Abstract
Ethanol (EtOH) tachyphylaxis (acute tolerance), a time-dependent decrease in apparent potency, is known in vivo and in some neuronal preparations. The present studies characterize EtOH tachyphylaxis in spinal motorneurons and test the hypothesis that metabotropic glutamate receptors (mGluRs) play a role.
Patch clamp studies were carried out in motorneurons in rat spinal cord slices. Currents were evoked by pulses of glutamate, alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) or N-methyl-D-aspartic acid (NMDA).
In nine of 15 cells, ethanol depression of glutamate-evoked currents was time-dependent. EtOH depressed current area 36.9±3% at 8–10 min, but only 16.8±3% at 20 min. Mean reduction in depression was 20.1±1%, N=9. Tachyphylaxis was less prominent in currents evoked by AMPA or NMDA, appearing in two of 10 AMPA and three of 11 NMDA currents.
The mGluR agonist trans-(1S,3R)-1-amino-1,3-cyclopentanedicarboxylic acid (ACPD) increased, the antagonist (±)-alpha-methyl-4-carboxyphenylglycine (MCPG) decreased the area of glutamate-evoked currents. ACPD also increased the area of NMDA- and AMPA-evoked currents.
ACPD increased the incidence of tachyphylaxis in glutamate-evoked currents to 100% (N=9); MCPG markedly reduced tachyphylaxis. ACPD also increased the incidence of tachyphylaxis in currents evoked by NMDA and AMPA to five of eight and four of seven neurons, respectively.
Block of G-protein pathways by intracellular GDP-β-s abolished tachyphylaxis in glutamate-evoked currents (N=8); however, currents recovered only partially following EtOH washout.
Activation of mGluRs contributes to neuronal tachyphylaxis to EtOH in spinal cord motorneurons, probably via G-protein pathways.
Keywords: Ethanol, acute tolerance, tachyphylaxis, metabotropic glutamate receptors, spinal cord, motor neurons, G-proteins
Introduction
Tachyphylaxis (acute tolerance) to ethanol (EtOH) is known from many in vivo studies. Following a single administration of an anesthetic dose of EtOH, blood alcohol level on recovery is higher than at loss of righting reflex. Acute tolerance has been described in some neuronal preparations (Grover et al., 1994; Pearson et al., 1997; Ludvig et al., 2001). We have previously reported that ethanol at general anesthetic concentrations depresses synaptic transmission to motorneurons in spinal cord (Wong et al., 1997; Wang et al., 2000) and that the depression is due in part to direct postsynaptic actions on glutamate currents in the motorneurons themselves (Wang et al., 1999). In the course of the latter studies, we observed that the actions of EtOH on glutamate-evoked currents are time-dependent, maximum depression at approximately 6-min exposure being succeeded by partial recovery at 15 min in the continued presence of EtOH. This phenomenon is properly described as tachyphylaxis.
In these studies, currents were evoked by puffs of glutamate from a pipette containing a high (100 mM) glutamate concentration. Tachyphylaxis was observed in all of the sample of five neurons. Tachyphylaxis was present when GABAA and glycine receptors were blocked by bicuculline (50 μM) and strychnine (5 μM) (four cells), and when either alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors were blocked by 6-cyano-7-nitroquinoxaline-2,3-dinone disodium (CNQX; 10 μM) or when N-methyl-D-aspartic acid (NMDA) receptors were blocked by D,L-2-amino-5-phosphonopentanoic acid (AP-5; 40 μM). Thus, when high glutamate concentrations were used, almost all neurons displayed tachyphylaxis as manifested by partial recovery during ethanol exposure. In a set of follow-up studies, glutamate concentration in the pipette was reduced to 5 mM. Under these conditions, only about half of the neurons displayed tachyphylaxis. The present studies were carried out to further characterize EtOH tachyphylaxis in motorneurons and to probe for its mechanism. In particular, since the incidence of tachyphylaxis appeared to depend on high glutamate concentrations, they were designed to test the hypothesis that metabotropic glutamate receptors (mGluRs) mediate the tachyphylaxis. In order to probe the role of mGluRs, bath applications were made of a broad spectrum mGluR agonist, trans-(1S,3R)-1-amino-1,3-cyclopentanedicarboxylic acid (ACPD), or antagonist, (±)-alpha-methyl-4-carboxyphenylglycine (MCPG). Portions of this work have been reported in abstract form (Kendig & Li, 2001).
Methods
Spinal cord motorneurons were studied using patch clamp techniques as we have previously described (Cheng & Kendig, 2000; Wang & Kendig, 2000). All experiments were carried out according to the protocols approved by the Stanford Institutional Animal Care and Use Committee. Sprague–Dawley rats aged P7–10 (P0=date of birth) were anesthetized with halothane and decapitated, and spinal cords quickly removed and placed in a cold (under 4°C) oxygenated artificial cerebrospinal fluid (ACSF), which contained (mM): NaCl2: 123; KCl: 4; NaH2PO4: 1.2; MgSO4: 1.3; NaHCO3: 26; dextrose: 10; and CaCl2: 2, pH 7.4. Slices 350 μM thick were sectioned from the lumbar region on a vibratome (Technical Products International, St Louis, MO, U.S.A.), and removed into oxygenated ACSF at room temperature for 1-h incubation. Individual slices were transferred to a perfusion chamber for recordings. All experiments were carried out at room temperature.
