Abstract
TXD258, a new taxoid antitumor agent, is a poor substrate for the P-glycoprotein (P-gp) in Caco-2 cells. In this study, we investigated the amount of drug accumulating in the brains of rats and mice under a variety of conditions (dose and infusion time, species and plasma concentration) using conventional in vivo pharmacokinetic techniques and in situ brain perfusion.
Mice were infused with radiolabeled TXD258 at 15, 30, 45 and 90 mg m−2 for 45 s or 1 h and rats were infused with 15 and 60 mg m−2 over 2.3 min. The radioactivity in the plasma and brains was measured. The brain concentrations of TXD258 in mice and rats were maximal from 2 min to 1 h postinfusion and radioactivity was still detectable at 168 h. While the plasma concentration of TXD258 increased linearly in mice with the infused dose, the brain content increased more than proportionally with the dose between 15 and 90 mg m−2. This nonlinear uptake of TXD258 also occurred in the plasma and brain of the rat.
These findings suggest that the protein-mediated efflux across the blood–brain barrier (BBB) becomes saturated. In situ brain perfusion studies confirmed that TXD258 is a P-gp substrate at the BBB of mice and rats. The P-gp of both species was saturated at the half-inhibitory concentration (∼13 μM) produced by i.v. infusion.
Thus, the observed nonlinear accumulation of TXD258 in the brain seems to occur by saturation of the P-gp at the rodent BBB. This saturation could have several advantages, such as overcoming a P-gp-mediated efflux, but the nonlinear pharmacokinetics could increase the risk of toxicity.
Keywords: Blood–brain barrier, in situ brain perfusion, multidrug resistance, P-glycoprotein, taxanes
Introduction
TXD258 is a new taxoid that has an in vivo spectrum of antitumor action similar to that of docetaxel; it stabilizes microtubules against cold-induced depolymerization (Bissery et al., 2000). TXD258 also inhibits the growth of tumor cells expressing the mdr1 gene in vitro. The pharmacological treatment of brain diseases is often complicated by the inability of potent drugs to pass across the blood–brain barrier (BBB), which is formed by the tight endothelial cell junctions of the brain capillaries. In vivo, intravenous TXD258 suppresses implanted B16/TXT-resistant melanomas and intracerebral glioblastoma, suggesting that the drug is able to cross the BBB and/or the blood–brain tumor barrier (Bissery et al., 2000; Dykes et al., 2000). Other pharmacokinetic studies indicate that TXD258 is active when given orally to mice and is recognized as a marginal substrate of P-glycoprotein (P-gp) efflux pump in human Caco-2 colon carcinoma cells, largely used to predict in vivo oral absorption (Bissery et al., 2000). These properties have prompted the development of TXD258 for clinical use, with potential to treat brain metastases. Therefore, more detailed pharmacokinetic studies are needed to investigate the amount of drug given systemically that reaches the brain, as this can be influenced by the dose and duration of the drug infusion. Our pharmacokinetic studies were performed in mice and rats, using two methods. In the first conventional method, we measured the drug concentrations in the plasma and brain over time. TXD258 was given intravenously at different doses and over various infusion times. In the second method, we used in situ brain perfusion to determine the parameters of TXD258 uptake across the BBB in rats and mdr1a-deficient or control mice. We showed that the transport of TXD258 across the BBB is mediated by P-gp. This transport could be saturated by vascular concentrations of TXD258 greater than 13 μM. These data explain why uptake by the brain was nonlinear in the conventional pharmacokinetic dose range and emphasize how a delivery rate above or below the plasma TXD258 concentration that saturates P-gp at BBB could be critical for controlling the amount of TXD258 in the brain parenchyma and consequently available to treat brain tumors. This new taxoid shows that the P-gp at the BBB can be saturated and that this saturation is responsible for the difference between the concentration of a drug in the brain and its systemic concentration.
Methods
Chemicals and reagents
TXD258 was manufactured by Aventis (Vitry, France). [14C]-TXD258 was provided by NEN™ Life Science Products (Boston, MA, U.S.A.). Its molecular weight of 835.95 g mol−1 and its structure is shown in Figure 1.
Figure 1.
Chemical structure of [14C]-TXD258.
The tracer had a specific activity of 1.99 GBq mmol−1, and a radiochemical purity of 97.7% determined by h.p.l.c. [3H]-sucrose (377.4 GBq mmol−1) was obtained from NEN™ Life Science Products (Paris, France). Polysorbate 80 (PS80) and ethanol were purchased from Prolabo (Paris, France). Glucose 5%, (±)-verapamil hydrochloride and dimethyl sulfoxide were purchased from Meram (Melun, France), Sigma (St. Quentin Fallavier, France) and Merck Eurolab (Strasbourg, France), respectively. Liquid scintillation cocktails were purchased from Packard (Rungis, France). All other chemicals were commercial products of analytical grade.
Animals
The studies were performed on female CD2F1/Crl BR mice (18–23 g, 6–8 weeks old) and female Crl:CD®-(SD)BR rats (180–220 g, 6–8 weeks old), provided by Charles River Laboratories (Elbeuf, France). Adult female CF-1 mice (mdr1a(+/+) and (−/−), 30–40 g, 6–8 weeks old) were bred in-house from progenitors genotyped for mdr1a P-gp that were initially obtained from Charles River Laboratories (Wilmington, MA, U.S.A.). Tap water and diet were provided ad libitum. The animals used in these studies were handled and maintained in accordance with the requirements of the E.E.C. Guideline (1986) and U.S. Federal Guidelines (1985). Compliance with the above legislation was ensured by adhering to the standards set forth in the Guide for the Care and Use of Laboratory Animals, DHHS Publication No. (NIH) 86–23, revised 1985.
Pharmacokinetic studies
Formulation, dose and administration
The poor TXD258 solubility prompted us to use a similar administration protocol used for preclinical taxoid drug docetaxel studies that involved PS80, ethanol and relative high amount of glucose 5% (Bissery et al., 1995). [14C]-TXD258 was diluted in PS80/ethanol/glucose 5% and was administered via the femoral vein using an infusion pump (Harvard PHD 2000, Harvard apparatus, Holliston, MA, U.S.A.), at 25 ml kg−1 for female mice to give doses of 15, 30, 45 or 90 mg m−2 and at 10 ml kg−1 for female rats to give doses of 15 and 60 mg m−2. The proportion of each vehicle PS80/ethanol/glucose 5% for TXD258 administration at 15 mg m−2 was 0.3/1.0/98.7% and 0.4/1.4/98.2% for mice and rats, respectively. Each dose was perfused at a constant rate of 1 ml min−1 and the duration of the perfusion was approximately 45 s for mice and 2.3 min for rats. TXD258 was also given to female mice at doses of 30 and 90 mg m−2 using a perfusion rate of 8.3 μl min−1 for approximately 1 h.
