Abstract
The effects of fluoxetine, a commonly used antidepressant drug, on G protein-activated inwardly rectifying K+ channels (GIRK, Kir3) were investigated using Xenopus oocyte expression assays.
In oocytes injected with mRNAs for GIRK1/GIRK2, GIRK2 or GIRK1/GIRK4 subunits, fluoxetine reversibly reduced inward currents through the basal GIRK activity. The inhibition by fluoxetine showed a concentration-dependence, a weak voltage-dependence and a slight time-dependence with a predominant effect on the instantaneous current elicited by voltage pulses and followed by slight further inhibition. Furthermore, in oocytes expressing GIRK1/2 channels and the cloned Xenopus A1 adenosine receptor, GIRK current responses activated by the receptor were inhibited by fluoxetine. In contrast, ROMK1 and IRK1 channels in other Kir channel subfamilies were insensitive to fluoxetine.
The inhibitory effect on GIRK channels was not obtained by intracellularly applied fluoxetine, and not affected by extracellular pH, which changed the proportion of the uncharged to protonated fluoxetine, suggesting that fluoxetine inhibits GIRK channels from the extracellular side.
The GIRK currents induced by ethanol were also attenuated in the presence of fluoxetine.
We demonstrate that fluoxetine, at low micromolar concentrations, inhibits GIRK channels that play an important role in the inhibitory regulation of neuronal excitability in most brain regions and the heart rate through activation of various G-protein-coupled receptors. The present results suggest that inhibition of GIRK channels by fluoxetine may contribute to some of its therapeutic effects and adverse side effects, particularly seizures in overdose, observed in clinical practice.
Keywords: Fluoxetine, antidepressant, GIRK, Kir channel, ethanol, Xenopus oocyte
Introduction
Fluoxetine, known by the trade name of Prozac, has been used widely in the treatment of depression and other psychiatric disorders, such as obsessive-compulsive disorder, bulimia nervosa, post-traumatic stress disorder and alcoholism (Wong et al., 1995; Baldessarini, 2001). Inhibition of serotonin (5-hydroxytryptamine; 5-HT) transporters in the brain is generally thought to have important implications in its therapeutic effects, and fluoxetine is classified as a selective serotonin reuptake inhibitor (SSRI) (Wong et al., 1995). Recent studies have also shown that fluoxetine inhibits the functions of several receptors and ion channels, such as 5-HT2C (Ni & Miledi, 1997) and 5-HT3 receptors (Fan, 1994), nicotinic acetylcholine receptors (García-Colunga et al., 1997; Maggi et al., 1998), voltage-gated Ca2+, Na+ and K+ channels (Pancrazio et al., 1998; Choi et al., 1999;2001; Yeung et al., 1999; Deák et al., 2000; Perchenet et al., 2001; Thomas et al., 2002) and Cl− channels (Maertens et al., 1999). In addition to inhibition of serotonin transporters, these effects might be involved in the molecular and cellular mechanisms underlying the multiple therapeutic effects and side effects of fluoxetine.
G protein-activated inwardly rectifying K+ (GIRK) channels (also known as Kir3 channels) are members of a family of inward-rectifier K+ (Kir) channels that includes seven subfamilies (Doupnik et al., 1995; Reimann & Ashcroft, 1999). Four GIRK channel subunits have been identified in mammals (Kubo et al., 1993b; Lesage et al., 1995; Wickman et al., 1997). Neuronal GIRK channels are predominantly heteromultimers composed of GIRK1 and GIRK2 subunits in most brain regions (Kobayashi et al., 1995; Lesage et al., 1995; Karschin et al., 1996; Liao et al., 1996) or homomultimers composed of GIRK2 subunits in the substantia nigra (Inanobe et al., 1999), and atrial GIRK channels are heteromultimers composed of GIRK1 and GIRK4 subunits (Krapivinsky et al., 1995). Various G-protein-coupled receptors, such as M2 muscarinic, α2 adrenergic, D2 dopamine, 5-HT1A, opioid, nociceptin/orphanin FQ receptors and A1 adenosine (Ado) receptor, activate GIRK channels (North, 1989; Ikeda et al., 1995,1996,1997) through direct action of G protein βγ-subunits released from pertussis toxin-sensitive G proteins (Reuveny et al., 1994). In addition, ethanol (EtOH) activates GIRK channels independent of G-protein-coupled signalling pathways (Kobayashi et al., 1999; Lewohl et al., 1999). Activation of GIRK channels causes membrane hyperpolarization, and thus the channels play an important role in the inhibitory regulation of the neuronal excitability and the heart rate (North, 1989; Signorini et al., 1997; Wickman et al., 1998). Therefore, modulators of GIRK channel activity may affect many brain and cardiac functions. Here we show that fluoxetine inhibits brain-type GIRK1/2 and GIRK2 channels and cardiac-type GIRK1/4 channels expressed in Xenopus oocytes, whereas two different SSRIs: fluvoxamine and zimelidine have no significant effect on the channels. In contrast, ROMK1 (Kir1.1) and IRK1 (Kir2.1) channels in other Kir channel subfamilies were insensitive to fluoxetine. Moreover, fluoxetine inhibited EtOH-induced GIRK currents. We propose that some effects of fluoxetine in clinical practice may involve inhibition of GIRK channels in the brain and heart.
Methods
Preparation of specific mRNAs
Plasmids containing the entire coding sequences for the mouse GIRK1, GIRK2, weaver (wv) GIRK2 and GIRK4 channel subunits and the Xenopus A1 (XA1) Ado receptor were obtained using the polymerase chain reaction method as described previously (Kobayashi et al., 1995,1999,2000,2002). In addition, cDNAs for rat ROMK1 in pSPORT and mouse IRK1 in pcDNA1 were provided by Dr Steven C. Hebert and Dr Lily Y. Jan, respectively. These plasmids were linearized by digestion with an appropriate enzyme as described previously (Ho et al., 1993; Kubo et al., 1993a; Kobayashi et al., 2000), and the specific mRNAs were synthesized in vitro using the mMESSAGE mMACHINE™ In Vitro Transcription Kit (Ambion, Austin, TX, U.S.A.).
