Abstract
Neurokinins contribute to the neural regulation of gastrointestinal (GI) smooth muscles. We studied responses of murine colonic smooth muscle cells to substance P (SP) and NK1 and NK2 agonists using confocal microscopy and the patch clamp technique.
Colonic myocytes generated localized Ca2+ transients that were coupled to spontaneous transient outward currents (STOCs). SP (10−10 M) increased Ca2+ transients and STOCs. Higher concentrations of SP (10−6 M) increased basal Ca2+ and inhibited Ca2+ transients and STOCs.
Effects of SP were due to increased Ca2+ entry via L-type Ca2+ channels, and were mediated by protein kinase C (PKC). Nifedipine (10−6 M) and the PKC inhibitor, GF 109203X (10−6 M) reduced L-type Ca2+ current and blocked the effects of SP.
SP responses depended upon parallel stimulation of NK1 and NK2 receptors. NK1 agonist ([Sar9,Met(O2)11]-substance P; SSP) and NK2 agonists (neurokinin A (NKA) or GR-64349) did not mimic the effects of SP alone, but NK1 and NK2 agonists were effective when added in combination (10−10–10−6 M). Consistent with this, either an NK1-specific antagonist (GR-82334; 10−7 M) or an NK2-specific antagonist (MEN 10,627; 10−7 M) blocked responses to SP (10−6 M).
Ryanodine (10−5 M) blocked the increase in Ca2+ transients and STOCs in response to SP (10−10 M).
Our findings show that low concentrations of SP, via PKC-dependent enhancement of L-type Ca2+ current and recruitment of ryanodine receptors, stimulate Ca2+ transients. At higher concentrations of SP (10−6 M), basal Ca2+ increases and spontaneous Ca2+ transients and STOCs are inhibited.
Keywords: Calcium puffs, L-type calcium channels, neurokinin receptors, protein kinase C, enteric neurotransmission
Introduction
Localized Ca2+ transients (‘sparks' or ‘puffs') regulate the open probabilities of Ca2+-dependent conductances in the plasma membranes of smooth muscle cells (Nelson et al., 1995; Gordienko et al., 1998; ZhuGe et al., 1998; Bayguinov et al., 2000). For example, in vascular smooth muscles, localized Ca2+ transients are because of release of Ca2+ from ryanodine receptors (Ca2+ sparks), and they activate large conductance Ca2+-activated K+ channels (BK channels; e.g. Perez et al., 1999). Activation of clusters of BK channels results in spontaneous transient outward currents (STOCs) that hyperpolarize smooth muscle cells and reduce excitability (Benham & Bolton, 1986; Nelson et al., 1995; ZhuGe et al., 1998).
Spontaneous Ca2+ transients also regulate the electrical and mechanical activity of gastrointestinal (GI) smooth muscles. We have shown in experiments on colonic smooth muscle cells that localized Ca2+ transients are due to release of Ca2+ from inositol 1,4,5-trisphosphate (IP3) receptor-operated stores (Ca2+ puffs), and the STOCs associated with these events are due to activation of BK channels and small conductance Ca2+-activated K+ channels (SK channels; see Bayguinov et al., 2000; Kong et al., 2000). The frequency and amplitudes of Ca2+ puffs in colonic muscle cells are modulated by enteric neurotransmitters. Thus, responses of GI smooth muscle cells to neurotransmitters depend, in part, on the coupling between Ca2+ transients and BK and SK channels (and possibly other Ca2+-dependent conductances).
We have previously characterized two examples of regulation of Ca2+ puffs and STOCs by enteric neurotransmitters. ATP, which mediates part of the enteric inhibitory neural response, binds to P2Y receptors, activates phospholipase Cβ, and increases IP3 production. Enhanced IP3 levels stimulate Ca2+ transients and STOCs causing hyperpolarization responses to ATP (Bayguinov et al., 2000). Acetylcholine (ACh), the major excitatory neurotransmitter in the GI tract, also couples to activation of phospholipase C and production of IP3. In contrast to the actions of ATP, ACh reduced Ca2+ transients and STOCs (Bayguinov et al., 2001b). Inhibition of Ca2+ transients was a result of a rise in basal (cytoplasmic) Ca2+ that resulted from Ca2+ entry through a nonselective cation conductance activated by ACh.
Other transmitter substances, such as substance P (SP) and neurokinin A, are coexpressed with ACh in enteric excitatory motor neurons and participate in neural responses of GI muscles (e.g. Brookes et al., 1991; Schmidt et al., 1991). In the present study, we have tested the effects of SP and specific neurokinin (NK1 and NK2) receptor agonists and antagonists on Ca2+ transients and STOCs in murine colonic myocytes using laser scanning confocal microscopy and the patch clamp technique. We have also investigated the second messenger coupling and ionic conductances linking NK1 and NK2 receptor activation to regulation of Ca2+ transients and STOCs.
Methods
Cell preparation
BALB/C mice (60–90 days old) of either sex were anesthetized with isoflurane inhalation (AErrane, Baxter Healthcare Corp., Deerfield, IL, U.S.A.) and killed by decapitation in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno. Colons were excised and opened along the mesenteric border. The luminal contents were removed with Krebs–Ringer bicarbonate buffer (KRB; see the section ‘Solutions and drugs'). Tissues were pinned to the base of a Sylgard-coated dish and the mucosa and submucosa were dissected away.
Strips of colonic muscle were cut and equilibrated in Ca2+-free solution for 60 min. Then the tissues were digested at 37°C for 16 min without agitation in an enzyme solution containing collagenase F (Sigma Chemical Corp., MO, U.S.A.) (Bayguinov et al., 2000). After the digestion period, the tissues were washed with Ca2+-free Hanks' solution to remove the enzyme. Then the tissues were triturated with blunt-tipped pipettes to free single smooth muscle cells.
Confocal microscopy
Suspensions of cells were placed in a 0.5 ml chamber with a glass bottom. The cells were incubated for 35 min at room temperature in Ca2+-free buffer containing fluo-4 acetoxymethylester (10 μg/ml; Molecular Probes, Eugene, OR, U.S.A.) and pluronic acid (2.5 μg/ml−1; Teflabs, Austin, TX, U.S.A.). Cell loading was followed by incubation in a solution containing 2 mM Ca2+ for 25 min to restore the normal concentration of extracellular Ca2+ and to allow the cells to adhere tightly to the bottom of the chambers during deesterification of fluo-4. All measurements were made within 45 min after restoring extracellular Ca2+.
An ODYSSEY XL confocal laser scanning head (NORAN Instruments Inc., Middleton, WI, U.S.A.) connected to a Nikon Diaphot 300 microscope with 60 × water immersion lens (NA=1.2) was used to image the cells. The cells were scanned using INTERVISION software (NORAN Instruments Inc., Middleton, WI, U.S.A.) running on an Indy workstation (Silicon Graphics, Inc., Mountain View, CA, U.S.A.). Changes in the fluo-4 fluorescence (indicating fluctuations in cytosolic Ca2+) were recorded for 20 s test periods using T-series acquisition and a laser of wavelength 488 nm (excitation for FITC). In all, 600 frames were acquired per test period (one frame every 33 ms), creating 20-s movie files.
