Abstract
Extracellular nucleotides are the focus of increasing attention for their role as extracellular mediators since they are released into the extracellular environment in a regulated manner and/or as a consequence of cell damage.
Here, we show that human eosinophils stimulated with different nucleotides release eosinophil cationic protein (ECP) and the chemokine interleukin 8 (IL-8), and that release of these two proteins has a different nucleotide requirement.
Release of ECP was triggered in a dose-dependent manner by ATP, UTP and UDP, but not by 2′-&3′-o-(4-benzoyl-benzoyl)adenosine 5′-triphosphate (BzATP), ADP and α,β-methylene adenosine 5′ triphosphate (α,β-meATP). Release of IL-8 was triggered by UDP, ATP, α,β-meATP and BzATP, but not by UTP or ADP. Pretreatment with pertussis toxin abrogated nucleotide-stimulated ECP but not IL-8 release.
Release of IL-8 stimulated by BzATP was fully blocked by the P2X7 blocker KN-62, while release triggered by ATP was only partially inhibited. IL-8 secretion due to UDP was fully insensitive to KN-62 inhibition.
Priming of eosinophils with GM-CSF increased IL-8 secretion irrespectively of the nucleotide used as a stimulant.
It is concluded that extracellular nucleotides trigger secretion of ECP by stimulating a receptor of the P2Y subfamily (possibly P2Y2), while, on the contrary, nucleotide-stimulated secretion of IL-8 can be due to activation of both P2Y (P2Y6) and P2X (P2X1 and P2X7) receptors.
Keywords: Human eosinophils, extracellular ATP, P2 receptors, ECP, IL-8
Introduction
Eosinophils are terminally differentiated effector cells involved in the defensive response against parasites (Butterworth et al., 1975) as well as in the pathogenesis of allergic diseases and immunological disorders (Leung, 1999). Upon recruitment at the site of inflammation, they release a broad panel of different mediators that can provoke tissue damage and contribute to the pathophysiology of diseases such as asthma (Bousquet et al., 1990; Gleich, 1990), atopic dermatitis (Leung, 1999) and rheumatoid arthritis (Mertens et al., 1993). Among granule-associated mediators produced by these cells, eosinophil cationic protein (ECP) is one of the most investigated. It is a single-chain, zinc-containing protein with a molecular weight ranging from 16 to 22 kDa, depending on the glycosylation level. ECP is also named “ribonuclease 3” because of its ribonuclease activity and homology (Olsson & Venge, 1972; Olsson et al., 1977). It is used as a marker of eosinophil activation in diseases since its level correlates with eosinophil counts (Marguet et al., 2001; Pronk-Admiraal & Bartels, 2001). Cytotoxic or cytostatic effects of ECP have been widely described (Maeda et al., 2002). Recently, in vitro degradation of cytoskeletal proteins by ECP was also shown, suggesting a role for the protein in the pathogenesis of “eosinophilic myopathies” (Sugihara et al., 2001).
Eosinophils have been reported to synthesize and release different cytokines such as interleukin (IL)-1α (Weller et al., 1993), IL-3 (Kita et al., 1991), IL-5 (Dubucquoi et al., 1994), IL-8, (Kita et al., 1995; Simon et al., 1995), granulocyte/macrophage colony-stimulating factor (GM-CSF) (Kita et al., 1991), IL-16 and RANTES (Lim et al., 1996). An increased secretion of IL-8 has been described in eosinophils from patients with bronchial asthma or atopic dermatitis (Yousefi et al., 1995). Furthermore, IL-8 concentration in BAL fluids from asthmatic patients is significantly increased in comparison to that of healthy subjects (Yousefi et al., 1995). Since IL-8 is a chemotaxin for neutrophils and CD16+ NK cells (Teran et al., 1996; Campbell et al., 2001), the increased IL-8 level in BAL fluids from asthmatic patients suggests the involvement of IL-8 in amplification of the inflammatory reaction.
Nucleotides play a role not only inside but also outside the cell as they can be released through different mechanisms. Extracellular nucleotides exert their effects by stimulating two subfamilies of plasma membrane receptors named P2 receptors: the metabotropic G-protein-coupled P2Y and the ionotropic ligand-gated ion channels P2X (Dubyak & El-Moatassim, 1993; Ralevic & Burnstock, 1998; Di Virgilio et al., 2001). ATP binds to both subfamilies with high affinity, while ADP activates P2Y1 and P2Y12, UTP primarily interacts with P2Y2 and P2Y4, and UDP binds to the P2Y6 subtype (Von Kuegelgen & Wetter, 2000). In addition, α,β-meATP and BzATP preferentially activate P2X subtypes (Dubyak & El-Moatassim, 1993; Ralevic & Burnstock, 1998; North & Surprenant, 2000). It has been recently shown that human eosinophils express both P2Y and P2X receptor subtypes (Ferrari et al., 2000; Mohanty et al., 2001) whose stimulation induces intracellular Ca2+ transients, oxygen radical production and expression of the adhesion molecule CD11b (Dichmann et al., 2000; Ferrari et al., 2000; Idzko et al., 2001).