Electrophysiological analyses
Adult female Xenopus laevis frogs were purchased from Copacetic (Soma, Aomori, Japan) and maintained in the laboratory until use. Frogs were anesthetized by immersion in water including 0.15% tricaine (Sigma Chemical Co., St Louis, MO, U.S.A.). A small incision was made on the abdomen to remove several ovarian lobes from the frogs that were humanely killed after the final collection. Oocytes (Stages V and VI) were isolated manually from the ovary and maintained in Barth's solution (Kobayashi et al., 2002). Xenopus laevis oocytes were injected with mRNA(s) for GIRK1/GIRK2 or GIRK1/GIRK4 combinations (each ∼0.4 ng), GIRK2 (∼5 ng), wvGIRK2 (∼12.5 ng), ROMK1 (∼5 ng) or IRK1 (∼0.5 ng) and/or XA1 (∼10 ng). The oocytes were incubated at 19°C in Barth's solution, and defolliculated following treatment with 0.8 mg ml−1 collagenase as described previously (Kobayashi et al., 2002). Whole-cell currents of the oocytes were recorded from 2 to 10 days after the injection with a conventional two-electrode voltage clamp (Kobayashi et al., 1999). The membrane potential was held at −70 mV, unless otherwise specified. Microelectrodes were filled with 3 M KCl. The oocytes were placed in a 0.05 ml narrow chamber and superfused continuously with a high-potassium (hK) solution (composition in mM: KCl 96, NaCl 2, MgCl2 1, CaCl2 1.5 and HEPES 5), a K+-free high-sodium (ND98) solution (composition in mM: NaCl 98, MgCl2 1, CaCl2 1.5 and HEPES 5) or a large organic cation N-methyl-D-glucamine (NMDG) solution (composition in mM: NMDG 98, MgCl2 1 and HEPES 5) at a flow rate of 2.5 ml min−1. The acidic or basic solutions were made by adding HCl or NaOH, respectively. For examining the effect of intracellular fluoxetine, 23 nl of 10 mM fluoxetine or 30 mM lidocaine N-ethyl bromide (QX-314) dissolved in distilled water was injected into an oocyte using a Nanoliter injector (World Precision Instruments, Sarasota, FL, U.S.A.) (Kobayashi et al., 1999) and the oocyte currents were continuously recorded for 30−40 min. Owing to a volume of ∼1 μl in the oocyte, the intracellular concentrations of fluoxetine or QX-314 were presumed as ∼225 or ∼674 μM, respectively. In the hK solution, the K+ equilibrium potential (EK) was close to 0 mV and inward K+ current flow through Kir channels was observed at negative holding potentials. Data were fitted to a standard logistic equation using SigmaPlot (SPSS Science, Chicago, IL, U.S.A.) in the analysis of concentration–response relations. The EC50 value, which is the concentration of a drug that produces 50% of the maximal current response for that drug, IC50 value which is the concentration of a drug that reduces control current responses by 50% and the Hill coefficient (nH) were obtained from the concentration–response relations.
Statistical analysis of results
The values obtained are expressed as mean±s.e.m., and n is the number of oocytes tested. Statistical analysis of differences between groups was carried out using paired t-test, Student's t-test or one-way ANOVA followed by Tukey–Kramer post hoc test. A probability of 0.05 was taken as the level of statistical significance.
Compounds
Fluoxetine hydrochloride and fluvoxamine maleate were purchased from Tocris Cookson Limited (Bristol, U.K.). Zimelidine dihydrochloride and Ado were purchased from Research Biochemical International (Natick, MA, U.S.A.). QX-314 was purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). Fluoxetine and fluvoxamine were dissolved in dimethyl sulfoxide (DMSO) or distilled water. Other drugs were dissolved in distilled water. The stock solutions of these compounds were stored at −30°C until use. EtOH was purchased from Wako Pure Chemical Industries (Osaka, Japan). Each compound was added to the perfusion solution in appropriate amounts immediately before the experiments.
Results
Inhibition of GIRK channels by fluoxetine
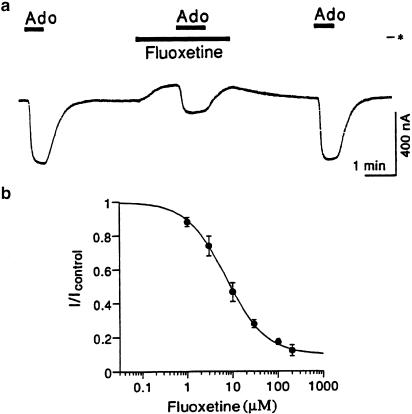
To investigate whether fluoxetine interacts with brain-type GIRK1/2 and cardiac-type GIRK1/4 channels, we used Xenopus oocyte expression assays. In oocytes coinjected with GIRK1 and GIRK2 mRNAs, basal GIRK currents, which are known to depend on free G protein βγ-subunits present in the oocytes because of the inherent activity of G proteins (Dascal, 1997), were observed under the conditions of a hK solution containing 96 mM K+ and negative membrane potentials (Kobayashi et al., 2000; Figure 1a). Application of fluoxetine, at 10 μM, immediately and reversibly caused a reduction of the inward currents through basally active channels in the hK solution (Figure 1a). The current responses were abolished in the presence of 3 mM Ba2+, which blocks the Kir channel family including GIRK channels (n=4; Figure 1a). Fluoxetine produced no significant response in a K+-free ND98 solution containing 98 mM Na+ instead of the hK solution (5±5 nA, n=3), suggesting that the fluoxetine-sensitive current components show K+ selectivity. In uninjected oocytes, fluoxetine and Ba2+ caused no significant response (0 nA at 200 μM and 5.5±0.7 nA at 3 mM at a membrane potential of −70 mV, n=4 and 29, respectively; Figure 1a), suggesting no effect of fluoxetine on intrinsic oocyte channels. In addition, application of DMSO, the solvent vehicle, at the highest concentration (0.3%) used had no significant effect on the current responses in oocytes coinjected with GIRK1 and GIRK2 mRNAs (n=4; data not shown). These results suggest that fluoxetine inhibits GIRK1/2 channels. However, the channels were insensitive to two different SSRIs: fluvoxamine and zimelidine (6.1±2.3% inhibition and 0.7±1.2% inhibition of the 3 mM Ba2+-sensitive current component at 100 μM, n=11 and 10, respectively). Furthermore, similar results were obtained in oocytes co-injected with GIRK1 and GIRK4 mRNAs (Figure 1a), suggesting that fluoxetine also inhibits GIRK1/4 channels.
Figure 1.
Inhibition by fluoxetine of brain-type GIRK1/2 channels and cardiac-type GIRK1/4 channels expressed in Xenopus oocytes. (a) Top: In an oocyte coinjected with GIRK1 and GIRK2 mRNAs, current responses to 10 μM fluoxetine and 10 μM fluoxetine in the presence of 3 mM Ba2+. Middle: In an oocyte coinjected with GIRK1 and GIRK4 mRNAs, current responses to 10 μM fluoxetine and 3 mM Ba2+. Bottom: In an uninjected oocyte, no significant current responses to 200 μM fluoxetine and 3 mM Ba2+. Asterisks show the zero current level. Bars show the duration of application. (b) Concentration–response relations for fluoxetine in regard to the effects on GIRK1/2 channels (left) and GIRK1/4 channels (right). The magnitudes of inhibition of GIRK current by fluoxetine were compared with the 3 mM Ba2+-sensitive current components, which were 741.6±118.0 nA (n=5) in oocytes expressing GIRK1/2 channels and 600.8±268.0 nA (n=5) in oocytes expressing GIRK1/4 channels, respectively. Current responses were measured at a membrane potential of −70 mV in a high-potassium solution containing 96 mM K+ (pH 7.4). Each point and error bar represents the mean and s.e.m. of the percentage responses obtained from five oocytes. Data points were fitted using a logistic equation.