Ionic currents of single cells
Ionic currents were measured in isolated muscle cells using the whole cell, perforated-patch (Amphotericin-B) configuration of the patch clamp technique. An Axopatch 200B amplifier with a CV 203BU head stage (Axon Instruments, Foster City, CA, U.S.A.) was used to measure ionic currents. Membrane currents were recorded using pClamp software (Version 7.0, Axon Instruments) while holding cells at –30 or −40 mV (after correction for a –11 mV junction potential). Currents were digitized at 1 kHz. In some experiments, cells were preloaded with fluo-4 and simultaneously voltage-clamped and scanned for fluorescence changes, as described above. In other experiments, whole-cell patch clamp experiments were performed to measure inward currents. In these experiments, cells were voltage-clamped with pipettes containing Amphotericin B and Cs+ (replacing K+) to block outward currents. The data were digitized at 2 kHz and filtered at 1 kHz using pClamp Software (Version 6.0, Axon Instruments).
Measuring membrane potential
We used two methods to measure the effects of SP on resting membrane potentials of smooth muscle cells. In the first series of experiments, circular muscle cells of intact colonic muscles were impaled with glass microelectrodes filled with 3 M KCl and having resistances of 50–70 MΩ. The electrical signals were digitized and recorded on a computerized data acquisition and analysis system (MP 100, Biopac Systems Inc., Santa Barbara, CA, U.S.A.). Membrane potentials of isolated myocytes were recorded using the current clamp mode of the patch clamp amplifier. Gigaseals were formed with cells using electrodes filled with an internal solution that included Amphotericin-B. A small, constant holding current (–10 to –30 pA) was applied to most cells to standardize the initial membrane potential at −50 mV (approximate physiological resting potential). Voltage responses were digitized at 1 kHz and filtered at 500 Hz using pClamp Software (Version 6.0, Axon Instruments).
Solutions and drugs
The standard KRB used in studies of intact muscles contained 120 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 15.5 mM NaHCO3, 1.2 mM NaH2PO4, and 11.5 mM dextrose. This solution had a final pH of 7.3–7.4 after equilibration with 97% O2–3% CO2. The enzyme solution used to disperse smooth muscle cells contained 1.3 mg ml−1 collagenase F, 2 mg ml−1 papain, 1 mg ml−1 BSA, 0.154 mg ml−1 L-DTT, 134 mM NaCl, 6 mM KCl, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES (pH 7.4). The bathing solution used in confocal microscopy studies and in all whole-cell patch clamp studies contained 134 mM NaCl, 6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES (pH 7.4). The pipette solution used in all whole-cell patch clamp experiments in which STOCs were recorded contained: 110 mM K-aspartate, 30 mM KCl, 10 mM NaCl, 1 mM MgCl2, 10 mM HEPES, 0.05 mM EGTA (pH 7.2), and 250 μg ml−1 Amphotericin B. The pipette solution for experiments in which whole-cell inward currents were recorded contained CsOH 110 mM, L-aspartic acid 110 mM, TEACl 30 mM, EGTA 1 mM, HEPES 10 mM, and Amphotericin B 200 μg ml−1. Ca2+ current records were corrected by digital subtraction of leak current. Currents are reported in percent change from control. All experiments were performed at room temperature (22–25°C).
Nicardipine, ryanodine, MEN-10,376, GR-64349 (Lis-Asp-Ser-Phe-Val-Gli-R-γ-lactam-Leu-Met-NH2)and GR-82334 (pGlu-Ala-Asp-Pro-Asn-Lis-Phe-Tyr-Pro(spiro-γ-lactam)Leu–Trp–NH2) were obtained from Sigma (MO, U.S.A.). Iberiotoxin, substance P (SP), [Sar9, Met(O2)11]-substance P (SSP), neurokinin A (NKA), 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl-1H-imidazole (SKF 96365 hydrochloride), and GF 109203X were obtained from Tocris Cookson Inc. (MO, U.S.A.). The concentrations of drugs used in experiments were ascertained from the literature or by empirical determinations of effective concentrations on murine colonic myocytes.
Analysis of data
Image analysis was performed using custom analysis programs developed with Interactive Data Language software (Research Systems Inc., Boulder, CO, U.S.A.), as previously described (Bayguinov et al., 2000). Baseline fluorescence (F0) was determined by averaging 10 images (of 600) with no activity. F0 images from control experiments were used to generate ratios with images during drug treatments (i.e. create F/F0 files). Ratio images were then constructed and replayed for careful examination to detect active areas where sudden increases in F/F0 occurred. F/F0 vs time traces were further analyzed in Microcal Origin (Microcal Software, Inc., Northampton, MA, U.S.A) and AcqKnowledge Software (Biopac Systems Inc., Santa Barbara, CA, U.S.A.), and represent the averaged F/F0 from a box region of 2.2 × 2.2 μm centered in an active area of interest to achieve the fastest and sharpest changes. Fluorescence records from single colonic myocytes were composed of Ca2+ transients of multiple characteristics (i.e. single Ca2+ puffs, clusters of puffs, and Ca2+ waves). In many cells, especially after stimulation with neurokinins, it was impossible to make measurements on single, discrete Ca2+ puffs. Therefore, as a measure of the Ca2+ released during the 20 s sampling periods, we integrated the area of signals above baseline fluorescence. This measurement incorporates both the amplitude and duration of Ca2+ transients. The amplitude and duration of the Ca2+ transients are both important parameters because either an increase in the amplitude or the duration of Ca2+ transients may cause more openings of Ca2+-activated K+ channels. Therefore, it is likely that the fluorescence integrals are a better representation of the elevation in local Ca2+ for the purposes of this study.
In fluorescence experiments, Ca2+ transients were compared before and 5 min after addition of the neurokinin agonists. Thus, it was not possible to determine the time course of the effects observed. In electrophysiological experiments, we were able to monitor responses as they developed. We did not notice significant differences in the latencies between addition of 10−10 and 10−6 M SP to the recording chamber and the development of responses (e.g. half-maximal changes in spontaneous transient outward currents were noted at 90.2±5.2 s and 103±6.4 s, respectively, after adding SP to the bath). It should be noted that since the time courses of responses were tabulated from the point of addition of the drug to the bath, the latencies in the development of the responses may be overestimated.
Statistical analysis
Results are expressed as means±s.e. where applicable. Statistical analysis was made with SigmaStat 2.03 software (Jandel Scientific Software, San Rafael, CA, U.S.A.). ANOVA on Ranks test was used to compare average I–V relations before and after application of test compounds. STOC amplitudes were measured using the Mini Analysis Program (Synaptosoft, Leonia, NJ, U.S.A.) with a threshold for detection set at 15 pA. The distributions of STOC amplitude were strongly skewed, resembling those of single channel dwell times or survival curves. Accordingly, we have illustrated changes in STOC amplitudes in control and test conditions as cumulative distributions where the y-axis is the fraction of STOCs of amplitude greater than the pA value on the x-axis (Bayguinov et al., 2001a). For statistical analysis of STOC amplitudes, we calculated the mean STOC amplitude observed in each cell during control and test conditions, and compared the means from all cells tested in a paired t-test and a log-rank test, a powerful method for determining if one group has a tendency towards larger values than another group (McGehee & Oxford, 1991). Both tests gave equivalent results. In the text we have reported P-values from the log-rank tests with ‘n' representing the number of cells in each experiment.