Patch pipettes were pulled on a Flaming–Brown pipette puller (Sutter Instruments, Novato, CA, U.S.A.) and had an impedance of 2–5 MΩ when filled with intracellular solution containing (mM): NaCl: 15; K-gluconate: 110; N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES): 10; MgCl2: 2; ethylene glycolbis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA): 11; CaCl2·2H2O: 1; ATP-Na: 2; and GTP: 0.4, pH 7.3 adjusted with KOH. The osmolarity of the pipette solution was adjusted to 285–295 mosM. Previous studies (Wang et al., 1999,2000) had identified the large cell bodies in the ventral horn as motorneurons by fluorescence labeling with dye injected into the gastrocnemius muscle. Whole-cell voltage clamp recordings were made from visually identified motorneurons using infrared video microscopy and a × 40 water-immersion lens (Zeiss Axioskop) and an Axopatch 200B amplifier (Axon Instruments) at a holding potential of −60 mV in perfusate containing bicuculline methiodide (BMI) 10 μM; strychnine 5 μM; tetrodotoxin (TTX) 0.5 μM. Currents were obtained by direct pressure application (8–10 psi, 100–150 ms) of 5 mM glutamate, 0.02 mM AMPA or 2 mM N-Methyl-D-Aspartic Acid (NMDA) from a pipette positioned near the recorded cell (Picospritzer, General Valve Division of Parker Hannefin, Fairfield, NJ, U.S.A.) at 1–3 min intervals. In some preliminary experiments, glutamate concentrations in the pipette were 100 mM. Responses to repeated agonist application were stable, with no evidence of receptor desensitization under any of the experimental conditions. Duration of the pressure pulse was adjusted to give a current of acceptable magnitude.
Ethanol (EtOH) was obtained from commercial sources (Gold Shield Chemical Company, Hayward, CA, U.S.A.) as the 95% pure compound, diluted to 100 mM (an anesthetic concentration) in ACSF. Concentrations of EtOH in the bath were verified by gas chromatography of the vapor phase in equilibrium with the solution in the chamber. Owing to reports that the DHPE plasticizer in polyethylene tubing would cause a rise in intracellular calcium, particularly when leached out by high EtOH concentrations (Tully et al., 2000), the perfusion system employed Teflon throughout. All other drugs used in the experiments were from Sigma, and included TTX, BMI, CNQX, AP-5, acid ACPD, and MCPG. These drugs were bath applied. In the case of ACPD and MCPG, after control recordings were made, the mGluR agents were applied until recordings were stable before pre-ethanol controls were made.
The area of evoked currents during EtOH application was measured and normalized to the average baseline current area during the 10 min preceding EtOH. Data are expressed as mean±s.e.m. Statistical significance of the differences in the extent of EtOH depression of currents evoked by glutamate, AMPA and NMDA, and in the difference between control, EtOH depression at 10 and at 20 min was assessed by paired t-test or one-way ANOVA followed by either Dunn's or Tukey's corrections for multiple comparisons. In the initial pilot study, currents were evoked by puffs from a pipette containing 100 mM glutamate (19 neurons). The data reported herein were obtained from 91 motorneurons in which currents were evoked by 5 mM glutamate puffs or by AMPA or NMDA as described above. A single motorneuron was studied in each slice.
Results
Depressant effect of EtOH on glutamate-evoked currents
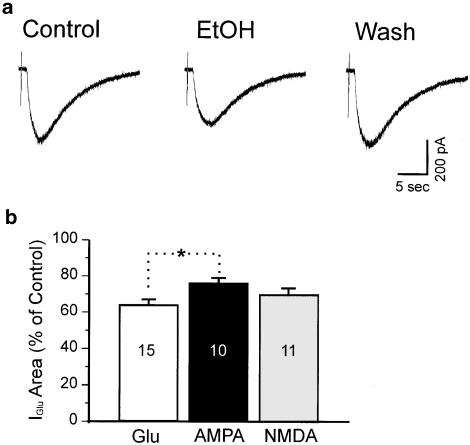
We arbitrarily set depression larger than 10% as a criterion for EtOH-sensitive cells. In all, 15 out of 19 motorneurons tested (79%) were depressed by more than 10% at 8–10 min after application of EtOH. Average depression was 35.6±2%, with a range from 11 to 52%. Figure 1a shows a representative example of the EtOH effect on glutamate-evoked currents.
Figure 1.
EtOH depresses glutamate-evoked currents recorded from a spinal cord motorneuron. (a) Individual traces from the same neuron showing predrug (control), 10 min after perfusion and 10 min after washout of 100 mM EtOH. (b) Histogram showing mean area of currents as a percent of control for depression at 8–10 min after 100 mM EtOH perfusion. Glu, AMPA and NMDA indicate: glutamate-, AMPA-, or NMDA-evoked currents. In each bar, 15, 10, and 11 indicate number of neurons. *The difference between glutamate and AMPA groups was significant (P<0.05, one-way ANOVA followed by Tukey's multiple comparison test).
EtOH depression was not accompanied by consistent changes in membrane input resistance or holding current. Of the 15 EtOH-sensitive neurons, 10 showed a slight depolarization averaging 1.87±0.37 mV, with a range from 0.46 to 3.8 mV; the remaining five showed either no change or a very slight hyperpolarization (0–0.35 mV). These changes were not significant.