Characteristics in fluoxetine inhibition of GIRK channels
We investigated the concentration–response relations of the inhibitory effects of fluoxetine on GIRK channels expressed in Xenopus oocytes, compared with the current components sensitive to 3 mM Ba2+ which blocked basal GIRK currents (Kobayashi et al., 2002). Inhibition of GIRK1/2 and GIRK1/4 channels by fluoxetine was concentration-dependent with similar potency and effectiveness at micromolar concentrations (Figure 1b). Fluoxetine reduced the GIRK currents to a limited extent even at high concentrations. Table 1 shows the EC50 and nH values obtained from the concentration–response relations for fluoxetine and the percentage inhibition of the GIRK currents by the drug at the highest concentrations tested. In addition, to further compare the effects of fluoxetine on GIRK channels, the drug concentrations required to inhibit the GIRK currents by 50% are shown in Table 1.
Table 1.
Inhibitory effects of fluoxetine on GIRK channels
| Channel | EC50 | IC50 | % inhibition (μM) | nH | (n) |
|---|---|---|---|---|---|
| GIRK1/2 | 7.89±0.60 | 16.9±4.0 | 73.6±6.1 (300) | 0.83±0.06 | (5) |
| GIRK1/4 | 7.05±0.65 | 18.4±4.3 | 63.3±8.5 (300) | 0.85±0.03 | (5) |
| GIRK2 | 13.03±1.89 | 89.5±24.3 | 57.5±7.5 (300) | 0.89±0.02 | (7) |
| wvGIRK2 | 7.85±2.24 | 9.37±2.81 | 92.0±3.4 (200) | 0.82±0.05 | (4) |
The mean±s.e.m. of the EC50 values and the drug concentrations required to reduce basal GIRK currents by 50% (IC50) are shown in μM. The values of % inhibition indicate the mean±s.e.m.% inhibition of basal GIRK currents by fluoxetine at the highest concentrations tested. The highest concentrations tested (μM) and the number of oocytes tested (n) are indicated in parentheses. The nH values indicate the mean±s.e.m. of the Hill coefficients.
The instantaneous GIRK1/2 currents elicited by the voltage step to −100 mV from a holding potential of 0 mV diminished in the presence of 10 μM fluoxetine (Figure 2a). The percentage inhibition of the steady-state GIRK current at the end of the voltage step by fluoxetine was slightly higher than that of the instantaneous current (4.6±0.2%, 4.6±0.1% and 5.2±0.1% at −80, −100 and −120 mV; paired t-test, P<0.05, 0.005 and 0.005; n=11, 10 and 12, respectively). These results suggest that the channels were primarily inhibited at the holding potential of 0 mV and in only a slight time-dependent manner by external fluoxetine.
Figure 2.
Characteristics in the effects of fluoxetine on GIRK currents. (a) Representative GIRK1/2 currents elicited by voltage step to −100 mV for 1 s from a holding potential of 0 mV in the absence and presence of 10 μM fluoxetine. Current responses were recorded in a high-potassium solution containing 96 mM K+. Arrow indicates the zero current level. (b) Current–voltage relations of 3 mM Ba2+-sensitive inward currents and 10 μM fluoxetine-sensitive inward currents in oocytes expressing GIRK1/2 channels or GIRK1/4 channels. Current responses were normalized to the 3 mM Ba2+-sensitive current component measured at a membrane potential of −100 mV. The Ba2+-sensitive current components were 2426.9±661.3 nA (n=7) in oocytes expressing GIRK1/2 channels and 1203.3±307.0 nA (n=4) in oocytes expressing GIRK1/4 channels. (c) The percentage inhibition of GIRK channels by fluoxetine over the voltage range of −120 to −20 mV. There was a significant interaction between the fluoxetine effect and the membrane potential effect (P<0.05 for GIRK1/2, n=5–16 for the groups, and P<0.001 for GIRK1/4, n=4 for each group; one-way ANOVA), and then there were significant differences between the effects at −80 or −100 mV and that at −20 mV for GIRK1/2 channels, and between the effects at −60, −80 or −100 mV and that at −20 mV and between the effects at −80 or −100 mV and that at −40 mV for GIRK1/4 channels (P<0.05; Tukey–Kramer post hoc test). All values are mean and s.e.m.
Like 3 mM Ba2+-sensitive currents corresponding to basal GIRK currents, fluoxetine-sensitive currents in oocytes expressing GIRK channels increased with negative membrane potentials and the current–voltage relations showed strong inward rectification (Figure 2b), indicating a characteristic of GIRK currents.
For GIRK1/2 channels, the percentage inhibition of GIRK currents by 10 μM fluoxetine tended to decrease with membrane potentials from −80 to −20 mV, although there was no significant difference in the percentage inhibition at membrane potentials between −120 and −40 mV (Figure 2c). For GIRK1/4 channels, the percentage inhibition of GIRK currents by 10 μM fluoxetine decreased with membrane potentials from −80 to −20 mV (Figure 2c). When membrane potentials were more negative than −100 mV, the percentage inhibitions by 10 μM fluoxetine were almost the same both for GIRK1/2 and GIRK1/4 channels, suggesting that some changes in the interaction between fluoxetine and GIRK channels may occur at very negative membrane potentials.
At physiological pH or below, fluoxetine exists mainly in a protonated form and the proportion of the uncharged form increases by increasing pH, because of a pKa value of 9.5 (Maertens et al., 1999). We examined whether changes in pH affect fluoxetine inhibition of GIRK channels. The effects of pH on the fluoxetine inhibition were not observed in the concentration–response relations for fluoxetine (Figure 3), suggesting that the inhibition is mediated by both forms of fluoxetine with almost the same effectiveness. It also appears unlikely that the inhibition by fluoxetine is caused by hydrophobic interactions with GIRK channels within the membrane bilayer.
Figure 3.
Concentration–response relations for inhibition of GIRK1/2 channels and GIRK1/4 channels by fluoxetine at three different pH values. The magnitudes of inhibition of GIRK current by fluoxetine were compared with the 3 mM Ba2+-sensitive current components, which were 1033.3±184.8 nA (n=6, pH 6.0), 741.6±118.0 nA (n=5, pH 7.4) and 822.0±166.3 nA (n=4, pH 9.0) in oocytes expressing GIRK1/2 channels (a), and 625.5±117.4 nA (n=6, pH 6.0), 600.8±268.0 nA (n=5, pH 7.4) and 328.8±114.0 nA (n=4, pH 9.0) in oocytes expressing GIRK1/4 channels (b), respectively. Current responses were measured at a membrane potential of −70 mV in a high-potassium solution. Each point and error bar represents the mean and s.e.m. of the percentage responses obtained. Data points were fitted using a logistic equation.
Moreover, we examined the effect of intracellular fluoxetine on GIRK channels. Injection of fluoxetine into oocytes expressing GIRK1/2 channels had no or little effect on the basal currents (3.8±1.8% inhibition of control at the presumed intracellular concentration of ∼225 μM, n=9). Therefore, the result suggests that fluoxetine directly inhibits GIRK channels from the extracellular side of the cell membrane.
