Abstract
The human orphan G-protein coupled receptor bombesin receptor subtype 3 (hBRS-3) was screened for peptide ligands by a Ca2+ mobilization assay resulting in the purification and identification of two specific ligands, the naturally occurring VV-hemorphin-7 (VV-H-7) and LVV-hemorphin-7 (LVV-H-7), from human placental tissue. These peptides were functionally characterized as full agonists with unique specificity albeit low affinity for hBRS-3 compared to other bombesin receptors.
VV-H-7 and LVV-H-7 induced a dose-dependent response in hBRS-3 overexpressing CHO cells, as well as in NCI-N417 cells expressing the hBRS-3 endogenously. The affinity of VV-H-7 was higher in NCI-N417 cells compared to overexpressing CHO cells. In detail, the EC50 values were 45±15 μM for VV-H-7 and 183±60 μM for LVV-H-7 in CHO cells, and 19±6 μM for VV-H-7 and 38±18 μM for LVV-H-7 in NCI-N417 cells. Other hemorphins had no effect. Gastrin-releasing peptide (GRP) and neuromedin B (NMB) showed similar EC50 values of 13–20 μM (GRP) and of 1–2 μM (NMB) on both cell lines.
Structure-function analysis revealed that both the N-terminal valine and the C-terminal phenylalanine residues of VV-H-7 are critical for the ligand-receptor interaction.
Endogenous hBRS-3 in NCI-N417 activated by VV-H-7 couples to phospholipase C resulting in changes of intracellular calcium, which is initially released from an inositol trisphosphate (IP3)-sensitive store followed by a capacitive calcium entry from extracellular space.
VV-H-7-induced hBRS-3 activation led to phosphorylation of p42/p44-MAP kinase in NCI-N417 cells, but did not stimulate cell proliferation. In contrast, phosphorylation of focal adhesion kinase (p125FAK) was not observed.
Keywords: Orphan receptor, hBRS-3, hemorphin, GPCR, signal transduction
Introduction
The human bombesin receptor subtype 3 (hBRS-3) exhibits about 50% amino-acid sequence homology with human neuromedin B (hNMB-R) and gastrin-releasing peptide (hGRP-R) receptors, and together they form the bombesin-like receptor group (Gorbulev et al., 1992). Gastrin-releasing peptide (GRP) and neuromedin B (NMB) are high-affinity ligands for hGRP-R and hNMB-R, respectively, while both peptides bind only with low affinity to hBRS-3. Recently, a synthetic peptide [DPhe6,β-Ala11,Phe13,Nle14]-bombesin (6-14) (B9P) was described to have high affinity for hBRS-3 (Mantey et al., 1997), but so far a natural high-affinity ligand for hBRS-3 has not been identified. The expression of hBRS-3 is limited to a few brain regions (Ohki-Hamazaki et al., 1997a), placenta (Whitley et al., 1996), pancreatic islets (Fleischmann et al., 2000), secondary spermatocytes and certain tumor cell lines (Fathi et al., 1993; Gorbulev et al., 1994). Targeted disruption of the hBRS-3 gene in mice leads to mild obesity, impaired glucose metabolism, and hypertension (Ohki-Hamazaki et al., 1997b). Therefore, hBRS-3 has been implicated in the regulation of neuroendocrine function and energy metabolism. Several studies described a role of bombesin-like peptides (NMB, GRP) in growth regulation of, for example, small cell lung carcinoma (SCLC) (Cuttitta et al., 1985), human breast cancer (Nelson et al., 1991), and gastrointestinal tissue (Lehy et al., 1983). They are also involved in development of the lung (Johnson et al., 1982) and induce the contraction of smooth muscle (Minamino et al., 1983). Bombesin-like peptides were postulated as mitogens for bronchial epithelial cells and SCLC, and lead to increased fetal lung growth and maturation in utero as well as in organ cultures (Sunday et al., 1998). Furthermore, it has been reported that bombesin-like peptides induce the phosphorylation of focal adhesion kinase (FAK) resulting in proliferation and invasion of cancer cells (Leyton et al., 2001). Since the native specific ligand of hBRS-3 is still unknown, the receptor is classified as orphan receptor and the pharmacology and physiology of the receptor remain speculative.
Here, we describe the isolation of two naturally derived ligands VV-H-7 and LVV-H-7 for the hBRS-3 from human placenta tissue. Originally, LVV-H-7 was isolated from pig hypothalami (Chang et al., 1980) characterized as nonclassical opioid peptides (Brantl et al., 1986; Piot et al., 1992) and binding to the AT4 receptor with affinity in the nanomolar range (Moeller et al., 1997; Garreau et al., 1998). In the present study, we characterized the structure–function relation between hemorphins and hBRS-3, and compare the biological activity with GRP and NMB. Additionally, we demonstrate the signal transduction mechanism of the interaction between hemorphin VV-H-7 and the hBRS-3 expressed in NCI-N417 lung cancer cells. We show that endogenous hBRS-3 receptor couples to phospholipase C resulting in changes of intracellular calcium which is initially released from an IP3-sensitive store followed by a capacitive calcium entry from extracellular space. We carried out a physiological characterization of the VV-H-7/hBRS-3 interaction regarding the involvement of kinases in proliferation and adhesion.
Materials
Cell lines and cell culture
Transfected CHO-Gα16 cells (Molecular Devices, Sunnyvale, CA, U.S.A.) were grown in nutrient mixture F12 (HAM) with 2 mM L-glutamine, 200 μg ml−1 hygromycin, and 400 μg ml−1 G418. NCI-N417 cells (ATCC, CRL-5809) were grown in RPMI-1640 with 2 mM L-glutamine, HT-29 (ATCC, HTB-38) in McCoy's 5a medium with 1.5 mM L-glutamine and BHY cells (DSMZ, ACC 404) in Dulbecco's MEM (4.5 g l−1 glucose) with 2 mM L-glutamine. All cells were cultivated with 100 units ml−1 penicillin/streptomycin and 5% FCS (BioWhittacker) at 37°C in a 5% CO2 atmosphere.
Molecular biological standard methods
RNA extraction, cDNA first-strand synthesis, polymerase chain reaction (PCR), reverse transcription/PCR (RT-PCR), and DNA sequencing were performed as described (Mägert et al., 1998). Expression analysis for hGRP receptor, hNMB receptor, and hBRS-3 receptor was performed by RT-PCR. The primer sets used for hBRS-3 were: 5′-primer: 5′-CAGAATCATCAAGCTCTGTG-3′; 3′-primer: 5′-AGTCTTTCAGGATGGCATTGG-3′; for hNMB-R: 5′-primer: 5′-CGGACTCTGCTGGAAAGGA-3′; 3′-primer: 5′-CCAGCAACACGGAGACCAC-3′; for hGRP-R: 5′-primer: 5′-CAAAGAGCCCGGCATAGA-3′; 3′-primer: 5′- AGCGCCGTGAGTGTGAAG-3′.
Preparation of hBRS-3 overexpressing Gα16 cell line
The coding region of human hBRS-3 cDNA (GenBank accession number: L08893) was subcloned into the expression vector pcDNA 3.1 and transfected into a CHO-Gα16 expressing cell line using Effectene (Qiagen, Hilden, Germany). Cells were selected by G418 and hygromycin, single-cell clones were propagated and tested for stable expression by Northern blotting using a digoxigenin kit (Roche, Mannheim, Germany).