Sampling
Mice and rats were anesthetized with isoflurane and exsanguinated via the abdominal aorta at times optimized for the metabolites profile: 0.03, 0.25, 1, 6, 24, 72 and 168 h after dosing. Blood and brain samples were collected from four mice and two rats at each time point. Blood samples were collected into heparinized syringes. The plasma was separated by centrifugation at 3000 × g for 15 min. The plasma samples obtained at each time point were pooled to obtain enough material for quantifying metabolites. Thus, the resulting data are averages. Brains were lyophilized and kept frozen at –80°C until analyzed.
Total radioactivity analysis
The total radioactivity in plasma and brain samples was determined by liquid spectrometry using a Beckman LS 6000 SC spectrometer equipped with an external standard system (number H). A quench curve was generated using [14C] quenched standards supplied by Beckman. Samples were counted for up to 10 min (0.5% precision). D.p.m. values of less than twice the background were considered to be insignificant. Plasma samples (0.5 – 1 ml) were added directly to the liquid scintillation cocktail Hionic-Fluor (Beckman). Duplicate samples of freeze-dried homogenized brain were processed in a sample oxidizer (Packard model 307) and the 14CO2 formed was trapped in 9 ml Carbosorb (Packard). The carbosorb was mixed with 12 ml of Permafluor E+ (Packard) liquid scintillation cocktail for counting.
Metabolite analysis
The parent drug and metabolites in plasma and brain extracts were measured at 1 and 6 h after the end of infusion by h.p.l.c. with on-line radiochemical detector. Aliquots of plasma were subject to solid phase extraction with Oasis HLB cartridges (Waters) equilibrated with methanol and demineralized water. The eluates were concentrated and aliquots (50 μl) were analyzed by h.p.l.c. A lyophilized sample of brain was weighed and sonicated for 15 min with 20.5 ml ethyl acetate, water/formic acid (0.1%), (100/2.5, v v−1). The homogenate was stirred and centrifuged at 13,000 r.p.m. for 10 min. The resulting supernatant was evaporated to dryness, taken up in 0.5 ml of a mixture containing dimethyl sulfoxide, methanol/acetone (15%), (50/50, v v−1), vortexed for 2 min and clarified by centrifugation at 15,000 r.p.m. for 10 min. The extraction procedure was repeated once, the supernatants were combined and a 50 μl aliquot analyzed by h.p.l.c. The Merck® h.p.l.c. analytical system consisted of a L6200A gradient elution system pump with autosampler model AS4000, a diode array detector (L7450) operating at 230 nm and a Berthold model LB507B on-line radioactivity detector equipped with a 500 μl flow cell. Samples were separated on a Symmetry C8 column (250 × 4.6 mm, 5 μm) connected to a Symmetry C8 guard column (Waters). Elution was performed under gradient conditions using succession of six steps from 0 to 170 min with a mobile phase of water/trifluoroacetic acid 0.01% and acetonitrile, at a flow rate of 0.6 ml min−1 and at room temperature. Peaks of radioactivity were quantified on the radioactivity detector by integrating the area under each peak. The calculated intra- and interday coefficients of variation were below 15%.
Pharmacokinetic analysis
The pharmacokinetic analysis was carried out using a noncompartmental model with WinNonlin® software (Version 1.0, Scientific Consulting Inc., U.S.A.). The following brain and plasma parameters were determined, Cmax, Tmax, and AUC(0–t). The area under the radioactivity concentration decay curves between 0 and t (h) were computed from the experimental points by the trapezoidal method. The partition coefficient Kp was calculated as the ratio of brain AUC0 – 168 h over plasma AUC0 – 168 h.
In situ brain perfusion studies
Surgical procedure and perfusion technique
The transport of [14C]-TXD258 into the brains of rats and mice was measured using the in situ brain perfusion method (Takasato et al., 1984; Smith, 1996; Dagenais et al., 2000). Animals were anesthetized by i.p. injection of a mixture of xylazine (Bayer, Puteaux, France) and ketamine (Panpharma, Fougères, France), at 8/140 mg kg−1 for mice and 4/70 mg kg−1 for rats.
Briefly, the right common carotid artery was catheterized with heparin-filled polyethylene tubing (0.30 mm i.d. × 0.70 mm o.d. for mice; 0.76 mm i.d. × 1.22 mm o.d. for rats, Biotrol Diagnostic, Chennevières-les–Louvre, France). The common carotid artery was ligated on the heart side before inserting the catheter. In mice, the external carotid was ligated rostral to the occipital artery at the bifurcation of the common carotid artery. In rats, the external carotid and occipital arteries were ligated. Body temperature was maintained at 37 – 38°C during surgery using a rectal thermistor connected to a temperature monitor. The syringe containing the perfusion fluid was placed in an infusion pump (Harvard pump PHD 2000, Harvard Apparatus) and connected to the catheter. Before perfusion, the thorax of the animal was opened, the heart was cut and perfusion immediately started with a flow rate of 2.5 ml min−1 for mice and 10 ml min−1 for rats. The perfusion fluid consisted of bicarbonate-buffered physiological saline (mM): 128 NaCl, 24 NaHCO3, 4.2 KCl, 2.4 NaH2PO4, 1.5 CaCl2, 0.9 MgCl2 and 9 D-glucose. The solution was gassed with 95% O2 and 5% CO2 to obtain a pH of 7.4 and warmed to 37°C in a water bath. Compounds were added to the perfusate at the appropriate concentration. Ethanol and PS80 did not exceed 0.08 and 0.02% in the perfusate, respectively. Each animal was perfused with [14C]-TXD258 plus [3H]-sucrose (11.1 kBq ml−1) to check the physical integrity of the BBB. Perfusion was terminated after 60 s by decapitating the animal. The brain was removed from the skull and dissected free on ice. The right cerebral hemisphere was placed in a tared vial and weighed. Aliquots of the perfusion fluid were also collected and weighed to determine tracer concentrations in the perfusate. Samples were digested in 2 ml of Solvable (Packard) at 50°C and mixed with 9 ml of Ultima gold XR scintillation cocktail (Packard). Both labels were counted simultaneously in a Packard Tri-Carb model 1900 TR (Packard).
