Abstract
The subtypes of α1- and α2-adrenoceptor mediating contractions of vas deferens have been examined in wild-type and α2A/D-adrenoceptor knockout mice.
Maximum contractions to noradrenaline but not phenylephrine were significantly greater in vas from wild-type. The α1A-adrenoceptor antagonist RS100329 (5-methyl-3-[3-[4-[2-(2,2,2,-trifluoroethoxy)phenyl]-1-piperazinyl]propyl]-2,4-(1H)-pyrimidinedione) (10 nM) significantly shifted the potency of noradrenaline. The α2D-adrenoceptor antagonist BRL 44408 (2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole) significantly reduced the maximum contraction to noradrenaline in wild-type but not in knockout.
Following prazosin (0.1 μM), a component of the contraction to noradrenaline in wild-type but not in knockout was sensitive to the α2-adrenoceptor antagonist yohimbine.
Nifedipine (10 μM) or suramin (100 μM) reduced the contraction to 10 Hz stimulation for 4 s to an early peak and small maintained response. The peak was abolished by the α1-adrenoceptor antagonist prazosin.
RS100329 or prazosin inhibited 10 Hz stimulation evoked contractions with a U-shaped concentration-response curve: inhibiting responses up to 0.1 μM, with a reversal of inhibition above this concentration. In the presence of suramin or nifedipine, the reversal of inhibition by high concentrations of prazosin was reduced.
The α1D-adrenoceptor selective antagonist BMY7378 (8-[2-(4-(2- methoxyphenyl)piperazin-1-yl)ethyl]-8-azaspiro[4,5]decane-7,9-dione) produced inhibition of 10 Hz evoked contractions only in high concentrations.
In conclusion, contractions to nerve stimulation in mouse vas deferens involve largely α1A-adrenoceptors and purinoceptors. α1-Adrenoceptor antagonists in high concentrations increase the purinergic response presumably by blocking prejunctional α2-adrenoceptor-mediated inhibition. In the presence of nifedipine, responses are predominantly α1-adrenoceptor mediated. Contractions to exogenous noradrenaline involved both α1A- and α2A/D-adrenoceptors in wild-type mice.
Keywords: Rat vas deferens, α1-adrenoceptors, α2A/D-adrenoceptors, prazosin, BMY 7378, BRL 44408, RS 100329
Introduction
Molecular cloning techniques revealed initially four subtypes of α1-adrenoceptor: α1a, α1b, α1c, α1d (Cotecchia et al., 1988; Schwinn et al., 1990; Lomasney et al., 1991; Perez et al., 1991; see Heible et al., 1995), but these clones have now been renamed to match the functional receptors: α1A (formerly α1c), α1B (formerly α1b), and α1D (formerly α1a/α1d). (Heible et al., 1995). α2-Adrenoceptors have been subdivided into three subtypes, α2A-, α2B- and α2C-adrenoceptors, based on ligand binding and molecular cloning studies (Lorenz et al., 1990; Bylund, 1992), and the rat α2D-adrenoceptor is a species orthologue of the human α2A-adrenoceptor (Lanier et al., 1991; Harrison et al., 1991a).
In both rat and mouse vas deferens, noradrenaline and ATP are cotransmitters mediating contractions (Allcorn et al., 1986), and acetylcholine may also be involved, at least in the mouse (Kaschube & Zetler, 1988). Both neurotransmitters are important in mediation of the function of vas deferens; hence, in mice lacking P2X1 receptors, contractions of vas deferens to nerve stimulation are reduced to 60% of normal, and male fertility is reduced by 90% (Mulryan et al., 2000). Contractions of rat vas deferens to exogenous noradrenaline or adrenaline are mediated predominantly by α1A -adrenoceptors (Han et al., 1987; Hanft & Gross, 1989; Aboud et al., 1993; Honner & Docherty, 1999), but contractions to endogenous noradrenaline involve α1D-adrenoceptors (Honner & Docherty, 1999). The receptors mediating contractions in mouse vas deferens have been less well characterised.
The advent of knockout technology has led many researchers to turn to the mouse, and in this study we wish to establish the basic parameters of the mouse vas deferens by comparing wild-type and α2A/D-adrenoceptor knockout mice. In mouse vas deferens, in addition to the predominant α1-adrenoceptors, there is also evidence for the involvement of α2-adrenoceptors in contraction to exogenous agonists (Bultmann et al., 1991): the present study examines the possible involvement of α2A/D-adrenoceptors in contraction and the relative importance of these in relation to α1-adrenoceptors. In addition, we have characterised the adrenoceptors involved in contractions to exogenous and endogenous noradrenaline.
Methods
Male C57 Black mice (18–30 g) (both wild-type and homozygous α2A/D-adrenoceptor knockouts) were obtained as breeding pairs from Jackson Laboratories. A small number of experiments were carried out employing Wistar rats (250–300 g) obtained from Trinity College, Dublin. Vas deferens was employed as described below.
Mouse vas deferens
Mice were killed by CO2 overdose, and whole vas deferens was obtained. Tissues were attached to myograph transducers under 0.5 × g tension in organ baths at 37°C in Krebs–Henseleit solution of the following composition (mM): NaCl 119; NaHCO3 25; D-glucose 11.1; KCl 4.7; CaCl2 2.5; KH2 PO4 1.2; MgSO4 1.0. In addition, cocaine (3 μM) was present throughout in studies of exogenous agonist-evoked responses.
