Abstract
Exposure of isolated skeletal muscle to troglitazone has resulted in inconsistent findings ranging from inhibition to stimulation of fuel oxidation and the glycogenic pathway. To better understand such variation in outcome, the present study used isolated rat soleus muscle strips to examine the interdependent influences of prolonged maintenance in vitro and of troglitazone exposure.
If freshly isolated muscle strips were exposed to troglitazone (1 μmol l−1) for 24 h, glucose oxidation was markedly reduced (−26±1%, P<0.0001), whereas glycogen synthesis remained unaffected (+9±7%, n.s.).
In contrast, extended exposure to troglitazone for 72 h increased both glucose oxidation (+65±28%, P<0.05) and glycogen synthesis (+46±11%, P<0.005), and a similar stimulatory effect was also observed in muscles exposed to troglitazone only during the last 24 h of their 72 h preincubation period (glucose oxidation: +61±15%, P<0.001; glycogen synthesis: +43±15%, P<0.01).
Troglitazone thus stimulated glucose utilization in long-term incubated muscle independent of the duration of exposure (24 or 72 h), whereas it inhibited glucose utilization in freshly isolated muscle.
The observed differences in troglitazone action on freshly isolated vs long-term incubated muscle suggest that findings on muscle tissue subject to prolonged maintenance in vitro cannot be extrapolated to native muscle in vivo.
Keywords: Thiazolidinedione, troglitazone, skeletal muscle, glycogen storage, glucose oxidation, in vitro
Introduction
Thiazolidinediones (TZDs) are potent agonists of the nuclear peroxisome proliferator-activated receptor γ (PPARγ), which is predominantly expressed in adipose tissue and mediates adipocyte differentiation and adipose tissue remodelling (Spiegelman, 1998; Olefsky, 2000; Rosen & Spiegelman, 2001). The associated antihyperglycemic effect of TZDs, however, is mainly attributed to improved insulin-induced glucose disposal into skeletal muscle (Inzucchi et al., 1998; Olefsky, 2000; Rosen & Spiegelman, 2001). It is as yet unclear, whether the observed improvement in muscle glucose disposal is due to direct TZD action on skeletal muscle or due to indirect action via substrate or hormone signals from adipose tissue (Spiegelman, 1998; Olefsky, 2000; Rosen & Spiegelman, 2001).
To separate direct from indirect effects, the interaction of TZDs with isolated specimens of skeletal muscle has been repeatedly examined in vitro. A bulk of studies showed that TZDs stimulate glucose transport into muscle cells independently of concomitant insulin stimulation (El-Kebbi et al., 1994; Ciaraldi et al., 1995; Arakawa et al., 1998; Park et al., 1998; Fürnsinn et al., 1999, 2000; Brunmair et al., 2001; Cha et al., 2001; Kausch et al., 2001; Yonemitsu et al., 2001). In contrast to the effects on glucose uptake, the observed TZD-induced changes in intracellular glucose fluxes were rather inconsistent. Whereas prolonged exposure (⩾48 h) to troglitazone increased glycogen synthase activity, glycogen synthesis, and mitochondrial palmitate oxidation in human muscle cell cultures (Park et al., 1998; Cha et al., 2001; Kausch et al., 2001), short-term exposure (⩽24 h) to identical troglitazone concentrations inhibited mitochondrial fuel oxidation and glycogen storage in native rat soleus muscle strips (Fürnsinn et al., 2000; Brunmair et al., 2001). Although the latter short-term effects were apparently independent of PPARγ (Brunmair et al., 2001), it cannot be excluded that this receptor could mediate delayed actions on skeletal muscle (Zierath et al., 1998; Loviscach et al., 2000).
The present study was to unravel the cause of controversial troglitazone effects on glucose utilization in vitro. It is shown that the metabolic characteristics and the response to troglitazone of freshly isolated native muscle deviate strongly from those of long-term incubated muscle. From this, it appears that findings on muscle tissue subject to prolonged maintenance in vitro are of limited value for our understanding of TZD action on native muscle in vivo.
Methods
Rats
Where not stated otherwise, male Sprague – Dawley (SD) rats (approximately 5 weeks old, 140 g body weight) from the breeding facilities of the University of Vienna (Himberg, Austria) were employed. In some experiments, male genetically obese Zucker rats (fa/fa; approximately 5 months old, 650 g body weight) from Harlan-Winkelmann (Borchen, Germany) were used. Rats were kept at an artificial 12-h-light/12-h-dark cycle at constant room temperature. They were provided with conventional laboratory diet and tap water ad libitum. Food, but not water, was withdrawn overnight before rats were killed by cervical dislocation between 8 : 30 and 9 : 30 a.m. All experiments were performed according to local law and according to the principles of good laboratory animal care.