Currents elicited by NMDA or AMPA
To examine whether EtOH has differential effects on glutamate receptor subtypes, we carried out a set of experiments to test the effect of EtOH on currents mediated either by NMDA receptors or AMPA receptors. Of 11 spinal cord motorneurons in which responses were elicited by AMPA, EtOH depressed 10 (91%) by the 10% criterion. Mean depression was 24±3%, with a range from 11 to 34%, 8–10 min after application of EtOH. In 15 neurons in which currents were elicited by NMDA, 11 (73%) were depressed by at least 10%. Average depression was 30±4%, with a range from 18 to 57%. No significant difference was found in the magnitudes of initial depression between AMPA- and NMDA-evoked currents (24±3 vs 30±4%, AMPA and NMDA, respectively, P>0.05), nor between glutamate- and NMDA-evoked currents (35.6±2 vs 30±4%, P>0.05, Figure 1b). However, the magnitude of depression of currents evoked by AMPA was significantly less than that of currents evoked by glutamate (24±3 vs 35.6±2%, P<0.05, Figure 1b) at 10 min.
EtOH depression of glutamate-evoked currents is time-dependent
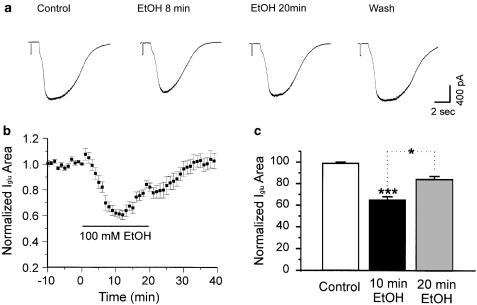
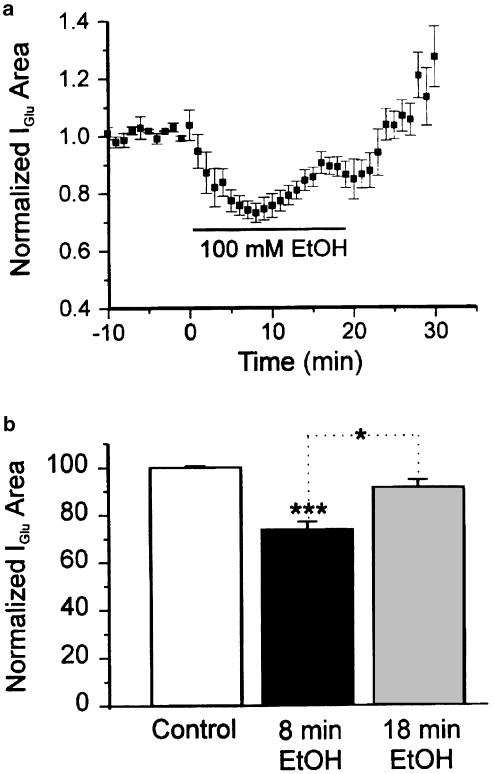
In the preliminary studies in which glutamate concentrations in the pipette were high, time dependence appeared to be universal. In later studies in which much lower glutamate concentrations were used, time dependence assumed a probabilistic character. We observed the time course of EtOH actions on 15 neurons sensitive to ethanol by the criterion of depression greater than 10%. Nine of the 15 (60%) showed a pronounced time-dependent effect of 100 mM EtOH on glutamate-evoked responses. Maximum depression in these nine cells was 36.9±3% at 8–10 min after application of EtOH. During the subsequent period in the continued presence of 100 mM ethanol, depression declined to 16.8±3% at 18–20 min. The mean reduction in percent depression was 20.1±1% (Figure 2b and c). The difference was significant (P<0.05) when analyzed by ANOVA followed by either Dunn's or Tukey's test. At 18–20 min, the average depression across the entire sample of 15 neurons was 25.1±3%, which was also significantly less than the average maximum level of depression at 8–10 min (35.6±2%, P<0.01 by one-way ANOVA followed by Tukey's tests). Tachyphylaxis to EtOH was not accompanied by corresponding changes in input resistance or holding current.
Figure 2.
EtOH depression of currents evoked by glutamate decreases over time. (a) Individual traces from a neuron showing tachyphylaxis. (b) Time course of the mean effect of EtOH on glutamate-evoked currents (N=9). (c) Histogram showing the initial depression by EtOH at 10 min and reduced depression EtOH at 20 min of continuous application of 100 mM EtOH (N=9). ***P< 0.001 between control and 10 min EtOH application, *P<0.05 between 10 and 20 min EtOH application. One-way ANOVA followed by Dunn's multiple comparison test.
Tachyphylaxis in AMPA- and NMDA-evoked currents
In preliminary studies with currents evoked by high glutamate concentrations in the pipette, AMPA currents were blocked by CNQX (N=5) or NMDA currents by AP-5 (N=5). Under these conditions, both types of the remaining currents were approximately equally sensitive to 100 mM EtOH. All of the neurons in which AMPA currents were blocked and four of the five in which NMDA currents were blocked displayed acute tachyphylaxis of approximately equal magnitudes, suggesting that both AMPA and NMDA receptors showed tachyphylaxis.
