Abstract
Erythropoietin (EPO) plays a significant role in the hematopoietic system, but the function of EPO as a neuroprotectant and anti-inflammatory mediator requires further definition. We therefore examined the cellular mechanisms that mediate protection by EPO during free radical injury in primary neurons and cerebral microglia.
Neuronal injury was evaluated by trypan blue, DNA fragmentation, phosphatidylserine (PS) exposure, Akt1 phosphorylation, Bad phosphorylation, mitochondrial membrane potential, and cysteine protease activity. Microglial activation was assessed through proliferating cell nuclear antigen and PS receptor expression.
EPO provides intrinsic neuronal protection that is both necessary and sufficient to prevent acute genomic DNA destruction and subsequent membrane PS exposure, since protection by EPO is completely abolished by cotreatment with an anti-EPO neutralizing antibody.
Extrinsic protection by EPO is offered through the inhibition of cerebral microglial activation and the suppression of microglial PS receptor expression for the prevention of neuronal phagocytosis. In regards to microglial chemotaxis, EPO modulates neuronal poptotic membrane PS exposure necessary for microglial activation primarily through the regulation of caspase 1.
EPO increases Akt1 activity, phosphorylates Bad, and maintains neuronal nuclear DNA integrity through the downstream modulation of mitochrondrial membrane potential, cytochrome c release, and caspase 1, 3, and 8-like activities.
Elucidating the intrinsic and extrinsic protective pathways of EPO that mediate both neuronal integrity and inflammatory microglial activation may enhance the development of future therapies directed against acute neuronal injury.
Keywords: Apoptosis, Bad, cytochrome c, cysteine proteases, erythropoietin, microglial activation, mitochondrial membrane potential, phosphatidylserine exposure, protein kinase B
Introduction
Initially considered to mediate primarily the proliferation and differentiation of erythroid progenitors, erythropoietin (EPO) has emerged as a versatile growth factor that may play a significant role in the nervous system. Both EPO and the erythropoietin receptor EPOR are expressed throughout the nervous system in neurons, endothelial cells, and astrocytes in the cerebral cortex, hippocampus, and the amygdala (Morishita et al., 1997; Nagai et al., 2001; Chong et al., 2002b). In neuronal injury paradigms, EPO has been shown to provide protection against toxic insults, such as ischemia and free radical injury (Bernaudin et al., 1999; Chong et al., 2002a; Wen et al., 2002). As a result, EPO has been identified as a possible candidate in the formulation of therapeutic strategies against neurodegenerative diseases.
To further the development of EPO as a novel neuroprotectant against both acute and chronic neurodegenerative disease, it is first critical to understand the cellular pathways that may mediate neuronal injury and are subsequently susceptible to modulation by EPO. Oxygen-free radicals, such as nitric oxide (NO), have been established as significant precipitants of neuronal degeneration (Maiese & Vincent, 2000; Anderson et al., 2001). NO can trigger the induction of two independent apoptotic pathways that consist of nuclear DNA degradation and the exposure of membrane phosphatidylserine (PS) residues (Maiese & Vincent, 2000; Dumont et al., 2001; Lin & Maiese, 2001). Although DNA degradation in neurons may immediately impact cellular integrity (Jessel et al., 2002), the exposure of membrane PS residues in neurons can precipitate a latent cellular inflammation (Dombroski et al., 2000) and microglial phagocytosis of viable neurons (Maiese & Vincent, 2000; Hoffmann et al., 2001).
Several downstream cellular pathways may ultimately determine the protective role of EPO. In particular, the serine/threonine kinase Akt1, a key determinant of cell survival, appears to be necessary for EPO to prevent apoptosis of erythroid progenitors (Uddin et al., 2000). Once activated through phosphorylation, Akt1 can inhibit the activity of several substrates that promote apoptosis, such as Bad (Blume-Jensen et al., 1998), IκB kinase α (IKKβ) (Romashkova & Makarov, 1999), the forkhead transcription factor (FHKRL1) (Brunet et al., 1999), and the glycogen synthase kinase-3β (Cross et al., 1995).
The protective role of Akt1 also may be dependent upon the preservation of mitochondrial membrane integrity and the modulation of cysteine protease activity through cytochrome c. The protein Akt1 may serve to stabilize mitochondrial membrane potential and prevent the release of cytochrome c (Kennedy et al., 1999). Cellular release of NO can directly lead to mitochondrial membrane depolarization and the opening of mitochondrial permeability transition pores (Bal-Price & Brown, 2000; Chong et al., 2002a). As a result, cytochrome c is released from mitochondria and subsequently leads to the activation of a family of cysteine proteases (caspases) that include caspase 8, caspase 1, and caspase 3. Together, these cysteine proteases can lead to both DNA fragmentation and membrane PS exposure (Lin & Maiese, 2001; Mandal et al., 2002). Given the strong neuroprotective potential of EPO, we investigated the underlying cellular mechanisms controlled by EPO that may determine both the maintenance of neuronal cellular integrity and the inhibition of microglial activation to gain greater insight for the development of future neurodegenerative therapeutic strategies.
Methods
Primary hippocampal neuronal cultures
The hippocampi were obtained from E-19 Sprague–Dawley rat pups and incubated in dissociation medium (90 mM Na2SO4, 30 mM K2SO4, 5.8 mM MgCl2, 0.25 mM CaCl2, 10 mM kynurenic acid, and 1 mM HEPES with the pH adjusted to 7.4) containing papain (10 U ml−1) and cysteine (3 mM) for two 20-min periods. The hippocampi were then rinsed in dissociation medium and incubated in dissociation medium containing trypsin inhibitor (10–20 U ml−1) for three 5-min periods. The cells were washed in growth medium (Leibovitz's L-15 medium, Gibco BRL, Gaithersburg, MD, U.S.A.) containing 6% sterile rat serum (ICN, Aurora, OH, U.S.A.), 150 mM NaHCO3, 2.25 mg ml−1 of transferrin, 2.5 μg ml−1 of insulin, 10 nM progesterone, 90 μM putrescine, 15 nM selenium, 35 mM glucose, 1 mM L-glutamine, penicillin and streptomycin (50 μg ml−1), and vitamins. The dissociated cells were plated at a density of ∼1.5 × 103 cells mm−2 in 35-mm polylysine/laminin-coated plates (Falcon Labware, Lincoln Park, NJ, U.S.A.). Neurons were maintained in growth medium at 37°C in a humidified atmosphere of 5% CO2 and 95% room air for 2 weeks.
Microglia cell cultures, assessment of microglial activation, and the microglial phosphatidylserine receptor (PSR)
Microglia were obtained from the cerebral cortex of E-19 Sprague–Dawley rat pups (Giulian & Baker, 1986). Briefly, cerebral cortex cells were mechanically dissociated with Dulbecco's modified Eagle's Medium (DMEM/F-12) (Gibco BRL, Gaithersburg, MD, U.S.A.) containing 10% fetal bovine serum (ICN, Aurora, OH, U.S.A.) and subsequently seeded in 75-cm2 plastic flasks at a density of 8.5 × 106 cells per flask. Microglia were purified from mixed cultures with rotary shaking at 180 rpm for 15 h and then re-seeded at 105 cells ml−1 for cell adhesion of 3 h duration to yield an almost pure preparation of microglia (98%). Microglial cells were identified by α-naphthyl acetate esterase (Sigma, St Louis, MO, U.S.A.). Microglia were conditioned for 3 h by media from neurons 24 h following NO exposure. Proliferating cell nuclear antigen (PCNA) staining for microglial activation (Williams et al., 2002) was performed with anti-mouse monoclonal antibody against PCNA (1 : 100) conjugated with biotinylated anti-mouse IgG (1 : 50) (Calbiochem, San Diego, CA, U.S.A.) and visualized through fluorescein avidin (1 : 50) (Vector Laboratories, Burlingame, CA, U.S.A.). For detection of microglial PSR (Hoffmann et al., 2001), microglia were incubated 12 h following exposure to neuronal media with mouse anti-human PSR (Cascade Bioscience, Winchester, MA, U.S.A.) overnight at 4°C. Biotinylated anti-mouse antibody was used as a secondary antibody (1 : 50) and subsequently visualized through fluorescein avidin (1 : 50) (Vector Laboratories, Burlingame, CA, U.S.A.).