We further investigated the effects of fluoxetine on GIRK channels activated by G-protein-coupled receptors. In oocytes coexpressing GIRK1/2 channels and XA1 receptor (Kobayashi et al., 2002), application of 10 nM Ado induced inward GIRK currents (Figure 4). Effects of fluoxetine were evaluated by measuring the amplitude of the Adoinduced current response during application of fluoxetine at different concentrations. The current responses to 10 nM Ado were reversibly inhibited by fluoxetine with an IC50 value of 9.26±2.00 μM and a nH value of 0.88±0.08 (n=5, Figure 4). The extent of inhibition by fluoxetine was similar to that of basally active GIRK1/2 channels (P>0.05 at each concentration, Student's t-test), suggesting interaction of fluoxetine with GIRK channels. In addition, the Ado-induced GIRK currents were not significantly affected by intracellularly applied fluoxetine (112.2±14.4% of pretreated control current, paired t-test, P>0.1, n=5), whereas the GIRK currents were significantly inhibited by intracellularly applied QX-314 (55.2±11.9% of pretreated control current, paired t-test, P<0.01, n=8) as reported previously (Zhou et al., 2001). The results, therefore, suggest that extracellular fluoxetine can inhibit the effect of GIRK channels activated by G-protein-coupled receptors.
Figure 4.
Inhibitory effect of fluoxetine on GIRK channels activated by a G-protein-coupled receptor. (a) In an oocyte coinjected with mRNAs for GIRK1 and GIRK2 channels and XA1 receptor, current responses to adenosine (Ado), Ado in the presence of 10 μM fluoxetine and Ado are shown. The concentration of Ado used was 10 nM. Bars show the duration of application. Asterisk indicates the zero current level. (b) Concentration-dependent inhibition of fluoxetine on Ado-induced GIRK currents. Icontrol is the amplitude of GIRK currents induced by 10 nM Ado (407.2±109.0 nA, n=5) and I is the current amplitude in the presence of fluoxetine. Current responses were measured at a membrane potential of −70 mV in a high-potassium solution. Each point and error bar represents the mean and s.e.m. of the relative responses. Data points were fitted using a logistic equation.
Comparison of the fluoxetine effects on members of Kir channels
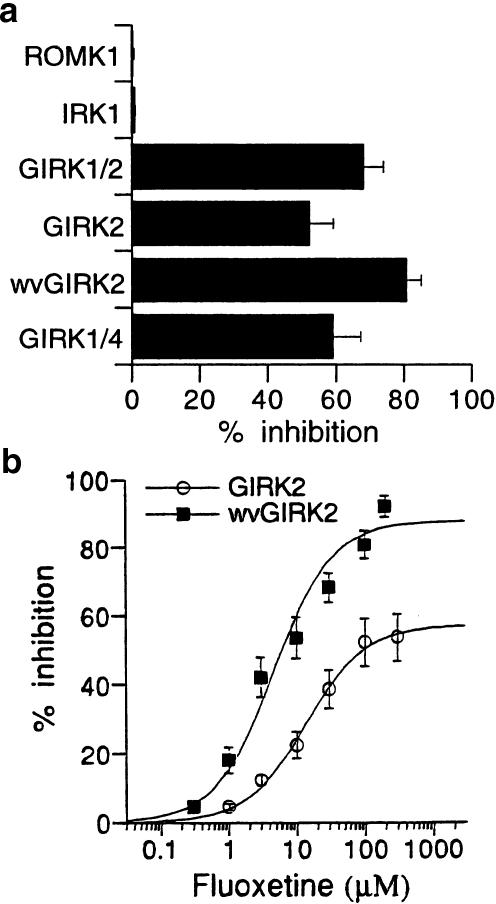
We examined whether fluoxetine interacts with ROMK1, an ATP-regulated inwardly rectifying K+ channel, and IRK1, a constitutively active Kir channel, among G-protein-insensitive Kir channels. In oocytes expressing ROMK1 or IRK1 channels, application of 100 μM fluoxetine had no significant effect on the inward currents through the channels in the hK solution (0.3±0.3 inhibition and 0.8±0.3% inhibition of the 3 mM Ba2+-sensitive current components that were 946.0±126.0 and 967.7±418.1 nA at a membrane potential of −70 mV, n=3 for ROMK1 and n=6 for IRK1, respectively; Figure 5a).
Figure 5.
Comparison of the fluoxetine effects on members of inwardly rectifying potassium channels. (a) Action of fluoxetine on GIRK, ROMK1 and IRK1 channels expressed as homomeric or heteromeric channels in Xenopus oocytes. The concentration of fluoxetine used was 100 μM. Current responses were measured at a membrane potential of −70 mV in a high-potassium solution. (b) Distinct concentration-dependent responses to fluoxetine for GIRK2 channels and weaver (wv) GIRK2 channels. The magnitudes of inhibition of GIRK current by fluoxetine were compared with the 3 mM Ba2+-sensitive current components (685.8±183.6 nA, n=5) in oocytes expressing GIRK2 channels, and with the hK current components (2857.8±321.0 nA, n=4) in oocytes expressing wvGIRK2 channels, respectively. Current responses were measured at a membrane potential of −70 mV in a high-potassium solution or a NMDG solution. Each point and error bar represents the mean and s.e.m. of the percentage responses obtained. Data points were fitted using a logistic equation.
GIRK2 channels form homomultimers (Lesage et al., 1995; Inanobe et al., 1999). The GIRK2 channels of wv mutant mice, which have a missense point mutation in the pore-forming region (Patil et al., 1995), show constitutive activation in a G-protein- and EtOH-independent manner and permeability to sodium as well as potassium ions because of the lack of K+ selectivity (Navarro et al., 1996; Slesinger et al., 1997; Kobayashi et al., 1999). To further address the relation between fluoxetine and GIRK channels, we investigated the effects of fluoxetine on these homomeric channels. In oocytes expressing GIRK2 channels, fluoxetine inhibited the channels to the similar extent when compared with GIRK heteromeric channels (P>0.05 at 300 μM, Student's t-test), although the IC50 value for GIRK2 channels was ∼5 times higher than those for the heteromeric channels (Table 1). On the other hand, in oocytes expressing wvGIRK2 channels, large inward currents were observed when the oocytes were perfused with either ND98 or hK solution instead of NMDG solution, which does not contain Na+ or K+ (3118.6±190.1 and 3094.6±364.4 nA, n=7 and 25, respectively). The addition of 100 μM fluoxetine to ND98 or hK solutions immediately reduced the inward currents (89.9±1.9% of the ND98 current component and 80.9±4.1% of the hK current component, n=4 and 7, respectively; Figure 5a). As shown in Figure 5 and Table 1, the inhibitory effect of fluoxetine on wvGIRK2 channels was more efficacious than that of GIRK2 channels (P<0.01, Student's t-test). Moreover, injection of fluoxetine inhibited only 8.4±2.3% of the hK current component, which was 1329.8±232.9 nA, by the presumed intracellular concentration of ∼225 μM (n=5). The results suggest the possibilities that extracellular fluoxetine may easily approach its action sites on wvGIRK2 channels because of a mutation in the pore-forming region and/or that it may easily cause a distinct conformational change in the channels with the different channel properties.