Preincubation period
Immediately after killing, two (SD rats) or three (Zucker rats) longitudinal soleus muscle strips per leg (i.e. four or six strips per rat) were prepared, weighed (approximately 25 mg strip−1), and tied under tension on stainless-steel clips as described previously (Crettaz et al., 1980). According to procedures employed earlier (Stace et al., 1990; Brunmair et al., 2001), muscles were immediately put into 50-ml-Erlenmeyer flasks coated with BlueSlick solution (Serva, Heidelberg, Germany) and were placed into a shaking waterbath (six strips per flask; 37°C; 130 cycles min−1). Each flask contained 20-ml cell culture medium 199 (pH 7.35, 5.5 mmol l−1 glucose; Sigma, St Louis, MO, U.S.A. Cat. No. M-4530) with additions of 0.3% (w v−1) fatty acid-free bovine serum albumin (BSA), 5 mmol l−1 HEPES, 25,000 U l−1 penicillin G, 25 mg l−1 streptomycin, and 0.2 mg/l ciprofloxacine. Palmitate was dissolved in ethanol and added to the medium to give final concentrations of 300 μmol l−1 palmitate and 0.25% ethanol (v v−1). Troglitazone (generously provided by Sankyo, Tokyo, Japan) was dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO, USA) and was added to give 0.1% (v v−1) DMSO and the indicated concentrations of troglitazone. The same concentration of DMSO was present in control media without troglitazone. All preincubations were in the absence of insulin and an atmosphere of 95% O2 : 5% CO2 was continuously provided within the flasks. Experimental protocols differed with regard to the periods of muscle preincubation and of troglitazone exposure (details described in the Results section and outlined in the respective figures). In experiments with preincubation periods of 48 or 72 h, fresh medium was provided every 24 h.
Measurement period
Immediately after preincubation, muscles were transferred for 1 h into 25-ml flasks (one strip per flask) containing 3 ml of medium additionally supplemented with trace amounts of D-[U-14C]glucose (Amersham, Amersham, U.K.). If not stated otherwise, measurements were performed under stimulation with 100 nmol l−1 human insulin (Actrapid, Novo, Bagsvaerd, Denmark). At the end of the 1-h-measurement period, muscles were quickly removed from the flasks, blotted, and frozen in liquid nitrogen.
Analytical procedures
Frozen muscle strips were lysed in 1 mol l−1 KOH at 70°C. The net rate of glucose incorporation into glycogen is referred to as glycogen synthesis and was calculated from the conversion of [14C]glucose into [14C]glycogen as described previously (Crettaz et al., 1980). Rates of CO2 production from glucose are referred to as glucose oxidation and were calculated from the conversion of [14C] glucose into 14CO2 which was trapped with a solution containing methanol and phenethylamine (1 : 1) (Fürnsinn et al., 1995). The applied methods give the amount of extracellular glucose converted into glycogen and CO2 but do not provide any information on glycogen cycling or on the oxidation of glucosyl units derived from the initially unlabelled glycogen pool.
For measurement of muscle glycogen content, glycogen in the muscle lysate was degraded to glucose with amyloglucosidase (Fürnsinn et al., 1995) and glucose was then measured enzymatically by a commercial kit (Human, Taunusstein, Germany).
Statistics
All results are given as means±s.e.m. Two-tailed paired or unpaired Student's t-test was used as appropriate, and a value of P<0.05 was considered as significant. The influence of troglitazone was always evaluated by paired comparison of muscle strips from the same rat that were incubated for the same time period in the presence vs the absence of troglitazone.
Results
Effects of the incubation per se
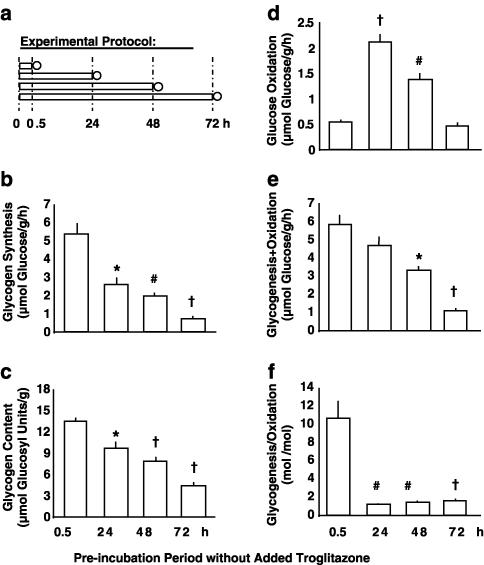
Figure 1.
Effects of incubation per se (no troglitazone added). Experimental protocol (a): immediately after preparation, soleus muscle strips from Sprague–Dawley rats were preincubated for 0.5, 24, 48, or 72 h in the absence of insulin (preincubation period; bars), which was followed by 1 h under insulin stimulation (measurement period; circles). During the measurement period, rates of conversion of glucose into glycogen (glycogen synthesis; (b)) and CO2 (glucose oxidation; (d)) were determined. Glycogen content as prevailing at the end of the experiments (c), as well as the sum and the quotient of glycogenesis plus/per glucose oxidation ((e) and (f), respectively) are also shown. Means±s.e.m.; n=6–12 each; *P<0.005, #P<0.001, †P<0.0001 vs preincubation for 0.5 h.
