Abstract
The aim of this study was to find taurinergic compounds that do not interact with brain GABA ergic systems.
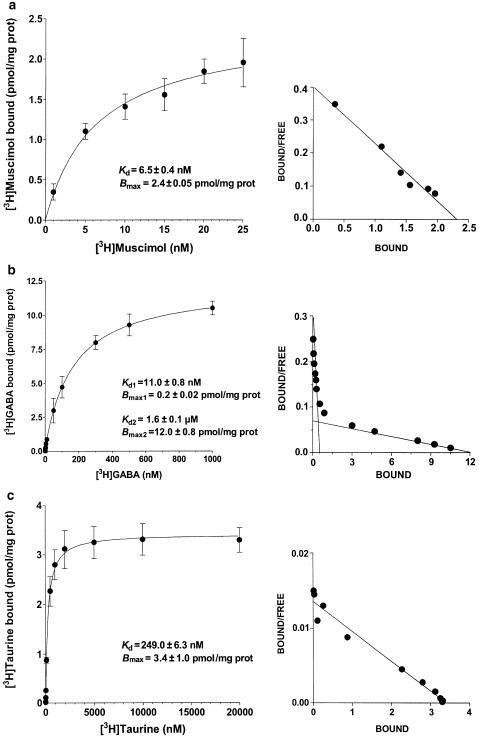
Washed synaptic membranes (SM) from whole rabbit brain were able to bind [3H]muscimol. Saturation experiments of the binding of [3H]GABA to GABAB receptors showed that SM possess two binding components; twice Triton X-100-treated SM contained 0.048 mmol endogenous taurine/kg protein and bound [3H]taurine in a saturable manner (Kd=249.0±6.3 nM and Bmax=3.4±1.0 pmol mg−1 prot).
Among the 19 structural analogues of taurine, 6-aminomethyl-3-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide (TAG), 2-aminoethylarsonic (AEA), 2-hydroxyethanesulfonic (ISE) and (±)cis-2-aminocyclohexane sulfonic acids (CAHS) displaced [3H]taurine binding (Ki=0.13, 0.13, 13.5 and 4.0 μM, respectively). These analogues did not interact with GABAA and GABAB receptors and did not affect taurine- and GABA-uptake systems and GABA-transaminase activity.
3-Aminopropanesulfonic acid (OMO), β-alanine, pyridine-3-sulfonic acid, N,N,N-trimethyltaurine (TMT), 2-(guanidino)ethanesulfonic acid (GES), ethanolamine-O-sulphate, N,N-dimethyltaurine (DMT), taurine and (±)piperidine-3-sulfonic acid (PSA) inhibited [3H]muscimol binding to GABAA receptors with different affinities (Ki=0.013, 7.9, 24.6, 47.5, 52.0, 91.0, 47.5, 118.1 and 166.3 μM, respectively). Taurine, 2-aminoethylphosphonic acid, DMT, TMT and OMO inhibited the binding of [3H]GABA to GABAB receptors with Ki's in the μM range (0.8, 3.5, 4.4, 11.3 and 5.0, respectively). GES inhibited taurine uptake (IC50=3.72 μM) and PSA GABA transaminase activity (IC50=103.0 μM).
In conclusion, AEA, TAG, ISE and CAHS fulfill the criteria for taurinergic agents.
Keywords: Taurine, GABA, taurine derivatives, GABA receptors, taurine binding site
Introduction
Taurine and GABA are recognized as major inhibitory amino acids distributed in large quantities in various areas of the central nervous system (CNS) (Barbeau et al.,, 1975; Yakimova, 1996). The role of GABA as an inhibitory neurotransmitter has been well established, whereas that of taurine is still under investigation. In the case of GABA, the structure and function of three types of GABA receptors, namely GABAA, GABAB and GABAC, have so far been identified, while the taurine receptor is not yet defined. Some very convincing evidence has outlined functional inter-relations between taurine and GABA in the brain at transporters and receptors of specific neuronal networks. From this it appears that GABA plays a major role as an inhibitory transmitter while taurine acts as a modulator of GABAergic function (Kuriyama & Hashimoto, 1998).
Since taurine binds both to GABAA and GABAB receptors (Krogsgaard-Larsen et al.,, 1980; Kontro & Oja, 1990), it has been suggested that it affects nervous functions by interacting with GABAergic systems. However, it appears that taurine possesses many actions distinct from those of GABA, such as growth promotion and enhancing the survival of neuronal cells (Hayes et al.,, 1975), modulation of calcium fluxes (Huxtable, 1989), transmitter release (Kamisaki et al.,, 1993) and regulation of osmolality in the mammalian brain (Hussy et al.,, 2001; Tuz et al.,, 2001). In particular, taurine is able to inhibit the depolarization-evoked release of aspartate and glutamate through a taurine-specific site(s) but not through GABA receptors (Kamisaki et al.,, 1993). Thus, it is therefore possible that taurine may exert its biologic activity by interacting with a specific taurine receptor. Wu et al., (1987) and Kontro & Oja (1987a),(1987b) looked for specific synaptic receptor sites for taurine and described a sodium-independent taurine binding to synaptic membranes: this could reflect binding by postsynaptic receptor sites.
In the present investigation, we studied 19 taurine analogues to see if they interact with (1) GABAA and GABAB receptors, (2) uptake systems for GABA and taurine, (3) taurine binding site(s), and (4) 4-aminobutyrate transaminase (i.e. GABA-transaminase, EC 2.6.1.19) activity. In the course of this study, the Kd and Bmax of the binding of different radioligands to GABAA, GABAB and taurine binding sites or the Km and Vmax values relative to GABA and taurine uptake systems in the rabbit brain, with the use of different preparations obtained from whole brains, have been systematically characterized. 2-Aminoethylarsonic acid (AEA), 6-aminomethyl-3-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide (TAG), 2-hydroxyethanesulfonic acid (ISE) and (±)cis-2-aminocyclohexane sulfonic acid (CAHS) fulfill the criteria of taurinergic agents by displacing [3H]taurine binding without interacting with the GABAergic system. Thus, they can represent useful probes to investigate the role of taurine in the CNS.
Methods
Materials
Taurine derivatives were selected by modifying the sulfo and the amino group of taurine, by changing the carbon chain length and by considering more restricted analogues such as the cyclic derivatives. The structures and sources of the 20 compounds used in the present study are reported in Table 1.
Table 1.
