1047–1051. doi: 10.1259/0007-1285-58-695-1047. [DOI] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ L.B. Tryptase, a mediator of human mast cells. J. Allergy Clin. Immunol. 1990;86:594–598. doi: 10.1016/s0091-6749(05)80222-2. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ L.B., IRANI A.A., ROLLER K., CASTELLS M.C., SCHECHTER N.M. Quantitation of histamine, tryptase and chymase in dispersed human T and TC mast cells. J. Immunol. 1987;138:2611–2615. [PubMed] [Google Scholar]
- SENDO T., KATAOKA Y., TAKEDA Y., FURUTA W., OISHI R. Nitric oxide protects against contrast media-increased pulmonary vascular permeability in rats. Invest. Radiol. 2000;35:472–478. doi: 10.1097/00004424-200008000-00003. [DOI] [PubMed] [Google Scholar]
- STELLATO C., DE CRESCENZO G., PATELLA V., MASTRONARDI P., MAZZARELLA B., MARONE G. Human basophil/mast cell releasability. XI. Heterogeneity of the effects of contrast media on mediator release. J. Allergy Clin. Immunol. 1996;97:838–850. doi: 10.1016/s0091-6749(96)80162-x. [DOI] [PubMed] [Google Scholar]
- SUEYASU M., KOMASU C., HARA T., FUTAGAMI K., KATAOKA Y., OISHI R. Enhancement by morphine of radiographic contrast media-induced histamine release in rat peritoneal mast cells. Jpn. J. Pharmacol. 1997;74:217–220. doi: 10.1254/jjp.74.217. [DOI] [PubMed] [Google Scholar]
- SWYSTUN V.A., GORDON J.R., DAVIS E.B., ZHANG X., COCKCROFT D.W. Mast cell tryptase release and asthmatic responses to allergen increase with regular use of salbutamol. J. Allergy Clin. Immunol. 2000;106:57–64. doi: 10.1067/mai.2000.107396. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., SHARKEY K.A., WALLACE J.L. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR-2)-activating peptides in the rat paw. Br. J. Pharmacol. 1999;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., WALLACE J.L., BUNNETT N.W., HOLLENBERG M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- WENZEL S.E., FOWLER A.A., SCHWARTZ L.B. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with or without asthma. Am. Rev. Respir. Dis. 1988;137:1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]


