Abstract
We have previously reported that the inhibitory gamma subunit of the rod photoreceptor type 6 cyclic GMP phosphodiesterase (PDEγ) is expressed in nonretinal tissues and is involved in the stimulation of the p42/p44 mitogen-activated protein kinase (MAPK) pathway by growth factors and G-protein-coupled receptor agonists. We have now investigated whether PDEγ plays a role in modulating chronic hypoxic-dependent mitogenic signalling pathways in pulmonary smooth muscle from rats with pulmonary hypertension (PHT).
We show for the first time that PDEγ is expressed in rat main, first, intrapulmonary and resistance pulmonary arteries. Moreover, its expression is increased in all the arteries to varying extents by chronic hypoxia. The extent of the increased expression of PDEγ is correlated with an enhanced activation of p42/p44 MAPK in these vessels.
We also report that PDEγ translation from mRNA transcript is increased in cultured human pulmonary artery smooth muscle cells subjected to chronic hypoxia for 14 days. This was correlated with hypoxic-dependent increase in p42/p44 MAPK activation.
In conclusion, our studies identify for the first time a major chronic hypoxic-dependent change in the phenotypic expression of an intermediate protein regulating mitogenic signalling in pulmonary arteries. This may have a significant effect on arterial remodelling in PHT. Future studies will focus on strategies designed to knockout rod PDEγ to assess whether this rescues rats from chronic hypoxic-dependent changes in arterial remodelling and PHT.
Keywords: Phosphodiesterase gamma subunit, mitogen-activated protein kinase, pulmonary hypertension, smooth muscle, vasodilator, vasoconstrictor
Introduction
The pulmonary vascular bed is a low-pressure system with a resistance approximately one-tenth of the systemic circulation. In the normal lung, pulmonary vascular tone is regulated by a balance between the effects of vasodilators/antiproliferative agents, such as prostacyclin and nitric oxide and vasoconstrictors/comitogens, such as serotonin and endothelin-1 (Fishman, 1998; Channick & Rubin, 2000). Primary pulmonary arterial hypertension (PPH), also referred to as idiopathic pulmonary hypertension (PHT), is the clinical term used to describe a rare condition associated with progressive elevation in pulmonary arterial pressure and pulmonary vascular remodelling, for which no underlying cause can be found. The familial form of PPH is associated with heterozygous germline mutations of the bone morphogenetic protein receptor type II gene, a component of transforming growth factor-α signalling pathway (Lane et al., 2000). This mutation is not present in all familial cases and is associated with only 32% of sporadic PPH patients. Hence, other factors are also believed to play a critical role in the aetiology of this disease. An objective of the current study is to investigate these other potential factors in the development of PHT. PHT occurs more frequently secondary to cardio-respiratory diseases, such as left ventricular failure, chronic obstructive pulmonary disease and exposure to hypoxic conditions. An imbalance between the vasoconstrictor/vasodilator and mitogenic/antimitogenic effects of endogenous mediators is thought to contribute to PHT (MacLean, 1999).
Vascular remodelling is brought about by excessive smooth muscle cell proliferation. Mitogenic stimuli initiate cell proliferation via different classes of cell surface receptors that include growth factor receptor tyrosine kinase receptors and G-protein-coupled receptors (GPCRs). This involves stimulation of the p42/p44 mitogen-activated protein kinase or extracellular signal regulated kinase (p42/p44 MAPK or ERK) pathway (Marshall, 1995; Van Biesen et al., 1995). The phototransduction cascade involving rhodopsin (GPCR), G-protein-coupled receptor kinase (GRK) and β-arrestin (Stryer, 1991) bears many similarities with signalling by growth factors and GPCRs in other mammalian cell systems. The phototransduction cascade involves cGMP phosphodiesterases that are expressed in rod and cone photoreceptors (termed PDE6) as tetrameric proteins composed of two catalytic subunits and two γ subunits phosphodiesterase (PDEγ). PDEγ inhibits cGMP hydrolysis at the catalytic sites. We are interested in the possibility that the PDEγ might be expressed in other mammalian tissues, where it may regulate distinct receptor-G-protein-mediated pathways. Indeed, we have found that rod PDEγ is expressed in lung, kidney testes, liver, heart, airway smooth muscle and human embryonic kidney 293 cells, and have shown its absence from these tissues in rod PDEγ knockout mice (Tate et al., 1998,2002; Frame et al., 2001). In recent studies, we showed that rod and cone PDEγ (termed here PDEγ1 and PDEγ2, respectively, and which differ only in their N-terminal region) interact with the G-protein-coupled receptor kinase-2 (GRK2) signalling system to regulate the epidermal growth factor- and thrombin-dependent stimulation of p42/p44 MAPK in human embryonic kidney 293 cells (Wan et al., 2001). Thrombin also stimulated the association of endogenous rod PDEγ with dynamin I, which plays a critical role in regulating endocytosis of receptor signal complexes required for activation of p42/p44 MAPK by certain growth factors and GPCR agonists. PDEγ1/2 appears to play an important role in promoting endocytosis of receptor signal complexes leading to the activation of p42/p44 MAPK and cell proliferation (Wan et al., 2001).