Intracellular Ca2+-measurement
The Ca2+-measurements were performed using the FLIPR-system (Fluorometric Imaging Plate Reader, Molecular Devices, Sunnyvale, U.S.A.). CHO-transfected cells were seeded in 96-black well plates (Costar, U.K.) at 20,000 cells/well and cultured overnight. After 30 min at 37°C in loading medium (HEPES-buffered HBSS), pH 7.4, containing 2.5 mM probenecid and 1 μM Fluo-4 AM (Molecular Probes, Leiden, The Netherlands), cells were washed in loading medium without Fluo-4 AM. NCI-N417 cells were loaded with medium containing 2 μM Fluo-4 (30 min; 37°C), washed (centrifugation at 250 × g, in HBSS/HEPES), seeded in 96-black well plates (107 cells ml−1) and centrifuged at 125 × g for 2 min. The cells were placed in the FLIPR and basal fluorescence was determined prior to agonist addition at room temperature (λex=488 nm, λem=540 nm). Changes in cellular fluorescence were recorded online after the addition of 50 μl tissue extract fractions or test compounds diluted in wash buffer. Cell plates were preincubated with inhibitors of signal transduction and Ca2+ modulators for 20 min at 37°C.
Isolation of ligands for hBRS-3 from human placenta
An 11 kg human placenta was prepared as described previously (Seiler et al., 1999). Stepwise batch elution was performed using six different buffers (pool 1–6) with increasing pH from 3.3 to 13. Each of these six eluates was applied onto a Source RPC column (15–20 μM, 300 Å, 20 cm × 15.5 cm, Pharmacia, Freiburg, Germany) and separated with a gradient from 0–50% B (solvent A: water, 10 mM HCl; solvent B: 80% acetonitrile, 10 mM HCl) at a flow rate of 400 ml min−1. Fractions of 600 ml were collected and aliquots corresponding to 250 mg equivalent of placental tissue were tested in the bioassay. Fractions inducing a fluorescence signal on CHO-Gα16-hBRS-3 cells in the FLIPR-system were further separated. The corresponding fractions were applied to a preparative RP C18 column (PrepPak, 300 Å, 15–30 μM, Baker, Phillipsburg, NJ, U.S.A.), with a gradient from 20 to 70% B in 45 min, solvent A: 30% MeOH, 10 mM HCl; solvent B: MeOH 100%, 10 mM HCl) fractionated and tested in the bioassay. The bioactive material was further purified using the same column with different eluents (solvent A: water, 0.1% trifluoroacetic acid; solvent B: 80% acetonitrile, 0.1% trifluoroacetic acid; gradient from 20 to 50% B in 45 min). Subsequently, a semipreparative RP C4 (20 × 250 mM, 100 Å, 5 μM, Biotek, Heidelberg, Germany, solvent A: 0.1% trifluoroacetic acid; solvent B: 80% acetonitrile, 0.1% trifluoroacetic acid; gradient from 30 to 65% B in 50 min) was applied. The final purification step was performed with an analytical RP C18 column (4.6 × 250 mM, Aqua RP C18, Phenomenex, Aschaffenburg, Germany, solvent A: 0.1% trifluoroacetic acid; solvent B: 80% acetonitrile, 0.085% trifluoroacetic acid, isocratic at 32.5% B, flow rate: 0.7 ml min−1). The purified fraction was freeze-dried and analyzed.
Peptide analysis and synthesis
Purity was confirmed by capillary zone electrophoresis (CZE) (P/ACE 2000, Beckman, München, Germany) at 220 nM. Molecular weight determination was carried out on a Sciex API III quadrupol mass spectrometer (Perkin-Elmer, Überlingen, Germany). Sequencing was performed on a 473 A gas-phase sequencer (Applied Biosystems, Weiterstadt, Germany). VV-H-7, LVV-H-7, and V-hemorphin-7 (V-H-7), were prepared by Fmoc solid-phase peptide synthesis.
Cell proliferation
Stimulation of cell proliferation was determined using the WST-1 proliferation assay kit (Roche Molecular Biochemicals, Mannheim, Germany). The survival assay was performed as described earlier (Ryan et al., 1998b).
Immunoblot analysis
Cells (105 cells ml−1) were seeded in RPMI medium with 0.25% BSA for 48 h. Cells were stimulated with VV-H-7 (50 μM–500 nM), B9P (1 μM–100 nM) alone and in combination with the MEK-1 inhibitor PD 98059 using FCS (10%) as positive control and buffer as negative control. For detection of p125FAK, cells were incubated with VV-H-7 (50 μM) or B9P alone or in combination with cytochalasin D for 5–30 min. Cell lysates were prepared, separated by SDS–PAGE and blotted as described previously (Ryan et al., 1998a). Membranes were incubated with antisera against pMAPK-42/44 (1 : 2000 diluted) or p125FAK overnight at 4°C and signals were detected as described (Maronde et al., 1999).
Statistical analysis
Responses were measured as peak fluorescence intensity (FI) minus basal FI, and were presented as percentage of maximum VV-H-7 (10 μM) induced response. Plotting and statistical analysis of the data were performed with PRISM software 3.0 (GraphPad Software, San Diego CA, U.S.A.). Student's t-test was used to determine statistical significance.
Materials
Fluo-4 AM was obtained from Molecular Probes (Leiden, The Netherlands) and [DPhe6,β-Ala11,Phe13,Nle14]-bombesin (6-14) (B9P) was obtained from PolyPeptide Laboratories (Wolfenbüttel, Germany). [(D-Phe6,Leu(®)-p-chloro-Phel4]-bombesin(6-14), [D-Phe,Leu-NHEt13,des-Met14]-bombesin(6-14), [(D)Nal-Cys-Tyr-(D)Trp-Lys-Val-Cys-Nal-NH2], Neuromedin B (NMB), and Neuromedin C (GRP) were from Bachem (Heidelberg, Germany). Miconazole, thapsigargin, cyclopiazonic acid, and chelerythrine were obtained from Alomone Labs (Israel). Verapamil, diltiazem, U73122, xestospongin C (XeC), LY294002, Gö6850, and D-erythro-sphingosine were from Calbiochem-Novabiochem GmbH (Bad Soden, Germany). MEK-1 inhibitor PD 98059 was from NEB (Bad Nauheim, Germany) and cytochalasin D from Sigma (Deisenhofen, Germany). Antibodies against pMAPK-42/44 were obtained from New England BioLabs (Beverly, MA, U.S.A.) and p125FAK from BD Bioscience (Franklin Lakes, NJ, U.S.A.).
Results
Isolation of ligands for hBRS-3 from human placenta tissue extracts
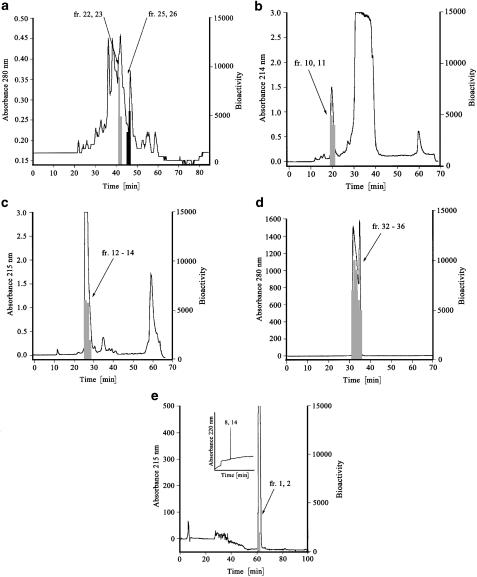
To identify endogenous ligands, hBRS-3 expressing CHO cells were generated and receptor activation was analyzed in the FLIPR assay. Based upon hBRS-3 expression in placenta, a placenta peptide library was prepared to screen for endogenous ligands. Testing 240 fractions of this library on CHO-Gα16-hBRS-3 cells, fractions 22/23 and fractions 25/26 from pH-pool 4 generated an increase in intracellular Ca2+ concentrations (Figure 1a). These fractions were not active with control cells (CHO cells expressing other orphan GPCRs). We isolated VV-hemorphin-7 and LVV-hemorphin-7 from human placenta in four chromatographic steps. Most inactive peptides were separated in the first isolation step using a preparative C18 reverse phase (RP) column, where the biological activity was detected in fractions 10/11 (Figure 1b). Second, by changing the mobile phase from methanol/HCl to acetonitrile/TFA we detected the specific biological activity in fractions 23/24 (Figure 1c). Finally, further purification steps using a semipreparative RP C4 column (Figure 1d) and an analytical RP C18 column resulted in two bioactive fractions (fractions 1/2) (Figure 1e). Fraction 1 was pure as shown by capillary zone electrophoresis (CZE; inset Figure 1e). Mass spectrometry combined with sequence analysis (LC/MS) revealed a molecular weight of 1194 Da with the amino-acid sequence VVYPWTQRF (VV-H-7), which was also confirmed by Edman sequencing. The corresponding purification strategy using fractions 25/26 of pH pool 4 as starting material resulted in the isolation of LVV-H-7. Finally, the biological activity of the identified hemorphin sequences as activators of the hBRS-3 receptor was confirmed by chemically synthesized peptides of VV-H-7 and LVV-H-7. We isolated pure VV-H-7 and LVV-H-7 from 11 kg placenta extract and calculated the enrichment factor as 7500 (Table 1).