Experimental treatments
NO administration was performed by replacing the culture media with media containing sodium nitroprusside (SNP) (300 μM) (Sigma, St Louis, MO, U.S.A.) or 6-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-hexanamine (NOC-9) (300 μM) (Calbiochem, San Diego, CA, U.S.A.) as per the experimental paradigm (Maiese & Vincent, 2000). More than one NO generator was used as a control to demonstrate that the neurons were responding to NO rather than to other by-products of these agents. During both pre- and post-paradigm applications, EPO or the EPO antibody (R&D Systems, Minneapolis, MN, U.S.A.) application was continuous.
Phosphatidylinositol-3-kinase (PI-3K) inhibition of Akt1
PI-3K inhibition was performed by administering wortmannin (Calbiochem, La Jolla, CA, U.S.A.) or LY294002 (Tocris, Ellisville, MO, U.S.A.) (Norman et al., 1996). Wortmannin or LY294002 was added directly to the cultures 1 h prior to NO application and the treatment of PI-3K inhibition was continuous.
Assessment of neuronal survival
Hippocampal neuronal injury was determined by bright-field microscopy using a 0.4% trypan blue dye exclusion method 24 h following NO exposure as per our previous protocols (Lin et al., 2000). Neurons were identified by morphology and the mean survival was determined by counting eight randomly selected nonoverlapping fields with each containing approximately 10–20 neurons (viable + nonviable) in each 35 mm2 Petri dish.
Assessment of DNA fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (Lin et al., 2000; Maiese & Vincent, 2000). Briefly, neurons were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3′-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI, U.S.A.) followed by streptavidin-peroxidase and visualized with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA, U.S.A). The mean number of cells positive for TUNEL was determined by counting eight randomly selected nonoverlapping fields with each containing approximately 20 cells (TUNEL (+) + TUNEL (−)).
Assessment of membrane PS residue externalization
As per our prior protocols (Lin et al., 2000; Maiese & Vincent, 2000), a 30 μg ml−1 stock solution of annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN, U.S.A.) was diluted to 3 μg ml−1 in warmed calcium-containing binding buffer (10 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2). Plates were incubated with 500 μl of diluted annexin V for 10 min. Images were acquired with ‘blinded' assessment with a Leitz DMIRB microscope (Leica, McHenry, IL, U.S.A.) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm. The mean number of cells positive for membrane PS exposure was determined by counting eight randomly selected nonoverlapping fields with each containing approximately 20 cells (PS (+) + PS (−)).
Immunocytochemistry for EPO and the EPOR
For detection of EPO and the EPOR, neurons were fixed in 4% paraformaldehyde, blocked with horse serum, and then incubated with primary rabbit polyclonal antibody against EPO (H-162; 1 : 1000) or EPOR (C-20; 1 : 1000) (Santa Cruz Biotechnologies, Santa Cruz, CA, U.S.A.) overnight at 4°C. Biotinylated horse anti-rabbit antibody was used as a secondary antibody (1 : 100). ABC reagent was applied to detect EPO and EPOR with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA, U.S.A.). Absence of primary antibodies was used as an initial negative control. The specificity of the antibodies were previously confirmed by prior studies (Acs et al., 2001). In addition, the specificity of the immunoreactivities for the antibodies was evaluated with antibody absorption tests. The primary antibody against EPO was preincubated with EPO (10:1 peptide:antibody ratio, Santa Cruz Biotechnology, CA, U.S.A.) or the primary antibody against EPOR was preincubated with blocking peptide for EPOR (Santa Cruz Biotechnologies, Santa Cruz, CA, U.S.A.). This resulted in the complete abolition of immunocytochemical staining for EPO and the EPOR.
Assessment of mitochondrial membrane potential
The fluorescent probe JC-1 (Molecular Probes, Eugene, OR, U.S.A.) was used to assess the mitochondrial mem-brane potential (Chong et al., 2002a). Neurons in 35-mm plates were incubated with 2 μg ml−1 JC-1 in growth medium for 30 min. After washing, neurons were analyzed immediately under a Leitz DMIRB microscope (Leica, McHenry, IL, U.S.A.) with a dual-emission fluorescence filter with 515–545 nm for green fluorescence and emission at 585–615 nm for red fluorescence. The relative ratio of red/green fluore-scent intensity of mitochondrial staining was measured in four independent experiments by counting eight randomly selected nonoverlapping fields with each containing approximately 20 cells.
Assessment of cysteine protease activity
At specific times following NO exposure, cysteine protease activities were determined as previously described (Lin et al., 2000; Chong et al., 2002a). Cell suspensions were prepared and an aliquot of supernatant containing 30 μg protein was incubated with a 250 μM colorimetric substrate for caspase 8 (Ac-IETD-pNA), caspase 1 (Ac-YVAD-pNA), or for caspase 3 (Ac-DEVD- pNA) (Calbiochem, San Diego, CA, U.S.A.). Absorbance was measured at 405 nm and substrate cleavage reported in micromoles per minute per gram protein (μmol min−1 g−1) against standard p-nitroaniline solutions.
Modulation of cysteine protease activity
Modulation of cysteine protease activity in neurons was performed by using the irreversible and cell-permeable caspase inhibitors (50 μM 1 h prior to NO exposure) Z-IETD-FMK for caspase 8 (IETD), Z-YVAD-FMK (YVAD) for caspase 1, and Z-DEVD-FMK (DEVD) for caspase 3 obtained from Pharmingen Inc (Livermore, CA, U.S.A.).
Western blot analysis for Akt1 and Bad phosphorylation and cytochrome c release
Cells were homogenized and following protein determination, each sample (50 μg per lane) was then subjected to 7.5% (Akt1) or 12.5% (Bad, cytochrome c) SDS-polyacrylamide gel electrophoresis. The membranes were incubated with a mouse monoclonal antibody against the active form of Akt1 (phospho-Akt1, Ser 473, 1 : 1000) (Active-Motif, Carlsbad, CA, U.S.A.), a goat polyclonal antibody against phosphorylated Bad (p-Bad, Ser 136, 1 : 100) (Santa Cruz Biotechnologies, Santa Cruz, CA, U.S.A.) and a mouse monoclonal antibody against cytochrome c (1 : 2000) (Pharmingen, San Diego, CA, U.S.A.). After washing, the membranes were incubated with a horseradish peroxidase conjugated secondary antibody (goat anti-mouse IgG, 1 : 2000) (Pierce, Rockford, IL, U.S.A.) or rabbit anti-goat IgG (1 : 5000) (Santa Cruz Biotechnologies, Santa Cruz, CA, U.S.A.). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech).
Preparation of mitochondria for the analysis of cytochrome c release
As per our prior protocols (Chong et al., 2002a), after washing once with ice-cold PBS, cells were harvested and resuspended in buffer A (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 phenylmethylsulfonylfluoride) containing 250 mM sucrose. The cells were homogenized and then centrifuged twice at 750 × g for 10 min at 4°C. The harvested supernatants were centrifuged at 10,000 × g for 10 min and the cytosolic fraction was centrifuged at 50,000 × g for 60 min at 4°C.
Statistical analysis
For each experiment involving assessment of neuronal cell survival, DNA degradation, membrane PS exposure, mitochondrial membrane potential, and caspase activity, the mean and standard error were determined from four to six replicate experiments. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) with the post hoc Student's t-test. Results are expressed as the mean ± s.e. Statistical significance was considered at P<0.05.
