Abstract
The present investigation was undertaken to characterize the Na+/K+ pump current in small (⩽25 μm in soma diameter) dorsal root ganglion (DRG) neurons isolated from lumbar L4-6 segments of adult rats.
The Na+/K+ pump current was identified as an ouabain-sensitive current during square voltage steps to membrane potentials between +40 and −120 mV, using the whole-cell patch-clamp technique in which Ca2+ and K+ channel currents and Na+/Ca2+ exchange currents were minimized. The Na+/K+ pump current was practically time-independent over the entire voltage range examined and exhibited a voltage-dependence; its current – voltage (I–V) relationship displayed a positive slope at potentials between −120 and 0 mV but nearly plateau levels at positive membrane potentials.
The concentration-dependent block of Na+/K+ pump current (activated by 30 mM pipette Na+) by ouabain at concentrations between 0.1 μM and 5 mM was biphasic and was well described using a two-binding site model with dissociation constants for high- and low-affinity binding sites of 0.20 and 140.1 μM, respectively. The relative amplitude of the Na+/K+ pump current produced by low- and high-affinity sites (probably α1β1 and α3β1 isozymes, respectively) was estimated to be 13 : 1 in the presence of 30 mM Na+ in the pipette solution.
Additionally, the activation of Na+/K+ pump current by pipette Na+ at concentrations ranging from 5 to 100 mM also exhibited a biphasic concentration dependence which can be reasonably well fitted by assuming the existence of two isozymes having high and low affinities for Na+ (6.7 and 67.6 mM, respectively).
Thus, the present investigation provides functional evidence to suggest that the Na+/K+ ATPase comprises two functionally distinct isozymes as expected for α1β1 and α3β1 in rat small DRG neurons.
Keywords: Na+/K+ pump current, dorsal root ganglion, neuron, ouabain, α1 isoform, α3 isoform
Introduction
The Na+/K+ ATPase or electrogenic Na+/K+ pump transports three Na+ out of the cell and two K+ into the cell by using the energy derived from hydrolysis of one molecule of ATP and thereby plays a crucial role in maintaining the Na+ and K+ gradients across the plasma membrane. The steep gradients of these ions are essential for regulating the osmotic balance and for maintaining the resting membrane potential and excitable properties in electrically active cells such as neurons and muscles. The Na+/K+ ATPase is a heterodimer composed of an α-catalytic subunit with 10 membrane-spanning domains and a β-glycoprotein subunit with a single membrane-spanning domain (for review, see Sweadner, 1989; Blanco & Mercer, 1998). The α subunit exhibits a molecular mass of about 110 kDa and contains the binding site for ATP, Na+, K+ and cardiac glycoside ouabain. Thus, the catalytic, transport and pharmacological properties of the Na+/K+ ATPase are generally considered to reside in its α subunit. The smaller β subunit has a molecular weight of about 55 kDa and is necessary for the structural and functional maturation of the α subunit (Hasler et al., 1998; Abriel et al., 1999). To date, four α isoforms (α1 – α4) and three β isoforms (β1 – β3) have been identified in mammalian tissues. These α and β isoforms have been shown to be expressed in a species- and tissues-dependent pattern (for a review, see Blanco & Mercer, 1998).
Dorsal root ganglion (DRG) neurons transmit various types of sensory information from peripheral tissues such as muscle and skin to the spinal cord. The DRG comprises functionally heterogeneous cell types and the size (diameter) of their soma closely correlates with their functions; small neurons transmit nociceptive information whereas large ones are closely related to the transmission of tactile and proprinoceptive impulses (Harper & Lawson, 1985). Previous in situ hybridization studies have shown that mRNAs for α1, α3 and β1 isoforms of the Na+/K+ ATPase are expressed in rat DRG (Mata et al., 1991; Fink et al., 1995), suggesting the existence of two isozymes of the Na+/K+ ATPase; namely, α1β1 and α3β1. Immunocytochemical studies using isoform-specific antibody have revealed that α isoforms are differentially expressed in subpopulations of DRG neurons; α1 isoform is equally expressed in small and large neurons, whereas the α3 isoform is predominantly distributed in large ones (Dobretsov et al., 1999a). Previous transfection studies using single, defined α isoforms have demonstrated that rat α1 isoforms exhibit a higher affinity for intracellular Na+ but a lower sensitivity to blockade by ouabain compared with α3 isoforms (Munzer et al., 1994; Therien et al., 1996; Zahler et al., 1997; Abriel et al., 1999; Horisberger & Kharoubi-Hess, 2002).
Using the whole-cell patch-clamp method, Dobretsov et al. (1999b) have measured the membrane current produced by the activity of Na+/K+ ATPase in rat DRG neurons and have shown that the Na+/K+ pump current in small and large neurons is primarily generated by the Na+/K+ ATPase isozyme having a low affinity for ouabain as expected for α1β1 isozyme. The presence of α3β1 isozymes of the Na+/K+ ATPase in rat DRG neurons has yet to be fully elucidated, especially in small neurons. In the present study, we used small (⩽25 μm in soma diameter) DRG neurons of adult rat to investigate the properties of the Na+/K+ pump current and found that ouabain inhibition as well as Na+ activation for the Na+/K+ pump current exhibit biphasic concentration–response relationship. Our results thus strongly suggest the presence of two functionally distinct components of the Na+/K+ pump current in rat small DRG neurons.
Methods
Isolation and culture of DRG neurons
DRG neurons were obtained from the L4-6 segments of 8- to 14-week old male Sprague–Dawley rats (250–400 g body weight) using an enzymatic dissociation procedure as described previously (Sanada et al., 2002). Rats were fully anesthetized with an overdose of sodium pentobarbital (50 mg kg−1, i.p.) and then were killed by decapitation. Six lumbar dorsal root ganglia were rapidly dissected and placed in Ham's F12 medium (Gibco BRL, Grand Island, NY, U.S.A.). After carefully removing the surrounding connective tissues, each ganglion was incubated for 90 min, in a shaking water bath at 37°C, in a Ham's F12 medium containing 1 mg ml−1 collagenase (class 3, Worthington Biochemical Corporation, Freehold, NJ, U.S.A.) and were then washed twice with Ca2+- and Mg2+-free Hanks' balanced salt solution (HBSS; Gibco BRL). The digested tissues were further incubated for 15 min at 37°C in HBSS containing 1 mg ml−1 trypsin (Type I, Sigma, St Louis, MO, U.S.A.). The trypsin digestion was then stopped by adding trypsin inhibitor (Type I-S, Sigma) and the enzyme-digested ganglia were mechanically agitated using a fire-polished Pasteur pipette to disperse the neurons. The dissociated neurons thus obtained were washed twice with Ham's F12 medium supplemented with 10% (v v−1) fetal bovine serum (Gibco BRL). The neurons were then plated onto poly-L-lysine (10 μg ml−1, Sigma)-coated rectangular (6 × 4 mm2) glass coverslips in Ham's F12 medium supplemented with penicillin (50 i.u. ml−1), streptomycin (0.1 mg ml−1) and 10% fetal bovine serum and were incubated at 37°C for 24–48 h in a humidified atmosphere of 95% air plus 5% CO2 prior to the experiments. DRG neurons that appeared practically spherical without neuronal processes were used for experiments.
All these experimental procedures were reviewed and approved by the Shiga University of Medical Science Animal Care Committee, Japan.