Results
Effect of SP on spontaneous Ca2+ puffs and STOCs in murine colonic myocytes
Colonic myocytes loaded with fluo-4 AM generated spontaneous elevations in intracellular Ca2+ ([Ca2+]i) that occurred either as highly localized events (Ca2+ puffs) or more widely spreading Ca2+ waves, as previously reported (Bayguinov et al., 2000). Imaging of cells under whole-cell voltage clamp conditions demonstrated that Ca2+ puffs were associated with STOCs, as shown previously (Bayguinov et al., 2000, 2001a, 2001b).
We tested the effects of SP on Ca2+ puffs and STOCs in murine colonic myocytes. In low concentrations (10−11–10−10 M) SP increased Ca2+ puffs and increased the frequency and amplitude of STOCs (Figure 1). The greatest increase was observed with 10−10 M SP (135±10% of control area, P<0.05). Higher concentrations of SP (10−8–10−6 M) reduced the areas of Ca2+ puffs and reduced STOC frequency and amplitude. For example, at 10−6 M, SP reduced Ca2+ puffs to 55±12% (n=18, P<0.001) of control activity. The reduction of Ca2+ puffs was accompanied by a significant tonic increase in F/F0 to 128±7% of control (n=18, P<0.001), indicating an increase in basal Ca2+.
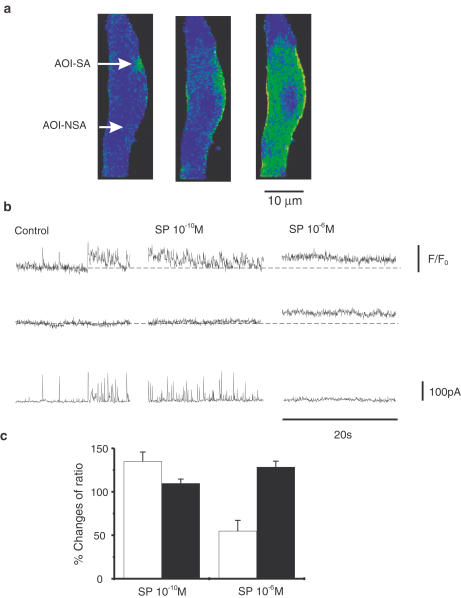
Figure 1.
Effects of SP on basal Ca2+, localized Ca2+ transients and STOCs. (a) Cell loaded with Fluo-4. Areas of interest AOI-NSA (not spontaneously active) and AOI-SA (spontaneously active) are denoted by arrows. (b) Ca2+ transients during 20 s scans from the two AOI (top traces in each segment are from AOI-SA; middle traces in each segment are AOI-NSA) depicted in (a) and STOCs (bottom traces in each segment) associated the Ca2+ transients. At the beginning of the control trace, discrete Ca2+ puffs associated with STOCs were observed, but late in the scan, a small Ca2+ wave occurred that was associated with a cluster of STOCs. SP (10−10 M) increased Ca2+ puffs and STOCs with no change in basal Ca2+. SP (10−6 M) increased basal Ca2+ and reduced Ca2+ puffs and STOCs. (c) Data from eight experiments. Open bars show changes in Ca2+ transients (in AOI-SA) in relation to control records, and closed bars show changes in basal Ca2+ (in AOI-NSA) in relation to control.
Parallel effects of SP were noted on STOCs. SP at 10−10 M increased STOC amplitude (n=6, P<0.005; Figure 2a,b and d) and frequency (i.e. to 316.6±64.1% above control frequency; Figure 2b). At 10−6 M, SP strongly reduced STOC amplitude (n=6, P<0.0001; Figure 2a,c and e) and frequency to 36.4±6.5% of control (n=6, P<0.05; Figure 2c). There was no significant desensitization of responses to either 10−10 M or 10−6 M SP during the 7 min exposure periods (e.g. comparing responses after 3 min with responses after 6–7 min yielded P-values of 0.45 and 0.69 for 10−10 and 10−6 M SP, respectively).
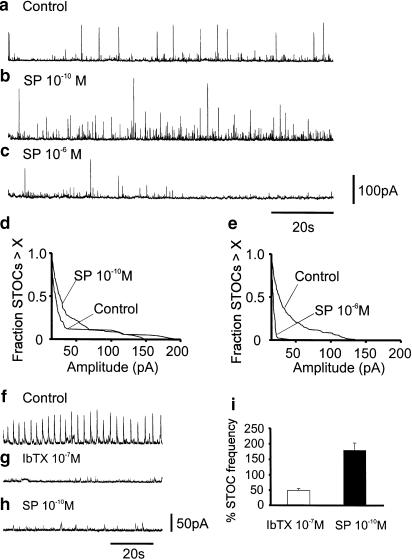
Figure 2.
Effects of SP on STOCs. Extended whole-cell voltage-clamp records before (a) and after application of SP (b,c). SP (10−10 M) increased STOC frequency and amplitude (b). SP (10−6 M) reduced STOC frequency and amplitude (c). (d,e) Survival curves summarizing the effects of SP on STOC amplitude (n=6). Note the increase in response to 10−10 M and the significant decrease in STOCs after 10−6 M. Pre-treatment with IbTX (f,h) reduced STOCs due to blockade of BK channels that contribute to these events in murine colonic myocytes (Bayguinov et al., 2000). Addition of SP (10−10 M) increased STOCs in the presence of IbTX due to activation of SK channels. (i) Effects of IbTX and SP (10−10 M) in the presence of IbTX in eight experiments.
STOCs in murine colonic myocytes result from activation of large conductance Ca2+-activated K+ channels (BK) and small conductance Ca2+-activated K+ channels (SK) (Kong et al., 2000). Pretreatment with BK channel blockers, charybdotoxin or iberiotoxin, did not prevent the activating effect of SP (10−10 M) on STOC activity. For example, pre-treatment with iberiotoxin (10−7 M) reduced STOCs to 49.8±5.4% of control (n=8, P<0.0005). Addition of SP (10−10 M) in the presence of iberiotoxin increased STOC frequency to 179.8±23.5% of the level after iberiotoxin (n=8, P<0.05; Figure 2f–h).
Influence of Ca2+ channel blockers on action of SP
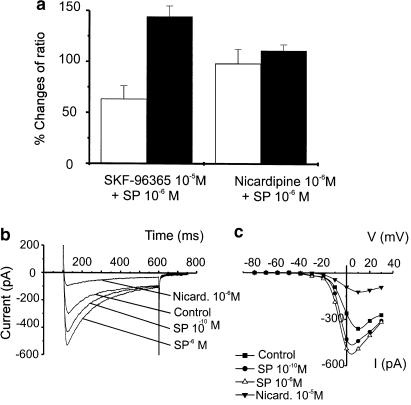
We reported previously that pretreatment of murine colonic myocytes with SKF-96365, a blocker of receptor-operated Ca2+ channels, prevented the rise in basal Ca2+ caused by ACh. In the present experiments, SKF-96365 did not prevent the rise in basal Ca2+ (i.e. F/F0 increased to 144.3±10.4% of control, n=6, p=0.005; Figure 3a) or the reduction in Ca2+ puffs (to 63.3±13.0% of control area, n=6; P<0.05) caused by 10−6 M SP. In contrast, nicardipine (10−6 M) prevented both the initial increase in puffs with SP (10−10 M) and the rise in basal [Ca2+]i and inhibition of puffs with 10−6 M SP (Figure 3a). These data suggest that the effects of SP require the function of L-type Ca2+ channels.
Figure 3.