Responses to exogenous agonists
In the first series of experiments investigating contractions produced by exogenous agonists, tissues were equilibrated for 30 min and then contracted with the α1-adrenoceptor agonists noradrenaline or phenylephrine (10 μM). Bathing fluid was then changed every 15 min for the next hour. Following 30-min exposure to antagonist (BRL 44408: 8-[2-(4-(2-methoxyphenyl) piperazin-1-yl)ethyl]-8-azaspiro[4,5]decane-7,9-dione) or vehicle, a single agonist concentration–response curve was obtained per tissue. Tissues were contracted with noradrenaline or phenylephrine cumulatively in 1.0 log unit increments beginning with 100 nM.
In the second series of experiments, combinations of antagonists were employed. Following exposure to noradrenaline (10 μM), bathing fluid was changed every 15 min for the next hour. Following 30-min exposure to prazosin (0.1 μM) and vehicle, prazosin (0.1 μM) and yohimbine (1 μM), RS 100329 (5-methyl-3-[3-[4-[2-(2,2,2,-trifluoroethoxy) phenyl]-1-piperazinyl]propyl]-2,4-(1H)-pyrimidinedione: 10 nM) and vehicle, or double vehicle, tissues were contracted with noradrenaline cumulatively in 1.0 log unit increments beginning with 100 nM.
Nerve-mediated responses
In experiments investigating the ability of competitive antagonists to inhibit the isometric contraction to electrical stimulation in mouse vas deferens (in the presence or absence of nifedipine, 10 μM or suramin, 10–100 μM), tissues were placed between platinum electrodes and stimulated every 5 min with trains of 40 pulses at 10 Hz (0.5 ms pulses, supramaximal pulses) to produce isometric contractions. Antagonists or vehicle were added cumulatively in 0.5 or 1 log unit increments at 5-min intervals. An isometric contractile response was obtained following 5-min exposure to each antagonist concentration, or following exposure to the vehicle. In vehicle experiments, stimulation-evoked contractions decreased by approximately 10% over 30 min. Antagonist responses were not corrected for these small changes.
Rat vas deferens
Rats were killed by CO2 overdose, and vas deferens was bisected into prostatic and epididymal portions. Tissues were attached to myograph transducers under 1 × g tension. Experiments were carried out exactly as described for mouse vas deferens.
Radioligand binding studies
Preparation of rat vas deferens membranes was carried out largely as described for rat kidney membranes in Connaughton & Docherty (1990). The resultant pellets were used immediately or stored at −20°C for later use. Pellets were reconstituted in10 volumes of incubation buffer. The homogenate was filtered through two layers of nylon 43 T-mesh gauze to remove the connective tissue.
In saturation experiments, aliquots of membrane suspension were incubated with various concentrations of [3H]prazosin (specific activity: 70–87 Ci mmol−1, New England Nuclear, U.S.A.) at 25°C (0.25–20 nM; incubation buffer: Tris-HCl 50 mM, EDTA 5 mM, pH 7.4 at 25°C). In competition studies [3H]prazosin (2 nM) was incubated with competing ligands in concentrations from 0.1 nM to 1 mM in 0.5 log unit increments for 30 min. Nonspecific binding was determined in the presence of phentolamine (10 μM). Specific binding of [3H]prazosin was 60–80% of total binding at the concentration used in displacement experiments. Assays were terminated by washing with ice-cold incubation buffer, followed by rapid vacuum filtration through Whatman GF/C filters, using a Brandel Cell Harvester. Radioactivity retained on filters was determined by liquid scintillation spectroscopy.
The inhibition constant (Ki) for displacement of radiolabelled ligand binding was determined from the formula:
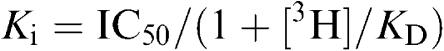 |
where IC50 is the concentration of competing ligand that inhibits radioligand specific binding by 50%, KD is the dissociation constant for the radioligand prazosin (0.33± 0.12 nM, n=6), and 3H is the concentration of tritiated prazosin (2 nM).
Drugs
8-[2-(4-(2-methoxyphenyl) piperazin-1-yl)ethyl]-8-azaspiro[4,5]decane-7,9-dione (BMY 7378); Research Biochemicals); 2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3- dihydro-1-methyl- 1H-isoindole maleate (BRL 44408); Tocris, U.K.); cocaine hydrochloride (Sigma, U.K.); nifedipine (Sigma, U.K.); noradrenaline bitartrate (Sigma, U.K.); phenylephrine hydrochloride (Sigma, U.K.); prazosin hydrochloride (gift; Pfizer, Sandwich, U.K.); (5-methyl-3-[3-[4-[2-(2,2,2,-trifluoroethoxy)phenyl]-1-piperazinyl]propyl]-2,4- (1H)-pyrimidinedione (RS 100329); Tocris); suramin, sodium salt (Sigma); yohimbine hydrochloride (Sigma).
Drugs were dissolved in distilled water except for nifedipine which was dissolved in 100% ethanol.
Statistics
Values are mean±s.e.m. from n experiments. Agonist pD2 (−log EC50) or antagonist pIC30 (concentration producing 30% inhibition of the stimulation-evoked contraction) values were obtained using GraphPad Prism, taking only responses in the steep part of the concentration–response curve. Antagonist pKB values were obtained from the equation KB=[B]/(DR-1), where [B] is the antagonist concentration and DR is the agonist dose ratio produced by the antagonist. Since not all experiments were carried out with paired vehicle, effects of antagonist were compared with vehicle mean. Agonist pD2 values and maximum responses after vehicle or test antagonist were compared, and effects of antagonist on nerve stimulation-evoked responses were compared with the effects of vehicle, by Student's t-test for paired or unpaired data where appropriate, and by analysis of variance. Statistical and graphical analysis was carried out using Instat for Macintosh and GraphPad Prism for PC.