Solutions and chemicals
Normal Tyrode solution contained (in mM): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5.5 glucose and 5.0 HEPES (pH adjusted to 7.4 with NaOH). The nominally Ca2+-free Tyrode solution was prepared by simply omitting CaCl2 from the normal Tyrode solution. The external solution used for recording the Na+/K+ pump current was nominally Ca2+-free Tyrode solution supplemented with 2 mM BaCl2, 1 mM CsCl, 0.2 mM CdCl2, 2 mM NiCl2 and 1 μM nisoldipine (a generous gift from Bayer, Germany). Various concentrations (0.1–5000 μM) of ouabain (Sigma) were added to the external solution to block the Na+/K+ pump current. The standard pipette solution contained (in mM): 110 tetramethylammonium hydroxide (TMA-OH), 100 aspartic acid, 20 tetraethylammonium chloride (TEA-Cl), 2 MgCl2, 5 EGTA, 5 Tris-ATP, 2.5 Tris2-creatine phosphate, 5 glucose and 10 HEPES (pH adjusted to 7.2 with TMA-OH). Since the amount of TMA-OH required for titration was 10 mM on average, the total TMA+ concentration was estimated to be 120 mM in the standard pipette solution. To make a Na+-containing pipette solution at concentrations between 5 and 100 mM, TMA-OH was replaced with an equimolar concentration of NaOH, thus keeping the total content of Na++TMA+constant (120 mM). In these pipette solutions, the osmolarity was 285–300 mOsm. The concentration of free Ca2+ in the pipette solution was calculated to be ∼10−10 M (Fabiato & Fabiato, 1979; Tsien & Rink, 1980), which is expected to abolish the Na+/Ca2+ exchange current (Kimura et al., 1986).
Under the present experimental conditions with selected external and pipette solutions, membrane currents through Ca2+ channels, K+ channels and Na+/Ca2+ exchanger were minimized. K+ channel currents (Gold et al., 1996) were suppressed by omission of K+ from internal solution and addition of BaCl2 to the external solution. Low- (T-type) and high-threshold (N-, L- and P-type) Ca2+ channel currents (Scroggs & Fox, 1992; Cardenas et al., 1995) were eliminated by removal of Ca2+ from the external solution, and any residual influx of Ba2+ through these voltage-gated Ca2+ channels was blocked by the addition of CdCl2, NiCl2 and nisoldipine to the external solution. The tetrodotoxin (TTX)-sensitive Na+ channel as well as T-type Ca2+ channel was inactivated by setting a holding potential to −40 mV. In most of the small DRG neurons dialyzed with a pipette solution containing Na+ at lower concentrations (⩽∼20 mM) the TTX-resistant Na+ channel current was activated during depolarizing voltage steps applied from a holding potential of −40 mV; however, its amplitude was reduced with an increase in the concentration of Na+ in the pipette solution due to the reduction of the electrochemical gradient for Na+ and was nearly abolished with 100 mM Na+ (see Figure 3). The Na+–Ca2+ exchange current (Verdru et al., 1997) was prevented by elimination of Ca2+ from internal and external solutions and the addition of NiCl2 to the external solution.
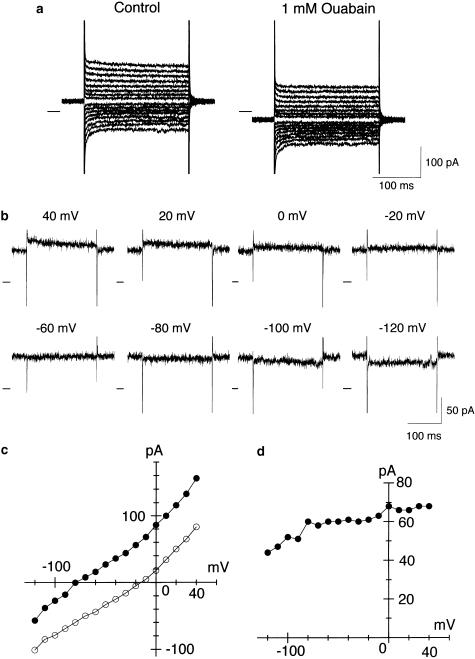
Figure 3.
Na+/K+ pump current determined as an ouabain-sensitive component in a rat small DRG neuron. (a) Superimposed current traces in response to 200 ms voltage steps to potential levels between +40 and −120 mV in 10 mV steps applied from a holding potential of −40 mV before (left-hand panel) and 3 min after (right-hand panel) exposure to 1 mM ouabain. The perfusate was nominally Ca2+-free Tyrode solution containing 5.4 mM K+ and also various blockers for ion channels and Na+/Ca2+ exchanger (see Methods). These currents were recorded from a small DRG neuron (diameter, 22.5 μm; Cm, 23.8 pF) equilibrated with a pipette solution containing 100 mM Na+. (b) Ouabain-sensitive difference currents at test potentials of +40, +20, 0, −20, −60, −80, −100 and −120 mV, obtained by digital subtraction of the membrane current in the presence of ouabain from that in its absence at each test potential. The zero-current level is indicated to the left of the current records by a horizontal line in panels (a) and (b). (c) I–V relationship for membrane currents measured in the absence and presence of ouabain, shown in panel (a). (d) I–V relationship for the Na+/K+ pump current determined as an ouabain-sensitive current, shown in panel (b). The steady-state current levels were measured near the end of 200 ms voltage step for I–V relationships shown in panels (c) and (d).
Whole-cell patch-clamp technique and data analysis
Rat DRG neurons were voltage-clamped using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) with a patch-clamp amplifier (CEZ-2400, Nihon Kohden, Tokyo, Japan). Patch pipettes were fabricated from glass capillaries (o.d., 1.5 mm; i.d., 0.9 mm; Narishige Scientific Instrument Laboratory, Tokyo, Japan) using a Sutter P-97 microelectrode puller (Novato, CA, U.S.A.), and the tips were then heat polished with a microforge. Pipettes manufactured in this way had a resistance of 1.0–2.5 MΩ when filled with the standard pipette solution used in these studies. These pipettes having relatively wide tips facilitated rapid equilibration of the cell interior with a Na+-containing pipette solution.
DRG neurons cultured on rectangular glass coverslips were transferred to a recording chamber mounted on the stage of an inverted Nikon Diaphot microscope (Tokyo, Japan) and were superfused at a constant flow rate of 2 ml min−1 with normal Tyrode solution. A tight seal (resistance, 5–50 GΩ) was then established between the electrode tip and the cell membrane in normal Tyrode solution, by applying negative pressure (∼−20 to −40 cm H2O). The patch membrane was then ruptured by a brief period of more vigorous suction, controlled manually with a 2.5 ml syringe, to establish the whole-cell mode. As a Na+-containing pipette solution was dialyzing into the cell at a holding potential of −40 mV, the membrane current rapidly shifted in inward direction, primarily due to replacement of intracellular K+ with TMA and TEA, and usually reached a steady-state level within 1–2 min of patch rupture. The bath solution was then switched from normal Tyrode solution to the external solution for the measurement of Na+/K+ pump current, which resulted in a further reduction of membrane conductance. Under these conditions in which membrane currents through Ca2+ and K+ channels and Na+/Ca2+ exchanger were mostly inhibited, Na+/K+ pump current was identified as the ouabain-sensitive current (see Figure 2 and Figure 3). Current and voltage signals as well as trigger pulses were stored on a digital audio tape (DM120, Hitachi Maxell, Tokyo, Japan) using a PCM data recorder (RD-120TE, TEAC, Tokyo, Japan). Current and voltage records were filtered with a low-pass 3 kHz filter (48 dB per octave, E-3201A, NF, Tokyo, Japan) and digitized at a sampling rate of 2–4 kHz using a 100 kHz A/D board (ADX-98, Canopus, Kobe, Japan) installed in a personal computer (PC98RL, NEC, Tokyo, Japan).