Results
EPO and the EPOR are constitutively expressed in neurons, but expression of the EPOR is diminished during NO exposure
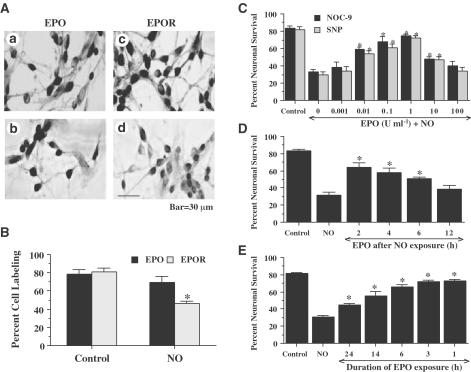
Neurons were exposed to the NO donors NOC-9 (300 μM) or SNP (300 μM) and expression of EPO and the EPOR was assessed 24 h later with immunocytochemistry. In untreated control neurons not exposed to a NO donor, both endogenous EPO and the EPOR were present in all neuronal populations examined (Figures 1A and B). Application of NO did not significantly alter the expression of EPO when examined at 24-h following NO application (Figures 1A and B). In contrast, expression of the EPOR was significantly altered over a 24-h time period and was decreased by approximately 45% when compared to untreated control neurons (Figures 1A and B).
Figure 1.
Protection by EPO is concentration and time dependent during diminished expression of the EPOR during NO exposure. (A) Primary hippocampal neuronal cultures were subjected to immunocytochemical detection for EPO (Aa, Ab) and the EPOR (Ac, Ad) by using a rabbit primary polyclonal anti-EPO and anti-EPOR antibodies. For EPO and EPOR detection, representative images are displayed for control cells (untreated neurons) (Aa, Ac) and for cells 24 h following exposure to the NO donor NOC-9 (300 μM) in the adjacent panels (Ab, Ad). (B) Quantitation of the percentage of neurons expressing EPO or the EPOR at 24 h following exposure to either NOC-9 (300 μM) or SNP (300 μM) is shown (*P<0.01 vs untreated control). (C) Neurons were pretreated with EPO (0.001–100 U ml−1) 1 h prior to exposure to a NO donor (NOC-9 or SNP, 300 μM) and cell survival was assessed 24 h later. Protection of EPO against NO toxicity was evident in cultures with EPO (0.01–10 U ml−1) when compared with cultures exposed to NO alone (*P<0.01 vs NO treated alone). (D) Protection of EPO was evident in post-treatment paradigms during NO toxicity. Neurons were treated with EPO (1 U ml−1) at 2, 4, 6, and 12 h following NO exposure (NOC-9 or SIN-1, 300 μM). Post-treatment with EPO at 2, 4, and 6 h following NO exposure increased neuronal survival significantly 24 h following NO exposure (*P<0.01 vs NO treated alone). (E) EPO (1 U ml−1) was pre-administered at 1, 3, 6, 14, and 24 h prior to NO exposure and neuronal survival was assessed 24 h following NO application (NOC-9 or SNP, 300 μM). Administration of EPO at 1, 3, and 6 h prior to NO exposure generated the highest levels of neuronal survival, but EPO applications provided at 14 and 24 h resulted in decreased efficacy for neuroprotection by EPO (*P<0.01 vs NO treated alone; †P<0.01 vs 24 h pretreated group). In (B, D, E), to simplify the figures, the results for the two NO donors were combined.
EPO provides neuroprotection during pre- and post-treatment protocols that is concentration and time dependent
Increasing concentrations of EPO (0.001–100 U ml−1) were administered directly to cultures and cell survival was evaluated by a trypan blue dye exclusion method 24 h later to examine the possible toxicity of EPO in neurons. No significant toxicity over a 24 h period was present in the cultures exposed to EPO in the concentrations of 0.001–100 U ml−1 (data not shown). Neuronal survival was significantly reduced to 33±3% following exposure to NOC-9 (300 μM) and to 30 ± 3% following exposure to SNP (300 μM) when compared with untreated control cultures (82±3%, P<0.01) (Figure 1C). In contrast, application of EPO with the concentrations of 0.01–10 U ml−1 significantly increased neuronal survival. A concentration of EPO of 1 U ml−1 achieved the maximum neuronal survival (75±2%, NOC-9; 72±3%, SNP), but concentrations lower than 0.01 U ml−1 or higher than 10 U ml−1 did not improve neuronal survival during NO exposure.
We next assessed whether changes in the temporal administration of EPO altered its protective ability during free radical exposure. As shown in Figure 1D, EPO applied at 2, 4, and 6 h following NO exposure significantly increased neuronal survival from 31±4% (NO alone) to 64±5% (P<0.01), 58±5% (P<0.01), and to 51±2% (P<0.01), respectively. In contrast, posttreatment with EPO at 12 h following NO exposure did not increase neuronal survival. The time of pretreatment of EPO also altered neuronal survival. EPO applied at time points closest to the application of NO yielded the greatest protection (Figure 1E). For example, administration of EPO at 1 h prior to NO exposure generated the highest levels of neuronal survival (74 ± 2%, P<0.01). EPO applications that were provided at 14 h (55±6%, P<0.01) and 24 h (45±2%, P<0.01) continued to significantly increase neuronal survival when compared to neurons exposed to NO only (30 ± 3%), but the efficacy of EPO to increase neuronal survival during these time periods was reduced (Figure 1E). In Figures 1D and E, data for the two NO donors was combined since no significant differences in cell injury were present between the two agents (see Figure 1C).
EPO is necessary and sufficient for neuronal protection
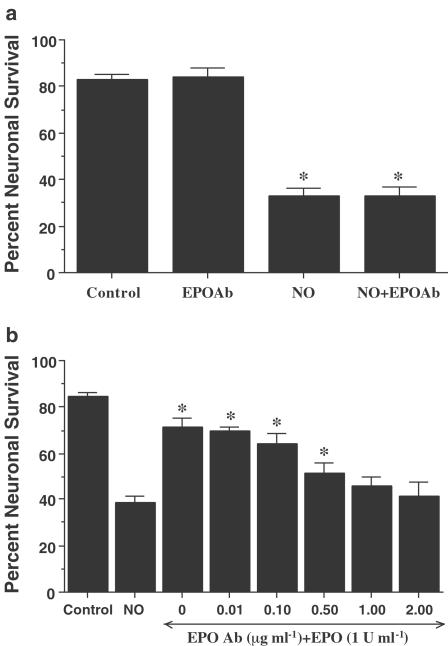
Administration of an antibody to EPO (EPO Ab) in a series of concentrations of 0.01–2.00 μg ml−1 did not significantly alter neuronal survival when compared to untreated control cultures (data not shown). In studies using NO (NOC-9 or SNP, 300 μM) as the toxic agent, application of the EPO Ab (2 μg ml−1) also did not alter neuronal survival when compared to cultures treated with NO alone, suggesting that constitutive expression of EPO is not sufficient to offer neuronal protection (Figure 2a).
Figure 2.
EPO is necessary and sufficient for neuronal protection during NO exposure. (a) To examine the ability of the EPO Ab to alter neuronal viability during NO exposure, EPO Ab (2 μg ml−1) was applied 1 h prior to increasing concentrations of a NO donor (NOC-9 or SNP, 300 μM). Neuronal survival was assessed 24 h following NO application. Administration of EPO Ab alone was not toxic. No significant changes in neuronal survival were observed following application of the EPO Ab when compared to cultures treated with NO alone (*P<0.01 vs untreated control). (b) Increasing concentrations of the EPO Ab (0.01–2.00 μg ml−1) were applied to neuronal cultures in conjunction with EPO (1 U ml−1) for 1 h prior to NO exposure (NOC-9 or SNP, 300 μM). Neuronal survival was assessed 24 h following NO application. Protection by EPO against NO toxicity was attenuated or abolished during applications of EPO Ab (0.50, 1.00, and 2.00 μg ml−1) (*P<0.01 vs NO treated alone). In (a) and (b), to simplify the figures, the results for the two NO donors were combined.