Results
Mouse vas deferens: responses to exogenous agonists
In mouse vas deferens in the presence of cocaine (3 μM), phenylephrine produced concentration-dependent contractions with pD2 values (−log M) of 4.64±0.21 and 4.53± 0.22, and a maximum contraction of 0.17±0.03 × g, n=8, and 0.14±0.05 × g, n=8, in wild-type and α2A/D-adrenoceptor knockout mice, respectively. There were no significant differences between wild-type and knockout in responses to phenylephrine.
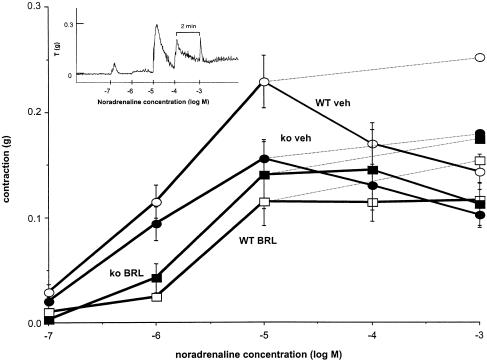
In the first series of experiments, noradrenaline produced concentration-dependent contractions with pD2 values (−log M) of 6.00±0.08, n=21 and 5.98±0.10, n=19, in vas from wild-type and α2A/D-adrenoceptor knockout mice, respectively (no significant difference) (see Figure 1). The inset (Figure 1) shows a typical concentration–response to noradrenaline: responses consisted of transient spikes and slower maintained contractions. However, the maximum contraction to noradrenaline was significantly greater in tissues from wild-type than in knockout (Figure 1). The α2D-adrenoceptor antagonist BRL 44408 (1 μM) significantly reduced the maximum contraction to noradrenaline in vas from wild-type but not from knockout (Figure 1). BRL 44408 (1 μM) significantly reduced the pD2 of noradrenaline to 5.18±0.15, n=11 and 5.54±0.11, n=7, in wild-type and knockout, respectively (P<0.001 and P<0.05 from their respective control experiments). In knockout animals, a pKB for BRL 44408 was calculated as 6.33±0.09 (n=7).
Figure 1.
Concentration–response curves to noradrenaline following vehicle (veh) or BRL 44408 (1 μM) (BRL) in vas deferens from wild-type (WT) and α2A/D-adrenoceptor knockout mice (ko) in the first series of experiments. The dashed lines indicate the maximum tensions obtained (sum of maximum tensions obtained in each individual tissue irrespective of concentration at which they were obtained). Vertical bars indicate s.e.m. from seven to 22 animals. Maximum responses to noradrenaline following vehicle in wild-type were significantly greater than in knockout. BRL 44408 significantly decreased the maximum contraction to noradrenaline only in wild-type. The inset shows a typical tension recording in a tissue from a wild-type animal, in which a maximum response is obtained to 10 μM, with subsequent decline (responses were qualitatively similar in tissues from knockout); tension and time scales have an arbitrary zero point.
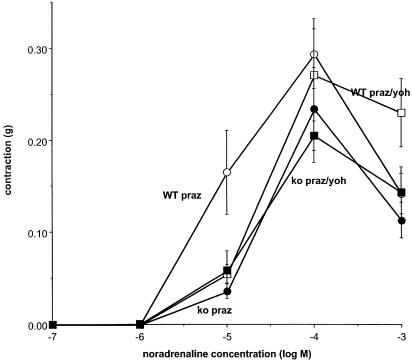
In the second series of experiments to identify a component of the contraction to noradrenaline resistant to prazosin, concentration–response curves to noradrenaline were constructed following exposure to prazosin (0.1 μM) and vehicle or prazosin (0.1 μM) and yohimbine (1 μM) (Figure 2 and Table 1). As in the first series of experiments, the maximum response to noradrenaline tended to be larger in tissues from wild-type animals, and this reached significance in the vehicle and prazosin/yohimbine groups (Table 1). The response to noradrenaline (10 μM) in the presence of prazosin (0.1 μM) was significantly larger in tissues from wild-type than knockout, and this difference was absent following prazosin (0.1 μM) and yohimbine (1 μM) (Figure 2). Hence, yohimbine revealed an α2-adrenoceptor-mediated component of the contraction to noradrenaline in wild-type which was absent in knockout.
Figure 2.
Concentration–response curves to noradrenaline following prazosin (0.1 μM) (praz) or prazosin (0.1 μM)/yohimbine (1 μM) (praz/yoh) in vas deferens from wild-type (WT) and α2A/D-adrenoceptor knockout mice (ko) in the second series of experiments. Vertical bars indicate s.e.m. from eight to 11 animals. The response to noradrenaline (10 μM) following prazosin in wild-type was significantly larger than in the other three groups.
Table 1.
Effects of antagonists on potency (pD2, −log M) and maximum contraction (g) of noradrenaline in vas deferens from wild-type and α2A/D-adrenoceptor knockout mice
| Wild-type | Knockout | |||
|---|---|---|---|---|
| pD2 | max (g) | pD2 | max (g) | |
| Vehicle | 6.06±0.23 | 0.318±0.038 | 6.13±0.22 | 0.160±0.020* |
| Prazosin (0.1 μM) | 5.00±0.17** | 0.320±0.035 | 4.73±0.11** | 0.236±0.045 |
| Praz/yoh | 4.59±0.13** | 0.329±0.039 | 4.74±0.09** | 0.216±0.032* |
| RS 100329 (10 nM) | 5.30±0.20** | 0.110±0.029** | — | — |
Values are mean±s.e.m., from six to 11 (wild-type) or seven to eight (knockout) experiments.Abbreviations: praz/yoh, prazosin (0.1 μM)/yohimbine (1 μM).
