Abstract
In phenylephrine (PHE) (1 μM)-precontracted superior mesenteric arteries from adult rats, low concentration of hydrogen peroxide (H2O2, 10–100 μM) caused only contraction, while high concentration of H2O2 (0.3–1 mM) caused a biphasic response: a transient contraction followed by a relaxation response.
Endothelium removal did not affect the biphasic response. 7,7-Dimethyl-(5Z,8Z)-eicosadienoic acid, diclofenac, furegrelate, or SQ 29548 greatly inhibited the contraction but did not affect the relaxation. 17-Octadecynoic acid, eicosatriynoic acid, ICI 198615, SQ 22536, or ODQ did not inhibit the biphasic response.
KCl at 40 mM inhibited the relaxation response to H2O2 by 98±24%. 4-Aminopyridine (4-AP) inhibited while tetraethylammonium chloride (TEA), charybdotoxin, or glibenclamide attenuated the relaxation response. A combination of 4-AP, TEA and glibenclamide mimicked the effects of 40 mM KCl. Iberiotoxin, apamin, or barium chloride did not inhibit the relaxation response.
H2O2 at 1 mM hyperpolarized membrane potential and reversibly augmented K+ current in smooth muscle cells of mesenteric artery. These effects of H2O2 were attenuated significantly by 4-AP.
In summary, in PHE-precontracted rat mesenteric artery: (1) the response to H2O2 shifted qualitatively from contraction to a biphasic response as H2O2 increased to 0.3 mM or higher; (2) the relaxation response is caused by the activation of K+ channels, with voltage-dependent K+ channels playing a primary role; and the contraction is likely to be mediated by thromboxane A2; (3) the K+ channel activation by H2O2 is independent of phospholipase A2, cyclooxygenase, lipoxygenase, cytochrome P450 monooxygenase, adenylate or guanylate cyclase.
Keywords: 4-Aminopyridine, contraction, hydrogen peroxide, mesenteric artery, potassium channels, rat, reactive oxygen species, relaxation
Introduction
Hydrogen peroxide (H2O2), a nonradical form of reactive oxygen species, can be produced by vascular endothelium, smooth muscle cells, fibroblasts, and activated polymorphonuclear leukocytes and macrophages (Griendling et al., 2000). H2O2 may participate in local regulation of blood flow, because H2O2 is a vasomotor agent, causing vessels to constrict (Hibino et al., 1999) or to relax (Fujimoto et al., 2001).
In quiescent vessels, H2O2 has been shown to cause vasoconstriction (Katusic et al., 1993; Rodriguez-Martinez et al., 1998). The mechanisms include the activation of phospholipase A2 (PLA2) (Chakraborti et al., 1989), cyclooxygenase (COX) (Rodriguez-Martinez et al., 1998), phospholipase C (Yang et al., 1998b), and tyrosine kinase (Jin & Rhoades, 1997). In rat mesenteric arteries, we have previously reported that H2O2-induced contraction was through the PLA2 – COX – thromboxane A2 (TXA2) synthase pathway mediated by TXA2 (Gao & Lee, 2001).
In vessels precontracted with agonists, H2O2 caused a relaxation response in rat and rabbit aorta (Zembowicz et al., 1993; Iesaki et al., 1994), porcine and canine coronary arteries (Rubanyi & Vanhoutte, 1986; Barlow & White, 1998), cat and canine cerebral arteries (Fraile et al., 1994; Yang et al., 1998a), and rabbit mesenteric artery (Fujimoto et al., 2001). There was no general agreement on the mechanisms involved in the relaxation by H2O2. For example, one study using rat aorta has attributed H2O2 vasorelaxation to an endothelium-dependent mechanism (Yang et al., 1999), whereas another report using porcine coronary arteries has found that the relaxation was endothelium-independent (Barlow & White, 1998). Some studies have shown that H2O2 activates K+ channels, but the results are conflicting regarding the subtype of K+ channels involved: Ca2+-dependent K+ channels (KCa) in porcine coronary arteries (Barlow & White, 1998) and ATP-dependent K+ channels (KATP) in feline pial arteries (Wei et al., 1996). The possible role of voltage-dependent K+ channels (KV) and inward rectifier K+ channels (KIR) in H2O2-induced relaxation was not fully elucidated.
In the present study, by precontracting rat mesenteric artery with phenylephrine (PHE) to a submaximal level, we found that higher concentration of H2O2 (≥0.3 mM) caused a biphasic response: a transient contraction followed by a relaxation, while lower concentrations of H2O2 (10 and 100 μM) only caused contraction. We hypothesized that the two components of the biphasic response, contraction and relaxation, are mediated by different mechanisms. This knowledge may further our understanding about the role H2O2 may play in the regulation of vascular functions under certain pathological conditions such as inflammation and ischemia-reperfusion injury, which may involve burst production of reactive oxygen species.
Methods
Animals
Male Wistar–Kyoto rats (WKY) at the age of 6–8 months were obtained from the rat colony maintained at the McMaster University Central Animal Facilities. The care and the use of these animals were in accordance with the guidelines of the Canadian Council on Animal Care.