We have previously shown that PDEγ1can also bind to the cGMP-binding cGMP-specific PDE (PDE5). This was evidenced by the fact that PDEγ1converts PDE5 to a conformation that is more sensitive to attack by proteases, such as caspase-3 (Frame et al., 2001). This is an important finding as it suggests that PDEγ1 may function to localise PDE5 in signalling complexes (containing dynamin II) that are organised to stimulate the p42/p44 MAPK pathway. This would have the effect of protecting the p42/p44 MAPK signalling pathway from the inhibitory action of cyclic GMP. We have also reported that the cGMP inhibited cAMP PDE (PDE3A/B) and PDE5 expression and activity are increased in pulmonary vessels from rats maintained under chronic hypoxic conditions (MacLean et al., 1997; Murray et al., 2002). This is predicted to afford more protection to mitogenic signalling pathways from the inhibitory action of cyclic nucleotides and might provide, in part, an explanation for enhanced pulmonary vessel remodelling in PHT.
Therefore, we have assessed whether PDEγ is regulated by chronic hypoxia in pulmonary vessels and smooth muscle cells. Since PDEγ governs the extent to which growth factor and GPCR stimulate the p42/p44 MAPK pathway (Wan et al., 2001), any change in its expression level might have a profound effect on mitogenic signalling and cellular proliferation in PHT. We have therefore investigated (a) whether pulmonary smooth muscle express PDEγ1/2 and (b) the effect of chronic hypoxia on the expression of PDEγ1/2 in rats and correlation with any change in p42/p44 MAPK activation. In addition, we have compared results from the animal model of PHT with a cultured human pulmonary artery smooth muscle cells (PASMC) cell model.
Methods
Materials
All biochemicals were from Boehringer Mannheim (Germany), while general chemicals were from Sigma Chemical Co. (Poole, U.K.). Nitrocellulose was from Amersham Pharmacia Biotech (Amersham, U.K.). Cell culture supplies were from Life Technologies (Paisley, U.K.). RNeasy total RNA isolation kit and QIA shredder were from Qiagen (Crawley, U.K.). Superscript II reverse transcriptase and primers were from Life Technologies (Paisley, U.K.). Dynazyme II DNA polymerase was from Flowgen (Shenstone, U.K.) and DNase was from Promega (Southampton, U.K.). GFX PCR and gel purification kits and dNTPS were from Amersham Pharmacia Biotech (U.K.). The BigDye Dye terminator cycle sequencing kit was from PE-Applied Biosystems (Warrington, U.K.). Anti-PDEγ antibody to the C-terminal domain of photoreceptor PDEγ was a kind gift from Dr R. Cote (University of New Hampshire, U.S.A.). Phospho-p42/p44 MAPK antibody was from Santa Cruz (U.S.A.).
Animal studies
Male Wistar rats of 28–30 days of age (at start of experiment) were placed in a specially designed perspex hypobaric chamber (Royal Hallamshire Hospital, Sheffield, U.K.). This was depressurised over 2 days to 550 mbar (pO2 of ∼110 mmHg (∼10% equivalent)). The temperature of the chamber was maintained at 21–22°C and the chamber was ventilated with air at approximately 45l min−1. Animals were maintained in these hypoxic/hypobaric conditions for 14 days. Age-matched controls were maintained under normoxic/normobaric room conditions (20% v v−1 oxygen). Following killing, the right ventricle (RV) of the heart was carefully dissected free of the septum and left ventricle, and this was blotted lightly and weighed. PHT was assessed by measuring the ratio of RV/total ventricular (TV) weight. This is a well-established index of the degree of PHT in rats (Welsh et al., 2001). Pulmonary arteries were then dissected and taken for biochemical analysis.
