Abstract
It is widely established that in rat mesenteric arteries, endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxation evoked by acetylcholine is abolished by a combination of charybdotoxin plus apamin. 4-Aminopyridine, an inhibitor of voltage-gated (Kv) K+-channels, in combination with apamin had moderate effects on the EDHF-mediated relaxation. Maurotoxin (MTX), an inhibitor of Kv and intermediate-conductance Ca2+-activated K+-channels (IK), had no effect on EDHF-mediated relaxation. However, MTX in combination with apamin completely abolished EDHF-mediated relaxation and endothelial cell hyperpolarization. The selective IK inhibitor 2-(2-chlorophenyl)-2,2-diphenyl acetonitrile (TRAM-39) had no significant effect on EDHF-mediated relaxation. EDHF-mediated vasorelaxation and hyperpolarization was abolished by a combination of TRAM-39 and apamin. These data demonstrate two new combinations of K+-channel inhibitors for the investigation of EDHF. Furthermore, by using TRAM-39, a potent selective inhibitor of IK channels, we provide the first direct evidence that abolition of EDHF requires the simultaneous presence of intermediate- and small-conductance Ca2+-activated K+-channel inhibitors.
Keywords: EDHF, endothelium, Ca2+-activated K+-channels, charybdotoxin, maurotoxin, apamin, TRAM-39
Introduction
In addition to nitric oxide (NO) and prostacyclin, endothelium-derived hyperpolarizing factor (EDHF) is an important mediator of vasodilatation in many vascular beds, including the rat mesenteric artery. EDHF-mediated relaxation of these arteries is inhibited by the simultaneous presence of apamin, an inhibitor of small-conductance Ca2+-activated K+-channels (SK), and charybdotoxin (ChTX), an inhibitor of large- (BK) and intermediate- (IK) conductance Ca2+-activated K+-channels, but not by either toxin alone (Waldron & Garland, 1994). Subsequent work demonstrated that the site of action of apamin and ChTX was on the endothelium, not the smooth muscle (Doughty et al., 1999).
The inhibitory action of ChTX may suggest that EDHF mediates relaxation by activating BK channels. However, iberiotoxin, a selective inhibitor of BK, cannot substitute for ChTX (Waldron & Garland, 1994). Voltage-dependent K+-channels (Kv) have been proposed to be involved in EDHF-mediated responses, as ChTX inhibits Kv1.2 and Kv1.3 (Garcia et al., 1995). Although Kv channels are expressed in endothelial cells from several arteries (Nilius & Droogmans, 2001), a role in EDHF-mediated relaxation remains unclear.
The present study uses the scorpion toxin, mauro-toxin (MTX) (Castle et al., 2003), a potent, synthetic, selective inhibitor of intermediate-conductance Ca2+-activated K+-channels, 2-(2-chlorophenyl)-2,2-diphenyl acetonitrile (TRAM-39) (Wulff et al., 2000), and a nonselective inhibitor of Kv, 4-aminopyridine (4-AP), to investigate their effects on endothelium-dependent relaxation and hyperpolarization. Preliminary data have been presented to The Physiological Society (Hinton & Langton, 2001) and The Biophysical Society (Hinton et al., 2002).
Methods
Male Wistar rats (225–250 g) were killed by stunning and cervical dislocation. Third-order mesenteric arteries were dissected free in physiological saline solution (PSS) gassed with 95%O2/5%CO2 containing (mM) NaCl 119, KCl 4.7, NaHCO3 25, KH2PO4 1.18, CaCl2 1.8, MgSO4 1.2, glucose 11 and EDTA 0.027. All experiments were performed in the presence of NG-nitro-L-arginine (100 μM) and indomethacin (2.8 μM), inhibitors of NO synthase and cyclooxygenase to suppress synthesis of NO and prostanoids, respectively.
Wire myography
Segments of mesenteric artery were mounted in a Mulvany – Halpern wire myograph under normalized tension for isometric recording. Arterial segments were initially contracted with phenylephrine (PE, 10 μM) to give a maximal contraction. Subsequently, PE was applied to give approximately 70% of maximal force (0.5–2 μM). Data are expressed as normalized force as a percentage of the 70% maximal response (100%). Sequential, cumulative concentration effect curves for acetyl-choline (ACh) were performed, firstly, in the absence of any inhibitors, then each inhibitor alone, and finally in the presence of the inhibitor combinations.
