Abstract
Available anticancer drugs do not seem to modify the prognosis of metastatic melanoma. Salicylate and acetyl salicylic acid (aspirin) were found to suppress growth in a number of transformed cells, that is, prostate and colon. Therefore, we studied the direct effects of aspirin on metastatic B16 melanoma cells.
Aspirin at a plasma-attainable and nontoxic level suppressed the proliferation of B16 cells.
Aspirin induced the activation of p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases.
Inhibition of JNK, but not p38, decreased the suppressive effect of aspirin upon the proliferation of B16 cells.
The aspirin-induced reduction in B16 proliferation was cumulative over time.
Aspirin and the chemotherapeutic drug 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) induced B16 cell death synergistically.
In addition to the murine B16 cell line, the proliferation of SK-28 human melanoma cells was also suppressed by aspirin.
In conclusion, aspirin suppresses the proliferation of metastatic B16 cells in a JNK-dependent mechanism.
Keywords: MAPK, melanoma, aspirin, BCNU, p38, JNK
Introduction
The incidence of cutaneous malignant melanoma is increasing and it is projected that Americans born in the year 2000 will have a one in 75 lifetime risk of developing melanoma. Long-term survival for patients with metastatic disease is only 5% and the commonly used anticancer drugs do not seem to modify the prognosis of metastatic disease (Brown & Nelson, 1999; Serrone & Hersey, 1999). Thus, new chemotherapeutic agents for melanoma are of great clinical interest.
A significant decrease in the relative risk of malignant melanoma was observed in women taking aspirin (Harris et al., 2001). We have recently shown that incubation with acetyl salicylic acid (aspirin) for 3 days reduced cellular proliferation by up to 35–55% in prostate cancer cell lines (Rotem et al., 2000), and that salicylic acid suppressed the proliferation of various types of human cancer cells (Fingrut & Flescher, 2002). Therefore, we studied the effects of aspirin on melanoma cells.
The mechanism through which aspirin affects the proliferation and viability of different cells is not clear. Mitogen-activated protein kinases (MAPK) are proline-directed serine–threonine kinases that are activated by upstream dual specificity kinases which produce a simultaneous phosphorylation on threonine and tyrosine residues. The p38 kinase and c-Jun N-terminal kinase (JNK) comprise a subfamily of MAPK that are strongly activated by stress signals (Robinson & Cobb, 1997). Sodium salicylate was found to induce p38 activation in human fibroblasts and in green monkey kidney cells (Schwenger et al., 1997). 1998The activation of p38 resulted in apoptosis (Schwenger et al., 1997). In addition, sodium salicylate and aspirin induced JNK activation in green monkey kidney cells and in colon adenocarcinoma human cells (Schwenger et al., 1999). Consequently, we evaluated the involvement of the stress-responsive MAPK in the effects of aspirin on melanoma cells.
Methods
Cells and reagents
The B16-F10.9 cell line used in this study is a metastatic variant of the B16 murine melanoma (Hart, 1979). It was grown in DMEM with 10% FCS. SK-28 are human melanoma cells grown in RPMI-1640 with 10% FCS (ATCC, Rockville, MD, U.S.A.).
All the reagents used were purchased from Sigma Chemicals (St Louis, MO, U.S.A.) unless otherwise stated. SB203580 (a specific p38 inhibitor that does not inhibit the activity of other MAPK, Turchi et al., 2000) and SB202190 (an agent that inhibits both JNK and p38, Turchi et al., 2000) were purchased from Calbiochem (La Jolla, CA, U.S.A.). 1,3-Bis(2-chloroethyl)-1-nitrosourea (BCNU) is a chemotherapeutic drug.
Cytotoxicity and cellular proliferation assays
Cellular proliferation and its inhibition were determined by the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, U.S.A.). Upon completion of a given experiment, MTS (a tetrazolium compound) at 333 μg ml−1+phenazine methosulfate (at 25 μM) were added to each well of the 96-well plate for 1 h at 37°C. This allowed for the development of the reaction in which dehydrogenases reduce the MTS in metabolically active cells. Since the cells were not washed before the addition of MTS, we did not have any problem with potentially loosely adherent melanoma cells. The soluble MTS formazan product was measured at 490 nm by the CERES 900 HDi ELISA reader (Bio-Tek Instruments, Inc, Highland Park, VT, U.S.A.). Optical density is directly proportional to the number of living cells in culture. Cytotoxicity (%) was calculated in the following way: [(OD of control cells −OD of drug-treated cells)/OD of control cells] × 100. Since the same number of cells was aliquoted into each well initially, decreased optical density in wells containing treated cells reflects cellular death and/or decrease in the rate of proliferation. To distinguish between these two possibilities, we employed an additional cytotoxicity assay that detects cell death by lack of trypan blue exclusion (see below).