Cell culture
Human pulmonary smooth muscle cells (Clontech, derived from main and first branch arteries) were maintained in Dulbecco's Eagle's Medium containing 10% (v v−1) foetal calf serum and 10% (v v−1) donor horse serum and grown to confluence on six-well plates. After this time, cells will be maintained under normoxic and hypoxic conditions (550 bar, 10% O2, 0.3% CO2, balance N2) for 1–14 days.
Homogenate preparation
Pulmonary vessels were combined with buffer containing 0.25 M sucrose, 10 mM Tris/HCl, pH 7.4, 1 mM EDTA, 0.1 mM phenylmethylsulphonyl fluoride and 2 mM benzamidine. The tissue was rinsed three times with this buffer and homogenised using a turrex homogeniser.
Total RNA extraction
RNeasy lysis buffer (600 μl) was added to pulmonary smooth muscle homogenates. These were passed through a QIAshredder. Total RNA was extracted according to the RNeasy protocol instructions. This included an incubation of the extracted RNA with 4 U of DNase at 37°C for 15 min to remove potential contaminating genomic DNA carried over during RNA purification.
Reverse transcriptase reaction
First strand synthesis was carried out using 5 μg extracted total RNA and Superscript II reverse transcriptase. The reaction was primed using 500 ng of oligo (dT)18. This mixture was heated to 70°C for 10 min and quick chilled on ice. The reverse transcriptase reaction was incubated at 42°C for 90 min and terminated by heating at 70°C for 15 min.
PCR
The PCR reaction was carried out using the following protocol: 95°C for 1 min and 25–40 cycles of 95°C for 30 s, 55°C for 30 s and 68°C for 2 min. This was followed by a final extension of 10 min at 65°C.
Reverse transcriptase polymerase chain reaction (RT-PCR) with specific forward and reverse oligonucleotide primers was used to amplify PDEγ1/2 transcripts. For PDEγ1, the forward primer used was ATGAACCTGGAGCCACCC, while the reverse primer was AAT GAT GCC ATA CTG GGC CAG. A restriction tagged PDEγ2 forward primer was used corresponding to CTACTCTGCCTGCTGCAGCTTC, while the reverse primer, GGCACTTCAGATAATCCCAAACTG (designed to amplify a 282 bp product containing the full ORF of PDEγ2 of 252 bp).
Sequence analysis
The purified amplicons were sequenced, in both directions, on a PE-Applied Biosystems Division Model 373A automated DNA sequencer using the PCR primers and a BigDye Dye terminator cycle sequencing kit.
Western blotting
Nitrocellulose sheets were blocked in 5% gelatin in phosphate-buffered serum (PBS) at 37°C for 1 h and then probed with antibodies in PBS containing 1% gelatin (w v−1) plus 0.05% (v v−1) NP40 at 37°C for 12 h. After this time, the nitrocellulose sheets were washed in PBS plus 0.05% (v v−1) NP40. Detection of immunoreactivity was by incubating nitrocellulose sheets for 2 h at 37°C with a reporter HRP-linked anti-rabbit antibody in PBS containing 1% gelatin (w v−1) plus 0.05% (v v−1) NP40. After washing the blots as described above, to remove excess reporter antibody, immunoreactive bands were detected using an enhanced chemiluminesence detection kit.
Quantification
RT-PCR and Western blotting results were quantified by densitometry (linear range of optical density between 0 and 1 arbitary unit).
Statistics
Student's t-test was performed for Rv/TV and densitometry results.
Results
PHT
Pulmonary hypertension was measured using RV/ TV ratio. These values were 0.249±0.005 and 0.399±0.005 for normobaric- versus hypobaric-treated animals (P<0.01, n=10).