Microelectrode experiments
Intact mesenteric vessels were opened longitudinally, pinned to the base of a bath, luminal side upward and superfused (2.5 ml min−1) with PSS. Individual endothelial cells were impaled using microelectrodes filled with 3 M KCl (resistance 35–80 Mω). Electrical responses at the tip of the electrode were monitored by injecting current pulses (0.1 nA, duration 40 ms). The electrode was advanced toward the endothelial cells at an angle of approximately 40°. Successful impalements were seen as a sudden change in voltage to a stable, negative level. If electrodes were advanced through the endothelial cell, the elastic membrane separating endothelial cells and smooth muscle cells, the internal elastic lamina, caused the electrode to bend and break. Owing to the long fine taper of the electrode tip and the shallow angle of approach advancing the electrode still further did not lead to impalement of smooth muscle cells. Furthermore, removal of the endothelium by gentle agitation did not give rise to impalement of either smooth muscle or endothelial cells. ACh, toxins and inhibitors were added to a gassed reservoir of PSS.
Calculations and statistics
Results are mean values±s.e. mean of n animals or cells impaled. Statistical analysis was performed using Mann–Whitney test; P<0.05 was considered to be statistically significant.
Chemicals and drugs
Synthetic ChTX and apamin were purchased from Calbiochem, 1-ethyl-2-benzimidazolinone (1-EBIO) was purchased from Aldrich and levcromakalin was supplied by SmithKline Beecham. All other drugs, except where stated, were from Sigma, U.K. ChTX was dissolved in 0.9% saline, and indomethacin was dissolved in ethanol. All other drugs were made up as stock solutions in distilled water and diluted in PSS. Stock solutions of 1-EBIO (0.1 M) and TRAM-39 (1 mM) were dissolved in dimethylsulfoxide (DMSO), final concentration 0.1%. Control experiments indicated that 0.1% DMSO had no direct effect on either relaxation or hyperpolarization at this concentration. All other drugs were made up as stock solutions in distilled water and diluted with PSS.
Results
Effects of inhibitors of Kv channels
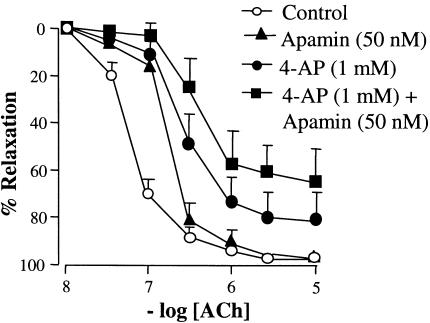
In isometrically mounted arteries contracted with PE (0.5–2 μM), ACh (0.01–10 μM) elicited concentration-dependent relaxation (Emax 97.5±2.2%; EC50 59±0.1 nM). Apamin (50 nM) alone had no significant effect on the maximal EDHF-mediated relaxation to increasing concentrations of ACh (Emax 98.1±1.8%; EC50 180±0.2 nM; n=6). The nonselective Kv inhibitor 4-AP (1 mM) produced a significant rightward shift in the concentration–response curve and reduced the maximal relaxation to ACh (Emax 78.9±12.1%; EC50 350±0.4 nM; n=6). 4-AP and apamin in combination produced a further rightward shift in the concentration–response curve and reduced the maximal relaxation (Emax 64.8±7.5%; EC50 660±0.3 nM; n=6; Figure 1).
Figure 1.
Concentration–response curve for ACh-induced relaxation in rat isolated mesenteric arteries precontracted with phenylephrine (PE). ACh-evoked relaxation (0.01–10 μM) was not significantly affected by 4-AP (1 mM; n=6) or apamin (50 nM; n=6). The combination of 4-AP and apamin (1 and 50 nM, respectively; n=6) shifted the curve to the right and reduced the maximal relaxation. Data are means and vertical lines s.e. mean.
