Abstract
This report analyses the intracellular and extracellular accumulation of cyclic AMP in primary rat skeletal muscle cultures, after direct and receptor-dependent stimulation of adenylyl cyclase (AC).
Isoprenaline, calcitonin gene-related peptide (CGRP) and forskolin induced a transient increase in the intracellular cyclic AMP that peaked 5 min after onset stimulation.
Under stimulation with isoprenaline or CGRP, the intracellular cyclic AMP initial rise was followed by an exponential decline, reaching 46 and 52% of peak levels in 10 min, respectively.
Conversely, the forskolin-dependent accumulation of intracellular cyclic AMP decreased slowly and linearly, reaching 49% of the peak level in 30 min.
The loss of intracellular cyclic AMP from peak levels, induced by direct or receptor-induced activation of AC, was followed by an increase in the extracellular cyclic AMP.
This effect was independent on PDEs, since it was obtained in the presence of 3-isobutyl-1-methylxanthine (IBMX).
Besides, in isoprenaline treated cells, the beta-adrenoceptor antagonist propranolol reduced both intra- and extracellular accumulation of cyclic AMP, whereas the organic anion transporter inhibitor probenecid reduced exclusively the extracellular accumulation.
Together our data show that direct or receptor-dependent activation of skeletal muscle AC results in a transient increase in the intracellular cyclic AMP, despite the continuous presence of the stimulus. The temporal declining of intracellular cyclic AMP was not dependent on the cyclic AMP breakdown but associated to the efflux of cyclic nucleotide to the extracellular compartment, by an active transport since it was prevented by probenecid.
Keywords: Calcitonin gene-related peptide, β-adrenoceptor, adenylyl cyclase, neuromuscular junction, skeletal muscle, cyclic AMP, organic anion transport, phosphodiesterase
Introduction
It is well established that cyclic adenosine 3′-, 5′-monophosphate (cyclic AMP) regulates a wide variety of skeletal muscle processes, mediating the biological action of many extracellular substances, such as catecholamines and adenosine. For example, activation of cell surface β2-adrenoceptors and adenosine receptors, linked to stimulatory G (Gs) protein, stimulates adenylyl cyclase (AC) influencing the skeletal muscle glucose transport (Roberts & Summers, 1998; Han et al., 1998), carbohydrate metabolism (Hespel & Richter, 1998; Fagher et al., 1986; Nagase et al., 2001) and contractility (van der heijden et al., 1998), through cyclic AMP dependent pathways.
The increase in intracellular cyclic AMP also mediates the effects of nerve-derived calcitonin gene-related peptide (CGRP). Once released at synaptic cleft, CGRP activates specific Gs protein-coupled receptors, highly expressed at the endplate sarcolemmal membrane (Popper & Micevych, 1989), providing a localized signal to subsynaptic nuclei that stimulates the synthesis of synaptic proteins, including nicotinic acetylcholine receptors (AChR) (Fontaine et al., 1986; New & Mudge, 1986) and acetylcholinesterase (AChE) (Choi et al., 1996; Fernandez et al., 1999; Da Costa et al., 2001). Besides, CGRP also accelerates the desensitization of AChRs by a mechanism that involves receptor subunit phosphorylation (Mulle et al., 1988). All these effects are mediated by cyclic AMP-dependent protein kinases (PKAs) accumulated at the end-plate area (Imaizumi-Scherrer et al., 1996; Barradeau et al., 2002), allowing a restricted but efficient response to transient and discrete CGRP-dependent elevation of cyclic AMP.
We have recently shown that acute but not chronic activation of CGRP receptors increases the synthesis of catalytically active AChE in rat cultured skeletal muscle cells (Da Costa et al., 2001), that was associated to a proportional increase in the intracellular cyclic AMP. The transient increase in AChE synthesis was also induced by direct activation of AC with forskolin, a natural diterpene extracted from Coleus forskolii, indicating that mechanisms downstream the AC activation might be responsible for the attenuation of cellular response.
It is generally accepted that termination of intracellular cyclic AMP signalling requires the degradation of cyclic AMP by phosphodiesterase (PDE) enzyme family (Soderling & Beavo, 2000), associated or not to desensitization of Gs protein-coupled receptors (Bunemann et al., 1999) and inhibition of AC, through Gi protein or phosphorylation-dependent pathways (Defer et al., 2000). Another mechanism that might influence the intracellular level of cyclic AMP in the skeletal muscle is its transport to the extracellular compartment. This type of control is particularly attractive because it can be triggered by increased intracellular cyclic AMP, preventing excessive accumulation of cyclic nucleotide inside the cell. However, there are no data that analyse the involvement of cyclic AMP efflux on the regulation skeletal muscle cyclic nucleotide, despite the demonstration in many tissues that the cyclic AMP egression contributes to the inactivation of cyclic AMP signalling (Dubey et al., 2000; Jackson & Dubey, 2001).
It was therefore our aim to investigate the mechanisms involved in the temporal regulation of intracellular accumulation of cyclic AMP, induced by direct activation of AC as well as by receptor-dependent mechanisms. For this purpose, we studied the effect of forskolin, β-adrenoceptor agonist isoprenaline and CGRP on the intracellular and extracellular accumulation of cyclic AMP in differentiated primary skeletal muscle cultures.
Methods
Skeletal muscle cultures
Primary skeletal muscle cultures were originally obtained from hindlimb muscles of newborn rats as described by Da Costa et al. (2001). Briefly, the myoblasts (3×105 cells ml−1) were grown on collagen-coated 35 mm dishes in 2 ml of Dulbecco's Modified Eagle Medium (DMEM, Gibco-BRL, Gaithersburg, U.S.A.) supplemented with 15% foetal calf serum (FCS) and 40 μg ml−1 gentamicin, at 37°C in humidified atmosphere of 90% air and 10% CO2. The medium was replaced on the third day and every other day by D-MEM supplemented with 10% HS and 2% FCS. All the experiments were performed on 7–8-day-old differentiated cultured skeletal muscle cells.