Compounds used in the present study
| Compound (abbreviation) | Structure | Origin | ||
|---|---|---|---|---|
| Group modified | 2-Aminoethanesulfonic acid, taurine (TAU) | +NH3–CH2–CH2–SO3− | Merck | |
| 6-Aminomethyl-3-methyl-4H-1,2,4-benzothiadiazine-1,1-dioxide (TAG) | 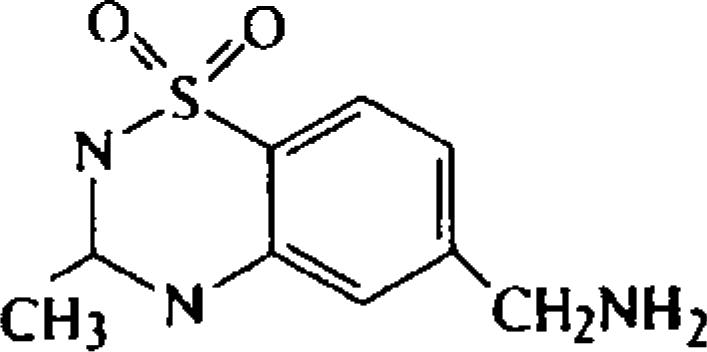 |
Gift | ||
| –SO3− | 2-Aminoethane phosphonic acid (AEP) | +NH3–CH2–CH2–PO3H− | Sigma | |
| 2-Aminoethylarsonic acid (AEA) | +NH3–CH2–CH2–AsO3H− | Synth. | ||
| β-Alanine (βALA) | +NH3–CH2–CH2–COO− | Sigma | ||
| Ethanolamine-O-sulphate (EOS) | +NH3–CH2–CH2–O–SO3− | Sigma | ||
| N-methyltaurine (MMT) | +NH2(CH3)–CH2–CH2–SO3− | Merck | ||
| N,N-dimethyltaurine (DMT) | +NH(CH3)2–CH2–CH2–SO3− | Synth. | ||
| –NH3+ | N,N,N-trimethyltaurine (TMT) | +N(CH3)3–CH2–CH2–SO3− | Synth | |
| 2-(Guanidine)ethanesulfonic acid (GES) | H2N+=C(NH)–NH–CH2–CH2–SO3− | Synth | ||
| 2-Hydroxyethanesulfonic acid (ISE) | HO–CH2–CH2–SO3− | Sigma | ||
| N-(Carbamoylmethyl)-2-aminoethane sulfonic acid (ACES) | H2N–CO–CH2–NH2+–CH2–CH2–SO3− | Sigma | ||
| Carbon chain length | 1C | Aminomethansulfonic acid (AMS) | +NH3–CH2–SO3− | Sigma |
| 3C | 3-Aminopropane sulfonic acid, homotaurine (OMO) | +NH3–CH2–CH2–CH2–SO3− | Sigma | |
| Cyclic derivatives | Piperazine-N,N′-bis(2-ethane sulfonic acid) (PIPES) | 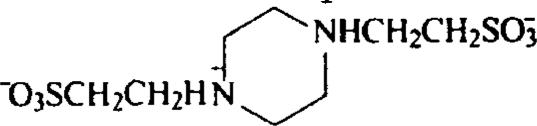 |
Sigma | |
| Pyridine-3-sulfonic acid (PYR) | 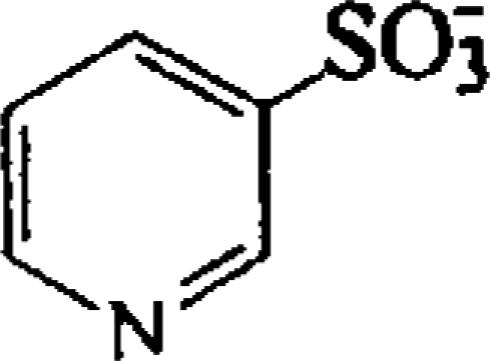 |
Aldrich | ||
| (±)Piperidine-3-sulfonic acid (PSA) | 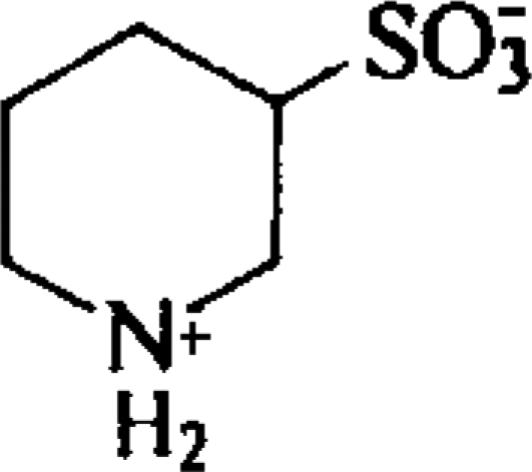 |
Synth. | ||
| 2-Aminobenzenesulfonate (ANSA) | 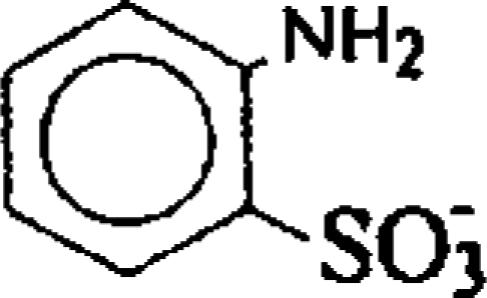 |
Aldrich | ||
| 2-Aminocyclohexane sulfonic acid | 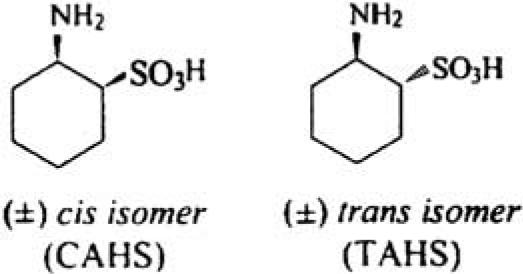 |
Synth. | ||
| Others | Glycine (GLY) | +NH3–CH2–COO− | Sigma |
[3H]muscimol (specific activity 19.1 Ci mmol−1), [3H]GABA (specific activity 40.0 Ci mmol−1) and [3H]taurine (specific activity 24.1 Ci mmol−1) were purchased from NEN™ Life Science Products, Inc. (Boston, U.S.A). All other materials were from standard local sources and of the highest grade commercially available.