Muscle specimens were preincubated under control conditions (i.e. without troglitazone) immediately after preparation for 0.5, 24, 48, or 72 h. Subsequently, the rates of insulin-stimulated glucose metabolism were measured (Figure 1a). Figures 1b–f show the time-dependent influence of the incubation procedure per se on glucose metabolism. After 24 h of maintenance in vitro, glucose incorporation into glycogen (Figure 1b) and glycogen content (Figure 1c) were significantly diminished. The oxidation of glucose derived from the ambient medium was increased about four-fold (Figure 1d), obviously because the considerable reduction in glycogenic flux caused more radiolabelled glucose from the extracellular medium to directly enter glycolysis. Consequently, the ratio of net glucose flux into glycogenesis to glucose flux into oxidation was very markedly reduced from 10.6 : 1 to 1.2 : 1 (Figure 1f) and the sum of glucose converted into glycogen and CO2 decreased during the first day of incubation (Figure 1e).
Maintenance of muscle strips in vitro for more than 24 h was associated with progressive reductions in glycogen synthesis, glucose oxidation, and glycogen content, whereas the ratio of glycogenesis/oxidation remained constant between 24 h and 72 h. Taken together, the incubation procedure per se caused a rapid and distinct reduction of glycogenic activity within 24 h, as well as a progressive reduction of the sum of glucose conversion into glycogen plus CO2.
Time-dependent effects of troglitazone
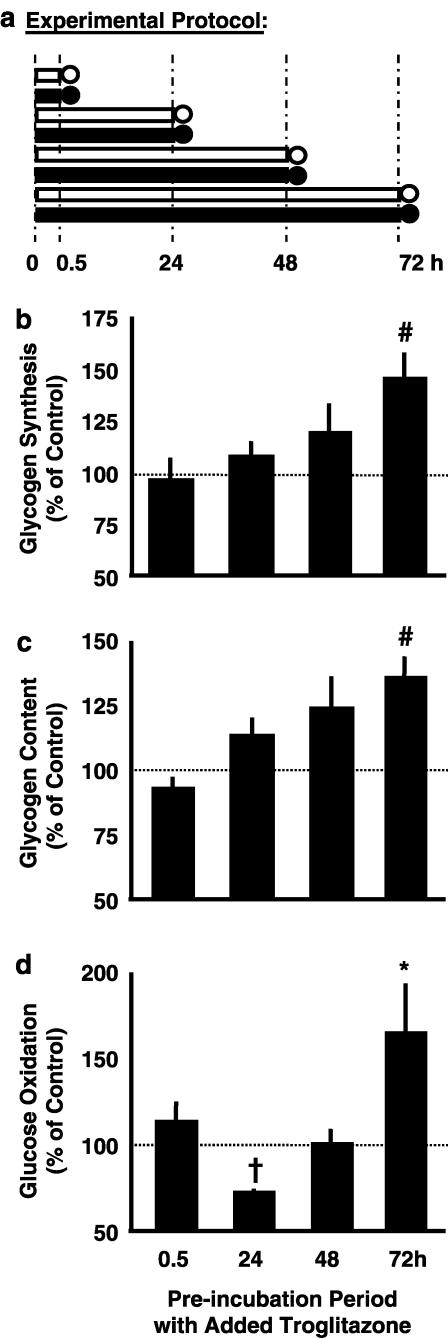
Figure 2.
Time-dependent effects of troglitazone. Experimental protocol (a): soleus muscle strips from Sprague–Dawley rats were preincubated without insulin in the absence (open symbols) or presence (full symbols) of 1 μmol l−1 troglitazone for 0.5, 24, 48, or 72 h (preincubation period; bars), which was followed by 1 h under insulin stimulation (measurement period; circles). During the measurement period, rates of conversion of glucose into glycogen (glycogen synthesis; (b)) and CO2 (glucose oxidation; (d)) were determined. Glycogen content is shown as prevailing at the end of the respective experiment (c). Data are given as a percentage of an intraindividual control muscle incubated in the absence of troglitazone (absolute rates under control conditions are given in Figure 1). Means±s.e.m.; n=6–12 each; *P<0.05, #P<0.005, †P<0.0001 vs control without troglitazone (=100%).
If troglitazone (1 μmol l−1) was added under the same experimental conditions (Figure 2a), insulin-stimulated glucose oxidation was markedly blunted vs the controls after 24 h (Figure 2d). At this time, glycogen storage was not significantly affected by troglitazone (Figures 2b and c). With more prolonged incubation, the troglitazone-induced reduction in glucose oxidation faded (48 h) and then turned into a troglitazone-dependent increase (72 h). After 72 h, insulin-stimulated glycogen synthesis, glycogen content, and glucose oxidation were higher in troglitazone-exposed vs control muscles. Troglitazone thus acted both as an inhibitor and as a stimulator of glucose utilization, depending on the duration of the preincubation period. Inhibitory action predominated during the initial 24 h and was progressively overcome by stimulatory action during more prolonged incubation.
Effects of 72 h troglitazone: dose- and insulin-dependency
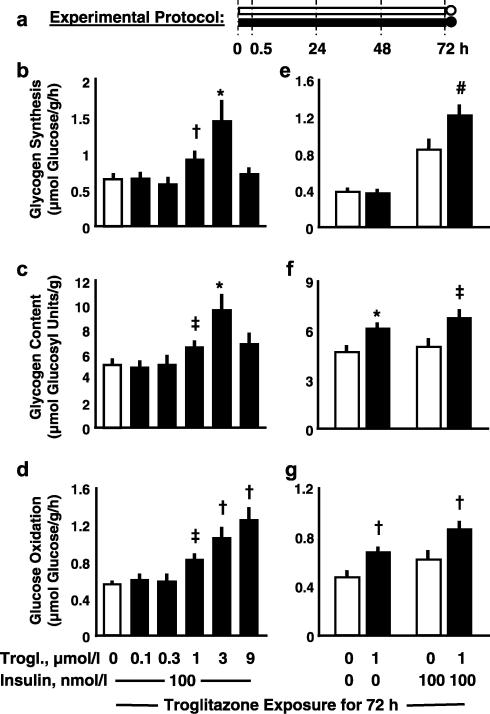
Figure 3.