Fluoxetine inhibits EtOH-induced GIRK currents
GIRK channels are also activated by EtOH independent of G-protein signalling pathways (Kobayashi et al., 1999). Several studies have shown that fluoxetine reduces EtOH consumption (Rockman et al., 1982; Naranjo & Knoke, 2001), suggesting a relation between the fluoxetine and EtOH effects. We next examined the effect of fluoxetine on EtOH-induced GIRK currents. In oocytes expressing GIRK1/2 channels, the EtOH-induced GIRK currents were attenuated in the presence of fluoxetine, with an IC50 value of 11.1±2.5 μM and a nH value of 0.78±0.02, in a reversible manner (n=5; Figure 6). Furthermore, we compared the EtOH-induced GIRK currents before and after injecting fluoxetine into the oocytes. The EtOH-induced GIRK currents were not significantly affected by intracellularly applied fluoxetine (105.9±4.6% of pretreated control current, paired t-test, P>0.1, n=5). The results, therefore, suggest that extracellular fluoxetine can inhibit the effect of GIRK channels induced by EtOH.
Figure 6.
Inhibitory effect of fluoxetine on the ethanol-induced GIRK currents in Xenopus oocytes expressing GIRK1/2 channels. (a) In an oocyte coinjected with GIRK1 and GIRK2 mRNAs, current responses to ethanol (EtOH), EtOH in the presence of 10 μM fluoxetine, EtOH and 3 mM Ba2+ are shown. The concentration of EtOH used was 100 mM. Asterisk indicates the zero current level. Bars show the duration of application. (b) Concentration-dependent inhibition of fluoxetine on EtOH-induced GIRK currents. Icontrol is the amplitude of GIRK currents induced by 100 mM EtOH (340.8±18.1 nA, n=5) and I is the current amplitude in the presence of fluoxetine. Current responses were measured at a membrane potential of −70 mV in a high-potassium solution containing 96 mM K+. Each point and error bar represents the mean and s.e.m. of the relative responses. Data points were fitted using a logistic equation.
Discussion
We have demonstrated that extracellular fluoxetine inhibits both brain-type GIRK1/2 and GIRK2 channels and cardiac-type GIRK1/4 channels to a limited extent at high concentrations. However, two different SSRIs fluvoxamine and zimelidine have no significant effect on the channels. The inhibition by fluoxetine showed a concentration-dependence, a weak voltage-dependence and a slight time-dependence with a predominant effect on the instantaneous current elicited by voltage pulses and followed by slight further inhibition. On the other hand, blockade by extracellular Ba2+ and Cs+, typical of Kir channel blockers that occlude the pore of the open channel, shows a concentration-dependence, a strong voltage-dependence and a time-dependence with a comparatively small effect on the instantaneous current, but a marked inhibition on the steady-state current at the end of voltage pulses (Lesage et al., 1995). These observations suggest that fluoxetine probably causes a conformational change in the channels, but does not act as typical open channel blockers of Kir channels like Ba2+ and Cs+, although all these molecules act at the channels from the extracellular side. The different mechanism from Ba2+ for GIRK channels may be involved in the incomplete blockade and slower time course of inhibition, compared with the inhibition by Ba2+ as shown in Figure 1. Since wvGIRK2 channels were highly sensitive to fluoxetine, but ROMK1 and IRK1 channels among members of the Kir channel family were insensitive to fluoxetine, further studies using chimeric GIRK channels replaced with the homologous regions from ROMK1 and IRK1 channels and mutant GIRK channels may clarify the critical sites mediating the effects of fluoxetine on GIRK channels. In addition, single channel analyses of wild-type GIRK channels and wvGIRK2 channels in the presence of fluoxetine may be useful for understanding the mechanisms of the action of fluoxetine on GIRK channels and the greater inhibition by fluoxetine of the mutant channels with different channel properties.
The therapeutic plasma concentrations of fluoxetine range approximately from 0.3 to 1.5 μM (Baldessarini, 2001). The brain concentrations of fluoxetine are approximately 10 or 20 times higher than the corresponding blood levels (Karson et al., 1993; Bolo et al., 2000). Therefore, the present findings suggest that GIRK channels in the brain may be inhibited by fluoxetine at clinically relevant concentrations.
Neuronal GIRK channels are widely present in various brain regions, such as the frontal cortex, hippocampus, amygdala, thalamus, substantia nigra, ventral tegmental area, brain stem and cerebellum (Kobayashi et al., 1995; Karschin et al., 1996; Liao et al., 1996). GIRK channels are thought to play an important role in inhibiting the neuronal excitability through activation of various G-protein-coupled receptors. Therefore, inhibition of GIRK channels by fluoxetine may affect many brain functions. Recent studies have demonstrated that there are regional abnormalities in the prefrontal cortex, hippocampus, amygdala, thalamus and ventral striatum in depressed individuals (Manji et al., 2001). It is generally thought that the therapeutic effects of fluoxetine and fluvoxamine are primarily because of the inhibition of the reuptake of serotonin in the brain. Although a comparative study of fluoxetine and fluvoxamine showed that both drugs were equally effective in the treatment of patients with major depression (Rapaport et al., 1996), cohort studies showed that fluvoxamine was significantly less effective in the treatment of depression, anxiety and other disorders than fluoxetine (Mackay et al., 1997). In addition, fluvoxamine is approved for the treatment of obsessive-compulsive disorder in the US, but not for the treatment of depression (Barbey & Roose, 1998). The present study demonstrates that in contrast to fluoxetine, fluvoxamine has no significant effect on GIRK channels. GIRK2-deficient mice show an increase in motor activity and less anxiety (Blednov et al., 2001). Taken together, inhibition of neuronal GIRK channels by fluoxetine may contribute to additive therapeutic effects for depression and other related psychiatric disorders.
On the other hand, the incidence of seizures during treatment with SSRIs including fluoxetine and fluvoxamine is a serious side effect in contrast with various benign side effects (Barbey & Roose, 1998; Neely, 1998; Baldessarini, 2001). The molecular mechanisms underlying seizures during treatment with SSRIs remains unclear. The serotonin syndrome, which is a toxic hyperserotonergic state, typically includes not only changes in mental state, myoclonus, tremor and various autonomic responses, but also seizures in severe cases (Sternbach, 1991; Baldessarini, 2001). Seizures associated with SSRIs may be related to a consequence of aberrant responses in serotonergic system. Moreover, overdoses of fluoxetine have been reported to be associated with seizures (Barbey & Roose, 1998; Neely, 1998). The plasma concentrations of fluoxetine in several patients who experienced seizures were reported to be approximately 3.3–7.1 μM (Barbey & Roose, 1998; Neely, 1998). Therefore, fluoxetine at the corresponding brain levels may potently inhibit neuronal GIRK channels. Bupivacaine, a local anesthetic, also inhibits GIRK channels but does not affect other Kir channels (Zhou et al., 2001). Overdoses of bupivacaine are associated with seizures, and the blood levels are close to the IC50 values for GIRK channels. In addition, fluoxetine inhibits two types of neuronal voltage-gated K+ channels, Kv1.1 and Kv3.1 channels (Yeung et al., 1999; Choi et al., 2001). GIRK2-deficient mice show spontaneous seizures (Signorini et al., 1997), and the inhibition of these K+ channels could lead to an increase in neuronal excitability. Therefore, potent inhibition of neuronal GIRK channels by fluoxetine together with the Kv channels may also contribute to the cause of seizures and some of other neuropsychiatric toxicity.