Effect of drugs on the intra and extracellular accumulation of cyclic AMP
Cultured skeletal muscle cells were rinsed twice with Krebs bicarbonate solution, preincubated with 1 mM 3-isobutyl-1-methylxanthine (IBMX) for 10 min and treated with CGRP (3–100 nM), isoprenaline (0.1–100 μM), forskolin (0.1–100 μM) or vehicle solutions, at 37°C. After 10 min, the medium was aspirated and the reaction stopped with 500 μl of cold Krebs solution containing 4 mM EDTA. The cyclic AMP content was determined using the cyclic AMP kit [3H]-assay system, as described by Da Costa et al. (2001). The results were expressed as pmol of cyclic AMP per culture dish or per mg of protein. Protein was measured by the method described by Bradford (1976), using BSA as standard.
To determine the temporal effect of direct or indirect stimulation of AC on the intracellular cyclic AMP, cultured skeletal muscle cells were stimulated with 10–30 μM forskolin, 30 μM isoprenaline or 10 nM CGRP for 5–60 min, in 800 μl buffer solution. In some experiments, the incubation medium was collected to determine the extracellular cyclic AMP. Then, the intracellular cyclic AMP was extracted from cells using additional 800 μl buffer solution. The total cyclic AMP was considered the sum of the intracellular and extracellular cyclic nucleotide content individually determined and was expressed as pmol of cyclic AMP per culture dish or per mg of protein. The total cyclic AMP calculated using this procedure was not significantly different from that determined in the homogenates containing both cells and incubation medium (data not shown).
In a different set of experiments, intracellular and extracellular cyclic AMP were determined in cultured skeletal muscle cells treated for 15 min with 10 μM forskolin or 30 μM isoprenaline at 37°C, in the presence or absence of 1 mM IBMX.
Finally, the effects of β-adrenoceptor and organic anion transport inhibitors were evaluated on the intracellular and extracellular accumulation of cyclic AMP, using IBMX pretreated cultures incubated with 10 nM propranolol or 100 μM probenecid, 15 min prior to incubation of cells with isoprenaline.
Cyclic AMP generation by isolated muscle cell membranes
The cyclic AMP generation by membrane fraction was determined using a modified method originally described by Carvalho et al. (1996). Cultured skeletal muscle cells were rinsed twice with ice-cold phosphate-buffered saline (PBS), pH 7.4, homogenized in 12.5 mM Tris-HCl, pH 7.4, using a Dounce tissue grinder and centrifuged twice at 20,000×g for 30 min, at 4°C. The membrane pellet containing 10 μg of protein were resuspended in 200 μl of 12.5 mM Tris- HCl, pH 7.4 (containing 1 mg ml−1 BSA, 2 mM MgCl2, 0.5 mM ATP, 1 μM GTP, 1 mM IBMX, 10 mM creatine phosphate and 25 u ml−1 creatine kinase) and incubated with 10 μM forskolin or vehicle for 5–60 min, at 37°C. The reaction was stopped by boiling the samples for 10 min. Then, the samples were vigorously mixed, centrifuged at 20,000×g for 10 min and the cyclic AMP from the supernatant was determined and expressed as pmol per mg of protein.
Cyclic AMP metabolism study
To analyse the contribution of PDEs activity on the regulation of cyclic AMP content, cultured skeletal muscle cells were rinsed twice with PBS and homogenized in ice cold Tris-HCl, pH 7.4. Immediately after homogenization, aliquots from crude cell extract, containing 10 μg of protein, were incubated for 30 min at 37°C in a total volume of 210 μl of 50 mM Tris-HCl, pH 8,0, 10.0 mM MgCl2, 150 μg ml−1 BSA (reaction mix) and 30 nM cyclic AMP, in the presence or absence of 1 mM IBMX. To precisely quantify the total amount of exogenous cyclic AMP added, in a different set of tubes the muscle cell extract was replaced by the same volume of reaction mix. The reaction was stopped by boiling the samples and the cyclic AMP content determinated using the same procedure described above.
Drugs
Adenosine 5′ triphosphate, bovine serum albumin (fraction V), creatine phosphate, creatine phosphokinase, forskolin, gentamicin, guanosine 5′ triphosphate, 3-isobutyl-1-methylxanthine, isoprenaline, probenecid, D-L-propranolol hydrochloride and rat alpha-calcitonin gene-related peptide were from Sigma Chemical CO., St. Louis U.S.A.; Dulbecco's Modified Eagle Medium, horse serum donor herd and foetal calf serum were from Gibco-BRL, Life Technologies, Grand Island, NY, U.S.A. and AMP kit [3H]-assay system was from Amershan-Pharmacia Biotech U.K. Limited, Buckinghamshire, U.K.
Statistical analysis
The results were expressed as mean values±s.e.mean of determinations obtained from at least three culture dishes. Differences between means were analysed by Student's t-test or one-way analysis of variance followed by Newman Keuls multiple comparison test. The level of significance was set at P<0.05.