Transport studies
The transport of [14C]-TXD258 into the brain was first measured in mice perfused with 4.5, 10, 12.5, 15, 20, 25 and 30 μg ml−1 drug and in rats perfused with 2.5, 4.5, 10, 12.5, 15, 25 and 30 μg ml −1 drug. These were the concen-trations measured in previous pharmacokinetic and toxicokinetic studies. We then measured the influence of P-gp on the uptake of [14C]-TXD258 by the brain using a drug concentration of 4.5 μg ml−1 (∼5.4 μM) and 150 μM (±)-verapamil, in the perfusion fluid. These studies were conducted on rats, wild-type mice and P-gp deficient mdr1a(−/−) mice.
A ‘wash-out' procedure was also used to study the trans-efflux zero and trans-inhibition of the compound (Stein, 1986). One syringe (syringe A) of a dual-syringe infusion pump (Harvard Apparatus) contained the bicarbonate-buffered physiological saline plus the radiotracer (11.1 kBq ml−1; ∼5.4 μM) and the other (syringe B) contained saline, no tracer, but with or without (±)-verapamil (150 μM). The carotid catheter was connected to a four-way valve (Hamilton, Bonnaduz, Switzerland). After the carotid cannulation was completed and the appropriate connections were made, syringe A was discharged at 2.5 ml min−1 (mice) or 10 ml min−1 (rats) for 60 s. Syringe A was switched off and syringe B was switched on simultaneously to wash-out the capillary space for 30 s. The animal was decapitated and the brain removed to measure its radioactivity.
Distribution in brain microvascular and parenchymal compartments
To distinguish molecules that are trapped in endothelial cells from those that reach the brain parenchyma by transcytosis, distribution of [14C]-TXD258 in the brain microvascular and parenchymal compartments was assessed in rats using the capillary depletion method of Triguero et al. (1990) as modified by Rousselle et al. (2000). We used the wash-out procedure described above with syringe B containing compound-free bicarbonate-buffered saline in order to remove nonspecific adsorption and free circulating labeled compounds from the vascular space (Triguero et al., 1990). At the end of the wash-out, the right cerebral hemisphere was rapidly removed, weighed and homogenized (15 pestel strokes) in 3.5 ml buffer (10 mM HEPES, 141 mM NaCl, 4 mM KCl, 1 mM NaH2PO4, 2.8 mM CaCl2, 1 mM MgSO4 and 10 mM D-glucose, pH 7.4) on ice. Chilled 37% dextran solution (4 ml) was added to obtain a final dextran concentration of 18.5%. All homogenizations were performed rapidly at 4°C. An aliquot of homogenate was removed and the remainder was centrifuged at 5400 × g for 15 min at 4°C in a swinging-bucket rotor. The pellet and supernatant were carefully separated and counted in the liquid scintillation counter. The pellet was composed mainly of brain capillaries and the supernatant reflected the parenchyma.
Calculation of BBB transport parameters
All calculations were made as described by Smith (1996). Brain vascular volume (Vvasc; μl g−1) was estimated from the tissue distribution of [3H]-sucrose, which diffuses very slowly across the BBB, using the following equation:
 |
where X* (d.p.m. g−1) is the amount of sucrose measured in the right brain hemisphere and C *perf (d.p.m. μl−1) is the concentration of labeled sucrose in the perfusion fluid. Transport across the BBB was expressed in terms of the apparent distribution volume (Vbrain) and the transport coefficient (Kin). The apparent distribution volume was calculated from the radioactivity in the right brain hemisphere using the following equation:
 |
where Xbrain (d.p.m. g−1) is the calculated amount of [14C]-TXD258 in the right cerebral hemisphere and Cperf (d.p.m. μl−1) is the labeled TXD258 tracer concentration in the perfusion fluid. Brain tissue radioactivity was corrected for vascular contamination with the following equation:
where Xtot (d.p.m. g−1) is the total quantity of tracer measured in the tissue sample (vascular+extravascular).
Brain uptake, expressed as Kin (μl s−1 g−1), was calculated from
 |
where T is the perfusion time (s).
The perfusion time used in single-time uptake studies was long enough to ensure that at least 40% of the total radioactivity in the tissue was outside the vascular space (Xbrain⩾0.4Xtot; Takasato et al., 1984).
BBB transport modeling
As the relation between Kin values and the TXD258 perfusate concentration was sigmoidal, an empirical Hill function was derived for assessing kinetic transport parameters. Kinetic analyses were carried out using nonlinear regression with the least-squares method of Systat 5.01 (Systat Inc., IL, U.S.A.) to fit the equation:
 |
where C is the TXD258 concentration in the perfusate, Kin,min is the minimal and Kin,max the maximal brain coefficient transport (Kin) value measured for [14C]-TXD258, n is the Hill coefficient, and IC50 is the concentration at which brain transport was half-maximally inhibited.
Statistical analysis
Data are presented as means ±s.d. for four to eight animals, unless specified otherwise. Student's unpaired t-test was used to identify significant differences between groups when appropriate. All the tests were two-tailed and statistical significance was set at P<0.05. The error values associated with the kinetic parameters (IC50, Kin,min, Kin,max) are asymptotic standard errors returned by a nonlinear regression routine and are a measure of the certainty of the best-fit value.
Results
Pharmacokinetics in plasma and brain
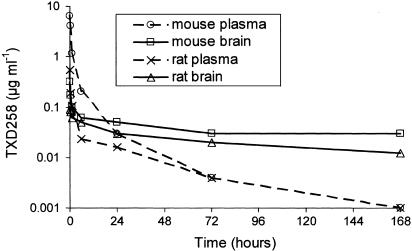
The plasma and brain total radioactivity kinetics in rats and mice following a single short intravenous administration of [14C]-TXD258 at 15 mg m−2 are shown in Figure 2. The other doses tested gave similar plasma and brain dispositions (data not shown). The maximal plasma concentration of TXD258 radioactivity was reached in the first sample, that is, at the end of infusion, after both short and long infusions. The concentration then rapidly decreased up to 6 h, and slowly thereafter up to 168 h, for all doses in both species. The TXD258-related radioactivity rapidly penetrated into the brain and the concentration was maximal in brain 2 min after the end of a short infusion at doses lower than 90 mg m−2 in both mice and rats, and 15 min after the end of short infusion of 90 mg m−2 in mice. The maximal brain concentration in mice occurred 15 min after the end of the 1 h infusion of TXD258 at 30 mg m−2 and 60 min after an infusion of 90 mg m−2 (Table 1). The brain radioactivity decreased slowly thereafter, but was still detectable for up to 168 h (Figure 2).