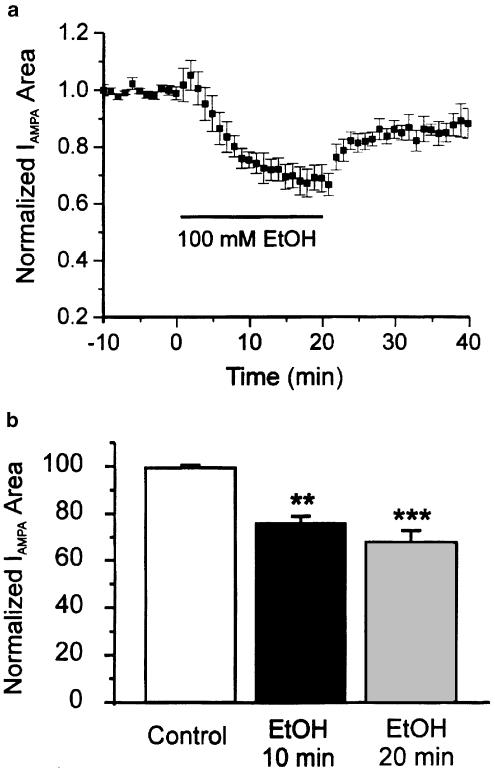
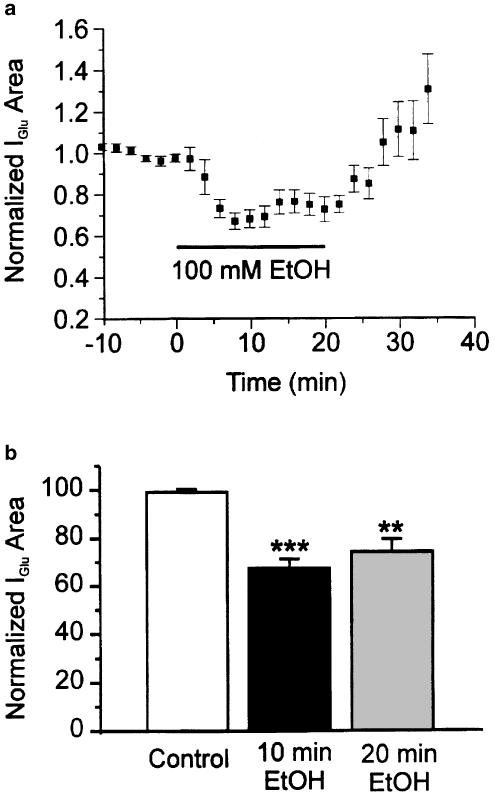
In the present set of studies, a different approach was taken; instead of isolating AMPA or NMDA currents evoked by glutamate, the respective currents were evoked by the selective agonists AMPA or NMDA. Compared to glutamate-evoked currents, AMPA- or NMDA-evoked currents were less likely to show acute tachyphylaxis. Among 10 neurons in which responses were evoked by AMPA, only two (20%) showed tolerance and that to a modest extent (mean reduction in depression at 18–20 min compared to 8–10 min was 7±1%, N=2). In the entire sample of 10, moreover, the mean magnitude of depression at 20 min of application of EtOH was larger (32±5%) compared to the initial depression at 8–10 min (24±3%, N=10, Figure 3); this difference did not reach the level of statistical significance (P>0.05, one-way ANOVA followed by Tukey's test). The magnitude of depression in AMPA-evoked currents at 20 min was close to the maximum depression of glutamate-evoked currents at 10 min, suggesting EtOH effects on currents evoked by AMPA take longer to develop than when currents are evoked by glutamate.
Figure 3.
EtOH depresses responses evoked by AMPA. (a) Time course of the mean effect of EtOH on AMPA-evoked currents (N=10). (b) Histogram showing no significant difference in EtOH depression at 10 and 20 min EtOH exposure. ***P<0.001 between control and 20 min EtOH; **P<0.01 between control and 10 min EtOH application. One-way ANOVA followed by Dunn's multiple comparison test.
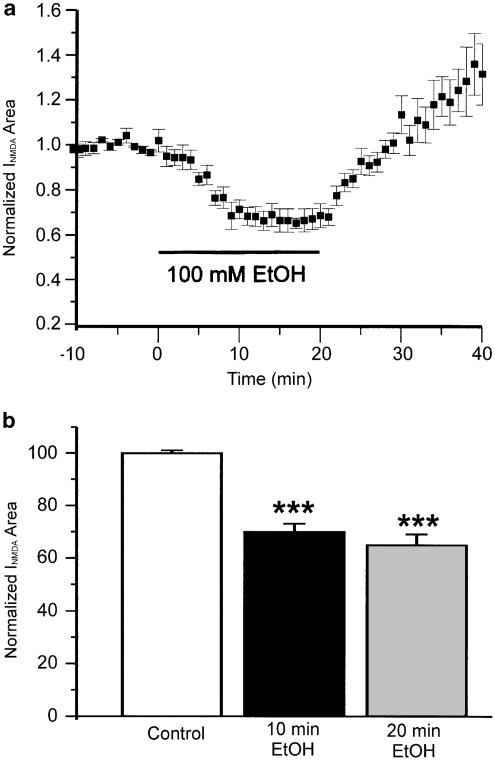
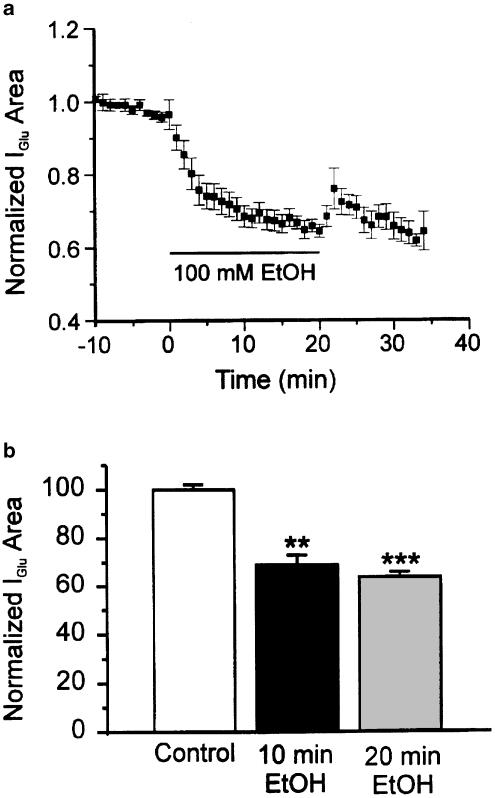
Similarly, in 11 neurons in which responses were evoked by NMDA, only three (27%) displayed tachyphylaxis to EtOH, again to a modest extent (average reduction in depression was 7.7±1.1%, N=3). In the entire sample of 11 neurons, the average magnitude of depression at 20 min after application of EtOH was 34.6±4%, not significantly different from the initial depression (30±4%, N=11, Figure 4). Note that on wash NMDA-evoked currents recover to levels well above controls (Figure 4). This withdrawal hyper-responsiveness is the subject of a separate study. Comparing the results from glutamate-, AMPA- and NMDA-evoked currents, and considering the reduction in the probability of tachyphylaxis when glutamate concentration was reduced, we then tested the hypothesis that mGluRs are involved in the time-dependent actions of EtOH.
Figure 4.
EtOH depresses currents evoked by NMDA. (a) Time course of the mean effect of EtOH on NMDA-evoked currents (N=11). On washout, currents increase to levels well above controls; this phenomenon of withdrawal hyper-responsiveness is the subject of a separate study. (b) Histogram of EtOH depression at 10 and 20 min. ***P<0.001 between control and EtOH application at 10 and 20 min. There was no significant difference between depression at 10 and 20 min. One-way ANOVA followed by Dunn's multiple comparison test.