Results
Effects of CGRP, isoprenaline and forskolin on the intracellular accumulation of cyclic AMP
Activation of receptors linked to Gs protein increased the generation of cyclic AMP in a concentration-dependent manner (Figure 1). Ten minutes incubation of cultured skeletal muscle cells with 3, 10, 30 and 100 nM CGRP increased the intracellular cyclic AMP content by 39% (35.7±3.0 pmol mg protein−1), 69% (43.4±2.5 pmol mg protein−1), 119% (56.3±5.8 pmol mg protein−1) and 204% (78.2±2.2 pmol mg protein−1), relative to the control values (25.7±2.6 pmol mg protein−1), respectively. Similarly, stimulation of β-adrenoceptors with 0.1, 1, 10 and 100 μM isoprenaline increased by 53% (39.5±0.9 pmol mg protein−1), 133% (60.0±3.8 pmol mg protein−1) and 213% (80.5±9.8 pmol mg protein−1) and 191% (74.8±7.1 pmol mg protein−1) the intracellular cyclic AMP, respectively. As expected, direct stimulation of AC with 0.1–100 μM forskolin also increased the intracellular cyclic AMP content by 1.8–27.4 folds, respectively (Figure 1).
Figure 1.
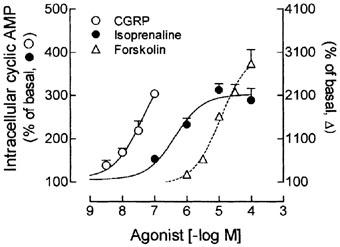
Direct and receptor-dependent stimulation of AC increases the intracellular cyclic AMP in skeletal muscle cells. Rat cultured skeletal muscle cells, pre-incubated with 1 mM IBMX, were treated with 3–100 nM CGRP, 0.1–100 μM isoprenaine, 1–100 μM forskolin or vehicle, for 10 min at 37°C. The cyclic AMP was measured using a cyclic AMP [3H]-assay kit and expressed as percentage of the basal values (100%). Each point represents the mean±s.e.mean; n=3–4.
Effects of forskolin on the accumulation of cyclic AMP in intact skeletal muscle cells and isolated muscle cell membrane
To analyse the temporal effect of forskolin on the intracellular cyclic AMP accumulation, cultured skeletal muscle cells were treated with 30 μM forskolin for 15, 30 and 60 min. The basal level of intracellular cyclic AMP, determined in the presence of 1 mM IBMX alone, was not significantly changed from 15 to 60 min (20.2±2.8 to 23.1±1.3 pmol dish−1). However, the increment of intracellular cyclic AMP induced by forskolin was inversely proportional to the period of treatment (Figure 2a). After 15 min, forskolin increased by 1958% the intracellular cyclic AMP content, whereas after 30 and 60 min treatment the increment in cyclic AMP represented 903 and 712% respectively, when compared to the values from skeletal muscle cells treated with IBMX alone.
Figure 2.
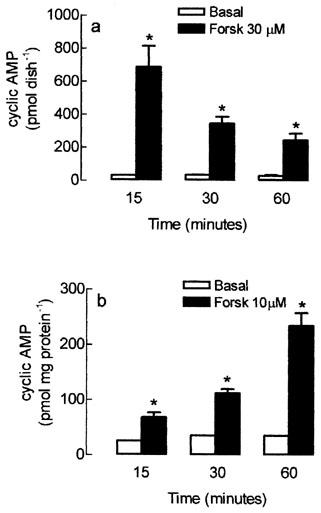
Time-course of forskolin-dependent activation of adenylyl cyclase on the intracellular cyclic AMP content in the rat cultured skeletal muscle cells (a) or in total cyclic AMP produced by membrane fraction (b). Rat cultured muscle fibres (a) or crude membrane fraction (b) were pre-incubated with 1 mM IBMX and treated with forskolin (Forsk) or vehicle (basal) for 15, 30 and 60 min at 37°C. The intracellular cyclic AMP was extracted and measured using a cyclic AMP [3H]-assay kit. Each column represents the mean±s.e.mean; n=3. *Significantly different from group treated with IBMX alone, P<0.05.
The time-dependent attenuation of forskolin effect on cyclic AMP levels was observed only in intact muscle cells. As shown in Figure 2b, when isolated cell membranes were treated with forskolin for 15–60 min, the cyclic AMP generation was linearly increased from 68.2±15.2 to 235.0±39.6 pmol mg protein−1, respectively (Figure 2b), whereas in non-stimulated membranes, the intracellular cyclic AMP content was kept constant during the whole experiment (30.2±4.2 pmol mg protein−1).
Time-course of CGRP and isoprenaline effects on the intracellular accumulation of cyclic AMP
To evaluate the time-course of cyclic AMP production induced by stimulation of Gs protein-coupled receptors, cultured skeletal muscle cells were treated with 10 nM CGRP or 30 μM isoprenaline for 5–60 min, in the presence of 1 mM IBMX. The maximal increase in the basal intracellular cyclic AMP (25.0±3.9 pmol mg protein−1) was obtained 5 min after treatment of the cultured cells with CGRP (63.0±2.2 pmol mg protein−1) or isoprenaline (309.5±30.3 pmol mg protein−1). However, 10 min after incubation of CGRP, the initial effect was attenuated by 52%, followed by the stabilization of intracellular cyclic AMP level (Figure 3a), whereas in isoprenaline treated cells, intracellular cyclic AMP content declined by 46, 69, 92 and 95% after 10, 15, 30 and 60 min, respectively (Figure 3b).
Figure 3.