Synthesis of AEA, DMT, TMT, PSA, CAHS, TAHS and GES
AEA was prepared by periodate oxidation of 2-[(2-hydroxyethyl)amino]ethylarsonic acid, itself made by treating 2-chloroethylarsonic acid with ethanolamine (Geoghegan & Dixon, 1989). (N,N-dimethyltaurine) (DMT) was prepared by methylation of taurine as described by Clarke et al., (1933). (N,N,N-trimethyltaurine) (TMT) was prepared starting from DMT, which was methylated with iodomethane in the presence of a hindered base (tributylamine) (Barnhurst, 1961). (±)Piperidine-3-sulfonic acid (PSA) was prepared by catalytic hydrogenation (nickel as a catalyst) of sodium pyridine-3-sulfonate as reported by Freifelder & Wright (1964). CAHS was prepared from 2-aminobenzenesulfonic acid by catalytic hydrogenation as reported by Egli & Eugster (1975). (±)trans-2-Aminocyclohexane sulfonic acid (TAHS) was prepared from cyclohexene by sulfur monochloride addition, followed by oxidation to 2-chlorosulfonic acid and substitution of chlorine as reported by Machetti et al., (2000). 2-(Guanidino)ethanesulfonic acid (GES) was prepared by the treatment of taurine with methyl thioisourea as reported by Fujii & Cook (1975). TAG, synthesized as described by Girard et al., (1982), was a generous gift of Dr G.G. Yarbrough from Merk Frosst Laboratories (Quebec, Canada). The purity of all compounds was evaluated above 95% by 1H NMR or high-performance liquid chromatography (HPLC).
Receptor binding assays
GABAA receptors
Rabbit-brain membranes were prepared and assayed for GABAA receptor binding by using the method described by Watabe et al., (1993) with slight modifications. The whole brain was homogenized in 10 vol. of cold 0.32 M sucrose and the homogenate was centrifuged at 2500 × g for 10 min. The supernatant was then centrifuged at 48,000 × g for 30 min and the pellet (crude synaptic membranes, CSM) was resuspended in Tris-HCl 50 mM pH 7.4 and frozen for 24 h. Thawed CSM were then resuspended in an appropriate volume of Tris-HCl containing Triton X-100 (0.05% w v−1) in order to obtain a final protein concentration of 1 mg ml−1. The mixture was incubated for 30 min at 37°C and then centrifuged at 48,000 × g for 20 min. The pellet (washed synaptic membranes, WSM) was then washed three times with buffer and frozen at –20°C before use. For saturation experiments, samples containing increasing concentrations of [3H]muscimol (1 × 10−9–2.5 × 10−8 M) were added to 100 μg of WSM (final volume=1 ml) and incubated for 30 min at 0°C. The incubation was followed by rapid filtration on Whatman GF/B glass fiber filters which were washed three times with 2 ml of cold buffer. 5 × 10−5 M GABA was used to assess nonspecific binding.
For displacement experiments, a fixed concentration of labelled muscimol (1 × 10−8 M) was incubated, as described above, with increasing concentrations of taurine or its derivatives (1 × 10−10–1 × 10−2 M).
GABAB receptors
Radioligand binding of [3H]GABA to CSM from rabbit brain was performed as described by Hill & Bowery (1981). Briefly, a crude mitochondrial fraction (P2) enriched in synaptic membranes was prepared from the whole brain of rabbit according to the method of Gray & Whittaker (1962). The P2 fraction was collected by centrifugation at 20,000 × g for 20 min and subjected to hypotonic shock by rehomogenization in water. The mixture was then recentrifuged for 20 min at 8000 × g and the supernatant was used to gently rinse the upper layer of the pellet. The combined suspension was recentrifuged for 20 min at 20,000 × g and washed twice by homogenization and centrifugation and then stored frozen at −18°C until use. Saturation and displacement studies were performed on thawed membranes resuspended in Tris-HCl (50 mM, pH 7.4)+CaCl2 (2.5 mM) (Tris-Ca) and incubated for 45 min at 20°C before centrifugation at 7000 × g for 10 min. This washing procedure was repeated three times allowing 15 min of incubation to remove endogenous GABA and other possible inhibitory substances. The final pellet (WSM) was resuspended in Tris-Ca for the assays. For saturation experiments, 900 μg of WSM (final volume=0.8 ml) was incubated for 10 min at room temperature in Tris-Ca containing a fixed concentration of [3H]GABA (2 × 10−8 M)+ increasing concentrations of unlabelled GABA (5 × 10−8–1 × 10−6 M) in the presence of 4 × 10−5 M isoguvacine to suppress any binding to GABAA sites. The incubation was terminated by a rapid filtration on Whatman GF/B glass fiber filters, which were washed three times with 2 ml of cold buffer. To study the displacement of radiolabelled GABA from GABAB receptors by taurine and its derivatives, a fixed concentration (2 × 10−8 M) of [3H]GABA+increasing concentrations (1 × 10−8–1 × 10−2 M) of the compounds were used. The assay was performed as described for saturation experiments.
Taurine binding sites
CSM, prepared as described for GABAA binding assay, were frozen, thawed after 3 days, incubated for 30 min at 37°C in Tris-HCl buffer (50 mM, pH 7.1) containing Triton X-100 (0.05%, v/v−1), centrifuged for 10 min at 48,000 × g, washed with distilled water and frozen again. This treatment was repeated not earlier than 5 days later when the binding and inhibition assays were carried out (Kontro & Oja, 1987a). In the binding experiments, 400 μg of WSM was incubated for 10 min at 4°C in 400 μl final volume of 50 mM Tris-HCl buffer (pH 7.1) with increasing concentrations of [3H]taurine (1 × 10−9–2 × 10−8 M) or with a fixed amount of [3H]taurine (2 × 10−8 M)+increasing concentrations of unlabelled taurine (1 × 10−7–2 × 10−5 M). The incubation was terminated by filtration on Whatman GF/B glass fiber filters, which were washed three times with 2 ml of cold buffer. When the effects of taurine derivatives on [3H]taurine binding were tested, 6 × 10−8 Mlabelled taurine+increasing amounts (1 × 10−9–1 × 10−3 M) of the compounds were used.
GABA and taurine uptake by crude synaptosomes
Uptake of [3H]GABA by crude synaptosomal preparation from rabbit whole brain was assayed by the method described by Suzdak et al., (1992) with some modifications. The crude synaptosomes were prepared by homogenizing the brain in 20 vol of ice-cold 0.32 Msucrose. The homogenate was then centrifuged for 20 min (17,000 × g at 4°C) and the resulting pellet resuspended in 20 vol of Krebs buffer pH 7.1. 300 μg-aliquots of synaptosomal suspension (final volume 0.8 ml) were incubated for 10 min at 30°C with [3H]GABA alone (1 × 10−9–1 × 10−8) or with [3H]GABA (1 × 10−8 M) +increasing concentrations of unlabelled GABA (5 × 10−8–1 × 10−4 M) to determine Km and Vmax. Synaptosomes were then recovered by rapid filtration through Whatman GF/B glass fiber filters under vacuum and the filters were washed three times with 2 ml cold buffer. To study the inhibition by taurine and its derivatives on carrier-mediated [3H]GABA uptake, 1 × 10−8 M [3H]GABA+increasing concentrations of the compounds (1 × 10−8–1 × 10−3 M) were used. Noncarrier-mediated uptake was determined in the presence of nipecotic acid (5 × 10−4 M) and was subtracted from total binding to give carrier-mediated [3H]GABA uptake.