Activation of metabotropic receptors increases the area of glutamate-evoked currents; there are no significant effects on the extent of EtOH depression
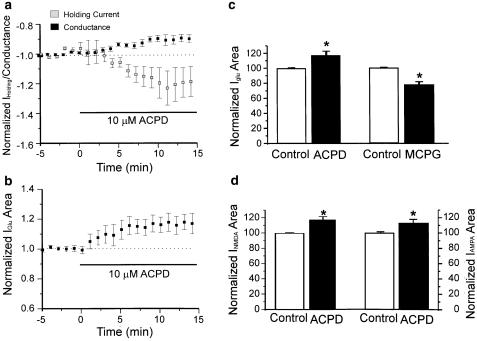
The mGluR agonist ACPD (10 μM) produced a small but significant decrease in conductance and a shift in holding current in the depolarizing direction that was not significant (Figure 5a). In some of the neurons, the small shift in holding current was transient. Activation of mGluRs by 10 μM ACPD increased the current area of glutamate-evoked currents in six out of eight cells tested (Figures 5b and c). The average increase is 18.7±6% (P<0.05, N=6). The current area stabilized during the application and there was no suggestion of time dependence (Figure 5b). Block of mGluRs by 800 μM MCPG decreased the area of currents by 21.6±4% (P<0.05, N=8) (Figure 5c). The increase in glutamate-evoked current area following ACPD application appears to be because of increases in both NMDA and AMPA receptor-mediated currents; ACPD also increased NMDA-evoked currents in five out of six neurons by 17.2±4% (paired t-test, P<0.05, N=5, Figure 5d) and AMPA-evoked currents in five out of eight neurons by 11±4% (paired t-test, P<0.05, N=5, Figure 5d). mGluR activation or block did not significantly alter the depressant effect of EtOH. The initial depression at 8–10 min produced by EtOH in the presence of ACPD was 27.7±3% (N=9), compared to 35.6±2% in control solution (N=15). When mGluRs were blocked by MCPG, the initial depression produced by EtOH (37.5±5%, N=9) was similar to that in control solution (35.6±2%). The differences among the three groups were not statistically significant (one-way ANOVA followed by Tukey's test, P>0.05 between each pair of groups).
Figure 5.
Activation and block of mGluRs act on motorneuron intrinsic properties and modulate responses evoked by glutamate, AMPA and NMDA. Currents were measured 15 min after application of ACPD or MCPG. (a) Time course of the mean effect of ACPD on holding currents and conductance (N=8). There is a small but significant decrease in conductance and a shift in holding current in the depolarizing direction which is not significant. (b) Time course of the mean effect of ACPD on glutamate-evoked currents (N=8). The increase in area is stable over the time of application. (c) Activation of mGluR receptors by ACPD enhanced the current area of glutamate-evoked currents by 18.7±6% (left, N=6). Block of mGluR receptors by MCPG decreased current area by 21.6±4% (right, N=8). *Paired t-test, P<0.05. (d) Activation of mGluR receptors by ACPD enhanced area of currents evoked by both NMDA (left, N=5) and AMPA (right, N=5). *Paired t-test, P<0.05.