Cell death was determined by trypan blue exclusion. Cells were incubated with 0.1% trypan blue for 2–5 min and the percentage of dead cells (those which did not exclude the dye) was determined microscopically.
Determination of p38 and c-Jun phosphorylation
For analysis of phospho-p38 (indicating p38 activation) levels, and c-Jun phosphorylation (indicating JNK activity), whole-cell lysates were prepared as described before (Samet et al., 1998). Protein samples (50 μg of whole-cell lysate) were separated by sodium dodecylsulfate-polyacrylamide gel electorphoresis (SDS-PAGE) on 14% tris-glycine gels, followed by immunoblotting using specific antibodies against phospho-p38 (1 : 1000, New England Biolabs, Cambridge, MA, U.S.A.) and phosphorylated c-Jun (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), respectively, at 4°C. Antigen&–antibody complexes were stained with HRP-conjugated antibody (1 : 2000, Bio-Rad, Richmond, CA, U.S.A.) and enhanced chemiluminescence reagent (ECL), and exposed to ECL film (both from Amersham, Arlington Heights, IL, U.S.A.). Immunoblot images were digitized and the optical densities of specific antigen–antibody complexes were quantified using the Gene Gnome Imaging System supported by the Gene Tools software package (both from Syngene, Frederick, MD, U.S.A.). The phosphorylation of p38 and c-Jun was determined following treatment with aspirin. No change in the total levels of these proteins was detected in the same cultures.
Statistical analysis
The statistical significance of the results was determined (where appropriate) by two-tailed Student's t-test.
Results
Suppression of B16 proliferation by aspirin
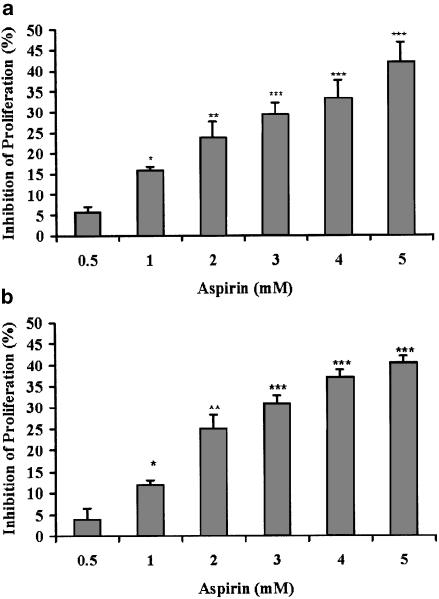
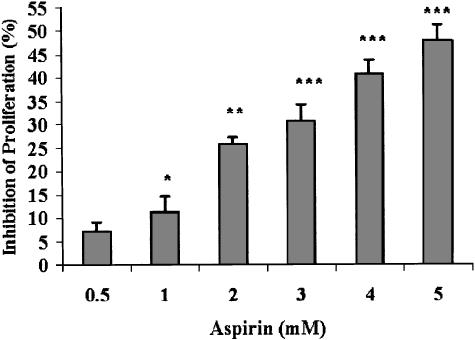
Since the direct effect of aspirin on melanoma cells has never been reported, we studied the ability of aspirin to modulate the viability and proliferative potential of a metastatic variant of B16 melanoma cells. Aspirin (at 0.5–5 mM) suppressed the ability of B16 cells to proliferate in a dose-dependent manner (Figure 1). The validity of the enzymatic assay (Figure 1, panel a), as a measure of cellular proliferation, was established by direct cell counts (Figure 1, panel b). This outcome could be the result of cell death. However, determining the fraction of dead cells in our cultures (by the trypan blue exclusion method), we did not find more than 5–10% dead cells in either control or any of the aspirin-treated cultures. These results strongly suggest that the reduction in cell number, depicted in Figure 1, reflects suppression of cellular proliferation rather than cell death.
Figure 1.
Aspirin suppresses B16 cellular proliferation. (a) B16 cells (at 4 × 103 well−1) were seeded in 96-well plates and allowed to adhere overnight. Aspirin at the indicated concentrations was added for 1 day. Optical density representing viable cells was determined by the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay. (b) B16 cells (at 4 × 105 well−1) were seeded in six-well plates and allowed to adhere overnight. Aspirin at the indicated concentrations was added for 1 day. The number of viable cells was determined by microscopic cell counts. Inhibition of proliferation is calculated as percentage of control cultures that were not treated with aspirin, mean±s.d. The inhibition was statistically significant at *P<0.025, **P<0.01, ***P<0.005 for panel a, and *P<0.025, **P<0.005, ***P<0.0005 for panel b; n=3.