Figure 1.
Purification of VV-H-7 from human placenta. Fractions inducing a Ca2+ signal in the bioassay are denoted with shading. (a) pH pool 4 was fractionated by a Source RPC column (20 cm × 15.5 cm) with a gradient from 0 to 50% B (solvent A: water, 10 mM HCl; solvent B: 80% acetonitrile, 10 mM HCl). (b) Preparative RP C18 column (4.7 cm × 30 cm) with a gradient from 20 to 70% B in 45 min (solvent A: 30% MeOH, 10 mM HCl; solvent B: MeOH 100%, 10 mM HCl). (c) RP-HPLC fractionation of the bioactive fractions using the identical column as in step 1, changing the mobile phase from MeOH to acetonitrile (solvent A: 0.1% TFA, solvent B: 80% acetonitrile, 0.1% TFA, gradient: from 20 to 5% B in 45 min). (d) A semipreparative RP C4 (20 × 250 mm2) (solvent A: 0.1% trifluoroacetic acid; solvent B: 80% acetonitrile, 0.1% trifluoroacetic acid; gradient from 30 to 65% B in 50 min). (e) Final purification step with an analytical RP C18 column (4.6 × 250 mm2) RP C18 (solvent A: 0.1% trifluoroacetic acid; solvent B: 80% acetonitrile, 0.085% trifluoroacetic acid, isocratic at 32.5% B, flow rate: 0.7 ml min−1). Inset in (e): CZE analysis of fraction 1 the last step.
Table 1.
Determination of the enrichment factor by calculation of the increasing specific activity (activity [fluorescence intensity units (FIU)] mg−1 peptide) after each isolation step
| Isolation step | Specific activity (FIU mg−1) | Enrichment factor |
|---|---|---|
| Starting material (placenta peptide bank) | ∼2 | — |
| Isolation step 1 | ∼20 | ∼10 |
| Isolation step 2 | ∼70 | ∼35 |
| Isolation step 3 | ∼200 | ∼100 |
| Isolation step 4 | ∼15000 | ∼7500 |
Expression of hBRS-3 on different cell lines
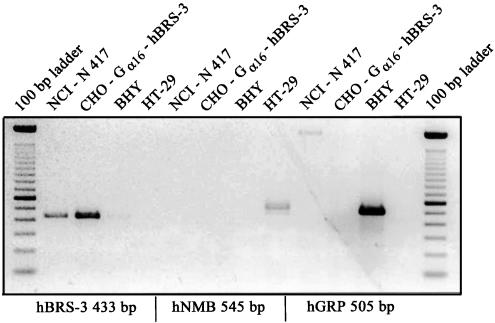
As bombesin peptides can bind with varying affinity to hGRP-R, hNMB-R, and hBRS-3 we examined the expression profile of these receptors on the human cell lines, NCI-N417, BHY, or HT-29 cells and on CHO-Gαl6-hBRS-3 by RT-PCR. As expected, the lung cancer cell line NCI-N417 and CHO-Gαl6-hBRS-3 were positive for hBRS-3, and negative for hGRP-R and hNMB-R. In contrast, hGRP-R or hNMB-R was only detected in BHY and HT-29 cells, respectively (Figure 2). The control cell lines BHY and HT-29 which expressed only the GRP- or NMB-receptor, respectively, but not the hBRS-3 receptor showed no Ca2+ increase in the FLIPR assay when treated with VV-H-7 or LVV-H-7 up to 10 μM (data not shown).
Figure 2.
Expression of bombesin receptor subtypes mRNA in NCI-N417, CHO-Gα16-hBRS-3, BHY, and HT-29 human tumor cell lines by RT-PCR.
Biological activity of VV-H-7 and LVV-H-7 on hBRS-3
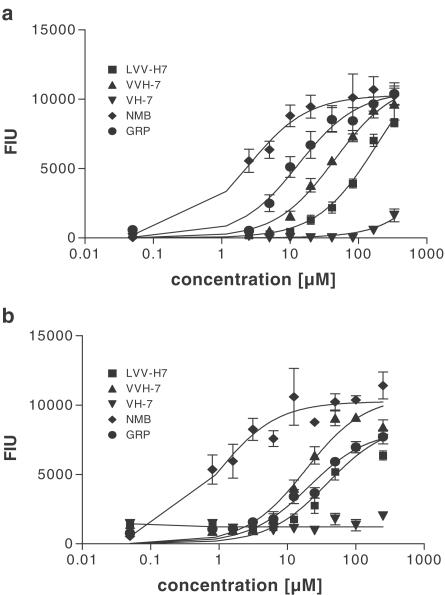
To analyze whether the dose-response curves of VV-H-7 and LVV-H-7 are similar to those of GRP and NMB, we performed FLIPR assays. All four peptides (Figure 3) exerted a Ca2+ release in a concentration-dependent manner in the CHO-Gα16-hBRS-3 cells with EC50 values of 45±15 μM for VV-H-7, 183±60 μM for LVV-H-7, 13±4.5 μM for GRP and 2±1 μM for NMB (Figure 3a), whereas V-H-7 had no detectable agonistic activity up to 100 μM (Figure 3a). Moreover, each of the active peptides stimulated Ca2+ release dose-dependently also in NCI-N417 cells with EC50 values of 19±6 μM for VV-H-7, 38±18 μM for LVV-H-7, 20±6 μM for GRP, and 0.9±0.5 μM for NMB (Figure 3b). As before, V-H-7 had no detectable agonistic activity up to 100 μM (Figure 3b). Comparison of the EC50 values in natively expressing versus hBRS-3 transfected cell lines indicated a two-fold lower value for VV-H-7 and a six-fold lower value for LVV-H-7 than in CHO-Gα16-hBRS-3 cells.
Figure 3.
Effect of VV-H-7, LVV-H-7, GRP, NMB, V-H-7 on intracellular Ca2+-changes in CHO-Gα16-hBRS-3 (a) and NCI-N417 (b) cells. [Ca2+]i-changes were monitored as fluorescence intensity units (FIU) using the FLIPR system. Values represent the maximal fluorescence change stimulated by the indicated peptides and are the means±s.e.mean from at least eight independent experiments.
Structure-function relation between hemorphins and hBRS-3
The N- and C-terminal amino acids responsible for ligand-receptor interaction were tested and compared to the activity of functionally and structurally related peptides (Table 2). We examined the intracellular Ca2+ changes ([Ca2+]i) with NCI-N417 cells and CHO-Gα16-hBRS-3 and found that only VV-H-7 and LVV-H-7 increased [Ca2+]i at 1–10 μM in both cell lines. The loss of the N-terminal valine to V-H-7 reduced the change of intracellular Ca2+ concentration significantly, while deletion of the C-terminal phenylalanine (VV-H-6: VVYPWTQR) led to a complete loss of biological activity. The amidated hemorphin showed neither activity on CHO-Gα16-hBRS-3 nor on NCI-N417 cells. Furthermore, neuropeptide FF and Met-enkephalin-RF showed no activity on hBRS-3 expressing cell lines.
Table 2.