Reactivity experiments
The procedure for preparing mesenteric arterial rings and the components of Krebs solution have been described in our previous report (Gao & Lee, 2001). Briefly, 4 mm long segments of the mesenteric artery (lumen diameter 1.5–2 mm) were equilibrated under a resting tension of 14.7 mN for 1 h in a computerized organ bath system for isometric tension recording. The endothelium was mechanically removed and successful removal was indicated by the absence of relaxation response to 1 μM carbamycholine chloride. A single concentration of H2O2 (10 μM–1 mM) was added when the precontraction to PHE (1 μM, 70–80% of the maximal contraction) had reached a plateau, and 5–10 min reaction time with the artery was allowed, followed by a thorough wash with Krebs solution. The precontraction by PHE was sustained for at least 30 min (data not shown). Each ring was exposed to only one concentration of H2O2, and the concentration–response relation for H2O2 was established using different rings. Catalase, or superoxide dismutase, or dimethylsulfoxide (DMSO) was introduced for 5–10 min, whereas enzyme inhibitors and receptor antagonists were added 25 min before exposure to H2O2. Generally, five to six rings were obtained from the middle part of the mesenteric arcade from each rat. There was no significant difference among the rings in their response to H2O2. We randomly took one ring as control, and the other rings to test various agents. An increase or decrease in tension (in mN) from the precontraction level and the time required for the relaxation to reach its maximum (in minutes), as indicated by a, b, and c, respectively, in Figure 1c, were used to quantify the contraction and the relaxation response to H2O2. The enzyme inhibitors employed and their targets (in parentheses) were: 7,7-dimethyl-5,8-eicosadienoic acid (DMDA, PLA2), diclofenac (COX), furegrelate (TXA2 synthase), 17-octadecynoic acid (17-ODA, cytochrome P 450 monooxygenase (P 450)), eicosatriynoic acid (ETI, lipoxygenase (LOX)), SQ 22536 (adenylate cyclase), ODQ (guanylate cyclase). Receptor antagonists were: SQ 29548 (TXA2/prostaglandin H2 (TP) receptor), ICI 198615 (leukotriene receptor). K+ channel blockers were: 4-aminopyridine (4-AP, KV), tetraethylammonium chloride (TEA, KCa), glibenclamide (KATP), barium chloride (KIR), and more selective KCa blockers: iberiotoxin, charybdotoxin, and apamin. The concentration chosen for each drug was based on the information from the literatures for studies involving vascular tissues, and our preliminary experiments in order to avoid nonspecific effects. In some cases, another inhibitor or antagonist that is different in structure was used to verify its pharmacological effects.
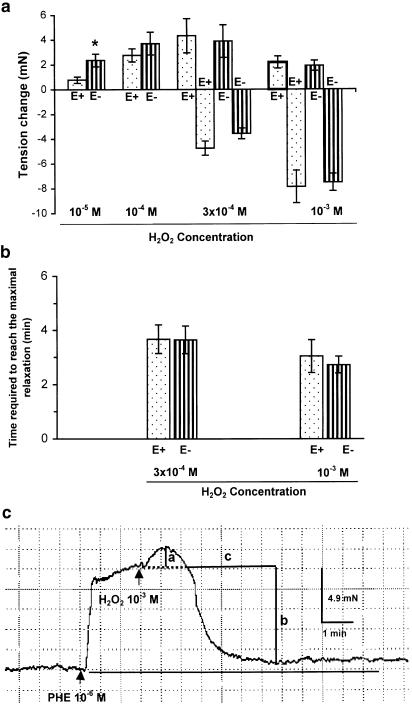
Figure 1.
(a) Concentration – response relation of H2O2 in PHE (10−6 M)-precontracted superior mesenteric arteries from WKY rats with (E+) or without (E−) endothelium. Low concentration of H2O2 (10−5 M, 10−4 M) caused contraction (positive), and high concentration (3 × 10−4 M, 10−3 M) caused a transient contraction followed by a profound relaxation (negative). Results (mean±s.e.) were from four to six rats. *P<0.05 compared with respective E+ arteries. Tension (in mN) generated by PHE was 8.7±1.6 (E+, n=6) and 8.6±0.9 (E−, n=6). (b) Time (in minutes) required for the relaxation to reach the maximal. (c) Typical tracing showing the biphasic response to H2O2 (10−3 M) in WKY mesenteric artery with endothelium removed. The contraction (a) and relaxation (b) were expressed in mN, and the time (c) required to reach the maximal relaxation was expressed in minutes.
Electrophysiological experiments
Microelectrode study
The arterial segments were superfused at a rate of 3 ml min−1 with the Krebs–Ringer's buffer (in mM: NaCl, 116; KC1, 4.2; CaCl2, 2.5; NaH2PO4, 1.6; MgSO4, 1.2; NaHCO3, 22; D-glucose, 11; pH 7.4), bubbled with 95% O2/5% CO2 and heated to 37°C. During incubation with channel blockers, superfusion was paused and the chamber was directly bubbled with 95% O2 and 5% CO2. The tip resistance of the microelectrodes used was within 30 – 100 MΩ when filled with 3 M KCl. Membrane potential changes were measured and amplified at 37°C on a Duo 773 Electrometer (World Precision Instruments Inc., Sarasota, FL, U.S.A.), digitally sampled at 5 Hz and analyzed using WinDaq 700 series data acquisition software (Dataq Instruments Inc., Akron, OH, U.S.A.).