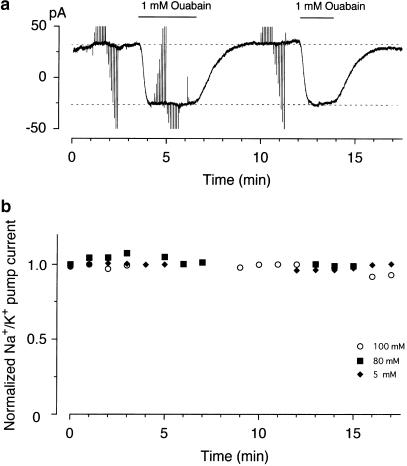
Figure 2.
Stability of the Na+/K+ pump current. (a) Chart record of whole-cell current recorded from a small DRG neuron dialyzed with a pipette solution containing 100 mM Na+. A small neuron (diameter, 22.5 μm; Cm, 23.8 pF) was held at −40 mV, and was twice exposed to 1 mMouabain for a period indicated by the horizontal bars over the chart record. Deflections in the current recordings reflect the imposition of 200 ms voltage steps to test potentials between +40 and −120 mV (data not shown). The dashed lines represent holding current levels recorded under control conditions (without ouabain) and in the presence of 1 mM ouabain. (b) Time course of changes in amplitude of the Na+/K+ pump current in three different neurons activated by 5, 80 and 100 mM Na+ in the pipette solution. The points represent the normalized amplitude of the Na+/K+ pump current with reference to its initial amplitude shortly after starting measurements (usually 3–5 min after establishment of whole-cell mode). The Na+/K+ pump current was defined as an ouabain (1 mM)-sensitive holding current at −40 mV every 1 min.
Cell membrane capacitance (Cm) was calculated from the capacitive transients elicited by 20-ms voltage-clamp steps (±5 mV) from a holding potential of −40 mV, using the following relationship (Bénitah et al., 1993):
where τc is the time constant of the capacitive transient, I0 is the initial peak current amplitude, ΔVm is the amplitude of voltage step (5 mV), and I∞ is the steady-state current value. The sampling rate for these measurements of Cm was 50 kHz with a low-pass 10 kHz filter. The average values for τc and Cm in small (⩽25 μm in diameter) DRG neurons were 0.148±0.006 ms and 21.7±0.7 pF (mean±s.e.m.; n=79 neurons), respectively. The amplitude of the Na+/K+ pump current was divided by Cm to provide an estimate of current density (pApF–1).
Results are expressed as the mean±s.e.m., and n indicates the number of neurons studied. Statistical comparisons were made using the Student's t-test, and differences were regarded as significant at the 95% confidence level.
Measurement of size of DRG neurons
The diameter of DRG neurons was measured using a micrometer (2.5 μm per division) under a microscope at × 400 magnification.
Results
Cell membrane capacitance of rat DRG neurons
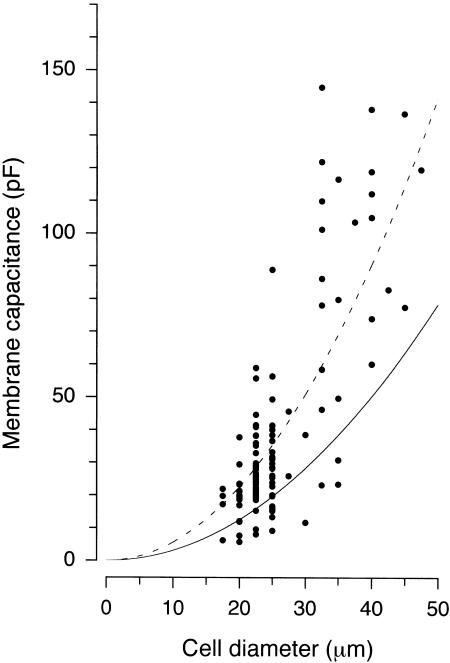
Each DRG neuron was initially tested for cell membrane capacitance (Cm) from capacitive transient in response to ±5 mV voltage-clamp steps applied from a holding potential of −40 mV (see Methods), prior to measuring the Na+/K+ pump current. As demonstrated in Figure 1, Cm ranged from 6.2 to 145.1 pF in DRG neurons with soma diameter of 17.5–47.5 μm (n=114). The solid line represents Cm values estimated by assuming the neuron to be a sphere with the specific membrane capacitance of 1 μF cm−2, and is found to rather deviate from data points, as has been demonstrated in isolated rat DRG neurons (Cardenas et al., 1995; Dobretsov et al., 1999b; Genzen et al., 2001). A more likely explanation is that the actual membrane area of DRG neurons is larger than the surface area calculated from optical measurements of the diameter (Genzen et al., 2001). In these DRG neurons, the ratio of actual membrane area to the apparent area of a smooth sphere is estimated to be 1.78 (dashed curve), assuming 1 μF cm−2 for specific membrane capacitance (see figure legend). In the present study, the Na+/K+ pump current was measured from DRG neurons with soma diameter of ⩽25 μm, and their diameter and Cm averaged 21.17±0.08 μm and 21.7±0.7 pF (n=79), respectively.
Figure 1.
Relationship between cell membrane capacitance (Cm) and diameter in DRG neurons. Cm, calculated using Equation (1) (see Methods) in each neuron, was plotted as a function of diameter of cell body measured using a micrometer (n=114). The solid curve through the data points represents the equation: Cm=4π (diameter/2)2 × 0.01 (pF μm−2), which predicts Cm values from the diameter of neuron, assuming that neuron is spherical with a specific membrane capacitance of 1 μF cm−2. The dashed curve was drawn by a least-squares fit of the equation: Cm=k × 4π (diameter/2)2 × 0.01 (pF μm−2), yielding k (ratio of actual membrane area to the apparent area of a smooth sphere) of 1.78.