Activation of metabotropic receptors increases the incidence of tachyphylaxis; block of metabotropic receptors reduces its magnitude
Of nine neurons treated with 10 μM ACPD in which currents were evoked by glutamate, all showed tachyphylaxis to EtOH. Figure 6a shows the time course of EtOH effects on glutamate-evoked responses in the presence of ACPD. EtOH decreased the current area of glutamate-evoked currents by 27.7±3% at 8–10 min; depression was reduced to 9.7±3% at 18–20 min in the continued presence of EtOH (P<0.05, one-way ANOVA followed by Dunn's test) (Figure 6b).
Figure 6.
Activation of mGluRs increases the incidence of tachyphylaxis. EtOH was applied 15–20 min after application of ACPD, the increases in current area because of the mGluR agonist had reached a steady state. (a) Time course of the mean effect of EtOH on glutamate-evoked currents (N=9) showing tachyphylaxis. (b) Histogram showing significant difference of EtOH depression at 8–10 and 18–20 min. ***P<0.001 between control and 10 min EtOH exposure; *P<0.05 between 10 and 20 min EtOH exposure. One-way ANOVA followed by Dunn's test.
We then tested whether ACPD also promotes tachyphylaxis in currents evoked by NMDA and AMPA. Five of eight neurons (62.5%) in which currents were evoked by NMDA displayed tachyphylaxis, the effect of EtOH changing from an initial depression of 27.0±4% at 8–10 min to 13.3±3% at 18–20 min (P<0.05, N=5, data not shown). Four of seven neurons (57.1%) in which currents were evoked by AMPA displayed tachyphylaxis; initial depression of 29.0±6% at 8–10 min decreasing to 18.0±3% at 18–20 min (P<0.05, N=4, data not shown).
Block of mGluRs did not completely eliminate the occurrence of tachyphylaxis. Of nine neurons treated with 500–800 μM MCPG, five showed tachyphylaxis. However, over the entire sample of nine neurons, there was no significant time dependence of EtOH-depressant actions (Figure 7).
Figure 7.
Block of mGluRs reduces tachyphylaxis to insignificant levels but does not completely eliminate it. EtOH was applied 15–20 min after application of MCPG, at which time the depressant action of the antagonist had reached a steady state. (a) Time course of the mean effect of EtOH on glutamate-evoked currents (N=9). (b) Histogram showing no significant difference between EtOH depression at 8–10 and 18–20 min. ***P<0.001; **P<0.01 compared to control. One-way ANOVA followed by Dunn's test.
Block of G-protein pathways eliminates tachyphylaxis
To examine whether G-protein pathways participate in acute tachyphylaxis to EtOH, we included GDP-β-s in the patch-pipette solution to block cytosolic G-protein pathways. Of 12 neurons tested, glutamate-evoked currents in eight (67%) were depressed more than 10% by 100 mM EtOH. The depression of glutamate-evoked currents was not significantly different at 18–20 min after application than at 8–10 min, 31±3% at 8–10 min and 34.6±2% at 18–20 min after application of EtOH (P>0.05, N=8). However, the current areas did not recover to control levels after 20-min wash. Figure 8a shows the time course of EtOH effects on glutamate-evoked currents in the presence of GDP-β-s.
Figure 8.
Block of G-protein pathways eliminates tachyphylaxis. GDP-β-s was in the pipette solution, and would be expected to dialyze into the cell within a minute or two following patch rupture. Recordings were begun 10–15 min after the patch was ruptured. (a) Time course of the mean effect of EtOH on glutamate-evoked currents (N=8). Recovery following washout is incomplete. (b) Histogram showing no difference between EtOH depression at 8–10 and 18–20 min. ***P<0.001; **P<0.01 compared to control. One-way ANOVA followed by Dunn's test.
Discussion
Currents in motorneurons
In spinal cord motorneurons, inward currents evoked either transsynaptically or by glutamate are mediated by both CNQX-sensitive AMPA receptors and by AP-5-sensitive NMDA receptors (Pook et al., 1993; Wang et al., 1999). When both the NMDA receptor antagonist AP-5 and the AMPA/kainate antagonist CNQX are coapplied, the response to dorsal root stimulation is completely blocked and to glutamate application nearly so (Cheng & Kendig, 2000; Wang & Kendig, 2000). Thus, no other receptor plays a significant role in generating fast synaptic or glutamate-evoked currents. Tachyphylaxis occurred in glutamate-evoked currents in the presence of bicuculline and strychnine to block inhibitory chloride channels. Although GABAA receptors have been proposed to be involved in mechanisms of tolerance and dependence (Harris et al., 1998), the tachyphylaxis observed in the present study is a property of motorneurons independent of GABAA or glycine receptors.
In preliminary studies, glutamate was used to evoke currents and AMPA and NMDA currents were isolated by application of AP-5 or CNQX, respectively; each component of the glutamate-evoked response displayed tachyphylaxis. However, when currents were evoked by the respective specific agonists AMPA or NMDA, the probability and magnitude of tachyphylaxis were markedly decreased. This finding, together with the higher probability of tachyphylaxis with high glutamate concentrations, led to the hypothesis that tachyphylaxis depended on activation of metabotropic glutamate receptors, although it was expressed by ionotropic glutamate currents.