Increase of basal Ca2+ caused by SP is a result of activation of L-type Ca2+ current. (a) Comparison of the effects of pretreatment with SKF-96365 (10−5 M; blocker of receptor operated Ca2+ channels) and nicardipine (10−6 M; L-type Ca2+ channel blocker). SKF-96365 did not prevent the effects of SP (10−6 M) on Ca2+ puffs or basal Ca2+ (open bars show changes in Ca2+ transients in relation to control records, and closed bars show changes in basal Ca2+ in relation to control). Nicardipine blocked the effects of SP. (b) Inward currents activated in a colonic myocyte in response to depolarization from –80 to 0 mV in control conditions and after addition of SP (10−10 M), SP (10−6 M), and nicardipine (10−6 M) in the continued presence of SP (10−6 M) in the same cell. Small residual current after treatment with nicardipine is a voltage-dependent non-selective cation current that has been characterized previously (see Koh et al., 2001). (c) I/V curves demonstrating the increase in inward current after application of SP and reduction in inward currents after application of nicardipine. The current remaining after addition of nicardipine in (b,c) likely to be the dihydropyridine-insensitive, voltage-dependent nonselective cation current previously described in murine colonic myocytes (Koh et al., 2001). Nicardipine blocked the inward current activated by SP.
We tested the effects of SP on inward currents of colonic cells using the whole cell patch clamp technique. Cells were held at −80 mV and stepped to potentials ranging from −80 to +30 mV for 500 ms (in 10 mV increments) every 20 s. This protocol was repeated before and after application of SP (10−10 and 10−6 M; Figure 3b). Nicardipine (10−6 M) reduced both the voltage-dependent inward current activated under control conditions and the current activated by SP. Figure 3C shows I/V curves summarizing the increase in Ca2+ current caused by SP and the inhibition of this current by nicardipine.
Previous experiments on canine colonic myocytes have observed an increase in holding current in response to SP that was due to activation of a nonselective cation current (Lee et al., 1995). In the present series of experiments, application of 10−10 M SP at a holding potential of −80 mV changed the holding current from –21.6±3.8 to −25.8±2.8 pA (P>0.1), and 10−6 M SP changed holding current to –38.8±3.0 pA (n=6; P<0.005). These observations suggest that activation of a nonselective cation current is a rather minor component of the response of murine colonic myocytes to SP, and the properties of the small inward current activated by 10−6 M SP were not investigated.
Activation of both NK1 and NK2 receptors is necessary for excitatory effect of SP
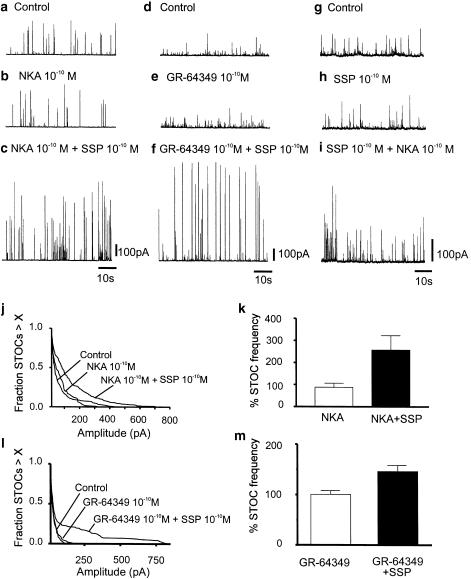
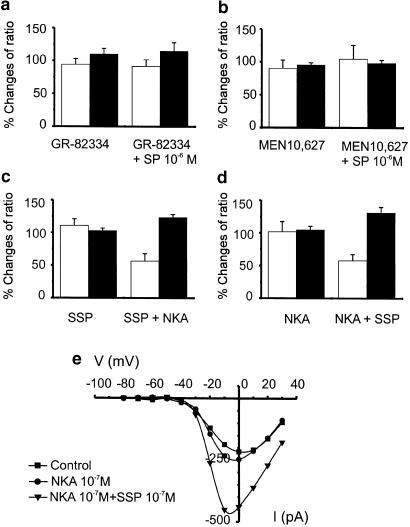
Both NK1 and NK2 receptors are expressed in GI smooth muscles (Burcher, 1989). Stimulation of murine colonic myocytes with the NK2 agonists, NKA (10−10 M) or GR-64349 (10−10 M), had no effect on STOC amplitude (P=0.54, n=5, and P=0.44, n=4, respectively) or frequency (P=0.29, n=5; and P=0.49, n=4, respectively) (Figure 4a & b and d & e). Similarly, treatment with the NK1 agonist SSP (10−10 M) had no effect on STOC amplitude (P=0.61) or frequency (P=0.37) (Figure 4g and h). Combination of NKA (10−10 M) or GR-64349 (10−10 M) and SSP (10−10 M), however, elicited effects equivalent to SP (10−10 M; Figure 4c, f and i). Data from five experiments with NKA and 4 experiments with GR-64349 are summarized in Figure 4j–m (P=0.025 for frequency and P<0.001 for amplitude with NKA and p=0.039 for frequency and P<0.0001 for amplitude with GR-64349).
Figure 4.
Simultaneous activation of NK1 and NK2 receptors increases STOC amplitude and frequency. Application of NK2 agonists NKA (10−10 M) (b,c) or.33 GR-64349 (10−10 M) (d,e) or NK1 agonist SSP (10−10 M) (g,h) did not significantly affect STOC amplitude and frequency. Combination of NK1 with NK2 agonists (c,f,i) mimicked the effect of SP (10−10 M) and increased STOC amplitude and frequency. (j,l) Survival curves summarizing the lack of effects of NKA and GR-64349 on STOC amplitude (P=0.54 and 0.44, respectively) and the increase in STOC amplitude following addition of SSP in both cases (P<0.001, n=5 and P<0.0001, n=4, respectively). (k) Effects of NKA alone (P=0.29) and in combination with SSP (P=0.025; n=5) on STOC frequency. (m) Effects of GR-64349 alone (P=0.49) and in combination with SSP (P=0.039; n=4) on STOC frequency.
Pretreatment of murine colonic myocytes with the NK1 antagonist, GR-82334 (10−7 M), did not significantly affect basal Ca2+ or spontaneous Ca2+ puffs (P>0.1; see Figure 5a); however, this compound blocked the increase in basal Ca2+ (P>0.1) and the reduction in Ca2+ puffs (P>0.1) caused by SP (10−6 M; Figure 5a). Pretreatment with the NK2 antagonist, MEN 10,627 (10−7 M), did not significantly affect spontaneous Ca2+ puffs (P>0.05) or basal Ca2+ (Figure 5b), but the NK2 antagonist also blocked the increase in basal Ca2+ (P>0.5) and the reduction in Ca2+ puffs (P>0.1) caused by 10−6 M SP (Figure 5b). These compounds also blocked the reduction in STOCs caused by 10−6 M SP under voltage-clamp conditions (data not shown).
Figure 5.
Effects of SP require simultaneous activation of NK1 and NK2 receptors. Pretreatment with either the NK1 antagonist, GR-82334 (10−7 M; (a), or the NK2 antagonist, MEN 10,627 (10−7 M; b), abolished the effects of SP on Ca2+ transients (open bars) and basal Ca2+ (closed bars) in relation to control. Application of NK1 agonist SSP (10−7 M) did not significantly affect spontaneous Ca2+ transients or basal Ca2+ level (c). Application of NK2 agonist, NKA (10−7 M) in the continued presence of SSP reduced Ca2+ puffs and increased basal Ca2+. Thus, combination of these agonists mimicked the effects of SP. (d) Effects when the order of application of NK1 and NK2 agonists was reversed. (e) Effects of SSP and NKA on inward currents. The I/V curves show that there was little or no effect on voltage-dependent inward currents after application of NKA (10−7 M), but the current was greatly enhanced by subsequent addition of SSP (10−7 M) in the continued presence of NKA. Thus, combination of NK1 and NK2 agonists mimicked the effects of SP on inward current.