As ATP is released upon tissue damage and/or in response to inflammatory stimuli (Cook & Mccleskey, 2002) such as bacterial products (Ferrari et al., 1997) or salivary histatin 5 (Edgerton & Koshlukova, 2000), we focused our interest on the question whether stimulation of P2 receptors expressed by human eosinophils could induce the release of cytotoxic granular mediators such as the ECP and IL-8.
Methods
Reagents
ATP, UTP, UDP, ADP, BzATP, α,β-meATP, suramin, Ficoll, and Triton X-100, were obtained from Sigma (Deisenhofen, Germany); pertussis toxin from Calbiochem (La Jolla, CA, U.S.A.); the calcium indicator (1-[2-(5-carboxy-oxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2′-amino-5′-methyl-phenoxy)- ethane-N,N,N,N′-tetraacetic acid, pentaacetoxymethyl-ester) (Fura-2/AM) was obtained from Molecular Probes (Leiden, Netherlands); immunomagnetic beads (Dynabeads M-450) were purchased from Dianova (Hamburg, Germany).
Preparation of human eosinophils
Human eosinophil granulocytes from healthy nonatopic volunteers were isolated from heparin-treated (10 U ml−1) blood by Ficoll separation, and negatively selected with anti-CD16 antibody-coated Dynabeads. Eosinophil purity was ⩾96% as judged by Pappenheim staining. Viability of purified eosinophils was measured by trypan blue exclusion and was more than 98%.
Cell viability
Survival of cultured eosinophils was assessed by propidium iodide staining and FACS analysis of at least 5000 cells. Briefly, cells were washed once in PBS plus 2% FCS and resuspended in 200 μl of a propidium iodide solution (0.5 μg ml−1 dissolved in PBS).
Intracellular Ca2+ measurements
Ca2+ transients were measured in eosinophils loaded with the Ca2+ indicator Fura-2/AM (Calbiochem, La Jolla, CA, U.S.A.) by using the digital fluorescence microscope unit Attofluor (Zeiss, Oberkochen, Germany). Briefly, cells were incubated with 2 μM Fura-2/AM for 30 min at 37°C in a Ca2+- and Mg2+-free Hanks' BSA solution. Cells were then washed twice and finally resuspended in the same buffer containing 1.5 mM CaCl2 and MgCl2. Traces were followed fluorospectrometrically and Ca2+ transients were determined by multiple cell acquisitions with the 340/380nm wavelength excitation ratio at an emission wavelength of 505 nm. Curves shown are representative of the whole cell population.
Permeabilization to YO-PRO
Human eosinophils were incubated in a saline solution containing 10 μM YO-PRO and 250 μM sulfinpyrazone, in the presence or absence of 3 mM ATP for 30 min at 37°C. At the end of the incubation time, 5 mM MgSO4 was added. Cells were centrifuged and resuspended in a YO-PRO, ATP-free solution. Fluorescence intensity was then measured in a luminescence spectrometer. Data are expressed as means±s.e.m. of experiments performed in triplicate.
ECP and IL-8 determination
ECP and IL-8 secretion were measured by ELISA. Kits were purchased from Amersham and R&D Systems, respectively.
Statistical analysis
Unless otherwise stated, data are expressed as the means ±s.e.m. Analysis of variance (ANOVA) was used to compare experimental groups and control values. When the global test of differences was significant at the 5% level, pairwise tests of differences between groups were applied (Tukey's multiple comparison test).