P<0.05 versus equivalent wild-type
P<0.05 versus equivalent vehicle.
The α1A-adrenoceptor selective antagonist RS 100329 (10 nM) significantly shifted the potency of noradrenaline in vas deferens from wild-type mice from 6.06±0.23 (n=8) to 5.30±0.20 (n=6), with a resultant pKB of 8.65±0.2 (Table 1). However, RS 100329 acted noncompetitively in that it significantly reduced the maximum response to noradrenaline (Table 1). The non-selective α1-adrenoceptor antagonist prazosin (0.1 μM) significantly shifted the potency of noradrenaline in vas deferens from wild-type mice to 5.00±0.17 (n=11) (see Figure 2 and Table 1), with a resultant pKB of 8.03±0.16. RS 100329 was significantly more potent than prazosin.
Mouse vas deferens: nerve-mediated responses
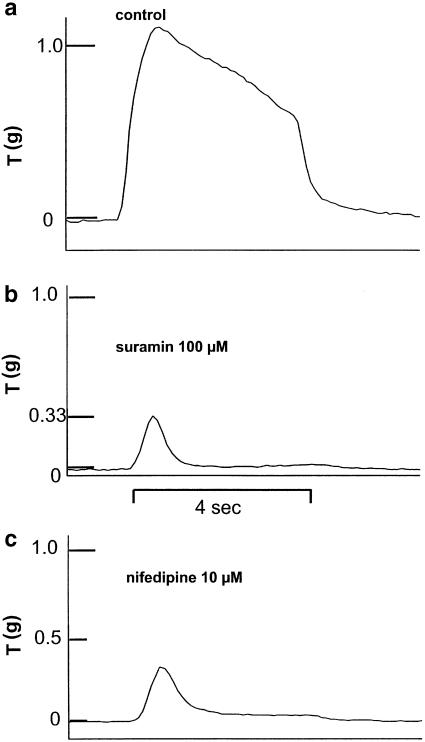
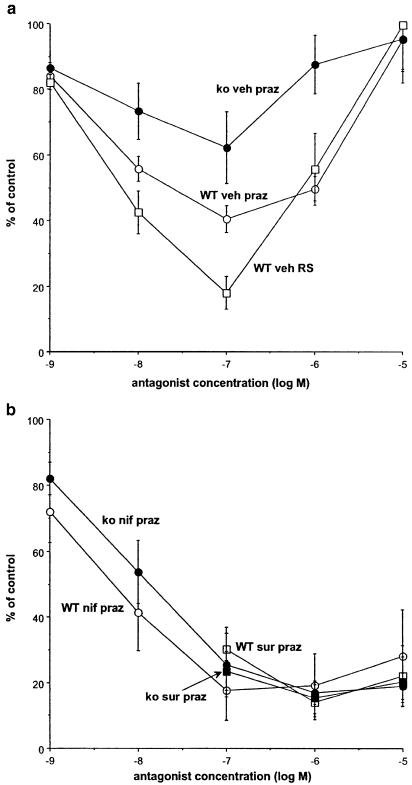
The isometric contractile response to 10 Hz for 4 s was 0.53±0.03 × g, n=80, and 0.72±0.04 × g, n=62, in vas from wild-type and α2A/D-adrenoceptor knockout mice, respectively (significantly different: P<0.001). A typical response to 10 Hz stimulation in a tissue from a knockout mouse is shown in Figure 4a. Prazosin at low concentrations produced a concentration-dependent inhibition of stimulation-evoked contractions, but the effects of prazosin (0.1 μM) were significantly greater in tissues from wild-type (Figure 3a). Prazosin at high concentrations restored contractions towards baseline in both wild-type and knockout. In the presence of the α2-adrenoceptor antagonist yohimbine (10 μM), prazosin inhibited stimulation-evoked contractions in tissues from wild-type mice but there was a reduced tendency for contractions to recover at high prazosin concentrations (e.g. with prazosin 10 μM: 59.8±5.2% of control, n=4). Hence, α2-adrenoceptor blockade with yohimbine prevented high concentrations of prazosin from restoring the contraction towards baseline. The α1A-adrenoceptor selective antagonist RS 100329 behaved like prazosin in that it inhibited nerve stimulation-evoked contractions in concentrations up to 0.1 μM, but the contraction returned to the control level by a concentration of 10 μM (Figure 3a). When antagonist potency was expressed as a pIC30, RS 100329 was significantly more potent than prazosin (8.78±0.10, n=5 and 8.33±0.19, n=11, respectively).
Figure 4.
Typical recordings of contractions evoked by 10-Hz stimulation for 4 s in vas from knockout mice: (a) control, (b) following suramin (100 μM) or (c) following nifedipine (10 μM) (qualitatively similar in tissues from wild-type). Tension and time scales have arbitrary zero points.
Figure 3.
Effects of prazosin (praz) or RS 100329 (RS) on isometric contractions evoked by field stimulation at 10 Hz for 4 s in vas deferens from wild-type (WT) and knockout mice (ko): (a) in the presence of vehicle (veh), or (b) in the presence of suramin (100 μM) (sur) or nifedipine (10 μM) (nif). Vertical bars indicate s.e.m. from three to 11 animals.