Patch-clamp studies
Mesenteric artery tissues were minced and transferred to Ringer's based solution (in mM: 130 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 20 HEPES, 10 D-glucose; pH 7.4) containing collagenase (0.9 U ml−1) and elastase (12.5 U ml−1) and incubated at 37°C for 1 h to liberate individual smooth muscle cells. These were allowed to settle and adhere to the bottom of a recording chamber (1 ml volume) and superfused with standard Ringer's solution at room temperature. Electrophysiological responses were tested in cells that were phase dense and appeared relaxed. Recordings were made at room temperature using the nystatin perforated-patch configuration of the conventional patch-clamp recording technique (Horn & Marty, 1988) and pipettes with tip resistance of 3–5 MΩ when filled with electrode solution containing (in mM) 140 KCl, 1 MgCl2, 0.4 CaCl2, 20 HEPES, 1 EGTA, and 150 U ml−1 nystatin (pH 7.2) were used. Membrane currents were filtered at 5 kHz and sampled at 2 Hz. Acquisition and analysis of data were accomplished using Axopatch 200B and pCLAMP8 software (Axon Instruments, Foster City, CA, USA).
Chemicals
The following chemicals were used: H2O2 and DMSO (BDH Inc., Toronto, Canada); 4-AP, apamin, barium chloride, catalase, carbamycholine chloride, charybdotoxin, furegrelate sodium salt, glibenclamide, iberiotoxin, superoxide dismutase, TEA (Sigma, U.S.A.); diclofenac sodium, SQ 29548 (RBI, Sigma, U.S.A.); DMDA, ETI, 17-ODA (Cayman Chemical, U.S.A.); ODQ, SQ 22536 (RBI, Sigma, U.S.A.); ICI 198615 (a gift from Zeneca, Alderley Park, U.K.). DMDA, ETI, 17-ODA, SQ 29548 were dissolved in absolute ethanol and diluted in 50% ethanol; glibenclamide, ICI 198615 were dissolved in DMSO; apamin, charybdotoxin, and iberiotoxin were dissolved in oxygen-free water. All other agents were dissolved in deionized water and prepared fresh daily.
Statistical analysis
The results were expressed as mean±s.e.m. where n represents the number of rats. Statistical analysis was performed by one-way ANOVA or unpaired Student's t-test. The differences were considered significant when P⩽0.05.
Results
In PHE (1 μM)-precontracted mesenteric artery, H2O2 caused contraction at the concentrations of 10–100 μM, but produced a biphasic response: a transient contraction followed by a profound and persistent relaxation, at the concentrations of 0.3–1 mM. Removal of endothelium increased the contraction at low concentration (10 μM), but did not affect the biphasic responses at high concentrations of H2O2 (0.3–1 mM) (Figure 1a–c).
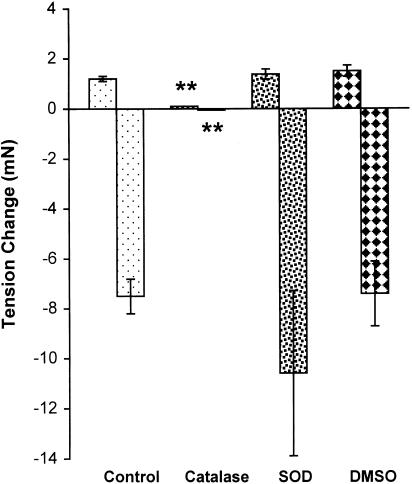
The experiments reported below were carried out with 1 mM H2O2 because this concentration induced an endothelium-independent biphasic response. Endothelium-denuded mesenteric artery rings were used in order to minimize the possible influence of endothelium-derived vasoactive factors. Catalase (1000 U ml−1), a H2O2-decomposing enzyme, inhibited the biphasic response to H2O2, while superoxide dismutase (150 U ml−1), a superoxide scavenger, and DMSO (5 mM), a hydroxyl radical scavenger, did not affect the response (Figure 2).
Figure 2.
Effects of catalase (1000 U ml−1), superoxide dismutase (SOD, 150 U ml−1), and DMSO (5 mM), on the biphasic response to H2O2 (10−3 M) in the PHE-precontracted mesenteric artery without endothelium. Results (mean±s.e.) were from four to six rats. **P<0.01 compared with control.
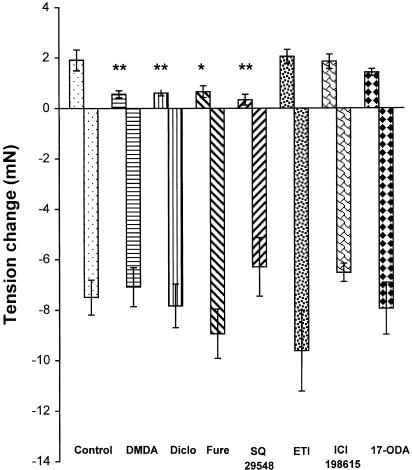
PLA2 inhibitor DMDA (100 μM), COX inhibitor diclofenac (10 μM), TXA2 synthase inhibitor furegrelate (100 μM), and TP receptor antagonist SQ 29548 (10 μM) significantly inhibited the contraction component, but did not affect the relaxation response. ETI (10 μM, a nonselective LOX inhibitor), 17-ODA (3 μM, a P 450 inhibitor), and ICI 198615 (1 μM, a leukotriene receptor antagonist) had no effect on either the contractions or the relaxations (Figure 3). The time required for the relaxation to reach its maximal was not affected by these enzyme inhibitors or receptor antagonists (data not shown). All these agents did not modify the baseline tension of the vessels.