Mediation of aspirin-induced suppression of B16 proliferation by JNK, but not p38, MAPK
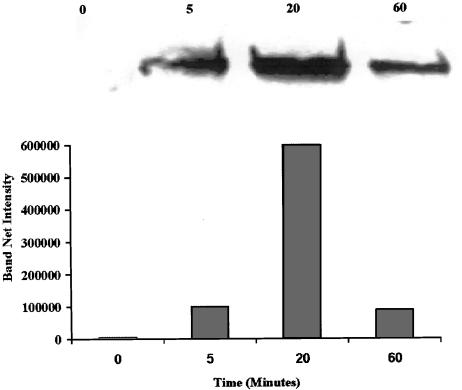
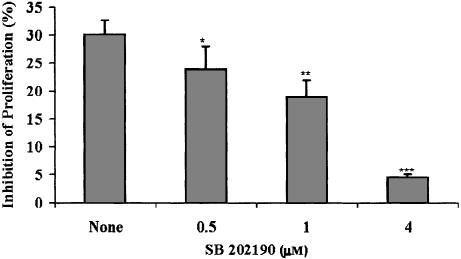
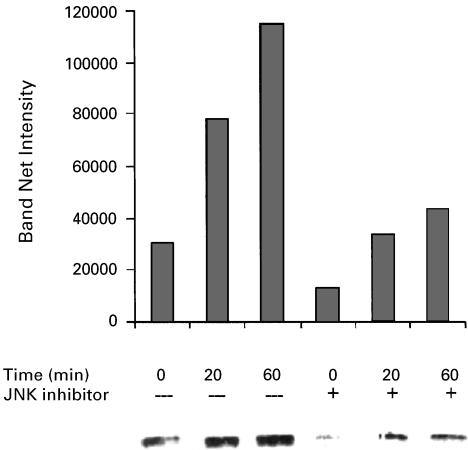
Since sodium salicylate was found to induce p38 activation in human fibroblasts and green monkey kidney cells (Schwenger et al., 1997), 1998we investigated the possible involvement of this stress-regulated kinase in the aspirin-induced suppression of B16 proliferation. Upon exposure to aspirin at 3 mM for 5–60 min, B16 cells exhibited a rise in the levels of activated p38 (Figure 2). The rise was maximal at 20 min. While these results clearly indicate an activation of p38 by aspirin in our system, a cause and effect relation had to be investigated. To that end, we employed a specific p38 inhibitor that does not affect other MAPK-SB203580. This inhibitor belongs to a series of pyridinyl-imidazole compounds that have a selective effect on the p38 MAPK activity, while not exhibiting inhibition of any other MAPK (JNK and ERK), protein kinase C and cAMP-dependent kinase activities (Lee et al., 1994). SB203580 did not exhibit any effect on aspirin-induced suppression of proliferation in B16 cells. On the other hand, SB202190 (an inhibitor of both p38 and JNK, Turchi et al., 2000) inhibited significantly, in a dose-dependent manner, the aspirin-induced suppression of B16 cells (Figure 3). SB202190 by itself did not affect B16 proliferation at the range of concentrations used (0.5–4 μM). These results point towards JNK as the MAPK that mediates the suppression of B16 proliferation induced by aspirin. Indeed, we found that aspirin induced JNK activity in B16 cells and that SB202190 decreased this effect (Figure 4).
Figure 2.
Aspirin induces p38 activation in B16 cells. B16 cells (at 4 × 105 well−1) were seeded in six-well plates and allowed to adhere overnight. The cells were then treated with or without (0) aspirin at 3 mMfor the indicated periods of time. For analysis of phospho-p38 (indicating p38 activation) levels, whole-cell lysates were prepared (Samet et al., 1998) and proteins were separated by SDS-PAGE, followed by immunoblotting using specific antibodies against phospho-p38. Antigen&–antibody complexes were stained with HRP-conjugated antibody and enhanced chemiluminescence reagent, and exposed to ECL film. Immunoblot images were digitized and the optical densities of specific antigen-antibody complexes were quantified. A typical experiment is shown, representing two experiments with similar results.
Figure 3.