Results
Nucleotides induce ECP release from human eosinophils
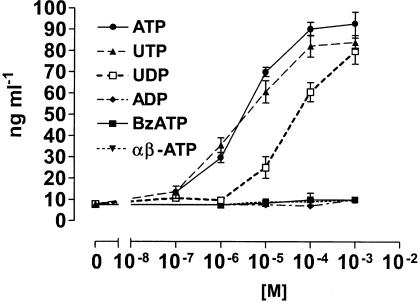
To study the involvement of P2 receptors in ECP release, eosinophils were stimulated with different nucleotides and secretion of the mediator into the supernatants was evaluated by ELISA. Figure 1 shows that the P2 agonist ATP induced a dose-dependent secretion of ECP. To characterize the involvement of P2Y receptors in this response, eosinophils were stimulated with UTP and UDP. These nucleotides activate different P2Y subtypes: UTP acts at P2Y2 and P2Y4 receptors, while UDP preferentially activates the P2Y6 subtype (Warny et al., 2001). Both nucleotides were active in stimulating ECP secretion from eosinophils, although with a different dose-dependency. UTP dose-dependency was similar to that of ATP, while UDP stimulated release of ECP at concentrations starting from 10−5 M. Interestingly, ADP which is an agonist at P2Y1 and P2Y12 receptors did not stimulate ECP release. To evaluate the contribution of the P2X subtypes in ECP secretion, eosinophils were stimulated with increasing concentrations of two ATP derivatives, α,β-meATP and BzATP, known to stimulate P2X receptors. In contrast to UTP and UDP, these agonists did not induce secretion of ECP at any of the concentrations tested.
Figure 1.
Effect of nucleotides on ECP secretion. Human eosinophils (106 cells ml−1) were stimulated in culture medium for 2 h, with the indicated concentrations of ATP, UTP, UDP, ADP, BzATP or α,β-meATP. ECP release was evaluated by ELISA. Data are expressed as mean±s.e.m. (n=5).
Nucleotide-stimulated-ECP release is pertussis toxin sensitive
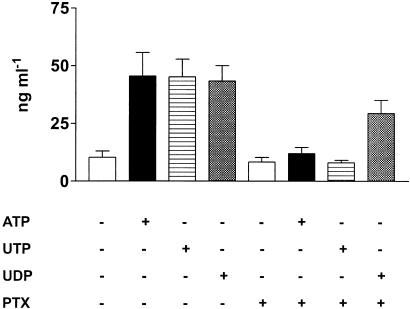
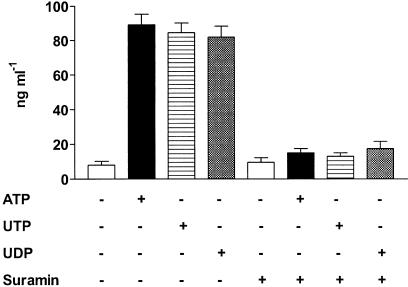
Stimulation of P2Y receptors induce activation of different G proteins (Ralevic & Burnstock, 1998) and regulates Ca2+ mobilization from intracellular stores as well Ca2+ influx from the extracellular medium. To dissect the intracellular signaling pathway of P2Y-mediated ECP release, eosinophils were incubated either with the Gi/o proteins inhibitor pertussis toxin (PTX) or in the presence of the Ca2+ chelator EGTA. As shown in Figure 2, pretreatment with PTX completely abrogated ATP- and UTP-induced ECP release while UDP-mediated response was reduced, showing that activation of Gi/o proteins was a step of purinergic-mediated ECP secretion. Release of ECP by ATP, UTP or UDP was also blocked by the broad-spectrum P2Y inhibitor suramin (Figure 3). Addition of EGTA prior to stimulation with the nucleotides did not significantly affect ECP release (not shown).
Figure 2.
Effect of pertussis toxin on nucleotide-induced ECP secretion. Eosinophils were incubated in the presence or absence of 4 μg ml−1 PTX for 2 h. Cells were then stimulated for 2 h with 1 mM ATP, UTP or UDP and ECP release was evaluated. Data are expressed as mean±s.e.m. (n=3).
Figure 3.
Effect of suramin on nucleotide-induced ECP secretion. Eosinophils were pretreated for 30 min with 100 μM suramin or medium alone. Thereafter, cells were stimulated with 1 mM ATP, UTP or UDP. Data are expressed as mean±s.e.m. (n=3).