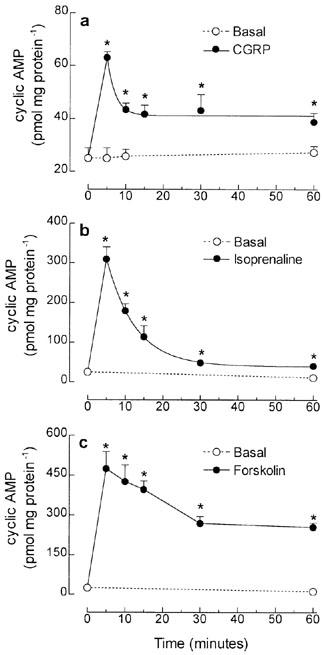
Time-course of intracellular cyclic AMP accumulation induced by direct or receptor-dependent activation of adenylyl cyclase in tissue cultured skeletal muscle cells. Rat cultured skeletal muscle cells, pre-incubated with 1 mM IBMX, were treated with 10 nM CGRP (a), 30 μM isoprenaline (b) or 10 μM forskolin (c), for 5–60 min at 37°C. The intracellular cyclic AMP was extracted and measured using a cyclic AMP [3H]-assay kit. Each point represents the mean±s.e.mean; n-3–5. *Significantly different from basal level, P<0.05.
In both cases, the reduction of cyclic AMP from peak levels fitted to single phase exponential decay (Figure 3a, b), contrasting with the slower linear decline in intracellular cyclic AMP from the 5 min peak level (405.8±10.4 pmol mg protein−1), detected in forskolin treated cells (Figure 3c).
Involvement of PDEs on the intracellular accumulation of cyclic AMP
The contribution of PDE activity on the degradation of cyclic AMP was evaluated on by incubating crude muscle cell extracts with 30 nM cyclic AMP, as substrate. After 30 min incubation, the cyclic AMP content in the control group represented only 27% of cyclic AMP added (Figure 4). On the other hand, when muscle cell extracts were pre-exposed to the PDE inhibitor IBMX, the cyclic AMP was not significantly different from the total amount of cyclic nucleotide added, indicating that 1 mM IBMX was sufficient to completely restrain the degradation of cyclic AMP, by PDEs.
Figure 4.
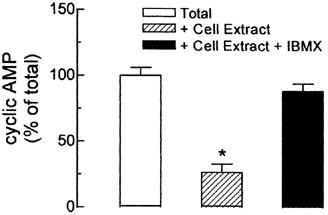
Effect of PDE inhibition on the degradation of cyclic AMP by crude muscle cell membranes. Crude extracts from cultured skeletal muscle were incubated with 30 nM cyclic AMP for 30 min, at 37°C, in the presence or absence of 1 mM IBMX. The total amount of cyclic AMP added into the tubes was considered as 100%. The cyclic AMP was measured using a cyclic AMP [3H]-assay kit. Each column represents the mean±s.e.mean; n=4. *Significantly different from total cyclic AMP, P<0.05.
Direct and receptor-dependent stimulation of AC induces the efflux of cyclic AMP from cultured skeletal muscle cells
In order to analyse the possible implication of cyclic AMP efflux on the regulation of intracellular cyclic AMP level, we quantified the extracellular cyclic AMP. As shown in Figure 5a, in IBMX treated skeletal muscle cells, the extracellular levels of cyclic AMP represented 52% of total cyclic AMP (47.5±4.2 pmol dish−1). Even in the absence of IBMX, 47% of total cyclic AMP was detected in the extracellular compartment of control skeletal muscle cells (data not shown).
Figure 5.
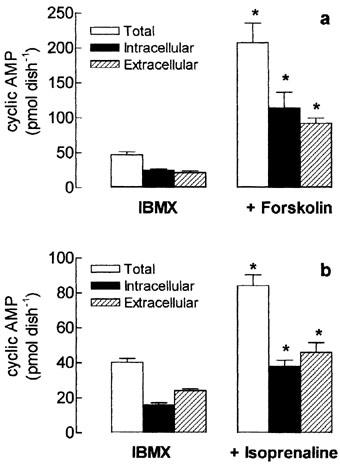
Effect of forskolin and isoprenaline on total, intracellular and extracellular cyclic AMP content in tissue-cultured skeletal muscle. Rat cultured skeletal muscle cells were treated with 10 μM forskolin (a) or 30 μM isoprenaline (b), in the presence of 1 mM IBMX, for 15 min at 37°C. The intracellular and extracellular cyclic AMP was extracted and measured using a cycle AMP [3H]-assay kit. Total cyclic AMP represents the sum of intra and extracellular cyclic nucleotide. Each column represents the mean±s.e.mean; n=4. *Significantly different from IBMX group, P<0.05.
When cultured cells were exposed to forskolin (Figure 5a) or isoprenaline (Figure 5b), the total amount of cyclic AMP increased by 338 and 78%, respectively, but the ratio of the extracellular cyclic AMP was kept constant in both forskolin and isoprenaline stimulated groups (45 and 55%, respectively) indicating that the efflux of cyclic AMP is proportional to the total cyclic AMP generated inside the cells.
The time-course effect of direct and indirect activation of AC on cyclic AMP secretion was evaluated on cultured skeletal muscle cells treated with 10 μM forskolin or 30 μM isoprenaline. Figure 6a shows that incubation of forskolin for 5–60 min increased by 203–599% the total cyclic AMP, when compared to the respective basal values. The maximum accumulation of intracellular cyclic AMP (109.7±12.0 pmol dish−1), detected after 5 min, was followed by the subsequent reduction in the accumulation cyclic AMP (Figure 6b) and the linear increase in the cyclic AMP released into the medium, that persisted for at least 60 min (Figure 6c).
Figure 6.