Stability of Na+/K+ pump current in the course of patch-clamp experiments
It has been demonstrated in several cell types (Gadsby & Nakao, 1989; Dobretsov & Stimers, 1996) including rat DRG neurons (Dobretsov et al., 1999b) that the Na+/K+ pump current exhibits a gradual rundown in the course of whole-cell patch-clamp experiments. We therefore checked the time course of changes in the amplitude of the Na+/K+ pump current in small DRG neurons under our experimental conditions. A small DRG neuron dialyzed with a pipette solution containing Na+ was superfused with nominally Ca2+ -free Tyrode solution supplemented with various blockers for Ca2+ and K+ channels and Na+/Ca2+ exchanger. About 3–5 min after the establishment of whole-cell configuration, the holding current level (at −40 mV) usually stabilized at levels between ∼+40 and −10 pA, depending upon the concentration of Na+ in the pipette solution, which reflects the equilibration of the cell interior with a Na+-containing pipette solution. Measurement of the Na+/K+ pump current was then started for a period of ∼17 min, using ouabain at 1 mM, a concentration known to produce a nearly maximal inhibition of the Na+/K+ pump current in rat DRG neurons (Dobretsov et al., 1999b; see also Figure 4). Figure 2a illustrates a representative example obtained from a small neuron dialyzed with 100 mM Na+. In this instance, 1 mM ouabain was applied at 3.5 and 12 min after starting the measurement, which evoked a similar magnitude of the inward shift in holding current due to the inhibition of the Na+/K+ pump current. After washing off the drug, the holding current level was restored to the predrug level, without an overshoot response. The amplitude of the Na+/K+ pump current in the course of experiments was determined as the ouabain-sensitive difference current, obtained by digitally subtracting the current level in the presence of ouabain from that in its absence, and was plotted against time after starting the pump current measurement (Figure 2b). The Na+/K+ pump current activated by various concentrations of Na+ did not exhibit an appreciable rundown in rat small DRG neurons under our experimental conditions; the current amplitude recorded at 15 min after starting current measurement was 92.4±2.8% of the initial value (n=3).
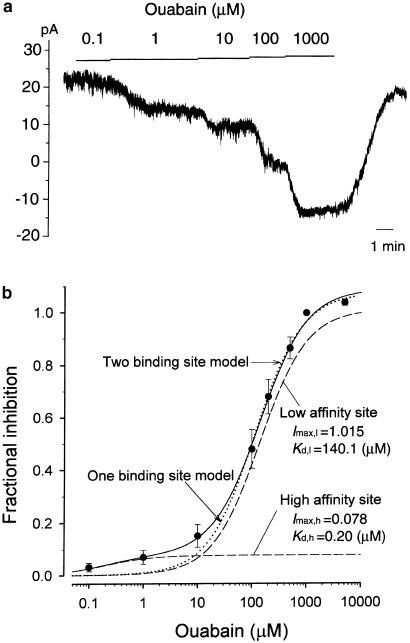
Figure 4.
Concentration-dependent block of Na+/K+ pump current by ouabain. (a) Chart record of the membrane current at a holding potential of −40 mV recorded from a small DRG neuron (diameter, 20.0 μm; Cm, 21.5 pF) dialyzed with a pipette solution containing 30 mM Na+. An isolated small DRG neuron was exposed to increasing concentrations of ouabain in a cumulative manner after inhibition by the previous concentration reached a steady state. The horizontal bar indicates the changes in ouabain concentration added to the bath. (b) Concentration–response relationship for the inhibition of the Na+/K+ pump current by ouabain in small DRG neurons dialyzed with 30 mM Na+. An inward shift of the holding current at −40 mV evoked by ouabain at various concentrations was normalized with reference to that induced by 1 mM ouabain and was plotted as a function of ouabain concentration. Data are expressed as mean±s.e.m. of 6 neurons (mean Cm=21.2±4.8 pF; mean diameter=22.5±1.4μm). Data points were fitted with either one- (dotted curve) or two-binding site models (solid curve). Dashed curves represent the theoretical curves for inhibition with dissociation constants of 0.20 and 140.1 μM. The relative amplitude of the Na+/K+ pump current generated by the isozymes having high- and low-affinity sites for ouabain (probably α3β1 and α1β1, respectively) is calculated to be approximately 1 : 13 in the presence of 30 mM Na+ in the pipette solution.
Na+/K+ pump current identified in small DRG neurons
Figure 3 demonstrates a representative example for the measurement of the Na+/K+ pump current from a small DRG neuron dialyzed with 100 mM Na+. Membrane currents in response to depolarizing (up to +40 mV) and hyperpolarizing (up to −120 mV) test potentials applied from a holding potential of −40 mV were of small amplitude and largely time-independent, except small amplitude of current relaxations observed at the initial part of moderate and strong hyperpolarizations (Figure 3a, left-hand panel), thus showing that ion channel currents known in small DRG neurons were largely (but not totally) abolished. An influx of Na+ through the TTX-resistant Na+ channels, known to be preferentially expressed in small neurons (Arbuckle & Docherty, 1995; Villiére & McLachlan, 1996), was considered to be practically abolished due to a significant reduction in electrochemical gradient for Na+ (predicted Na+ equilibrium potential of −9.8 mV). The DRG neuron was then exposed to ouabain at 1 mM, which caused an inward shift of membrane currents at all test potentials between +40 and −120 mV (Figure 3a, right-hand panel; Figure 3c). Upon moderate and strong hyperpolarizations, a decay of inward currents was still present within the initial 40 ms of the hyperpolarizing pulses and remained practically unaltered by exposure to ouabain, thus indicating that these smaller current relaxations were mostly unrelated to the Na+/K+ pump current. We therefore did not attempt further to analyze their nature.
The Na+/K+ pump current was then determined as an ouabain-sensitive current, which was obtained by digitally subtracting current trace in the presence of ouabain from that in its absence at each test potential. The Na+/K+ pump current thus isolated in a rat small DRG neuron was practically time-independent at all test potentials between +40 and −120 mV (Figure 3b) and its I–V relationship exhibited a voltage-dependence (Figure 3d); the amplitude of the Na+/K+ pump current was increased with membrane depolarization from −120 to 0 mV and reached a plateau level at potentials ⩾0 mV, as has been demonstrated in various mammalian cardiac myocytes (Gadsby et al., 1985; Gadsby & Nakao, 1989; Glitsch et al., 1989; Nakao & Gadsby, 1989; Shattock & Matsuura, 1993; Stimers et al., 1993).
It should be noted that the TTX-resistant Na+ channel was not blocked under the present experimental conditions. A rapidly activating and inactivating inward current associated with an activation of the TTX-resistant Na+ channel was usually evoked in response to depolarizing voltage steps to potentials positive to ∼−10 mV applied from a holding potential of −40 mV, when small DRG neurons were dialyzed with a pipette solution containing low or intermediate concentrations (⩽20 mM) of Na+ (data not shown). However, even in the presence of the TTX-resistant Na+ current, the Na+/K+ pump current, defined as ouabain-sensitive current, was found to be virtually time-independent at all test potentials between +40 and −120 mV (data not shown), thus suggesting that measurement of the Na+/K+ pump current was not appreciably distorted by the presence of the TTX-resistant Na+ current.
Ouabain blockade of Na+/K+ pump current in small DRG neurons
Rat DRG neurons have been demonstrated to express two distinct Na+/K+ ATPase isozymes; namely, α1β1 and α3β1 (Mata et al., 1991; Fink et al., 1995; Dobretsov et al., 1999a). These two isozymes exhibit distinct kinetic characteristics with regard to activation by intracellular Na+ and inhibition by cardiac glycoside ouabain (for review see Sweadner, 1989; Blanco & Mercer, 1998). We functionally assessed whether the Na+/K+ pump current in rat small DRG neurons arises from single or multiple isozyme by testing the sensitivity to blockade by ouabain (Figure 4) or activation by intracellular Na+ (Figure 5). Figure 4a demonstrates a representative experiment showing an ouabain inhibition of the Na+/K+ pump current activated by 30 mM Na+ in a small DRG neuron. Bath application of ouabain evoked an inward shift of the holding current at −40 mV in a concentration-dependent manner and the Na+/K+ pump current blocked by each concentration of ouabain was determined as the difference in holding current levels in the absence and presence of ouabain. It should be noted that whereas the holding current level was slightly affected by 0.1 μM ouabain, there was a substantial (∼9 pA) inward shift of holding current by exposure to 1 μM ouabain. In rat small DRG neurons, increasing the concentration of ouabain above 1 mM did not produce any further discernible inward shift of holding current, thus suggesting that a maximal inhibition was achieved at a concentration of 1 mM. An inward shift of holding current was mostly restored within a few minutes after washing off the drug, even when a maximal effect was achieved at high drug concentration (1 mM; see also Figure 2a), consistent with a previous observation in rat DRG neurons (Dobretsov et al., 1999b).