Effects of inhibitors of Ca2+-activated K+-channels
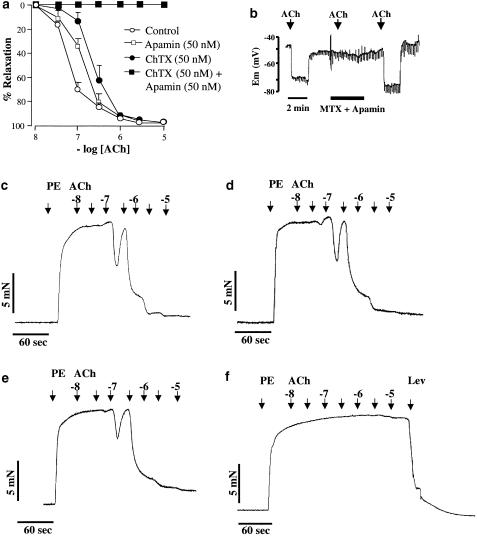
Charybdotoxin (50 nM), an inhibitor of BK, IK, Kv1.2 and Kv1.3 channels (see Garcia et al., 1995), was without effect on vasorelaxation (Emax 98.2 ± 1.1%; EC50 228±0.2 nM; P>0.05; n=6). However, in combination with apamin (50 nM) ChTX abolished EDHF-mediated relaxation of rat mesenteric arteries (P<0.05; n=6; Figure 2a).
Figure 2.
(a) Effects of apamin and ChTX on EDHF relaxations induced by ACh in arteries precontracted with phenylephrine (PE). ChTX alone (50 nM; n=6) and apamin alone (50 nM; n=6) were without significant effect on EDHF relaxations. ChTX and apamin in combination (each 50 nM; n=6) abolished EDHF-mediated relaxation. Data are means ± s.e.mean. (b) Continuous recording of endothelial cell membrane potential (Em). Apamin and MTX in combination completely, and reversibly, abolished the hyperpolarization to ACh (10 μM). (c) Representative trace to demonstrate concentration-dependent relaxation to ACh (0.01–10 μM) in precontracted arteries. (d) and (e) Representative traces demonstrating concentration-dependent relaxation to ACh (0.01–10 μM) in the presence of apamin (50 nM) and MTX (50 nM), respectively. (f) Representative record to demonstrate abolition of relaxation in the presence of apamin and MTX. The ability of the smooth muscle to relax in the presence of the toxin combination was demonstrated by maximal relaxation to levcromakalin (lev; 10 μM). As evidenced on original traces, relaxations to lower concentrations of ACh were transient. For the construction of concentration–effect curves, the maximal relaxations to each applied dose of ACh were recorded.
MTX (50 nM), a peptide toxin known to block cloned Kv1.2 and Kv1.3 channels and IK channels (Kharrat et al., 1997; Castle et al., 2003), was without effect on vasorelaxation to ACh (Emax 99.4±2.1%; EC50 210±0.2 nM; P>0.05; n=6; Figure 2e). However, the combination of this toxin and apamin (each 50 nM) completely abolished EDHF-mediated relaxation of these arteries (P<0.05; n=6). Maximal relaxation in the presence of the toxin combination was achieved by application of 10 μM levcromakalin (Figure 2f).
In the presence of NG-nitro-L-arginine and indomethacin (100 and 2.8 μM, respectively), the resting membrane potential of endothelial cells was –51.3±2.7 mV (n=6). Application of ACh (10 μM) induced a rapid, reversible hyperpolarization of the endothelial cells, –23.5±2.8 mV (n=6). In the presence of apamin and MTX in combination, hyperpolarization to application of ACh (10 μM) was completely and reversibly abolished (n=3; Figure 2b).
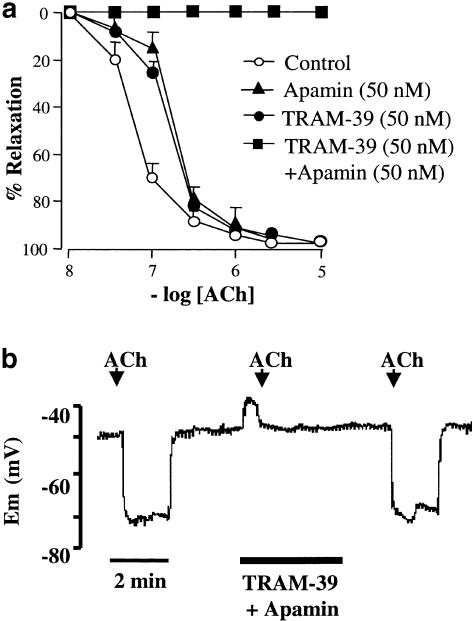
TRAM-39, a potent, selective inhibitor of IKCa channels (Kd 60 nM; Wulff et al., 2000), alone had no effect on relaxation to ACh (Emax 98.8±1.1%; EC50 165±0.1 nM; P>0.05; n=6; Figure 3a). A combination of TRAM-39 (100 nM) and apamin (50 nM) abolished EDHF-mediated relaxation of the rat isolated mediated relaxation (P>0.05; n=6; Figure 3a); however, in the presence of the toxin combination, smooth muscle relaxation was elicited by levcromakalin (10 μM). The combination of TRAM-39 (100 nM) and apamin (50 nM) evoked a small, transient depolarization of endothelial cells ∼8 mV. Hyperpolarization to application of ACh (10 μM; n=3) was completely abolished in the presence of these inhibitors (n=3); hyperpolarization to ACh was restored after the K+-channel inhibitors had washed off (Figure 3b).