We next examined whether specific antagonism against exogenous EPO application with the EPO Ab could neutralize the protective capacity of EPO during NO exposure. In the presence of the EPO Ab, the concentrations of EPO Ab of 0.50, 1.00, and 2.00 μg ml−1 significantly decreased the protective capacity of EPO, yielding neuronal survivals of 52±4% (P<0.01), 46±4% (P<0.01), and 41±6% (P<0.01), respectively (Figure 2b).
EPO prevents DNA fragmentation and membrane PS exposure in neurons
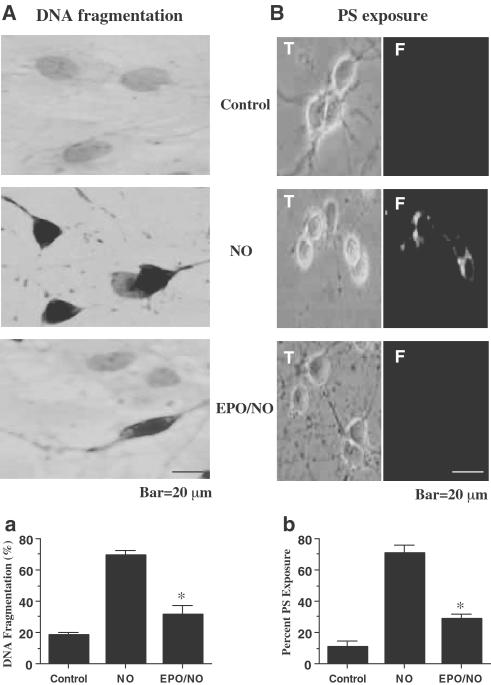
At 24 h following NO exposure (NOC-9 or SNP, 300 μM), nuclear chromatin condensation was observed in neurons (Figure 3A). In contrast, neurons pretreated with EPO (1 U ml−1) 1 h prior to NO exposure were without nuclear fragmentation. As shown in Figure 3Aa, NO resulted in a significant increase in percent DNA fragmentation (69±3%) when compared to untreated control cultures (18±1%). DNA fragmentation was reduced to 32±6% in the neuronal cultures during the application of EPO over a 24 h period.
Figure 3.
EPO prevents DNA fragmentation and externalization of membrane PS residues in neurons. (A) Neurons were exposed to NO (NOC-9 or SNP, 300 μM) and DNA fragmentation was determined 24 h later using the TUNEL assay. Pretreatment with EPO (1 U ml−1) decreased DNA fragmentation significantly during NO exposure (*P<0.01 vs NO, panel Aa). (B) Neurons were labeled with annexin V PE to visualize PS exposure 24 h following exposure to NO (NOC-9 or SNP, 300 μM) and were imaged using transmitted (T) light and corresponding fluorescence (F) images of the same microscopy field. Pretreatment with EPO (1 U ml−1) 1 h prior to NO significantly prevented membrane PS externalization (*P<0.01.3 vs NO, panel Bb). In (Aa) and (Bb), to simplify the figures, the results of the two NO donors were combined. In all cases, control = untreated neurons.
In Figure 3B, NO exposure resulted in the marked induction of membrane PS exposure that is present throughout the membrane of neurons. Administration of EPO (1 U ml−1) 1 h prior to NO prevented the externalization of membrane PS residues in neurons. In Figure 3Bb, a significant increase in membrane PS residue exposure was observed in neuronal cultures at 24 h following NO (71±5%) when compared to untreated control cultures (11±4%). Application of EPO (1 U ml−1) 1 h prior to NO significantly inhibited externalization of membrane PS residues to 29±5% at 24 h following NO exposure.
EPO inhibits microglial activation in neurons and expression of the PSR in microglia
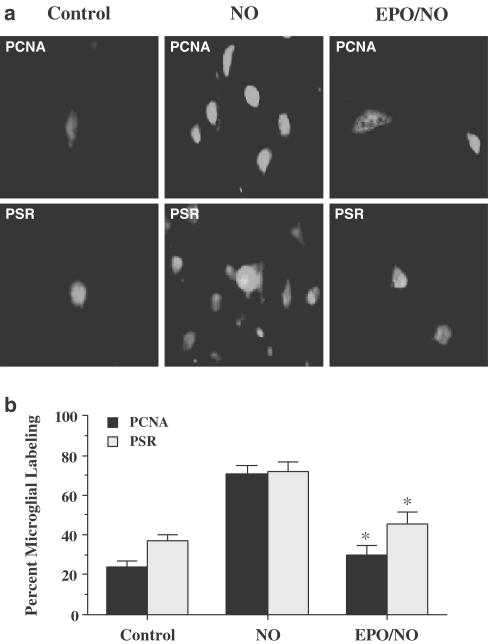
At 24 h following exposure to NO (NOC-9 or SNP, 300 μM), media from neuronal cultures were applied to pure cultures of microglia. Following 3 h of incubation, microglial activation was assessed through PCNA (Williams et al., 2002) expression and PSR expression. In Figure 4a, representative microglial cultures illustrate a marked induction of microglial activation during treatment with media from neuron cells as evidenced by significant PCNA expression. To a similar degree, treatment of microglia cultures with NO-exposed neuronal media had a significant increase in PSR expression when compared to microglia treated with neuronal media not exposed to NO (control). In contrast, administration of EPO (1 U ml−1) 1 h prior to NO prevented both PCNA expression and PSR expression in microglia.
Figure 4.
EPO protects against microglial activation and microglial PSR expression during NO exposure. Pure microglial cultures were treated for 3 h with media with or without 1 h EPO (1 U ml−1) pretreatment that had been exposed to NO (NOC-9 or SNP, 300 μM) 24 h prior. (a) A representative image illustrates that PCNA or PSR expression was significantly increased in microglia treated with media from NO exposed neurons. In contrast, PCNA expression or PSR expression was significantly less in microglia treated with media from EPO (1 U ml−1) and NO-treated neurons. (b) PCNA expression or PSR expression in microglia treated with media from NO-exposed neurons was significantly increased, but PCNA or PSR expression was significantly diminished in microglia treated with media from EPO (1 U ml−1) and NO-treated neurons EPO/NO vs NO, *P<0.01). To simplify the figures, the results of the two NO donors were combined. Control = cultures without NO exposure.
In Figure 4b, quantitation of PCNA and PSR labeling revealed that a significant expression in PCNA (71±4%) and PSR (72±5%) was present in microglia cultures following the application of NO-treated media when compared to untreated control cultures (24±3%, PCNA; 37±3%, PSR). Application of media from cells with EPO (1 U ml−1) 1 h prior to NO administration resulted in significantly less microglial activation with reduced PCNA (30±5%) expression and PSR (45±6%) expression. Administration of EPO (1 U ml−1) alone to microglia cultures did not significantly alter PCNA expression or PSR expression when compared to untreated control cultures (data not shown).
Neuronal protection by EPO is dependent on the enhanced activity of Akt1 and the phosphorylation of Bad
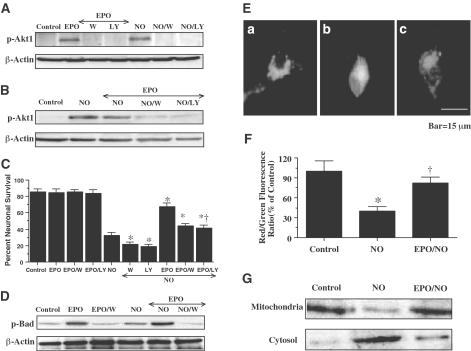
Western blot analysis was performed for phospho-Akt1 (activated form of Akt1, p-Akt1) 12 h following NO exposure. In Figure 5A, EPO and NO (NOC-9 or SNP, 300 μM) independently increased the expression of p-Akt1. This increased expression of p-Akt1 was blocked by the agents wortmannin (100 nM), specifically inhibits Akt1 phosphorylation (Norman et al., 1996), and LY294002 (10 μM). In combination with NO exposure, EPO continued to enhance the expression of p-Akt1 (Figure 5B). Increased expression of p-Akt1 by EPO was blocked by wortmannin (100 nM) and LY294002 (10 μM). In Figure 5C, application of EPO (1 U ml−1) 1 h prior to NO exposure significantly increased neuronal survival to 68±4%. Yet, coapplication of wortmannin (100 nM) or LY294002 (10 μM) at a concentration that blocks activation of p-Akt1 during NO exposure (Figures 5A and B) with EPO (1 U ml−1) significantly reduced the ability of EPO to protect neurons against free radical injury, suggesting that EPO required some level of Akt1 activation to offer neuroprotection. Application of EPO and wortmannin (100 nM) or LY294002 (10 μM) without NO exposure was not toxic to neurons, but enhanced injury during NO exposure, suggesting that endogenous Akt1 activation provides a small level of protection during toxic insults (Figure 5C).