To study the uptake of labelled taurine by rabbit whole-brain crude synaptosomes, the method of Hruska et al., (1978) was used. Briefly, the brain was homogenized in 9 vol of 0.32 M sucrose and then centrifuged at 1000 × g for 10 min. The supernatant was centrifuged again at 17,500 × g for 20 min. The pellet was resuspended in the original volume of sucrose. Samples of the tissue suspension (crude synaptosomal fraction) were used in subsequent experiments within 6 h. To determine Km and Vmax of [3H]taurine uptake, 300 μg of crude synaptosomes was resuspended in Krebs' phosphate-buffered medium (final volume=1 ml) containing a constant amount of [3H]taurine (2 × 10−8 M) and increasing concentrations of non-labelled taurine (2 × 10−8–1 × 10−2 M). The tubes were incubated for 10 min at 4°C. The reaction was stopped by rapid filtration on Whatman GF/B glass fiber filters, which were washed three times with 2 ml of cold buffer. The inhibition of labelled taurine uptake by taurine derivatives was studied by using a fixed amount of [3H]taurine (2 × 10−8 M)+increasing concentrations of the derivatives (1 × 10−9–1 × 10−2 M). Noncarrier-mediated uptake was determined in the presence of GES (1 × 10−3 M) and was subtracted from total binding to give carrier-mediated [3H]taurine uptake.
GABA-transaminase activity
Rabbit brain was homogenized in 2 vol of cold distilled water as described by Qume & Fowler (1997) and stored at –20°C before use. The inhibition by either taurine or its derivatives of GABA-transaminase activity was studied by using the fluorimetric method of Salvador & Albers (1959).
Determination of taurine and GABA levels in WSM
WSM (3.2 mg) were solubilized with Triton X-100 (0.1%v.v−1) and the resulting solution was analysed for taurine and GABA concentrations by reversed-phase HPLC with o-phthalaldehyde precolumn derivatization (Bianchi et al.,, 1999).
Data analysis
All the experiments were performed in triplicate or quadruplicate. Saturable binding constants relative to the binding of [3H]muscimol to GABAA receptors were determined by computer-assisted nonlinear regression analysis (GraphPad Prism 3.02, GraphPad Software Inc., San Diego, CA, USA), assuming binding to either a single site or a population of binding sites or two noninteracting populations and by choosing the best fit. When a mixture of hot/cold radioligand was used (i.e. for GABAB binding and [3H]taurine binding assays and for GABA and taurine uptake), the analysis of data was performed by using LIGAND Program (Munson & Rodbard, 1980; Unnerstall, 1990).
When studying the ability of taurine derivatives to inhibit the binding of [3H]muscimol, [3H]GABA or [3H]taurine to GABAA, GABAB and taurine receptors, respectively, the half-maximal concentration (i.e. the IC50 value) for inhibition was obtained by plotting specific binding (percentage of control) vs the inhibitor concentration (M) and fitted with a nonlinear (sigmoidal) analysis (GraphPad Prism 3.02, GraphPad Software Inc., San Diego, CA, U.S.A). The Ki was calculated according to the method of Cheng & Prusoff (1973).
Results
Kd and Bmax of the binding of different radioligands to GABAA and GABAB receptors and taurine binding sites
Whole rabbit-brain WSM were able to bind [3H]muscimol with affinity constants (Kd) of 6.5±0.4 nM and binding capacities (Bmax) of 2.4±0.5 pmol mg−1 prot., that is quite close to those reported for cow, pig, rat and mouse brain (Figure 1a). Saturation experiments of the binding of [3H]GABA to GABAB receptors showed that rabbit-brain synaptic membranes possess two binding components with Kd values of 11.0±0.8 nM (Kd1) and 1.6±0.1 μM (Kd2), respectively, and Bmax values of 0.2±0.02 and 12.0±0.8 pmol mg−1 prot., respectively, (Figure 1b). Moreover, twice Triton X-100-treated SM were shown to bind in a saturable manner [3H]taurine with a Kd of 249.0±6.3 nM and a Bmax of 3.4±1.0 pmol mg−1 prot. (Figure 1c). In this preparation, taurine amounted to 0.048±0.026 mmol kg−1 prot. (n=3), while GABA was not detectable.
Figure 1.
Binding of [3H]muscimol (a), [3H]GABA (b) and [3H]taurine (c) to GABAA, GABAB and taurine binding sites, respectively, present in different preparations of synaptic membranes of rabbit brain. Depicted saturation curves (left) and Scatchard plots (right) were obtained from a representative experiment performed in triplicate. Kd and Bmax values are reported as mean±s.e.m. and are obtained by at least five different experiments. For further details, see Methods section.
Km and Vmax values of GABA and taurine uptake systems
The kinetics of [3H]GABA uptake by crude synaptosomes of the rabbit whole brain indicated both a high- and a low-affinity uptake system, with relative Km values differing by one order of magnitude (Km1=1.6±0.2 and Km2=18.9±1.5 μM), while corresponding Vmax values were quite similar (Vmax1=100.6±10.0 and Vmax2=452.3±13.5 pmol mg−1 prot. min−1). By contrast, [3H]taurine uptake exhibited a single high affinity system (Km=15.5±3.1 μM, Vmax=146.0±18.9 pmol mg−1 prot. min−1).
Displacement of specific [3H]muscimol and [3H]GABA binding to GABAA and GABAB receptors
Taurine and its analogues have been examined for their ability to displace [3H]muscimol from GABAA receptors. As reported in Table 2, OMO was the most potent displacer with a Ki at nanomolar range, that is much lower than that of GABA. Among the other derivatives, β-ALA and PYR inhibited [3H]muscimol binding with Ki values in the μM range, while TMT, GES, EOS, DMT and taurine were very weak inhibitors. CAHS, MMT, AEP, AEA, ISE, TAG, AMS, ACES, PIPES, ANSA, GLY and TAHS did not affect [3H]muscimol binding. To displace specific [3H]GABA binding from GABAB receptors, taurine was, after GABA, the most potent agent, followed by DMT and β-ALA with Ki values of the same order of magnitude (μM). Also, AEP, TMT and OMO displaced the binding of [3H]GABA, even though less effectively than the previous compounds. The other taurine derivatives were almost without effect.
Table 2.