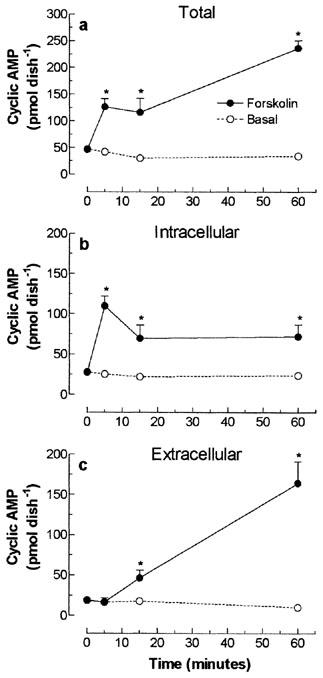
Temporal effect of direct stimulation of cultured skeletal muscle adenylyl cyclase on total (a), intracellular (b) and extracellular (c) cyclic AMP content. Rat cultured skeletal muscle cells, pre-incubated with 1 mM IBMX, were treated with 10 μM forskolin or vehicle (basal), for 5–60 min at 37°C. The cyclic AMP was extracted and measured using a cyclic AMP [3H]-assay kit. Each point represents the mean±s.e.mean; n=3. *Significantly different from IBMX, P<0.05.
Treatment of skeletal muscle cells with isoprenaline induced a transient increase in both total and intracellular cyclic AMP levels that peaked at 5 min after the onset stimulation (104.8±9.2 and 84.8±11.2 pmol dish−1, respectively, Figure 7a,b). After the initial rise, the 74 and 90% reduction of intracellular cyclic AMP levels, observed after 15–60 min, respectively, was followed by a parallel increase in the extracellular cyclic AMP (Figure 7c), indicating that the reduction in intracellular cyclic AMP was a consequence of cyclic AMP egression. However, differently from forskolin-treated groups, activation of β-adrenoceptors with isoprenaline significantly reduced total cyclic AMP after 15 min, indicating that other mechanisms, upstream AC activation, might contribute to the regulation of intracellular cyclic AMP.
Figure 7.
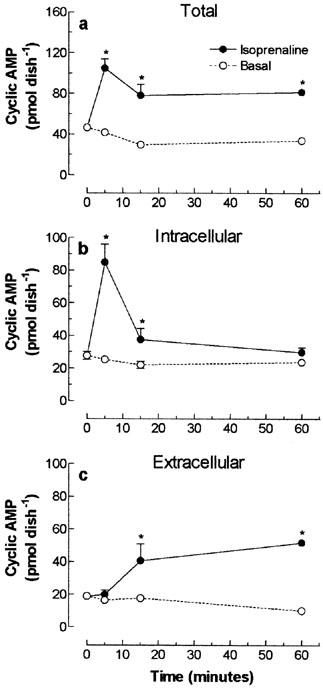
Temporal effect of β-adrenoceptor dependent stimulation of cultured skeletal muscle adenylyl cyclase on total (a), intracellular (b) and extracellular (c) cyclic AMP content. Rat cultured skeletal muscle cells, pre-incubated with 1 mM IBMX, were treated with 30 μM isoprenaline or vehicle (basal), for 5–50 min at 37°C. The cyclic AMP was extracted and determined using a cyclic AMP [3H]-assay kit. Each point represents the mean±s.e.mean; n=3. *Significantly different from IBMX, P<0.05.
Finally, the effects of the β-adrenoceptor antagonist propranolol and the organic anion transport inhibitor probenecid were evaluated on the accumulation of cyclic AMP induced by isoprenaline. As shown in Figure 8, the increase in both intracellular and extracellular cyclic AMP induced by isoprenaline was reduced by pre-treatment of cells with 10 nM propranolol. In contrast, 100 μM probenecid exclusively depressed the extracellular accumulation of cyclic AMP, demonstrating that the extrusion of cyclic nucleotide from skeletal muscle cells is mediated by a probenecid-sensitive anion transporter.
Figure 8.
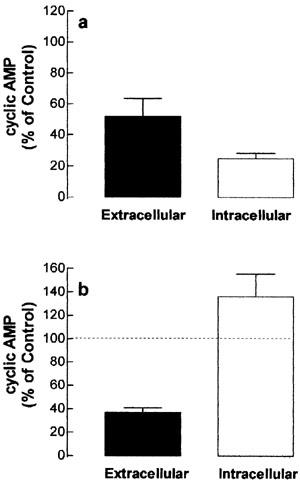
Effect of propranolol (a) and probenecid (b) on the intracellular and extracellular cyclic AMP content in tissue-cultured skeletal muscle. Rat cultured skeletal muscle cells were incubated with 10 mM propranolol, 100 μM probenecid or vehicle (control group). After 30 min, the cells were exposed to 3 μM (a) or 30 μM (b) isoprenaline for 15 min, in the presence of 1 mM IBMX, at 37°C. The intracellular and extracellular cyclic AMP was measured using a cyclic AMP [3H]-assay kit and expressed as percentage of control values. Each column represents the mean±s.e.mean; n=4. *Significantly different from control group (100%, dashed lines), P<0.05.
Discussion
The intracellular cyclic AMP level is regulated by different macromolecules, including AC, cell surface receptors, G proteins and PDE isoenzymes (Antoni, 2000). Previous studies from our laboratory showed that stimulation of skeletal muscle CGRP receptor induced a transient increase in the intracellular cyclic AMP, that was discontinued after 60 min stimulation (Da Costa et al., 2001). The depression of CGRP effect was obtained in IBMX-treated cultures, indicating that modulation of skeletal muscle cyclic AMP signalling cascade might include other mechanisms downstream AC, but unrelated to its degradation by PDEs.