Threshold concentration of agonist−evoked Ca2+ release in cells naturally expressing hBRS−3 (NCI−N417) or cells stably transfected with hBRS−3 (CHO−Gα16−hBRS−3)
| Peptides | Sequence | Threshold concentration (μM) NCI−N417 | Threshold concentration (μM) CHO−Gα16−hBRS−3 |
|---|---|---|---|
| VV−H−7 | VVYPWTQRF | 1 μM | 3 μM |
| LVV−H−7 | LVVYPWTQRF | 1 μM | 10 μM |
| V−H−7 | VYPWTQRF | 10 μM | 300 μM |
| VV−H−7 amide | VVYPWTQRF−NH2 | >300 μM* | >300 μM* |
| VV−H−6 | VVYPWTQR | >300 μM* | >300 μM* |
| VV−H−5 | VVYPWTQ | >300 μM* | >300 μM* |
| Ang II | DRVYIHPF | >300 μM* | >300 μM* |
| Ang III | RVYIHPF | >300 μM* | >300 μM* |
| Ang IV | VYIHPF | >300 μM* | >300 μM* |
| Neuropeptid FF | FLFQPQRF−NH2 | >300 μM* | >300 μM* |
| Met−Enkephalin−RF | YGGFMRF | >300 μM* | >300 μM* |
Values represent the means from at least eight independent experiments. The concentrations inducing a Ca2+ response are indicated.
The highest concentration used in the bioassay (300 μM) which induced no specific Ca2+ signal.
Beside the well-established function of hemorphins as opioid peptides (Piot et al., 1992), Garreau et al. (1998) and Moeller et al. (1997) described the inhibition of Ang-IV binding to a putative Ang IV receptor by LVV-H-7 and VV-H-7. To test the possible connection between the hBRS-3 receptor, Ang IV binding sites and LVV-H-7/VV-H-7, FLIPR experiments were performed. Angiotensin peptides (Ang II, Ang III, and Ang IV) did not induce [Ca2+]i when applied in concentrations up to 300 μM (Table 2). Moreover, Ang IV showed no inhibitory effect on VV-H-7 binding to hBRS-3 (data not shown) indicating that the putative Ang IV receptor is not identical to hBRS-3.
The synthetic high-affinity ligand [DPhe6,βAla11,Phe13,Nle14]-bombesin(6-14) (BP9) for hBRS-3 subtype (Mantey et al., 1997) induced a concentration-dependent release of Ca2+ (EC50: 20 nM) on NCI-N417 cells. A possible additive effect of VV-H-7 and BP9 on the stimulation of hBRS-3 was analyzed by FLIPR experiments. Both peptides were applied in concentrations according to their EC50 values of 20 nM for BP9 and 25 μM for VV-H-7. The combination of VV-H-7 and the synthetic ligand induced no significant further increase in Ca2+-release indicating no additivity. Furthermore, since the maximum Ca2+ signal induced by hemorphins alone is comparable to that by the synthetic ligand, VV-H-7/LVV-H-7 are full agonists for hBRS-3.
Functional coupling of the hBRS-3
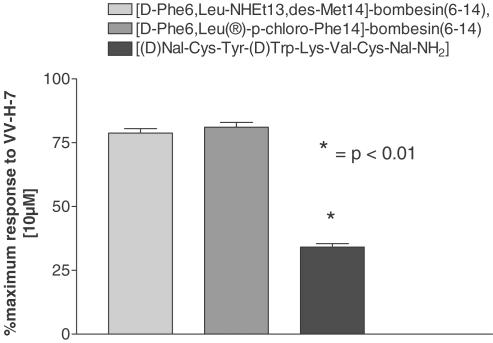
The functional coupling of the hBRS-3 was analyzed with specific hBRS-3 antagonists in hBRS-3 overexpressing CHO-cells and a hBRS-3 naturally expressing cell line (NCI-N417). The GRP-R antagonists [(D-Phe6,Leu®-p-chloro-Phe14]-bombesin(6-14) and [D-Phe,Leu-NHEt13,des-Met14]-bombesin(6-14) did not inhibit Ca2+ elevation induced by VV-H-7. In contrast, the somatostatin analog [(D)Nal-Cys-Tyr-(D)Trp-Lys-Val-Cys-Nal-NH2], an NMB-R-specific antagonist (Orbuch et al., 1993) with high affinity to hBRS-3 (Ryan et al., 1998a), inhibited the VV-H-7-induced response significantly (Figure 4). To exclude that the isolated hemorphins bind to opioid binding sites described to be present on lung carcinoma cell lines (Maneckjee & Minna, 1990) we applied naloxone, an antagonist of opioid receptors, and VV-H-7 on NCI-N417 cells, without any effect on increasing intracellular Ca2+ [Ca2+]i compared to VV-H-7 alone, indicating that VV-H-7 did not bind to opioid binding sites (data not shown).
Figure 4.
Inhibition of the transient Ca2+ increase by hBRS-3 antagonists. The indicated antagonist (1 μM) was applied for 20 min prior to addition of VV-H-7 (10 μM). Responses were measured as peak increase in fluorescence minus basal, expressed relative to the maximum VV-H-7 (10 μM) response. Values represent the means±s.e.mean from eight independent experiments using the FLIPR system.
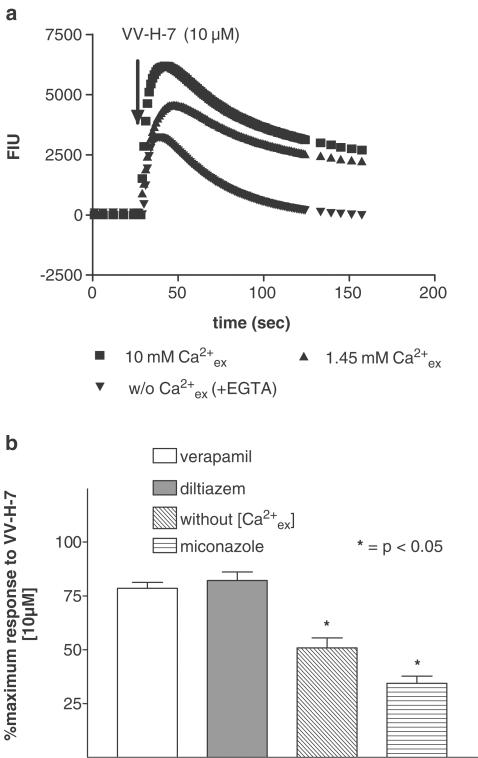
To determine the dependence of ([Ca2+]i) transients on extracellular Ca2+ ([Ca2+]ex), cells were stimulated with (i) VV-H-7 in the presence of the Ca2+-chelating agent EGTA, (ii) at physiological Ca2+ concentration (1.45 mM), and (iii) high Ca2+ HBSS-buffer (10 mM). In the absence of [Ca2+]ex, the magnitude of the response was reduced by 25% and the return to basal levels was faster than in Ca2+-containing buffer (Figure 5a). High [Ca2+]ex resulted in an increase of [Ca2+]i and a delay in returning to the basal level. Furthermore, the relation between extracellular and intracellular Ca2+ concentration was tested with the L-type Ca2+ channel inhibitors verapamil and diltiazem, and miconazole as an inhibitor of the ‘Ca2+-release-activated Ca2+-channels' (CRAC). We show that miconazole reduced the maximal Ca2+ increase similar to conditions without extracellular Ca2+ (Figure 5a), whereas verapamil and diltiazem did not reduce [Ca2+]i.
Figure 5.
Influence of extracellular Ca2+ on VV-H-7-induced Ca2+ release in NCI-N417 cells. (a) Cells stimulated with 10 μM VV-H-7 under high or physiological Ca2+ concentration and in the presence of 5 mM EGTA without Ca2+. [Ca2+]i (as fluorescence intensity units) was monitored using Fluo-4 AM. One typical experiment out of 10 replicates is shown. (b) Effect of Ca2+ inhibitors: Verapamil (10 μM), Diltiazem (10 μM); Miconazole (10 μM), were added for 20 min prior to application of VV-H-7 (10 μM), application of VV-H-7 (10 μM) in the presence of 5 mM EGTA without extracellular Ca2+. Responses were measured as peak increase in fluorescence minus basal, expressed relative to the maximum VV-H-7 (10 μM) response under physiological Ca2+ concentration. Values represent the means±s.e.mean from at least eight independent experiments.