Figure 2.
Average changes in the plasma and brain concentrations of TXD258 over time. [14C]-TXD258 (15 mg m−2) was infused into mice for 45 s and into rats for 2.3 min. Samples were taken from four mice and two rats at each time and pooled to obtain an average analytical determination.
Table 1.
Brain and plasma pharmacokinetic parameters obtained after the intravenous infusion of mice and rats with [14C]-TXD258
| Mice | Rats | |||||||
|---|---|---|---|---|---|---|---|---|
| Infusion time | 45 s | 1 h | 2.3 min | |||||
| Dose (mg m−2) | 15 | 30 | 45 | 90 | 30 | 90 | 15 | 60 |
| Cmax plasma (μg ml−1) | 6.4 | 8.4 | 13.6 | 32.2 | 6 | 6.98 | 0.55 | 4.31 |
| Cmax brain (μg ml−1) | 0.31 | 0.39 | 0.92 | 6.1 | 0.39 | 0.45 | 0.09 | 0.49 |
| Tmax brain (min) | 2 | 2 | 2 | 15 | 15 | 60 | 2 | 2 |
| AUC0–168 h plasma (μg h ml−1) | 9.7 | 15.4 | 24.7 | 52.1 | 16 | 26.9 | 1.6 | 11 |
| AUC0–168 h brain (μg h ml−1) | 6.8 | 16.5 | 57 | 339 | 10.6 | 45.4 | 3.9 | 39.1 |
| Kp | 0.70 | 1.07 | 2.31 | 6.51 | 0.66 | 1.69 | 2.44 | 3.55 |
The parameters (Cmax, Tmax, AUC0–168 h) were calculated by a noncompartmental model using WinNonlin software. The partition coefficient (Kp) was calculated as the ratio of the brain AUC0–168 h to the plasma AUC0–168 h.
Radio-h.p.l.c. analysis of plasma showed that unchanged drug was the major compound, accounting for at least 60% of the radioactivity at 1 h and 100% at 6 h after infusion in mice, and about 84% at 1 h and 76% at 6 h in rats, at all the perfusion times and doses studied. One of the five metabolites, docetaxel, accounted for 2–11% of the total plasma radioactivity 1 h after the end of both short or long infusions for all the doses studied in both species. Only TXD258 was detected in the brains of mice after infusion of the lowest dose. Unchanged drug represented about 90% of the total radioactivity in the brain at the end of the short infusion of TXD258 at 45 and 90 mg m−2, and about 72% of the brain radioactivity after a long infusion at 90 mg m−2. Docetaxel was not detected in the brains of either rats or mice at any of the doses of TXD258 studied.
Dose ranging effect on plasma and brain Cmax and AUC
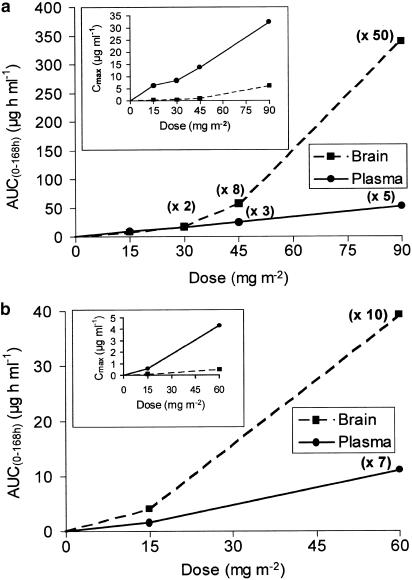
The values of Cmax and AUC0 – 168 h determined from the total plasma radioactivity increased proportionally with the dose in mice between 15 and 90 mg m−2, but greater than proportionally with the dose in rats between 15 and 60 mg m−2 (Table 1 and Figure 3). The brain AUC0 – 168 h increased eight-fold greater than proportionally with the dose in mice and 2.5-fold greater in rats, at the highest dose investigated compared to the lowest dose. However, higher doses were not investigated in rats because of the toxicity of TXD258, which possibly prevented a clear demonstration of the nonlinear brain uptake of TXD258. The brain-to-plasma AUC0–168 h ratios also increased more than proportionally as the doses increased (Table 1).
Figure 3.
(a) Relations between the average areas under the curves (AUC0 – 168 h) or maximal concentration (Cmax; inset) in the brains and plasma of mice infused intravenously with [14C]-TXD258 at 15, 30, 45 and 90 mg m−2 for 45 s. Values in parentheses represent the increases in the AUC obtained at 30, 45 or 90 mg m−2 compared to the value measured at 15 mg m−2. (b) Rat brain and plasma AUC0 – 168 h or Cmax (inset) values. The rats were given an intra-venous infusion of [14C]-TXD258 at 15 and 60 mg m−2 lasting 2.3 min.
Effect of doses and infusion times on [14C]-TXD258 in the brain and plasma of mice
These plasma concentrations produced AUC brain-to-plasma ratios of ∼1.1 for the short infusion and 0.7 for the long infusion. Doses of 90 mg m−2 infused for 45 s or 1 h resulted in dramatically higher Kp values of 2.3 and 1.7, respectively (Table 1). When Kp values are compared between 30 and 90 mg m−2, the values are increased 6.1- and 2.6-fold for 45 s and 1 h infusion, respectively. This increase in Kp could be attributed to the saturation of an active efflux transport at the BBB. The lower Kp values obtained following the 1 h infusions could, in part, be explained by a lower maximal peak plasma after the long infusion; it was 7 μg ml−1 after the long infusion and 32 μg ml−1 after the short infusion of 90 mg m−2.