Comparative Ki values (μM) for displacement of specific [3H]muscimol, [3H]GABA and [3H]taurine from GABAA, GABAB receptors and taurine binding site (TAU) of rabbit brain by GABA, taurine and some taurine analogues
| Compound | GABAA | GABAB | TAU |
|---|---|---|---|
| GABA | 0.05±0.006 | 0.014±0.001 | 2.38±0.2 |
| TAU | 118.1±8.4 | 0.8±0.06 | 0.23±0.01 |
| GES | 52.0±3.6 | N.A. | N.T. |
| OMO | 0.013±0.001 | 5.0±0.3 | N.I. |
| PSA | 166.3±9.8 | N.A. | N.T. |
| CAHS | N.A. | N.A. | 4.0±0.3 |
| MMT | N.A. | N.A. | N.I. |
| AEP | N.A. | 3.5±0.2 | N.T. |
| AEA | N.A. | N.A. | 0.13±0.01 |
| EOS | 69.1±4.4 | N.A. | N.T. |
| PYR | 24.6±1.7 | N.A. | N.T. |
| ISE | N.A. | N.A. | 13.5±0.6 |
| DMT | 91.0±13.6 | 1.6±0.1 | N.I. |
| TMT | 47.5±3.4 | 4.0±0.3 | N.I. |
| TAG | N.A. | N.A. | 0.13±0.01 |
| AMS | N.A. | N.A. | N.T. |
| β-ALA | 7.9±0.6 | 1.6±0.1 | N.I. |
| ACES | N.A. | N.A. | N.I. |
| PIPES | N.A. | N.A. | N.I. |
| ANSA | N.A. | N.A. | N.T. |
| GLY | N.A. | N.A. | N.T. |
| TAHS | N.A. | N.A. | N.I. |
N.A. (not active)=IC50>500 μM. N.I.: no inhibition at 1 × 10−3 M. Ki values are reported as mean±s.e.m. of data from three or more experiments for each analogue (concentration range: 0.1 nM–1000 μM). The concentration of [3H]muscimol and [3H]GABA were 10 and 20 nM, respectively, while that of [3H]taurine was 60 nM. For further details, see Methods section.
Displacement of specific [3H]taurine binding from taurine binding sites
As reported in Table 2, AEA, TAG, taurine, CAHS, GABA and ISE inhibited [3H]taurine binding with corresponding Ki values ranging between 0.13±0.01 (AEA) and 13.5±0.6 μM (ISE).
Inhibition of [3H]taurine and [3H]GABA uptake by crude synaptosomes
The effects of taurine derivatives on both taurine and GABA uptake systems were investigated. Only GES, the reported taurine uptake inhibitor in rat tissues (Huxtable 1989), was shown to inhibit [3H]taurine uptake by rabbit-brain synaptosomes with an IC50 of 3.7±0.2 μM, while none of the other compounds affected it (data not shown). Similarly, none of the compounds tested revealed any effect on [3H]GABA uptake by rabbit-brain synaptosomes. On the contrary, nipecotic acid, an inhibitor of [3H]GABA uptake in many mammalian species including the rabbit, was able to inhibit with an IC50 of 7.8±0.1 μM.
Effects on GABA-transaminase activity
As reported in Table 3, among the compounds studied, PSA was the most potent inhibitor of rabbit-brain GABA-transaminase activity with an IC50 of 103.0±3.9 μM. Vigabatrin, the GABA-transaminase inhibitor, in clinical use, is effective towards the enzymes of many species (Suzdak et al.,, 1992), including the rabbit (IC50=287.1±17.3 μM). AEP, ANSA and AMS were weak inhibitors (IC50 in the mM range), while the other derivatives were inactive at 1000 μM concentration.
Table 3.
Comparative IC50 values (μM) of taurine and some of its derivatives toward GABA transaminase activity in rabbit brain crude homogenate
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| PSA | 103.5±3.9 | EOS | N.I. |
| AEP | 2494.5±74.8 | PYR | N.I. |
| ANSA | 2023.0±172.7 | ISE | N.I. |
| AMS | 3572.7±588.4 | DMT | N.I. |
| TAU | N.I. | TMT | N.I. |
| GES | N.I. | TAG | N.I. |
| OMO | N.I. | β-ALA | N.I. |
| PIP | N.I. | ACES | N.I. |
| CAHS | N.I. | PIPES | N.I. |
| MMT | N.I. | GLY | N.I. |
| AEA | N.I. | TAHS | N.I. |
N.I.=no inhibition of the enzyme at 1000 μM concentration. The concentration of GABA used in the assay was 12.5 mM. IC50 values are reported as mean±s.e.m. from three or more experiments for each analogue (concentration range: 1 nM–1 mM). In the same assay, IC50 value of vigabatrin was 287.1±17.3 μM.
Discussion
In the present study, the binding characteristics of GABAA and GABAB receptors, GABA and taurine uptake and GABA-transaminase activity in different rabbit-brain preparations were investigated. Data for rat, mouse, pig and cow brain are already present in the literature. Equilibrium binding experiments on GABAA and GABAB receptors carried out in the present study have shown that the relative Kd and Bmax found in rabbit-brain preparations are very similar to those reported for rat, mouse and pig (Krogsgaard-Larsen et al.,, 1980; Bowery et al.,, 1985; Yang & Olsen, 1987; Bureau & Olsen, 1991; Facklam & Bowery, 1993). Also findings on the uptake of [3H]GABA, by rabbit whole-brain crude synaptosomes, match those reported for rat brain by some authors (Cupello et al.,, 1993), but are at variance with those obtained by Debler & Lajtha (1987), which indicated only one high-affinity system in rat brain cortex synaptosomes. Furthermore, nipecotic acid was shown here to inhibit GABA uptake with an IC50 value close to those already described for rat and mouse (IC50=3.6 and 2.79 μM, respectively) (Suzdak et al.,, 1992; Mantz et al.,, 1994).
Meiners et al., (1980) found two saturable processes for the uptake of [3H]taurine by different brain preparations and cell types. These were characterized by Km values in the μM (high affinity) and mM (low affinity) ranges, respectively. Nevertheless, we found only a single type of taurine uptake with a Km of 15.5±3.1μM.
It seems that the ability or affinity of taurine to displace [3H]muscimol binding from GABAA receptors and [3H]GABA from GABAB receptors varies according to the animal species considered. Thus taurine affinity for GABAA receptors differs significantly among cow (IC50=2.2 μM) (Krogsgaard-Larsen et al.,, 1981), rat (IC50=50.0 μM) (Bureau & Oslen, 1991) and rabbit (IC50=300.0 μM, this study). Hill & Bowery (1981) found a taurine IC50 value relative to its ability to displace [3H]baclofen from GABAB receptors greater than 800 μM, while Kontro & Oja (1990) showed that it inhibits the binding of [3H]GABA to GABAB receptors of mouse brain with an IC50 value of 5.12 μM. This is very close to that reported in the present study with rabbit. Among taurine derivatives, OMO was shown to interact with GABAA receptors with a very high affinity (IC50 and Ki values in the nM range) similarly to what was reported by Robinson et al., (1989) for rat (IC50=0.049 μM) and by Krogsgaard-Larsen et al., (1981) for cow (IC50=0.080 μM). Furthermore, OMO was able to displace the binding of [3H]GABA from GABAB receptors with an IC50 value of 14.2 μM (Ki=5.0±0.3 μM), close to that reported by Hill & Bowery for rat (1981). Among the other compounds, only the interactions of β-ALA and PSA have been tested as to their interaction with rat brain GABAA and GABAB receptors and GABAA receptors, respectively. The resulting data (Krogsgaard-Larsen et al.,, 1980,1981; Hill & Bowery, 1981) are very close to those found in the present study for rabbit-brain GABA receptors.