Application of NK1 agonist SSP (10−7 or 10−6 M) did not significantly affect spontaneous Ca2+ puffs (both P>0.1) or change basal Ca2+ (both P>0.1). Addition of the NK2 agonist NKA (10−7 M), in the continued presence of SSP, however, reduced Ca2+ puffs to 56.7±1.2% of control (P<0.01, n=6) and increased basal F/F0 to 123.7±4.4% of control (P<0.005, n=6) (Figure 5c). The NK1 and NK2 agonists were also tested sequentially in the opposite order. NKA (10−7 M or 10−6 M) did not significantly affect basal F/F0 (i.e. 104.8±5.6 % of control) or spontaneous Ca2+ puffs (i.e. 101.9±15.5% of control; both P>0.1, n=6). Similar effects were noted with GR-64349; GR-64349 (10−6 M) did not significantly affect basal F/F0 (i.e. 101.3 ± 8.0 % of control; P=0.99) or spontaneous Ca2+ puffs (i.e. 83.5±5.4% of control; P>0.6, n=5). Addition of SSP (10−7 M), in the continued presence of NKA or GR-64349, reduced Ca2+ puffs to 58±9.3% (P<0.005, n=6) and 43.9±7.6% (P=0.007) and increased F/F0 to 131.65±8.2% (P<0.01, n=6; Figure 5d) and 145±17% of control P=0.045; n=5), respectively.
We also tested the effects of the NK1 and NK2 agonists on voltage-dependent inward currents in voltage-clamp experiments. Application of NKA (10−7 M) or SSP (10−7 M) had no significant effect on the magnitude of the inward current, but subsequent addition of the second agonist caused a significant increase in voltage-dependent inward current. I–V curves after application of NKA and after NKA and SSP are shown in Figure 5e. These data demonstrate that combination of NK1 and NK2 agonists is necessary to mimic the increase in L-type Ca2+ current caused by SP.
Intracellular mechanisms of SP action
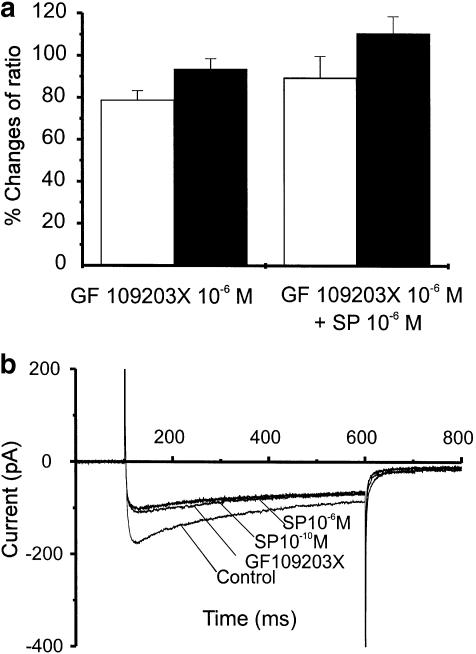
NK1 and NK2 receptors are coupled via Gq/11 to phospholipase Cβ (PLC) and synthesis of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). DAG has been reported to enhance voltage-dependent Ca2+ channels via activation of protein kinase C (PKC) in vascular smooth muscles (Mironneau & Macrez-Lepretre, 1995). Thus, we tested inhibitors of PKC on the stimulation of Ca2+ currents, inhibition of spontaneous Ca2+ transients, and increase in basal Ca2+ caused by SP (10−6 M). We previously reported that the PKC inhibitor GF 109203X did not significantly affect spontaneous Ca2+ transients or basal Ca2+ (Bayguinov et al., 2001a). In the present series of cells exposed to GF 109203X (10−6 M), we noted a small, but significant, decrease in spontaneous Ca2+ transients (i.e. 78.9±4.4% of control, n=6 P<0.05; Figure 6a). This compound had no significant effect on basal F/F0 (93.7±5% of control, n=6; P>0.1; Figure 6a). However, in the presence of GF 109203X, SP (10−6 M) did not significantly affect spontaneous Ca2+ transients (89.7±10% of control, n=6, P>0.1) or F/F0 (110±8% of control, n=6, P>0.1; Figure 6a). When cells were studied under voltage-clamp condition, GF 109203X reduced peak voltage-dependent inward current to 28.9±3.3% of control (P<0.05, n=4), and addition of 10−10 or 10−6 M SP in the presence of GF 109203X did not affect the inward current (Figure 6b). These data suggest that L-type Ca2+ currents are enhanced under basal conditions by PKC in murine colonic myocytes, and blockade of this pathway inhibited the increase in this conductance caused by SP.
Figure 6.
PKC participates in activation of L-type currents by SP. (a): Pre-treatment with GF 109203X (10−6 M) slightly reduced spontaneous Ca2+ transients (open bars) and had no effect on basal Ca2+ (solid bars) in relation to control. After GF 109203X, SP had no significant effect. (b) Raw data from stepping a cell from −80 to 0 mV. GF 109203X alone significantly reduced inward currents, and in the presence of GF 109203X, SP (10−10 or 10−6 M) did not enhance inward current.
Role of ryanodine receptors in mediating responses to SP
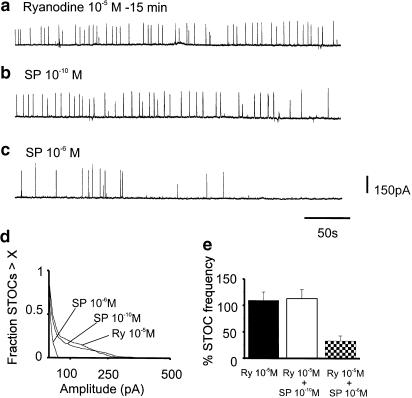
We have shown in previous studies that ryanodine receptors do not directly participate in the generation of spontaneous Ca2+ transients and STOCs in murine colonic myocytes (Bayguinov et al., 2000). In the same study, we found that ryanodine (10−5 M) blocked amplification of Ca2+ transients and STOCs caused by ATP. In the present study, we considered whether the amplification of Ca2+ transients and STOCs by SP involved recruitment of Ca2+ release from ryanodine receptors. As previously observed, pretreatment of cells with ryanodine (10−5 M) had no significant effect on spontaneous Ca2+ transients. However, ryanodine blocked the increase in Ca2+ transients in response to SP (10−10 M; after SP Ca2+ transients were 86.3±7.0% of control; n=6, P>0.05). In the presence of ryanodine, SP (10−6 M) increased basal Ca2+ to 152.5 ± 4.2% of control (n=6, P<0.001) and reduced Ca2+ puffs to 59.2±10.4% of control (n=6, P<0.01). Pretreatment with ryanodine had no effect on STOCs under control conditions, but this compound prevented the increase in STOCs caused by SP (10−10 M; Figure 7a–b, d–e). Ryanodine, however, did not block the reduction in STOCs caused by SP (10−6 M; Figure 7c–e).
Figure 7.