Nucleotides induce IL-8 secretion from human eosinophils
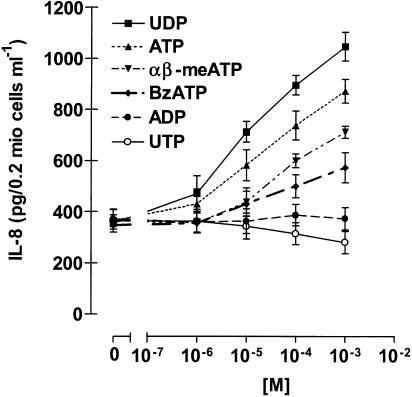
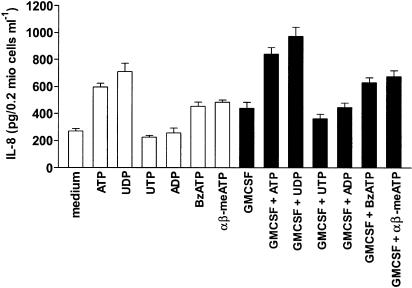
IL-8 is an important chemotactic factor and a central mediator in inflammation, eliciting various responses in different cells (Mukaida, 2000; Baggiolini, 2001). Stimulation of eosinophils with the P2 agonist ATP, as well as with the P2X agonists α,β-meATP and BzATP, dose-dependently induced secretion of the cytokine. Interestingly, in addition to α,β-meATP and BzATP, only the P2Y6 agonist UDP was able to induce the production of IL-8, while UTP and ADP were ineffective (Figure 4). To exclude the possibility that release of the cytokine was due to the cytotoxic effect of nucleotides, we performed a viability test. Eosinophils were incubated with the different nucleotides and vitality measured by propidium iodide and FACS analysis. No significant difference between control and nucleotide-stimulated cells was detected (Table 1).
Figure 4.
Effect of nucleotides on IL-8 release. Eosinophils were stimulated with the indicated concentrations of ATP, UTP, UDP, ADP, α,β-meATP and BzATP. After 24 h, supernatants were collected and IL-8 content was measured by ELISA. Data are expressed as mean±s.e.m. (n=6).
Table 1.
Effect of nucleotides on viability of human eosinophils
| Control | ATP | UDP | α,β-meATP | BzATP | |
|---|---|---|---|---|---|
| 0 h | 96.52±3.81 | 97.25±2.68 | 95.90±2.63 | 97.56±3.34 | 94.15±2.23 |
| 2 h | 94.79±2.84 | 93.92±4.20 | 93.07±3.12 | 95.13±2.59 | 92.55±3.42 |
| 6 h | 92.55±5.02 | 92.21±3.12 | 94.81±4.84 | 94.34±3.35 | 89.79±5.74 |
| 12 h | 89.49±4.46 | 92.05±4.31 | 92.22±3.79 | 92.34±2.78 | 88.09±4.32 |
| 24 h | 82.79±5.84 | 89.92±4.20 | 90.07±4.89 | 88.55±4.66 | 85.69±3.84 |
Eosinophils (106 cells ml−1) were cultured in the absence or presence of the different nucleotides (1 mM) for the indicated time. Viability was detected as described in the Methods section. Data are shown as percentage of viable eosinophils±s.e.m. obtained in five independent experiments. No significant difference between control and nucleotide-stimulated cells was detected.
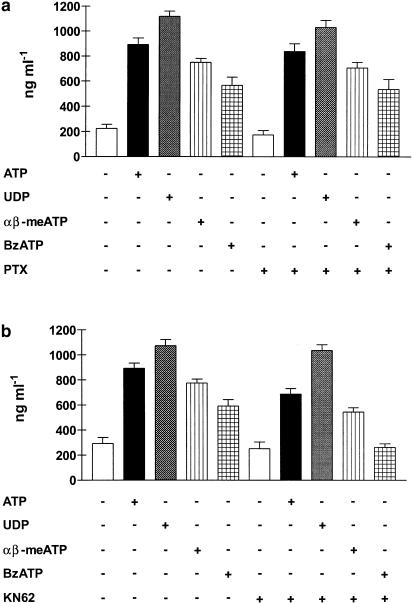
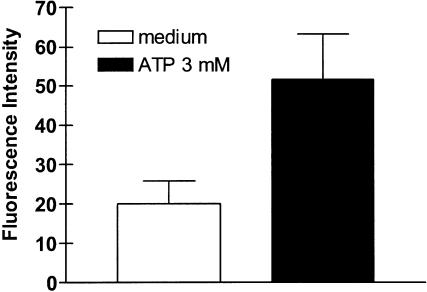
Pretreatment of eosinophils with PTX did not significantly affect ATP-, UDP-, α,β-meATP- or BzATP-induced IL-8 production (Figure 5a). In contrast, preincubation of eosinophils with KN-62 (25 nM) for 10 min, completely abrogated BzATP- and reduced ATP- and α,β-meATP-induced IL-8 production; however, it had no influence on the UDP-mediated response (Figure 5b). Stimulation by BzATP and inhibition by KN-62 (Figure 5b) suggested that IL-8 production also involved stimulation of the P2X7 subtype. We previously showed that human eosinophils expressed mRNA for P2X7 receptor, but it is yet unknown whether the receptor is functional in these cells. To test this hypothesis, we performed the YO-PRO uptake assay. Figure 6 shows that eosinophils stimulated with ATP became permeable to the extracellular dye.