The one taurine analogue effective in inhibiting GABA transaminase was PSA (Table 3), which is similar in effectiveness to vigabatrin, used clinically to inhibit this enzyme. Furthermore, EOS, an inhibitor of rat and mouse brain GABA-transaminase activity (Phillips & Fowler, 1982; Qume & Flower, 1997) was found to be ineffective towards the rabbit enzyme.
It has been suggested that taurine acts as a neurotransmitter in the CNS (Kontro & Oja, 1987a). This implies its interaction with a postsynaptic receptor. The demonstration of a sodium-independent binding of taurine to brain synaptic membranes has been controversial. Some authors failed to find a specific taurine binding to synaptic membranes from different brain regions of rat (Lopez-Colomè & Pasantes-Morales, 1981) and calf (Lähdesmäki et al.,, 1977). They, however, did not use detergents for preparing membranes. Detergents, such as Triton X-100, make the receptor sites more accessible, both by removing extrajunctional plasma membranes and by diminishing nonspecific binding as Enna & Snyder (1977) showed for GABA. Moreover, detergents aid in washing out inhibiting compounds, such as endogenous taurine and GABA, and by breaking resealed membrane pouches. Kontro & Oja (1987a), in fact, could detect a saturable taurine binding in synaptic membranes isolated from mouse brain only after Triton X-100 treatment; the binding was maximal in those preparations treated twice with this detergent. Although this treatment completely removed endogenous GABA from the mouse synaptic membranes, removal of endogenous taurine was incomplete (Kontro & Oja, 1987a); we similarly found incomplete removal of endogenous taurine from the rabbit-brain synaptic membranes. In rabbit WSM, the persistence of small amounts of taurine, albeit much lower than that found in mouse WSM (i.e. 1.6±0.2 mmol kg−1 prot.), could lead to underestimate the number of taurine binding sites. Kontro & Oja (1987a) found a sodium-independent, taurine binding with outlines of positive cooperativity, suggesting two or more taurine molecules interacting at a single binding site and an apparent Kd value of 270 nM and a Bmax of 5.8 pmol mg−1 prot. By contrast, Kontro & Oja (1983) had found that, in the rat, the binding fitted a one-site curve with a Kd value of 539 nM and a Bmax of 4.6 pmol mg−1 prot. Furthermore, GABA was able to displace taurine binding with an IC50 value of 3.0 μM (Kontro & Oja, 1987a). Other authors, however, have demonstrated the existence of a GABA-insensitive taurine binding in pig brain (Wu et al.,, 1992) with a Kd value of 92 nM and a Bmax of 6.0 pmol mg−1 prot. The membrane preparation used in the latter study, however, required a procedure involving cycles of thawing and freezing and extensive washing of the membranes with buffers, but not the use of detergents. Our procedure, based on the use of Triton X-100, is less time-consuming and gave similar results. The Kd value for [3H]taurine binding found in the present study is very similar to that found by Kontro & Oja in the mouse (1987a), and both values are one order of magnitude higher than those reported for several labelled GABAA agonists for GABAA receptors (Krogsgaard-Larsen et al.,, 1980,1981; Yang & Olsen, 1987; Bureau & Olsen, 1991). This may be because of some unremovable endogenous binding inhibitors such as taurine itself. The same happened for the earlier GABA binding studies, wherein endogenous GABA hampered the characterization of GABAA receptors until detergents were introduced into the procedure (Napias et al.,, 1980). In the present study, GABA was shown to displace bound [3H]taurine, thus giving rise to the possibility that GABA receptors were responsible for taurine binding. However, the fact that OMO, DMT, TMT and β-ALA displaced both [3H]muscimol and [3H]GABA binding to GABAA and GABAB receptors, respectively, but did not affect [3H]taurine binding, allows one to exclude that GABA receptors were responsible for [3H]taurine binding. Finally, since CAHS, AEA, ISE and TAG did not interact either with GABAergic receptors or with the taurine uptake system but were able to displace [3H]taurine binding from rabbit WSM, although with different affinities – AEA and TAG exhibiting an affinity two orders of magnitude higher than ISE and CAHS – they fulfill the criteria for taurinergic agents and represent useful probes to investigate the role of taurine in the CNS.
This paper is dedicated to Alberto Giotti Professor Emeritus of Pharmacology, University of Florence.
Acknowledgments
This work was supported by contributions of Ministero degli Affari Esteri (Rome, Italy) under law 212/92 and by MURST, Cofin. '98, EU COST Action D13 (WG Number 13-0011-00) and Fondazione Monte dei Paschi di Siena. The authors thank Dr Yarbrough for supplying TAG, Prof. L. Della Corte and Dr A. Colivicchi for taurine and GABA determinations, and Dr R. Matucci for helpful discussions about LIGAND. The technical assistance of Dr A. Benocci is gratefully acknowledged.
Abbreviations
- ACES
N-(carbamoylmethyl)-2-aminoethanesulfonic acid
- AEA
2-aminoethylarsonic acid
- AEP
2-aminoethanephosphonic acid
- β-ALA
β-alanine
- AMS
aminomethanesulfonic acid
- ANSA
2-aminobenzenesulfonate
- CAHS
(±)cis-2-aminocyclohexane sulfonic acid
- DMT
N,N-dimethyltaurine
- EOS
ethanolamine O-sulfate
- GES
2-(guanidino)ethanesulfonic acid
- GLY
glycine
- ISE
2-hydroxyethanesulfonic acid
- MMT
N-methyltaurine
- OMO
3-aminopropanesulfonic acid, homotaurine
- PIPES
piperazine-N,N′-bis-(2-ethanesulfonic acid)
- PSA
(±)piperidine-3-sulfonic acid
- PYR
pyridine-3-sulfonic acid
- TAG
6-aminomethyl-3-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide
- TAHS
(±)trans-2-aminocyclohexane sulfonic acid
- TAU
2-aminoethanesulfonic acid, taurine
- TMT
N,N,N-trimethyltaurine.