Figure 5.
Neuronal protection by EPO is mediated by the activation of Akt1, phosphorylation of Bad, and the prevention of mitochondrial membrane depolarization and cytochrome c release. In (A) and (B), equal amounts of neuronal protein extracts (50 μg per lane) were immunoblotted with antiphospho-Akt1 (p-Akt1, active Akt1, Ser 473) antibody. Exposure to EPO (1 U ml−1) or NO significantly increased p-Akt1 expression. Application of the PI-3K inhibitor wortmannin (100 nM) or LY294002 (LY) (10 μM) was sufficient to block the expression of active p-Akt1 in the presence of EPO during NO (NOC-9, 300 μM) exposure. (C) At a concentration that blocks activation of p-Akt1 during NO administration (NOC-9 or SNP, 300 μM), wortmannin (100 nM) or LY294002 (10 μM) applied 1 h prior to NO significantly reduced the protective capacity of EPO (1 U ml−1) during NO exposure (*P<0.01 vs NO; †P<0.01 vs EPO). (D) Equal amounts of neuronal protein extracts (50 μg per lane) were immunoblotted with antiphosphorylated Bad (p-Bad, Ser 136) antibody. Exposure to EPO (1 U ml−1) or NO significantly increased p-Bad expression. EPO application further increased phosphorylation of Bad during NO exposure. Application o of the PI-3K inhibitor wortmannin (100 nM) was sufficient to block the expression of p-Bad in the presence of EPO during NO (NOC-9, 300 μM) exposure. (E, F) Exposure to NO (NOC-9, 300 μM) produced a significant decrease in the red/green fluorescence intensity ratio using a cationic membrane potential indicator JC-1 within 3 h when compared with untreated control cultures, suggesting that NO results in mitochondrial membrane depolarization. Application of EPO (1 U ml−1) 1 h prior to NO exposure significantly increased the red/green fluorescence intensity of neurons, indicating that mitochondrial permeability transition pore membrane potential was restored to baseline (E,F). (G) A representative Western blot with equal amounts of mitochondrial or cytosol protein extracts (50 μg per lane) were immunoblotted demonstrating that application of EPO (1 U ml−1) significantly prevented cytochrome c release from mitochondria during NO exposure. In (C) and (F), to simplify the figures, the results of the two NO donors were combined. In all cases, control=untreated neurons.
Since wortmannin and LY294002 function at the level of PI-3K and may not directly inhibit Akt1 phosphorylation, we also examined whether EPO altered the phosphorylation of Bad, a downstream substrate of Akt1 at Ser136 (Blume-Jensen et al., 1998). Western blot analysis was performed for phosphorylated Bad (p-Bad) 3 h following NO exposure. In Figure 5D, EPO significantly increased the phosphorylation of Bad. Exposure to NO (NOC-9 or SNP, 300 μM) also increased Bad phosphorylation, but to a lesser degree than compared to EPO. In combination with NO exposure, EPO significantly enhanced the phosphorylation of Bad (Figure 5D). Phosphorylation of Bad by EPO was blocked by wortmannin (100 nM) either with or without NO exposure.
EPO prevents mitochondrial membrane depolarization and the release of cytochrome c during NO exposure
Exposure to NO (NOC-9 or SNP, 300 μM) produced a significant decrease in the red/green fluorescence intensity ratio using a cationic membrane potential indicator JC-1 within 3 h when compared with untreated control cultures (Figures 5E and 5F), suggesting that NO results in mitochondrial membrane depolarization. Application of EPO (1 U ml−1) 1 h prior to NO exposure significantly increased the red/green fluorescence intensity of the neurons, indicating that mitochondrial permeability transition pore membrane potential was restored to baseline (Figures 5E and 5F). Administration of EPO (1 U ml−1) 1 h prior to NO maintained mitochondrial permeability transition pore membrane function and prevented mitochondrial cytochrome c release as demonstrated by Western analysis (Figure 5G).
EPO protects neurons from DNA fragmentation and PS exposure through the modulation of caspase 8, caspase 1, and caspase 3 - like activities
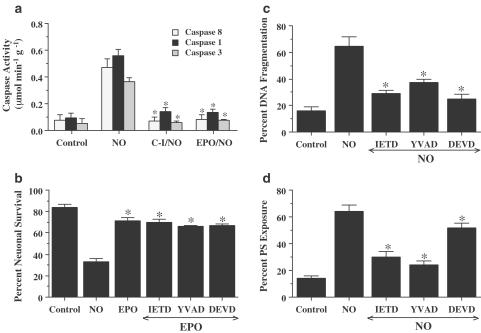
In Figure 6a, EPO (1 U ml−1) was applied to the neuronal cultures 1 h prior to a NO donor (NOC-9 or SNP, 300 μM) and data for caspase 8, caspase 1, and caspase 3 activities were obtained 12 h post-NO exposure, since this time period represented the peak activities for these cysteine proteases (Lin et al., 2000, Lin & Maiese, 2001). Administration of EPO significantly decreased caspase 8-like activity to 0.08± 0.04 μmol min−1 g−1 (P<0.01). Similarly, EPO pre-treatment significantly reduced the activity of caspase 1-like activity (0.14±0.02 μmol min−1 g−1) and caspase 3-like activity (0.07± 0.01 μmol min−1 g−1) when compared to cultures treated with NO alone (0.56±0.05 and 0.36±0.03 μmol min−1 g−1, respectively).
Figure 6.
EPO protects neurons from injury through the modulation of caspase 8, caspase 1, and caspase 3-like activities. (a) Neurons were exposed to NO (NOC-9 or SNP, 300 μM) and caspase 8-, caspase 1-, and caspase 3-like activities were assessed 12 h later through their respective colorimetric substrates. Pretreatment with EPO (1 U ml−1) or the caspase inhibitors (C-I, 50 μM) for caspase 8 (IETD), caspase 1 (YVAD), and caspase 3 (DEVD) 1 h prior to NO significantly inhibited the increase in the activity of caspase 8, caspase 1, and caspase 3 induced by NO (*P<0.01 vs NO). (b) Neurons were pretreated with EPO (1 U ml−1) alone or in combination with an inhibitor of caspase 8 (IETD, 50 μM), caspase 1 (YVAD, 50 μM), or caspase 3 (DEVD, 50 μM). No enhanced or synergistic protection was observed during the application of each caspase inhibitor combined with EPO when compared with cultures exposed to EPO and NO alone. (c) Neurons were pretreated with a caspase 8 inhibitor (IETD, 50 μM), a caspase 1 inhibitor (YVAD, 50 μM), or a caspase 3 inhibitor (DEVD, 50 μM) 1 h prior to NO (NOC-9 or SNP, 300 μM) and DNA fragmentation with TUNEL was determined 24 h following NO exposure (*P<0.01 vs NO). (d) Neurons were pretreated with a caspase 8 inhibitor (IETD, 50 μM), a caspase 1 inhibitor (YVAD, 50 μM), or a caspase 3 inhibitor (DEVD, 50 μM) 1 h prior to NO (NOC-9 or SNP, 300 μM) and membrane PS exposure with annexin V PE was determined 24 h following NO exposure (*P<0.01 vs NO). In all cases, to simplify the figures, the results of the two NO donors were combined and control = untreated neurons.