Participation of ryanodine receptors in regulation of Ca2+ puffs and STOCs by SP. Ryanodine had no effect on STOCs in murine colonic myocytes (a), but pre-treatment with ryanodine blocked the increase in STOCs caused by SP (10−10 M; (b). Ryanodine did not block the reduction in STOCs caused by higher concentrations of SP (10−6 M) (c); see text for details). (d,e) Summary of the effects of ryanodine on STOC amplitude (d) and frequency (e) in five experiments.
Effects of SP on membrane potentials in intact colonic muscles and isolated myocytes
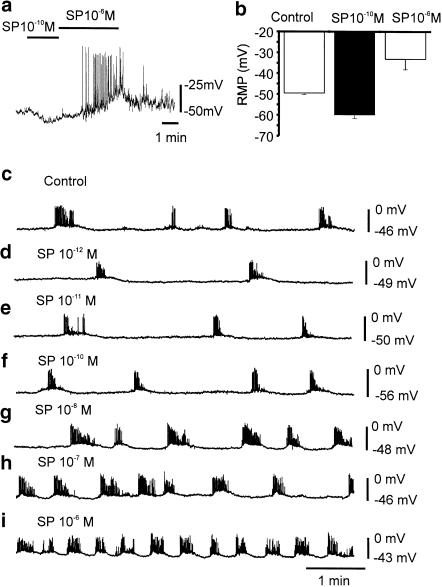
Our data predict a biphasic membrane potential response to SP in colonic muscles and myocytes. Therefore, we tested low and high concentrations of SP to determine whether its effects on Ca2+ transients and STOCs translate into integrated membrane potential responses. In isolated myocytes, membrane potentials averaged −49±0.8 mV after application of small holding currents (see the section Methods). Application of SP (10−10 M) hyperpolarized membrane potential to −60±1.7 mV (n=5, P<0.05). Increasing SP to 10−6 M depolarized cells to −33±5 mV (n=5, P<0.05) and induced repetitive spiking (Figure 8a). Figure 8b summarizes the effects of SP (10−10 and 10−6 M) on resting potential.
Figure 8.
Effects of SP on the membrane potentials of murine colonic smooth muscle cells and intact muscles. In isolated myocytes under current clamp, SP (10−10 M; black bar) caused hyperpolerization of membrane potential. Raising SP to 10−6 M caused depolarisation and generation of action potentials (a). (b) Changes in membrane potentials of five cells in response to SP (10−10 and 10−6 M). SP had similar effect on intact muscles. From 10−12 to 10−10 M SP caused hyperpolarization of membrane potentials. As the concentration of SP was raised above 10−8 M, however, this trend reversed and membrane potential depolarized (c–i). The frequency of action potential clusters decreased and increased with the changes in membrane potential caused by SP.
The effects of SP were also tested on intact colonic muscles. After impalement of circular muscle cells, SP (10−10 M) caused hyperpolarization of membrane potential from –49.8±1.7 to –56.4±1.6 mV (at approximately the second minute of the response) and to −56.0±1.5 (after the fifth minute; n=5; P<0.05 comparing control with maximum response, and P=0.89 between time points during response). After washout of SP for 5 min and restoration of control membrane potential, application of SP (10−6 M) depolarized membrane potential from –50.2±1.8 to –37.2±2.2 mV (at approximately the second minute of the response) and to −36.6±2.3 (after the fifth minute; n=5; P<0.005 comparing control with maximum response, and P=0.86 between time points during response). In two experiments we were able to maintain impalements of single cells during exposures to a range of SP concentrations (10−12–10−6 M). After each concentration of SP tested, the muscle was perfused with KRB for 5 min before testing the next dose. An example of one of these experiments is shown in Figure 8c–i.
Discussion
In the present study, we found that low concentrations of SP (i.e. 10−10 M) enhanced Ca2+ transients and STOCs in murine colonic myocytes. This suggests that a pure response of colonic muscles to SP at low concentrations would be hyperpolarization. When this hypothesis was tested on isolated colonic myocytes and intact muscles, we found that SP (10−12–10−10 M) hyperpolarized the muscle cells (see Figure 7). In situ it is unlikely that stimulation of excitatory motor neurons would ever result in a situation in which SP is released in isolation of other transmitters, and the hyperpolarizing phase of SP responses may be masked by superimposed cholinergic responses. At higher concentrations, SP increased basal cytoplasmic Ca2+ concentration by increasing Ca2+ influx through L-type Ca2+ channels. We have previously demonstrated that enhanced basal cytoplasmic Ca2+, which also occurs during muscarinic stimulation, inhibits Ca2+ puffs and STOCs (Bayguinov et al., 2001b). Thus, at higher concentrations, SP and muscarinic stimulation lead to a common response in colonic myocytes, albeit by different mechanisms.
The range of responses to SP (i.e. from increased Ca2+ transients and STOCs at low concentrations to increased basal Ca2+ and inhibition of Ca2+ transients and STOCs at high concentrations) can all be attributed to effects on L-type Ca2+ current that are mediated by activation of PKC. At low concentrations, the enhancement of Ca2+ transients appeared to involve increased Ca2+ entry and recruitment of ryanodine receptors. Ryanodine receptors in the sarcoplasmic reticulum are closely apposed to dihydropyridine-sensitive (L-type) Ca2+ channels in the plasma membranes of smooth muscles (Carrington et al., 1995). Ryanodine receptors are activated by cytoplasmic Ca2+ (i.e. Ca2+-induced Ca2+ release; Coronado et al., 1994). When colonic smooth muscle cells are stimulated with SP, Ca2+ entry through L-type Ca2+ channels may summate with Ca2+ released from IP3 receptors and cause Ca2+-induced Ca2+ release from ryanodine receptors. It appears that increased IP3 production, which might also result from SP (10−10 M) stimulation, was not sufficient to significantly increase IP3 receptor-operated Ca2+ release since ryanodine blocked the increase in Ca2+ transients in response to SP.
The enhancement in Ca2+ transients by SP (10−10 M) coupled to activation of Ca2+-activated K+ channels (BK and SK; Bayguinov et al., 2000). As expected, activation of K+ conductances by low concentrations of SP resulted in membrane hyperpolarization in isolated myocytes and intact colonic muscles. At higher SP concentrations, increased dihydropyridine-sensitive Ca2+ current increased basal Ca2+, inhibited Ca2+ puffs, and abolished STOCs. The outward current activated by low concentrations of SP was apparently exceeded by inward currents when the cells were stimulated by 10−6 M SP, because membrane potential depolarized. Experiments in which intact muscles were stimulated by a range of SP concentrations suggest that 10−7 M is the ‘cross-over' concentration at which the hyperpolarizing trend of SP gives way to depolarization (see Figure 8).
Responses to SP required parallel stimulation of both NK1 and NK2 receptors. Neither NK1-specific nor NK2-specific agonists were effective in eliciting responses equivalent to SP, but combination of NK1 and NK2 agonists mimicked the effects of SP. Consistent with this observation, both NK1- and NK2-specific antagonists blocked responses to SP. Both NK1 and NK2 receptors are coupled through pertussis toxin-insensitive G proteins (Gq/G11) to phosphoinositide metabolism by activation of phospholipase Cβ (PLCβ; Khawaja & Rogers, 1996). DAG, produced by activation of PLCβ, activates PKC and increases L-type Ca2+ current. It is unclear at the present time why activation of both receptors is necessary. Parallel activation of NK1 and NK2 receptors may not be a common requirement in all colonic muscles. For example, in human colonic muscles, contractile responses to SP were inhibited by an NK2 receptor antagonist (SR48968), but not by an NK1 antagonist CP99994 (Liu et al., 2002). Thus, NK2 receptors alone were sufficient to elicit responses to SP in the human colon.
