Abstract
This study examines the activity of the antioxidant N-acetylcysteine on bleomycin-induced pulmonary fibrosis in rats with emphasis on the early inflammatory phase.
Rats receiving N-acetylcysteine (300 mg kg−1 day−1, intraperitoneal) had less augmented lung wet weight, and lower levels of proteins, lactate dehydrogenase, neutrophil and macrophage counts in bronchoalveolar lavage fluid and lung myeloperoxidase activity with a betterment of histological score at 3 days postbleomycin.
A diminished lung GSH/GSSG ratio and augmented lipid hydroperoxides were observed 3 days postbleomycin. These changes were attenuated by N-acetylcysteine. Alveolar macrophages from bleomycin-exposed rats released augmented amounts of superoxide anion and nitric oxide. N-Acetylcysteine did not modify superoxide anion generation but reduced the increased production of nitric oxide.
N-Acetylcysteine suppressed the bleomycin-induced increased activation of lung NF-κB (shift assay and immunohistochemistry), and decreased the augmented levels of the early inflammatory cytokines, tumour necrosis factor-α, interleukin-β, interleukin-6 and macrophage inflammatory protein-2 observed in bronchoalveolar lavage fluid at 1 and 3 days postbleomycin exposure.
At 15 days postbleomycin, N-acetylcysteine decreased collagen deposition in bleomycin-exposed rats (hydroxyproline content: 6351±669 and 4626±288 μg per lung in drug vehicle- and N-acetylcysteine-treated rats, respectively; P<0.05). Semiquantitative histological assessment at this stage showed less collagen deposition in N-acetylcysteine-treated rats compared to those receiving bleomycin alone.
These results indicate that N-acetylcysteine reduces the primary inflammatory events, thus preventing cellular damage and the subsequent development of pulmonary fibrosis in the bleomycin rat model.
Keywords: Pulmonary fibrosis, bleomycin, rat, N-acetylcysteine, inflammation
Introduction
Pulmonary fibrosis is a chronic inflammatory interstitial lung disease of potential fatal prognosis and poor response to available medical therapy. It has been hypothesized that activated inflammatory cells which accumulate in the lower airways, release harmful amounts of reactive oxygen species (ROS) that result in parenchymal injury, and interstitial and alveolar fibrosis (Strausz et al., 1990). This oxidant burden may be even more consequential due to a deficiency in glutathione, a major component of the lung antioxidant defence system (Rahman & MacNee, 2000). This pathogenetically relevant redox imbalance suggests that the lung antioxidant defences may be improved with glutathione-modulating agents like sulphydryl compounds. N-Acetylcysteine (NAC) is one of the most widely investigated antioxidants, having shown beneficial effects in various disease states in which free radicals have been involved (Cotgreave, 1997; Tepel et al., 2000). The results of short-term studies using oral and intravenous NAC in patients with pulmonary fibrosis have demonstrated its ability to improve the antioxidant screen of the lung by elevating glutathione levels, as well as restoring pulmonary function tests with a low incidence of adverse effects (Meyer et al., 1994, 1995; Behr et al., 1997).
One of the clinically relevant causative agents of pulmonary fibrosis is bleomycin, a glycopeptide antibiotic used in the chemotherapy of different types of cancer that produces its antineoplastic effect by causing oxidant damage to DNA (Hay et al., 1991). Bleomycin produces pulmonary fibrosis as an adverse effect due to the low levels in lung tissue of the cysteine hydrolase that inactivates bleomycin; therefore, bleomycin is widely used in experimental models to cause lung injury leading to oxidant-induced inflammatory and fibrotic lesions in the lung interstitium of various animal species (Hay et al., 1991). This animal model of pulmonary fibrosis resembles that seen in humans (Giri, 1990) and it is useful to assess the effects of potential therapeutic agents including antioxidants (Punithavathi et al., 2000; Cortijo et al., 2001).
The present study was undertaken to evaluate the beneficial effects of NAC in a rat model of lung injury produced by endotracheal instillation of bleomycin with emphasis on the changes observed at an early stage (1 and 3 days after bleomycin exposure) when inflammatory changes predominate by contrast with the later stage (15 days postbleomycin) where fibrosis is evident (Lazenby et al., 1990). Morphological changes were assessed by a semiquantitative histological score, and lung hydroxyproline content was used as a marker of collagen deposition. Markers of lung cell damage, airway microvascular leakage and oxidant stress were evaluated at this early inflammatory phase. In addition, initial events after exposure to bleomycin are the activation of the nuclear factor κB (NF-κB) and the release of a variety of cytokines that modulate the inflammatory and fibroproliferative response (Coker & Laurent, 1998; Gurujeyalakshmi et al., 2000). Key factors of this cytokine network in bleomycin-induced pulmonary fibrosis are the macrophage cytokines tumour necrosis factor (TNF)-α, the interleukin (IL)-1β and IL-6 (Elias et al., 1990; Piguet, 1990; Smith et al., 1998), and the macrophage inflammatory protein-2 (MIP-2) (Keane et al., 1999). Therefore, we determined the effect of NAC on lung NF-κB activation and on the levels of proinflammatory cytokines in bronchoalveolar lavage fluid.
Methods
Materials and animals
Bleomycin sulphate, N-acetylcysteine, halothane and sodium pentobarbital were obtained from Almirall-Prodesfarma (Barcelona, Spain), Zambon Group (Barcelona, Spain), Zeneca Farma (Pontevedra, Spain) and Normon (Madrid, Spain), respectively. Other chemicals and reagents were purchased from standard suppliers.
Pathogen-free, male Sprague–Dawley rats, weighing 200–250 g at the start of experiments, were obtained from Criffa (Iffa Credo, France). Rats were housed in a controlled environment and fed rodent chow (A04; Panlab, Barcelona, Spain) and tap water ad libitum. This study conformed to European Community (Directive 86/609/EEC) and Spanish guidelines for the use of experimental animals and it was approved by the institutional committees of animal care and research.
Experimental model
To produce pulmonary fibrosis, animals received endotracheally, by the transoral route, a single sublethal dose of 2.5 U kg−1 of bleomycin dissolved in 0.25 ml saline (0.9% NaCl). The dose of bleomycin was selected from previous experiments in this laboratory to cause no mortality but consistent biochemical and histological damage (Cortijo et al., 2001). Control animals received the same volume of intratracheal saline instead of bleomycin. Tracheal instillation was carried out under halothane anaesthesia. At days 1, 3 or 15 after endotracheal bleomycin or saline, the animals were killed by a lethal injection of sodium pentobarbital (100 mg kg−1, intraperitoneal (i.p.)) followed by exsanguination from abdominal aorta. Bronchoalveolar lavage was performed and lungs were weighed and processed separately for biochemical (immediately frozen in liquid nitrogen) and histological studies as indicated below.
Experimental groups
Animals were randomly distributed into four experimental groups for each time point: Group A–drug vehicle+saline; group B–NAC+saline; group C–drug vehicle+bleomycin; group D–NAC+bleomycin. Treatments (vehicle or NAC) were administered i.p. on a daily basis (at 9:00 a.m.) starting 7 days prior to the intratracheal instillation of bleomycin up to the conclusion of the experiments. The dose of NAC was 300 mg kg−1 body weight per day. NAC solution was made fresh every day in sterile saline and its pH adjusted within the range of 6.8–7.2 with 6 N NaOH immediately before administration. NAC was given to animals in a final volume of 1 ml of vehicle. The route of administration, dose level and schedule were based on previous studies (Giri et al., 1988; Blackwell et al., 1996). In a group of additional experiments where lung glutathione status was measured (see below), the same daily dose of NAC was administered orally by gavage instead of intraperitoneally. The oral route was selected as the usual in the clinical setting.