To further address the relation between protection of EPO and caspase activity, we examined the ability of caspase inhibitors combined with EPO to prevent NO-induced neuronal injury (Figure 6b). Coapplication of EPO with either the caspase 8-like inhibitor (IETD), caspase 1-like inhibitor (YVAD), or the caspase 3-like inhibitor (DEVD) did not provide a synergistic level of protection against NO free radical injury, suggesting that cytoprotection by EPO employs similar pathways that inhibit caspase 8-, caspase 1-, and caspase 3-like activities.
We next examined whether the induction of caspase 1-, caspase 3-, and caspase 8-like activities were required for NO-induced cell injury through neuronal DNA fragmentation and membrane PS exposure. As shown in Figure 6c, neurons exposed to NO (NOC-9 or SNP, 300 μM) resulted in neuronal DNA fragmentation of 65±7%. Pretreatment of neurons with 50 μM of YVAD, DEVD, and IETD to inhibit caspase 1-, caspase 3-, and caspase 8-like activities significantly decreased DNA fragmentation to 37±3, 25±3, and 29±2%, respectively. In addition, inhibition of caspase 1 (YVAD/NO), caspase 3 (DEVD/NO), or caspase 8 (IETD/NO) activities maintained cellular membrane asymmetry and significantly prevented PS externalization (Figure 6d). NO exposure (NOC-9 or SNP, 300 μM) in neurons resulted in a membrane PS exposure of 64±5%. Although inhibition of each of the caspases significantly decreased membrane PS exposure to 24±3% (caspase 1), 52±3% (caspase 3), and 30±4% (caspase 8), modulation of caspase 1 was most effective in preventing the induction of membrane PS exposure.
Discussion
Interest in EPO as a novel neuroprotectant for the central nervous system disease continues to gain significant momentum. Yet, it is the knowledge of the underlying cellular mechanisms employed by EPO that will serve to lay the foundation for future drug development against neurodegenerative disorders. As a result, we identified some of the cellular pathways modulated by the novel neuroprotectant EPO that were critical for protection against neuronal apoptosis and neuronal phagocytosis.
The expression of either endogenous brain EPO or the EPOR in neurons during free radical injury has not been investigated. We demonstrate that EPO and the EPOR are constitutively expressed in primary neurons and that only the expression of the EPOR is altered over a 24-h time period following NO application. Expression of the EPOR becomes significantly decreased by approximately 45% over a following 24 h course. As a result, the ability of EPO to offer neuronal protection following a toxic insult may be linked to the temporal presence of EPO and its receptor.
Neuroprotection with EPO was achieved only in a limited concentration range that is dependent upon the temporal exposure of EPO. Concentrations of EPO less than 0.01 U ml−1 or greater than 10 U ml−1 did not enhance neuronal survival during free radical exposure. This concentration range for neuroprotection with EPO is similar to other injury paradigms in both in vitro (Wen et al., 2002) and in vivo models (Grasso et al., 2002) that illustrate a tight therapeutic concentration range for EPO.
Posttreatment strategies with EPO illustrate that neuronal injury is reversible in nature, but resides in a fixed time frame. Our results suggest that EPO, within a 6 h period after the onset of a toxic exposure, can modulate critical cellular pathways prior to the induction of cellular mechanisms that can destine a cell to die. This fixed time frame for protection by EPO most likely coincides with the progressive induction of secondary cellular pathways during this 6 h time span, such as cytochrome c release (Figure 5) and cysteine protease induction in neurons (Figure 6) (Uehara et al., 1999; Lin & Maiese, 2001; Chong et al., 2002a). Furthermore, prolonged administration of EPO during acute injury paradigms also reduces the ability of EPO to provide neuroprotection. We show that with preadministration of EPO, the greatest neuronal survival was achieved with administration periods closest to the application of NO exposure, such as at 1, 3, and 6 h prior to NO administration. Several factors may determine both the concentration and temporal parameters that regulate the neuroprotective ability of EPO. During chronic administration, EPO can result in the formation of anti EPO antibodies (Casadevall et al., 2002) and decrease the expression of the EPOR on the cell surface (Verdier et al., 2000). As a result, biological function of EPO can be blocked at any concentration level.
The presence of constitutive EPO in neurons appears to be insufficient to provide a significant level of neuronal protection. Yet, application of exogenous EPO is both necessary and sufficient to protect neurons from NO-induced injury. In the presence of NO exposure, application of the EPO Ab, which can bind to EPO and block its biological activities in neurons (Koshimura et al., 1999), did not alter neuronal survival when compared to cultures treated with NO alone, suggesting that constitutive EPO in neurons is insufficient to protect cells from injury. Yet, administration of exogenous EPO offers robust protection against NO exposure that is prevented only with coapplication of the EPO Ab, illustrating that EPO provides necessary and sufficient protection against neuronal injury. The work also demonstrates that the inability of constitutive EPO to provide neuronal protection is not a result of a functionally deficient EPOR following a neuronal insult, but more likely associated with insufficient concentrations of constitutive EPO since exogenous EPO can utilize the EPOR to achieve neuroprotection.
EPO offers intrinsic neuronal protection not only through the maintenance of intact genomic DNA, but also through extrinsic mechanisms by inhibiting neuronal membrane PS exposure. In several cell systems, membrane PS externalization functions to identify cells that have entered the early stages of apoptosis and to expedite the elimination of these cells through phagocytosis (Rucker-Martin et al., 1999; Maiese & Vincent, 2000; Fadok et al., 2001). Exposure of neuronal membrane PS residues can promote cell-to-cell interactions and lead to the ‘tagging' of neurons for removal by microglia that require increased PSR expression on their cell surface (Hoffmann et al., 2001). Prevention of membrane PS exposure provides an additional mechanism to avert neuronal cell injury and death. Our present work provides further insight into the ability of EPO to protect cells from inflammatory injury and phagocytic removal. Prior studies have demonstrated the early shedding of annexin V-binding membrane particles that are complementary to membrane PS residues during apoptotic cellular injury (Simak et al., 2002). We demonstrate that EPO provides strong protection against neuronal membrane PS exposure and possible shedding of membrane PS particles during free radical injury. In addition, we illustrate that media from cells exposed to a toxic injury with NO directly lead to the induction of both microglial activation and microglial PSR expression. Yet, media taken from cells that have been treated with EPO during NO exposure lead to a significant reduction in the activation of microglia and the expression of microglial PSRs. Taken together, our work provides strong evidence that modulation of neuronal membrane PS exposure and complementary microglial activation with PSR expression by EPO is biologically relevant and enables EPO to inhibit phagocytosis of neurons.
Protection by EPO in the central nervous system is mediated through a series of cellular pathways that maintain intricate links between one another. At an initial level, the protein Akt1 appears to be important to preserve neuronal function and integrity during toxic injury paradigms. In our neuronal cultures, endogenous activation of Akt1 during NO provides a minimum level of protection, since inhibitors of PI-3K activity, such as wortmannin or LY294002, can lessen survival during these injury paradigms. In regards to EPO, we demonstrate that it independently phosphorylates and increases the activation of Akt1 in neurons similar to its capacity to activate Akt1 in erythroid progenitors (Haseyama et al., 1999; Uddin et al., 2000). EPO appears to be dependent upon the activation of Akt1 to prevent neuronal injury, since prevention of Akt1 phosphorylation with inhibitors of PI-3K activity significantly reduces the ability of EPO to protect neurons. This protection by EPO also is closely linked to the phosphorylation of Bad at Ser136, a downstream substrate of Akt1. In other cell systems, phosphorylation of Bad at Ser136 precipitates an interaction with the cytosolic 14-3-3 protein resulting in the liberation of the antiapoptotic protein Bcl-2/Bcl-xL (Hsu et al., 1997; Blume-Jensen et al., 1998). Either alone or during NO exposure, we illustrate that EPO in neurons significantly increases the phosphorylation of Bad that can be prevented by PI-3K inhibition. Taken together, these results support the premise that EPO fosters neuronal survival, at least in part, through the enhanced activity of Akt1.