Previous studies of the effects of SP and NKA on colonic muscles and cells have revealed a variety of pathways linked to NK1 and NK2 receptors. For example, the contractile responses of guinea pig colonic muscles to NK1 receptor stimulation were greatly attenuated by nifedipine, but responses to an NK2-specific agonist were minimally affected (Zagorodnyuk et al., 1994). In rabbit longitudinal colonic myocytes, SP enhanced L-type Ca2+ current (Mayer et al., 1990), but in canine colonic muscles, SP and NKA suppressed dihydropyridine-sensitive Ca2+ current (Lee et al., 1995). In canine myocytes, neurokinins activated a nonselective cation conductance (Lee et al., 1995). In the present study, using perforated patched cells to preserve intracellular second messenger pathways, SP or a combination of NK1 and NK2 agonists increased dihydropyridine-sensitive Ca2+ current. These compounds activated only a minor nonselective cation conductance (which was apparent under the conditions of our experiments as a small increase in holding current between test potentials). These data suggest that a variety of pathways are activated by neurokinins, and there may be significant interspecies differences in the mechanisms of action of these peptides.
Production of DAG enhances the activity of PKC (Liu & Heckman, 1998), and activation of PKC has previously been shown to enhance L-type Ca2+ currents in a variety of smooth muscle cells (for review see Keef et al., 2001). SP increased nicardipine-sensitive Ca2+ current in the present study. The PKC inhibitor, GF 109203X, reduced peak Ca2+ current to about 30% of the control level, suggesting ongoing stimulation of Ca2+ current by PKC in colonic cells. Addition of SP, 10−10 or 10−6 M, did not enhance Ca2+ current in the presence of the PKC inhibitor. Thus, it is likely that enhancement of L-type Ca2+ current in response to SP was due to DAG stimulation of PKC.
Low levels of stimulation of enteric excitatory motor neurons (even single pulses) produce atropine-sensitive postjunctional effects in GI muscles. Higher stimulus frequencies recruit the release of excitatory peptides (Holzer, 1984), and thus at some levels of neural firing, both ACh and neurokinins would be expected to be released. Thus, the question might arise about how regulation of Ca2+ puffs by muscarinic stimulation (Bayguinov et al., 2001b) integrates with the mechanisms described in the present study. There are reports of synergism between muscarinic and neurokinin receptor stimulation in GI muscles (e.g. Holzer & Maggi, 1994). Of course to fully understand the integrated responses of two or more transmitters (i.e. to produce a realistic model), one needs to know more than the basic characteristics of the responses to the individual transmitters. Transmitter concentrations at postjunctional receptors as a function of time must be evaluated, and responses to transmitter combinations over the physiological concentration range must be determined. Currently, spatio-temporal profiles of transmitter concentrations within GI muscles are not available, so it is hard to perform realistic experiments to investigate responses to multiple transmitter stimulation.
To further complicate our understanding of the integrated response to SP and ACh, recent work has suggested that cholinergic responses in GI muscles may be largely mediated through actions on intramuscular interstitial cells of Cajal (IC-IM; see Ward et al., 2000; Beckett et al., 2002). It appears that very little of the ACh released from motor nerve terminals in situ escapes the tight, synaptic-like junctions between enteric nerve varicosities and IC-IM due to rapid metabolism of the ACh released from nerve terminals (Ward et al., 2000). Although, IC-IM (also referred to as interstitial cells of Cajal of the deep muscular plexus or IC-DMP in the small intestine) express neurokinin (NK1) receptors (cf. Grady et al., 1996; Lavin et al., 1998; Vannucchi et al., 2000; Epperson et al., 2000; Rettenbacher & Reubi, 2001) and may mediate part of the post functional response to neurally released neurokinins in normal tissues, a substantial, atropine-resistant contractile capacity remains in GI tissues lacking IC-IM (Beckett et al., 2002). For example, atropine-resistant, neurally mediated contractile responses of the murine fundus were not significantly different in wild-type and Sl/Sld mice that lack IC-IM (Beckett et al., 2002). Thus, a significant portion of the peptide-dependent, postjunctional response in GI muscles may be due to stimulation of receptors on smooth muscle cells, and integration of cholinergic and peptidergic postjunctional responses may result from a summation of responses in interstitial cells of Cajal and smooth muscle cells. In guinea pig taenia coli muscles, close relationships between interstitial cells of Cajal and nerve varicosities are not as apparent as in some GI muscles (e.g. Gabella, 2001). In these tissues, it is likely that direct stimulation of muscle fibers by excitatory neurotransmitters is prevalent.
In summary, neurokinins regulate Ca2+ puffs and STOCs in murine colonic myocytes by PKC-dependent regulation of L-type Ca2+ current. Parallel activation of NK1 and NK2 receptors is necessary for these effects. At low levels of SP, Ca2+ transients were enhanced due to recruitment of Ca2+ release from ryanodine receptors. The increase in localized Ca2+ transients enhanced STOCs and hyperpolarized colonic muscles. At higher levels of SP, basal Ca2+ increased and this, as previously shown, inhibited Ca2+ transients and STOCs. Thus, at high SP levels the hyperpolarizing effects of SP were blocked, and responses became purely excitatory. The responses to high SP concentrations are equivalent to and would function in parallel with cholinergic stimulation. Regulation of spontaneous Ca2+ transients by SP and the mechanisms we have described in the present study linking stimulation of neurokinin receptors to membrane currents represent novel pathways activated by excitatory neuropeptides in visceral smooth muscles. These mechanisms may contribute to the neural regulation of GI muscles.
Acknowledgments
This study was supported by a Program Project Grant from NIDDK – DK41315. The Noran Confocal microscope was purchased by a shared equipment grant from the NIH - HL44455. We are grateful for technical support from Salah Abu Baker in performing the intracellular experiments on intact colonic muscles and to Dr James Kenyon for critical reading of the manuscript.
Abbreviations
- BK channels
Large conductance Ca2+-activated K+ channels
- DAG
diacylglycerol
- GI
gastrointestinal
- IC-IM
intramuscular interstitial cells of Cajal
- IP3
inositol 1*4*5-trisphosphate
- NKA
neurokinin A
- PKC
protein kinase C
- PLC
phospholipase C
- SK channels
small conductance Ca2−-activated K+ channels
- SP
substance P
- SSP
[Sar9, Met(O2)11]-substance P
- STOCs
spontaneous transient outward currents.