The purinergic antagonist suramin (100 μM) and the calcium entry blocker nifedipine (10 μM) had essentially the same action to reduce markedly the contraction to 10 Hz stimulation in tissues from both wild-type and knockout (Figure 4). The response was reduced to an early peak, which was prazosin sensitive (see below), and a small maintained contraction. The contractile response was reduced to 0.16±0.04 × g, n=8, and 0.23±0.05 × g, n=3, in the presence of suramin and to 0.12±0.02 × g, n=24, and 0.22±0.04 × g, n=24, in the presence of nifedipine, in tissues from wild-type and knockout mice, respectively. Responses in the presence of nifedipine, but not in the presence of suramin, were significantly larger in tissues from knockout mice (P<0.05).
In tissues from wild-type and knockout, suramin (100 μM) greatly reduced the tendency to potentiate by high concentrations of prazosin (Figure 3b). Likewise, in the presence of nifedipine (10 μM), the tendency to potentiate by high concentrations of prazosin was greatly reduced (Figure 3b).
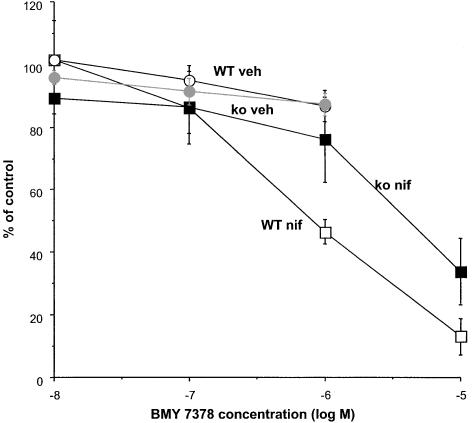
The α1D-adrenoceptor antagonist BMY 7378 showed low potency in mouse vas deferens, consistent with α1A-adrenoceptor potency (Figure 5). Concentrations of BMY 7378 up to 1 μM in the absence of nifedipine did not significantly inhibit stimulation-evoked contractions as compared with the effects of vehicle (Figure 5), and in the presence of nifedipine (10 μM), significant inhibition (P<0.05) only occurred at a concentration of 1 μM in tissues from wild-type, and 10 μM in tissues from knockout (Figure 5).
Figure 5.
Effects of BMY 7378 on isometric contractions evoked by field stimulation at 10 Hz for 4 s in the absence (veh) or presence of nifedipine (10 μM) (nif) in vas deferens from wild-type (WT) or knockout mice (ko). Vertical bars indicate s.e.m. from six animals.
Rat vas deferens: nerve-mediated responses
The isometric contractile response to 10 Hz for 4 s was 3.38±0. 25 × g, n=6 and 1.72±0.40 × g, n=6, in epididymal and prostatic portions of rat vas deferens, respectively. Prazosin (0.1 μM) significantly inhibited contractions in both epididymal and prostatic portions, but higher concentrations restored contractions towards baseline only in prostatic portions (data not shown). The combination of prazosin (0.1 μM) and suramin (100 μM) significantly reduced the contraction to 10 Hz stimulation in both epididymal and prostatic portions to 53.1±10.4%, n=6, and 53.4±3.9%, n=5, respectively (15 min after addition). However, unlike in the mouse, the contraction was well maintained over the 4-s stimulation period (data not shown).
Ligand-binding studies
Table 2 shows the affinities of antagonists used in this study for α1A-adrenoceptor ligand binding sites in rat vas deferens. RS 100329 was at least as potent as prazosin, whereas BRL 44408 was 1000 times less potent (Table 2).
Table 2.
Affinity of antagonists for α1A-adrenoceptor ligand binding sites in rat vas deferens
| Antagonist | |
| BRL 44408 | 6.59±0.18 |
| Prazosin | 9.43±0.02 |
| RS 100329 | 9.61±0.19 |
| Values are pKi (−log M)±s.e.m. from at least three experiments. |
Discussion
In this study, we have looked at α1- and α2-adrenoceptors mediating contractions of the mouse vas deferens to exogenous agonists (noradrenaline and phenylephrine) and to endogenous noradrenaline. These will be discussed separately, beginning with contractions to exogenous agonists. First, we must consider the possible locations of α-adrenoceptors in the vas deferens. Prejunctional α2A/D-adrenoceptors on nerve terminals mediate an inhibitory modulation of both noradrenaline and ATP release in vas deferens of wild-type mice, and these receptors are replaced by α2C-adrenoceptors in α2A/D-adrenoceptor knockout mice (Altman et al., 1999; Rajamani et al., 2001). Postjunctional receptors may be of the α1 or α2-adrenoceptor subtypes, and there may be differences between the receptor subtypes involved in contractions to the endogenous neurotransmitter (receptors in the junctional region) and exogenous agonist (receptors in the junctional region and elsewhere).