Figure 3.
Effects of enzyme inhibitors and receptor antagonists on H2O2 (10−3 M)-induced contraction and relaxation in endothelium-denuded mesenteric artery. DMDA, 7,7-dimethyl-(5Z, 8Z)-eicosadienoic acid (0.1 mM); Diclo, diclofenac (10 μM); Fure, furegrelate (0.1 mM); SQ 29548 (10 μM); ETI, eicosatriynoic acid (10 μM); ICI 198615 (1 μM); 17–ODA, 17-octadecynoic acid (3 μM). Results (mean±s.e.) were from four to 10 rats. *P<0.05, **P<0.01 compared with control.
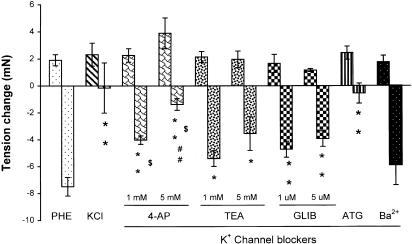
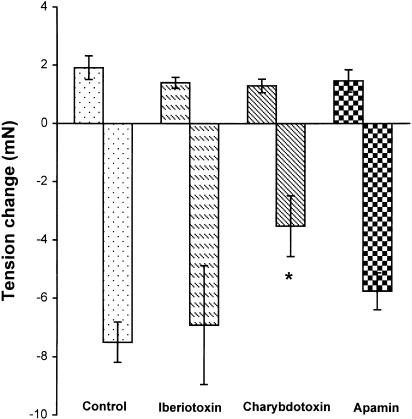
In arteries precontracted with KCl (40 mM), the relaxation component of the response to H2O2 was inhibited by 98±24%. This result indicated the involvement of K+ channels in the relaxation. To identify which subtype of K+ channels was involved, K+ channel blockers were used. 4-AP, a delayed rectifier K+ channel blocker, which initialized a transient or a sustained increase of vascular tone at 1 or 5 mM (data not shown), inhibited the relaxation component by 46±4 and 82±6%, respectively. TEA (1 and 5 mM, a nonselective KCa channel blocker), which also increased vessel tone to a similar degree as 4-AP (data not shown), attenuated the relaxation by 28±8 and 53±16%, respectively. The KATP channel blocker glibenclamide (that did not affect vessel tone) attenuated the relaxation by 37±8 and 48±8% at 1 and 10 μM, respectively, but KIR channel blocker barium chloride (100 μM) did not inhibit the relaxation. The inhibition by 4-AP was significantly greater than those by TEA, and the inhibition by 5 mM 4-AP was greater than that by 10 μM glibenclamide. A combination of 4-AP (5 mM), TEA (5 mM), and glibenclamide (10 μM), which caused a sustained increase of the tone (data not shown), inhibited the relaxation by 93±8%. These results are summarized in Figure 4. 4-AP and TEA at 5 mM prolonged the time required for the relaxation to reach its maximum (in minutes, control: 2.7±0.3, n=12; 4-AP: 3.8±0.3, n=6, P<0.05 versus control; TEA: 4.3±0.4, n=4, P<0.05 versus control). To further differentiate the subtypes of KCa channels involved, more selective blockers were employed. Charybdotoxin (100 nM, an intermediate large conductance KCa blocker), which increased vessel tone transiently (data not shown), reduced the relaxation by 53±14%; however, neither iberiotoxin (1 μM, a large conductance KCa channel blocker) nor apamin (100 nM, a small conductance KCa channel blocker) inhibited the relaxation significantly (Figure 5).
Figure 4.
Effects of KCl (40 mM) and a series of K+ channel blockers on H2O2 (10−3 M)-induced relaxation in endothelium-denuded mesenteric artery precontracted with PHE. 4-AP; TEA; GLIB, glibenclamide; ATG, a combination of 4-AP (5 mM), TEA (5 mM) and GLIB (10 μM); and Ba2+, barium chloride (0.1 mM). Results (mean±s.e.) were from four to 12 rats. *P<0.05, **P<0.01 compared with control; $P<0.05 versus respective TEA; ##P<0.01 versus GLIB (10−5 M).
Figure 5.
Effects of iberiotoxin (1 μM), charybdotoxin (100 nM), and apamin (100 nM) on H2O2 (10−3 M)-induced relaxation in endothelium-denuded rat mesenteric artery precontracted with PHE (1 μM). Results (mean±s.e.) were from four to six rats. *P<0.05 compared with control. The time required for the relaxation to reach the maximal was not affected by these agents (data not shown).
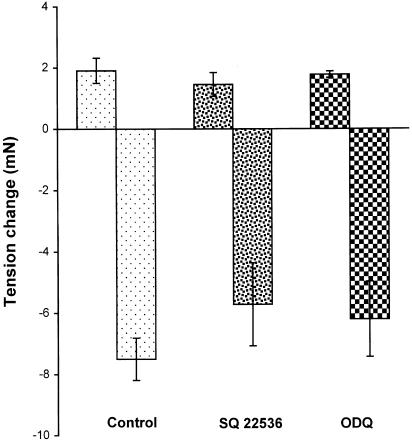
The possible involvement of cAMP and cGMP in H2O2-induced relaxation was investigated. Neither the adenylate cyclase inhibitor SQ 22536 (100 μM) nor the guanylate cyclase inhibitor ODQ (100 μM) had any effect on the biphasic responses to H2O2 (Figure 6).