The JNK and p38 inhibitor SB202190 decreases aspirin-induced suppression of B16 cellular proliferation. B16 cells (at 4 × 103 well−1) were seeded in 96-well plates and allowed to adhere overnight. Aspirin at 3 mMwas added for 1 day. SB202190 was added at the indicated concentrations, 30 min before the addition of aspirin, and was removed 2.5 h afterwards. Optical density representing viable cells was determined by the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay. Inhibition of proliferation is calculated as percentage of control cultures that were not treated with aspirin, mean±s.d. The effect of SB202190 (in comparison with None=only aspirin) was statistically significant at *P<0.05, **P<0.005, ***P<0.0005; n=3.
Figure 4.
Aspirin induces JNK activity in B16 cells. B16 cells (at 4 × 105 well−1) were seeded in six-well plates and allowed to adhere overnight. The cells were then treated with or without (0) aspirin at 3 mMfor the indicated periods of time. SB202190 (JNK inhibitor) was added at 4 μM, 30 min before the addition of aspirin. For analysis of phospho-c-Jun levels (indicating JNK activity), whole-cell lysates were prepared (Samet et al., 1998) and proteins were separated by SDS-PAGE, followed by immunoblotting using specific antibodies against phospho-c-Jun. Antigen–antibody complexes were stained with HRP-conjugated antibody and enhanced chemiluminescence reagent, and exposed to ECL film. Immunoblot images were digitized and the optical densities of specific antigen–antibody complexes were quantified. A typical experiment is shown, representing two experiments with similar results.
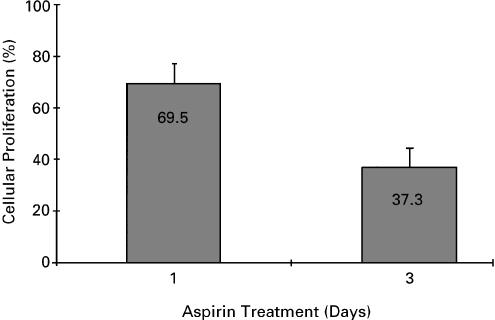
B16 proliferation during prolonged exposure to aspirin
Aspirin at 3 mM reduces B16 proliferation by approximately 30% (Figure 1). We prolonged the exposure to aspirin for 3 days and determined if the effect is cumulative. After 3 days in the presence of aspirin at 3 mM, the number of B16 cells was reduced by about 63% in comparison with untreated cells (Figure 5), suggesting that the suppressive effect of aspirin is cumulative. Growing the cells for three additional days with aspirin further decreased their number, reaching an inhibition level of 91%. Similar to 1-day exposure, the effect of aspirin during prolonged exposure was also on cell proliferation, but did not cause cell death, as assessed by trypan blue exclusion. When the aspirin was removed, the cells resumed their normal rate of proliferation (data not shown).
Figure 5.
Effect of prolonged exposure to aspirin on B16 proliferation. B16 cells (at 4 × 103 well−1) were seeded in 96-well plates and allowed to adhere overnight. Aspirin at 3 mMwas added for 1 and 3 days. Optical density representing viable cells was determined by the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay. Cellular proliferation is calculated as percentage of control cultures that were not treated with aspirin (defined as 100%), mean±s.d; n=3.
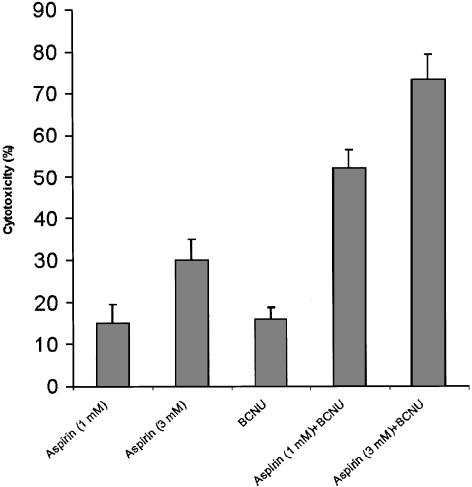
Synergistic effect of aspirin and a chemotherapeutic drug on B16 proliferation
To evaluate the efficiency of aspirin in enhancing the anticancer effect of currently available chemotherapy, we determined the cytotoxic effect of aspirin+BCNU. BCNU is a chemotherapeutic drug which has been shown to kill B16 cells (Poo et al., 1997). As can be seen in Figure 6, aspirin and BCNU acted synergistically (P<0.05) in exerting a cytotoxic effect towards B16 cells. While aspirin by itself does not kill B16 cells, we confirmed that the drug combination induced cell death, by the trypan blue exclusion assay.
Figure 6.