Figure 5.
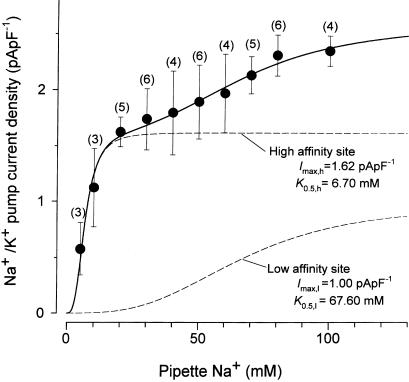
Concentration–response relationship for the activation of Na+/K+ pump current by pipette Na+. Small DRG neurons were dialyzed with a pipette solution containing Na+ at concentrations ranging from 5 to 100 mM. The amplitude of the Na+/K+ pump current, determined as an ouabain (1 mM)-sensitive current at −40 mV, was normalized with reference to Cm and plotted as a function of Na+ concentration in the pipette solution ([Na+]pip). Data points represent means±s.e.m. with the number of measurements given in parentheses. The solid curve through the data points represents a least-squares fit of the sum of two Hill equations with a Hill coefficient of 3.0: I=Imax,h/(1+(K0.5,h/[Na+]pip)3)+Imax,l/(1+(K0.5,l/[Na+]pip)3), with Imax,h (fitted maximal current density generated by high-affinity site)=1.6222 pA pF−1, K0.5,h (apparent Na+ affinity for high-affinity site)=6.7 mM, Imax,l (fitted maximal current density generated by low-affinity site)=0.9998 pA pF−1 and K0.5,l (apparent Na+ affinity for low-affinity site)=67.6 mM. Dashed curves show the theoretical curves for activation generated by high- and low-affinity sites for Na+.
Figure 4b illustrates the concentration–response relationship for the inhibition of the Na+/K+ pump current by ouabain measured in rat small DRG neurons dialyzed with a pipette solution containing 30 mM Na+. The Na+/K+ pump current inhibition by ouabain at each concentration, represented by an inward shift of holding current, was normalized with reference to the maximum effect obtained by 1 mM ouabain (fractional inhibition) and plotted as a function of ouabain concentration. The dotted curve through the data points represents a least-squares fit of one Michaelis–Menten equation, assuming a single-binding site:
where I is the fractional inhibition, Imax is the fitted maximum degree of inhibition (1.1) and Kd is the dissociation constant (115.0 μM). It is evident that data points at lower concentrations of ouabain (0.1, 1 and 10 μM) are not well described by this single-binding site model. The data were then fitted using the sum of two Michaelis–Menten equations, assuming the existence of two isozymes exhibiting distinct sensitivities to ouabain (two-binding site model):
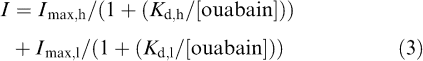 |
where Imax,h and Imax,l are the fractional inhibition of the pump current evoked by isozymes showing high and low ouabain affinity (0.08 and 1.02), respectively, Kd,h and Kd,l are the respective dissociation constants of the interaction between ouabain and two distinct isozymes having high or low affinity to ouabain (0.20 and 140.1 μM, respectively). All data points were found to be reasonably well described by using this two-binding site models, suggesting that the Na+/K+ pump current in rat small DRG neurons arises from two distinct isozymes displaying different sensitivities to inhibition by ouabain. One of the components corresponding to most (∼93%) of the total pump currents has a calculated Kd,l of 140.1 μM and is very likely to represent the α1β1 isozyme. The second component exhibiting a high sensitivity to ouabain (Kd,h of 0.20 μM) comprises ∼7% of the total activity and is presumably associated with α3β1 isozyme.
Activation of Na+/K+ pump current by intracellular Na+
It has been demonstrated in various tissue and cell types that α1β1 and α3β1 isozymes of Na+/K+ ATPase have distinct affinities for intracellular Na+ (Munzer et al., 1994; Therien et al., 1996; Zahler et al., 1997). If the Na+/K+ pump current is generated by both of α1β1 and α3β1 isozymes in rat small DRG neurons, intracellular Na+ dependence of pump current activation can be expressed by the sum of two binding curves. The concentration–response relationship for the activation of the Na+/K+ pump current by pipette Na+ was therefore obtained by dialyzing different small DRG neurons with different pipette solutions containing Na+ at concentrations ranging between 5 and 100 mM. The amplitude of the Na+/K+ pump current, determined as an ouabain (1 mM)-sensitive current at −40 mV, was normalized with reference to Cm (see also Figure 6a) and was then plotted against Na+ concentration in the pipette solution ([Na+ ]pip) (Figure 5). The solid curve through the data points represents a best fit of the sum of two Hill equations in which Hill coefficients for both components are set to 3, as described previously (Munzer et al., 1994; Blanco et al., 1995; Zahler et al., 1997):
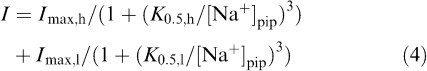 |
where K0.5,h and K0.5,l represent the apparent Na+ affinity at the high- and low-affinity sites, respectively, and Imax,h and Imax,l are the fitted maximal current density produced by high- and low-affinity sites, respectively. All data points were reasonably well fitted by Equation (4) with the following parameters (K0.5,h of 6.7 mM, K0.5,l of 67.6 mM, Imax,h of 1.62 pApF−1 and Imax,l of 0.99 pApF−1), supporting the hypothesis that the Na+/K+ pump current in rat small DRG neurons are generated by two functionally distinct isozymes exhibiting different affinities for intracellular Na+. Assuming that α1β1 and α3β1 isozymes, respectively, correspond to the high- and low-Na+ affinity sites, relative contributions of α1β1 and α3β1 isozymes to total Na+/K+ pump current activated by 30 mM Na+ are estimated to be approximately 19 : 1.
Figure 6.