Effect of the concentration of TXD258 on transport into the brain of wild-type mice and rats
The effect of the TXD258 concentration on drug transport into the brain was investigated by in situ brain perfusion. More [14C]-TXD258 was taken up by the brain when the perfusate concentrations were over 10 μg ml−1 (∼11 μM) of TXD258 in both rats and mice, suggesting the saturation of an active efflux process at the BBB (Figure 4). The variation in the blood – brain transport coefficient Kin with the TXD258 concentration fitted a sigmoid equation with a Hill coefficient of 23 and TXD258 IC50 values of 12.9±0.4 μM in rats and 13.0±0.4 μM in mice. The Kin values at lower concentrations (Kin,min) were 0.63±0.01 and 0.83±0.01 μl s−1 g−1 and Kin values at higher concentrations (Kin,max) were 1.97±0.01 and 2.76±0.01 μl s−1 g−1 in mice and rats, respectively (Figure 4). The differences between the Kin in mice and rats were statistically significant (P<0.001), whereas the IC50 values in the two species were not different. The Kin ratios between the high and low concentrations were about 3. The amount of drug in the brain after infusions of concentrations from 2.5 to 30 μg ml−1 showed that the BBB was not damaged by TXD258 as the [3H]-sucrose vascular volumes remained within the normal range (data not shown).
Figure 4.
Concentration-dependent brain transport of [14C]-TXD258 (expressed as a brain transport coefficient Kin) measured by in situ brain perfusion in rats and mice. Animals were perfused with TXD258 via the common carotid artery for 60 s. Dotted (rats) and solid (mice) curves represent data fitted with the Hill equation and an apparent TXD258 IC50 value of ∼13 μM. Data are presented as means±s.d. of n=4 – 8 animals per point.
Involvement of P-glycoprotein in the transport of [14C]-TXD258 across the BBB
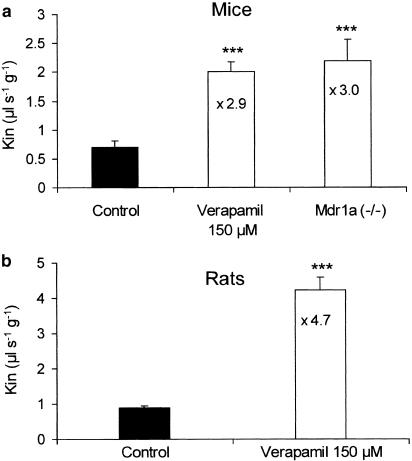
We evaluated the importance of P-gp for the transport of TXD258 across the BBB using two approaches. First, brain trans-influx zero experiments were performed in rats and mice with 4.5 μg ml−1 [14C]-TXD258, as this concentration does not saturate P-gp. The Kin in mdr1a(−/−) mice was about three-fold higher than in the wild-type mice (Figure 5). Perfusion of rats and wild-type mice with [14C]-TXD258 (4.5 μg ml−1) plus the P-gp modulator (±)-verapamil (150 μM) increased the brain uptake 2.9-fold in mice and 4.7-fold in rats over the values for control animals perfused with TXD258 alone (no verapamil) (Figure 5). Moreover, [14C]-TXD258 Kin at a saturating dose (30 μg ml−1) in mdr1a(−/−) mice was not different as compared to the [14C]-TXD258 Kin observed with a nonsaturating concentration (4.5 μg ml−1) in mdr1a(−/−) mice (data not shown). The trans-efflux zero process was also investigated in wild-type mice and rats after 60 s of accumulation of [14C]-TXD258 followed by a 30 s wash-out with a drug-free and tracer-free bicarbonate-buffered saline. The brain distribution volume was 1.2-fold lower in mice and 2.5-fold lower in rats (Figure 6) than in control animals not given the wash-out. Finally, a trans-inhibition study was conducted on mice and rats by adding (±)-verapamil (150 μM) to the bicarbonate-buffered saline during the 30 s wash-out. The brain distribution volumes measured after this treatment were not different from the control values observed in rat and mouse not given the wash-out (Figure 6). We therefore conclude that P-gp at the BBB controls the uptake of TXD258 across the BBB.
Figure 5.
The brain transport coefficient (Kin; μl s−1 g−1) was measured by in situ brain perfusion in P-gp-proficient mice (a) and rats (b) with or without the chemical P-gp modulator (±)-verapamil (150 μM) and in P-gp-deficient mdr1a(−/−) mice. Each group of animals was perfused with [14C]-TXD258 at a noninhibiting concentration (5.4 μM). P-gp chemical modulation or disruption produced an approximately three-fold increase in the brain TXD258 transport in mice and a 4.7-fold increase in rats. Data are presented as means ±s.d. of n=5 – 8 animals per group. ***P<0.001 compared to the control group.
Figure 6.
The apparent distribution volume (Vd; μl g−1) of [14C]-TXD258 was measured in controls after [14C]-TXD258 had been accumulated for 60 s. The animals in the two other groups, underwent a 30 s tracer-free ‘wash-out' with or without (±)-verapamil (150 μM) after the 60 s of brain accumulation of [14C]-TXD258. Both mice and rats were perfused with [14C]-TXD258 at 5.4 μM. Data are presented as means ±s.d. of n=5 – 6 animals per group. *P<0.05, ***P<0.001, compared to their respective controls.
Distribution in the brain microvascular and parenchymal compartments of rats
These brain perfusion experiments were performed to determine the percentage of TXD258 in the brain parenchyma. The distributions of [14C]-TXD258 in brain capillaries and parenchyma were measured in rats subjected to perfusion and a 30 s wash-out using the capillary depletion method of Triguero et al. (1990). This procedure distinguishes between the fraction of TXD258 remaining in the endothelial cells and the drug that has crossed the abluminal endothelial membrane to enter the brain parenchyma. The washing procedure removed radioactive compounds from the vessel lumen space. About 6% of the [14C]-TXD258 was associated with the brain capillaries, and about 94% of [14C]-TXD258 was in the brain parenchyma.
Discussion
The taxanes are a new family of antineoplastic drugs. The family includes the natural compound paclitaxel (Taxol®) and the more recently prepared hemisynthetic docetaxel (Taxotere®), which are remarkably potent against various cancers (for review, see Lin & Ojima, 2000). Despite their clinical success and the fact that docetaxel is more effective than paclitaxel in inhibiting the growth of tumoral cells (Clarke & Rivory, 1999; Ferlini et al., 2000), some tumors such as brain malignancies respond poorly to these drugs. Moreover, despite its lipophilicity at a log P(octanol/water) of 3.5, paclitaxel does not readily cross the intact BBB of either rodents (Eiseman et al., 1994; Klecker et al., 1994) or humans (Heimans et al., 1994). The physiologically abundant P-gp at the luminal surface of the brain endothelial cells that form the BBB might be the main reason why paclitaxel and docetaxel are poorly diffusing into the brain. P-gp is involved in the active efflux of a broad spectrum of substrates from the brain to the blood vessel lumen (Ambudkar et al., 1999; for review, see Tsuji & Tamai, 1997; Schinkel, 1999).