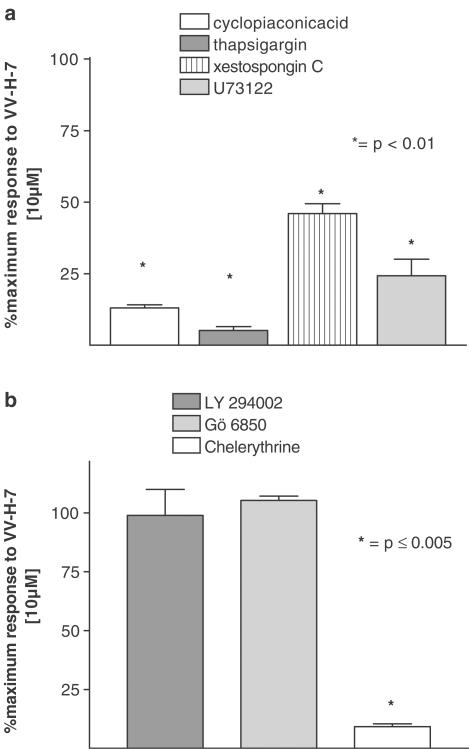
The signal transduction was further examined using thapsigargin (3 μM) and cyclopiazonic acid (10 μM), both sarcoplasmatic Ca2+/ATPase inhibitors. Both substances abolished the VV-H-7-induced response. Additionally, the VV-H-7-evoked response was inhibited by either pretreatment with the phospholipase C inhibitor, U73122 (Smith et al., 1990), or the antagonist of the IP3-receptor inhibitor, xestospongin C (XeC) (Figure 6a).
Figure 6.
Inhibition of transient Ca2+ increase by different inhibitors of signal transducing elements. Values represent maximal fluorescence change stimulated by VV-H-7 (10 μM), expressed relative to the maximum VV-H-7 (10 μM), response and are the means±s.e.mean from at least eight independent experiments. (a) Specific inhibitors of the Ca2+/ATPase: cyclopiazonic acid (10 μM) and thapsigargin (1 μM), the IP3-receptor-antagonist xestospongin C (20 μM) and the PLC-inhibitor U 73122 (3 μM) were incubated for 20 min prior to VV-H-7 application. (b) Phosphoinositid-3-kinase inhibitor LY 294002 (3 μM) and inhibitor of the PKC: Gö 6850 (3 μM) and Chelerythrine (3 μM) were incubated for 20 min prior to VV-H-7 application.
Neither LY 294002, a phosphoinositid-3-kinase inhibitor (Smith et al., 1990) nor Gö6850 or D-erythro-sphingosine, both PKC inhibitors (Hannun et al., 1986; Vlahos et al., 1994), reduced the VV-H-7- mediated increase of [Ca2+]i. Interestingly, only chelerythrine, another PKC inhibitor (Herbert et al., 1990), prevented change of [Ca2+]i (Figure 6b). Thus, the mobilized calcium after activation of hBRS-3 with VV-H-7 is recruited from intracellular stores via a PLC and PKC-dependent signal transduction pathway and from the extracellular space via CRAC.
Phosphorylation of cytoplasmatic kinases
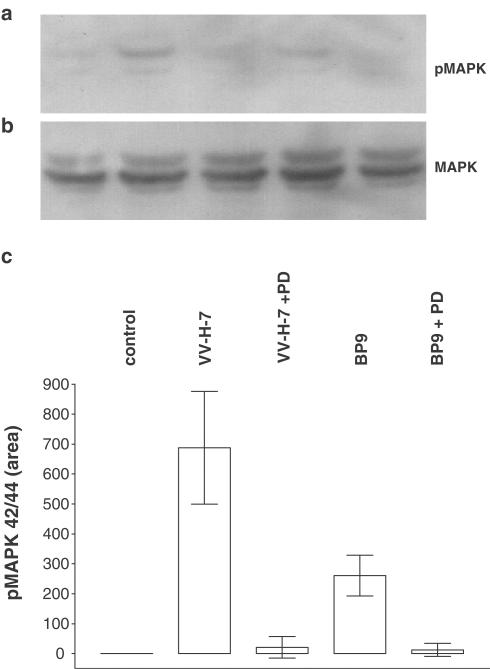
Since bombesin-like peptides and their receptors display a wide tissue distribution and are potential mitogens for gastrointestinal and lung cancer cells, we examined proliferation of NCI-N417 cells. The number of 48 h serum-deprived NCI-N417 cells was not changed after stimulation with VV-H-7 or B9P up to 10 μM. As a widely used biochemical correlate of proliferation induction, we also investigated phosphorylation of pMAPK 42/44 induced by VV-H-7. In contrast, to the results on proliferation mentioned above, the tyrosine/threonine phosphorylation of pMAPK42/44 with VV-H-7 or B9P compared to unstimulated cells was seven- and 2.5-fold increased, respectively. This increase in phosphorylation was abolished by prior application of the pMAPK42/44 inhibitor PD98059 (Figure 7). We conclude that VV-H-7 induces MAPK42/44 activation, but does not result in elevated proliferation.
Figure 7.
MAPK42/44 phosphorylation. NCI-N417 cells were grown in DMEM/0.25% BSA. Cells were stimulated for 45 min with the indicated substances. A measure of 10 μg of protein per lane was subjected to SDS–PAGE, and subsequently transferred to PVDF membranes. (a) Active MAPK42/44 was visualized by probing with a specific antiserum against phosphoMAPK42/44. (b) Specific antisera against total MAPK42/44 were used as control. (c) Densitometric analysis of phosphoMAPK42/44 immunoblots. The cells were treated as described above. Membranes were subjected to densitometry (Quantiscan, BioSoft, Cambridge, U.K.) and presented as area values. Experiments were performed in triplicate.
Recent studies suggested that several neuropeptides and bombesin-like peptides can stimulate tyrosine phosphorylation of, for example, cytosolic focal adhesion kinase (p125FAK), which is involved in tumor development (Ryan et al., 1998a). Since phosphorylation of p125FAK depends on cell type or ligands and occurs after various times, we tested time- and dose-dependency of p125FAK phosphorylation. Neither increasing the incubation time nor increasing VV-H-7 concentration resulted in elevated phosphorylation of p125FAK (data not shown).
Discussion
In the present study, we have identified and characterized VV-H-7 and LVV-H-7 as low-affinity ligands for hBRS-3 receptor. The isolation strategy used was based on the increase of intracellular Ca2+-concentration after application of potential ligands, collected in a human placenta peptide library. In addition, we have characterized the pharmacology and signal transduction of ligand-receptor interaction by FLIPR-assays and by analyses of intracellular signaling kinases in cells with recombinant and native hBRS-3 expression.
The screening for ligands that specifically activate hBRS-3 was performed using a CHO cell line transfected with the receptor. Exclusively hBRS-3 expressing cell lines showed an increased Ca2+ influx after application of the indicated biologically active fractions and the isolated ligands VV-H-7 and LVV-H-7. The specific binding of hemorphins to hBRS-3 was further demonstrated by analyzing the hemorphin activity on cell lines expressing single members of the bombesin receptor family. As shown by RT-PCR, overlapping effects caused by the coexpression of more than one bombesin-like receptor could be excluded. This demonstrated a unique specificity of the isolated ligands for the hBRS-3 receptor compared to other bombesin receptors.
Specificity of receptor-ligand interaction was further examined by dose-response analyses with bombesin like peptides and hemorphins in hBRS-3 natively expressing and in overexpressing cell lines. As described GRP and NMB were shown to be low-affinity ligands for hBRS-3 and bind with EC50 values in a similar range as VV-H-7 and LVV-H-7 (Mantey et al., 1997). Notably, the EC50 values for VV-H-7 and LVV-H-7 were two to six-fold lower in NCI-N417 cells which express hBRS-3 natively compared to recombinant expression, indicating a more effective coupling and activation of the receptor in these cells.