Figure 6.
Effects of SQ 22536 (100 μM) or ODQ (100 μM) on H2O2 (10−3 M)-induced biphasic response in endothelium-denuded mesenteric artery precontracted with PHE. Results (mean±s.e.) were from three to six rats.
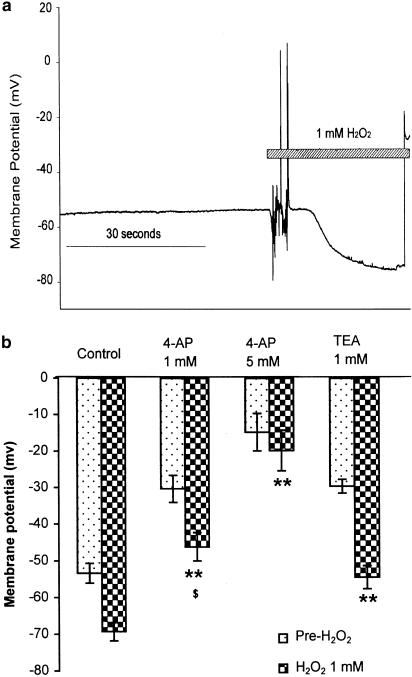
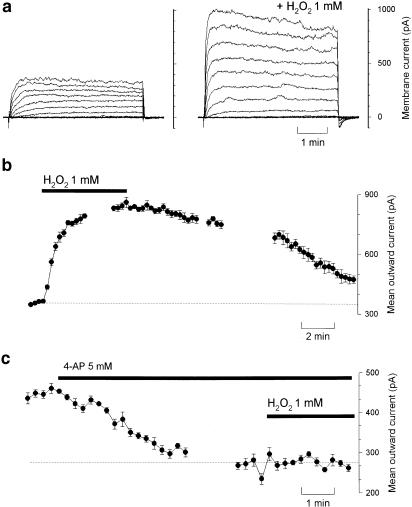
The electrophysiological experiments were performed in the absence of phenylephrine. H2O2 (1 mM) induced a significant hyperpolarization of smooth muscle cells of the mesenteric artery (Figure 7). Treatment with 4-AP (1 and 5 mM), which depolarized the smooth muscle cells by 28±8 and 44±4 mV respectively, reduced the hyperpolarization induced by H2O2 by 34±5 and 72±7%, respectively. The attenuation of H2O2-induced hyperpolarization by TEA (1 mM, 21±3%) was less than that by 4-AP (P<0.05), although the depolarizations by TEA (24±4 mV) and 4-AP (28±8 mV) were similar. In whole-cell patch-clamp studies using single smooth muscle cells, H2O2 (1 mM in application pipette brought into proximity with the cell) augmented outward K+ currents evoked by incrementing step depolarizations (10 mV increments from the holding potential of −70 mV, 250 ms duration, Figure 8a). The time-course of this effect was investigated using step depolarization to +10 mV delivered at 15 s intervals: the augmentation developed over the course of 2–3 min and this was reversed upon wash-out of H2O2 (Figure 8b). On average, the K+ currents were augmented by 92±25% (Figure 8b). Application of water itself had essentially no effect (outward currents were 102±5% of control). When voltage-dependent K+ currents were first abolished by pretreating the cells with 4-AP (5 mM), the augmentation of outward currents by H2O2 was significantly reduced (to only 112±34% of control, Figure 8c).
Figure 7.
(a) H2O2 (10−3 M)-induced hyperpolarization of smooth muscle of mesenteric artery and (b) blocking effects of 4-AP and TEA. Results (mean±s.e.) were from four to 11 rats. **P<0.01 compared with control; $P<0.05 versus TEA (1 mM).
Figure 8.
H2O2-augmented K+ current of a freshly isolated superior mesenteric artery smooth muscle cell. (a) K+ currents were evoked using depolarizing pulses (10 mV increments from a holding potential of −70 mV; pulse duration 250 ms) before and after the application of H2O2 (10−3 M in the application pipette). (b) Simple stepwise depolarization from −70 to +10 mV delivered at 15 s intervals were used to monitor the time-course of the augmentation caused by H2O2 in this cell, showing both the progressive enhancement of the current as well as the restoration toward baseline levels upon wash-out of H2O2. (c) This same strategy was used in a different cell that had been exposed to 4-AP (5 mM). Under these conditions, H2O2 had no effect on the 4-AP-resistant K+ currents.
Discussion
H2O2 (≥0.3 mM) produced a biphasic response: a transient contraction component and a profound and persistent relaxation response in rat mesenteric artery. This study was designed to explore the underlying mechanisms of this biphasic response. The major findings of this study are: (1) TXA2 generated from PLA2 – COX – TXA2 synthase pathway mediates the transient contractile component; (2) activation of delayed rectifier K+ channels plays a primary role in H2O2-induced relaxation; and (3) activation of K+ channels appears to be independent of PLA2, COX, LOX, P 450, adenylate cyclase and guanylate cyclase; therefore, the relaxation effects of H2O2 is probably a direct action of H2O2 on K+ channels.