Figure 5.
Effect of pertussis toxin (PTX) and KN-62 on nucleotide-induced IL-8 release. Eosinophils were preincubated in the presence or in the absence of 4 μg ml−1 PTX for 2 h (a); KN-62 (25 nM) was added to eosinophils 10 min prior to stimulation (b). Cells were then stimulated for 24 h with the indicated nucleotides and IL-8 release was measured by ELISA. Data are expressed as mean±s.e.m. (n=3).
Figure 6.
ATP-dependent YO-PRO uptake by human eosinophils. Cells were incubated in a 10 μM YO-PRO-containing saline solution and stimulated with 3 mM ATP for 30 min (see Methods for details). Fluorescence intensity was then measured as reported in the Methods. Data are expressed as means±s.e.m. of experiments performed in triplicate.
Virtually all cell types express ecto-nucleotidases (Zimmermann, 1992; Mizumoto et al., 2002); therefore, responses to extracellular nucleotides could also be due to stimulation of adenosine receptors by nucleotide degradation products. Table 2 shows that incubation of eosinophils with the A1-receptor antagonist DPCPX, the A2a-receptor antagonist CSC or A3-receptor antagonist MRS-1220, did not affect nucleotide-stimulated IL-8 secretion.
Table 2.
Lack of effect of adenosine receptor antagonists on nucleotide-induced IL-8 secretion
| DPCPX | CSC | MRS-1220 | ||
|---|---|---|---|---|
| Control | 332±48 | 357±38 | 292±68 | 360±56 |
| ATP | 925±43 | 893±53 | 932±73 | 1025±73 |
| UDP | 1073±52 | 1002±63 | 1127±59 | 999±102 |
| αβ-meATP | 774±35 | 743±29 | 704±53 | 798±86 |
| BzATP | 610±53 | 595±59 | 621±47 | 600±34 |
Cells were pretreated for 30 min with the A1-receptor antagonist DPCPX or the A2a-receptor antagonist CSC or the A3-receptor antagonist MRS-1220 at a concentration of 1 μM, and were then stimulated for 24 h with 1 mM of the indicated nucleotide. Supernatants were then collected and IL-8 content was determined by ELISA. Data are expressed as mean±s.e.m. (n=3).
GM-CSF has been recently shown to modulate eosinophil functions and survival by reducing spontaneous apoptosis (Esnault & Malter, 2002; Hoontrakoon et al., 2002). We asked whether GM-CSF would also modulate nucleotide-induced IL-8 secretion. Pretreatment of eosinophils with GM-CSF increased nucleotide-induced IL-8 production without changing nucleotide stimulation pattern (Figure 7).
Figure 7.
Nucleotide-induced IL-8 production is potentiated by GM-CSF. Cells were incubated for 30 min in the presence or absence of 10 ng ml−1 GM-CSF. Eosinophils were then stimulated with 1 mM of the indicated nucleotides and cultured for 24 h. Supernatants were then collected and IL-8 content was determined by ELISA. Data are expressed as mean±s.e.m. (n=4).
Discussion
ECP released by eosinophils can cause respiratory, epithelial and cardiovascular tissue damage and consequently inflammation (Venge et al., 1999). Cell cytoplasm contains huge amounts (5–10 mM) of ATP, which can be released into the extracellular milieu as a consequence of shear stress forces, membrane stretching or following cell injury/death. However, ATP can also be accumulated into specific intracellular granules or vesicles such as platelets, dense granules or neuronal vesicles and secreted in a regulated manner (Jo & Role, 2002; Vonend et al., 2002). Many inflammatory cells (e.g. macrophages, dendritic cells, microglia) chemotact to ATP. Thus, it is conceivable that ATP and/or other intracellular nucleotides could function as an early alarm signal alerting the immune cells of damage to tissue. ATP release could also represent an amplification system spreading the initial alarm by generating additional mediators such as the proinflammatory cytokines IL-1, IL-6, IL-8, IL-18 (Ferrari et al., 1997; Perregaux et al., 2000; Shigemoto-Mogami et al., 2001; Warny et al., 2001). ATP is a chemotactic factor for eosinophils (Burgers et al., 1993) and purinergic stimulation of eosinophils elicit activatory responses such as production of reactive oxygen species and CD11b expression (Idzko et al., 2001). These findings suggest that ATP could function as an amplification signal to potentiate the killing capacity of eosinophils and recruitment of inflammatory cells.