EtOH and mGluRs
The mGluR agonist ACPD itself increased the area of glutamate-evoked currents and also increased the proportion of cells that displayed tachyphylaxis. Application of ACPD was accompanied by a shift in holding current in the direction of depolarization that was not significant. Although in some cells the shift was transient, overall the shift was persistent rather than transient. There was also a small stable decrease in conductance. mGluR-induced transient depolarization has also been reported by others (Ugolini et al., 1997), as has an increase in both NMDA and AMPA responses by mGluR agonists (Ugolini et al., 1997,1999). The increase in area induced by ACPD stabilized before the application of EtOH and thus was not responsible for the tachyphylaxis. The mGluR antagonist MCPG markedly reduced the magnitude of tachyphylaxis but did not block it completely. ACPD is a nonselective agonist at group I (mGluRs 1 and 5) and group II metabotropic receptors (mGluRs 2 and 3) but not group III; MCPG is a nonselective antagonist at all three groups of receptors. Thus, the actions of agonist and antagonist are not completely congruent. Furthermore, it is not certain that all mGluRs were blocked by the antagonist, an uncertainty which may account for the inability of MCPG to block tachyphylaxis completely. EtOH is known to act on some but not all mGluRs (Minami et al., 1998); the actions of EtOH that evoke tachyphylaxis appear to be amplified by mGluR receptor activation, but the subtypes responsible cannot be determined by the present results because of the broad spectrum nature of the agonist and antagonist employed.
mGluRs are members of the class of receptors coupled to G-proteins. When cytosolic G-protein pathways were blocked, tachyphylaxis was abolished. However, the results were complicated by the incomplete recovery displayed by these neurons following EtOH washout. In view of the importance of G-protein pathways in regulating many cellular targets, it is possible that the nonselective inhibitor affected other mechanisms than those involved in tachyphylaxis and led to rundown of glutamate currents. If mGluR promotion of tachyphylaxis is dependent on G-proteins, there are many transduction mechanisms that could mediate the effect, including different mechanisms for group I and group II receptors such as phospholipase C and adenylyl cyclase, respectively. The results of the present studies do not speak to this.
The end effector involved in the pathway from metabotropic receptors to tachyphylaxis is not known. G-protein-mediated phosphorylation of one or both glutamate receptors is a plausible target, as is increase in intracellular calcium. The presence of the calcium chelator EGTA in the pipette solution would be expected to attenuate but not abolish an increase in intracellular calcium (Tymianski et al., 1994). Changes in membrane resistance following EtOH application were not consistent and thus provided little in the way of evidence.
Other studies of tachyphylaxis (acute neuronal tolerance)
Only a few studies have been carried out demonstrating tachyphylaxis or acute neuronal tolerance in individual CNS cell types. Tolerance has, however, been observed in several different areas including hippocampus (Grover et al., 1994; Tymianski et al., 1994; Ludvig et al., 2001), cerebellum (Pearson et al., 1997) and locus coeruleus (Poelchen et al., 1997). In no case has the mechanism been determined, although in locus coeruleus the tolerance is expressed by NMDA receptors (Poelchen et al., 1997). In all cases the tolerance is acute, developing within minutes of continued exposure, although the reported time courses vary somewhat.
Relations to tachyphylaxis in vivo
EtOH tachyphylaxis is well known in experimental animals, manifested as a lower blood EtOH concentration at the time of loss of righting reflex than that obtained when the animal regains righting reflex. The temporal characteristics of this tachyphylaxis are such that the phenomenon we describe could contribute to it, but it may not be the only contributor. We have previously shown that there is a pronounced increase in EtOH anesthetic potency with increasing age, as measured by immobility in response to a noxious stimulus in both rats (Fang et al., 1997a) and mice (Fang et al., 1997b). Age-related differences in acute tolerance might account for this. There is evidence in support of this suggestion (Silveri & Spear, 1998); in rats initial sensitivities to EtOH as measured by loss of righting reflex increased with age, concomitantly with a decrease in acute tolerance. Similarly, the genetic difference in EtOH sensitivity between long sleep and short sleep mice may be related to differences in acute tolerance; Purkinje neurons isolated from the two strains displayed differences in acute tolerance manifested as depression followed by recovery of impulse frequency in the continued presence of ethanol (Pearson et al., 1997).
Summary
Motorneurons display tachyphylaxis to EtOH, with a time course of minutes. Activation of mGluRs mediates the occurrence of tachyphylaxis, probably via G-protein pathways. The neuronal tachyphylaxis observed in vitro probably contributes to acute EtOH tolerance in vivo and to age- and strain-related differences in sensitivity to EtOH.
Acknowledgments
Supported by NIH Grants NS13108 and GM47818 to JJK. Ethanol concentrations were measured in the laboratory of Dr E.I. Eger in the Department of Anesthesia at the University of California, San Francisco. We are indebted to the members of Program Project Group GM47818 for many helpful discussions.