PDEγ expression
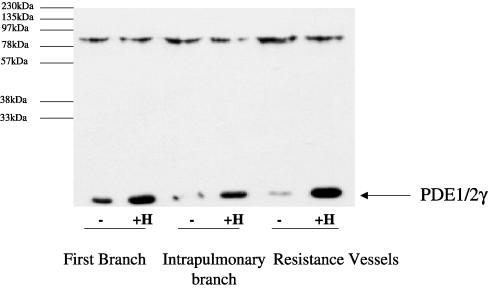
Homogenates from main, first branch, intrapulmonary and small resistance vessels were subjected to Western blotting with anti-PDEγ antibody. PDEγ (Mr=14 kDa) was expressed in the main, first, intrapulmonary and resistance arteries (Figure 1). The anti-C-terminal PDEγ antibody does not discriminate between PDEγ1 and PDEγ2. Chronic hypoxia for 14 days increased PDEγ1/2 protein levels above basal in all the arteries (Figure 1). The percentage increase in PDEγ1/2 protein expression was 26±10% in the main branch, 97±8% in the first branch, 110±9% in the intrapulmonary artery and 127±9% in the resistance vessels (n=3, P<0.05 versus normoxic animals, Student's t-test). The stimulation of PDEγ1/2 expression by chronic hypoxia is most marked in the resistance vessels. These are the first results to show that pulmonary smooth muscle express the retinal forms of PDEγ1/2, and that chronic hypoxia induced a marked increase in their protein expression. An 85 kDa protein, the identity of which is unknown, was also immunostained by the anti-PDEγ antibody (Figure 1). Significantly, chronic hypoxia did not alter the levels of the 85 kDa protein in any of the vessels, and therefore this protein serves as a useful internal control for the hypoxic-dependent changes in PDEγ expression.
Figure 1.
Western blot analysis of PDEγ1/2 in pulmonary vessels from rats maintained under chronic hypoxic conditions. Western blot analysis using an anti-PDEγ1/2 antibody showing the expression level of PDEγ1/2 in homogenates from first branch, intrapulmonary and resistance vessels from rat maintained under normoxic (−) and chronic hypoxic (+H) conditions for 14 days. In all, 10 μg protein/sample was loaded onto SDS-PAGE. Above is a representative result of three individual experiments, quantified by densitometry.
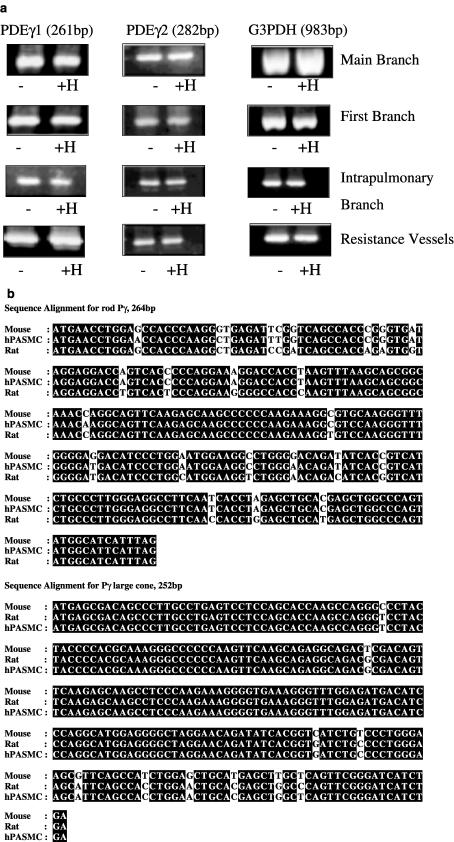
We next investigated whether changes in the protein expression of PDEγ1/2 were because of hypoxic-dependent modulation of mRNA transcription or protein translation. This was assessed by RT-PCR. RT-PCR with gene specific primers separately amplified 261 and 282 bp product corresponding to PDEγ1 and PDEγ2 (ORF of 252 bp), respectively, from all the vessels (Figure 2a). The amplicons were not obtained when reverse transcriptase was omitted from first-strand DNA synthesis (data not shown). The products did not contain intronic sequence, indicating that they are derived from authentic mRNA transcript. The alignment of the 261 and 252 bp products with the mouse rod and cone photoreceptor PDEγ, respectively, revealed >90% similarity in their nucleotide sequences (Figure 2b). These results confirm that the amplicons correspond to PDEγ1/2 mRNA transcripts obtained from all the vessels. Chronic hypoxia had no effect on PDEγ transcript levels in any of the vessels (Figure 2a). As an additional control for the RT-PCR, we amplified G3PDH transcript from the same total RNA samples with specific primers to ensure that equal amounts of total RNA had been used for the amplification of PDEγ transcripts. Identical amounts of G3PDH transcript were amplified under normoxic and hypoxic conditions from each individual vessel (Figure 2a). Using these RT-PCR conditions, we have previously observed a chronic hypoxic-dependent increase in PDE3 and PDE5 transcript levels from the same samples used for RT-PCR of the PDEγ1/2 transcripts (Murray et al., 2002). In addition, as no significant differences could be seen in the levels of G3PDH, this confirms that equal amounts of total RNA had been used for the amplification of the PDEγ1 and PDEγ2 transcripts.