Cytotoxic effect of aspirin and BCNU towards B16 cells. B16 cells (at 4 × 103 well−1) were seeded in 96-well plates and allowed to adhere overnight. Aspirin at 1 and 3 mM, and/or BCNU at 50 μM, were added for 1 day. Optical density representing viable cells was determined by the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay. Cytotoxicity is calculated as percentage of control cultures that were not treated, mean±s.d., n=3. The synergistic effect (the difference between cytotoxicity in the presence of both drugs together, and the sum of the cytotoxicities of each drug administered separately) was significant at P<0.05, for each combination of aspirin and BCNU.
Proliferation of human melanoma cells (SK-28) in the presence of aspirin
In order to evaluate the relevance of aspirin-induced suppression of cellular proliferation in the B16 murine system to human cells, we determined the effect of aspirin on the human cell line – SK-28. As can be seen in Figure 7, aspirin suppressed the proliferation of SK-28 cells in a dose-dependent manner.
Figure 7.
Aspirin suppresses SK-28 cellular proliferation. SK-28 cells (at 4 × 103 well−1) were seeded in 96-well plates and allowed to adhere overnight. Aspirin at the indicated concentrations was added for 1 day. Optical density representing viable cells was determined by the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay. Inhibition of proliferation is calculated as percentage of control cultures that were not treated with aspirin, mean±s.d. The inhibition was statistically significant at *P<0.05, **P<0.005, ***P<0.0005; n=3.
Discussion
The purpose of this study was to investigate for the first time the direct effect of aspirin on melanoma cells and to explore its mechanism. Using the B16-F10.9 metastatic melanoma cell line, we found that aspirin suppresses cellular proliferation without killing the cells. The inhibitory effect of aspirin is mediated through the activation of the JNK MAPK. Prolonged exposure to aspirin produces a cumulative effect. While aspirin does not kill the B16 cells by itself, it enhances the cytocidal effect of BCNU synergistically. The antiproliferative effect of aspirin is also exerted on the human melanoma cells – SK-28.
Our novel finding that aspirin suppresses murine and human melanoma cell proliferation is not unique in that aspirin was found to exert the same effect on colon and prostate cancer cells (Fulton, 1987; Ara & Teicher, 1996; Hanif et al., 1996; Rotem et al., 2000). However, aspirin in the same range of concentrations (0.25–4 mM) did not interfere with the proliferation of nontransformed mouse epidermal cells (Huang et al., 1997). Thus, while the antiproliferative effect of aspirin was demonstrated against three types of transformed cells (colon, prostate and melanoma), the drug does not affect the proliferation of nontransformed epidermal cells, nor does salicylic acid affect the viability or proliferation of normal activated human blood lymphocytes (Fingrut & Flescher, 2002). While aspirin was cytostatic to melanoma cells, it did not kill these cells, similar to its effect on colon and prostate carcinoma cells (Rotem et al., 2000; Shiff et al., 1996). On the other hand, in combination with BCNU, aspirin enhanced B16 cell death. Thus, the type of effect of aspirin on the cells (suppression of proliferation versus death) depends on the context, that is, whether BCNU is present. BCNU was found to enhance the activities of JNK and p38 in T lymphocytes (Hehner et al., 2000). We hypothesize that these activities play a role in the cytocidal effect of BCNU towards melanoma cells, and that aspirin (by inducing these activities in B16 cells) primes the cells to the cytotoxic action of BCNU, resulting in a synergistic effect of aspirin and BCNU. Whereas aspirin suppressed B16-F10.9 cellular proliferation at clinically relevant concentrations (Katzung, 1998), the proliferation of canine melanoma cells was only suppressed at levels of aspirin that are not achievable in vivo (Knapp et al., 1995). This different response results probably from interspecies variation.