References
- BARBEAU A., INOUE N., TSUKADA Y., BUTTERWORTH R.F. The neuropharmacology of taurine. Life Sci. 1975;17:669–677. doi: 10.1016/0024-3205(75)90520-2. [DOI] [PubMed] [Google Scholar]
- BARNHURST J.D. Dipolar ions related to taurine. J. Org. Chem. 1961;26:4520–4522. [Google Scholar]
- BIANCHI L., DELLA CORTE L., TIPTON K.F. Simultaneous determination of basal and evoked output levels of aspartate, glutamate, taurine and 4-aminobutyric acid during microdialysis and from superfused brain slices. J. Chromatogr. B: Biomed. Sci. Appl. 1999;723:47–59. doi: 10.1016/s0378-4347(98)00519-2. [DOI] [PubMed] [Google Scholar]
- BOWERY N.G., HILL D.R., HUDSON A.L. [3H](−)Baclofen: an improved ligand for GABAB sites. Neuropharmacology. 1985;24:207–210. doi: 10.1016/0028-3908(85)90075-9. [DOI] [PubMed] [Google Scholar]
- BUREAU M.H., OLSEN R.W. Taurine acts on a subclass of GABAA receptors in mammalian brain in vitro. Eur. J. Pharmacol. 1991;207:9–16. doi: 10.1016/s0922-4106(05)80031-8. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibitory constant (Ki) and the concentrations of an inhibitor which causes 50 per cent inhibition (IC50) of an enzyme reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CLARKE H.T., GILLESPIE H.B., WEISSHAUS S.Z. The action of formaldehyde on amines and amino acids. J. Am. Chem. Soc. 1933;55:4571–4578. [Google Scholar]
- CUPELLO A., GASPARETTO B., MAINARDI P., VIGNOLO L., ROBELLO M. Effect of protein kinase C activators on the uptake of GABA by rat brain synaptosomes. J. Neurosci. 1993;69:131–136. doi: 10.3109/00207459309003323. [DOI] [PubMed] [Google Scholar]
- DEBLER E.A., LAJTHA A. High-affinity transport of gamma-aminobutyric acid, glycine, taurine, L-aspartic acid and L-glutamic acid in synaptosomal (P2) tissue: a kinetic and substrate specificity analysis. J. Neurochem. 1987;48:1851–1856. doi: 10.1111/j.1471-4159.1987.tb05747.x. [DOI] [PubMed] [Google Scholar]
- EGLI VON R., EUGSTER C.D. Über die selektive katalytische Reduktion von substituierten Anilinen zu substituierten Cyclohexylaminen und von Benzol-bwz. Phenyl-alkan –sulfonsäuren zu Cyclohexan-bzw. Cyclohexylalkan-sulfonsäuren. Helv. Chim. Acta. 1975;58:2321–2346. [Google Scholar]
- ENNA S.J., SNYDER S.H. Influences ions, enzymes, and detergents on gamma-aminobutyric acid-receptor binding in synaptic membranes of rat brain. Mol. Pharmacol. 1977;13:442–453. [PubMed] [Google Scholar]
- FACKLAM M., BOWERY N.G. Solubilization and characterization of GABAB receptor binding sites from porcine brain synaptic membranes. Br. J. Pharmacol. 1993;110:1291–1296. doi: 10.1111/j.1476-5381.1993.tb13958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIFELDER M., WRIGHT H.B. The hydrogenation of some pyridinesulphonic and pyridinealkanesulphonic acids. J. Med. Chem. 1964;7:664–665. doi: 10.1021/jm00335a024. [DOI] [PubMed] [Google Scholar]
- FUJII A., COOK E.S. Probiotics. Antistaphylococcal and antifibrinolytic activities of omega-amino- and omega-guanidinoalkanisulfonic acids. J. Med. Chem. 1975;18:502–505. doi: 10.1021/jm00239a013. [DOI] [PubMed] [Google Scholar]
- GEOGHEGAN K.F., DIXON H.B. Synthesis of 2-aminoethylarsonic acid. A new synthesis of primary amines. Biochem. J. 1989;15:295–296. doi: 10.1042/bj2600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD Y., ATKINSON J.G., HAUBRICH D.R., WILLIAMS M., YARBROUGH G.G. Aminomethyl-1,2,4-benzothiadiazines as potential analogues of gamma-aminobutyric acid. Unexpected discovery of a taurine antagonist. J. Med. Chem. 1982;25:113–116. doi: 10.1021/jm00344a004. [DOI] [PubMed] [Google Scholar]
- GRAY E.G., WHITTAKER V.P. The isolation of nerve endings from brain: an electron microscopy study of cell fragments derived by homogenisation and centrifugation. J. Anat. 1962;96:79–87. [PMC free article] [PubMed] [Google Scholar]
- HAYES K.C., CAREY R.E., SCHMIDT S.Y. Retinal degeneration associated with taurine deficiency in the cat. Science. 1975;188:949–951. doi: 10.1126/science.1138364. [DOI] [PubMed] [Google Scholar]
- HILL D.R., BOWERY N.G. 3H-Baclofen and [3H]-GABA bind to bicuculline-insensitive GABAB sites in rat brain. Nature. 1981;290:149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- HRUSKA R.E., PADJEN A., BRESSLER R., YAMAMURA H.I. Taurine: sodium-dependent, high-affinity transport into rat brain synaptosomes. Mol. Pharmacol. 1978;14:77–85. [PubMed] [Google Scholar]
- HUSSY N., BRES V., ROCHETTE M., DUVOID A., ALONSO G., DAYANITHI G., MOOS F.C. Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. J. Neurosci. 2001;21:7110–7116. doi: 10.1523/JNEUROSCI.21-18-07110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXTABLE R.J. Taurine in the central nervous system and the mammalian actions of taurine. Prog. Neurobiol. 1989;32:471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- KAMISAKI Y., MAEDA K., ISHIMURA M., OMURA H., ITOH T. Effects of taurine on depolarization-evoked release of amino acids from rat cortical synaptosomes. Brain. Res. 1993;627:181–185. doi: 10.1016/0006-8993(93)90318-h. [DOI] [PubMed] [Google Scholar]
- KONTRO P., OJA S.S. Sodium-independent taurine binding to brain synaptic membranes. Cell Mol. Neurobiol. 1983;3:183–187. doi: 10.1007/BF00735281. [DOI] [PubMed] [Google Scholar]
- KONTRO P., OJA S.S. Co-operativity in sodium-independent taurine binding to brain membranes in the mouse. Neuroscience. 1987a;23:567–570. doi: 10.1016/0306-4522(87)90076-5. [DOI] [PubMed] [Google Scholar]
- KONTRO P., OJA S.S. Taurine and GABA binding in mouse brain: effects of freezing, washing and Triton X-100 treatment on membranes. Int. J. Neurosci. 1987b;32:881–889. doi: 10.3109/00207458709043343. [DOI] [PubMed] [Google Scholar]
- KONTRO P., OJA S.S. Interactions of taurine with GABAB binding sites in mouse brain. Neuropharmacology. 1990;29:243–247. doi: 10.1016/0028-3908(90)90008-f. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P., FALCH E., SCHOUSBOE A., CURTIS D.R., LODGE D. Piperidine-4-sulphonic acid, a new specific GABA agonist. J. Neurochem. 1980;34:756–759. doi: 10.1111/j.1471-4159.1980.tb11211.x. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P., SNOWMAN A., LUMMIS S.C., OLSEN R.W. Characterization of the binding of the GABA agonist [3H]piperidine-4-sulphonic acid to bovine brain synaptic membranes. J. Neurochem. 1981;37:401–409. doi: 10.1111/j.1471-4159.1981.tb00469.x. [DOI] [PubMed] [Google Scholar]
- KURIYAMA K., HASHIMOTO T. Interrelationship between taurine and GABA. Adv. Exp. Med. Biol. 1998;442:329–337. doi: 10.1007/978-1-4899-0117-0_41. [DOI] [PubMed] [Google Scholar]
- LÄHDESMÄKI P., KUMPULAINEN E., RAASAKKA O., KYRKI P. Interaction of taurine, GABA and glutamic acid with synaptic membranes. J. Neurochem. 1977;29:819–826. doi: 10.1111/j.1471-4159.1977.tb10724.x. [DOI] [PubMed] [Google Scholar]
- LOPEZ-COLOMÉ A.M., PASANTES-MORALES H. Taurine binding to membranes from rat brain regions. J. Neurosci. Res. 1981;6:475–485. doi: 10.1002/jnr.490060405. [DOI] [PubMed] [Google Scholar]
- MACHETTI F., CACCIARINI M., CATRAMBONE F., CORDERO F.M., ROMOLI S., DE SARLO F. Synthesis of taurine analogues. Part 1: 2-aminosulfonic acids from alkene–sulfur monochloride adducts. J. Chem. Res. 2000. pp. 120–121.