Figure 2.
RT-PCR of PDEγ1, PDEγ2 and G3PDH transcripts in pulmonary vessels from rats maintained under normoxic and chronic hypoxic conditions. (a) RT-PCR with specific primers for PDEγ1, 261 bp; PDEγ2, 282 bp and G3PDH, 983 bp, from main, first branch, intrapulmonary and resistance vessels from Wistar rats maintained under normoxic (−) and chronic hypoxic (+H) conditions for 14 days. Total RNA/sample (1 μg) was used as a template for cDNA synthesis, of which one-fifth was used for each RT-PCR. This is a representative result of four individual experiments, quantified by densitometry. (b) cDNA sequence analysis of PDEγ1 and 2 from pulmonary smooth muscle.
Effect of chronic hypoxia on the phosphorylation of p42/p44 MAPK in rat PA
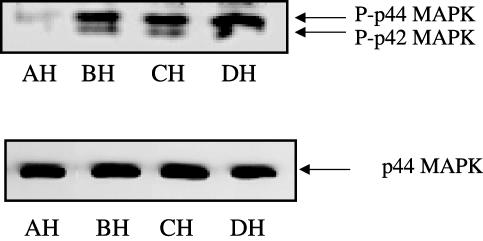
We next investigated whether the chronic hypoxic-dependent increase in PDEγ1/2 expression could be correlated with similar changes in the activation status of p42/p44 MAPK in the vessels. Figure 3 shows that phosphorylated p42/p44 MAPK (Mr=42/44 kDa) was present in the main pulmonary artery, the first branch pulmonary artery, the intrapulmonary artery and the resistance vessels. Interestingly, the extent to which p42/p44 MAPK was phosphorylated increased in vessels where PDEγ1/2 protein expression was also elevated by chronic hypoxia (Figure 3). The percentage increase in phosphorylated p42/p44 MAPK in each vessel versus main branch (100±4%) was 146±9% in the first branch, 154±7% in the intrapulmonary artery and 184±11% in the resistance vessels (n=3, P<0.05 versus main branch vessel, Student's t-test). The expression of p44 MAPK was shown to be very similar in each vessel. Therefore, chronic hypoxic-dependent changes in the phosphorylation status of p42/p44 MAPK is not because of an increase in the expression of this kinase. The largest increase in the phosphorylation of p42/p44 MAPK was observed in the resistance vessels, and correlated with the most pronounced increase in PDEγ1/2 expression.
Figure 3.
Phosphorylation of p42/p44 MAPK in pulmonary vessels from rats maintained under chronic hypoxic conditions. Western blot analysis showing phosphorylated p42/p44 MAPK in homogenates from main, first branch, intrapulmonary and resistance vessels from rat maintained under chronic hypoxic (H) conditions for 14 days. In all, 10 μg protein/sample was loaded onto SDS-PAGE. This is a representative result of three individual experiments, quantified by densitometry. A=main branch, B=first branch, C=intrapulmonary arteries, and D=resistance arteries.
Cell culture model
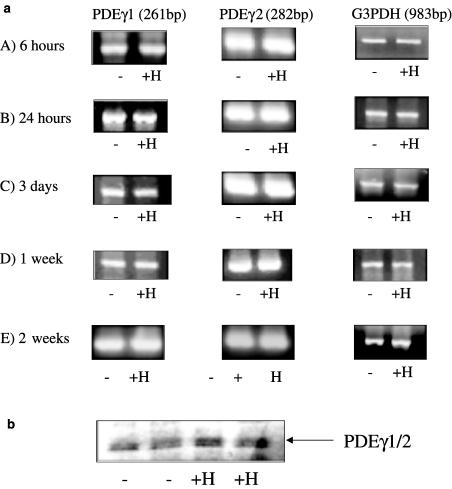
We next investigated whether similar changes in PDEγ1/2 expression and p42/p44 MAPK phosphorylation was evident in cultured hPASMC maintained under chronic hypoxic conditions for 14 days. Figure 4a shows that hPASMC contain PDEγ1 and PDEγ2 transcripts. Figure 4a shows that there is no significant difference in PDEγ1, PDEγ2 or G3PDH transcript levels after 6 h, 24 h, 7 or 14 days of chronic hypoxia. Alignment of the PDEγ1 and PDEγ2 from hPASMC with the published mouse sequence and the rat pulmonary artery sequence all revealed >90% similarity (see Figure 2b). However, the PDEγ1/2 protein expression, detected by Western blotting cell lysates with anti-C-terminal PDEγ antibody, was significantly increased by 65±7% (n=3, P<0.05 versus normoxic hPASMC, Student's t-test) after 14 days of chronic hypoxia (Figure 4b). These results are therefore correlated with the rat model of PHT.