Effects of 72 h troglitazone; dose-dependency and insulin-dependency. Experimental protocol (a): soleus muscle strips from Sprague–Dawley rats were preincubated without insulin in the absence (open symbols) or presence (full symbols) of various concentrations of troglitazone for 72 h (preincubation period; bars), which was followed by 1 h under basal conditions or under insulin stimulation (measurement period; circles). During the measurement period, rates of conversion of glucose into glycogen (glycogen synthesis; (b), (e)) and CO2 (glucose oxidation; (d), (g)) were determined. Glycogen content is shown as prevailing at the end of the respective experiment ((c), (f)). Means±s.e.m.; n=6–18 each; *P<0.02, #P<0.01, †P<0.005, ‡P<0.001 vs control without troglitazone.
In muscle strips exposed to troglitazone for 72 h (Figure 3a), increases in insulin-stimulated net glycogen synthesis and glycogen content were observed at 1 and 3 μmol l−1 troglitazone (Figures 3b and c). At 9 μmol l−1 troglitazone, insulin-stimulated glycogenesis and glycogen content were back to control values. Furthermore, troglitazone dose-dependently stimulated glucose oxidation which, in contrast to glycogen storage, increased further at 9 μmol l−1 troglitazone (Figure 3d).
Basal and insulin-stimulated glucose oxidation increased to the same extent in response to incubation with 1 μmol l−1 troglitazone for 72 h (Figure 3g). Basal glycogen synthesis prevailing during the last hour of the experiment (measurement period) was the same in control and troglitazone-exposed muscle (Figure 3e), whereas glycogen content, which reflects net glycogen storage during the whole experiment (including the preincubation period), was elevated in troglitazone-exposed vs control muscles (Figure 3f). Troglitazone thus affected glucose oxidation as well as glycogen storage independent of insulin.
Effects of 72 h troglitazone: insulin-resistant muscle
Figure 4.
Effects of 72 h troglitazone; insulin resistant muscle. Experimental protocol (a): soleus muscle strips from obese Zucker rats (fa/fa) were preincubated without insulin in the absence (open symbols) or presence (full symbols) of various concentrations of troglitazone for 72 h (preincubation period; bars), which was followed by 1 h under basal conditions or under insulin stimulation (measurement period; circles). During the measurement period, rates of conversion of glucose into glycogen (glycogen synthesis; (b)) and CO2 (glucose oxidation; (d)) were determined. Glycogen content is shown as prevailing at the end of the respective experiment (c). Means±s.e.m.; n=9–12 each; *P<0.05, #P<0.01 vs control without troglitazone.
Specimens of soleus muscle from obese Zucker rats, which exhibit marked insulin resistance (Crettaz et al., 1980), were continuously exposed to troglitazone for 72 h (Figure 4a). At 1 μmol l−1 troglitazone, basal and insulin-stimulated glucose oxidation were increased to a similar extent (Figure 4d). Basal and insulin-stimulated glycogen synthesis were increased by approximately 25 and 45%, respectively, but the latter effect was not significant (P=0.06; Figure 4b). Glycogen content was not significantly affected (Figure 4c). Hence, incubation with 1 μmol l−1 troglitazone for 72 h caused similar increases in glucose utilization by healthy and insulin-resistant muscle strips (Figure 3 and Figure 4, respectively).