The idea of hemorphins functioning as opioid peptides was supported by several studies (Brantl et al., 1986; Piot et al., 1992). Sanderson et al. (1998) postulated a role of hemorphins in inhibiting the inflammatory response in acute and chronic inflammation, an effect thought to be mediated by an activation of opioid receptors after VV-H-7 binding. To examine whether our isolated hemorphins bind to opioid binding sites described to be present on lung carcinoma cell lines (Maneckjee & Minna, 1990) inhibitory experiments with naloxone, an antagonist for opioid receptors, were performed. Naloxone showed no effect on [Ca2+]i induced by VV-H-7, while the VV-H-7 response was abolished by prior application of a specific antagonist for hBRS-3. This implies that VV-H-7 binds to hBRS-3, that this receptor does not contain opioid binding sites and that binding to endogenous opioid receptors is negligible.
The hBRS-3 receptor was investigated as a putative AT4 receptor because binding of LVV-H-7 and VV-H-7 has been reported to an Ang IV binding site in nanomolar range (Moeller et al., 1997; Garreau et al., 1998) with so far unknown molecular identity. None of the tested angiotensin peptides showed agonist activity nor inhibition of VV-H-7 binding to hBRS-3, indicating that the hBRS-3 receptor is not the putative Ang IV binding site. Recently, the identification of the putative AT4 receptor was reported as insulin-regulated aminopeptidase (IRAP). It was proposed that the AT4 receptor ligands, Ang IV and LVV-H-7, may exert their effects by inhibiting the catalytic activity of IRAP and thereby extending the half-life of its neuropeptide substrates (Albiston et al., 2001).
Comparison of the sequence between the isolated hemorphins ((L)-VVYPWTQRF) and other peptides indicates similarity to Met-enkephalin-RF (YGGFMRF) and RFamide peptides like neuropeptide FF (FLFQPQRF-NH2). Szikra et al. (2001) tested the binding characteristics of VV-H-7 on opioid receptors and showed a binding profile similar to Met-enkephalin-RF. In contrast, Met-enkephalin-RF showed no effect in our assay system. To investigate a possible increase in efficacy by amidation, we tested amidated VV-H-7 and other amidated RF-peptides like neuropeptide FF. None of these peptides showed activity and notably the amidation of the C-terminal phenylalanine led to a complete loss of biological activity. Since N-terminal and C-terminal truncated VV-H-7 lost their biological activity, we conclude that both the N-terminal valine and the C-terminal phenylalanine residues are critical for the ligand-receptor interaction. The reduction of the biological activity by an additional leucine at the N-terminus (LVV-H-7) may be explained by steric hindrance.
The activation of phospholipase C (PLC)-mediated signaling pathways in nonexcitable cells causes the release of Ca2+ from intracellular stores and activation of Ca2+ influx across the plasma membrane by means of capacitative Ca2+ entry or store-operated Ca2+ entry processes. The inhibition of the VV-H-7-induced response by sarcoplasmatic Ca2+/ATPase inhibitors suggests that signaling via endogenous hBRS-3 involves the mobilization of Ca2+ from intracellular stores. Additionally, the VV-H-7-evoked responses were inhibited by either pretreatment with a phospholipase C inhibitor or an antagonist of the IP3-receptor.
Capacitative Ca2+ entry is mediated by plasma membrane Ca2+ channels termed intracellular Ca2+-release activated channels (ICRAC) (Berridge, 1995; Parekh & Penner, 1997), members of the TRP-protein family (Kanki et al., 2001). We show that an inhibitor of CRAC reduced the maximal Ca2+ increase similar to conditions without extracellular Ca2+, whereas L-type Ca2+ channel inhibitors did not reduce [Ca2+]i. These results suggest the involvement of CRAC channels in the VV-H-7-induced Ca2+ release in NCI-N417 cells and imply dependence from extracellular calcium concentration, which is in contrast to data presented by Ryan et al. (1998b). Consistent with previous studies using hBRS-3-transfected cells (Fathi et al., 1993; Wu et al., 1996), the endogenously expressed hBRS-3 couples to PLC, resulting in intracellular calcium changes. Our data and those from others using B9P-stimulated hBRS-3-transfected cells (Ryan et al., 1998a) suggest that the initial release of [Ca2+]i is from an IP3-sensitive calcium pool with a subsequent capacitive calcium entry, a mechanism previously described for hGRP and hNMB receptors (Ryan et al., 1993).
Another part of this work was the characterization of the VV-H-7/hBRS-3 interaction regarding the involvement of kinases in proliferation and adhesion. Bombesin-like peptides were postulated as mitogens for bronchial epithelial cells and SCLC (Sunday et al., 1998). In our experimental setup we could not detect any effect of VV-H-7 on NCI-N417 cell proliferation. In contrast, when treated with VV-H-7 or B9P, an increase in tyrosine/threonine phosphorylation of pMAPK42/44 compared to unstimulated cells was observed. This increase in phosphorylation was inhibited by prior application of a MEK-1 (MAP kinase kinase 1) inhibitor. This is in sound with the observation that phosphorylation of MAPK42/44 is also elevated after B9P-stimulation in hBRS-3 transfected cells without any effect on proliferation (Weber et al., 2001). However, phosphorylation of structural proteins by MAPK can induce changes in cellular morphology (Erickson et al., 1990; Ray & Sturgill, 1987) or GPCR desensitization may lead to phosphorylation of MAPK (Luttrell et al., 2001). Stimulation of hBRS-3 by VV-H-7 and subsequent phosphorylation of MAPK42/44 may therefore induce secretion of mitogenic or angiogenic factors or proteases supporting the growth of the lung tumor, as postulated by Hellmich et al. (1999) for GRP and GRP-receptor. FAK, a nonreceptor protein tyrosine kinase with downstream influences on cell cycle regulation, cytoskeletal dynamics, and cell attachment, is activated by integrin binding and aggregation. Numerous studies implicated a participation of p125FAK in cancer invasiveness and motility of cells (Kohno et al., 2002). In contrast to recent studies that several neuropeptides can stimulate tyrosine phosphorylation of cytosolic focal adhesion kinase (p125FAK), we cannot show an activation of p125FAK in nonadherent NCI N417 cells. Rodriguez-Fernandez & Rozengurt (1996) showed that phosphorylation of p125FAK caused by treatment with bombesin is abolished in Swiss 3T3 which have been placed in suspension. This indicates that adherence of cells might be a prerequisite for p125FAK phosphorylation. Our newly discovered ligands VV-H-7/LVV-H-7 are naturally derived low-affinity ligands for the hBRS-3 receptor. We cannot exclude that other high-affinity ligands exist which we did not detect within our peptide banks or assay systems. Importantly, it has to be noted that GRP and NMB the two other described endogenous low-affinity ligands are present only in low concentrations (pM) in plasma and tissue extracts (Haraguchi et al., 1988; Namba et al., 1985), and therefore may not play any physiological role in signaling via the hBRS-3 receptor. In contrast, LVV-H-7 was found in high concentrations (1.5 μM) in bronchoalveolar lavage fluid of one patient with non small cell lung cancer (Duethman et al., 2000). The minimum concentration of VV-H-7 evoking an increase of [Ca2+]i in the FLIPR-assay is about 1 μM. In consideration of the described processing of LVV-H-7 to VV-H-7 (Fruitier et al., 1998), we suggest that a sufficient ligand concentration to activate hBRS-3 could be present in vivo under certain pathological conditions.
In conclusion, we isolated two low-affinity ligands specifically binding to the orphan receptor hBRS-3 and show that stimulation of hBRS-3 with VV-H-7 and LVV-H-7 leads to an elevation of [Ca2+]i from IP3-sensitive stores, a subsequent influx of [Ca2+]ex via CRAC, an induction of PLC and PKC activity and phosphorylation of MAPK42/44. A pathophysiological role of hemorphin/hBRS-3 in vivo is possible but has to be confirmed in future studies.