Here we show that stimulation of eosinophils with nucleotides induced the release of ECP and secretion of IL-8. The two processes had different nucleotide requirements and involved different P2 receptor subtypes. Release of ECP was triggered by stimulation of P2Y receptors, possibly of the P2Y2 subtype. In contrast to ECP, IL-8 secretion was due to activation of both P2X and P2Y receptors, as in addition to UDP, the P2X agonists α,β-meATP and BzATP also were able to induce this response. Interestingly, UDP (a P2Y6 agonist) but not the other P2Y agonists UTP and ADP also induced IL-8 secretion. Further support to involvement of both P2 receptor families in IL-8 secretion came from the experiments with KN-62. This reversible P2X7 inhibitor, originally described as a calmodulin blocker and subsequently shown to powerfully inhibit the human P2X7 subtype (Blanchard et al., 1995; Gargett & Wiley, 1997; Idzko et al., 2001) completely abrogated BzATP-mediated response, while it did not affect the UDP-induced IL-8 release. Partial inhibition by KN-62 of ATP- and α,β-meATP-stimulated IL-8 secretion indicated that the P2X1 and P2X7 receptor subtypes play a role in stimulating this response; this observation also shows that the P2X7 receptor in eosinophils, at variance with neutrophils (Gu et al., 2000), is functional.
GM-CSF has been involved in the pathogenesis of chronic allergic inflammation since it reduces eosinophil apoptosis; therefore, inhibition of GM-CSF-mediated effects would reduce eosinophil inflammation (Allam & Renzi, 2001). Here we demonstrated that GM-CSF was able to upregulate responses of eosinophils to extracellular nucleotides, showing that GM-CSF could act as a proinflammatory cytokine also by inducing the release of ECP and IL-8.
In summary, our data provide evidence that nucleo-tides modulate eosinophil functions, showing a novel role for these extracellular mediators in the activation of human eosinophils.
Acknowledgments
We gratefully acknowledge the assistance of A. Komann. Furthermore we thank the Italian University Ministry (MURST), the Italian Space Agency (ASI) and the National Research Council (CNR) for financial support.
Abbreviations
- BzATP
2′-&3′-O-(4-benzoyl-benzoyl)adenosine 5′-triphosphate
- α,β-meATP
α,β-methylene adenosine 5′ triphosphate
- ECP
eosinophil cationic protein
- IL-8
interleukin 8
- PTX
pertussis toxin
References
- ALLAM M., RENZI P.M. Inhibition of GM-CSF/IL-3/IL-5 signaling by antisense oligodeoxynucleotides targeting the common beta chain of their receptors. Antisense Nucl. Acid Drug Dev. 2001;11:289–300. doi: 10.1089/108729001753231678. [DOI] [PubMed] [Google Scholar]
- BAGGIOLINI M. Chemokines in pathology and medicine. J. Intern. Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- BLANCHARD D.K., HOFFMAN S.L., DJEU J.Y. Inhibition of extracellular ATP-mediated lysis of human macrophages by calmodulin antagonists. J. Cell Biochem. 1995;57:452–464. doi: 10.1002/jcb.240570311. [DOI] [PubMed] [Google Scholar]
- BOUSQUET J., CHANEZ P., LACOSTE J.Y., BARNEON G., GHAVANIAN N., ENANDER I., VENGE P., AHLSTEDT S., SIMONY-LAFONTAINE J., GODARD P. Eosimophilic inflammation in asthma. N. Engl. J. Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- BURGERS J.A., SCHWEIZER R.C., KOENDERMAN L., BRUIJNZEEL P.L., AKKERMAN J.W. Human platelets secrete chemotactic activity for eosinophils. Blood. 1993;81:49–55. [PubMed] [Google Scholar]
- BUTTERWORTH A.E., STURROCK R.F., HOUBA V.A., MAHMOUD A., SHER A., REES P.H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- CAMPBELL J.J., QIN S., UNUTMAZ D., SOLER D., MURPHY K.E., HODGE M.R., WU L., BUTCHER E.C. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J. Immunol. 2001;167:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- COOK S.P., MCCLESKEY E.W. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- DICHMANN S., IDZKO M., ZIMPFER U., HOFMANN C., FERRARI D., LUTTMANN W., VIRCHOW C., DI VIRGILIO F., NORGAUER J. Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood. 2000;95:973–988. [PubMed] [Google Scholar]