Antidepressant drugs including fluoxetine, fluvoxamine and tricyclic antidepressants show analgesic activity (Messing et al., 1975; Lin et al., 1980; Schreiber et al., 1996; Korzeniewska-Rybicka & Plaznik, 2000; Galeotti et al., 2001). Studies using wv mutant mice or GIRK2-deficient mice have shown that activation of GIRK channels by opioids or EtOH may be involved in analgesia induced by the drugs, suggesting that the GIRK channel is one of the key molecules in analgesia (Kobayashi et al., 1999; Ikeda et al., 2000; 2002; Mitrovic et al., 2000). However, the present study demonstrates that fluoxetine inhibits GIRK channels, while fluvoxamine has no significant effect on GIRK channels. Therefore, the analgesic effect of fluoxetine and fluvoxamine may be caused by interaction with some of other several targets. Previous studies have shown that the actions of 5-HT may contribute to the analgesic effect (Messing et al., 1975; Lin et al., 1980; Tura & Tura, 1990). Various antidepressant drugs including fluoxetine and tricyclic antidepressants inhibit voltage-gated Na+ channels like local anesthetics (Pancrazio et al., 1998). Therefore, the analgesic effect of the antidepressant drugs may share these common mechanisms. In addition, Galeotti et al. (2001) suggest that the analgesic effects of tricyclic antidepressants may involve the opening of voltage-gated, ATP-sensitive and Ca2+-activated K+ channels. Further studies are required to clarify whether the analgesic effects of SSRIs are also mediated by these channels.
In the heart, acetylcholine released from the stimulated vagus nerve opens atrial GIRK channels via activation of M2 muscarinic acetylcholine receptors, and ultimately causes slowing of the heart rate (Brown & Birnbaumer, 1990). The binding affinity of fluoxetine for the receptor exhibits low micromolar concentrations (Stanton et al., 1993). In the present study, micromolar concentrations of fluoxetine inhibited cardiac-type GIRK1/4 channels. In clinical practice, fluoxetine overdoses are associated with the incidence of sinus tachycardia (Barbey & Roose, 1998; Neely, 1998). The clinically relevant plasma concentrations become significantly higher than the micromolar concentrations in the therapeutic use, although the corresponding heart concentrations have not been determined in humans. Sinus tachycardia during fluoxetine treatment may be related to not only antagonism of M2 muscarinic acetylcholine receptors, but also inhibition of atrial GIRK channels. In addition, 5-HT can elicit tachycardia in humans, although it is known that 5-HT elicits complex changes in the cardiovascular system comprising brady-cardia or tachycardia, hypotension or hypertension and vasodilatation or vasoconstriction (Saxena & Villalon, 1990). The 5-HT effect caused by inhibition of 5-HT uptake might also be related to the incidence of tachycardia during treatment with SSRIs. Sinus tachycardia is also observed in fluvoxamine overdose (Barbey & Roose, 1998). Although fluvoxamine has no significant effect on GIRK channels, it has less affinity for muscarinic acetylcholine receptors than fluoxetine (Baldessarini, 2001). Therefore, sinus tachycardia observed in fluvoxamine overdose might be related to the effects of 5-HT and antagonism of muscarinic acetylcholine receptors.
Interestingly, we also demonstrate that extracellar fluoxetine at low micromolar concentrations can inhibit the effect of brain-type GIRK channels induced by ethanol. Fluoxetine reduces EtOH consumption (Rockman et al., 1982; Naranjo & Knoke, 2001). However, GIRK2-deficient mice display no significant change in EtOH consumption (Blednov et al., 2001). So far, there is no evidence to help clarify the in vivo effects of fluoxetine on GIRK channel activation by EtOH. Further studies using mice treated with antisense oligodeoxynucleotides to the GIRK mRNAs, mutant mice with GIRK channels insensitive to EtOH alone and a selective unidentified GIRK channel activator or inhibitor may be useful for the understanding of the antagonism of EtOH-induced GIRK effects by fluoxetine in vivo. In addition, mutagenesis studies of GIRK channels may clarify the molecular mechanism underlying the inhibitory action of fluoxetine on EtOH activation of GIRK channels.
In conclusion, we demonstrate that fluoxetine, at clinically relevant brain concentrations, acts as an inhibitor at GIRK channels, which play an important role in the inhibitory regulation of neuronal excitability in most brain regions. Moreover, fluoxetine can inhibit the effect of GIRK channels induced by EtOH. Our results suggest that inhibition of GIRK channels by fluoxetine may contribute to some of the therapeutic effects and the adverse side effects, particularly seizures in overdose, observed in clinical practice.
Acknowledgments
We thank Drs Toshiro Kumanishi and Kansaku Baba for their cooperation, and Tomio Ichikawa and Kazuo Kobayashi for their assistance. We also thank Dr Steven C. Hebert for providing the ROMK1 cDNA, and Dr Lily Y. Jan for providing the IRK1 cDNA. This work was supported by research grants from the Ministry of Education, Science, Sports and Culture of Japan and the RIKEN Brain Science Institute.