Aspirin induced p38 activation and JNK activity. A specific p38 inhibitor – SB203580 (Lee et al., 1994; Turchi et al., 2000), did not affect the suppressive activity of aspirin on B16 proliferation. On the other hand, SB202190 that inhibits both p38 and JNK (Turchi et al., 2000), decreased the ability of aspirin to suppress proliferation and to induce JNK activity in B16 cells. Therefore, we conclude that JNK mediates the suppressive effect of aspirin on B16 proliferation. Evidence for the ability of p38 to mediate the response to cellular damage has been demonstrated in a variety of cells exhibiting apoptotic death. These include neurons (Horstman et al., 1998), PC-12 pheochromocytoma cells (Xia et al., 1995; Kummer et al., 1997), rat and human fibroblasts (Kummer et al., 1997; Schwenger et al., 1997), cardiac muscle cells (Wang et al., 1998; Ma et al., 1999) and artery endothelial cells (Yue et al., 1999). Also, p38 mediated suppression of proliferation in melanoma cells, induced by α-melanocyte-stimulating hormone (Smalley & Eisen, 2000), 2002aIL-1 (Itoh et al., 1999) and TNF (Hattori et al., 2001). However, p38 did not mediate salicylate-induced apoptosis in leukemia cells (Hall et al., 2001), in accordance with our findings. Moreover, inhibition of p38 increased farensyl thiosalicylic acid-induced apoptosis in Colo 853 melanoma cells (Smalley & Eisen, 2002b). Thus, whether p38 mediates the perturbation of normal cellular functioning or not, depends on the exogenous stimulus. Vitamin D may induce apoptosis through various pathways including JNK, and it has been shown to suppress the proliferation of malignant melanoma cells (Osborne & Hutchinson, 2002), pointing towards a possible connection between JNK activity and a decrease in melanoma cellular proliferation, following treatment with vitamin D. Our findings with aspirin provide evidence for the plausibility of this possibility. The mechanism underlying the effect of JNK on B16 cellular proliferation is yet to be elucidated. The downstream effects of JNK include activation of protein kinases and transcription factors, including AP-1 (Whitmarsh & Davis, 1996). The identified JNK substrates and genes they control are plausible targets for a research effort aiming at analyzing the pathway through which JNK activation results in suppression of cellular proliferation.
While we studied the effect of aspirin up to 5 mM, the highest clinically relevant concentration of plasma salicylate is 3 mM. Higher levels cause various degrees of intoxication (Katzung, 1998). Since aspirin at 3 mM caused approximately 30% suppression of B16 proliferation, we assessed whether this effect would increase over time and found it to be the case (Figure 5). If one assumes that every day of exposure to aspirin reduces cellular proliferation by 30%, the residual proliferative potential after 3 days would be 0.7 × 0.7 × 0.7=0.34. This is in close agreement to the 37.3% of cellular proliferation that we measured after 3 days of aspirin treatment (Figure 5). These results fit the hypothesis that aspirin reduces the number of cells in the population at the same rate each day. Thus, aspirin treatment over time would decrease the total number of melanoma cells very significantly. The issue of aspirin stability in vitro and in vivo deserves consideration. The half-life of aspirin in aqueous solution at 37°C and pH 7 is about 20 h (Connors et al., 1979). Hydrolysis of aspirin leads to the generation of salicylic acid (Connors et al., 1979). In the plasma, the half-life of aspirin is about 15 min, while that of salicylic acid can be up to about 12 h at antiinflammatory dosage (Katzung, 1998). Since we found (Ordan & Flescher, preliminary observations) that salicylic acid is as effective in suppressing the proliferation of melanoma cells as aspirin, we propose that aspirin and its major metabolite salicylic acid are present at relevant levels for periods much longer than those derived solely from the half-life of aspirin. Nevertheless, both in vitro, and even more so in vivo, the relevant levels cannot last for 3 days. Therefore, we propose an explanation based on the following: SB202190 can be removed after the first 2 h of exposure to aspirin and still be effective in decreasing the effect of aspirin on proliferation (Figure 3). Thus, it appears that while JNK mediates the suppressive effects of aspirin within 2 h postexposure (when the effective concentrations of aspirin and salicylic acid are still very high) additional events (which occur later, when lower concentrations of aspirin and salicylic acid prevail) are instrumental in mediating the effect of aspirin on melanoma cells. In other words, the high concentrations of the drug are only required during the early period of exposure, while lower concentrations of aspirin and its major metabolite salicylic acid are sufficient to further suppress the proliferation of melanoma cells. As for the practical ability to prolong the period during which high concentrations of aspirin and salicylic acid occur in vivo (where the half-life of aspirin is much shorter than in vitro), it can be achieved by daily administration of aspirin at antiinflammatory dosage. Finally, the synergism between aspirin and BCNU suggests that aspirin may enhance the effects of chemotherapeutic drugs, and possibly allow for the administration of the latter at lower (and less toxic) levels than are currently used. We are initiating the assessment of the therapeutic potential of aspirin by evaluating the effect of treatment with aspirin, with and without chemotherapeutic drugs, in vivo on the growth of B16 cells in syngeneic mice.
In conclusion, our results indicate that aspirin suppresses the proliferation of metastatic B16 melanoma cells in a JNK-dependent mechanism.
Acknowledgments
This work was supported by the Israel cancer association and by the Nofar program of the Israeli ministry of industry and trade.