Cell counts and biochemical studies in bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) was obtained by washing the lung four times with 5 ml aliquots of saline through a tracheal cannula. Typically, total fluid recovery exceeded 85%, and the percentages of fluid recovered did not significantly differ among experimental groups. The BALF was centrifuged (300 × g, 20 min, 4°C). The cell pellet was resuspended for total cell count (haemocytometer) and differential cell counts from cytospin preparations as previously outlined (Cortijo et al., 2001). The cell-free supernatant was used for biochemical determinations. Total protein concentration was measured by a standard dye technique from BioRad (Munich, Germany). Lactate dehydrogenase (LDH) activity was measured by using a commercial kit from Boehringer Manheimm (Munich, Germany). The total amount of TNF-α was measured by a competitive enzyme-immunoassay kit from Chemicon International (Temecula, CA, U.S.A.), and the levels of TNF-α soluble receptor (type I) were measured by a commercial solid-phase sandwich enzyme-linked immuno-sorbent assay (ELISA) from R&D Systems (Minneapolis, MN, U.S.A.) following the manufacturer's protocol. IL-1β and IL-6 concentrations were measured with commercial ELISA kits as indicated by the manufacturer (rat IL-1β and rat IL-6, Biotrack™, Amersham Pharmacia Biotech, Freiburg, Germany). MIP-2 concentration was measured with an ELISA kit (rat MIP-2, Cytoscreen™ Biosource Int., Camarillo, CA, U.S.A.). Lipid hydroperoxide (LPO) in BALF was measured by the Lipid Hydroperoxide Assay kit as indicated by the manufacturer (Cayman Chemical, Ann Arbor, MI, U.S.A.).
Biochemical assays in alveolar macrophage culture
At 3 days after bleomycin administration, groups of five rats per group were killed according to standard ethical procedures (see above). The lungs were then lavaged (4 × 5 ml) with calcium- and magnesium-free phosphate-buffered saline, pH 7.4. The pooled BALF was filtered through sterile gauze and then centrifuged at 300 × g for 10 min, the erythrocytes were lysed, and after washing three times in RPMI-1640 media, the pelleted cells were resuspended in RPMI-1640 media containing 25 mM HEPES buffer. Aliquots of the cell suspension were counted by a haemocytometer and cell viability was measured by trypan blue exclusion (>95%). The BAL cells were resuspended in RPMI-1640 media supplemented with 2 mM glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 10% heat-inactivated foetal bovine serum. The cells were seeded (1 × 106 cells ml−1) to a 24-well tissue culture plate and allowed to adhere for 2 h at 37°C in a humidified atmosphere in 95% O2 : 5% CO2; then, the nonadherent cells were removed by washing three times with RPMI-1640 media. The alveolar macrophage-enriched monolayers were incubated in fresh RPMI-1640 media for an additional 24 h. The superoxide anion release by alveolar macrophages was measured spectrophotometrically using the ferricytochrome C reduction technique (Johnston, 1984). To assess nitric oxide production, alveolar macrophages were cultured in DMEM in 96-well plates (1 × 106 cells ml−1) for 24 h and the accumulation of nitrite in the culture medium was assayed using the Greiss reaction with sodium nitrite as the standard (Closa et al., 1999).
Biochemical studies in lung tissue
Lung hydroxyproline was determined spectrophotometrically as previously reported (Woessner, 1961), and the results were expressed as micrograms of hydroxyproline per lung (Shahzeidi et al., 1991). Tissue myeloperoxidase was measured photometrically, employing 3,3′,5,5′-tetramethylbenzidine as a substrate as previously reported (Trush et al., 1994) with some modifications (Folch et al., 1998). Reduced glutathione (GSH) in lung tissue was measured by the GSH-transferase method (Brigelius et al., 1983) and oxidized glutathione (GSSG) by a method previously described in our laboratory (Asensi et al., 1994).
Nuclear protein extraction and determination of NF-κB binding activity by EMSA
Nuclear protein extracts were prepared from lung tissue by the method of Deryckere & Gannon (1994). Aliquots of frozen tissue (200–250 mg) were mixed with liquid nitrogen and ground to powder using a mortar and pestle. A volume of 1.5 ml of solution A (0.6% Nonidet P-40, 150 mM NaCl, 10 mM HEPES, pH 7.9, 1 mM EDTA, 0.5 mM phenylmethylsulphonylfluoride (PMSF), 0.2 mM benzamidine, 0.2 μg ml−1 pepstatin, 0.2 μg ml−1 leupeptin and 0.2 μg ml−1 aprotinin) was added to the mortar. The contents of the mortar were placed in a Dounce tissue homogenizer (Afora, Madrid, Spain), and the cells were lysed with five strokes of the pestle. After transfer to a 1.5 ml Eppendorf, debris was pelleted by centrifuging at 100 × g for 60 s. The supernatant containing intact nuclei was transferred to a 1.5 ml Eppendorf, incubated on ice for 5 min, and centrifuged for 30 s at 17,000 × g. The nuclear pellet was resuspended in 50 μl of solution B (25% glycerol, 20 mM HEPES, pH 7.9, 420 mM NaCl, 1.2 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol (DTT), 0.5 mM PMSF, 2 mM benzamidine, 2 μg ml−1 pepstatin, 2 μg ml−1 leupeptin and 2 μg ml−1 aprotinin) and incubated on ice for 30 min with occasional vortex. The mixture was transferred to microcentrifuge tubes, and the nuclei were pelleted by centrifugation at 17,000 × g for 2 min. Supernatants containing nuclear proteins were saved, aliquoted and stored at −80°C. Protein quantitation was performed using the Bradford assay (1976).
Aliquots of nuclear extracts with equal amounts of protein (10 μg) were mixed in a total volume of 20 μl with a buffer containing 100 mM HEPES (pH 7.6), 5 mM EDTA, 50 mM (NH4)2SO4, 5 mM DTT, 1% Tween 20, 150 mM KCl, 0.1 μg μl−1poly-L-Lys, and 1 μg μl−1 poly dI-dC according to the manufacturer's instructions (DIG gel shift kit from Boehringer Mannheim and Enzo Diagnostics Inc., Germany). After aliquots were gently mixed and equilibrated on ice for 10 min, binding reactions were started by the addition of 30 fmol of double-stranded digoxygenine-labelled oligonucleotide for NF-κB (the sense-strand sequence is 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; the underlined sequence corresponds to a κB-binding motif) obtained from Promega Co. (Madison, WI, U.S.A.), and allowed to proceed for 30 min at 4°C. Samples were analysed on a 6% nondenaturating polyacrylamide gel. After electrophoretical transfer to a positively charged nylon membrane (Hybond-N+; Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany), complexes were visualized by a chemiluminescence detection system (DIG gel shift kit from Boehringer Mannheim and Enzo Diagnostics Inc., Germany). The intensity of bands was quantified by using the image analysis system AnalySIS® 3.0 (Soft Imaging System GmbH, Münster, Germany). To ascertain the specificity of the binding reaction, competition assays were performed in the presence of excess (100-fold, i.e. 3000 fmol) unlabelled oligonucleotide.
Histology and immunohistochemistry
For histological studies, the lung was first perfused through its main bronchus with a fixative solution (10% neutral-buffered formalin) at a pressure of 25 cm H2O, immersed in the fixative for 12–24 h, and blocks were taken. Tissue blocks were placed in formalin, dehydrated in a graded series of ethanol, embedded in paraffin, cut into 4-μm-thick serial sections, and stained with haematoxylin–eosin and Masson's trichrome to identify inflammatory cells, connective tissue and collagen deposition. Histologic grading of lesions was performed by using a blinded semiquantitative scoring system for extent and severity of inflammation and fibrosis in lung parenchyma based on previous studies (Greco et al., 1997) with modifications (Cortijo et al., 2001).