Acknowledgments
This work was supported by the BMBF (FKZ: 0311797). We thank U. Block, C. Fiedler, A. Gasperina, R. Kopittke, K. Listemann, I. Uhrlandt for excellent technical assistance.
Abbreviations
- B9P
[DPhe6,β-Ala11,Phel3,Nle14]-bombesin (6-14)
- CHO-Gα16-hBRS-3
chinese hamster ovary cells transfected with Gα16 and hBRS-3
- FAK
focal adhesion kinase
- FIU
fluorescence intensity units
- FLIPR
fluorimetric imaging plate reader
- GPCR
G-protein coupled receptor
- GRP
gastrin-releasing peptide (neuromedin C)
- hBRS-3
human bombesin receptor subtype 3
- IP3
inositol(1,4,5)trisphosphate
- IRAP
insulin-regulated aminopeptidase
- LVV-H-7
LVV-hemorphin-7
- MAPK
mitogen-activated protein kinase
- NMB
neuromedin B
- PD98059
2′-amino-3′-methyoxyflavone (mitogen activated protein kinase kinase (MEK-1) inhibitor)
- SCLC
human small cell lung carcinoma
- VV-H-7
VV-hemorphin-7
References
- ALBISTON A.L., MC DOWALL S.G, MATSACOS D., SIM P., CLUNE E., MUSTAFA T., LEE J., MENDELSOHN F.A., SIMPSON R.J., CONNOLLY L.M., CHAI S.Y. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated amino-peptidase. J. Biol. Chem. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- BRANTL V., GRAMSCH C., LOTTSPEICH F., MERTZ R., JAEGER K.H., HERZ A. Novel opioid peptides derived from hemoglobin: hemorphins. Eur. J. Pharmacol. 1986;125:309–310. doi: 10.1016/0014-2999(86)90044-0. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R.C., HUANG W.Y., REDDING T.W., ARIMURA A., COY D.H., SCHALLY A.V. Isolation and structure of several peptides from porcine hypothalami. Biochim. Biophys. Acta. 1980;625:266–273. doi: 10.1016/0005-2795(80)90290-1. [DOI] [PubMed] [Google Scholar]
- CUTTITTA F., CARNEY D.N., MULSHINE J., MOODY T.W., FEDORKO J., FISCHLER A., MINNA J.D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- DUETHMAN D., DEWAN N., CONLON J.M. Isolation of the opioid peptide Leu-Val-Val-hemorphin-7 from bronchoalveolar lavage fluid of a patient with non-small cell lung cancer. Peptides. 2000;21:137–142. doi: 10.1016/s0196-9781(99)00186-2. [DOI] [PubMed] [Google Scholar]
- ERICKSON A.K., PAYNE D.M., MARTINO P.A., ROSSOMANDO A.J., SHABANOWITZ J., WEBER M.J., HUNT D.F., STURGILL T.W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J. Biol. Chem. 1990;265:19728–19735. [PubMed] [Google Scholar]
- FATHI Z., CORJAY M.H., SHAPIRA H., WADA E., BENYA R., JENSEN R., VIALLET J., SAUSVILLE E.A., BATTEY J.F. BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J. Biol. Chem. 1993;268:5979–5984. [PubMed] [Google Scholar]
- FLEISCHMANN A., LADERACH U., FRIESS H., BUECHLER M.W., REUBI J.C. Bombesin receptors in distinct tissue compartments of human pancreatic diseases. Lab. Invest. 2000;80:1807–1817. doi: 10.1038/labinvest.3780192. [DOI] [PubMed] [Google Scholar]
- FRUITIER I., GARREAU I., PIOT J.M. Cathepsin D is a good candidate for the specific release of a stable hemorphin from hemoglobin in vivo: VV-hemorphin-7. Biochem. Biophys. Res. Commun. 1998;246:719–724. doi: 10.1006/bbrc.1998.8614. [DOI] [PubMed] [Google Scholar]
- GARREAU I., CHANSEL D., VANDERMEERSCH S., FRUITIER I., PIOT J.M., ARDAILLOU R. Hemorphins inhibit angiotensin IV binding and interact with aminopeptidase N. Peptides. 1998;19:1339–1348. doi: 10.1016/s0196-9781(98)00075-8. [DOI] [PubMed] [Google Scholar]
- GORBULEV V., AKHUNDOVA A., BUCHNER H., FAHRENHOLZ F. Molecular cloning of a new bombesin receptor subtype expressed in uterus during pregnancy. Eur. J. Biochem. 1992;208:405–410. doi: 10.1111/j.1432-1033.1992.tb17201.x. [DOI] [PubMed] [Google Scholar]
- GORBULEV V., AKHUNDOVA A., GRZESCHIK K.H., FAHRENHOLZ F. Organization and chromosomal localization of the gene for the human bombesin receptor subtype expressed in pregnant uterus. FEBS Lett. 1994;340:260–264. doi: 10.1016/0014-5793(94)80150-9. [DOI] [PubMed] [Google Scholar]
- HANNUN Y.A., LOOMIS C.R., MERRILL A.H., JR, BELL R.M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J. Biol. Chem. 1986;261:12604–12609. [PubMed] [Google Scholar]
- HARAGUCHI Y., SAKAMOTO A., YOSHIDA T., TANAKA K. Plasma GRP-like immunoreactivity in healthy and diseased subjects. Gastroenterol. Japan. 1988;23:247–250. doi: 10.1007/BF02779466. [DOI] [PubMed] [Google Scholar]
- HELLMICH M.R., IVES K.L., UDUPI V., SOLOFF M.S., GREELEY G.H., CHRISTENSEN B.N., TOWNSEND C.M. Multiple protein kinase pathways are involved in gastrin-releasing peptide receptor-regulated secretion. J. Biol. Chem. 1999;274:23901–23909. doi: 10.1074/jbc.274.34.23901. [DOI] [PubMed] [Google Scholar]
- HERBERT J.M., AUGEREAU J.M., GLEYE J., MAFFRAND J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- JOHNSON D.E., LOCK J.E., ELDE R.P., THOMPSON T.R. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatr. Res. 1982;16:446–454. doi: 10.1203/00006450-198206000-00009. [DOI] [PubMed] [Google Scholar]
- KANKI H., KINOSHITA M., AKAIKE A., SATOH M., MORI Y., KANEKO S. Activation of inositol 1,4,5-trisphosphate receptor is essential for the opening of mouse TRP5 channels. Mol. Pharmacol. 2001;60:989–998. doi: 10.1124/mol.60.5.989. [DOI] [PubMed] [Google Scholar]
- KOHNO M., HASEGAWA H., MIYAKE M., YAMAMOTO T., FUJITA S. D151 enhances cell motility and metastasis of cancer cells in the presence of focal adhesion kinase. Int. J. Cancer. 2002;97:336–343. doi: 10.1002/ijc.1605. [DOI] [PubMed] [Google Scholar]
- LEHY T., ACCARY J.P., LABEILLE D., DUBRASQUET M. Chronic administration of bombesin stimulates antral gastrin cell proliferation in the rat. Gastroenterology. 1983;84:914–919. [PubMed] [Google Scholar]
- LEYTON J., GARCIA-MARIN L.J., TAPIA J.A., JENSEN R.T., MOODY T.W. Bombesin and gastrin releasing peptide increase tyrosine phosphorylation of focal adhesion kinase and paxillin in non-small cell lung cancer cells. Cancer Lett. 2001;162:87–95. doi: 10.1016/s0304-3835(00)00639-x. [DOI] [PubMed] [Google Scholar]
- LUTTRELL L.M., ROUDABUSH F.L., CHOY E.W., MILLER W.E., FIELD M.E., PIERCE K.L., LEFKOWITZ R.J. Activation and targeting of extracellular signal-regulated kinases by beta -arrestin scaffolds. Proc. Natl. Acad. Sci. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTEY S.A., WEBER H.C., SAINZ E., AKESON M., RYAN R.R., PRADHAN T.K., SEARLES R.P., SPINDEL E.R., BATTEY J.F., COY D.H., JENSEN RT. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates that it has a unique pharmacology compared with other mammalian bombesin receptors. J. Biol. Chem. 1997;272:26062–26071. doi: 10.1074/jbc.272.41.26062. [DOI] [PubMed] [Google Scholar]