Figure 3.
(a) Effects of apamin and TRAM-39 on EDHF relaxations induced by ACh in arteries precontracted with phenylephrine (PE). TRAM-39 alone (50 nM; n=6) and apamin alone (50 nM; n=6) were without effect on EDHF relaxations evoked by increasing concentrations of ACh. In contrast, when TRAM-39 and apamin were combined (each 50 nM) these inhibitors abolished EDHF-mediated relaxation. Data are means and vertical lines s.e. mean. (b) Endothelial cell membrane potential (Em) hyperpolarization to ACh. Representative data trace illustrates hyperpolarization of Em to application of ACh. A combination of apamin and TRAM-39 completely, and reversibly, abolished ACh hyperpolarization.
Effects of 1-EBIO: an activator of K+-channels
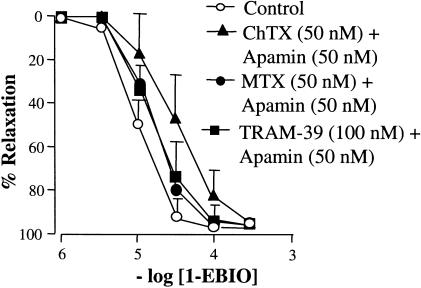
1-EBIO, an activator of IK and SK channels (Wickenden et al., 2000), evoked concentration-dependent vasorelaxation (1–1000 μM; Emax 98.4±1.3%; EC50 10.5±0.4 μM; n=18; Figure 4), in rat mesenteric arteries. However, this relaxation was unaffected by apamin in combination with either ChTX, MTX or TRAM-39 (P>0.05; each n=6; Figure 4). In preparations with-out endothelium, as evidenced by the lack of response to ACh, there was no change in response to those arteries with an intact endothelium (P>0.05; each n=6) (data not shown in Figure 4, as overlapping curves obscured the clarity of the figure).
Figure 4.
Concentration–response curves for 1-EBIO-induced relaxation in arteries precontracted with phenylephrine (PE). In endothelium-intact arteries, 1-EBIO evoked concentration-dependent relaxation that was unaffected by combinations of ChTX and apamin (each 50 nM), MTX and apamin (each 50 nM), and TRAM-39 and apamin (100 and 50 nM, respectively). Removal of the endothelium had no significant effect on the relaxation to 1-EBIO (data not shown, as overlapping curves obscured the clarity of the figure).
Discussion
It is widely established that inhibition of EDHF-mediated relaxation of rat isolated mesenteric arteries requires the simultaneous presence of apamin and ChTX. This study identifies two new combinations of K+-channel inhibitors that abolish both the relaxation and endothelial cell hyperpolarization evoked by EDHF in this artery.
Inhibitors of Kv channels
ChTX is a potent inhibitor of Kv1.2. and Kv1.3 channels (see Garcia et al., 1995). Some studies have speculated that inhibition of Kv channels may underlie the action of ChTX. 4-AP, a non-specific Kv channel blocker, inhibits ACh-induced endothelium-dependent hyperpolarization of guinea pig coronary artery (Eckman et al., 1998); however, this is not observed in all arteries. Agonist-induced hyperpolarization of endothelial cells is dependent upon the influx of extracellular Ca2+ and the release of Ca2+ from intracellular pools. It has been proposed that 4-AP may inhibit intracellular Ca2+ release evoked by ACh in endothelial cells (Ghisdal & Morel, 2001). Interpretation of these data is further complicated by the ability of micromolar concentrations of 4-AP to block cloned SK3 channels (Grunnet et al., 2001). Although Kv-channels are expressed in endothelial cells (see Nilius & Droogmans, 2001), it is unclear if the effects of Kv-inhibitors can be attributed to block of channels expressed in endothelial or smooth muscle cells.