Absence of Akt1 activation results in a partial decrease in the protective capacity of EPO, suggesting that alternate cellular pathways are responsible for the mediation of neuronal protection by EPO. One pathway that is closely associated with Akt1 activation is the modulation of mitochondrial membrane potential (Kennedy et al., 1999). Mitochondrial-mediated apoptosis can be initiated by free radical injury and result in the cytoplasmic release of cytochrome c (Bal-Price & Brown, 2000; Chong et al., 2002b). In our present studies, we demonstrate that the free radical NO leads to the depolarization of the mitochondrial membrane in neurons with the subsequent release of cytochrome c. Consistent with clinical studies that demonstrate preserved mitochondrial function as a result of EPO administration (Miro et al., 2002), our studies illustrate that EPO directly maintains mitochondrial membrane potential and prevents the release of cytochrome c.
Central to the changes in mitochondrial membrane potential during neuronal injury is the induction cysteine protease activity. Earlier studies suggested that prevention of apoptosis in erythroid progenitor cells may be associated with modulation of caspase activity by EPO (Gregoli & Bondurant, 1999). The ability of EPO to modulate caspase 1-, caspase 3-, and caspase 8-like activities appears to play a critical role in the protection conferred by EPO. Each of these cysteine proteases is associated with the independent apoptotic pathways of genomic DNA cleavage and cellular membrane PS exposure (Takahashi et al., 1999; Lin & Maiese, 2001). In particular, caspase 8 can cleave Bid and allow Bid to translocate to mitochondria to release cytochrome c (Tang et al., 2000). Subsequently, genomic DNA degradation and membrane PS exposure can ensue through the activation of caspase 3 and caspase 1 (Takahashi et al., 1999). Following caspase 8 activation, caspase 3 becomes a prominent mediator of genomic DNA degradation. Experimental models that use caspase 3 gene deletions or pharmacological inhibition illustrate little or no DNA fragmentation following toxic cellular insults (Keramaris et al., 2000; Lin & Maiese, 2001). Interestingly, we illustrate that EPO prevents membrane PS exposure primarily through the inhibition of caspase 8- and caspase 1-like activities and, to a lesser degree, through caspase 3-like activity. Given that caspase 8 can result in the downstream activation of caspase 1, caspase 1 is believed to be principally responsible for the externalization of membrane PS residues in several cell systems through the digestion of cytoskeletal proteins, such as fodrin and to be responsible for microglial phagocytosis (Vanags et al., 1996; Maiese & Vincent, 2000).
In conclusion, we illustrate that EPO is necessary and sufficient for the provision of both intrinsic and extrinsic neuronal protection through a series of specific cellular pathways (Figure 7). Although endogenous EPO is not sufficient to maintain cell survival during an acute injury such as free radical exposure, the administration of exogenous EPO is critical for significant neuronal protection. EPO provides broad neuroprotection through the maintenance of both genomic DNA integrity and cellular membrane asymmetry that prevents acute cellular injury and subsequent microglial activation that may mediate phagocytic demise. Intimately tied to this neuronal protection by EPO is the modulation of Akt1 activity, phosphorylation of Bad, mitochondrial membrane permeability, cytochrome c release, and caspase 1-, caspase 3-, and caspase 8-like activities.
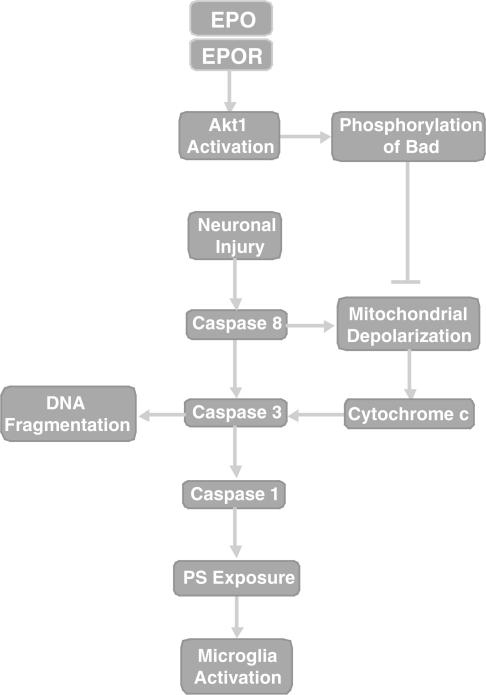
Figure 7.
EPO prevents neuronal injury through a series of pathways that involve Akt1, Bad, and cysteine protease activity. Following neuronal injury, both caspase 8 and Bad can lead to the depolarization of the mitochondrial membrane resulting in the release of cytochrome c. Concurrently, caspase 8 also can activate caspase 3 to precipitate DNA fragmentation and potentially activate caspase 1 to yield membrane PS externalization, microglial activation, and the phagocytic destruction of neurons. The prevention of neuronal apoptosis and microglial phagocytosis by EPO following its binding to the EPOR during NO.6 exposure can occur through cellular pathways that involve enhanced Akt1 activity, Bad phosphorylation, and the maintenance of mitochondrial membrane stability. Alternatively, EPO may act directly upon cytochrome c, caspase 8, caspase 3, or caspase 1 to promote neuronal survival during toxic insults.
Acknowledgments
This research was supported by the following grants (KM): American Heart Association (National), Janssen Neuroscience Award, Johnson and Johnson Focused Investigator Award, LEARN Foundation Award, MI Life Sciences Challenge Award, and NIH NIEHS (P30 ES06639).