Prior to immunohistochemical detection of NF-κB in lung sections, paraffin was removed and sections were boiled in 10 mM citrate buffer (pH 6) for 5 min as this procedure was found to enhance the immunoreactions. Endogenous peroxidases were blocked with methanol–H2O2 and nonspecific binding sites were blocked with 3% normal goat serum for 2 h. Sections were then incubated overnight in a humidified chamber at 4°C with a rabbit polyclonal anti-p65 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, U.S.A.) diluted 1 : 500 for paraffin sections. This was followed by biotinylated goat anti-rabbit antibody (Vectastain, Vector Laboratories Inc., Burlingame, CA, U.S.A.) diluted 1 : 200 for 1 h, and the avidin–biotin complex (ABC kit, Vector) 1 : 100 for 1 h. The reaction was developed with 0.05% diaminobenzidine (Sigma Chemical Co., St Louis, MO, U.S.A.) and 0.03% H2O2. The specificity of the immunoreactions was tested by incubation of the primary antibody against NF-κB p65 with their corresponding antigenic peptide (Santa Cruz).
Statistical analysis of results
Data are expressed as means±s.e.m. of n experiments. Statistical analysis was carried out by using a software package (GraphPad, San Diego, CA, U.S.A.). Analysis of variance followed by post hoc tests or nonparametric test was applied as appropriate. A P value of less than 0.05 was considered to be significant.
Results
Lung weight and hydroxyproline levels
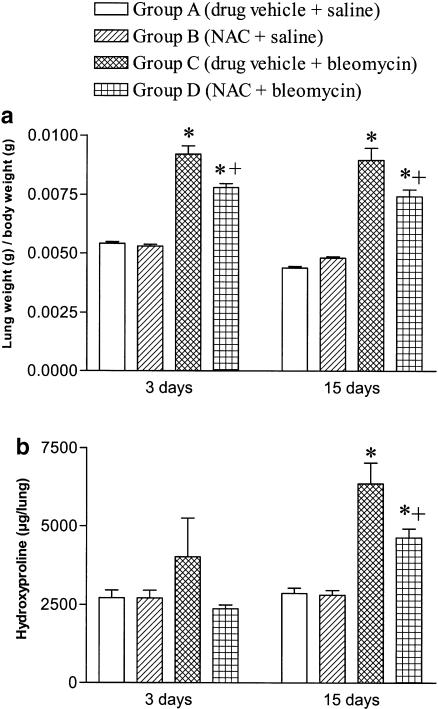
Bleomycin produced a significant increase in lung weight at 3 and 15 days postexposure that was partially reduced in animals treated with NAC (Figure 1a). Lung hydroxyproline levels, a marker of collagen deposition, were increased approximately two-fold at 15 days after bleomycin exposure. Treatment with NAC significantly reduced the hydroxyproline content in bleomycin-exposed rats although levels remained higher than those found in animals not exposed to bleomycin (Figure 1b).
Figure 1.
Lung weight/body weight (panel a) and lung hydroxyproline levels (panel b) in experimental groups and time points as indicated. Treatment with N-acetylcysteine (NAC; 300 mg kg−1 per day, i.p.) reduced the lung weight and the lung content of hydroxyproline. Data are means±s.e.m. of 10 animals per group; *P<0.05 from group A; +P<0.05 from group B.
Biochemical markers of lung inflammation and damage
The pulmonary inflammatory response 3 days after bleomycin administration was reflected by early increases of inflammatory cells in BALF, tissue myeloperoxidase activity, and LDH and proteins in BALF, thus indicating airway cell influx, cell damage and microvascular leakage. NAC was active to attenuate most of these early inflammatory changes (Table 1).
Table 1.
Effects of N-acetylcysteine (NAC) on total and differential cell counts in bronchoalveolar lavage fluid (BALF), myeloperoxidase (MPO) activity in lung tissue, and lactate dehydrogenase (LDH) and protein in BALF measured at 3 days after endotracheal instillation of bleomycin or saline as indicated
| Group A (drug vehicle + saline) | Group B (NAC + saline) | Group C (drug vehicle + bleomycin) | Group D (NAC + bleomycin) | |
| Total cells | 0.36±0.02 | 0.34±0.05 | 1.78±0.37* | 0.86±0.06*** |
| Neutrophils | 1.74±0.71 | 1.99±0.70 | 41.97±8.23* | 21.16±3.00*** |
| Eosinophils | 0.17±0.10 | 0.16±0.10 | 4.02±1.36* | 3.27±0.54* |
| Lymphocytes | 1.22±0.55 | 0.73±0.23 | 15.87±6.13* | 4.32±1.00* |
| Macrophages | 33.31±1.91 | 31.06±3.86 | 115.84±24.38* | 56.89±2.97*** |
| MPO activity | 90.4±8.1 | 91.6±7.7 | 260.4±17.1* | 151.6±16.8*** |
| LDH activity | 0.41±0.06 | 0.44±0.05 | 0.90±0.11* | 0.58±0.05*** |
| Proteins | 1.13±0.18 | 1.11±0.13 | 8.96±0.84* | 5.10±0.46** |
Total cells are expressed as × 106 ml−1 BALF; differential cell counts are expressed as × 104 ml−1 BALF; MPO activity as U lung−1; LDH activity as U 100 g−1 body weight; and protein as mg 100 g−1 body weight. Data are mean±s.e.m. of five animals in each group
P<0.05 vs group A
P<0.05 vs group C.
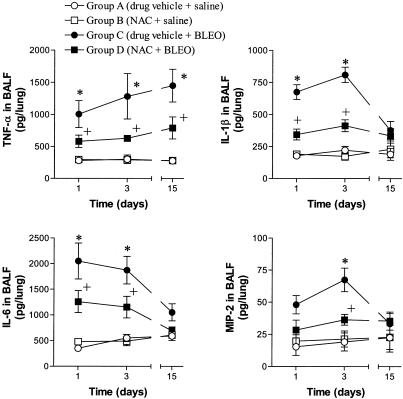
As signs of acute inflammation, early increases of TNF-α, IL-1β, IL-6 and MIP-2 levels in BALF were observed at 1 and 3 days postbleomycin (Figure 2). At 15 days postbleomycin, only TNF-α showed increased BALF levels. The augmented levels of these inflammatory cytokines were consistently reduced by NAC (Figure 2).
Figure 2.
Time-course changes in the levels of tumour necrosis factor (TNF)-α, IL-1β, IL-6 and MIP-2 in bronchoalveolar lavage fluid (BALF) at 1, 3 and 15 days postbleomycin or saline in experimental groups as indicated. Treatment with N-acetylcysteine (NAC; 300 mg kg−1 per day, i.p.) reduced the augmented levels of inflammatory cytokines in bleomycin-exposed rats. Data are means±s.e.m. of four to seven animals; *P<0.05 from group A; +P<0.05 from group C.
In additional experiments carried out at 3 days postbleomycin, the increased levels of TNF-α in BALF were accompanied by an elevation of its physiologic inhibitor, the sTNF-R; this augmentation was not affected by NAC (values in rats receiving endotracheal saline were 15±8 and 17±7 pg per lung for vehicle- and NAC-treated groups; values in bleomycin-exposed animals were 755±97 and 903±135 pg per lung for vehicle- and NAC-treated groups; nonsignificant between bleomycin-challenged groups; P<0.05 vs saline groups; n=5 for each group).
Glutathione status and oxidant stress markers
GSH and GSSG were measured in lung tissue at 3 days after bleomycin exposure. A decrease in GSH accompanied by an increase in GSSG, which translates into a decrease of the GSH/GSSG ratio, an index of the tissue redox state, were observed in rats instilled with bleomycin. These changes were partially reversed in the rats treated with intraperitoneal NAC (Table 2). Additional experiments confirmed the ability of oral NAC to produce similar effects on lung glutathione redox status (Table 2).
Table 2.