In the present study, we provide strong evidences for a functional process that drives the cyclic AMP out of skeletal muscle cell and therefore restricts the intracellular accumulation of cyclic AMP. This conclusion is supported by the following findings: (1) Activation of β-adrenoceptors, CGRP receptors or adenylyl cyclase increased the intracellular cyclic AMP in a concentration dependent manner. However, this effect was temporary, peaking after 5 min onset stimulation and declining thereafter, despite the continued presence of the stimulus. (2) Oppositely, in crude muscle membrane extracts, direct activation of AC with forskolin induced a progressive and sustained increase in the cyclic AMP, indicating that the reduction of cyclic AMP from peak levels depends on the integrity of cellular membrane. (3) In control or IBMX-treated cultured cells, approximately 50% of total cyclic AMP was detected in the extracellular compartment, demonstrating a basal release of cyclic AMP in non-stimulated conditions. Furthermore, the forskolin-dependent raise in intracellular cyclic nucleotide was rapid, whereas the rate of appearance in the medium increased with time, suggesting that increment in extracellular cyclic nucleotide was secondary to the accumulation of nucleotide within the cell. (4) An increased accumulation of cyclic AMP in the extracellular space was also observed after stimulation of β-adrenoceptor. (5) More importantly, the isoprenaline-induced accumulation of extracellular cyclic AMP was substantially reduced by the organic anion transport inhibitor probenecid, with no effect on the total cyclic AMP, indicating that generation and efflux of cyclic AMP are dissociated processes. Finally, the inhibition of β-adrenoceptor with propranolol reduced both intra- and extracellular accumulation of cyclic AMP, supporting the idea that isoprenaline-induced elevation of extracellular cyclic AMP depends on the accumulation of cyclic nucleotide inside the cell.
The proposal of a cyclic AMP transport in skeletal muscle cells is agreement with several reports showing the cellular secretion of cyclic nucleotide in several tissues and organs, including rat liver (Kuster et al., 1973), superior cervical ganglia (Cramer & Lindl, 1974), heart (O'brien & Strange, 1975), cultured bovine adrenal medullary cells (Marley & Thomson, 1992), rat UMR-106 osteoblast-like cells (Ahlstrom & Lamberg-Allardt, 1999), preglomerular microvessels (Jackson & Mi, 2000) and perfused rabbit atria (Cui et al., 2000). Described first almost 40 years ago in avian erythrocytes (Davoren et al., 1963), the existence of a cyclic AMP egress is also supported by the recent identification and characterization of a probenecid-sensitive transporter for cyclic GMP and cyclic AMP, member of ATP-binding cassette (ABC) protein superfamily (van aubel et al., 2002).
On the other hand, the involvement of phosphodiesterases on the phasic increase of intracellular cyclic AMP was discarded, considering that the reduction of intracellular cyclic AMP from peak levels was obtained in IBMX-treated cells, in a concentration high enough to totally suppress the degradation of exogenous cyclic AMP added into cell extracts. The possible involvement of desensitization or feedback inhibition of AC was also eliminated, since direct activation of AC with forskolin induced a sustained generation of cyclic AMP either in whole cells (total=intra+extracellular cyclic AMP) or in isolated membrane preparation, through the 60 min analysed.
Our data also indicate that, after stimulation of Gs protein-coupled receptors, mechanisms upstream the activation of AC may contribute to the regulation of skeletal muscle cyclic AMP. Differently from the results obtained with forskolin, the CGRP- and isoprenaline-dependent increase in intracellular cyclic AMP was exponentially and rapidly reduced, reaching ∼50% of peak level in 5 min. Moreover, in isoprenaline-treated cultures, the decreased accumulation of intracellular cyclic AMP was followed by a reduction of total cyclic AMP (Figure 7), indicating a decreased generation of cyclic AMP. The quick attenuation of agonist-dependent effect may be related to desensitization of receptor protein, as predicted for β-adrenoceptor and CGRP receptors. These two members of seven transmembrane domain receptor superfamily are subject to rapid desensitization process, induced by agonist-dependent phosphorylation and subsequent uncoupling of the receptors from G proteins (Bunemann et al., 1999; Aiyar et al., 2000). According to Yu et al. (1993), incubat ion of CHO cells with isoprenaline induces the sequestration of β2-adrenoceptor with a t1/2<10 min, which may explain the reduced generation of cyclic AMP in isoprenaline treated skeletal muscle cells (Figure 7a). Then, after sustained receptor-dependent stimulation of skeletal muscle AC, receptor desensitization and cyclic AMP degradation, combined with the cyclic AMP efflux featured here, could synergistically limit the intracellular accumulation of cyclic nucleotide, preserving the cell from excessive levels of intracellular cyclic AMP.
An alternative role for the efflux of cyclic AMP may be associated to the extracellular generation of adenosine (Cunha & Sebastiao, 1991; Han et al., 1998; Hespel & Richter, 1998). In this case, extracellular cyclic AMP must be sequentially metabolized to AMP and adenosine, by ecto-PDEs and ecto-5-nucleotidases expressed outside skeletal muscle fibers (Delgado et al., 1997; Garcia-Ayllon et al., 2001).
In fact, interstitial generation of adenosine from ATP or AMP has already been described in rat cultured skeletal muscle cells (Hellsten & Frandsen, 1997) and in human skeletal muscle (Hellsten et al., 1998). The increased formation of adenosine during muscle contractions has been associated to the regulation of skeletal muscle carbohydrate metabolism and stimulation of insulin-mediated glucose transport in oxidative muscle cells (Hespel & Richter, 1998), via activation of postsynaptic adenosine receptors (Lynge & Hellsten, 2000). On the other hand, by interacting with presynaptic receptors, adenosine may contributes to the modulation of neuromuscular transmission, since it modulates the quantal release of ACh (Correia-De-sa et al., 1996).
Taken together, our results show that regulation of intracellular cyclic AMP in skeletal muscle cells is a multi-mediated process that involves not only the classical degradation of cyclic AMP and desensitization of G-protein-coupled receptors but also the efflux of cyclic AMP to the extracellular space. The physiological significance of cyclic AMP egression from muscle cells is under investigation in our lab and current results have substantiated the hypothesized extracellular degradation of cyclic AMP, qualifying the cyclic nucleotide as a potential extracellular source of adenosine, that would expand the signalling of cyclic AMP beyond its degradation.