Inhibitors of Ca2+-activated K+-channels
The scorpion toxins MTX and ChTX share significant similarities in secondary structure and the profile of channels they block. Both toxins are potent inhibitors of IK and Kv-channels (Garcia et al., 1995; Kharrat et al., 1997; Castle et al., 2003). Each of these toxins, MTX and ChTX, in combination with apamin abolish both EDHF-mediated relaxation and endothelial cell hyperpolarization. However, unlike ChTX, MTX has no effect on BK channels (Castle et al., 2003), thus providing further evidence that the ability of ChTX to abolish EDHF-mediated effects in combination with apamin is not due to an effect on endothelial or smooth muscle cell BK channels.
The inability of the Kv-inhibitor, 4-AP, in combination with apamin to abolish EDHF-mediated relaxation led us to investigate the IK channel as the target for ChTX and MTX. Several studies investigating IK channels have used clotrimazole, which in addition to directly blocking IK channels also inhibits mammalian cytochrome P450 enzymes (CYP). However, metabolites of CYP have been suggested as candidates for the identity of EDHF (Fisslthaler et al., 1999). To investigate the contribution of the IK channel, we used TRAM-39, which is structurally related to clotrimazole, but without an activity at CYP (Wulff et al., 2000). The combination of TRAM-39 and apamin inhibited both EDHF-mediated relaxation and endothelial cell hyperpolarization, thus providing the strongest evidence to date that endothelial cell IK channels are directly involved in EDHF-mediated responses.
1-EBIO: an activator of Ca2+-activated K+-channels
To mimic the action of EDHF in these arteries, we used 1-EBIO, an activator of both IK and SK channels. In precontracted arteries, EBIO-1 evoked concentration-dependent relaxation. Relaxation to 1-EBIO was unaffected by removal of the endothelium or raising extracellular K+ concentration to 25 mM, previously shown to abolish EDHF-mediated relaxation and hyperpolarization (Waldron & Garland, 1994) (data not shown). Therefore, the relaxations evoked by 1-EBIO are by a mechanism that is not dependent on endothelial cell K+-channel activation. This apparent lack of specificity for K+-channels questions the usefulness of 1-EBIO for studies using intact arteries. However, 1-EBIO has been used to activate K+-currents in cultured (Edwards et al., 1999) and acutely isolated endothelial cells (Walker et al., 2001). In these studies, 1-EBIO induced a ChTX-sensitive, iberiotoxin-insensitive current. Most interestingly, the 1-EBIO-activated K+-current was totally abolished by ChTX. Although 1-EBIO opens IK and SK channels with equal potency (Wickenden et al., 2000), apamin in combination with ChTX was not required to abolish this current, despite the apamin-sensitivity of EDHF responses. These data suggest that apamin-sensitive SK channels, if expressed in endothelial cells, may not share the same characteristics as cloned, expressed SK channels. The characterization and identification of apamin-sensitive SK channels remains to be established in these arteries.
Conclusions
These data demonstrate two novel combinations of K+ channel inhibitors that abolish EDHF-mediated relaxation in the rat isolated mesenteric artery. Furthermore, using a potent, selective inhibitor of IK channels we provide the first direct evidence that endothelial cell IK channels are crucial for EDHF-mediated responses in these blood vessels.
Scorpion toxins, such as ChTX, are poorly selective, inhibiting BK, IK and Kv channels in both the smooth muscle and endothelial cells. The apparent selectivity of nonpeptide inhibitors, such as TRAM-39, provides a useful pharmacological tool for further defining the physiological role of IK channels and their role in EDHF-mediated responses.
Although the distribution of SK and IK channels in the vascular wall and their precise role in EDHF remain to be established, these data provide the strongest evidence to date that channels displaying the pharmacological characteristics of SK and IK are involved in EDHF responses of the rat mesenteric artery.
Acknowledgments
This research was funded by the BHF (PG/99/70). We are indebted to Professor K.G. Chandy and Dr H. Wulff for the gift of TRAM-39 and Professor J.-M. Sabatier for the gift of maurotoxin. We are especially grateful to Dr C.R. Hiley and Dr H. Mistry for sharing their expertise of membrane potential recording.