Figure 4.
RT-PCR of PDEγ1, PDEγ2 and G3PDH transcripts from hPASMCs exposed to chronic hypoxic (10% O2) or normoxic conditions. (a) RT-PCR amplification using specific primers for PDEγ1, 261 bp; PDEγ2, 282 bp and G3PDH, 983 bp from hPASMC maintained under normoxic (−) and chronic hypoxic (+H) conditions for (A) 6 h, (B) 24 h, (C) 3 days, (D) 1 or (E) 2 weeks. Total RNA/sample (1 μg) was used as a template for cDNA synthesis, of which one-fifth was used for each RT-PCR. (b) Western blot analysis using an anti-PDEγ1/2 antibody showing the expression level of PDEγ1/2 in lysates from cells maintained under normoxic (−) and chronic hypoxic (+H) conditions for 14 days. These are representative results of three individual experiments, quantified by densitometry.
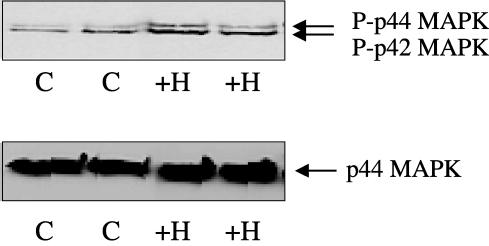
We also observed a 54±3%; increase (n=3) in phosphorylated p42/p44 MAPK levels versus normoxic cells (Figure 5). Thus, chronic hypoxia increases basal p42/p44 MAPK activation, correlated with increased PDEγ1/2 protein expression. There was no modulation of p44 MAPK expression by chronic hypoxia.
Figure 5.
Phosphorylation of p42/p44 MAPK in hPASMCs exposed to chronic hypoxic (10% O2) or normoxic conditions. Western blot analysis showing phosphorylated p42/p44 MAPK in hPASMC maintained under normoxic (−) and chronic hypoxic (+H) conditions for 14 days. In all, 10 μg protein/sample was loaded onto SDS-PAGE. Above is a representative result of three individual experiments, quantified by densitometry.
Discussion
A major finding of this study is that chronic hypoxia induced a substantial increase in the expression of PDEγ1/2 in the first branch, intrapulmonary and resistance vessels. It appears that chronic hypoxia increases translation of PDEγ1/2 transcript in these vessels. This might be because of activation of a translation pathway per se or increased mRNA stability, although in the latter case there was no change in absolute mRNA levels. These findings are significant because we have shown that PDEγ1 and PDEγ2 govern the extent to which p42/p44 MAPK is activated by both tyrosine kinase- and G-protein-coupled receptor-dependent stimulation of p42/p44 MAPK in mammalian cells (Wan et al., 2001). Therefore, increased expression of PDEγ1/2 improves the efficiency of mitogenic signalling from these receptor classes. In this regard, we have shown for the first time that a chronic hypoxic-dependent increase in p42/p44 MAPK phosphorylation/activation was correlated with the increase in PDEγ1/2 expression in all the pulmonary vessels, with the most pronounced effect in the resistance arteries. This is a significant finding, as p42/p44 MAPK activation plays a key role in regulating cell proliferation and is therefore likely to have an important role in pulmonary vessel remodelling in PHT. This conclusion is supported by several lines of previous evidence regarding the key role of p42/p44 MAPK in hypoxic-dependent PHT. First, others have shown that chronic and acute hypoxia induced temporal activation of p42/p44 MAPK (Jin et al., 2000; Das et al., 2001). In the case of chronic treatment, the hypoxic-dependent increase in p42/p44 MAPK activation peaked at 7 days and declined thereafter. Second, Premkumar et al. (2000) showed that hypoxia activates p42/p44 MAPK and that this was essential for stimulation of c-fos expression via the cis serum response element, a critical immediate early gene involved in regulating mitogenesis. Third, Minet et al. (2000) demonstrated that hypoxic-dependent stimulation of p42/p44 MAPK was essential for hypoxia-inducible factor-1 transactivation activity. The hypoxic-dependent induction of endothelial growth factor receptor, PDGF receptor and egr-1 mRNA was correlated with p42/p44 MAPK activation. These authors also concluded that the temporal activation of the p42/p44 MAPK pathway appears to be associated with hypoxia-induced pulmonary arterial remodelling.