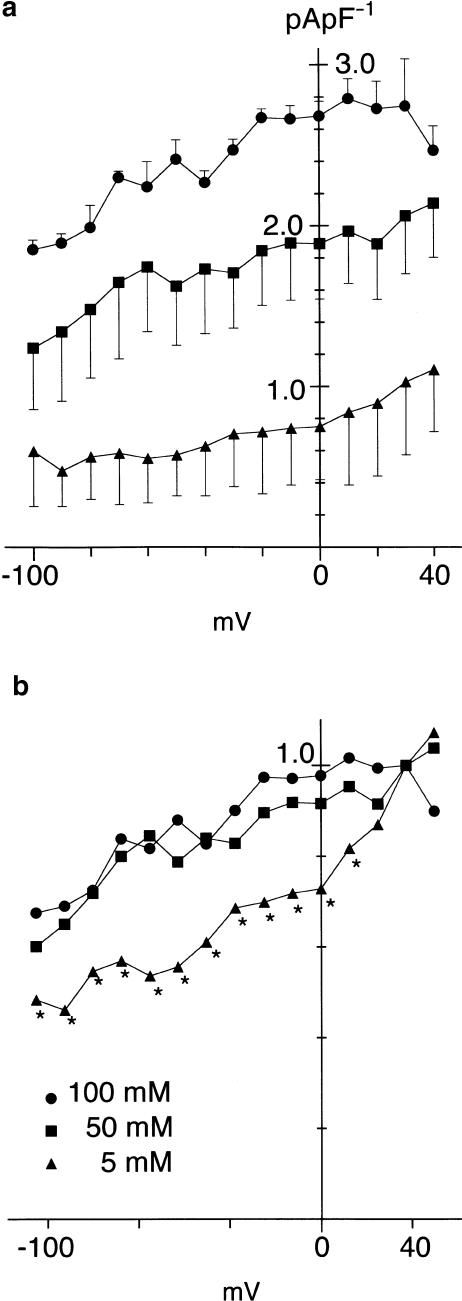
I–V relationships for Na+/K+ pump current activated by different concentrations of Na+ in pipette solutions. (a) I–V relationships for the Na+/K+ pump current activated by 5, 50 and 100 mM Na+ in pipette solution. The Na+/K+ pump current was measured as ouabain (1 mM)-sensitive current at each test potential and its amplitude was normalized with reference to the Cm. Each value is the mean, and error bars represent the s.e.m. of three (5 mM) or four (50 and 100 mM) neurons. (b) Same I–V relationships, with same symbols as in panel (a), but after normalizing each current amplitude with respect to its amplitude at +30 mV. For clarity, the mean value is only represented. The difference is significant at potentials ⩽+10 mV when the value at 5 mM pipette Na+ is compared with that at 100 mM Na+, as indicated by *.
Voltage dependence of Na+/K+ pump current activation at different Na+ concentrations
We compared the I–V relationships for the Na+/K+ pump current activated by 5, 50 and 100 mMNa+ in the pipette solution (Figure 6). The Na+/K+ pump currents at various test potentials were measured as the ouabain-sensitive difference currents and then normalized with reference to Cm. The current density of the Na+/K+ pump current thus obtained was plotted as a function of test potential (Figure 6a). To compare the voltage dependence in these I–V relationships, the amplitude of pump current at each test potential was normalized with reference to its magnitude at +30 mV (Figure 6b). The I–V relationships for the pump current activated by 5 mM Na+ was found to be shifted along the voltage axis to positive membrane potentials, compared with those activated by 50 and 100 mM Na+.
Discussion
The present experiments clearly detect the ouabain-sensitive Na+/K+ pump current, which is practically time-independent over a wide range of membrane potentials (between +40 and −120 mV) in rat small DRG neurons (⩽25 μm in diameter) dialyzed with a pipette solution containing various concentrations of Na+ ranging between 5 and 100 mM (Figure 3). Since the Na+/K+ pump current was determined as an ouabain-sensitive current, obtained by digital subtraction of the current traces in the absence and presence of the drug, it is important to block other membrane currents that frequently exhibit time-dependent variations (usually rundown) in the course of experiments. The present measurement of the Na+/K+ pump current was therefore conducted under conditions designed to minimize Ca2+ channel currents, K+ channel currents and Na+/Ca2+ exchange current using appropriate inhibitors and ion substitutions. When small DRG neurons were loaded intracellularly with higher concentrations of Na+ (⩾∼50 mM), membrane currents during 200-ms square voltage pulses to potential levels between +40 and −120 mV applied from a holding potential of −40 mV were largely time-independent except a small amplitude of current decay observed upon moderate and strong hyperpolarizations (Figure 3), indicating that voltage-gated ion channels present in small DRG neurons were largely suppressed under these conditions. The TTX-resistant Na+ current was usually evoked during depolarizing voltage steps in small neurons dialyzed with low or intermediate concentrations of Na+ (⩽∼20 mM) and was relatively stable for a period of at least 5 min after starting the measurement of the Na+/K+ pump current. Even in the presence of this current, an ouabain-sensitive current obtained by digital subtraction was also nearly time-independent at all test potentials between +40 and −120 mV (data not shown), suggesting that the presence of the TTX-resistant Na+ current did not appreciably distort an isolation of the Na+/K+ pump current using ouabain. Thus, the present measurement of the Na+/K+ pump current in small DRG neurons appears to be virtually free of contamination of nonpump currents over a wide range of membrane potentials.
The Na+/K+ pump current in small DRG neurons exhibits a voltage-dependence; there is a positive slope between −120 and 0 mV but a rather flat level at positive membrane potentials (Figure 3 and Figure 6). The present investigation has also demonstrated that lowering Na+ concentration in the pipette to 5 mM is associated with a shift of the normalized pump I–V relationship along the voltage axis to more positive potentials in small DRG neurons (Figure 6). A similar rightward shift of the normalized I–V relationshipship for pump current during dialysis with lower Na+ concentrations has previously been described for sheep cardiac Purkinje fibers (5 mM Na+, Glitsch et al., 1989) and guinea-pig cardiac myocytes (8 mM Na+, Nakao & Gadsby, 1989), which is ascribed, at least in part, to a reduction in voltage-dependent binding of cytoplasmic Na+ to its internal transport site (Glitsch, 2001). In recent years, it has been demonstrated that Na+/K+ pump current produced by α1β1 isozyme is voltage-dependent, whereas the Na+/K+ pump current associated with α3β1 isozyme is not significantly affected by membrane potentials in the range of −130 and +50 mV (Crambert et al., 2000). These differences in voltage-dependency between α1β1 and α3β1 isozymes might contribute at least partly to differences in normalized I–V relationships of the Na+/K+ pump current activated by lower and higher concentrations of internal Na+ (Figure 6), because the pump current activated by 5 mM pipette Na+ is expected to be primarily contributed by α1β1 isozyme, while that activated by higher concentrations of pipette Na+ (50 and 100 mM) is considered to be the sum of α1β1 and α3β1 isozymes (Figure 5).
Previous studies have suggested that α1β1 and α3β1 isozymes of the Na+/K+ ATPase are expressed in DRG neurons of adult rat (Mata et al., 1991; Fink et al., 1995; Dobretsov et al., 1999a). The rat α1β1 isozyme exhibits a higher Na+ affinity but a lower sensitivity toward ouabain than the α3β1 isozyme (for a review see Blanco & Mercer, 1998). The present study has revealed that the sum of two Michaelis–Menten curves is needed to describe the blockade of the Na+/K+ pump current (activated by 30 mM Na+ in the pipette solution) by ouabain at concentrations between 0.1 μM and 5 mM (Figure 4), which strongly suggests the presence of two functionally distinct components that have different sensitivities to ouabain in rat small DRG neurons. The dissociation constants for high- and low-affinity sites for ouabain were estimated to be 0.20 and 140.1 μM, respectively (Figure 4). This difference in ouabain sensitivity suggests that the low- and high-affinity bindings are likely to reflect the activities of α1 and α3 isoforms, respectively. It has been reported in rat kidney cells, brain synaptosomes, pineal glands and astrocytes that dissociation constants for ouabain inhibition in α1 isoforms is in the range of 32–170 μM, whereas ouabain inhibits the pump current produced by α3 isoforms with dissociation constants in the range of 0.1–1 μM (Sweadner, 1985; Brodsky and Guidotti, 1990; Shyjan et al., 1990; Matsuda et al., 1993). Thus, dissociation constants estimated for ouabain block of α1 and α3 isoforms in the present study are comparable to those reported for other cell types.