Abbreviations
- 4-AP
4-aminopyridine
- BK
large-conductance Ca2+-activated K+-channel
- ChTX
charybdotoxin
- CYP
cytochrome P450
- 1-EBIO
1-ethyl-2-benzimidazolinone
- EDHF
endothelium-derived hyperpolarizing factor
- IK
intermediate-conductance Ca2+-activated K+-channel
- Kv
voltage-gated K+-channel
- MTX
maurotoxin
- SK
small-conductance Ca2+-activated K+-channel
- TRAM-39
2-(2-chlorophenyl)-2,2-diphenyl acetonitrile
References
- CASTLE N.A., LONDON D.O., CREECH C., FAJLOUN Z., STOCKER J.W., SABATIER J.-M. Maurotoxin – a potent inhibitor of intermediate conductance Ca2+-activated potassium channels. Mol. Pharmacol. 2003;63:409–418. doi: 10.1124/mol.63.2.409. [DOI] [PubMed] [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- ECKMAN D.M., HOPKINS N., MCBRIDE C., KEEF K.D. Endothelium-dependent relaxation and hyperpolarization in guinea-pig coronary artery: role of epoxyeicosatrienoic acid. Br. J. Pharmacol. 1998;124:181–189. doi: 10.1038/sj.bjp.0701778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., GARDENER M.J., FELETOU M., BRADY G., VANHOUTTE P.M., WESTON A.H. Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br. J. Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENLE M., HARDER D.R., FLEMMING I., BUSSE R. Cytochrome P4502C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- GARCIA M.L., KNAUS H.-G., MUNUJOS P., SLAUGHTER R.S., KACZOROWSKI G.J. Charybdotoxin and its effects on potassium channels. Am. J. Physiol. 1995;269:C1–C10. doi: 10.1152/ajpcell.1995.269.1.C1. [DOI] [PubMed] [Google Scholar]
- GHISDAL P., MOREL N. Cellular target of voltage and calcium-dependent K+ channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery. Br. J. Pharmacol. 2001;134:1021–1028. doi: 10.1038/sj.bjp.0704348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNNET M., JESPERSEN T., ANGELO K., FROKJAER-JENSEN C., KLAERKE D.A., OLESEN S.P., JENSEN B.S. Pharmacological modulation of SK3 channels. Neuropharmacol. 2001;40:879–887. doi: 10.1016/s0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- HINTON J.M., LANGTON P.D.Possible involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of rat mesenteric artery J. Physiol. 2001536P
- HINTON J.M., SABATIER J.-M., LANGTON P.D. Maurotoxin and apamin in combination abolish EDHF-mediated relaxation of the rat mesenteric artery. Biophys. J. 2002;82:2740. [Google Scholar]
- KHARRAT R., MANSUELLE P., SAMPIERI F., CREST M., OUGHIDENI R., VANRIETSCHOTEN J., MARTINEAUCLAIRE M.F., ROCHAT H., ELAYEB M. Maurotoxin, a four disulfide bridge toxin from Scorpio maurus venom: purification, structure and action on potassium channels. FEBS Lett. 1997;406:284–290. doi: 10.1016/s0014-5793(97)00285-8. [DOI] [PubMed] [Google Scholar]
- NILIUS B., DROOGMANS G. Ion channels and their functional role in vascular endothelium. Phys. Rev. 2001;81:1416–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- WALDRON G.J., GARLAND C.J. Effect of potassium channel blockers on L-NAME insensitive relaxations in rat small mesenteric artery. Can. J. Physiol. Pharmacol. 1994;72:115. [Google Scholar]
- WALKER S.D., DORA K.A., INGS N.T., CRANE G.J., GARLAND C.J. Activation of endothelial cell IKCa with 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery. Br. J. Pharmacol. 2001;134:1548–1554. doi: 10.1038/sj.bjp.0704415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKENDEN A.D., STOCKER J., SILVIA C.P., WAGONER P.K. 1-EBIO activates human small conductance calcium-activated potassium channels. Biophys. J. 2000;78:1231. [Google Scholar]
- WULFF H., MILLER M.J., HANSEL W., GRISSMER S., CAHALAN M.D., CHANDY K.G. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]