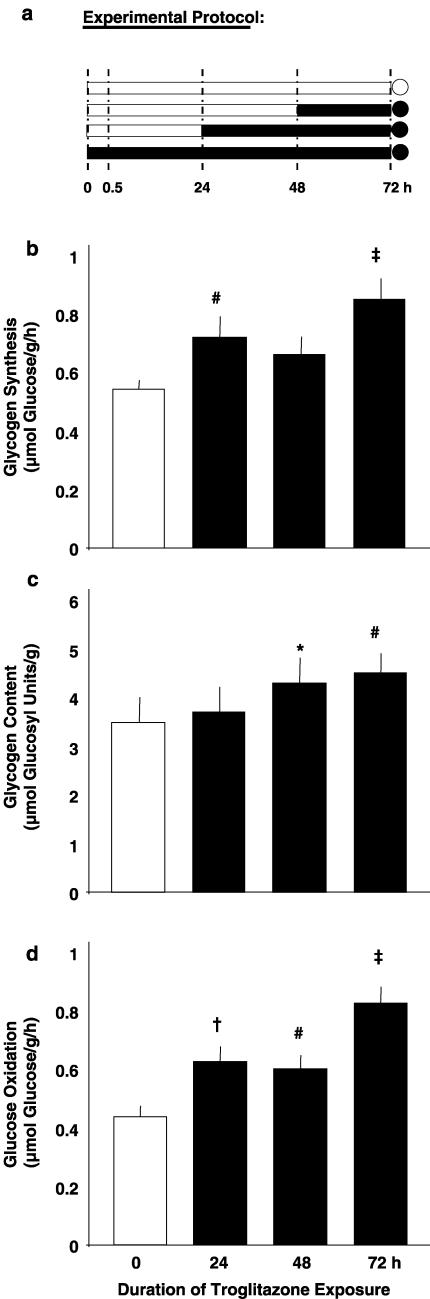
Effects of duration of troglitazone exposure on muscle strips incubated for 72 h
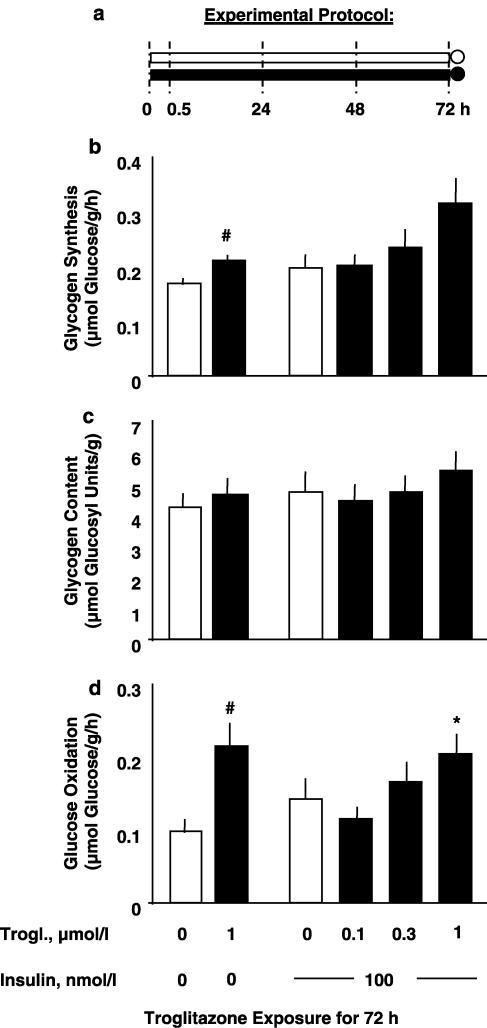
Figure 5.
Effects of duration of troglitazone exposure on muscle strips incubated for 72 h. Experimental protocol (a): soleus muscle strips from Sprague–Dawley rats were preincubated without insulin for a total period of 72 h (preincubation period, bars) without troglitazone or with 1 μmol l−1 troglitazone added during the last 24 h, the last 48 h, or the whole preincubation period (absence of troglitazone: open symbols; presence of troglitazone: full symbols), which was followed by 1 h under insulin stimulation (measurement period; circles). During the measurement period, rates of conversion of glucose into glycogen (glycogen synthesis; (b) and CO2 (glucose oxidation; (d)) were determined. Glycogen content is shown as prevailing at the end of the respective experiment (c). Means±s.e.m.; n=23 or 24 each; *P<0.05, #P<0.03, †P<0.002, ‡P<0.001 vs control without troglitazone.
The effects of 24, 48, and 72 h of troglitazone exposure were compared in muscle strips kept in vitro for the same total period of 72 h (Figure 5a). Addition of 1 μmol l−1 troglitazone during the last 24 h of the 72 h preincubation period resulted in increased rates of insulin-stimulated glycogen synthesis and glucose oxidation (Figures 5b and d). Thus, a dose and duration of troglitazone exposure (1 μmol l−1 for 24 h), which distinctly inhibited glucose oxidation in freshly isolated skeletal muscle (Figure 2d), exerted stimulatory action in muscle strips preadapted to the in vitro environment for 48 h (Figure 5d).
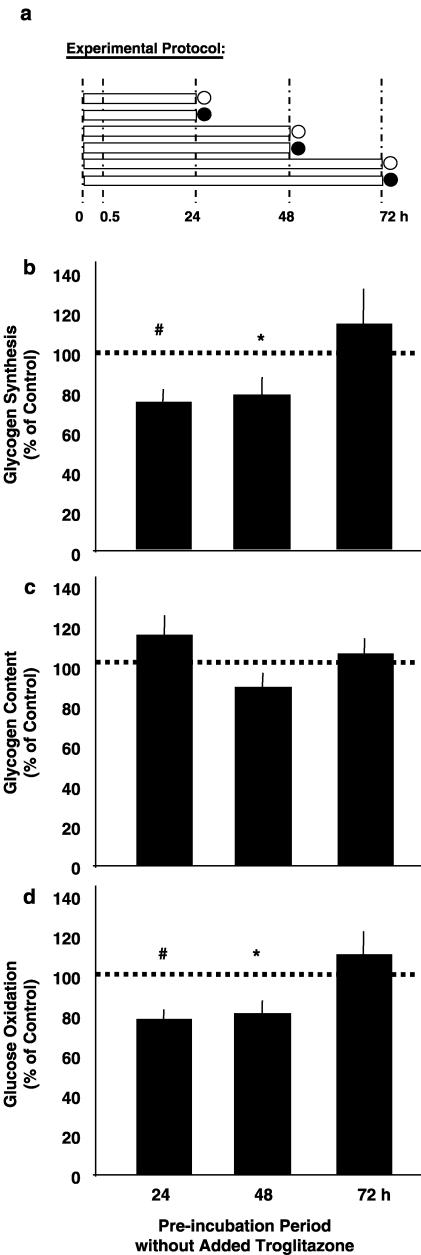
Acute effects of troglitazone after different periods of preincubation
Figure 6.