- MÄGERT H.J., REINECKE M., DAVID I., RAAB H.R., ADERMANN K., ZUCHT H.D., HILL O., HESS R., FORSSMANN W.G. Uroguanylin: gene structure, expression, processing as a peptide hormone, and co-storage with somatostatin in gastrointestinal D-cells. Regul. Pept. 1998;7:165–176. doi: 10.1016/s0167-0115(97)01078-1. [DOI] [PubMed] [Google Scholar]
- MANECKJEE R., MINNA J.D. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc. Natl. Acad. Sci. 1990;87:3294–3298. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARONDE E., WICHT H., TASKEN K., GENIESER H.G., DEHGHANI F., OLCESE J., KORF H.W. CREB phosphorylation and melatonin biosynthesis in the rat pineal gland: involvement of cyclic AMP dependent protein kinase type II. J. Pineal. Res. 1999;27:170–182. doi: 10.1111/j.1600-079x.1999.tb00613.x. [DOI] [PubMed] [Google Scholar]
- MINAMINO N., KANGAWA K., MATSUO H. Neuromedin B: a novel bombesin-like peptide identified in porcine spinal cord. Biochem. Biophys. Res. Commun. 1983;114:541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- MOELLER I., LEW R.A., MENDELSOHN F.A., SMITH A.I., BRENNAN M.E., TETAZ T.J., CHAI S.Y. The globin fragment LVV-H-7 is an endogenous ligand for the AT4 receptor in the brain. J Neurochem. 1997;68:2530–2537. doi: 10.1046/j.1471-4159.1997.68062530.x. [DOI] [PubMed] [Google Scholar]
- NAMBA M., GHATEI M.A., GIBSON S.J., POLAK J.M., BLOOM S.R. Distribution and localization of neuromedin B-like immunoreactivity in pig, cat and rat spinal cord. Neuroscience. 1985;15:1217–1226. doi: 10.1016/0306-4522(85)90264-7. [DOI] [PubMed] [Google Scholar]
- NELSON J., DONNELLY M., WALKER B., GRAY J., SHAW C., MURPHY R.F. Bombesin stimulates proliferation of human breast cancer cells in culture. Br. J. Cancer. 1991;63:933–936. doi: 10.1038/bjc.1991.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHKI-HAMAZAKI H., WADA E., MATSUI K., WADA K. Cloning and expression of the neuromedin B receptor and the third subtype of bombesin receptor genes in the mouse. Brain Res. 1997a;762:165–172. doi: 10.1016/s0006-8993(97)00380-6. [DOI] [PubMed] [Google Scholar]
- OHKI-HAMAZAKI H., WATASE K., YAMAMOTO K., OGURA H., YAMANO M., YAMADA K., MAENO H., IMAKI J., KIKUYAMA S., WADA E., WADA K. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature. 1997b;390:165–169. doi: 10.1038/36568. [DOI] [PubMed] [Google Scholar]
- ORBUCH M., TAYLOR J.E., COY D.H., MROZINSKI J.E., JR, MANTEY S.A., BATTEY J.F., MOREAU J.P., JENSEN R.T. Discovery of a novel class of neuromedin B receptor antagonists, substituted somatostatin analogues. Mol. Pharmacol. 1993;44:841–850. [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PIOT J.M., ZHAO Q., GUILLOCHON D., RICART G., THOMAS D. Isolation and characterization of two opioid peptides from a bovine hemoglobin peptic hydrolysate. Biochem. Biophys. Res. Commun. 1992;189:101–110. doi: 10.1016/0006-291x(92)91531-t. [DOI] [PubMed] [Google Scholar]
- RAY L.B., STURGILL T.W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc. Natl. Acad. Sci. 1987;84:1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-FERNANDEZ J.L., ROZENGURT E. Bombesin, bradykinin, vasopressin, and phorbol esters rapidly and transiently activate Src family tyrosine kinases in Swiss 3T3 cells. Dissociation from tyrosine phosphorylation of p125 focal adhesion kinase. J. Biol. Chem. 1996;271:27895–278901. doi: 10.1074/jbc.271.44.27895. [DOI] [PubMed] [Google Scholar]
- RYAN R.R., DANIEL J.L., COWAN A. Two bombesin analogues discriminate between neuromedin B- and bombesin-induced calcium flux in a lung cancer cell line. Peptides. 1993;14:1231–1235. doi: 10.1016/0196-9781(93)90181-f. [DOI] [PubMed] [Google Scholar]
- RYAN R.R., WEBER H.C., HOU W., SAINZ E., MANTEY S.A., BATTEY J.F., COY D.H., JENSEN R.T. Ability of various bombesin receptor agonists and antagonists to alter intracellular signaling of the human orphan receptor BRS-3. J. Biol. Chem. 1998a;273:13613–13624. doi: 10.1074/jbc.273.22.13613. [DOI] [PubMed] [Google Scholar]
- RYAN R.R., WEBER H.C., MANTEY S.A., HOU W., HILBURGER M.E., PRADHAN T.K., COY D.H., JENSEN R.T. Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J. Pharmacol. Exp. Ther. 1998b;287:366–380. [PubMed] [Google Scholar]
- SANDERSON K., NYBERG F., KHALIL Z. Modulation of peripheral inflammation by locally administered hemorphin-7. Inflamm. Res. 1998;47:49–55. doi: 10.1007/s000110050266. [DOI] [PubMed] [Google Scholar]
- SEILER P., STÄNDKER L., MARK S., HAHN W., FORSSMANN W.G., MEYER M. Application of a peptide bank from porcine brain in isolation of regulatory peptides. J. Chromatogr. A. 1999;852:273–283. doi: 10.1016/s0021-9673(99)00466-5. [DOI] [PubMed] [Google Scholar]
- SMITH R.J., SAM L.M., JUSTEN J.M., BUNDY G.L., BALA G.A., BLEASDALE J.E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- SUNDAY M.E., KAPLAN L.M., MOTOYAMA E., CHIN W.W., SPINDEL E.R. Biology of disease: gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest. 1998;59:5–24. [PubMed] [Google Scholar]
- SZIKRA J., BENYHE S., OROSZ G., DARULA Z., PIOT J.M., FRUITIER I., MONORY K., HANOUNE J., BORSODI A. Radioligand binding properties of VV-hemorphin 7, an atypical opioid peptide. Biochem. Biophys. Res. Commun. 2001;281:670–677. doi: 10.1006/bbrc.2001.4397. [DOI] [PubMed] [Google Scholar]
- VLAHOS C.J., MATTER W.F., HUI K.Y., BROWN R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- WEBER H.C., WALTERS J., LEYTON J., CASIBANG M., PURDOM S., JENSEN R.T., COY D.H., ELLIS C., CLARK G., MOODY T.W. A bombesin receptor subtype-3 peptide increases nuclear oncogene expression in a MEK-1 dependent manner in human lung cancer cells. Eur. J. Pharmacol. 2001;412:13–20. doi: 10.1016/s0014-2999(00)00941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITLEY J.C., GIRAUD A.S., SHULKES A. Expression of gastrin-releasing peptide (GRP) and GRP receptors in the pregnant human uterus at term. J. Clin. Endocrinol. Metab. 1996;81:3944–3950. doi: 10.1210/jcem.81.11.8923842. [DOI] [PubMed] [Google Scholar]
- WU J.M., NITECKI D.E., BIANCALANA S., FELDMAN R.I. Discovery of high affinity bombesin receptor subtype 3 agonists. Mol. Pharmacol. 1996;50:1355–1363. [PubMed] [Google Scholar]