- DI VIRGILIO F., CHIOZZI P., FERRARI D., FALZONI S., SANZ J.M., MORELLI A., TORBOLI M., BOLOGNESI G., BARICORDI O.R. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- DUBUCQUOI S., DESREUMAUX P., JANIN A., KLEIN O., GOLDMAN M., TAVERNIER J., CAPRON A., CAPRON M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J. Exp. Med. 1994;179:703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBYAK G.R., EL-MOATASSIM C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- EDGERTON M., KOSHLUKOVA S.E. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv. Dent. Res. 2000;14:16–21. doi: 10.1177/08959374000140010201. [DOI] [PubMed] [Google Scholar]
- ESNAULT S., MALTER J.S. GM-CSF regulation in eosinophils. Arch. Immunol. Ther. Exp. (Warsz) 2002;50:121–130. [PubMed] [Google Scholar]
- FERRARI D., CHIOZZI P., FALZONI S., HANAU S., DI VIRGILIO F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI D., IDZKO M., DICHMANN S., PURLIS D., VIRCHOW C., NORGAUER J., CHIOZZI P., DI VIRGILIO F., LUTTMANN W. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000;486:217–224. doi: 10.1016/s0014-5793(00)02306-1. [DOI] [PubMed] [Google Scholar]
- GARGETT C.E., WILEY J.S. The isoquinoline derivate KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br. J. Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLEICH G.-J. The eosinophil and bronchial asthma: current understanding. J. Allergy Clin. Immunol. 1990;85:422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- GU B.J., ZHANG W.Y., BENDALL L.J., CHESSELL I.P., BUELL G.N., WILEY J.S. Expression of P2X(7) purinoceptors on human lymphocytes and monocytes: evidence for non-functional P2X(7) receptors. Am. J. Physiol. Cell Physiol. 2000;279:C1189–C1197. doi: 10.1152/ajpcell.2000.279.4.C1189. [DOI] [PubMed] [Google Scholar]
- HOONTRAKOON R., CHU H.W., GARDAI S.J., WENZEL S.E., MCDONALD P., FADOK V.A., HENSON P.M., BRATTON D.L. Interleukin-15 inhibits spontaneous apoptosis in human eosinophils via autocrine production of granulocyte macrophage-colony stimulating factor and nuclear factor-kappaB activation. Am. J. Respir. Cell Mol. Biol. 2002;26:404–412. doi: 10.1165/ajrcmb.26.4.4517. [DOI] [PubMed] [Google Scholar]
- IDZKO M., DICHMANN S., PANTHER E., FERRARI D., HEROUY Y., VIRCHOW C., Jr, LUTTMANN W., DI VIRGILIO F., NORGAUER J. Functional characterization of P2Y and P2X receptors in human eosinophils. J. Cell Physiol. 2001;188:329–336. doi: 10.1002/jcp.1129. [DOI] [PubMed] [Google Scholar]
- JO Y.H., ROLE L.W. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J. Neurosci. 2002;22:4794–4804. doi: 10.1523/JNEUROSCI.22-12-04794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITA H., ABU-GHAZALEH R.I., SUR S., GLEICH G.J. Eosinophil major basic protein induces degranulation and IL-8 production by human eosinophils. J. Immunol. 1995;154:4749–4758. [PubMed] [Google Scholar]
- KITA H., OHNISHI T., OKUBO Y., WEILER D., ABRAMS J.S., GLEICH G.J. Granulocyte/macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J. Exp. Med. 1991;174:745–748. doi: 10.1084/jem.174.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG D.Y. Pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 1999;104:S99–S108. doi: 10.1016/s0091-6749(99)70051-5. [DOI] [PubMed] [Google Scholar]
- LIM K.G., WAN H.C., BOZZA P.T., RESNICK M.B., WONG D.T., CRUIKSHANK W.W., KORNFELD H., CENTER D.M., WELLER P.F. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte chemoattractant factor) and RANTES. J. Immunol. 1996;156:2566–2570. [PubMed] [Google Scholar]
- MAEDA T., KITAZO E.M., TADA H., DE LLORENS R., SALOMON D.S., UEDA M., YAMADA H., SENO M. Growth inhibition of mammalian cells by eosinophil cationic protein. Eur. J. Biochem. 2002;269:307–316. doi: 10.1046/j.0014-2956.2001.02653.x. [DOI] [PubMed] [Google Scholar]
- MARGUET C., DEAN T.P., BASUYAU J.P., WARNER J.O. Eosinophil cationic protein and interleukin-8 levels in bronchial lavage fluid from children with asthma and infantile wheeze. Pediatr. Allergy Immunol. 2001;12:27–33. doi: 10.1034/j.1399-3038.2001.012001027.x. [DOI] [PubMed] [Google Scholar]
- MERTENS A.V., DE CLERCK L.S., MOENS M.M., BRIDTS C.H., STEVENS W.J. Study of eosinophil–endothelial adhesion, production of oxygen radicals and release of eosinophil cationic protein by peripheral blood eosinophils of patients with rheumatoid arthritis. Clin. Exp. Allergy. 1993;23:868–873. doi: 10.1111/j.1365-2222.1993.tb00266.x. [DOI] [PubMed] [Google Scholar]