Responses to exogenous agonists
In mouse whole vas deferens, the agonists noradrenaline and phenylephrine produced concentration-dependent contractions consisting of phasic contractions with a smaller tonic component. Phasic responses were not sustained so that the magnitude of the contraction tended to fall at higher agonist concentrations. Experiments were carried out in the presence of the noradrenaline re-uptake blocker cocaine to prevent loss of administered noradrenaline but also to allow greater agonist access to postjunctional receptors in the neuroeffector junction region. The maximum response to noradrenaline and the response to noradrenaline (10 μM) were significantly greater in wild-type than knockout, but this was not the case with phenylephrine. The α2D-adrenoceptor antagonist BRL 44408 (1 μM) significantly reduced the maximum response to noradrenaline only in wild-type. This concentration of BRL 44408 should produce a marked blockade of α2A/D-adrenoceptors but have little effect on α2B or α2C (Ho et al., 1998). Admittedly, a small effect of BRL 44408 at α1-adrenoceptors was also demonstrated, consistent with the results of our α1-adrenoceptor ligand-binding studies, given that the potency of noradrenaline was reduced in vas deferens from both wild-type and knockout mice. In vas deferens from knockout mice, a pKB of 6.33 was obtained for BRL 44408, and this represents its potency at α1-adrenoceptors in the absence of α2A/D-adrenoceptors. In further studies to identify a component of the contraction to noradrenaline resistant to prazosin, concentration–response curves to noradrenaline were constructed following exposure to prazosin (0.1 μM) and vehicle or prazosin (0.1 μM) and yohimbine (1 μM). Yohimbine revealed an α2-adrenoceptor mediated component of the contraction to noradrenaline in wild-type which was absent in knockout, so that presumably only an α2A/D-adrenoceptor is involved in α2-adrenoceptor-mediated contractions in wild-type. These results suggest that phenylephrine acts primarily at α1-adrenoceptors in both wild-type and knockout, but that noradrenaline acts both at α1-adrenoceptors and α2AD-adrenoceptors in wild-type. In α2A/D-adrenoceptor knockout mice, noradrenaline acts primarily on α1-adrenoceptors, given that the maximum response is similar to that to phenylephrine in the same animals, and similar to that to the noradrenaline following BRL 44408 in wild-type. One apparent anomaly in the present study was that BRL 44408 (1 μM) reduced the maximum response to noradrenaline in wild-type mice, whereas yohimbine (1 μM) merely reduced potency. However, BRL 44408 has higher affinity than yohimbine at α2D-adrenoceptors (Ho et al., 1998), and may possibly act noncompetitively. In both series of experiments, the α2-adrenoceptor-mediated component of responses was seen with noradrenaline (10 μM). A previous study reported α2-adrenoceptor involvement in contractions of mouse vas deferens to the α2-adrenoceptor agonist UK 14,304, but not to phenylephrine (Bultmann et al., 1991). In that study, the receptor was tentatively identified as an α2A/D-adrenoceptor. We can now confirm that identification.
Given that RS 100329 was significantly more potent than prazosin at shifting the potency of noradrenaline in mouse vas deferens, it can be concluded that, since RS 100329 has 100 times lower affinity than prazosin for α1B and α1D-subtypes (Williams et al., 1999), the postjunctional α1-adrenoceptor is likely to be an α1A-subtype. Admittedly, RS 100329 acted noncompetitively, reducing the maximum contraction to noradrenaline, but this still suggests action at α1A-adrenoceptors. Although we did not investigate contractions to exogenous agonists in rat vas deferens in the present study, our previous results suggest that contractions to four agonists including noradrenaline are largely α1A-adrenoceptor mediated, with no clear evidence for an α2-adrenoceptor mediated component (Honner et al., 1999).
Responses to endogenous noradrenaline
In rat whole vas deferens, the contraction to a single electrical stimulus is biphasic (see Docherty & McGrath, 1984). The second α-noradrenergic phase can be examined in isolation in the rat in the presence of nifedipine, which eliminates the first nonnoradrenergic response. However, in the mouse, the response to a single stimulus proved to be small and variable (see Rajamani et al., 2001), so that responses to 10 Hz for 4 s were investigated. Nifedipine (10 μM) or the purinoceptor antagonist suramin (100 μM) (Dunn & Blakely, 1988) produced essentially the same effects: the response to 10-Hz stimulation was reduced to an early, prazosin sensitive, peak and a small plateau. Nifedipine produced essentially the same effect against trains of pulses in the rat vas deferens (see Warnock et al., 1985). This suggests that, as in the rat, the purinergic component of the response depends on calcium entry. However, since both suramin and α1-adrenoceptor antagonists had marked effects on the contraction to 10-Hz stimulation, it can be assumed that there is synergy between the two transmitters in producing contractions: block of either can greatly reduce the contraction.
The α1-adrenoceptor antagonist prazosin at low concentrations produced a concentration-dependent inhibition of 10-Hz stimulation-evoked contractions, but the effects were significantly greater in tissues from wild-type. Prazosin at high concentrations restored contractions towards baseline in both wild-type and knockout. Suramin or nifedipine greatly reduced this tendency to potentiate by high concentrations of prazosin. These results suggest that contractions to 10-Hz stimulation in mouse vas deferens from wild-type mice are largely α1-adrenoceptor mediated, but that prejunctional α2-adrenoceptor blockade by high concentrations of prazosin increases the release of the purinergic cotransmitter, presumably ATP. Since contractions to 10-Hz stimulation are significantly greater in vas deferens from knockout mice, presumably because of diminished α2-adrenoceptor-mediated prejunctional inhibition, one might expect that the purinergic response is increased in these animals. This would explain the diminished ability of prazosin in low concentration to inhibit contractions in tissues from knockout. However, high concentrations of prazosin also potentiate in knockout, suggesting that, in α2A/D-adrenoceptor knockout mice, blockade of the replacement α2C-adrenoceptors (see Altman et al., 1999; Rajamani et al., 2001) further increases the purinergic response. Indeed, since prazosin is more potent at α2C- than α2D-adrenoceptors (see Ho et al., 1998), an earlier activation of α2C-adrenoceptors may be an alternative explanation of why prazosin produces less inhibition of stimulation-evoked contractions in tissues from knockout animals.