Abbreviations
- ACPD
trans-(1S,3R)-1-amino-1,3-cyclopentanedicarboxylic acid
- AMPA
alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- AP-5
D,l-2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dinonedisodium
- MCPG
(±)-alpha-methyl-4-carboxyphenylglycine
- NMDA
N-methyl-D-aspartic acid
- TTX
Tetrodotoxin
References
- CHENG G., KENDIG J.J. Enflurane directly depresses glutamate AMPA and NMDA currents in mouse spinal cord motor neurons independent of actions on GABAA or glycine receptors. Anesthesiology. 2000;93:1075–1084. doi: 10.1097/00000542-200010000-00032. [DOI] [PubMed] [Google Scholar]
- FANG Z., GONG D., IONESCU P., LASTER M.J., EGER E.I., II, KENDIG J. Maturation decreases ethanol minimum alveolar anesthetic concentration (MAC) more than desflurane MAC in rats. Anesth. Analg. 1997a;84:852–858. doi: 10.1097/00000539-199704000-00028. [DOI] [PubMed] [Google Scholar]
- FANG Z., IONESCU P., GONG D., KENDIG J., HARRIS A., EGER E.I., II Maturation decreases ethanol minimum alveolar anesthetic consentration in mice as previously demonstrated in rats: there is no species difference. Anesth. Analg. 1997b;85:160–163. doi: 10.1097/00000539-199707000-00029. [DOI] [PubMed] [Google Scholar]
- GROVER C.A., FRYE G.D., GRIFFITH W.H. Acute tolerance to ethanol inhibition of NMDA-mediated EPSPs in the CA1 region of the rat hippocampus. Brain Res. 1994;642:70–76. doi: 10.1016/0006-8993(94)90906-7. [DOI] [PubMed] [Google Scholar]
- HARRIS R.A., VALENZUELA C.F., BROZOWSKI S., CHUANG L., HADINGHAM K., WHITING P.J. Adaptation of gamma-aminobutyric acid type A receptors to alcohol exposure: studies with stably transfected cells. J. Pharmacol. Exp. Ther. 1998;284:180–188. [PubMed] [Google Scholar]
- KENDIG J.J., LI H.-F.Acute tolerance to ethanol in glutamate-evoked currents in spinal cord motor neurons Society for Neuroscience Annual Meeting Abstracts 2001. Vol. 27, Program No. 225.4
- LUDVIG N., GEORGE M.A., TANG H.M., GONZALES R.A., BUNGAY P.M. Evidence for the ability of hippocampal neurons to develop acute tolerance to ethanol in behaving rats. Brain Res. 2001;900:252–260. doi: 10.1016/s0006-8993(01)02319-8. [DOI] [PubMed] [Google Scholar]
- MINAMI K., GEREAU R.W.t., MINAMI M., HEINEMANN S.F., HARRIS R.A. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol. Pharmacol. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- PEARSON B.J., DONATELLI D.P., FREUND R.K., PALMER M.R. Differential development and characterization of rapid acute neuronal tolerance to the depressant effects of ethanol on cerebellar Purkinje neurons of low-alcohol-sensitive and high-alcohol-sensitive rats. J. Pharmacol. Exp. Ther. 1997;280:739–746. [PubMed] [Google Scholar]
- POELCHEN W., NIEBER K., ILLES P. Tolerance to inhibition by ethanol of N-methyl-D-aspartate-induced depolarization in rat locus coeruleus neurons in vitro. Eur. J. Pharmacol. 1997;332:267–271. doi: 10.1016/s0014-2999(97)01113-8. [DOI] [PubMed] [Google Scholar]
- POOK P., BRUGGER F., HAWKINS N.S., CLARK K.C., WATKINS J.C., EVANS R.H. A comparison of the actions of agonists and antagonists at non-NMDA receptors of C fibres and motorneurones of the immature rat spinal cord in vitro. Br. J. Pharmacol. 1993;108:179–184. doi: 10.1111/j.1476-5381.1993.tb13459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERI M.M., SPEAR L.P. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin. Exp. Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- TULLY K., KUPFER D., DOPICO A.M., TREISTMAN S.N. A plasticizer released from IV drip chambers elevates calcium levels in neurosecretory terminals. Toxicol. Appl. Pharmacol. 2000;168:183–188. doi: 10.1006/taap.2000.9036. [DOI] [PubMed] [Google Scholar]
- TYMIANSKI M., CHARLTON M.P., CARLEN P.L., TATOR C.H. Properties of neuroprotective cell-permeant Ca2+ chelators: effects on [Ca2+]i and glutamate neurotoxicity in vitro. J. Neurophysiol. 1994;72:1973–1992. doi: 10.1152/jn.1994.72.4.1973. [DOI] [PubMed] [Google Scholar]
- UGOLINI A., CORSI M., BORDI F. Potentiation of NMDA and AMPA responses by group I mGluR in spinal cord motoneurons. Neuropharmacology. 1997;36:1047–1055. doi: 10.1016/s0028-3908(97)00103-2. [DOI] [PubMed] [Google Scholar]
- UGOLINI A., CORSI M., BORDI F. Potentiation of NMDA and AMPA responses by the specific mGluR5 agonist CHPG in spinal cord motoneurons. Neuropharmacology. 1999;38:1569–1576. doi: 10.1016/s0028-3908(99)00095-7. [DOI] [PubMed] [Google Scholar]
- WANG M.Y., KENDIG J.J. Patch clamp studies of motor neurons in spinal cord slices: a tool for high-resolution analysis of drug actions. Acta Pharmacol. Sin. 2000;21:507–515. [PubMed] [Google Scholar]
- WANG M.Y., RAMPIL I.J., KENDIG J.J. Ethanol directly depresses AMPA and NMDA glutamate currents in spinal cord motor neurons independent of actions on GABAA or glycine receptors. J. Pharmacol. Exp. Ther. 1999;290:362–367. [PubMed] [Google Scholar]
- WONG S.M., FONG E., TAUCK D.L., KENDIG J.J. Ethanol as a general anesthetic: actions in spinal cord. Eur. J. Pharmacol. 1997;329:121–127. [PubMed] [Google Scholar]