- MANTZ J., LAUDENBACH V., LECHARNY J.B., HENZEL D., DESMONTS J.M. Riluzole, a novel antiglutamate, blocks GABA uptake by striatal synaptosomes. Eur. J. Pharmacol. 1994;257:R7–R8. doi: 10.1016/0014-2999(94)90716-1. [DOI] [PubMed] [Google Scholar]
- MEINERS B.A., SPETH R.C., BRESOLIN N., HUXTABLE R.J., YAMAMAURA H.I. Sodium-dependent, high affinity taurine transport into rat brain synaptosomes. Fed. Proc. 1980;39:2695–2700. [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- NAMIMA M., OKAMOTO K., SAKAI Y. Modulatory action of taurine on the release of GABA in cerebellar slices of the guinea pig. J. Neurochem. 1983;40:1–9. doi: 10.1111/j.1471-4159.1983.tb12645.x. [DOI] [PubMed] [Google Scholar]
- NAPIAS C., BERGMAN M.O., VAN NESS P.C., GREENLEE D.V., OLSEN R.W. GABA binding in mammalian brain: inhibition by endogenous GABA. Life. Sci. 1980;27:1001–1011. doi: 10.1016/0024-3205(80)90111-3. [DOI] [PubMed] [Google Scholar]
- PHILLIPS N.I., FOWLER L.J. The effects of sodium valproate on gamma-aminobutyrate metabolism and behaviour in naive and ethanolamine-O-sulphate pretreated rats and mice. Biochem. Pharmacol. 1982;31:2257–2261. doi: 10.1016/0006-2952(82)90111-3. [DOI] [PubMed] [Google Scholar]
- QUME M., FLOWER L.J. Effect of chronic treatment with the GABA-transaminase inhibitors γ-vinylGABA and ethanolamine-O-sulphate on the in vitro release from rat hippocampus. Br. J. Pharmacol. 1997;122:539–545. doi: 10.1038/sj.bjp.0701383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON T.N., CROSS A.J., GREEN A.R., TOCZEK J.M., BOAR B.R. Effects of the putative antagonists phaclofen and delta-aminovaleric acid on GABAB receptor biochemistry. Br. J. Pharmacol. 1989;98:833–840. doi: 10.1111/j.1476-5381.1989.tb14612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVADOR R.A., ALBERS R.W. The distribution of glutamic-acid-γ-aminobutyric transaminase in the nervous system of the rhesus monkey. J. Biol. Chem. 1959;234:922–925. [PubMed] [Google Scholar]
- SUZDAK P.D., FREDERIKSEN K., ANDERSEN K.E., SØRENSEN P.O., KNUTSEN L.J.S., NIELSEN E.B. NNC-711 a novel potent and selective γ-aminobutyric acid uptake inhibitor: pharmacological characterization. Eur. J. Pharmacol. 1992;223:189–198. doi: 10.1016/0014-2999(92)90804-d. [DOI] [PubMed] [Google Scholar]
- TUZ K., ORDAZ B., VAA L., QUESADA O., PASANTES-MORALES H. Isovolumetric regulation mechanisms in cultured cerebellar granule neurons. J. Neurochem. 2001;79:143–151. doi: 10.1046/j.1471-4159.2001.00546.x. [DOI] [PubMed] [Google Scholar]
- UNNERSTALL J.R.Computer analysis of binding data Methods in Neurotransmitter Receptor Analysis 1990New York: Raven Press; 37–68.eds. Yamamura, H.I., Enna, S.J. & Kuhar, M.J. pp [Google Scholar]
- WATABE S., YAMAGUCHI H., ASHIDA S. DM-9384 a new cognition-enhancing agent, increases the turnover of components of the GABAergic system in the rat cerebral cortex. Eur. J. Pharmacol. 1993;238:303–309. doi: 10.1016/0014-2999(93)90861-b. [DOI] [PubMed] [Google Scholar]
- WU J.Y., LIAO C.C., LIN C.J., LEE Y.H., HO J.Y., TSAI W.H. Taurine receptor in the mammalian brain. Prog. Clin. Biol. Res. 1987;351:147–156. [PubMed] [Google Scholar]
- WU J.Y., TANG X.W., TSAI W.H. Taurine receptor: kinetic analysis and pharmacological studies. Adv. Exp. Med. Biol. 1992;315:263–268. doi: 10.1007/978-1-4615-3436-5_31. [DOI] [PubMed] [Google Scholar]
- YAKIMOVA K., SANN H., SCHMID H.A., PIERAU F.K. Effects of GABA agonists and antagonists on temperature-sensitive neurones in the rat hypothalamus. J. Physiol. 1996;494:217–230. doi: 10.1113/jphysiol.1996.sp021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG J.S., OLSEN R.W. Gamma-Aminobutyric acid receptor binding in fresh mouse brain membranes at 22°C: ligand-induced changes in affinity. Mol. Pharmacol. 1987;32:266–277. [PubMed] [Google Scholar]