Effect of N-acetylcysteine (NAC) on the glutathione content of rat lung tissue at 3 days postsaline or bleomycin exposure (NAC was daily administered at the same dose level (300 mg kg−1) by intraperitoneal (i.p.) or oral route)
| GSH (μmol g−1 lung tissue) | GSSG (nmol g−1 lung tissue) | GSH/GSSG ratio | |
| Drug vehicle+saline | 2.22±0.12 | 22.19±2.49 | 103.70±8.70 |
| NAC (i.p.)+saline | 1.98±0.21 | 24.97±3.16 | 83.27±15.22 |
| Drug vehicle+bleomycin | 1.75±0.12* | 44.77±6.94* | 41.22±3.90* |
| NAC (i.p.)+bleomycin | 1.81±0.11 | 25.90±1.65** | 71.86±8.45*** |
| Drug vehicle+saline | 2.07±0.14 | 27.88±3.59 | 78.89±10.00 |
| NAC (oral)+saline | 2.10±0.12 | 27.97±2.78 | 77.08±10.29 |
| Drug vehicle+bleomycin | 1.61±0.12* | 45.62±3.20* | 36.06±3.71* |
| NAC (oral)+bleomycin | 1.70±0.19 | 32.06±2.62** | 53.9±6.31** |
Values are expressed as mean±s.e.m. of five animals in each group except four in ‘NAC (i.p.) + saline' and three in ‘NAC (oral) + saline'
P<0.05 from the corresponding ‘drug vehicle+saline'
P<0.05 from the corresponding ‘drug vehicle+bleomycin'.
The amount of LPO in BALF was significantly increased at 3 days postbleomycin (3.4±0.6 nmol ml−1; n=5) compared to saline control (0.8±0.3 nmol ml−1; n=5). Treatment with intraperitoneal NAC did not alter the control values in saline-challenged animals (0.9±0.3; n=3) but attenuated the levels detected in bleomycin-exposed rats (1.7±0.4 nmol ml−1; n=5, P<0.05 compared to drug vehicle+bleomycin).
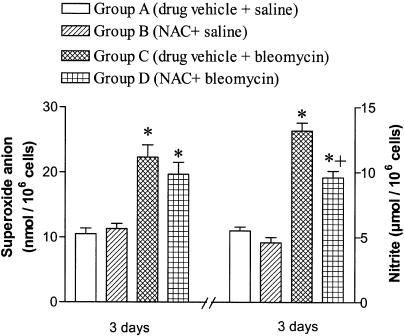
The production of superoxide anion and nitric oxide by alveolar macrophages at 3 days postchallenge was higher in bleomycin-exposed rats compared to saline controls, thus indicating the presence of oxidative and nitrosative stress in this model. The generation of superoxide anion by alveolar macrophages was not diminished by NAC but a slight, albeit significant, reduction of nitrite production was detected (Figure 3).
Figure 3.
Effect of N-acetylcysteine (NAC; 300 mg kg−1 per day, i.p.) on alveolar macrophage release of superoxide anion and nitrite in the culture medium. NAC did not significantly change superoxide anion release but attenuated nitric oxide production. Data are means±s.e.m. of five independent observations in each group. *P<0.05 from group A; +P<0.05 from group C.
Nuclear factor-κB binding activity and immunostaining
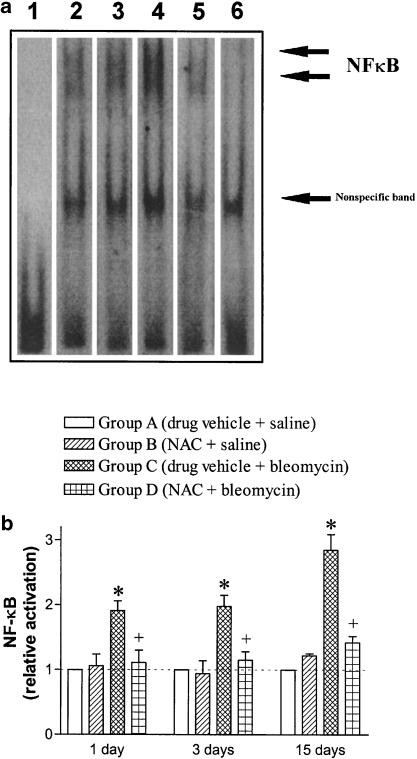
NF-κB binding activity determined by shift assay was increased in nuclear extracts from lungs obtained at 1, 3 and 15 days postbleomycin challenge compared to saline control animals. This augmentation of NF-κB activity was suppressed in NAC-treated rats (Figure 4).
Figure 4.
Effect of N-acetylcysteine (NAC; 300 mg kg−1 per day, i.p.) on the bleomycin-induced increase of NF-κB-binding activity assessed at 1, 3 and 15 days postexposure. Panel (a) An electrophoretic gel mobility shift assay showing NF-κB-binding activity in rat lung cell nuclear proteins from individual animals at 1 day postbleomycin. Lane 1: Free probe. Lanes 2, 3, 4 and 5 correspond to experimental groups A (drug vehicle + saline), B (NAC + saline), C (drug vehicle + bleomycin) and D (NAC + bleomycin), respectively. The specificity of the binding was confirmed by adding excess unlabelled NF-κB oligonucleotide (lane 6). Panel (b) The densitometric scanning of the band shift data of NF-κB-binding activity is expressed as relative values compared to their corresponding values in group A taken as unity. There was a significant increase in NF-κB-binding activity that was suppressed in NAC-treated rats. Columns are means±s.e.m. of three to four independent experiments for each group. *P<0.05 compared to group A; +P<0.05 from group B.
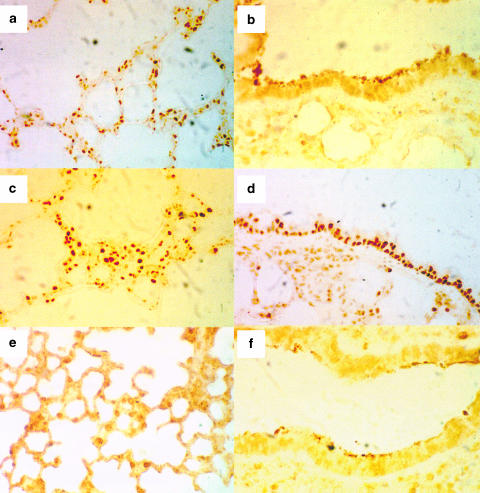
Lung sections from vehicle+vehicle rats showed a small degree of immunostaining for NF-κB at 3 days postbleomycin exposure, while an intense nuclear staining was noticed in airway epithelial cells, macrophages and other inflammatory cells (Figure 5). The immunostaining for NF-κB was diminished in NAC-treated rats (Figure 5).
Figure 5.
Immunohistochemical analysis of NF-κB in lung sections obtained at 3 days postbleomycin or saline exposure in vehicle- or N-acetylcysteine (NAC, 300 mg kg−1 per day, i.p.)-treated rats. Lung sections were obtained from control rats (vehicle+vehicle; (a, b)), and from rats exposed to bleomycin and receiving drug vehicle (c, d) or NAC (e, f). Panels show sections stained with anti-p65 antibody followed by the avidin–biotin complex. The immunohistochemical localization of NF-κB appears as dark-brown positive nuclear staining in airway epithelial cells as well as macrophages and other inflammatory cells like polymorphonuclear leukocytes in interalveolar septa. There is a low staining level in control lungs. Note the high level of staining in the epithelium of bleomycin-exposed rats (panel (d) compared to the virtual absence in epithelial cells from control animals (panel (b). The increased expression of p65 in vehicle+bleomycin rats was markedly diminished in NAC+bleomycin samples (cf. (c,d) and (e,f)). Original magnification × 25 (a, c, e) and × 40 (b, d, f).