References
- BAYGUINOV O., HAGEN B., BONEV A.D., NELSON M.T., SANDERS K.M. Intracellular calcium events activated by ATP in murine colonic myocytes. Am. J. Physiol. Cell Physiol. 2000;279:C126–C135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- BAYGUINOV O., HAGEN B., KENYON J.L., SANDERS K.M. Coupling strength between localized Ca(2+) transients and K(+) channels is regulated by protein kinase C. Am. J. Physiol. Cell. Physiol. 2001a;281:C1512–C1523. doi: 10.1152/ajpcell.2001.281.5.C1512. [DOI] [PubMed] [Google Scholar]
- BAYGUINOV O., HAGEN B., SANDERS K.M. Muscarinic stimulation increases basal Ca2+ and inhibits spontaneous Ca2+ transients in murine colonic myocytes. Am. J. Physiol. Cell Physiol. 2001b;280:C689–C700. doi: 10.1152/ajpcell.2001.280.3.C689. [DOI] [PubMed] [Google Scholar]
- BECKETT E.A.H, HORIGUCHI K, KHOYI M., SANDERS K.M., WARD S.M. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sld mice. J. Physiol. (Lond.) 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J. Physiol.(Lond.) 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKES S.J., STEELE P.A., COSTA M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42:863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- BURCHER E. The study of tachykinin receptors. Clin. Exp. Pharmacol. Physiol. 1989;16:539–543. doi: 10.1111/j.1440-1681.1989.tb01602.x. [DOI] [PubMed] [Google Scholar]
- CARRINGTON W.A., LYNCH R.M., MOORE E.D., ISENBERG G., FOGARTY K.E., FAY F.S. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science. 1995;268:1483–1487. doi: 10.1126/science.7770772. [DOI] [PubMed] [Google Scholar]
- CORONADO R., MORRISSETTE J., SUKHAREVA M., VAUGHAN D.M.Structure and function of ryanodine receptors Am. J. Physiol. Cell Physiol. 1994266C1485–C1504.(Part 1) [DOI] [PubMed] [Google Scholar]
- EPPERSON A., HATTON W.J., CALLAGHAN B., DOHERTY P., WALKER R.L., SANDERS K.M., WARD S.M., HOROWITZ B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am. J. Physiol. Cell Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- GABELLA G. Development and ageing of intestinal musculature and nerves: the guinea-pig taenia coli. J. Neurocytol. 2001;30:733–766. doi: 10.1023/a:1019660519961. [DOI] [PubMed] [Google Scholar]
- GORDIENKO D.V., BOLTON T.B., CANNELL M.B. Variability in spontaneous subcellular calcium release in guinea-pig ileum smooth muscle cells. J. Physiol. (Lond.) 1998;507:707–720. doi: 10.1111/j.1469-7793.1998.707bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADY E.F., BALUK P., BOHM S., GAMP P.D., WONG H., PAYAN D.G., ANSEL J., PORTBURY A.L., FURNESS J.B., MCDONALD D.M., BUNNETT N.W. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P. Characterization of the stimulus-induced release of immunoreactive substance P from the myenteric plexus of the guinea-pig small intestine. Brain Res. 1984;297:127–136. doi: 10.1016/0006-8993(84)90549-3. [DOI] [PubMed] [Google Scholar]
- HOLZER P., MAGGI C.A. Synergistic role of muscarinic acetylcholine and tachykinin NK-2 receptors in intestinal peristalsis. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:194–201. doi: 10.1007/BF00169837. [DOI] [PubMed] [Google Scholar]
- KEEF K.D, HUME J.R., ZHONG J. Regulation of cardiac and smooth muscle Ca2+ channels (CaV1.2a, b) by protein kinases. Am. J. Physiol. Cell Physiol. 2001;281:C1743–C1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- KHAWAJA A.M., ROGERS D.F. Tachykinins: receptor to effector. Int. J. Biochem. Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- KOH S.D., MONAHAGN K., MASON H.S., KENYON J.L., SANDERS K.M. Novel voltage-dependent non-selective cation conductance in murine colonic myocytes. J. Physiol. (Lond.) 2001;533:341–355. doi: 10.1111/j.1469-7793.2001.0341a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG I.D., KOH S.D., SANDERS K.M. Purinergic activation of spontaneous transient outward currents in guinea pig taenia colonic myocytes. Am. J. Physiol. Cell Physiol. 2000;278:C352–C362. doi: 10.1152/ajpcell.2000.278.2.C352. [DOI] [PubMed] [Google Scholar]
- LAVIN S.T., SOUTHWELL B.R., MURPHY R., JENKINSON K.M., FURNESS J.B. Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochem. Cell Biol. 1998;110:263–271. doi: 10.1007/s004180050288. [DOI] [PubMed] [Google Scholar]
- LEE H.K., SHUTTLEWORTH C.W., SANDERS K.M.Tachykinins activate nonselective cation currents in canine colonic myocytes Am. J. Physiol. Cell Physiol. 1995269C1394–C1401.(Part 1) [DOI] [PubMed] [Google Scholar]
- LIU L., SHANG F., MARKUS I., BURCHER E. Roles of substance P receptors in human colon circular muscle: alterations in diverticular disease. J. Pharmacol. Exp. Ther. 2002;302:627–635. doi: 10.1124/jpet.102.034702. [DOI] [PubMed] [Google Scholar]
- LIU W.S., HECKMAN C.A. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- MAYER E.A., LOO D.D., SNAPE W.J. , Jr., SACHS G. The activation of calcium and calcium-activated potassium channels in mammalian colonic smooth muscle by substance P. J. Physiol. (Lond.) 1990;420:47–71. doi: 10.1113/jphysiol.1990.sp017901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGEHEE D.S., OXFORD G.S. Bradykinin modulates the electrophysiology of cultured rat sensory neurons through pertussis toxin-insensitive G protein. Mol. Cell. Neurosci. 1991;2:21–30. doi: 10.1016/1044-7431(91)90036-n. [DOI] [PubMed] [Google Scholar]
- MIRONNEAU J., MACREZ-LEPRETRE N. Modulation of Ca2+ channels by alpha 1A- and alpha 2A-adrenoceptors in vascular myocytes: involvement of different transduction pathways. Cell Signal. 1995;7:471–479. doi: 10.1016/0898-6568(95)00014-g. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., CHENG H., RUBART M., SANTANA L.F., BONEV A.D., KNOT H.J., LEDERER W.J. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- PEREZ G.J., BONEV A.D., PATLAK J.B., NELSON M.T. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J. Gen. Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RETTENBACHER M., REUBI J.C. Localization and characterization of neuropeptide receptors in human colon. Naunyn Schmiedebergs Arch. Pharmacol. 2001;364:291–304. doi: 10.1007/s002100100454. [DOI] [PubMed] [Google Scholar]
- SCHMIDT P., POULSEN S.S., RASMUSSEN T.N., BERSANI M., HOLST J.J. Substance P and neurokinin A are codistributed and colocalized in the porcine gastrointestinal tract. Peptidesm. 1991;12:963–973. doi: 10.1016/0196-9781(91)90045-q. [DOI] [PubMed] [Google Scholar]
- VANNUCCHI M.G., FAUSSONE-PELLEGRINI M.S. NK1, NK2 and NK3 tachykinin receptor localization and tachykinin distribution in the ileum of the mouse. Anat. Embryol. (Berl.) 2000;202:247–255. doi: 10.1007/s004290000106. [DOI] [PubMed] [Google Scholar]
- WARD S.M., BECKETT E.A., WANG X., BAKER F., KHOYI M., SANDERS K.M. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAGORODNYUK V., SANTICIOLI P., MAGGI C.A. Different Ca2+ influx pathways mediate tachykinin receptor-induced contraction in circular muscle of guinea-pig colon. Eur. J. Pharmacol. 1994;255:9–15. doi: 10.1016/0014-2999(94)90076-0. [DOI] [PubMed] [Google Scholar]
- ZHUGE R., SIMS S.M., TUFT R.A., FOGARTY K.E., WALSH J.V., Jr Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J. Physiol. (Lond.) 1998;513:711–718. doi: 10.1111/j.1469-7793.1998.711ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]