Acknowledgments
This work was supported by research grant from Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP ( FAPESP # 01/01714-0). V. L. Costa-Jr is a PhD fellow of CAPES.
Abbreviations
- AC
adenylyl cyclase
- ATP
adenosine 5′ triphosphate
- BSA
bovine serum albumin
- CGRP
calcitonin gene-related peptide
- D-MEM
Dulbecco's Modified Eagle Medium
- FCS
foetal calf serum
- Forsk
forskolin
- GTP
guanosine 5′ triphosphate
- HBSS
Hanks' balanced salt solution
- HS
horse serum
- IBMX
3-isobutyl-1-methylxanthine
- PDE
phosphodiesterase
- PKA
cyclic AMP-dependent protein kinase
References
- AHLSTROM M., LAMBERG-ALLARDT C. Regulation of adenosine 3′,5′-cyclic monophosphate (cAMP) accumulation in UMR-106 osteoblast-like cells: role of cAMP-phosphodiesterase and cAMP efflux. Biochem. Pharmacol. 1999;58:1335–1340. doi: 10.1016/s0006-2952(99)00199-9. [DOI] [PubMed] [Google Scholar]
- AIYAR N., DISA J., DANG K., PRONIN A.N., BENOVIC J.L., NAMBI P. Involvement of G protein-coupled receptor kinase-6 in desensitization of CGRP receptors. Eur. J. Pharmacol. 2000;403:1–7. doi: 10.1016/s0014-2999(00)00419-2. [DOI] [PubMed] [Google Scholar]
- ANTONI F.A. Molecular diversity of cyclic AMP signaling. Front. Neuroendocrinol. 2000;21:103–132. doi: 10.1006/frne.1999.0193. [DOI] [PubMed] [Google Scholar]
- BARRADEAU S., IMAIZUMI-SCHERRER T., WEISS M.C., FAUST D.M. Intracellular Targeting of the Type-I[alpha] Regulatory Subunit of cAMP-Dependent Protein Kinase. Trends in Cardiovascular Medicine. 2002;12:235–241. doi: 10.1016/s1050-1738(02)00167-6. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BUNEMANN M., LEE K.B., PALS-RYLAARSDAM R., ROSEBERRY A.G., HOSEY M.M. Desensitization of G-protein-coupled receptors in the cardiovascular system. Annu. Rev. Physiol. 1999;61:169–192. doi: 10.1146/annurev.physiol.61.1.169. [DOI] [PubMed] [Google Scholar]
- CARVALHO S.D., BIANCO A.C., SILVA J.E. Effects of hypothyroidism on brown adipose tissue adenylyl cyclase activity. Endocrinology. 1996;137:5519–5529. doi: 10.1210/endo.137.12.8940379. [DOI] [PubMed] [Google Scholar]
- CHOI R.C., LEUNG P.W., DONG T.T., WAN D.C., TSIM K.W. Calcitonin generelated peptide increases the expression of acetylcholinesterase in cultured chick myotubes. Neurosci. Lett. 1996;217:165–168. [PubMed] [Google Scholar]
- CORREIA-DE-SA P., TIMOTEO M.A., RIBEIRO J.A. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J. Neurophysiol. 1996;76:3910–3919. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- CRAMER H., LINDL T. Release of cyclic AMP from rat superior cervical ganglia after stimulation of synthesis in vitro. Nature. 1974;249:380–382. doi: 10.1038/249380a0. [DOI] [PubMed] [Google Scholar]
- CUI X., LEE S.J., KIM S.Z., KIM S.H., CHO K.W. Effects of pituitary adenylate cyclase activating polypeptide27 on cyclic AMP efflux and atrial dynamics in perfused beating atria. Eur. J. Pharmacol. 2000;402:129–137. doi: 10.1016/s0014-2999(00)00514-8. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., SEBASTIAO A.M. Extracellular metabolism of adenine nucleotides and adenosine in the innervated skeletal muscle of the frog. Eur. J. Pharmacol. 1991;197:83–92. doi: 10.1016/0014-2999(91)90368-z. [DOI] [PubMed] [Google Scholar]
- DA COSTA V.L., LAPA A.J., GODINHO R.O. Short- and long-term influences of calcitonin gene-related peptide on the synthesis of acetylcholinesterase in mammalian myotubes. Br. J. Pharmacol. 2001;133:229–236. doi: 10.1038/sj.bjp.0704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVOREN P.R., SUTHERLAND E.W., MAXWELL A.M. The effect of L-epinephrine and other agents on the synthesis and release of adenosine 3′,5′-phosphate by whole pigeon erythrocytes. J. Biol. Chem. 1963;238:3009–3015. [PubMed] [Google Scholar]
- DEFER N., BEST-BELPOMME M., HANOUNE J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am. J. Physiol. Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- DELGADO J., MORO G., SABORIDO A., MEGIAS A. T-tubule membranes from chicken skeletal muscle possess an enzymic cascade for degradation of extracellular ATP. Biochem. J. 1997;327:899–907. doi: 10.1042/bj3270899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBEY R.K., GILLESPIE D.G., MI Z., JACKSON E.K. Cardiac Fibroblasts Express the cAMP-Adenosine Pathway. Hypertension. 2000;36:337–342. doi: 10.1161/01.hyp.36.3.337. [DOI] [PubMed] [Google Scholar]
- FAGHER B., LIEDHOLM H., MONTI M., MORITZ U. Thermogenesis in human skeletal muscle as measured by direct microcalorimetry and muscle contractile performance during beta-adrenoceptor blockade. Clin. Sci.(Colch.) 1986;70:435–441. doi: 10.1042/cs0700435. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ H.L., ROSS G.S., NADELHAFT I. Neurogenic calcitonin generelated peptide: a neurotrophic factor in the maintenance of acetylcholinesterase molecular forms in adult skeletal muscles. Brain Res. 1999;844:83–97. doi: 10.1016/s0006-8993(99)01891-0. [DOI] [PubMed] [Google Scholar]