Histopathology
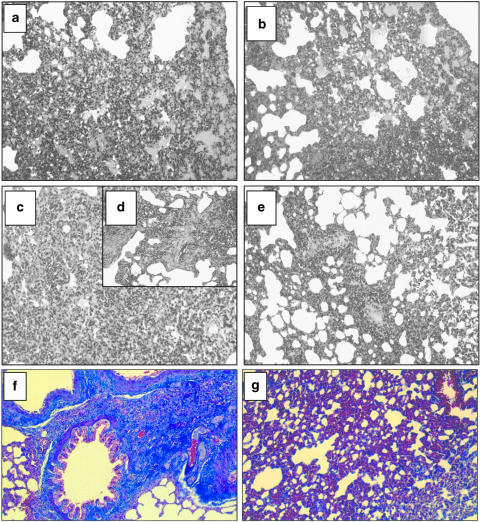
Lungs from rats in groups A (drug vehicle+saline) and B (NAC+saline) were histologically normal (not shown). Lungs from rats in group C (drug vehicle+bleomycin), killed at 3 days postbleomycin, showed extensive areas of oedema, congestion of alveolar walls and a moderate alveolar exudate composed mainly of polymorphonuclear leukocytes. Within the alveolar interstitium, an accumulation of polymorphonuclear neutrophils and scattered lymphocytes and macrophages was observed (Figure 6a). These areas were randomly distributed throughout the pulmonary parenchyma. No signs of fibrotic lesions were observed. Pulmonary lesions in group D (NAC+bleomycin) at 3 days postbleomycin were similar to those described for group C (Figure 6b) but better histological scores were obtained (Table 3).
Figure 6.
Representative photomicrographs of lung histopathology in groups B (vehicle + bleomycin) and C (N-acetylcysteine (NAC)+ bleomycin) at 3 days (a) and (b), respectively) and 15 days (c, d) and (e), respectively) following endotracheal bleomycin. Lung sections were stained with haematoxylin–eosin. NAC dose was of 300 mg kg−1 per day given intraperitoneally. Normal lungs observed for group A (vehicle + vehicle) are not shown. Three days after intratracheal bleomycin, a marked peribronchial interstitial infiltration of inflammatory cells and alveolar oedema were patent in vehicle-treated animals (a). These pulmonary lesions were not improved by NAC (b). At 15 days postbleomycin, inflammation and fibrosis were present in vehicle-treated rats ((c,d)), but NAC ameliorated the pulmonary lesions (e). Panels (f) (group B) and (g) (group C) show lung sections stained with Masson-trichrome at 15 days postbleomycin. The presence of interstitial collagen was diminished by NAC. Original magnification of panels × 10 (except (d) × 20).
Table 3.
Histologic lesion scores of lungs from rats receiving endotracheal bleomycin (BL) in the absence (group C) and presence of treatment with N-acetylcysteine (NAC; group D)
| Inflammation | Fibrosis | |||
| Severity | Extension | Severity | Extension | |
| 3 days postexposure | ||||
| Drug vehicle+bleomycin | 2.40±0.24 | 2.20±0.20 | Nil | Nil |
| NAC+bleomycin | 1.80±0.20* | 1.60±0.24* | Nil | Nil |
| 15 days postexposure | ||||
| Drug vehicle+bleomycin | 2.00+0.32 | 2.20±0.37 | 2.40±0.24 | 2.80±0.20 |
| NAC+bleomycin | 1.67±0.21 | 1.83±0.17 | 1.50±0.22* | 2.00±0.26* |
Lung sections from bleomycin-injured rats were coded and then blindly scored for inflammation and fibrosis. Lung inflammation was scored in tissue sections stained with haematoxylin and eosin on a scale of 0 – 4. Lung fibrosis was scored in trichrome-stained sections on a scale of 0 – 4. Data are means±s.e.m. of five to six animals per group.
P<0.05 from the corresponding group exposed to bleomycin without NAC.
The pulmonary lesions observed at 15 days postbleomycin in group C consisted of multifocal areas of severe inflammation and intense fibrosis (Figure 6c,d). In these areas, an intense thickening of alveolar septa with evident infiltration of macrophages, lymphocytes, polymorphonuclear neutrophils and some eosinophils was observed. Masson-trichrome staining confirmed the presence of an intense fibrosis in the inflammatory focal areas (Figure 6f). In contrast, NAC-treated animals at 15 days postbleomycin (group D) showed a less severe pattern of pulmonary lesion, consisting of multifocal areas of moderate inflammation and slight fibrosis (Figure 6e; Table 3). Although the type of inflammatory infiltrate was similar to that observed in group C, the pulmonary parenchyma was less affected. The reduced intensity of the fibrosis was confirmed by Masson-trichrome staining (Figure 6g).
Discussion
The results of the present study indicate that pretreatment with NAC was effective in reducing lung damage produced by intratracheal instillation of bleomycin in rats. This beneficial effect was based on the improvement of early inflammatory changes following endotracheal bleomycin as well as on the diminished accumulation of collagen and betterment of histologic lesion scores. This finding adds to a substantial amount of previous work showing the activity of NAC, either systemically (intraperitoneal or oral) or locally (aerosolized or endotracheal) administered, to prevent lung damage and subsequent fibrosis in rats and mice (Berend, 1985; Jamieson et al., 1987; Shahzeidi et al., 1991; Hagiwara et al., 2000; Cortijo et al., 2001). Yet, Ward et al. (1987) reported that subcutaneous NAC failed to ameliorate bleomycin-induced lung toxicity in rat, and Giri et al. (1988) found that intraperitoneal NAC did not reduce the lung damage in hamsters. Similarly, the activity of other antioxidants against pulmonary fibrosis produced by bleomycin has been recently demonstrated (Gurujeyalakshmi et al., 2000; Punithavathi et al., 2000; Tamagawa et al., 2000). The effectiveness of NAC in this experimental model would be consistent with the beneficial effect shown for this thiol compound in patients with idiopathic pulmonary fibrosis (Meyer et al., 1994, 1995; Behr et al., 1997).
The mechanism through which NAC could limit fibrosis is still uncertain, but is likely to be related to its ability to reduce damage to lung structures in the early inflammatory stage of the disease process. The pulmonary injury produced by bleomycin involves, as an initial event, the generation of oxidant species by an iron-dependent mechanism (Hay et al., 1991). Further damage is probably elicited by increased amounts of ROS produced by activated inflammatory cells, which accumulate in the bleomycin-induced pulmonary lesions (Strausz et al., 1990; MacNee & Rahman, 1995). In this scenario, two potential mechanisms for this lower degree of lesion would be possible. Either NAC reduces the ROS production or the ROS-induced aggression, leading to the early inflammatory phase. Our results would be in favour of a combination of effects at this early stage of the process. Thus, in the acute stage of the lesion (3 days), NAC attenuated bleomycin-induced exudation and cell damage (protein and LDH levels in BALF) as well as decreased the severity of the inflammatory infiltrate (myeloperoxidase activity, differential cell counts and histologic scores) and the oxidant stress burden (see below). NAC has demonstrated antiexudative effects in the airways in vivo (Bernareggi et al., 1999), as well as a capacity to inhibit the expression of redox-sensitive cell adhesion molecules in vitro (Faruqi et al., 1997) and in vivo (Alvarez & Sanz, 2001; Cuzzocrea et al., 2001). In particular, NAC has been demonstrated to suppress the early upregulation of P-selectin in bleomycin-induced lung injury in rats (Serrano-Mollar et al., 2002).
The fact that these events have been observed in the early inflammatory phase, in which fibrosis-related biochemical and histological changes are still imperceptible, suggests that the effect of NAC may be related to tissue preservation, that is, a decreased extent of lung lesion at the initial phase in NAC-treated animals would imply a reduced fibrotic process at later stages. This hypothesis does not exclude a direct effect of NAC on fibrogenesis since it has been reported that NAC inhibits fibroblast proliferation in vitro (Cantin et al., 1990).