Acute effects of troglitazone after different periods of pre-incubation. Experimental protocol (a): soleus muscle strips from Sprague–Dawley rats were preincubated in the absence of insulin and troglitazone for 24, 48, or 72 h (preincubation period, bars), which was followed by 1 h of insulin stimulation in the absence or presence of 20 μmol l−1 troglitazone (measurement period; absence of troglitazone: open circles; presence of troglitazone: full circles). During the measurement period, rates of conversion of glucose into glycogen (glycogen synthesis; (b)) and CO2 (glucose oxidation; (d)) were determined. Glycogen content is shown as prevailing at the end of the respective experiment (c). Data are given as a percentage of an intraindividual control muscle that was incubated in the absence of troglitazone. Means±s.e.m.; n=12–14 each; *P<0.03, #P<0.003 vs control (=100%).
Muscles were preincubated for different time periods, before the acute effects of 20 μmol l−1 troglitazone were determined during the last hour of the experiment (=measurement period; Figure 6a). In muscle strips allowed to adapt to the in vitro environment for the preceding 24 or 48 h, acute treatment with troglitazone blunted both insulin-stimulated glycogen synthesis and glucose oxidation. In muscle strips subjected to prolonged preincubation for 72 h, however, troglitazone failed to elicit a comparable response (Figures 6b and d). Glycogen content was not affected within 1 h of exposure to 20 μmol l−1 troglitazone independent of the preincubation period (Figure 6c). Thus, responsiveness to the acute inhibitory action of troglitazone was lost during prolonged maintenance in vitro.
Discussion and conclusions
The results confirm the finding of 24-h exposure to 1 μmol l−1 troglitazone to distinctly inhibit mitochondrial fuel oxidation but not glycogen synthesis in freshly isolated soleus muscle strips obtained from SD rats (Fürnsinn et al., 2000). Higher concentrations of the TZD (⩾2.5 μmol l−1) are known to reduce glycogen synthesis in parallel with glucose oxidation (Fürnsinn et al., 2000; Brunmair et al., 2001). It is of note, however, that inhibitory action of short-term troglitazone exposure was reversed to stimulatory action during prolonged incubation exceeding 24 h. After 72 h, both glycogen synthesis and glucose oxidation were markedly increased in troglitazone-treated vs control muscles. Low glycogen content, a potent stimulator of glycogen synthesis in skeletal muscle (Nielsen et al., 2000), cannot explain our findings because glycogen content was elevated after 72 h troglitazone. Such delayed increase in glucose utilization is in line with reports on muscle-derived cell cultures that exhibit increased glycogen synthase activity, glycogen synthesis, and mitochondrial palmitate oxidation after long-term troglitazone exposure (Park et al., 1998; Cha et al., 2001; Kausch et al., 2001).
The delayed stimulatory effects on glucose metabolism seen after 72 h of troglitazone exposure of rat soleus muscle in vitro exhibit several parallels with changes in glucose metabolism as associated with oral TZD treatment in vivo. They include that (i) glucose lowering action in vivo and increased utilization in vitro are delayed responses; (ii) the effects in vivo and in vitro are seen at troglitazone concentrations in the low micromolar range (prevailing in the incubation medium and in circulating plasma, respectively; Plosker & Faulds, 1999); and (iii) in rat soleus muscle exposed to troglitazone for 72 h in vitro as well as in soleus muscle from orally TZD-treated obese rodents, glucose oxidation is elevated independent of concomitant insulin stimulation, whereas glycogen synthesis is increased in an insulin-dependent manner (Stevenson et al., 1990; Fürnsinn et al., 1999; Sreenan et al., 1999).
On the first glimpse, these parallels in troglitazone action in vitro and in vivo make it tempting to speculate that the same mechanism(s) could be responsible for the delayed actions of troglitazone under both conditions, and that isolated muscle could be ideal to investigate the mechanisms of antidiabetic TZD action. However, the presented data suggest a very different conclusion.
We show that the simple maintenance of isolated skeletal muscle in vitro causes very distinct changes in its glucose handling. The most salient effect of the employed incubation procedure was a considerable reduction in glycogenic activity within only 24 h, which could be caused by the absence of regular cycles of glycogen depletion and restorage under in vitro conditions. Prolonged exposure of skeletal muscle to its artificial environment within the test flask also caused major changes in how the tissue specimens responded to troglitazone. Exposure to 1 μmol l−1 troglitazone for 24 h markedly inhibited glucose oxidation and failed to affect glycogen synthesis in freshly isolated muscle, but the same troglitazone concentration and the same duration of exposure exerted stimulatory action in muscle strips that were already adapted to the in vitro situation for a prolonged period of time. Furthermore, acute inhibition of glucose oxidation by 20 μmol l−1 troglitazone within 1 h is seen in freshly prepared muscles (Brunmair et al., 2001) and in muscle strips kept in vitro since 24 or 48 h (this study), but is absent after more prolonged maintenance in vitro for 72 h. Hence, the delay in troglitazone-induced stimulation of glucose utilization is obviously not because such response to troglitazone requires prolonged exposure to the agent, but rather because such response characterizes long-term incubated muscle and cultured muscle cells, but not freshly isolated native muscle.