- MIZUMOTO N., KUMAMOTO T., ROBSON S.C., SEVIGNY J., MATSUE H., ENJYOJI K., TAKASHIMA A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat. Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- MOHANTY J.G., RAIBLE D.G., MCDERMOTT L.J., PELLEG A., SCHULMAN E.S. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: activation of purinergic P2Y receptors. J. Allergy Clin. Immunol. 2001;107:849–855. doi: 10.1067/mai.2001.114658. [DOI] [PubMed] [Google Scholar]
- MUKAIDA N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int. J. Hematol. 2000;72:391–398. [PubMed] [Google Scholar]
- NORTH R.A., SURPRENANT A. Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- OLSSON I., VENGE P. Cationic proteins of human granulocytes. I. Isolation of the cationic proteins from the granules of leukaemic myeloid cells. Scand. J. Haematol. 1972;9:204–214. doi: 10.1111/j.1600-0609.1972.tb00932.x. [DOI] [PubMed] [Google Scholar]
- OLSSON I., VENGE P., SPITZNAGEL J.K., LEHRER R.I. Arginine-rich cationic proteins of human eosinophil granules: comparison of the constituents of eosinophilic and neutrophilic leukocytes. Lab. Invest. 1977;36:493–500. [PubMed] [Google Scholar]
- PERREGAUX D.G., MCNIFF P., LALIBERTE R., CONKLYN M., GABEL C.A. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J. Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- PRONK-ADMIRAAL C.J., BARTELS P.C. Total amount of ECP per eosinophil as indicator for the activity state of eosinophils. Scand. J. Clin. Lab. Invest. 2001;61:453–457. doi: 10.1080/00365510152567095. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SHIGEMOTO-MOGAMI Y., KOIZUMI S., TSUDA M., OHSAWA K., KOHSAKA S., INOUE K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J. Neurochem. 2001;78:1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- SIMON H.U., YOUSEFI S., WEBER M., SIMON D., HOLZER C., HARTUNG K., BLASER K. Human peripheral blood eosinophils express and release interleukin-8. Int. Arch. Allergy Immunol. 1995;107:124–126. doi: 10.1159/000236950. [DOI] [PubMed] [Google Scholar]
- SUGIHARA R., KUMAMOTO T., ITO T., UEYAMA H., TOYOSHIMA I., TSUDA T. Human muscle protein degradation in vitro by eosinophil cationic protein (ECP) Muscle Nerve. 2001;24:1627–1634. doi: 10.1002/mus.1198. [DOI] [PubMed] [Google Scholar]
- TERAN L.M., CARROLL M.P., FREW A.J., REDINGTON A.E., DAVIES D.E., LINDLEY I., HOWARTH P.H., CHURCH M.K., HOLGATE S.T. Leukocyte recruitment after local endobronchial allergen challenge in asthma. Relationship to procedure and to airway interleukin-8 release. Am. J. Respir. Crit. Care Med. 1996;154:469–476. doi: 10.1164/ajrccm.154.2.8756824. [DOI] [PubMed] [Google Scholar]
- VENGE P., BYSTROM J., CARLSON M., HAKANSSON L., KARAWACJZYK M., PETERSON C., SEVEUS L., TRULSON A. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin. Exp. Allergy. 1999;29:1172–1186. doi: 10.1046/j.1365-2222.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- VONEND O., OBERHAUSER V., VON KUGELGEN I., APEL T.W., AMANN K., RITZ E., RUMP L.C. ATP release in human kidney cortex and its mitogenic effects in visceral glomerular epithelial cells. Kidney Int. 2002;61:1617–1626. doi: 10.1046/j.1523-1755.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- VON KUEGELGEN I., WETTER A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WARNY M., ABOUDOLA S., ROBSON S.C., SEVIGNY J., COMMUNI D., SOLTOFF S.P., KELLY C.P. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- WELLER P.F., RAND T.H., BARRETT T., ELOVIC A., WONG D.T., FINBERG R.W. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J. Immunol. 1993;150:2554–2562. [PubMed] [Google Scholar]
- YOUSEFI S., HEMMANN S., WEBER M., HOLZER C., HARTUNG K., BLASER K., SIMON H.U. IL-8 is expressed by human peripheral blood eosinophils. Evidence for increased secretion in asthma. J. Immunol. 1995;154:5481–5490. [PubMed] [Google Scholar]
- ZIMMERMANN H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem. J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]