PDEγ1/2 might increase activation of components involved in p42/p44 MAPK signalling (e.g. increased rate of endocytic signalling) and/or reduce MAPK phosphatase activity. The increase in PDEγ1/2 expression and its ability to prolong temporal activation of p42/p44 MAPK, along with other hypoxic-dependent factors, might have a significant effect on gene induction and pulmonary artery remodelling. Indeed, prolongation of the activation of p42/p44 MAPK is required for mitogenesis in smooth muscle (Marshall, 1995; Orsini et al., 1999). Welsh et al. (2001) have also shown that p38 MAPK and p42/p44 MAPK are activated in fibroblasts derived from remodelled arteries, but not from systemic circulation from rats exposed to chronic hypoxic conditions. Moreover, such fibroblasts show sustained proliferative responses that can be prevented by p38 MAPK inhibitors. In this regard, we are currently assessing whether rod PDEγ is also involved in regulating p38 MAPK signalling.
We also report in this article that PDEγ1/2 translation from mRNA transcript is also increased in hPASMC subjected to chronic hypoxia for 14 days. This was correlated with hypoxic-dependent increase in basal p42/p44 MAPK activation. These results suggest that chronic hypoxia may induce release of a factor (e.g. growth factor) that can act back on smooth muscle receptors to regulate p42/p44 MAPK via a pathway involving PDEγ1/2. This would explain how hypoxic-dependent increased expression of PDEγ1/2 might elevate so-called ‘basal' p42/p44 MAPK activation.
In conclusion, our studies identify for the first time a major hypoxic-dependent change in the phenotypic expression of a novel intermediate protein regulating mitogenic signalling in pulmonary arteries. This may have a significant effect on arterial remodelling in PHT. Future studies will focus on strategies designed to knockout rod PDEγ to assess whether this rescues rats from chronic hypoxic-dependent changes in arterial remodelling and reverses transcription factor activation.
Acknowledgments
We thank the Universities of Glasgow and Strathclyde for awarding a University Studentship to FM.
Abbreviations
- ERK
extracellular signal regulated kinase
- GRK
G-protein-coupled receptor kinase
- GPCR
G-protein-coupled receptor
- H
chronic hypoxia
- MAPK
mitogen-activated protein kinase
- PDE
phosphodiesterase
- PHT
pulmonary hypertension
- RT-PCR
reverse transcriptase polymerase chain reaction
References
- CHANNICK R.N., RUBIN L.J. Combination therapy for pulmonary hypertension: a glimpse into the future. Crit. Care Med. 2000;28:896–897. doi: 10.1097/00003246-200003000-00055. [DOI] [PubMed] [Google Scholar]
- DAS M., BOUCHEY D.M., MOORE M.J., HOPKINS D.C., NEMENOFF R.A., STENMARK K.R. Hypoxia-induced proliferative response of vascular adventitial fibroblast is dependent on G-protein activation of mitogen-activated protein kinases. J. Biol. Chem. 2001;276:15631–15640. doi: 10.1074/jbc.M010690200. [DOI] [PubMed] [Google Scholar]
- FISHMAN A.L.Etiology and pathogenesis of primary pulmonary hypertension: a perspective Chest 1998114242S–247S.suppl [DOI] [PubMed] [Google Scholar]
- FRAME M., WAN K-F., TATE R., VANDENABEELE P., PYNE N.J. The gamma subunit of the rod photoreceptor cGMP phosphodiesterase can modulate the proteolysis of two cGMP binding cGMP-specific phosphodiesterases (PDE6 and PDE5) by caspase 3. Cell. Signal. 2001;13:735–741. doi: 10.1016/s0898-6568(01)00193-0. [DOI] [PubMed] [Google Scholar]