Abbreviations
- BCNU
1,3-bis(2-chloroethyl)-1-nitrosourea
- ECL
enhanced chemiluminescence reagent
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinases
- SDS-PAGE
sodium dodecylsulfate-polyacrylamide gel electorphoresis
References
- ARA G., TEICHER B.A. Cyclooxygenase and lipoxygenase inhibitors in cancer therapy. Prostaglandins, Leukot. Essent. Fatty Acids. 1996;54:3–16. doi: 10.1016/s0952-3278(96)90075-7. [DOI] [PubMed] [Google Scholar]
- BROWN T.J., NELSON B.R. Malignant melanoma: a clinical review. Cutis. 1999;63:275–284. [PubMed] [Google Scholar]
- CONNORS K.A., AMIDON G.L., KENNON L. Chemical Stability of Pharmaceuticals. A Handbook for Pharmacists. USA: John Wiley and Sons; 1979. pp. 151–160. [Google Scholar]
- FINGRUT O., FLESCHER E. Plant stress hormones suppress the proliferation and induce apoptosis in human cancer cells. Leukemia. 2002;16:608–616. doi: 10.1038/sj.leu.2402419. [DOI] [PubMed] [Google Scholar]
- FULTON A.M. Interactions of natural effector cells and prostaglandins in the control of metastasis. J. Natl. Cancer. Inst. 1987;78:735–741. [PubMed] [Google Scholar]
- HALL J.G., CORY A.H., HICKERSON D.H., CORY J.G. Increased sensitivity to sodium salicylate-induced apoptosis in drug-resistant leukemia L1210 cells. Anticancer Res. 2001;21:173–180. [PubMed] [Google Scholar]
- HANIF R., PITTAS A., FENG Y., KOUTSOS M.I., QIAO L., STAIANO-COICO L., SHIFF S.I., RIGAS B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- HARRIS R.E., BEEBE-DONK J., NAMBOODIRI K.K. Inverse association of non-steroidal anti-inflammatory drugs and malignant melanoma among women. Oncol. Rep. 2001;8:655–657. doi: 10.3892/or.8.3.655. [DOI] [PubMed] [Google Scholar]
- HART R.R. The selection and characterization of an invasive variant of the B16 melanoma. Am. J. Pathol. 1979;97:587–600. [PMC free article] [PubMed] [Google Scholar]
- HATTORI T., HAYASHI H., CHIBA T., ONOZAKI K. Activation of two distinct anti-proliferative pathways, apoptosis and p38 MAP kinase-dependent cell cycle arrest, by tumor necrosis factor in human melanoma cell line A375. Eur. Cytokine Netw. 2001;12:244–252. [PubMed] [Google Scholar]
- HEHNER S.P., BREITKREUTZ Z., SHUBINSKY G., UNSOELD H., SCHULZE-OSTHOFF K., SCHMITZ M.L., DROGE W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J. Immunol. 2000;165:4319–4328. doi: 10.4049/jimmunol.165.8.4319. [DOI] [PubMed] [Google Scholar]
- HORSTMAN S., KAHLE P.J., BORASIO G.D. Inhibitors of p38 mitogen-activated protein kinase promote neuronal survival in vitro. J. Neurosci. Res. 1998;52:483–490. doi: 10.1002/(SICI)1097-4547(19980515)52:4<483::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- HUANG C., MA W.-Y., HANENBERGER D., CLEARY M.P., BOWDEN G.T., DONG Z. Inhibition of ultraviolet B-induced activator protein-1 (AP-1) activity by aspirin in AP-1-luciferase transgenic mice. J. Biol. Chem. 1997;272:26325–26331. doi: 10.1074/jbc.272.42.26325. [DOI] [PubMed] [Google Scholar]
- ITOH S., HATTORI T., HAYASHI H., MIZUTANI Y., TODO M., TAKII T., YANG D., LEE J.C., MATSUFUJI S., MURAKAMI Y., CHIBA T., ONOZAKI K. Antiproliferative effect of IL-1 is mediated by p38 mitogen-activated protein kinase in human melanoma cell A375. J. Immunol. 1999;162:7434–7440. [PubMed] [Google Scholar]
- KATZUNG B.G. Basic and Clinical Pharmacology. Stamford, CT: Appleton and Lange; 1998. pp. 580–583. [Google Scholar]
- KNAPP D.W., CHAN T.C.K., KUCZEK T., REAGAN W.J., PARK B. Evaluation of in vitro cytotoxicity of nonsteroidal anti-inflammatory drugs against canine tumor cells. Am. J. Vet. Res. 1995;56:801–805. [PubMed] [Google Scholar]