As insulin resistance has been reported to develop in denervated skeletal muscle (Burant et al., 1984), it could be speculated that the different responses to troglitazone by fresh vs long-term incubated muscle are related to prevailing insulin sensitivity. This, however, is excluded by our previous observation that 24 h of incubation with troglitazone inhibits glucose oxidation not only in normal but also in insulin resistant muscle from obese Zucker rats (Brunmair et al., 2001), which opposes stimulatory action on long-term incubated muscle. As troglitazone's stimulatory effect after 72 h of incubation is also seen in both normal and insulin-resistant muscle, the response to troglitazone is obviously determined by the period of maintenance in vitro independently of prevailing insulin sensitivity.
Taken together, we show that glucose handling by isolated muscle progressively changes in response to its artificial environment, which necessarily implies that extrapolations from in vitro to in vivo become increasingly doubtful the longer the employed muscle specimens or muscle cells are separated from their physiologic environment. Adaptions to the in vitro conditions include that of troglitazone, which acts as an inhibitor of glucose utilization in freshly isolated native muscle, stimulates glucose utilization in long-term incubated or cultured muscle. It is therefore doubtful whether the demonstration of direct stimulatory action of troglitazone on fuel oxidation and glycogenic activity in long-term incubated muscle or cultured muscle cells (Park et al., 1998; Cha et al., 2001; Kausch et al., 2001; and this study) is appropriate evidence for such actions in vivo. Although it cannot be principally excluded that the mechanism responsible for troglitazone-induced stimulation of glucose utilization in long-term incubated muscle is relevant for the agent's antidiabetic action in vivo, our findings clearly define the limits in drawing conclusions from long-term incubated skeletal muscle or muscle-derived cell lines.
Acknowledgments
We thank Sankyo (Tokyo, Japan) for generously providing troglitazone. We thank the staff at the Biomedical Research Centre, University of Vienna, for taking care of the rats. This work was supported by the Austrian Science Fund (Grant No. P13049-MED).
Abbreviations
- TZD
thiazolidinedione
- PPARγ
peroxisome proliferator-activated receptor
- SD rats
Sprague–Dawley rats
- DMSO
dimethyl sulfoxide
References
- ARAKAWA K., ISHIHARA T., AOTO M., INAMUSA M., SAITO A., IKEZAWA K. Actions of novel antidiabetic thiazolidinedione, T-174, in animal models of non-insulin-dependent diabetes mellitus (NIDDM) and in cultured muscle cells. Br. J. Pharmacol. 1998;125:429–436. doi: 10.1038/sj.bjp.0702066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNMAIR B., GRAS F., NESCHEN S., RODEN M., WAGNER L., WALDHÄUSL W., FÜRNSINN C. Direct thiazolidinedione action on isolated rat skeletal muscle fuel handling is independent of peroxisome proliferator-activated receptor-γ-mediated changes in gene expression. Diabetes. 2001;50:2309–2315. doi: 10.2337/diabetes.50.10.2309. [DOI] [PubMed] [Google Scholar]
- BURANT C.F., LEMMON S.K., TREUTELAAR M.K., BUSE M.G. Insulin resistance of denervated rat muscle: a model for impaired receptor – function coupling. Am. J. Physiol. 1984;247:E657–E666. doi: 10.1152/ajpendo.1984.247.5.E657. [DOI] [PubMed] [Google Scholar]
- CHA B.S., CIARALDI T.P., CARTER L., NIKOULINA S.E., MUDALIAR S., MUKHERJEE R., PATERNITI J.R., HENRY R.R. Peroxisome proliferator-activated receptor (PPAR) γ and retinoid X receptor (RXR) agonists have complemetary effects on glucose and lipid metabolism in human skeletal muscle. Diabetologia. 2001;44:444–452. doi: 10.1007/s001250051642. [DOI] [PubMed] [Google Scholar]
- CIARALDI T.P., HUBER-KNUDSEN K., HICKMAN M., OLEFSKY J.M. Regulation of glucose transport in cultured muscle cells by novel hypoglycemic agents. Metabolism. 1995;44:976–982. doi: 10.1016/0026-0495(95)90092-6. [DOI] [PubMed] [Google Scholar]
- CRETTAZ M., PRENTKI M., ZANIETTI D., JEANRENAUD B. Insulin resistance in soleus muscle from obese Zucker rats. Involvement of several defective sites. Biochem. J. 1980;186:525–534. doi: 10.1042/bj1860525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-KEBBI I.M., ROSER S., POLLET R.J. Regulation of glucose transport by pioglitazone in cultured muscle cells. Metabolism. 1994;43:953–958. doi: 10.1016/0026-0495(94)90173-2. [DOI] [PubMed] [Google Scholar]