Abbreviations
- Ado
adenosine
- DMSO
dimethyl sulfoxide
- EK
K+ equilibrium potential
- EtOH
ethanol
- GIRK
G protein-activated inwardly rectifying K+ channel
- hK
high potassium
- 5-HT
serotonin
- Kir channel
inward-rectifier K+ channel
- ND98
K+-free high sodium
- nH
Hill coefficient
- NMDG
N-methyl-D-glucamine
- QX-314
lidocaine N-ethyl bromide
- SSRI
selective serotonin reuptake inhibitor
- wv
weaver
- XA1 receptor
Xenopus A1 adenosine receptor
References
- BALDESSARINI R.J.Drugs and the treatment of psychiatric disorders: depression and anxiety disorder Goodman & Gilman's The Pharmacological Basis of Therapeutics 2001New York: McGraw-Hill; 447–483.10th edn., ed. Hardman, J.G., Limbird, L.E. & Gilman, A.G. pp [Google Scholar]
- BARBEY J.T., ROOSE S.P. SSRI safety in overdose. J. Clin. Psychiatry. 1998;59 Suppl. 15:42–48. [PubMed] [Google Scholar]
- BLEDNOV Y.A., STOFFEL M., CHANG S.R., HARRIS R.A. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J. Pharmacol. Exp. Ther. 2001;298:521–530. [PubMed] [Google Scholar]
- BOLO N.R., HODE Y., NEDELEC J.-F., LAINE E., WAGNER G., MACHER J.-P. Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology. 2000;23:428–438. doi: 10.1016/S0893-133X(00)00116-0. [DOI] [PubMed] [Google Scholar]
- BROWN A.M., BIRNBAUMER L. Ionic channels and their regulation by G protein subunits. Annu. Rev. Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- CHOI B.H., CHOI J.-S., YOON S.H., RHIE D.-J., MIN D.S., JO Y.-H., KIM M.-S., HAHN S.J. Effects of norfluoxetine, the major metabolite of fluoxetine, on the cloned neuronal potassium channel Kv3.1. Neuropharmacology. 2001;41:443–453. doi: 10.1016/s0028-3908(01)00088-0. [DOI] [PubMed] [Google Scholar]
- CHOI J.-S., HAHN S.J., RHIE D.-J., YOON S.-H., JO Y.-H., KIM M.-S. Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J. Pharmacol. Exp. Ther. 1999;291:1–6. [PubMed] [Google Scholar]
- DASCAL N. Signalling via the G protein-activated K+ channels. Cell Signal. 1997;9:551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- DEÁK F., LASZTOCZI B., PACHER P., PETHEO G.L., KECSKEMETI V., SPAT A. Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology. 2000;39:1029–1036. doi: 10.1016/s0028-3908(99)00206-3. [DOI] [PubMed] [Google Scholar]
- DOUPNIK C.A., DAVIDSON N., LESTER H.A. The inward rectifier potassium channel family. Curr. Opin. Neurobiol. 1995;5:268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- FAN P. Effects of antidepressants on the inward current mediated by 5-HT3 receptors in rat nodese ganglion neurons. Br. J. Pharmacol. 1994;112:741–744. doi: 10.1111/j.1476-5381.1994.tb13140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALEOTTI N., GHELARDINI C., BARTOLINI A. Involvement of potassium channels in amitryptyline and clomipramine analgesia. Neuropharmacology. 2001;40:75–84. doi: 10.1016/s0028-3908(00)00097-6. [DOI] [PubMed] [Google Scholar]
- GARCÍA-COLUNGA J., AWAD J.N., MILEDI R. Blockage of muscule and neuronal nicotinic acetylcholine receptors by fluoxetine (Prozac) Proc. Natl. Acad. Sci. U.S.A. 1997;94:2041–2044. doi: 10.1073/pnas.94.5.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO K., NICHOLS C.G., LEDERER W.J., LYTTON J., VASSILEV P.M., KANAZIRSKA M.V., HEBERT S.C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI T., ICHIKAWA T., USUI H., ABE S., KUMANISHI T. Comparison of the three mouse G-protein-activated K+ (GIRK) channels and functional couplings of the opioid receptors with the GIRK1 channel. Ann. NY Acad. Sci. 1996;801:95–109. doi: 10.1111/j.1749-6632.1996.tb17434.x. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI T., ICHIKAWA T., USUI H., KUMANISHI T. Functional couplings of the δ- and the κ-opioid receptors with the G-protein-activated K+ channel. Biochem. Biophys. Res. Commun. 1995;208:302–308. doi: 10.1006/bbrc.1995.1338. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI K., KOBAYASHI T., ICHIKAWA T., KUMANISHI T., KISHIDA H., YANO R., MANABE T. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Mol. Brain Res. 1997;45:117–126. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI T., KUMANISHI T., NIKI H., YANO R. Involvement of G-protein-activated inwardly rectifying K+ (GIRK) channels in opioid-induced analgesia. Neurosci. Res. 2000;38:113–116. doi: 10.1016/s0168-0102(00)00144-9. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI T., KUMANISHI T., YANO R., SORA I., NIKI H. Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys. Neurosci. Res. 2002;44:121–131. doi: 10.1016/s0168-0102(02)00094-9. [DOI] [PubMed] [Google Scholar]
- INANOBE A., YOSHIMOTA Y., HORIO Y., MORISHIGE K.-I., HIBINO H., MATSUMOTO S., TOKUNAGA Y., MAEDA T., HATA Y., TAKAI Y., KURACHI Y. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J. Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARSCHIN C., DIßMANN E., STUHMER W., KARSCHIN A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J. Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARSON C.N., NEWTON J.E.O., LIVINGSTON R., JOLLY J.B., COOPER T.B., SPRIGG J., KOMOROSKI R.A. Human brain fluoxetine concentrations. J. Neuropsychiatry Clin. Neurosci. 1993;5:322–329. doi: 10.1176/jnp.5.3.322. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., IKEDA K., ICHIKAWA T., ABE S., TOGASHI S., KUMANISHI T. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem. Biophys. Res. Commun. 1995;208:1166–1173. doi: 10.1006/bbrc.1995.1456. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., IKEDA K., KOJIMA H., NIKI H., YANO R., YOSHIOKA T., KUMANISHI T. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat. Neuro-sci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., IKEDA K., KUMANISHI T. Inhibition of various antipsychotic drugs on the G-protein-activated inwardly rectifying K+ (GIRK) channels expressed in Xenopus oocytes. Br. J. Pharmacol. 2000;129:1716–1722. doi: 10.1038/sj.bjp.0703224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI T., IKEDA K., KUMANISHI T. Functional characterization of an endogenous Xenopus oocyte adenosine receptor. Br. J. Pharmacol. 2002;135:313–322. doi: 10.1038/sj.bjp.0704475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORZENIEWSKA-RYBICKA I., PLAZNIK A. Supraspinally mediated analgesic effect of antidepressant drugs. Pol. J. Pharmacol. 2000;52:93–99. [PubMed] [Google Scholar]