- KUMMER J.L., RAO P.K., HEIDENREICH K.A. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J. Biol. Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- LEE J.C., LAYDON J.T., MCDONNELL P.C., GALLAGHER T.F., KUMAR S., GREEN D., MCNULTY D., BLUMENTHAL M.J., HEYS J.R., LANDVATTER S.W., STRICKLER J.E., MCLAUGHLIN M.M., SIEMENS R.R., FISHER S.M., LIVI G.P., WHITE J.R., ADAMS J.L., YOUNG P.R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- MA X.L., KUMAR S., GAO F., LOUDEN C.S., LOPEZ B.L., CHRISTOPHER T.A., WANG C., LEE J.C., FEUERSTEIN G.Z., YUE T.-L. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- OSBORNE J.E., HUTCHINSON P.E. Vitamin D and systemic cancer: is this relevant to malignant melanoma. Br. J. Dermatol. 2002;147:197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- POO W.J., GUO X., HASLUND B., MOZDZIESZ D.E. Immunomodulation and enhancement of antitumor activity by co-administration of 1,3-bis(2-chloroethyl)-1-nitrosourea and thymidine. Biochem. Pharmacol. 1997;53:705–713. doi: 10.1016/s0006-2952(96)00905-7. [DOI] [PubMed] [Google Scholar]
- ROBINSON M.J., COBB M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- ROTEM R., TZIVONY Y., FLESCHER E. Contrasting effects of aspirin on prostate cancer cells: suppression of proliferation and induction of drug resistance. Prostate. 2000;42:172–180. doi: 10.1002/(sici)1097-0045(20000215)42:3<172::aid-pros2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- SAMET J.M., GRAVES L.M., QUAY J., DAILEY L.A., DEVLIN R.B. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- SCHWENGER P., ALPERT D., SKOLNIK E.Y., VILCEK J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IκBα phosphorylation and degradation. Mol. Cell. Biol. 1998;18:78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWENGER P., ALPERT D., SKOLNIK E.Y., VILCEK J. Cell-type-specific activation of c-Jun N-terminal kinase by salicylates. J. Cell. Physiol. 1999;179:109–114. doi: 10.1002/(SICI)1097-4652(199904)179:1<109::AID-JCP13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- SCHWENGER P., BELLOSTA P., VIETOR I., BASILICO C., SKOLNIK E.Y., VILCEK J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERRONE L., HERSEY P. The chemoresistance of human malignant melanoma: an update. Melanoma Res. 1999;9:51–58. doi: 10.1097/00008390-199902000-00007. [DOI] [PubMed] [Google Scholar]
- SHIFF S.J., KOUTSOS M.I., QIAO L., RIGAS B. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Exp. Cell Res. 1996;222:179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- SMALLEY K., EISEN T. The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. FEBS Lett. 2000;476:198–202. doi: 10.1016/s0014-5793(00)01726-9. [DOI] [PubMed] [Google Scholar]
- SMALLEY K.S., EISEN T.G. Differentiation of human melanoma cells through p38 MAP kinase is associated with decreased retinoblastoma protein phosphorylation and cell cycle arrest. Melanoma Res. 2002a;12:187–192. doi: 10.1097/00008390-200206000-00001. [DOI] [PubMed] [Google Scholar]
- SMALLEY K.S., EISEN T.G. Farnesyl thiosalicylic acid inhibits the growth of melanoma cells through a combi-nation of cytostatic and pro-apoptotic effects. Int. J. Cancer. 2002b;98:514–522. doi: 10.1002/ijc.10213. [DOI] [PubMed] [Google Scholar]
- TURCHI L., LOUBAT A., ROCHET N., ROSSI B., PONZIO G. Evidence for a direct correlation between c-Jun NH2 terminal kinase 1 activation, cyclin D2 expression, and G1/S phase transition in the murine hybridoma 7TD1 cells. Exp. Cell Res. 2000;261:220–228. doi: 10.1006/excr.2000.5060. [DOI] [PubMed] [Google Scholar]
- WANG Y., HUANG S., SAH V.P., ROSS J.R., BROWN J.H., HAN J., CHIEN K.R. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- WHITMARSH A.J., DAVIS R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. Mol. Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- XIA Z., DICKENS M., RAINGEAUD J., DAVIS R.J., GREENBERG M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- YUE T.-L., NI J., ROMAQNIC A.M., GU J.L., KELLER P., WANG C., KUMAR S., YU G.L., HART T.K., WANG X., XIA Z., DEWOLF W.E., Jr, FEUERSTEIN G.Z. TL1, a novel tumor necrosis factor-like cytokine, induces apoptosis in endothelial cells. Involvement of activation of stress protein kinases (stress-activated protein kinase and p38 mitogen-activated protein kinase) and caspase-3-like protease. J. Biol. Chem. 1999;274:1479–1486. doi: 10.1074/jbc.274.3.1479. [DOI] [PubMed] [Google Scholar]