- FÜRNSINN C., BRUNMAIR B., MEYER M., NESCHEN S., FURTMÜLLER R., RODEN M., KÜHNLE H.F., NOWOTNY P., SCHNEIDER B., WALDHÄUSL W. Chronic and acute effects of thiazolidinediones BM13.1258 and BM15.2054 on rat skeletal muscle glucose metabolism. Br. J. Pharmacol. 1999;128:1141–1148. doi: 10.1038/sj.bjp.0702886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FÜRNSINN C., BRUNMAIR B., NESCHEN S., RODEN M., WALDHÄUSL W. Troglitazone directly inhibits CO2 production from glucose and palmitate in isolated rat skeletal muscle. J. Pharmacol. Exp. Ther. 2000;293:487–493. [PubMed] [Google Scholar]
- FÜRNSINN C., EBNER K., WALDHÄUSL W. Failure of GLP-1(7-36)amide to affect glycogenesis in rat skeletal muscle. Diabetologia. 1995;38:864–867. doi: 10.1007/s001250050365. [DOI] [PubMed] [Google Scholar]
- INZUCCHI S.E., MAGGS D.G., SPOLLETT G.R., PAGE S.L., RIFE F.S., WALTON V., SHULMAN G.I. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N. Engl. J. Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- KAUSCH C., KRÜTZFELDT J., WITKE A., RETTIG A., BACHMANN O., RETT K., MATTHAEI S., MACHICAO F., HÄRING H.-U., STUMVOLL M. Effects of troglitazone on cellular differentiation, insulin signaling, and muscle glucose metabolism in cultured human skeletal muscle cells. Biochem. Biophys. Res. Commun. 2001;280:664–674. doi: 10.1006/bbrc.2000.4216. [DOI] [PubMed] [Google Scholar]
- LOVISCACH M., REHMANN N., CARTER L., MUDALIAR S., MOHADEEN P., CIARALDI T.P., VEERKAMP J.H., HENRY R.R. Distribution of peroxisome proliferator-activated receptors (PPARs) in human skeletal muscle and adipose tissue: relation to insulin action. Diabetologia. 2000;43:304–311. doi: 10.1007/s001250050048. [DOI] [PubMed] [Google Scholar]
- NIELSEN J.N., DERAVE W., KRISTIANSEN S., RALSTON E., PLOUG T., RICHTER E.A. Glycogen synthase localization and activity in rat skeletal muscle is strongly dependent on glycogen content. J. Physiol. 2000;531:757–769. doi: 10.1111/j.1469-7793.2001.0757h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLEFSKY J.M. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J. Clin. Invest. 2000;106:467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK K.S., CIARALDI T.P., ABRAMS-CARTER L., MUDALIAR S., NIKOULINA S.E., HENRY R.R. Troglitazone regulation of glucose metabolism in human skeletal muscle cultures from obese type II diabetic subjects. J. Clin. Endocrinol. Metab. 1998;83:1636–1643. doi: 10.1210/jcem.83.5.4764. [DOI] [PubMed] [Google Scholar]
- PLOSKER G.L, FAULDS D. Troglitazone. A review of its use in the management of type 2 diabetes mellitus. Drugs. 1999;57:279–462. doi: 10.2165/00003495-199957030-00014. [DOI] [PubMed] [Google Scholar]
- ROSEN E.D., SPIEGELMAN B.M. PPAR-γ: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- SPIEGELMAN B.M. PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- SREENAN S., KECK S., FULLER T., COCKBURN B., BURANT C.F. Effects of troglitazone on substrate storage and utilization in insulin-resistant rats. Am. J. Physiol. 1999;276:E1119–E1129. doi: 10.1152/ajpendo.1999.276.6.E1119. [DOI] [PubMed] [Google Scholar]
- STACE P.B., MARCHINGTON D.R., KERBEY A.L., RANDLE P.J. Long term culture of rat soleus muscle in vitro. Its effects on glucose utilization and insulin sensitivity. FEBS Lett. 1990;273:91–94. doi: 10.1016/0014-5793(90)81058-v. [DOI] [PubMed] [Google Scholar]
- STEVENSON R.W., HUTSON N., KRUPP M.N., VOLKMANN R.A., HOLLAND G.F., EGGLER J.F., CLARK D.A., MCPHERSON R.K., HALL K.L., DANBURY B.H., GIBBS E.M., KREUTTER D.K. Actions of novel antidiabetic agent englitazone in hyperglycemic hyperinsulinemic ob/ob mice. Diabetes. 1990;39:1218–1227. doi: 10.2337/diab.39.10.1218. [DOI] [PubMed] [Google Scholar]
- YONEMITSU S., NISHIMURA H., SHINTANI M., INOUE R., YAMAMOTO Y., MASUZAKI H., OGAWA Y., HOSODA K., INOUE G., HAYASHI T., NAKAO K. Troglitazone induces GLUT4 translocation in L6 myotubes. Diabetes. 2001;50:1093–1101. doi: 10.2337/diabetes.50.5.1093. [DOI] [PubMed] [Google Scholar]
- ZIERATH J.R., RYDER J.W., DOEBBER T., WOODS J., WU M., VENTRE J., LI Z., MCCRARY C., BERGER J., ZHANG B., MOLLER D.E. Role of skeletal muscle in thiazolidinedione insulin sensitizer (PPARγ agonist) action. Endocrinology. 1998;139:5034–5041. doi: 10.1210/endo.139.12.6364. [DOI] [PubMed] [Google Scholar]