NAC may act directly as an oxygen radical scavenger (Aruoma et al., 1989) but also, since it is a cell-permeable sulphydryl compound, readily enters cells and could promote the production of GSH by furnishing its limiting precursor, L-cysteine (Cotgreave, 1997). Both mechanisms may account for providing protection against bleomycin- and leukocyte-mediated cytotoxicity in the lung. The glutathione status has been studied in patients with idiopathic pulmonary fibrosis. Meyer et al. (1994, 1995) reported a deficiency in total glutathione levels in epithelial lining fluid but not in BALF from these patients. Oral treatment with NAC increased the total glutathione levels in epithelial lining and BALF of patients with idiopathic pulmonary fibrosis accompanied by an improvement of pulmonary function tests (Meyer et al., 1994; Behr et al., 1997). In the present study, we found a decrease of GSH accompanied by an increase of GSSG levels in lung tissue which translates into lower GSH/GSSG ratios at 3 days after exposure to bleomycin, thus confirming the early oxidative stress present in this model.
At the same time, the amount of LPO in BALF was increased, and alveolar macrophages release larger amounts of superoxide anion and nitric oxide, which is in keeping with the findings of other studies (Yamazaki et al., 1998; Hagiwara et al., 2000; Punithavathi et al., 2000), and indicates the existence of an important oxidant burden imposed on pulmonary tissues. NAC (oral or i.p.) did not significantly change the GSH levels but partly diminished levels of GSSG, thus increasing the GSH/GSSG ratio, which indicates an attenuation of oxidant stress. Giri et al. (1988) also reported that NAC (200 mg kg−1 per day for 13 days; i.p.) improved the lung glutathione redox state in bleomycin-exposed hamsters, and we also found higher glutathione levels in BALF of bleomycin-exposed rats orally treated with NAC (Cortijo et al., 2001). NAC did not inhibit the superoxide anion release by alveolar macrophages but the amount of nitric oxide was decreased. The capacity of NAC to decrease nitric oxide production in rat macrophages has been previously demonstrated (Pahan et al., 1998; Cuzzocrea et al., 2001). Taken together, these data indicate that NAC is endowed with direct and indirect antioxidant properties, which result in a beneficial effect against the oxidant-induced pulmonary damage elicited by bleomycin.
Another target for ROS generated by bleomycin is the activation of NF-κB, a transcription factor that regulates many cytokine genes, including those examined in this study, TNF-α, IL-1β and IL-6, and MIP-2 (Pahl, 1999). TNF-α and IL-1β may in turn also activate NF-κB (Barnes & Karin, 1997). Bleomycin augmented the NF-κB binding activity assessed as shift assay at 1, 3 and 15 days postexposure, a finding consistent with results from the mouse model (Gurujeyalakshmi et al., 2000). This sustained activation suggests a crucial role of this transcription factor in the sequential expression of pathogenetically relevant mediators of inflammation and fibrosis in this experimental model. The relevance of this target has been demonstrated with antisense oligonucleotides to NF-κB that ameliorate bleomycin-induced lung fibrosis in mice (Zhang et al., 2000). The immunohistochemical detection of NF-κB at 3 days postbleomycin indicates a small degree of immunostaining in control preparations (Figure 5), a finding consistent with the existence of constitutive NF-κB levels in alveolar macrophages from normal lung (Farver et al., 1998). Bleomycin exposure augmented the immunostaining for NF-κB in different cell types including airway epithelial cells, alveolar macrophages and polymorphonuclear cells in interalveolar septa. Staining for NF-κB in airway epithelium and inflammatory cells has also been reported in other inflammatory lung diseases (Hart et al., 1998).
We found that NAC suppressed this activation of NF-κB assessed as shift assay and immunostaining, and therefore this blocking effect may be crucial for reducing the upregulation of proinflammatory and fibrogenic cytokine genes in the bleomycin model of lung fibrosis. This finding would be consistent with the report by Blackwell et al. (1996) that NAC (200 mg kg−1, i.p.) blocks NF-κB activation in lung tissue in the acute lung injury produced by lipopolysaccharide, and also with findings in other in vivo animal models of oxidant stress (Ho et al., 1999; Cuzzocrea et al., 2001). Blocking of the activation of NF-κB has also been suggested to be the mechanism of the antifibrotic effect of other antioxidants in the bleomycin model (Gurujeyalakshmi et al., 2000).
TNF-α is an inflammatory cytokine secreted by activated alveolar macrophages that has been strongly implicated in the pathogenesis of pulmonary fibrosis and considered a potential target for therapeutic interventions (Piguet, 1990; Zhang et al., 1993; Smith et al., 1998; Ishii et al., 2000). In the present study, we confirmed that bleomycin exposure increased TNF-α levels in BALF both at early (1–3 days) and late (15 days) stages of the process initiated after bleomycin exposure. In addition, increased levels of its physiologic inhibitor, sTNF-R, were also found at the time point studied (3 days). Augmented levels of sTNF-R have been reported for other inflammatory lung diseases in which it may influence the local bioactivity of TNF-α (Suter et al., 1992; Hino et al., 1997). Our results show that NAC remarkably reduced the augmented levels of TNF-α in BALF without affecting those of sTNF-R. This result is consistent with decreases of elevated TNF-α levels recently reported for other antioxidants in the bleomycin model of pulmonary fibrosis and may be related to its blocking effect on the activation of NF-κB (Gurujeyalakshmi et al., 2000; Punithavathi et al., 2000). This inhibitory effect on TNF-α production may certainly contribute to the beneficial effect of these antioxidants including NAC against bleomycin-induced lung fibrosis.
Recent work demonstrates that IL-1β induces acute lung inflammation that converts to pulmonary fibrosis, and this cytokine is considered a valid target for therapeutic intervention in this disease (Kolb et al., 2001). TNF-α has been shown to stimulate IL-1β secretion in human alveolar macrophages (Scheule et al., 1992), and in turn IL-1β increases the expression of TNF-α and IL-6 (Kolb et al., 2001). IL-6 released from alveolar macrophages has also been implicated in the pathogenesis of human and experimental pulmonary fibrosis (Elias et al., 1990). We found augmented BALF levels of IL-β and IL-6 at the early inflammatory phase (1–3 days postbleomycin) but not at the fibrotic phase (15 days after bleomycin), which is consistent with the transient expression of these macrophage cytokines and confirms the findings in a bleomycin mouse model (Gurujeyalakshmi et al., 2000). Rats treated with NAC showed lower levels of IL-1β and IL-6 in BALF (this study), a finding consistent with the demonstrated ability of NAC to inhibit IL-1β release in carrageenan-induced pleuresy (Cuzzocrea et al., 2001) and IL-6 in lung epithelial cells (Simeonova et al., 1997). This inhibitory activity of NAC may contribute to its beneficial effect in this experimental model.
Another inflammatory cytokine relevant to the bleomycin model is the CXC chemokine MIP-2, a murine functional homologue of interleukin-8 that may also be regulated by NF-κB (Barnes & Karin, 1997; Keane et al., 1999). Recent studies indicate the importance of MIP-2 as a possible chemoattractant factor for neutrophils (Hagiwara et al., 2000) but also involved in the angiogenesis and fibrotic process (Keane et al., 1999). NAC diminished the elevated MIP-2 levels, and this effect may also contribute to the effectiveness of NAC in the bleomycin model of lung fibrosis.
NAC has also been reported in in vitro studies to interfere with other inflammatory mediators involved in lung fibrogenesis (Galis et al., 1998; Gon et al., 2000), but the influence of these mechanisms on the effects of NAC on bleomycin-induced lung fibrosis remains to be investigated. Under the conditions of the present study, the results obtained suggest that, in the acute phase of inflammation, NAC has a protective effect by acting as an antioxidant. Additional studies are required to specify further the main mechanism(s) of the beneficial effect of NAC in this model as well as the potential for NAC administration as a concomitant therapy for patients with fibrosing alveolitis, including that produced during bleomycin treatment.