TXD258 is a new semisynthetic compound that has interesting pharmacological properties. It is active against many tumors in vivo, including human glioblastoma cells implanted in the brain of mice (Dykes et al., 2000; Vrignaud et al., 2000) and interacts less strongly with P-gp than the older taxanes. We therefore determined the brain and plasma pharmacokinetics of TXD258 in rodents. TXD258 was prepared in the same excipients as the commercially available form of Taxotere®, which is free of cremophor EL. Pharmacokinetic studies in rodents indicate that TXD258 enters the brain more readily than paclitaxel (Eiseman et al., 1994; Klecker et al., 1994; Lesser et al., 1995). We have used in situ brain perfusion to study the transport of labeled taxanes across the BBB and found that TXD258 was taken up by the brain of wild-type mice approximately two- to three-fold better than docetaxel and paclitaxel (Cisternino, unpublished data). The capillary depletion studies also show that TXD258 is not trapped in the endothelial cells of the BBB, but enters the brain parenchyma.
Our conventional pharmacokinetic studies designed to evaluate the disposition of TXD258 in the plasma and brain show that the amount of TXD258 in the brain increases nonproportionally with the dose range exposure in mice and rats. The origin of this nonlinear brain distribution could be a nonlinear distribution in the plasma, as was reported in some pharmacokinetic studies with paclitaxel (Eiseman et al., 1994; Gianni et al., 1995). Recent studies indicate that the nonionic surfactant cremophor EL used to dissolve paclitaxel in the commercial formulation is responsible for nonlinear pharmacokinetics in mice (Sparreboom et al., 1996) and humans (van Tellingen et al., 1999b). Paclitaxel formulated in PS80, which is used to dissolve docetaxel and TXD258 does not show this nonlinear distribution (Sparreboom et al., 1996; van Tellingen et al., 1999a), suggesting that cremophor EL modifies blood – plasma partitioning of paclitaxel (van Zuylen et al., 2001). We have found that TXD258 tends to be nonlinearly distributed in the plasma of rats, but the nonlinear plasma disposition in rats cannot be fully characterized as the toxicity of TXD258 in rats precludes investigation of higher doses. Nevertheless, as the nonlinearity of the uptake by the brain is greater in mice than in rats, the nonlinear distribution of TXD258 could be due more to a nonlinear brain process at the BBB. This non-proportional increase in brain TXD258 appears when infused dose of TXD258 is higher than 30 mg m−2 in mice, which corresponds to a plasma concentration of ∼8.4 μg ml−1. This threshold value is extremely close to that found in the brain in situ. Thus, the in situ brain perfusion study confirms the saturation of the BBB by TXD258 with an apparent IC50 of ∼11 μg ml−1 (∼13 μM) and approximately three-fold greater transport of [14C]-TXD258 into the brain in both species. This is supported by the extremely sigmoid relation between Kin and the plasma concentrations of TXD258. The coefficient of sigmoidicity (n) was 23 for both mice and rats, demonstrating that P-gp rapidly becomes saturated as the plasma concentration of TXD258 increases. The shape of this curve is not owing to the disruption of the BBB; vascular space of the integrity marker [3H]-sucrose was close to normal in all the in situ brain perfusion experiments. Therefore, the greater concentrations of TXD258 in the brain indicated by the pharmacokinetic and in situ brain perfusion studies cannot be due to an opening of the BBB. The uptake of TXD258 by the brains of rodents is much lower than that predicted from its lipophilicity, as its log P(octanol/water) is 3.88. This could be partly because of the interaction of taxanes with P-gp.
We investigated the influence of the P-gp-mediated efflux across the BBB on the accumulation of [14C]-TXD258 in the brain by perfusing the brains of wild-type and P-gp deficient mdr1a(−/−) mice. The concentration of TXD258 in the brain of P-gp-deficient mice was 3.0-fold higher than in the brains of wild-type mice. The P-gp modulator (±)-verapamil enhanced the concentration of [14C]-TXD258 in the brains of wild-type mice ∼3.0-fold over that of the brains of wild-type mice not given verapamil. In addition, the brains of rats given verapamil had 4.7-times more [14C]-TXD258 than those of rats not perfused with verapamil. Thus, the increase in the transport of TXD258 across the BBB is due to saturation of P-gp. This saturation is complete in mice and almost complete in rats as the highest TXD258 concentration was approximately three-times greater than controls, while verapamil produced a 4.7-fold increase in brain TXD258 in rats. We carried out a trans-efflux zero study in rats and mice to look at the mechanics of efflux. Different rates of efflux led to a 1.2-fold decrease in the apparent brain [14C]-TXD258 distribution in mice and a 2.5-fold decrease in the brains of rats that were loaded with [14C]-TXD258 for 60 s and washed out for 30 s. This efflux could be as a result of nonspecific binding of TXD258 to the luminal membrane of the endothelial cells, which was removed by the wash-out, and/or to passive retro-diffusion and/or mediated efflux of TXD258 from the brain to the blood vessel lumen. The observed efflux of TXD258 was completely inhibited by (±)-verapamil in both rats and mice, suggesting that the efflux is mainly because of a P-gp activity at the BBB.
We are now faced with the question of whether this function of rodent P-gp at the BBB can be extrapolated to the human BBB. Previous studies have reported that P-gp-mediated drug resistance is well correlated with the human P-gp isoform expressed in Caco-2 or transfected L-MDR1 cells and the mdr1a isoform at the mice BBB (Adachi et al., 2001; Yamazaki et al., 2001). However, the transport of two of the 14 compounds studied by Yamazaki et al. (2001) was significantly different using human and mouse P-gp, suggesting that the maximum velocity (Vmax) of P-gp varied from one species to another. By contrast, another study found that the IC50 of the P-gp-mediated efflux of digoxin from the rat ileum, human colon and Caco-2 cells were not different (Stephens et al., 2001). However, only the Vmax values for P-gp-mediated digoxin efflux from the rat and human colons were shown to be similar. Stephens et al. (2001) therefore suggested that ‘the rat might well be a quantitatively predictive model of human intestinal efflux' mediated by P-gp. These studies thus suggest that it is possible to extrapolate the action of P-gp in rodents to humans. This may in part be because the sequences of mdr1a and the MDR1 P-gp isoform are 88% identical (Gottesman & Pastan, 1993). However, despite this great similarity, several factors such as differences between tissues in membrane lipid composition and P-gp density can significantly modulate the transport kinetics of P-gp (Romsicki & Sharom, 1999; Stephens et al., 2001). Finally, the presence of several distinct drug-binding sites on P-gp (Martin et al., 2000) may further confuse any attempt at defining a single rule for interspecies extrapolation. It is therefore difficult to extrapolate data on TXD258 in rodents to humans. We have found similar IC50 values for the transport of TXD258 by P-gp at the BBB of rats and mice, but the velocity of P-gp efflux appears to be higher in the rat than in the mouse, as suggested by the resistance obtained with verapamil and the wash-out rates in the two species. Further in situ studies at the human BBB are needed to indicate whether it is possible to extrapolate P-gp mediated transport at the rodent BBB to that of humans.