H2O2 has been used to induce experimental oxidative stress in isolated arteries. Most of the previous studies showed either a contractile or a relaxation response, depending on tissue origin, animal species, and contractile state (quiescent or precontracted). There is only one study (Leffler et al., 1990), as far as we know, which reported the biphasic pattern of response to 10 mM H2O2 in porcine cerebral arteries; however, the concentration – response relation and the underlying mechanisms, especially for the relaxation component, were not fully documented. In the current study, we found that in rat mesenteric artery precontracted with PHE, the type of response to H2O2 was related to the concentrations applied: a contractile response at low concentration (10–100 μM), and a biphasic response at high concentration (≥0.3 mM). Rat mesenteric artery studied under isobaric pressure also showed a similar biphasic pattern to 1 mM H2O2 (data not shown), indicating that this type of response is not dependent on the type of study method. Our result is probably the first to show such a qualitative shift of vascular response (from a contraction-dominated response to a relaxation-prevailing reaction) in the same artery as local H2O2 increases to higher concentrations.
H2O2 may generate other reactive oxygen species such as hydroxyl radical (Roychoudhury et al., 1996), which in turn can cause vasorelaxation (Prasad & Bharadwaj, 1996). However, our results showed that the endothelium-independent biphasic response to 1 mM H2O2 was specific to H2O2 because catalase inhibited the biphasic response, while DMSO, a OH• scavenger, or superoxide dismutase, a O2− scavenger, did not. We have also excluded the possibility that H2O2 may oxidize PHE, thereby affecting its action. We found that incubation of PHE with l mM H2O2 (37°C, 30 min) did not attenuate the ability of PHE to contract the artery (data not shown).
The transient contractile component of PHE precontracted mesenteric artery to H2O2, which preceded the relaxation response, was significantly attenuated by inhibitors of PLA2, COX, and TXA2 synthase (DMDA, diclofenac, and furegrelate), and a TP receptor antagonist (SQ 29548), suggesting that TXA2 generated from PLA2 – COX – TXA2 synthase pathway was the mediator. This is similar to the results on quiescent rat mesenteric artery (Gao & Lee, 2001), porcine cerebral arteries (Leffler et al., 1990) and rat aorta (Rodriguez-Martinez et al., 1998).
In this study, we have used electrophysiological experiments to confirm and extend our results from the pharmacological and functional studies. The relaxation response of rat mesenteric artery to H2O2 was prevented by 40 mM KCl, suggesting the involvement of a membrane potential sensitive mechanism. Our electrophysiological data did show that H2O2 markedly hyperpolarized the mesenteric artery (microelectrode study), and significantly augmented outward K+ currents (patch-clamp study), thus corroborating our functional results.
An important finding of this study is that KV channels are playing a major role in the relaxation to H2O2 in rat mesenteric artery, because 5 mM 4-AP (a KV channel blocker) inhibited H2O2-induced relaxation, membrane hyperpolarization, and the augmentation of K+ currents by 82, 72, and 89%, respectively, and the inhibition of relaxation by 4-AP was greater than that by TEA (a nonselective KCa channel blocker) or by glibenclamide (a KATP channel blocker). The existence of KV channels in various arteries including rat mesenteric artery is well known (Nelson & Quayle, 1995; Xu et al., 1999), but the pathological role of these channels in vascular function is still less understood. Our results showed that KV channels play a role in mediating vascular relaxation response to H2O2. This finding is supported by a recent functional study with canine cerebral artery (Iida & Katusic, 2000). Our finding that KV channels play a major role in the relaxation to H2O2 is different from the reports by Barlow & White (1998) and Wei et al. (1996), who proposed that KCa or KATP channels were the target of H2O2. This discrepancy may be a result of the differences in tissue origin (mesenteric artery versus coronary artery and cerebral artery) or because of different animal species (rat versus pig and cat).
The KCa channels, involved in H2O2-induced relaxation, are probably an intermediate conductance type, because charybdotoxin, an intermediate conductance KCa channel blocker, attenuated the relaxation, while iberiotoxin or apamin had no effects. This is consistent with the report by Hayabuchi et al. (1998). KIR channels are probably not involved because barium chloride, an inhibitor for KIR channels, did not exhibit any inhibition of H2O2-induced relaxation response.
Taken together, H2O2-induced relaxation response in rat mesenteric artery involves activation of KV, KCa, and KATP channels, with KV playing a major role. Further support for this conclusion is that a combination of 4-AP (5 mM), TEA (5 mM), and glibenclamide (10 μM) inhibited the relaxation response, which mimicked the effects of 40 mM KCl.
Some studies have proposed that LOX (Barlow & White, 1998; Barlow et al., 2000), or COX (Iida & Katusic, 2000), or P450 (Yang et al., 1999) metabolites of arachidonic acid may be involved in H2O2-induced relaxation response. Our results showed that PLA2 – arachidonic acid cascade does not seem to be involved in H2O2-induced relaxation response of rat mesenteric artery, because inhibitors of these agents did not affect the relaxation response. Inhibitors of adenylate or guanylate cyclase also did not affect the relaxation response to H2O2. We speculate that activation of K+ channels is probably a direct action of H2O2, although other mechanisms such as the involvement of ceramides (Czyborra et al., 2002) need to be excluded.