Acknowledgments
This work was supported by Grant 1FD97-1143 from the European Union (Regional Development Funds, FEDER), CICYT (Spanish Government), Regional Government (Generalitat Valenciana) and Grant FIS98/1367 (Spanish Ministry of Health). We thank Dr M. Cerdá for help with immunohistochemistry.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- EMSA
electrophoretic mobility shift assay
- GSH
reduced gluthathione
- GSSG
oxidized glutathione
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- i.p.
intraperitoneal
- LDH
lactate dehydrogenase
- LPO
lipid hydroperoxide
- MIP-2
macrophage inflammatory protein-2
- NAC
N-acetylcysteine
- NF-κB
nuclear factor κB
- ROS
reactive oxygen species
- sTNF-R
soluble receptor for TNF-α
- TNF-α
tumour necrosis factor-α.
References
- ALVAREZ A., SANZ M.J. Reactive oxygen species mediate angiotensin II-induced leukocyte–endothelial cell interactions in vivo. J. Leukoc. Biol. 2001;70:199–206. [PubMed] [Google Scholar]
- ARUOMA O.I., HALLIWELL B., HOEY B.M., BUTLER J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- ASENSI M., SASTRE J., PALLARDÓ J.V., GARCÍA DE LA ASUNCIÓN J., ESTRELA J.M., VIÑA J. A high-performance liquid chromatography method for measurement of oxidized glutathione in biological samples. Anal. Biochem. 1994;217:323–328. doi: 10.1006/abio.1994.1126. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-κB—A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BEHR J., MAIER K., DEGENKOLB B., KROMBACH F., VOGELMEIR C. Antioxidative and clinical effects of high dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am. J. Respir. Crit. Care Med. 1997;156:1897–1901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- BEREND N. Inhibition of bleomycin lung toxicity by N-acetylcysteine in the rat. Pathology. 1985;17:108–110. doi: 10.3109/00313028509063736. [DOI] [PubMed] [Google Scholar]
- BERNAREGGI M., RADICE S., ROSSONI G., ORIANI G., CHIESARA E., BERTI F. Hyperbaric oxygen increases plasma exudation in rat trachea: involvement of nitric oxide. Br. J. Pharmacol. 1999;126:794–800. doi: 10.1038/sj.bjp.0702354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKWELL T.S., BLACKWELL T.R., HOLDEN E.P., CHRISTMAN B.W., CHRISTMAN J.W. In vivo antioxidant treatment suppresses nuclear factor-κB activation and neutrophilic lung inflammation. J. Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRIGELIUS R., MUCKEL C., AKERBOOM T.P.M., SIES H. Identification and quantitation of glutathione in hepatic protein mixed disulfides and its relationships to glutathione disulfide. Biochem. Pharmacol. 1983;32:2529–2534. doi: 10.1016/0006-2952(83)90014-x. [DOI] [PubMed] [Google Scholar]
- CANTIN A.M., LARIVEE P., BEGIN R.O. Extracellular glutathione suppress human lung fibroblast proliferation. Am. J. Respir. Cell Mol. Biol. 1990;3:79–85. doi: 10.1165/ajrcmb/3.1.79. [DOI] [PubMed] [Google Scholar]
- CLOSA D., SABATER L., FERNÁNDEZ CRUZ L., PRATS N., GELPI E., ROSELLÓ-CATAFAU J. Activation of alveolar macrophages in lung injury associated with experimental acute pancreatitis is mediated by the liver. Ann. Surg. 1999;229:230–236. doi: 10.1097/00000658-199902000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COKER R.K., LAURENT G.J. Pulmonary fibrosis: cytokines in the balance. Eur. Respir. J. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- CORTIJO J., CERDA-NICOLAS M., SERRANO A., BIOQUE G., ESTRELA J.M., SANTANGELO F., ESTERAS A., LLOMBART-BOSCH A., MORCILLO E.J. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur. Respir. J. 2001;17:1228–1235. doi: 10.1183/09031936.01.00049701. [DOI] [PubMed] [Google Scholar]
- COTGREAVE I.A.N-acetylcysteine: pharmacological considerations and experimental and clinical applications Antioxidants in Disease: Mechanisms and Therapy. Advances in Pharmacology 1997New York, NY: Academic Press; 205–227.Vol. 38. ed. Sies, H. pp [PubMed] [Google Scholar]
- CUZZOCREA S., MAZZON E., DUGO L., SERRAINO I., CICCOLO A., CENTORRINO T., DE SARRO A., CAPUTI A.P. Protective effects of n-acetylcysteine on lung injury and red blood cell modification induced by carrageenan in the rat. FASEB J. 2001;15:1187–1200. doi: 10.1096/fj.00-0526hyp. [DOI] [PubMed] [Google Scholar]
- DERYCKERE F., GANNON F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994;16:405. [PubMed] [Google Scholar]
- ELIAS J.A., FREUNDLICH B., KERN J.A., ROSENBLOOM J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990;97:1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- FARUQI R.M., POPTIC E.J., FARUQI T.R., DE LA MOTTE D., DICORLETO P.E. Distinct mechanisms for N-acetylcysteine inhibition of cytokine-induced E-selectin and VCAM-1 expression. Am. J. Physiol. 1997;273:H817–H826. doi: 10.1152/ajpheart.1997.273.2.H817. [DOI] [PubMed] [Google Scholar]
- FARVER C.F., RAYCHAUDHURI B., BUHROW L.T., CONNORS M.J., THOMASSEN M.J. Constitutive NF-κB levels in human alveolar macrophages from normal volunteers. Cytokine. 1998;10:868–871. doi: 10.1006/cyto.1998.0373. [DOI] [PubMed] [Google Scholar]
- FOLCH E., CLOSA D., PRATS N., GELPÍ E., ROSELLÓ-CATAFAU J. Leukotriene generation and neutrophil infiltration after experimental acute pancreatitis. Inflammation. 1998;22:83–93. doi: 10.1023/a:1022399824880. [DOI] [PubMed] [Google Scholar]
- GALIS Z.S., ASANUMA K., GODIN D., MENG X. N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells. New target for antioxidant therapy. Circulation. 1998;97:2445–2453. doi: 10.1161/01.cir.97.24.2445. [DOI] [PubMed] [Google Scholar]
- GIRI S.N.Pharmacologic perspective in pulmonary fibrosis research Focus on Pulmonary Pharmacology and Toxicology 1990Boca Raton, FL: CRC Press; 19–55.ed. Hollinger, M.A. pp [Google Scholar]
- GIRI S.N., HYDE D.M., SCHIEDT M.J. Effects of repeated administration of N-acetyl-L-cysteine on sulfhydryl levels of different tissues and bleomycin-induced lung fibrosis in hamsters. J. Lab. Clin. Med. 1988;111:715–724. [PubMed] [Google Scholar]
- GON Y., HASHIMOTO S., NAKAYAMA T., MATSUMOTO K., KOURA T., TAKESHITA I., HORIE T. N-acetyl-L-cysteine inhibits bleomycin-induced interleukin-8 secretion by bronchial epithelial cells. Respirology. 2000;5:309–313. [PubMed] [Google Scholar]
- GRECO M.J., KEMNITZER J.E., FOX J.D., CHOE J.K., KOHN J., RILEY D.J., POIANI G.J. Polymer of proline analogue with sustained antifibrotic activity in lung fibrosis. Am. J. Respir. Crit. Care Med. 1997;155:1391–1397. doi: 10.1164/ajrccm.155.4.9105084. [DOI] [PubMed] [Google Scholar]
- GURUJEYALAKSHMI G., WANG Y., GIRI S.N. Taurine and niacin block lung injury and fibrosis by down-regulating bleomycin-induced activation of transcription nuclear factor-κB in mice. J. Exp. Pharmacol. Ther. 2000;293:82–90. [PubMed] [Google Scholar]