Abbreviations
- EPO
erythropoietin
- EPOR
Erythropoietin receptor
- PCNA
proliferating cell nuclear antigen
- PI-3K
phosphatidylinositol-3-kinase
- PS
phosphatidylserine
- PSR
phosphatidylserine receptor
References
- ACS G., ACS P., BECKWITH S.M., PITTS R.L., CLEMENTS E., WONG K., VERMA A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- ANDERSON I., ADINOLFI C., DOCTROW S., HUFFMAN K., JOY K.A., MALFROY B., SODEN P., RUPNIAK H.T., BARNES J.C. Oxidative signalling and inflammatory pathways in Alzheimer's disease. Biochem. Soc. Symp. 2001;67:141–149. doi: 10.1042/bss0670141. [DOI] [PubMed] [Google Scholar]
- BAL-PRICE A., BROWN G.C. Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J. Neurochem. 2000;75:1455–1464. doi: 10.1046/j.1471-4159.2000.0751455.x. [DOI] [PubMed] [Google Scholar]
- BERNAUDIN M., MARTI H.H., ROUSSEL S., DIVOUX D., NOUVELOT A., MACKENZIE E.T., PETIT E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J. Cerer. Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- BLUME-JENSEN P., JANKNECHT R., HUNTER T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 1998;8:779–782. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- BRUNET A., BONNI A., ZIGMOND M.J., LIN M.Z., JUO P., HU L.S., ANDERSON M.J., ARDEN K.C., BLENIS J., GREENBERG M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- CASADEVALL N., NATAF J., VIRON B., KOLTA A., KILADJIAN J.-J., MARTIN-DUPONT P., MICHAUD P., PAPO T., UGO V., TEYSSANDIER I., VARET B., MAYEUX P. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 2002;346:469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- CHONG Z.Z., KANG J., MAIESE K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002a;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- CHONG Z.Z., KANG J.Q., MAIESE K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J. Cerebr. Blood Flow Metab. 2002b;22:503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- CROSS D.A., ALESSI D.R., COHEN P., ANDJELKOVICH M., HEMMINGS B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- DOMBROSKI D., BALASUBRAMANIAN K., SCHROIT A.J. Phosphatidylserine expression on cell surfaces promotes antibody-dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J. Autoimmun. 2000;14:221–229. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- DUMONT E.A., REUTELINGSPERGER C.P., SMITS J.F., DAEMEN M.J., DOEVENDANS P.A., WELLENS H.J., HOFSTRA L. Real-time imaging of apoptotic cell – membrane changes at the single-cell level in the beating murine heart. Nat. Med. 2001;7:1352–1355. doi: 10.1038/nm1201-1352. [DOI] [PubMed] [Google Scholar]
- FADOK V.A., DE CATHELINEAU A., DALEKE D.L., HENSON P.M., BRATTON D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- GIULIAN D., BAKER T.J. Characterization of ameboid microglia isolated from developing mammalian brain. J. Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRASSO G., BUEMI M., ALAFACI C., SFACTERIA A., PASSALACQUA M., STURIALE A., CALAPAI G., DE VICO G., PIEDIMONTE G., SALPIETRO F.M., TOMASELLO F. Beneficial effects of systemic administration of recombinant human erythropoietin in rabbits subjected to subarachnoid hemorrhage. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5627–5631. doi: 10.1073/pnas.082097299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGOLI P.A., BONDURANT M.C. Function of caspases in regulating apoptosis caused by erythropoietin deprivation in erythroid progenitors. J. Cell Physiol. 1999;178:133–143. doi: 10.1002/(SICI)1097-4652(199902)178:2<133::AID-JCP2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- HASEYAMA Y., SAWADA K., ODA A., KOIZUMI K., TAKANO H., TARUMI T., NISHIO M., HANDA M., IKEDA Y., KOIKE T. Phosphatidylinositol 3-kinase is involved in the protection of primary cultured human erythroid precursor cells from apoptosis. Blood. 1999;94:1568–1577. [PubMed] [Google Scholar]
- HOFFMANN P.R., DECATHELINEAU A.M., OGDEN C.A., LEVERRIER Y., BRATTON D.L., DALEKE D.L., RIDLEY A.J., FADOK V.A., HENSON P.M. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU S.Y., KAIPIA A., ZHU L., HSUEH A.J. Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11. Mol. Endocrinol. 1997;11:1858–1867. doi: 10.1210/mend.11.12.0023. [DOI] [PubMed] [Google Scholar]
- JESSEL R., HAERTEL S., SOCACIU C., TYKHONOVA S., DIEHL H.A. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J. Cell Mol. Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY S.G., KANDEL E.S., CROSS T.K., HAY N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERAMARIS E., STEFANIS L., MACLAURIN J., HARADA N., TAKAKU K., ISHIKAWA T., TAKETO M.M., ROBERTSON G.S., NICHOLSON D.W., SLACK R.S., PARK D.S. Involvement of caspase 3 in apoptotic death of cortical neurons evoked by DNA damage. Mol. Cell Neurosci. 2000;15:368–379. doi: 10.1006/mcne.2000.0838. [DOI] [PubMed] [Google Scholar]
- KOSHIMURA K., MURAKAMI Y., SOHMIYA M., TANAKA J., KATO Y. Effects of erythropoietin on neuronal activity. J. Neurochem. 1999;72:2565–2572. doi: 10.1046/j.1471-4159.1999.0722565.x. [DOI] [PubMed] [Google Scholar]
- LIN S.H., MAIESE K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J. Cerebr. Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- LIN S.H., VINCENT A., SHAW T., MAYNARD K.I., MAIESE K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J. Cerebr. Blood Flow Metab. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- MAIESE K., VINCENT A.M. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J. Neurosci. Res. 2000;59:568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- MANDAL D., MOITRA P.K., SAHA S., BASU J. Caspase 3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett. 2002;513:184–188. doi: 10.1016/s0014-5793(02)02294-9. [DOI] [PubMed] [Google Scholar]
- MIRO O., MARRADES R.M., ROCA J., SALA E., MASANES F., CAMPISTOL J.M., TORREGROSA J.V., CASADEMONT J., WAGNER P.D., CARDELLACH F. Skeletal muscle mitochondrial function is preserved in young patients with chronic renal failure. Am. J. Kidney Dis. 2002;39:1025–1031. doi: 10.1053/ajkd.2002.32776. [DOI] [PubMed] [Google Scholar]
- MORISHITA E., MASUDA S., NAGAO M., YASUDA Y., SASAKI R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- NAGAI A., NAKAGAWA E., CHOI H.B., HATORI K., KOBAYASHI S., KIM S.U. Erythropoietin and erythropoietin receptors in human CNS neurons astrocytes microglia and oligodendrocytes grown in culture. J. Neuropathol. Exp. Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- NORMAN B.H., SHIH C., TOTH J.E., RAY J.E., DODGE J.A., JOHNSON D.W., RUTHERFORD P.G., SCHULTZ R.M., WORZALLA J.F., VLAHOS C.J. Studies on the mechanism of phosphatidylinositol 3-kinase inhibition by wortmannin and related analogs. J. Med. Chem. 1996;39:1106–1111. doi: 10.1021/jm950619p. [DOI] [PubMed] [Google Scholar]
- ROMASHKOVA J.A., MAKAROV S.S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- RUCKER-MARTIN C., HENAFF M., HATEM S.N., DELPY E., MERCADIER J.J. Early redistribution of plasma membrane phosphatidylserine during apoptosis of adult rat ventricular myocytes in vitro. Basic Res. Cardiol. 1999;94:171–179. doi: 10.1007/s003950050140. [DOI] [PubMed] [Google Scholar]
- SIMAK J., HOLADA K., VOSTAL J.G. Release of annexin V-binding membrane microparticles from cultured human umbilical vein endothelial cells after treatment with camptothecin. BMC Cell Biol. 2002;3:11. doi: 10.1186/1471-2121-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI H., NAKAMURA S., ASANO K., KINOUCHI M., ISHIDA-YAMAMOTO A., IIZUKA H. Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp. Cell Res. 1999;249:291–298. doi: 10.1006/excr.1999.4476. [DOI] [PubMed] [Google Scholar]
- TANG D., LAHTI J.M., KIDD V.J. Caspase-8 activation and bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis. J. Biol. Chem. 2000;275:9303–9307. doi: 10.1074/jbc.275.13.9303. [DOI] [PubMed] [Google Scholar]
- UDDIN S., KOTTEGODA S., STIGGER D., PLATANIAS L.C., WICKREMA A. Activation of the Akt/FKHRL1 pathway mediates the antiapoptotic effects of erythropoietin in primary human erythroid progenitors. Biochem. Biophys. Res. Commun. 2000;275:16–19. doi: 10.1006/bbrc.2000.3266. [DOI] [PubMed] [Google Scholar]
- UEHARA T., KIKUCHI Y., NOMURA Y. Caspase activation accompanying cytochrome c release from mitochondria is possibly involved in nitric oxide-induced neuronal apoptosis in SH-SY5Y cells. J. Neurochem. 1999;72:196–205. doi: 10.1046/j.1471-4159.1999.0720196.x. [DOI] [PubMed] [Google Scholar]
- VANAGS D.M., PORN-ARES M.I., COPPOLA S., BURGESS D.H., ORRENIUS S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J. Biol. Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- VERDIER F., WALRAFEN P., HUBERT N., CHRETIEN S., GISSELBRECHT S., LACOMBE C., MAYEUX P. Proteasomes regulate the duration of erythropoietin receptor activation by controlling down-regulation of cell surface receptors. J. Biol. Chem. 2000;275:18375–18381. doi: 10.1074/jbc.275.24.18375. [DOI] [PubMed] [Google Scholar]
- WEN T.C., SADAMOTO Y., TANAKA J., ZHU P.X., NAKATA K., MA Y.J., HATA R., SAKANAKA M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J. Neurosci. Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- WILLIAMS K., SCHWARTZ A., COREY S., ORANDLE M., KENNEDY W., THOMPSON B., ALVAREZ X., BROWN C., GARTNER S., LACKNER A. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am. J. Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]