The present study also demonstrates that intracellular Na+ activation of the pump current is reasonably well fitted with the sum of two binding curves (Figure 5). The apparent affinities for intracellular Na+ are calculated to be 6.7 and 67.6 mM for high- and low-affinity sites, respectively. These two values are also comparable to those reported for Na+ in rat α1 (7.1–17.6 mM) and α3 (30.3–63.5 mM) isoforms (Munzer et al., 1994; Blanco et al., 1995; Therien et al., 1996; Zahler et al., 1997), respectively. The relative contribution of the low ouabain affinity isozyme to the total Na+/K+ pump current activated by 30 mM Na+ is estimated to be 93%, by fitting the data points to the sum of two Michaelis–Menten equations (Figure 4). On the other hand, analysis of the dose–response curves for Na+ activation using the two-binding site model (Figure 5) predicts that the high Na+ affinity isozyme contributes to 95% of total Na+ pump current activated by 30 mM Na+. Thus, our data of dose–response curves for ouabain inhibition (Figure 4) and Na+ activation (Figure 5) are consistent, assuming the presence of two functionally distinct isozymes of the Na+/K+ ATPase; one isozyme which has high ouabain and low Na+ affinities and another which shows low ouabain and high Na+ affinities.
One of the limitations in the use of whole-cell patch-clamp technique to measure the Na+/K+ pump current is the difficulty in controlling the intracellular Na+ concentrations. It has been demonstrated that an activation of the Na+/K+ pump during dialysis with a Na+-containing pipette solution is accompanied by a substantial concentration gradient of Na+ between the cell interior ([Na+]i) and the pipette ([Na+]pip) due to the limited diffusion at the tip of recording pipette (Oliva et al., 1988; Pusch & Neher, 1988). At steady state, Na+ diffusion from pipette to cell must equal the net transmembrane Na+ fluxes, which is stated as follows (Nakao & Gadsby, 1989; Mathias et al., 1990; Gao et al., 1995):
where ρ represents the resistivity of the pipette filling solution (∼60 Ω cm), DNa is the diffusion coefficient for Na+ (1.5 × 10−5 cm2 s−1), Rp is the resistance of the pipette tip, Ip is the amplitude of the whole-cell pump current, Il is the amplitude of passive leak of Na+ into the cell and F is Faraday's constant. When small DRG neurons were loaded with 50 mM [Na+]pip, the Na+/K+ pump was strongly activated and its amplitude reached ∼40 pA at a holding potential of −40 mV (Figure 5). Assuming Il to be absent, the concentration gradient, [Na+]pip – [Na+]i, can be estimated to be ∼8 mM. If the passive leak of Na+ influx Il was substantial, then the Na+ gradient can be estimated to be smaller, although Il was not characterized in the present study. It is therefore reasonable to speculate that the activation of the Na+/K+ pump current by pipette Na+ shown in Figure 5 may be shifted to the right of the correct position for the pump activation by [Na+]i, as has been clearly shown by Gao et al. (1995). The apparent Na+ affinity at the high- and low-affinity sites given in this study (6.7 and 67.6 mM, respectively, Figure 5) is also likely to be an overestimate. It has been suggested that if [Na+]i is not adequately controlled in whole-cell voltage-clamp experiments, a characteristic overshoot of the Na+/K+ pump current is observed following the washout of ouabain, caused by an accumulation of [Na+]i during the period of pump inhibition (Gao et al., 1994). However, it should be noted that such a transient overshoot of pump current after a period of pump inhibition by ouabain was scarcely detected in our measurement in small DRG neurons, irrespective of [Na+]pip (Figure 2). This finding suggests that intracellular Na+ was fairly well controlled in the present experiments.
It has been demonstrated that α as well as β isoforms of the Na+/K+ ATPase are distributed in a tissue-specific pattern; α1 is expressed ubiquitously and is considered to assume the role of ‘housekeeping' to respond to normal physiological demands. The α3 is predominantly distributed in neurons and other excitable tissues (Blanco & Mercer, 1998), where an intracellular accumulation of Na+ can be evoked under a number of physiological or pathophysiological conditions including the repetitive discharges of action potentials. Under such conditions, the α3 can be substantially activated and thereby contributes to expel an excess of intracellular Na+, probably in cooperation with α1. It is thus of interest to elucidate the functional role of the α3 in small DRG neurons and to further investigate whether the expression and function of the α3 isoform of the Na+/K+ ATPase in small DRG neurons are altered under various pathophysiological conditions associated with the development of neuropathic pain.
Acknowledgments
We thank Dr Takao Shioya (Saga Medical School, Japan) for providing the computer programs used for the data analysis. This study was supported by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Nos. 13670042 and 14570590) and a grant from the Ministry of Health, Labor and Welfare of Japan.
Abbreviations
- DRG
dorsal root ganglion
- HBSS
Hanks' balanced salt solution
- TEA
tetraethylammonium
- TMA
tetramethylammonium
- TTX
tetrodotoxin
References
- ABRIEL H., HASLER U., GEERING K., HORISBERGER J.-D. Role of the intracellular domain of the β subunit in Na,K pump function. Biochim. Biophys. Acta. 1999;1418:85–96. doi: 10.1016/s0005-2736(99)00025-5. [DOI] [PubMed] [Google Scholar]
- ARBUCKLE J.B., DOCHERTY R.J. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurones of adult rats. Neurosci. Lett. 1995;185:70–73. doi: 10.1016/0304-3940(94)11227-a. [DOI] [PubMed] [Google Scholar]
- BÉNITAH J.-P., GOMEZ A.M., BAILLY P., DA PONTE J.-P., BERSON G., DELGADO C., LORENTE P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. J. Physiol. (London) 1993;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCO G., MERCER R.W. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- BLANCO G., SÁNCHEZ G., MERCER R.W. Comparison of the enzymatic properties of the Na,K-ATPase α3β1 and α32 isozymes. Biochemistry-US. 1995;34:9897–9903. doi: 10.1021/bi00031a011. [DOI] [PubMed] [Google Scholar]
- BRODSKY J.L., GUIDOTTI G. Sodium affinity of brain Na+-K+-ATPase is dependent on isozyme and environment of the pump. Am. J. Physiol. 1990;258:C803–822. doi: 10.1152/ajpcell.1990.258.5.C803. [DOI] [PubMed] [Google Scholar]
- CARDENAS C.G., DEL MAR L.P., SCROGGS R.S. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J. Neurophysiol. 1995;74:1870–1879. doi: 10.1152/jn.1995.74.5.1870. [DOI] [PubMed] [Google Scholar]
- CRAMBERT G., HASLER U., BEGGAH A.T., YU C., MODYANOV N.N., HORIABERGER J.-D., LELIÉVRE L., GEERING K. Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J. Biol. Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- DOBRETSOV M., HASTINGS S.L., STIMERS J.R. Non-uniform expression of α subunit isoforms of the Na+/K+ pump in rat dorsal root ganglia neurons. Brain Res. 1999a;821:212–217. doi: 10.1016/s0006-8993(98)01361-4. [DOI] [PubMed] [Google Scholar]