- FONTAINE B., KLARSFELD A., HOKFELT T., CHANGEUX J.P. Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci. Lett. 1986;71:59–65. doi: 10.1016/0304-3940(86)90257-0. [DOI] [PubMed] [Google Scholar]
- GARCIA-AYLLON M.S., CAMPOY F.J., VIDAL C.J., MUNOZ-DELGADO E. Identification of inactive ecto-5′-nucleotidase in normal mouse muscle and its increased activity in dystrophic Lama2(dy) mice. J. Neurosci. Res. 2001;66:656–665. doi: 10.1002/jnr.10014. [DOI] [PubMed] [Google Scholar]
- HAN D.H., HANSEN P.A., NOLTE L.A., HOLLOSZY J.O. Removal of adenosine decreases the responsiveness of muscle glucose transport to insulin and contractions. Diabetes. 1998;47:1671–1675. doi: 10.2337/diabetes.47.11.1671. [DOI] [PubMed] [Google Scholar]
- HELLSTEN Y., FRANDSEN U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. J. Physiol. 1997;504:695–704. doi: 10.1111/j.1469-7793.1997.695bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLSTEN Y., MACLEAN D., RADEGRAN G., SALTIN B., BANGSBO J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- HESPEL P., RICHTER E.A. Role of adenosine in regulation of carbohydrate metabolism in contracting muscle. Adv. Exp. Med. Biol. 1998;441:97–106. doi: 10.1007/978-1-4899-1928-1_9. [DOI] [PubMed] [Google Scholar]
- IMAIZUMI-SCHERRER T., FAUST D.M., BENICHOU J.C., HELLIO R., WEISS M.C. Accumulation in fetal muscle and localization to the neuromuscular junction of cAMP-dependent protein kinase A regulatory and catalytic subunits RI alpha and C alpha. J. Cell Biol. 1996;134:1241–1254. doi: 10.1083/jcb.134.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON E.K., DUBEY R.K. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am. J. Physiol. Renal Physiol. 2001;281:F597–F612. doi: 10.1152/ajprenal.2001.281.4.F597. [DOI] [PubMed] [Google Scholar]
- JACKSON E.K., MI Z. Preglomerular Microcirculation Expresses the cAMPAdenosine Pathway. J. Pharmacol. Exp. Ther. 2000;295:23–28. [PubMed] [Google Scholar]
- KUSTER J., ZAPF J., JAKOB A. Effects of hormones on cyclic AMP release in perfused rat livers. FEBS Lett. 1973;32:73–77. doi: 10.1016/0014-5793(73)80740-9. [DOI] [PubMed] [Google Scholar]
- LYNGE J., HELLSTEN Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta Physiol. Scand. 2000;169:283–290. doi: 10.1046/j.1365-201x.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- MARLEY P.D., THOMSON K.A. Regulation of cyclic AMP metabolism in bovine adrenal medullary cells. Biochem. Pharmacol. 1992;44:2105–2110. doi: 10.1016/0006-2952(92)90335-g. [DOI] [PubMed] [Google Scholar]
- MULLE C., BENOIT P., PINSET C., ROA M., CHANGEUX J.P. Calcitonin gene-related peptide enhances the rate of desensitization of the nicotinic acetylcholine receptor in cultured mouse muscle cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5728–5732. doi: 10.1073/pnas.85.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGASE I., YOSHIDA T., SAITO M. Up-regulation of uncoupling proteins by beta-adrenergic stimulation in L6 myotubes. FEBS Lett. 2001;494:175–180. doi: 10.1016/s0014-5793(01)02341-9. [DOI] [PubMed] [Google Scholar]
- NEW H.V., MUDGE A.W. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. Nature. 1986;323:809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- O'BRIEN J.A., STRANGE R.C. The release of adenosine 3′:5′-cyclic monophosphate from the isolated perfused rat heart. Biochem. J. 1975;152:429–432. doi: 10.1042/bj1520429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPPER P., MICEVYCH P.E. Localization of calcitonin gene-related peptide and its receptors in a striated muscle. Brain Res. 1989;496:180–186. doi: 10.1016/0006-8993(89)91064-0. [DOI] [PubMed] [Google Scholar]
- ROBERTS S.J., SUMMERS R.J. Cyclic AMP accumulation in rat soleus muscle: stimulation by beta2- but not beta3-adrenoceptors. Eur. J. Pharmacol. 1998;348:53–60. doi: 10.1016/s0014-2999(98)00021-1. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BEAVO J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- VAN AUBEL R.A., SMEETS P.H., PETERS J.G., BINDELS R.J., RUSSEL F.G. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J. Am. Soc. Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- VAN DER HEIJDEN H.F., ZHAN W.Z., PRAKASH Y.S., DEKHUIJZEN P.N., SIECK G.C. Salbutamol enhances isotonic contractile properties of rat diaphragm muscle. J. Appl. Physiol. 1998;85:525–529. doi: 10.1152/jappl.1998.85.2.525. [DOI] [PubMed] [Google Scholar]
- YU S.S., LEFKOWITZ R.J., HAUSDORFF W.P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J. Biol. Chem. 1993;268:337–341. [PubMed] [Google Scholar]