- JIN N., HATTON N., SWARTZ D.R., XIA X.I., HARRINGTON M.A., LARSEN S.H., RHOADES R.A. Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinases, and p38 kinase in pulmonary vessels (2000) Am. J. Respir. Cell. Mol. Biol. 2000;23:593–601. doi: 10.1165/ajrcmb.23.5.3921. [DOI] [PubMed] [Google Scholar]
- LANE K.B., MACHADO R.D., PAUCIULO M.W., THOMSON J.R., PHILLIPS III J.A., LOYD J.E., NICHOLS W.C., TREMBATH R.C. Heterozygous germline mutations in BMPR2, encoding a TGF-α receptor, cause familial pulmonary hypertension. Nature. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R. Endothelin and serotonin in pulmonary hypertension. J. Lab. Clin. Med. 1999;134:105–114. doi: 10.1016/s0022-2143(99)90114-2. [DOI] [PubMed] [Google Scholar]
- MARSHALL C.J. Specificity of receptor tyrosine kinase signalling: transient versus sustained extracellular signal regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- MINET E., ARNOULD T., MICHEL G., ROLAND I., MOTTET D., RAES M., REMACLE J., MICHIELS C. ERK activation upon hypoxia:involvement of HIF-1 activation. FEBS Lett. 2000;468:53–58. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- MURRAY F., MACLEAN M.R., PYNE N.J. Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in animal and cellular models of pulmonary hypertension. Br. J. Pharmacol. 2002;137:1187–1194. doi: 10.1038/sj.bjp.0704984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSINI M.J., KRYMSKAYA V.P., ESZTERHAS A.J., BENOVIC J.L., PANETTIERI R.A., PENN R.B. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged activation of p42/p44 activation. Am. J. Physiol. 1999;277:L479–L488. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]
- PREMKUMAR D.R., ADHIKARY G., OVERHOLT J.L., SIMONSON M.S., CHERNIAK N.S., PRABHAKAR N.R. Intracellular pathways linking hypoxia to activation of c-fos and AP-1. Adv. Exp. Med. Biol. 2000;475:101–109. doi: 10.1007/0-306-46825-5_10. [DOI] [PubMed] [Google Scholar]
- STRYER L. Visual transduction. J. Biol. Chem. 1991;266:10711–10714. [PubMed] [Google Scholar]
- TATE R., LOCHHEAD A., BRZESKI H., ARSHAVSKY V.Y., PYNE N.J. The γ subunit of the rod photoreceptor cGMP-binding cGMP-specific PDE is expressed in lung. Cell Biochem. Biophys. (U.S.A.) 1998;29:133–144. doi: 10.1007/BF02737832. [DOI] [PubMed] [Google Scholar]
- TATE R.J., ARSHAVSKY V.Y., PYNE N.J. The identification of the inhibitory γ subunit of the type 6 retinal cyclic GMP phosphodiesterase in non retinal tissues: differential processing of mRNA transcripts. Genomics. 2002;79:582–586. doi: 10.1006/geno.2002.6740. [DOI] [PubMed] [Google Scholar]
- WAN K.-F., SAMBI B., FRAME M., TATE T., PYNE N.J. The inhibitory gamma sub unit of the type 6 retinal cyclic guanosine monophosphate phosphodiesteraee is a novel intermediate regulating p42/p44 mitogen-activated protein kinase signalling in human embryonic kidney 293 cells. J. Biol. Chem. 2001;276:37802–37808. doi: 10.1074/jbc.M105087200. [DOI] [PubMed] [Google Scholar]
- WELSH D.J., PEACOCK A.J., MACLEAN M., HARNETT M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am. J. Respir. Crit. Care Med. 2001;164:282–289. doi: 10.1164/ajrccm.164.2.2008054. [DOI] [PubMed] [Google Scholar]
- VAN BIESEN T., HAWES B.E., LUTTRELL D., KREUGER K.M., TOUHARA K., PORFIRI E., SAKAUE M., LUTTRELL L.M., LEFKOWITZ R.J.Receptor tyrosine kinase and Gbetagamma mediated MAPK activation by a common signalling pathway Nature 1995376781–784.(Lond) [DOI] [PubMed] [Google Scholar]