- DOBRETSOV M., HASTINGS S.L., STIMERS J.R. Functional Na+/K+ pump in rat dorsal root ganglia neurons. Neuroscience. 1999b;93:723–729. doi: 10.1016/s0306-4522(99)00122-0. [DOI] [PubMed] [Google Scholar]
- DOBRETSOV M., STIMERS J.R. Characterization of the Na/K pump current in N20.1 oligodendrocytes. Brain Res. 1996;724:103–111. doi: 10.1016/0006-8993(96)00171-0. [DOI] [PubMed] [Google Scholar]
- FABIATO A., FABIATO F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- FINK D.J., FANG D., LI T., MATA M. Na,K-ATPase beta subunit isoform expression in the peripheral nervous system of the rat. Neurosci. Lett. 1995;183:206–209. doi: 10.1016/0304-3940(94)11152-9. [DOI] [PubMed] [Google Scholar]
- GADSBY D.C., KIMURA J., NOMA A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985;315:63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- GADSBY D.C., NAKAO M. Steady-state current–voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J. Gen. Physiol. 1989;94:511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO J., COHEN I.S., MATHIAS R.T., BALDO G.J. Regulation of the β -stimulation of the Na+–K+ pump current in guinea-pig ventricular myocytes by a cAMP-dependent PKA pathway. J. Physiol. (London) 1994;477:373–380. doi: 10.1113/jphysiol.1994.sp020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO J., MATHIAS R.T., COHEN I.S., BALDO G.J. Two functionally different Na/K pumps in cardiac ventricular myocytes. J. Gen. Physiol. 1995;106:995–1030. doi: 10.1085/jgp.106.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENZEN J.R., VAN CLEVE W., MCGEHEE D.S. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J. Neurophysiol. 2001;86:1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- GLITSCH H.G. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol. Rev. 2001;81:1791–1826. doi: 10.1152/physrev.2001.81.4.1791. [DOI] [PubMed] [Google Scholar]
- GLITSCH H.G., KRAHN T., PUSCH H. The dependence of sodium pump current on internal Na concentration and membrane potential in cardioballs from sheep Purkinje fibres. Pflügers Arch. 1989;414:52–58. doi: 10.1007/BF00585626. [DOI] [PubMed] [Google Scholar]
- GOLD M.S., SHUSTER M.J., LEVINE J.D. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J. Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARPER A.A., LAWSON S.N. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. J. Physiol. (London) 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER U., WANG X., CRAMBERT G., BEGUIN P., JASSER F., HORISBERGER J.D., GREEING K. Role of β -subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J. Biol. Chem. 1998;273:30826–30835. doi: 10.1074/jbc.273.46.30826. [DOI] [PubMed] [Google Scholar]
- HORISBERGER J.-D., KHAROUBI-HESS S. Functional differences between α subunit isoforms of the rat Na,K-ATPase expressed in Xenopus oocytes. J. Physiol. (London) 2002;539:669–680. doi: 10.1113/jphysiol.2001.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA J., NOMA A., IRISAWA H. Na–Ca exchange current in mammalian heart cells. Nature. 1986;319:596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- MATA M., SIEGEL G.J., HIEBER V., BEATY M.W., FINK D.J. Differential distribution of (Na,K)-ATPase alpha isoform mRNAs in the peripheral nervous system. Brain Res. 1991;546:47–54. doi: 10.1016/0006-8993(91)91157-v. [DOI] [PubMed] [Google Scholar]
- MATHIAS R.T., COHEN I.S., OLIVA C. Limitations of the whole cell patch clamp technique in the control of intracellular concentrations. Biophys. J. 1990;58:759–770. doi: 10.1016/S0006-3495(90)82418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA T., MURATA Y., KAWAMURA N., HAYASHI M., TAMADA K., TAKUMA K., MAEDA S., BABA A. Selective induction of α1 isoform of (Na+,K+)-ATPase by insulin/insulin-like growth factor-1 in cultured rat astrocytes. Arch. Biochem. Biophys. 1993;307:175–182. doi: 10.1006/abbi.1993.1576. [DOI] [PubMed] [Google Scholar]
- MUNZER J.S., DALY S.E., JEWELL-MOTZ E.A., LINGREL J.B., BLOSTEIN R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J. Biol. Chem. 1994;269:16668–16676. [PubMed] [Google Scholar]
- NAKAO M., GADSBY D.C. [Na] and [K] dependence of the Na/K pump current–voltage relationship in guinea pig ventricular myocytes. J. Gen. Physiol. 1989;94:539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVA C., COHEN I.S., MATHIAS R.T. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophysis. J. 1988;54:791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUSCH M., NEHER E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- SANADA M., YASUDA H., OMATSU-KANBE M., SANGO K., ISONO T., MATSUURA H., KIKKAWA R. Increase in intracellular Ca2+ and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111:413–422. doi: 10.1016/s0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- SCROGGS R.S., FOX A.P. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J. Physiol. (London) 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHATTOCK M.J., MATSUURA H. Measurement of Na+-K+ pump current in isolated rabbit ventricular myocytes using the whole-cell voltage-clamp technique. Inhibition of the pump by oxidant stress. Circ. Res. 1993;72:91–101. doi: 10.1161/01.res.72.1.91. [DOI] [PubMed] [Google Scholar]
- SHYJAN A.W., CENA V., KLEIN D.C., LEVENSON R. Differential expression and enzymatic properties of the Na+,K+-ATPase α3 isozyme in rat pineal glands. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1178–11182. doi: 10.1073/pnas.87.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STIMERS J.R., LIU S., KINARD T.A. Effect of Nai on activity and voltage dependence of the Na/K pump in adult rat cardiac myocytes. J. Membr. Biol. 1993;135:39–47. doi: 10.1007/BF00234650. [DOI] [PubMed] [Google Scholar]
- SWEADNER K.J. Enzymatic properties of separated isozymes of the Na,K-ATPase. Substrate affinities, kinetic cooperativity, and ion transport stoichiometry. J. Biol. Chem. 1985;260:11508–11513. [PubMed] [Google Scholar]
- SWEADNER K.J. Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- THERIEN A.G., NESTOR N.B., BALL W.J., BLOSTEIN R. Tissue-specific versus isoform-specific differences in cation activation kinetics of the Na,K-ATPase. J. Biol. Chem. 1996;271:7104–7112. doi: 10.1074/jbc.271.12.7104. [DOI] [PubMed] [Google Scholar]
- TSIEN R.Y., RINK T.J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim. Biophys. Acta. 1980;599:623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- VERDRU P., DE GREEF C., MERTENS L., CARMELIET E., CALLEWAERT G. Na+–Ca2+ exchange in rat dorsal root ganglion neurons. J. Neurophysiol. 1997;77:484–490. doi: 10.1152/jn.1997.77.1.484. [DOI] [PubMed] [Google Scholar]
- VILLIÉRE V., MCLACHLAN E.M. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J. Neurophysiol. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- ZAHLER R., ZHANG Z.-T., MANOR M., BORON W.F. Sodium kinetics of Na,K-ATPase α isoforms in intact transfected HeLa cells. J. Gen. Physiol. 1997;110:201–213. doi: 10.1085/jgp.110.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]