These data provide new insights into the way cytotoxic agents like TXD258 are delivered to the brain. The brain-drug exposure can be increased as long as the plasma concentrations exceed the concentration needed to saturate the P-gp. Rapid or short infusions that raise the plasma peak concentration above this threshold helped to increase the AUC for brain TXD258 7.5-fold compared to slow infusion. From a pharmaceutical point of view, the rate at which this new antitumor drug can be delivered to the CNS is critical for its antitumor action. These data extend the definition of Pardridge, which defines the brain uptake by the product of the drug permeability through the BBB with the AUC (Pardridge et al., 1975). Our data clearly demonstrate that plasma Cmax has to be considered as much as AUC when transporters regulate the transport of the drug into the brain. The critical pharmacokinetic parameter limiting the uptake of a P-gp-sensitive compound like TXD258 into the brain is that the drug AUC must remain above the saturating threshold of P-gp for a certain time. This could have several advantages, such as overcoming P-gp efflux, and allowing this taxoid to work more effectively by increasing the amount of antitumor agent delivered to the brain tumour environment. This P-gp-mediated efflux might also be circumvented at the brain tumor level itself as suggested by recent in vitro studies on human cancer cell lines with rhodamine-123 transport and taxoids (Ferlini et al., 2000). It was found that other novel antitumor taxanes could inhibit P-gp-mediated transport (Ferlini et al., 2000). However, these may also have disadvantages, in terms of safety, for use in humans. The risks of neurotoxicity caused by the greater exposure of the brain to TXD258 require further investigation. The etiology of the neurotoxicity caused by taxanes remains unclear as autoradiographic studies have shown no detectable concentration of paclitaxel in the peripheral and central nervous system when it is dissolved in dimethyl sulfoxide instead of cremophor EL (Lesser et al., 1995). Cremophor EL can cause neurotoxicity, which is reported to be one of the main side effects of paclitaxel, causing both axon degeneration and demyelination (Onetto et al., 1993). This suggests that the neurotoxicity of paclitaxel is enhanced by cremophor EL, as docetaxel causes less neurotoxicity than paclitaxel (Verweij et al., 1994).
In conclusion, P-gp-mediated transport at the BBB can be saturated when the circulating concentration of compound like TXD258 is high enough to be therapeutically useful. This raises important questions as to how drug delivery can be optimized to ensure an adequate concentration in the brain while avoiding the risks of drug – drug interaction and the variability in efficacy.
Acknowledgments
We thank Dr Marcel Debray (Laboratory of Biomathematics, Faculty of Pharmacy, Paris V) for mathematical help, Christophe Gros and Alain Grelier for technical assistance for the kinetic study and M. Owen Parkes for editing the English text. This study was supported by Aventis Pharma Contract No. DMPK/FR99- 0096.
Abbreviations
- AUC
area under the curve
- BBB
blood–brain barrier
- CNS
central nervous system
- Kp
brain-to-plasma area under the curve ratio
- MDR
multidrug resistance
- P-gp
P-glycoprotein
- PS80
polysorbate 80
References
- ADACHI Y., SUZUKI H., SUGIYAMA Y. Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharma. Res. 2001;18:1660–1668. doi: 10.1023/a:1013358126640. [DOI] [PubMed] [Google Scholar]
- AMBUDKAR S.V., DEY S., HRYCYNA C.A., RAMACHANDRA M., PASTAN I., GOTTESMAN M.M. Biochemical, cellular and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- BISSERY M.C., BOUCHARD H., RIOU J.F., VRIGNAUD C., COMBEAU J., BOURZAT J.D., COMMERCON A., LAVELLE F.Preclinical evaluation of TXD258, a new taxoid. Proceedings of the 91st American Association for Cancer Research 2000San Francisco, CA; 214April 1 – 541p [Google Scholar]
- BISSERY M.C., NOHYNEK G., SANDERINK G.J., LAVELLE F. Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: preclinical experience. Anticancer Drugs. 1995;6:339, 363–355. doi: 10.1097/00001813-199506000-00001. [DOI] [PubMed] [Google Scholar]
- CLARKE S.J., RIVORY L.P. Clinical pharmacokinetics of docetaxel. Clin. Pharmacokinet. 1999;36:99–114. doi: 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- DAGENAIS C., ROUSSELLE C., POLLACK G.M., SCHERRMANN J.M. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein deficient mice. J. Cerebr. Blood Flow Metab. 2000;20:381–386. doi: 10.1097/00004647-200002000-00020. [DOI] [PubMed] [Google Scholar]
- DYKES D.J., SARSAT J.P., BISSERY M.C.Efficacy evalua-tion of TXD258, a taxoid compound against orthotopic and subcutaneous glioblastomas. Proceedings of the 91st American Association for Cancer Research 2000San Francisco, CA; 301April 1–541p [Google Scholar]
- EISEMAN J.L., EDDINGTON N.D., LESLIE J., MACAULEY C., SENTZ D.L., ZUHOWSKI M., KUJAWA J.M., YOUNG D., EGORIN M.J. Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemother. Pharmacol. 1994;34:465–471. doi: 10.1007/BF00685656. [DOI] [PubMed] [Google Scholar]