Our concentration–response data showed that the relaxation occurs at the concentration of ≥300 μM H2O2. The local H2O2 concentration in vascular tissue is not known. However, according to Schraufstatter & Cochrane (1997), local concentration of superoxide can reach 5–10 mM when polymorphonuclear leukocytes are stimulated. Superoxide can be rapidly converted to H2O2 by superoxide dismutase. Furthermore, vascular tissues are rich sources of reactive oxygen species including H2O2 (Griendling et al., 2000). Therefore, the local concentration of H2O2 may reach a high level under some pathological conditions such as local acute inflammation. It was also reported that H2O2 improved postischemic functional recovery and coronary blood flow in isolated hearts (Hegstad et al., 1997), which may also be related to H2O2-induced vasodilation. In spontaneously hypertensive rats, the relaxation response of the mesenteric artery to H2O2 was less as compared with that from normotensive rats (unpublished data). Together with these findings, the present results suggest that H2O2 may act as an endogenous vasodilator particularly in some pathological conditions such as inflammation, and an attenuation of its response may be involved in some diseased states such as hypertension. An understanding of the mechanisms involved in the vascular actions of reactive oxygen species may help to develop strategies in the management of some pathological conditions, such as choosing appropriate antioxidants or specific channel blockers in the management of hypertension.
Some recent reports had suggested that H2O2 might be an endothelium-derived hyperpolarizing factor (EDHF) in mouse and human mesenteric artery (Matoba et al., 2000,2002). Our finding that H2O2 relaxes rat mesenteric artery through membrane hyperpolarization by opening K+ channels favors the notion that H2O2 may function as an EDHF in rat mesenteric artery, but the endothelial origin of H2O2 in this vessel needs to be proven, and the vasocontractile action of H2O2 does not fit the characteristics of an EDHF.
In conclusion, we have found that in rat mesenteric artery precontracted with PHE, the vasomotor effects of H2O2 depends on the concentration of H2O2 used. At submicromolar concentrations it causes contraction, whereas at higher concentrations it causes a transient contraction followed by a sustained relaxation response. The contractile response is mediated by TXA2 generated from PLA2–COX–TXA2 synthase pathway, while the relaxation response is caused by the activation of KV channels (KCa and KATP as well) through a mechanism independent of the PLA2 – arachidonic cascade or cyclic nucleotides.
Acknowledgments
We thank Drs E.E. Daniel, C.Y. Kwan, and A.K. Grover for their discussion; Dr D. Crankshaw for providing ICI 198615, and Ms Nermin Attia, Nada Albatish, and Angela Demeter for their technical assistance. Dr Yu-Jing Gao is a Research Fellow supported by the Joint Canadian Hypertension Society/Medical Research Council of Canada Special Initiative Program. This study was supported by the Heart and Stroke Foundation of Ontario, Canada.
Abbreviations
- 4-AP
4-aminopyridine
- COX
cyclooxygenase
- P 450
cytochrome P 450 monooxygenase
- DMDA
7,7-dimethyl-5,8-eicosadienoic acid
- DMSO
dimethyl sulfoxide
- EDHF
endothelium-derived hyperpolarizing factor
- ETI
eicosatriynoic acid
- H2O2
hydrogen peroxide
- K+ channels
potassium channels
- KCa
calcium-dependent potassium channels
- KATP
ATP-dependent potassium channels
- KIR
inward rectifier potassium channels
- KV
voltage-dependent potassium channels
- LOX
lipoxygenase
- MA
mesenteric artery
- 17-ODA
17-octadecynoic acid
- PHE
phenylephrine
- PLA2
phospholipase A2
- TEA
tetraethylammonium chloride
- TP
thromboxane/prostaglandin H2
- TXA2
thromboxane A2
- WKY
Wistar–Kyoto rats
References
- BARLOW R.S., EL-MOWAFY A.M., WHITE R.E. H2O2 opens BkCa, channels via the PLA2 – arachidonic acid signaling cascade in coronary artery smooth muscle. Am. J. Physiol. 2000;279:H475–H483. doi: 10.1152/ajpheart.2000.279.2.H475. [DOI] [PubMed] [Google Scholar]
- BARLOW R.S., WHITE R.E. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am. J. Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- CHAKRABORTI S., GURTNER G.H., MICHAEL J.R. Oxidant-mediated activation of phospholipase A2 in pulmonary endothelium. Am. J. Physiol. 1989;257:L430–L437. doi: 10.1152/ajplung.1989.257.6.L430. [DOI] [PubMed] [Google Scholar]
- CZYBORRA P., SAXE M., FETSCHER C., ZU HERINGDORF D.M., HERZIG S., JAKOBS K.H., MICHEL M.C., BISCHOFF A. Transient relaxation of rat mesenteric microvessels by ceramides. Br. J. Pharmacol. 2002;135:417–426. doi: 10.1038/sj.bjp.0704498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAILE M.L., CONDE M.V., SANZ L., MORENO M.J., MARCO E.J., LOPEZ d.P.A. Different influence of superoxide anions and hydrogen peroxide on endothelial function of isolated cat cerebral and pulmonary arteries. Gen. Pharmacol. 1994;25:1197–1205. doi: 10.1016/0306-3623(94)90138-4. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO S., ASANO T., SAKAI M., SAKURAI K., TAKAGI D., YOSHIMOTO N., ITOH T. Mechanisms of hydrogen peroxide- induced relaxation in rabbit mesenteric small artery. Eur. J. Pharmacol. 2001;412:291–300. doi: 10.1016/s0014-2999(00)00940-7. [DOI] [PubMed] [Google Scholar]