- HAGIWARA S.I., ISHII Y., KITAMURA S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am. J. Respir. Crit. Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- HART L.A., KRISHNAN V.L., ADCOCK I.M., BARNES P.J., CHUNG K.F. Activation and localization of transcription factor, nuclear factor-κB, in asthma. Am. J. Respir. Crit. Care Med. 1998;158:1585–1592. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- HAY J., SHAHZEIDI S., LAURENT G. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- HINO T., NAKAMURA H., SHIBATA Y., ABE S., KATO S., TOMOIKE H. Elevated levels of type II soluble tumor necrosis factor receptors in the bronchoalveolar lavage fluids of patients with sarcoidosis. Lung. 1997;175:187–193. doi: 10.1007/pl00007566. [DOI] [PubMed] [Google Scholar]
- HO E., CHEN G., BRAY T.M. Supplementation of N-acetylcysteine inhibits NF-κB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J. 1999;13:1845–1854. [PubMed] [Google Scholar]
- ISHII Y., HIRANO K., MORISHIMA Y., MASUYAMA K., GOTO Y., NOMURA A., SAKAMOTO T., UCHIDA Y., SAGAI M., SEKIZAWA K. Early molecular and cellular events of oxidant-induced pulmonary fibrosis in rats. Toxicol. Appl. Pharmacol. 2000;167:173–181. doi: 10.1006/taap.2000.8990. [DOI] [PubMed] [Google Scholar]
- JAMIESON D.D., KERR D.R., UNSWORTH I. Interaction of N-acetylcysteine and bleomycin on hyperbaric oxygen-induced lung damage in mice. Lung. 1987;165:239–247. doi: 10.1007/BF02714441. [DOI] [PubMed] [Google Scholar]
- JOHNSTON R.B. Measurement of O2− secreted by monocytes and macrophages. Meth. Enzymol. 1984;105:365–369. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
- KEANE M.P., BELPERIO J.A., MOORE T.A., MOORE B.B., ARENBERG D.A., SMITH R.E., BURDICK M.D., KUNKEL S.L., STRIETER R.M. Neuralization of the CXC chemokine, macrophage inflammatory protein-2, attenuates bleomycin-induced pulmonary fibrosis. J. Immunol. 1999;162:5511–5518. [PubMed] [Google Scholar]
- KOLB M., MARGETTS P.J., ANTHONY D.C., PITOSSI F., GAULDIE J. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZENBY A.J., CROUCH E.C., MCDONALD J.A., KUHN C., III Remodeling of the lung in bleomycin-induced pulmonary fibrosis in the rat. Am. Rev. Respir. Dis. 1990;142:206–214. doi: 10.1164/ajrccm/142.1.206. [DOI] [PubMed] [Google Scholar]
- MACNEE W., RAHMAN I. Oxidants/antioxidants in idiopathic pulmonary fibrosis. Thorax. 1995;50 Suppl 1:S53–S58. doi: 10.1136/thx.50.suppl_1.s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER A., BUHL R., KAMPF S., MAGNUSSEN H. Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals. Am. J. Respir. Crit. Care Med. 1995;152:1055–1060. doi: 10.1164/ajrccm.152.3.7663783. [DOI] [PubMed] [Google Scholar]
- MEYER A., BUHL R., MAGNUSSEN H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur. Respir. J. 1994;7:431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- PAHAN K., SHEIKH F.G., NAMBOODIRI A.M.S., SINGH I. N-acetylcysteine inhibits induction of NO production by endotoxin or cytokine stimulated rat peritoneal macrophages, C6 glial cells and astrocytes. Free Radic. Biol. Med. 1998;24:39–48. doi: 10.1016/s0891-5849(97)00137-8. [DOI] [PubMed] [Google Scholar]
- PAHL H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6856. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- PIGUET P.F. Is ‘tumor necrosis factor' the major effector of pulmonary fibrosis. Eur. Cytokine Netw. 1990;1:257–258. [PubMed] [Google Scholar]
- PUNITHAVATHI D., VENKATESAN N., BABU M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHMAN I., MACNEE W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- SCHEULE R.K., PERKINS R.C., HAMILTON R., HOLIAN A. Bleomycin stimulation of cytokine secretion by the human alveolar macrophage. Am. J. Physiol. 1992;262:L386–L391. doi: 10.1152/ajplung.1992.262.4.L386. [DOI] [PubMed] [Google Scholar]
- SERRANO-MOLLAR A., CLOSA D., CORTIJO J., MORCILLO E.J., PRATS N., GIRONELLA M., PANES J., ROSELLO-CATAFAU J., BULBENA O. P-selectin upregulation in bleomycin induced lung injury in rats: effect of N-acetyl-L- cysteine. Thorax. 2002;57:629–634. doi: 10.1136/thorax.57.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAHZEIDI S., SARNSTRAND B., JEFFREY P.K., MCANULTY R.J., LAURENT G.J. Oral N-acetylcysteine reduces bleomycin-induced collagen deposition in the lungs of mice. Eur. Respir. J. 1991;4:845–852. [PubMed] [Google Scholar]
- SIMEONOVA P.P., TORIUMI W., KOMMINENI C., ERKAN M., MUNSON A.E., ROM W.N., LUSTER M.I. Molecular regulation of IL-6 activation by asbestos in lung epithelial cells. Role of reactive oxygen species. J. Immunol. 1997;159:3921–3928. [PubMed] [Google Scholar]
- SMITH R.E., STRIETER R.M., PHAN S.H., LUKACS N., KUNKEL S.L. TNF and IL-6 mediate MIP-1α expression in bleomycin-induced lung injury. J. Leukoc. Biol. 1998;64:528–536. [PubMed] [Google Scholar]
- STRAUSZ J., MÜLLER-QUERNHEIM J., STEPPLING H., FERLINZ R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- SUTER P.M., SUTER S., GIRARDIN E., OUX-LOMBARD P., GRAU G.E., DAYER J.M. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am. Rev. Respir. Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- TAMAGAWA K., TAOOKA Y., MAEDA A., HIYAMA K., ISHIOKA S., YAMAKIDO M. Inhibitory effects of a lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 2000;161:1279–1284. doi: 10.1164/ajrccm.161.4.9906099. [DOI] [PubMed] [Google Scholar]
- TEPEL M., VAN DER GIET M., SCHWARZFELD C., LAUFER U., LIERMANN D., ZIDEK W. Prevention of radiographic contrast agent-induced reductions in renal function by acetylcysteine. N. Engl. J. Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- TRUSH M.A., EGNER P.A., KENSLER T.W. Myeloperoxidase as a biomarker of skin irritation and inflammation. Food Chem. Toxicol. 1994;32:143–147. doi: 10.1016/0278-6915(94)90175-9. [DOI] [PubMed] [Google Scholar]
- WARD H.E., NICHOLSON A., BEREND N. Failure of N-acetylcysteine to protect the rat lung against bleomycin toxicity. Pathology. 1987;19:358–360. doi: 10.3109/00313028709103883. [DOI] [PubMed] [Google Scholar]
- WOESSNER J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this ammino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI Y., HOSHINO J., SEKIGUCHI T., HORI Y., MIYAUCHI S., MIZUNO S., HORIE K. Production of superoxide and nitric oxide by alveolar macrophages in the bleomycin-induced interstitial pneumonia mice model. Jpn. J. Pharmacol. 1998;78:69–73. doi: 10.1254/jjp.78.69. [DOI] [PubMed] [Google Scholar]
- ZHANG X.Y., SHIMURA S., MASUDA T., SAITOH H., SHIRATO K. Antisense oligonucleotides to NF-κB improve survival in bleomycin-induced pneumopathy of the mouse. Am. J. Respir. Crit. Care Med. 2000;162:1561–1568. doi: 10.1164/ajrccm.162.4.9908093. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., LEE T.C., GUILLEMIN B., YU M., ROM W.N. Enhanced IL-1β and tumor necrosis factor-α release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J. Immunol. 1993;150:4188–4196. [PubMed] [Google Scholar]