- FERLINI C., DISTEFANO M., PIGNATELLI F., LIN S., RIVA A., BOMBARDELLI E., MANCUSO S., OJIMA I., SCAMBIA G. Antitumour activity of novel taxanes that act at the same time as cytotoxic agents and P-glycoprotein inhibitors. Br. J. Cancer. 2000;83:1762–1768. doi: 10.1054/bjoc.2000.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANNI L., KEARNS C.M., GIANI A., CAPRI G., VIGANO L., LACATELLI A., BONADONNA G., EGORIN M.J. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J. Clin. Oncol. 1995;13:180–190. doi: 10.1200/JCO.1995.13.1.180. [DOI] [PubMed] [Google Scholar]
- GOTTESMAN M.M., PASTAN I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- HEIMANS J.J., VERMORKEN J.B., WOLBERS J.G., EELTINK C.M., MEIJER O.W., TAPHOORN M.J., BEIJNEN J.H. Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann. Oncol. 1994;5:951–953. doi: 10.1093/oxfordjournals.annonc.a058736. [DOI] [PubMed] [Google Scholar]
- KLECKER R.W., JAMIS-DOW C.A., EGORIN M.J., ERKMEN K., PARKER R.J., STEVENS R., COLLINS J.M. Effect of cimetidine, probenecid, and ketoconazole on the distribution, biliary secretion, and metabolism of [3H]taxol in the Sprague – Dawley rat. Drug Metab. Dispos. 1994;22:254–258. [PubMed] [Google Scholar]
- LESSER G.J., GROSSMAN S.A., ELLER S., ROWINSKY E.K. The distribution of systemically administered [3H]-paclitaxel in rats: a quantitative autoradiographic study. Cancer Chemother. Pharmacol. 1995;37:173–178. doi: 10.1007/BF00685646. [DOI] [PubMed] [Google Scholar]
- LIN S., OJIMA I. Recent strategies in the development of taxane anticancer drugs. Exp. Opin. Ther. Patents. 2000;10:869–889. [Google Scholar]
- MARTIN C., BERRIDGE G., HIGGINS C.F., MISTRY P., CHARLTON P., CALLAGHAN R. Communication between multiple drug binding sites on P-glycoprotein. Mol. Pharmacol. 2000;58:624–632. doi: 10.1124/mol.58.3.624. [DOI] [PubMed] [Google Scholar]
- ONETTO N., CANETTA R., WINOGRAD B., CATANE R., DOUGAN M., GRECHKO J., BURROUGHS J., ROZENCWEIG M. Overview of Taxol safety. J. Natl. Cancer Inst. Monogr. 1993;15:131–139. [PubMed] [Google Scholar]
- PARDRIDGE W.M., CONNOR J.D., CRAWFORD I.L. Permeability changes in the blood – brain barrier: causes and consequences. C.R.C. Crit. Rev. Toxicol. 1975;2:159–199. doi: 10.3109/10408447509079857. [DOI] [PubMed] [Google Scholar]
- ROMSICKI Y., SHAROM F.J. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry. 1999;38:6887–6896. doi: 10.1021/bi990064q. [DOI] [PubMed] [Google Scholar]
- ROUSSELLE C., CLAIR P., LEFAUCONNIER J.M., KACZOREK M., SCHERRMANN J.M., TEMSAMANI J. New advances in the transport of doxorubicin through the blood – brain barrier by a peptide vector-mediated strategy. Mol. Pharmacol. 2000;57:679–686. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- SCHINKEL A.H. P-glycoprotein, a gatekeeper in the blood – brain barrier. Adv. Drug. Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- SMITH Q.R.Brain perfusion systems for studies of drug uptake and metabolism in the central nervous system Models for Assessing Drug Absorption and Metabolism 1996New York: Plenum Press; 285–307.ed. Borchardt, R.T., Smith, P.L. & Wilson, G. pp [DOI] [PubMed] [Google Scholar]
- SPARREBOOM A., VAN TELLINGEN O., NOOIJEN W.J., BEIJNEN J.H. Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res. 1996;56:2112–2115. [PubMed] [Google Scholar]
- STEIN W.D. Orlando, FL: Academic Press; 1986. Transport and Diffusion Across Cell Membranes. [Google Scholar]
- STEPHENS R.H., O'NEILL C.A., WARHURST A., CARLSON G.L., ROWLAND M., WARHURST G. Kinetic profiling of P-glycoprotein-mediated drug efflux in rat and human intestinal epithelia. J. Pharmacol. Exp. Ther. 2001;296:584–591. [PubMed] [Google Scholar]
- TAKASATO Y., RAPOPORT S.I., SMITH Q.R. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol. 1984;247:H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- TRIGUERO D., BUCIAK J., PARDRIDGE W.M. Comparison of in vitro and in vivo models of drug transcytosis through the blood – brain barrier. J. Pharmacol. Exp. Ther. 1990;253:884–891. [PubMed] [Google Scholar]
- TSUJI A., TAMAI I. Blood – brain barrier function of P-glycoprotein. Adv. Drug Del. Rev. 1997;25:287–298. [Google Scholar]
- VAN TELLINGEN O., BEIJNEN J.H., VERWEIJ J., SCHERRENBURG E.J., NOOIJEN W.J., SPARREBOOM A. Rapid esterase-sensitive breakdown of polysorbate 80 and its impact on the plasma pharmacokinetics of docetaxel and metabolites in mice. Clin. Cancer Res. 1999a;5:2918–2924. [PubMed] [Google Scholar]
- VAN TELLINGEN O., HUIZING M.T., PANDAY V.R., SCHELLENS J.H., NOOIJEN W.J., BEIJNEN J.H. Cremophor EL causes (pseudo-) non-linear pharmacokinetics of paclitaxel in patients. Br. J. Cancer. 1999b;81:330–335. doi: 10.1038/sj.bjc.6690696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ZUYLEN L., VERWEIJ J., SPARREBOOM A. Role of formulation vehicles in taxane pharmacology. Invest. New Drugs. 2001;19:125–141. doi: 10.1023/a:1010618632738. [DOI] [PubMed] [Google Scholar]
- VERWEIJ J., CLAVEL M., CHEVALIER B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann. Oncol. 1994;5:495–505. doi: 10.1093/oxfordjournals.annonc.a058903. [DOI] [PubMed] [Google Scholar]
- VRIGNAUD P., LEJEUNE P., CHAPLIN D., LAVELLE F., BISSERY M.C.In vivo efficacy of TXD258, a new taxoid, against human tumor xenografts. Proceedings of the 91st American Association for Cancer Research 2000San Francisco, CA; 214April 1–5, Vol.41p [Google Scholar]
- YAMAZAKI M., NEWAY W.E., OHE T., CHEN I., ROWE J.F., HOCHMAN J.H., CHIBA M., LIN J.H. In vitro substrate identification studies for P-glycoprotein-mediated transport: species difference and predictability of in vivo results. J. Pharmacol. Exp. Ther. 2001;296:723–735. [PubMed] [Google Scholar]