If there are postjunctional α2A/D-adrenoceptors involved in stimulation-evoked contractions in wild-type, these would be impossible to identify with antagonists: prejunctional actions would mask postjunctional actions.
The low potency of the α1D-adrenoceptor selective antagonist BMY 7378 (Goetz et al., 1995; Buckner et al., 1996) in the present study suggests that the major receptor involved in adrenergic contractions is not α1D. Furthermore, the α1A-adrenoceptor antagonist RS 100329 was significantly more potent than prazosin at inhibiting 10-Hz nerve-stimulation-evoked contractions in vas deferens from wild-type mice. This potency of RS 100329 in relation to prazosin (e.g. RS 100329 is three times more potent than prazosin at α1A, but 100 times less potent at α1B; Williams et al., 1999), suggests that the major adrenoceptor involved in contractions is an α1A-adrenoceptor. This contrasts with the rat vas deferens, where a clear component of the contractile response to single-pulse nerve stimulation is α1D-adrenoceptor mediated (Honner & Docherty, 1999).
We have also compared 10-Hz nerve stimulation-evoked responses between mouse and rat vas deferens. In epididymal portions of rat vas deferens, prazosin (0.1 μM) produced approximately 50% inhibition of the contraction, with no further effect with prazosin (1–10 μM). In contrast, prostatic portions of rat vas deferens behaved similarly to mouse whole vas deferens in that prazosin (0.1 μM) produced significant inhibition which was reversed by prazosin (1–10 μM); hence, prazosin (10 μM) did not significantly effect the stimulation-evoked contraction. Since nerve-stimulation-evoked contractile responses in prostatic portions have a smaller α1-adrenoceptor mediated and larger purinergic component, prejunctional α2-adrenoceptor-mediated actions of prazosin (1–10 μM) to increase ATP release cancel out postjunctional actions in this portion of the vas. The combination of suramin and prazosin produced less effect than in the mouse vas deferens. It is worth noting that, in both mouse and rat vas deferens, the use of a high concentration of prazosin might lead to an underestimation of the true importance of α1-adrenoceptors in the nerve-stimulation-evoked contraction, and might lead to the suggestion of prazosin resistant contractions.
In conclusion, contractions to nerve stimulation in mouse vas deferens involve mainly α1A-adrenoceptors and purinoceptors. Contractions to exogenous noradrenaline involved both α1A- and α2A/D-adrenoceptors in wild-type mice.
Acknowledgments
Supported by The Health Research Board and The Irish Heart Foundation.
Abbreviations
- BMY 7378
8-[2-(4-(2-methoxyphenyl) piperazin-1-yl)ethyl]-8-azaspiro[4,5]decane-7,9-dione
- BRL 44408
2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole
- RS 100329
5-methyl-3-[3-[4-[2-(2,2,2-trifluoroethoxy)phenyl]-1-piperazinyl]propyl]-2,4-(1H)-pyrimidinedione
References
- ABOUD R.W., SHAFII M., DOCHERTY J.R. Investigation of the subtypes of α1-adrenoceptor mediating contraction of rat aorta, vas deferens and spleen. Br. J. Pharmacol. 1993;109:80–87. doi: 10.1111/j.1476-5381.1993.tb13534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLCORN R.J., CUNNANE T.C., KIRKPATRICK K. Actions of α,β-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for co-transmission. Br. J. Pharmacol. 1986;89:647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTMAN J.D., TRENDELENBURG A.U., MACMILLAN L., BERNSTEIN D., LIMBIRD L., STARKE K., KOBILKA B.K., HEIN L. Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Mol. Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- BUCKNER S.A., OHEIM K.W., MORSE P.A., KNEPPER S.M., HANCOCK A.A. α1-Adrenoceptor-induced contractility in rat aorta is mediated by the α1D-subtype. Eur. J. Pharmacol. 1996;297:241–248. doi: 10.1016/0014-2999(95)00755-5. [DOI] [PubMed] [Google Scholar]
- BULTMANN R., VON KUGELGEN I., STARKE K. Contraction-mediating alpha2-adrenoceptors in the mouse vas deferens. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:623–632. doi: 10.1007/BF00184294. [DOI] [PubMed] [Google Scholar]
- BYLUND D.B. Subtypes of α1- and α2-adrenergic receptors. FASEB J. 1992;6:832–839. doi: 10.1096/fasebj.6.3.1346768. [DOI] [PubMed] [Google Scholar]