- GAO Y.J., LEE R.M.K.W. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. Br. J. Pharmacol. 2001;134:1639–1646. doi: 10.1038/sj.bjp.0704420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIENDLING K.K., SORESCU D., USHIO-FUKAI M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- HAYABUCHI Y., NAKAYA Y., MATSUOKA S, KURODA Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- HEGSTAD A.C., ANTONSEN O.H., YTREHUS K. Low concentrations of hydrogen peroxide improve post-ischemic metabolic and functional recovery in isolated perfused rat hearts. J. Mol. Cell Cardiol. 1997;29:2779–2787. doi: 10.1006/jmcc.1997.0513. [DOI] [PubMed] [Google Scholar]
- HIBINO M., OKUMURA K., IWAMA Y., MOKUNO S., OSANAI H., MATSUI H., TOKI Y., ITO T. Oxygen-derived free radical-induced vasoconstriction by thromboxane A2 in aorta of the spontaneously hypertensive rat. J. Cardiovasc. Pharmacol. 1999;33:605–610. doi: 10.1097/00005344-199904000-00013. [DOI] [PubMed] [Google Scholar]
- HORN R., MARTY A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IESAKI T., OKADA T., YAMAGUCHI H., OCH R. Inhibition of vasoactive amine induced contractions of vascular smooth muscle by hydrogen peroxide in rabbit aorta. Cardiovasc. Res. 1994;28:963–968. doi: 10.1093/cvr/28.7.963. [DOI] [PubMed] [Google Scholar]
- IIDA Y., KATUSIC Z.S. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- JIN N., RHOADES R.A. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am. J. Physiol. 1997;212:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- KATUSIC Z.S., SCHUGEL J., COSENTINO F., VANHOUTTE P.M. Endothelium-dependent contractions to oxygen-derived free radicals in the canine basilar artery. Am. J. Physiol. 1993;264:H859–H864. doi: 10.1152/ajpheart.1993.264.3.H859. [DOI] [PubMed] [Google Scholar]
- LEFFLER C.W., BUSIJA D.W., ARMSTEAD W.M., MIRRO R. H2O2 effects on cerebral prostanoids and pial arteriolar diameter in piglets. Am. J. Physiol. 1990;258:H1382–H1387. doi: 10.1152/ajpheart.1990.258.5.H1382. [DOI] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., KUBOTA H., MORIKAWA K., FUJIKI T., KUNIHIRO I., MUKAI Y., HIRAKAWA Y., TAKESHITA A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem. Biophys. Res. Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., NAKASHIMA M., HIRAKAWA Y., MUKAI Y., HIRANO K., KANAIDE H., TAKESHIT AA. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- PRASAD K., BHHARADWAJ L.A. Hydroxyl radical – a mediator of acetylcholine-induced vascular relaxation. J. Mol. Cell Cardiol. 1996;28:2033–2041. doi: 10.1006/jmcc.1996.0196. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTINEZ M.A, GARCIA-COHEN E.G., BAENA A.B., GONZALEZ R., SALAICES M., MARIN J. Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. Br. J. Pharmacol. 1998;125:1329–1335. doi: 10.1038/sj.bjp.0702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROYCHOUDHURY S., GHOSH S.K., CHAKRABORTI T., CHAKRABORTI S. Role of hydroxyl radical in the oxidant H2O2-mediated Ca2+ release from pulmonary smooth muscle mitochondria. Mol. Cell Biochem. 1996;159:95–103. doi: 10.1007/BF00420911. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am. J. Physiol. 1986;250:H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., COCHRANE C.G.Oxidants: types, sources, and mechanisms of injury The Lung: Scientific Foundation 1997New York: Raven; 2251–2258.2nd edn. ed. Crystal, R.G., West, J.B., Weibel, J.B. & Barnes, P.J. pp [Google Scholar]
- WEI E.P., KONTOS H.A., BECKMAN J.S. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am. J. Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- XU C., LU Y., TANG G., WANG R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am. J. Physiol. 1999;277:G1055–G1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]
- YANG Z., ZHANG A., ALTURA B.T., ALTURA B.M. Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta involvement of Ca2+ and other cellular metabolites. Gen. Pharmacol. 1999;33:325–336. doi: 10.1016/s0306-3623(99)00019-1. [DOI] [PubMed] [Google Scholar]
- YANG Z.W., ZHANG A., ALTURA B.T., ALTURA B.M. Endothelium-dependent relaxation to hydrogen peroxide in canine basilar artery a potential new cerebral dilator mechanism. Brain Res. Bull. 1998a;47:257–263. doi: 10.1016/s0361-9230(98)00120-8. [DOI] [PubMed] [Google Scholar]
- YANG Z.W., ZHENG T., ZHANG A., ALTURA B.T., ALTURA B.M. Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur. J. Pharmacol. 1998b;344:169–181. doi: 10.1016/s0014-2999(97)01576-8. [DOI] [PubMed] [Google Scholar]
- ZEMBOWICZ A., HATCHETT R.J., JAKUBOWSKI A.M., GRYGLEWSKI R.J. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br. J. Pharmacol. 1993;110:151–158. doi: 10.1111/j.1476-5381.1993.tb13785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]