- KRAPIVINSKY G., GORDON E.A., WICKMAN K., VELIMIROVIC B., KRAPIVINSKY L., CLAPHAM D.E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- KUBO Y., BALDWIN T.J., JAN Y.N., JAN L.Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993a;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- KUBO Y., REUVENY E., SLESINGER P.A., JAN Y.N., JAN L.Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993b;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- LESAGE F., GUILLEMARE E., FINK M., DUPRAT F., HEURTEAUX C., FOSSET M., ROMEY G., BARHANIN J., LAZDUNSKI M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J. Biol. Chem. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- LEWOHL J.M., WILSON W.R., MAYFIELD R.D., BROZOWSKI S.J., MORRISETT R.A., HARRIS R.A. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat. Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- LIAO Y.J., JAN Y.N., JAN L.Y. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J. Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN M.-T., CHANDRA A., CHI M.-L., KAU C.-L. Effects of increasing serotonergic receptor activity in brain on analgesic activity in rats. Exp. Neurol. 1980;68:548–554. doi: 10.1016/0014-4886(80)90108-9. [DOI] [PubMed] [Google Scholar]
- MACKAY F.J., DUNN N.R., WILTON L.V., PEARCE G.L., FREEMANTLE S.N., MANN R.D. A comparison of fluvoxamine, fluoxetine, sertraline and paroxetine examined by observational cohort studies. Pharmacoepidem. Dr. S. 1997;6:235–246. doi: 10.1002/(SICI)1099-1557(199707)6:4<235::AID-PDS293>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- MAERTENS C., WEI L., VOETS T., DROOGMANS G., NILIUS B. Block by fluoxetine of volume-regulated anion channels. Br. J. Pharmacol. 1999;126:508–514. doi: 10.1038/sj.bjp.0702314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI L., PALMA E., MILEDI R., EUSEBI F. Effects of fluoxetine on wild and mutant neuronal α7 nicotinic receptors. Mol. Psychiatry. 1998;3:350–355. doi: 10.1038/sj.mp.4000392. [DOI] [PubMed] [Google Scholar]
- MANJI H.K., DREVETS W.C., CHARNEY D.S. The cellular neurobiology of depression. Nat. Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- MESSING R.B., PHEBUS L., FISHER L.A., LYTLE L.D. Analgesic effects of fluoxetine hydrochloride (Lilly 110140) a specific inhibitor of serotonin uptake. Psychopharmacol. Commun. 1975;1:511–521. [PubMed] [Google Scholar]
- MITROVIC I., YI E., MARGETA-MITROVIC M., STOFFEL M., BASBAUM A.I. Contribution of GIRK2 channels to morphine and clonidine antinociception: a possible mediator of sex differences. Soc. Neurosci. Abstr. 2000;26:2188. [Google Scholar]
- NARANJO C.A., KNOKE D.M. The role of selective serotonin reuptake inhibitors in reducing alcohol consumption. J. Clin. Psychiatry. 2001;62 Suppl. 20:18–25. [PubMed] [Google Scholar]
- NAVARRO B., KENNEDY M.E., VELIMIROVIC B., BHAT D., PETERSON A.S., CLAPHAM D.E. Nonselective and Gβγ-insensitive weaver K+ channels. Science. 1996;272:1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- NEELY J.L. Tonic clonic seizures and tachycardia induced by fluoxetine (ProzacR) overdose. W. Vir. Med. J. 1998;94:283–285. [PubMed] [Google Scholar]
- NI Y.G., MILEDI R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac) Proc. Natl. Acad. Sci. U.S.A. 1997;94:2036–2049. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. Drug receptors and the inhibition of nerve cells. Br. J. Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANCRAZIO J.J., KAMATCHI G.L., ROSCOE A.K., LYNCH CIII. Inhibition of neuronal Na+ channels by antidepressant drugs. J. Pharmacol. Exp. Ther. 1998;284:208–214. [PubMed] [Google Scholar]
- PATIL N., COX D.R., BHAT D., FAHAM M., MYERS R.M., PETERSON A.S. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat. Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- PERCHENET L., HILFIGER L., MIZRAHI J., CLEMENT-CHOMIENNE O. Effects of anorexinogen agents on cloned voltage-gated K+ channel hKv1.5. J. Pharmacol. Exp. Ther. 2001;298:1108–1119. [PubMed] [Google Scholar]
- RAPAPORT M., COCCARO E., SHELINE Y., PERSE T., HOLLAND P., FABRE L., BRADFORD D. A comparison of fluvoxamine and fluoxetine in the treatment of major depression. J. Clin. Psychopharmacol. 1996;16:373–378. doi: 10.1097/00004714-199610000-00005. [DOI] [PubMed] [Google Scholar]
- REIMANN F., ASHCROFT F.M. Inwardly rectifying potassium channels. Curr. Opin. Cell Biol. 1999;11:503–508. doi: 10.1016/S0955-0674(99)80073-8. [DOI] [PubMed] [Google Scholar]
- REUVENY E., SLESINGER P.A., INGLESE J., MORALES J.M., INIGUEZ-LLUHI J.A., LEFKOWITZ R.J., BOURNE H.R., JAN Y.N., JAN L.Y. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- ROCKMAN G.E., AMIT Z., BROWN Z.W., BOURQUE C., OGREN S-.O. An investigation of the mechanisms of action of 5-hydroxytryptamine in the suppression of ethanol intake. Neuropharmacology. 1982;21:341–347. doi: 10.1016/0028-3908(82)90098-3. [DOI] [PubMed] [Google Scholar]
- SAXENA P.R., VILLALON C.M. Cardiovascular effects of serotonin agonists and antagonists. J. Cardiovasc. Pharmacol. 1990;15 Suppl. 7:S17–S34. [PubMed] [Google Scholar]
- SCHREIBER S., BACKER M.M., YANAI J., PICK C.G. The antinociceptive effect of fluvoxamine. Eur. Neuropsychopharmacol. 1996;6:281–284. doi: 10.1016/s0924-977x(96)00031-4. [DOI] [PubMed] [Google Scholar]
- SIGNORINI S., LIAO Y.J., DUNCAN S.A., JAN L.Y., STOFFEL M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc. Natl. Acad. Sci. U.S.A. 1997;94:923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLESINGER P.A., STOFFEL M., JAN Y.N., JAN L.Y. Defective γ-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANTON T., BOLDEN-WATSON C., CUSACK B., RICHELSON E. Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistamines. Biochem. Pharmacol. 1993;45:2352–2354. doi: 10.1016/0006-2952(93)90211-e. [DOI] [PubMed] [Google Scholar]
- STERNBACH H. The serotonin syndrome. Am. J. Psychiatry. 1991;148:705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- THOMAS D., GUT B., WENDT-NORDAHL G., KIEHN J. The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J. Pharmacol. Exp. Ther. 2002;300:543–548. doi: 10.1124/jpet.300.2.543. [DOI] [PubMed] [Google Scholar]
- TURA B., TURA S.M. The analgesic effect of tricyclic antidepressants. Brain Res. 1990;518:19–22. doi: 10.1016/0006-8993(90)90948-b. [DOI] [PubMed] [Google Scholar]
- WICKMAN K., NEMEC J., GENDLER S.J., CLAPHAM D.E. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- WICKMAN K., SELDIN M.F., GENDLER S.J., CLAPHAM D.E. Partial structure, chromosome localization, and expression of the mouse Girk4 gene. Genomics. 1997;40:395–401. doi: 10.1006/geno.1997.4599. [DOI] [PubMed] [Google Scholar]
- WONG D.T., BYMASTER F.P., ENGLEMAN E.A. Prozac (Fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- YEUNG S.Y., MILLAR J.A., MATHIE A. Inhibition of neuronal Kv potassium currents by the antidepressant drug, fluoxetine. Br. J. Pharmacol. 1999;128:1609–1615. doi: 10.1038/sj.bjp.0702955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU W., ARRABIT C., CHOE S., SLESINGER P.A.Mechanism underlying bupivacaine inhibition of G protein-gated inwardly rectifying K+ channels Proc. Natl. Acad. Sci. U.S.A. 2001986482–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]