- CONNAUGHTON S., DOCHERTY J.R. No evidence for differences between pre- and postjunctional alpha-2 adrenoceptors in the periphery. Br. J. Pharmacol. 1990;99:97–102. doi: 10.1111/j.1476-5381.1990.tb14660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTECCHIA S., SCHWINN D.A., RANDALL R.R., LEFKOWITZ R.J., CARON M.G., KOBILKA B.K. Molecular cloning and expression of the cDNA for the hamster α1-adrenergic receptor. Proc. Natl. Acad. Sci. USA. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCHERTY J.R., MCGRATH J.C. The actions of cirazoline on the rat vas deferens. Br. J. Pharmacol. 1984;82:9–14. doi: 10.1111/j.1476-5381.1984.tb16436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN P.M., BLAKELY A.G.H. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br. J. Pharmacol. 1988;93:243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOETZ A.S., KING H.K., WARD S.D.C., TRUE T.A., RIMELE T.J., SAUSSY D.L. BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur. J. Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- HAN C., ABEL P.W., MINNEMAN K.P. α1-Adrenoceptor subtypes linked to different mechanisms for increasing intracellular Ca2+ in smooth muscle. Nature. 1987;329:333–335. doi: 10.1038/329333a0. [DOI] [PubMed] [Google Scholar]
- HANFT G., GROSS G. Subclassification of α1-adrenoceptor recognition sites by urapidil derivatives and other selective antagonists. Br. J. Pharmacol. 1989;97:691–700. doi: 10.1111/j.1476-5381.1989.tb12005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON J.K., PEARSON W.R., LYNCH K.R. Molecular characterization of α1- and α2-adrenoceptors. Trends Pharmacol. Sci. 1991a;12:62–67. doi: 10.1016/0165-6147(91)90499-i. [DOI] [PubMed] [Google Scholar]
- HEIBLE J.P., BYLUND D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R.R. International Union of Pharmacology recommendation or nomenclature of α1-adrenoceptors: consensus update. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- HEIN L., ALTMAN J.D., KOBILKA B.K. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- HO S.L., HONNER V., DOCHERTY J.R. Investigation of the subtypes of α2-adrenoceptor mediating prejunctional inhibition in rat atrium and cerebral cortex. Naunyn-Schmeideberg's Arch. Pharmacol. 1998;357:634–639. doi: 10.1007/pl00005218. [DOI] [PubMed] [Google Scholar]
- HONNER V., DOCHERTY J.R. Investigation of the subtypes of α1–adrenoceptor ediating contractions of rat vas deferens. Br. J. Pharmacol. 1999;128:1323–1331. doi: 10.1038/sj.bjp.0702913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASCHUBE M., ZETLER G. Noradrenergic, purinergic and cholinergic transmission in the mouse vas deferens: influence of field-stimulation parameters, reserpinization, 6-hydroxydopamine and 4-aminopyridine. J. Neural Transmission. 1988;76:39–53. doi: 10.1007/BF01244990. [DOI] [PubMed] [Google Scholar]
- KAVA M.S., BLUE D.R., VIMONT R.L., CLARKE D.E., FORD A.P.D.W. α1L-Adrenoceptor mediation of smooth muscle contraction in rabbit bladder neck: a model for lower urinary tract tissues of man. Br. J. Pharmacol. 1998;123:1359–1366. doi: 10.1038/sj.bjp.0701748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANIER S.M., DOWNING S., DUZIE E., HOMEY C.J. Isolation of rat genomic clones encoding subtypes of the α2-adrenergic receptor. J. Biol. Chem. 1991;266:10470–10478. [PubMed] [Google Scholar]
- LOMASNEY J.W., COTECCHIA S., LORENZ W., LEUNG W.Y., SCHWINN D.A., YANG-FENG T.L., BROWNSTEIN M., LEFKOWITZ R.J., CARON M.G. Molecular cloning and expression of the cDNA for the α1A-adrenergic receptor. J. Biol. Chem. 1991;266:6365–6369. [PubMed] [Google Scholar]
- LORENZ W., LOMASNEY J.W., COLLINS S., REGAN J.W., CARON M.G., LEFKOWITZ R.J. Expression of three α2-adrenergic receptor subtypes in rat tissues: Implications for α2-receptor classification. Mol. Pharmacol. 1990;38:599–603. [PubMed] [Google Scholar]
- MULRYAN K., GITTERMAN D.P., LEWIS C.J., VIAL C., LECKIE B.J., COBB A.L., BROWN J.E., CONLEY E.C., BUELL G., PRITCHARD C.A., EVANS R.J. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- PEREZ D.M., PIASCIK M.T., GRAHAM R.M. Solution-phase library screening for the identification of rare clones: Isolation of an α1D -adrenergic receptor cDNA. Mol. Pharmacol. 1991;40:876–883. [PubMed] [Google Scholar]
- RAJAMANI K., LEONG S., LAVELLE A., DOCHERTY J.R. Prejunctional actions of methylenedioxymethamphetamine in vas deferens from wild-type and α2A/D knockout mice. Eur. J. Pharmacol. 2001;423:223–228. doi: 10.1016/s0014-2999(01)01118-9. [DOI] [PubMed] [Google Scholar]
- SCHWINN D.A., LOMASNEY J.W., LORENZ W., SZKLUT P.J., FREMEAU R.T., YANG-FENG T.L., CARON M.G., LEFKOWITZ R.J., COTECCHIA S. Molecular cloning and expression of the cDNA for a novel α1-adrenergic receptor subtype. J. Biol. Chem. 1990;265:8183–8189. [PubMed] [Google Scholar]
- WARNOCK P., HYLAND L., DOCHERTY J.R. Further examination of the inhibitory actions of α1-adrenoceptor agonists in rat vas deferens. Eur. J. Pharmacol. 1985;113:239–246. doi: 10.1016/0014-2999(85)90741-1. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., BLUE D.R., DANIELS D.V., DAVIS B., ELWORTHY T., GEVER J.R., KAVA M.S., ORGANS D., PADILLA F., TASSA S., VIMONT R.L., CHAPPLE C.R., CHESS-WILLIAMS R., EGLEN R.M., CLARKE D.E., FORD A.P.D.W. In vitroα1-adrenoceptor pharmacology of Ro 70-0004 and RS-100329, novel α1A-adrenoceptor selective antagonists. Br. J. Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]