Abstract
The marine product cacospongionolide B, a sesterterpene isolated from the Mediterranean sponge Fasciospongia cavernosa, is an inhibitor of secretory phospholipase A2 with anti-inflammatory properties. In this work, we have studied the mechanism of action of this compound in the inflammatory response induced by zymosan in primary cells and in the mouse air pouch.
In mouse peritoneal macrophages, cacospongionolide B was able to downregulate the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), resulting in decreased production of NO and prostaglandin E2 (PGE2). This compound also reduced tumour necrosis factor-α (TNF-α) mRNA expression and TNF-α levels.
Cacospongionolide B inhibited nuclear factor-κB (NF-κB)-DNA binding activity and the nuclear translocation of this transcription factor.
Treatment of cells with cacospongionolide B impaired NF-κB inhibitory protein (IκB-α) phosphorylation and enhanced IκB-α expression.
Inhibition of iNOS, COX-2 and inflammatory mediators was confirmed in the mouse air pouch.
These results show that cacospongionolide B is able to control NO, PGE2 and TNF-α production in vitro and in vivo, effects likely dependent on NF-κB inhibition.
Keywords: Inducible nitric oxide synthase, cyclooxygenase-2, tumour necrosis factor-α, nuclear factor-κB, cacospongionolide B
Introduction
Activated macrophages play a critical role in immune and inflammatory responses, through the release of a variety of mediators including active lipids, reactive oxygen species, nitric oxide (NO), chemokines and cytokines. These agents can in turn recruit and modulate the functions of additional inflammatory cells amplifying the ongoing response. Tumour necrosis factor-α (TNF-α) is a major factor in the development of chronic inflammatory conditions (Maini & Taylor, 2000). This cytokine elicits a wide spectrum of cellular responses including leukocyte adhesion and migration as well as activation of inflammatory cells, with enhanced secretion of additional mediators such as interleukin-1 and interleukin-8 (Vassalli, 1992; Vlahopoulos et al., 1999).
Inflammatory cytokines induce cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression, as has been demonstrated in cell systems, models of inflammation and rheumatoid joints (Arias-Negrete et al., 1995; Miyasaka & Hirata, 1997; Clancy et al., 1998). Cytokine production is in turn modulated by products of this enzyme activity in stimulated macrophages (Deakin et al., 1995). Although cyclooxygenase-1 (COX-1) may play a role in some inflammatory responses (Wallace et al., 1998), it is widely accepted that COX-2 is the predominant cyclooxygenase isoform in all stages of inflammation, where it can be responsible for the production of proinflammatory prostanoids (Vane & Botting, 1998). Accordingly, high levels of prostaglandin E2 (PGE2) and NO produced from COX-2 and iNOS, respectively, have been detected in rheumatoid synovial tissues, suggesting a pathogenic role (Miyasaka & Hirata, 1997; Koch, 1998).
Transcription factors belonging to the nuclear factor-κB (NF-κB)/rel family regulate a range of genes that mediate inflammation and cell survival (Pahl, 1999). This transcription factor is usually present in the cytoplasm of cells as homodimeric or heterodimeric complexes of p50 and p65 subunits, associated with the NF-κB inhibitory protein (IκB-α) as an inactive complex. Many stimuli can activate NF-κB by phosphorylation of IκB followed by ubiquitination and degradation in a proteasome-dependent way, which allows the translocation of free NF-κB to bind specific DNA motifs activating transcription of target genes (Karin, 1999; Abraham, 2000). This transcription factor participates in the activation of iNOS, COX-2 and TNF-α promoters in different cells (Drouet et al., 1991; Abraham, 2000) and plays an important role in cytokine expression in the rheumatoid synovium (Handel et al., 1995).
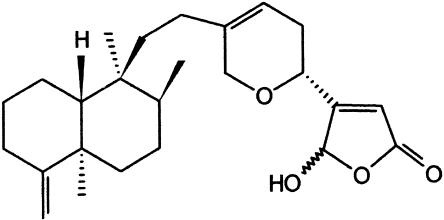
Cacospongionolide B (Figure 1) is an inhibitor of secretory phospholipase A2 and anti-inflammatory sesterterpene isolated from the sponge Fasciospongia cavernosa (García Pastor et al., 1999). However, the molecular mechanisms by which cacospongionolide B exerts its anti-inflammatory effects are not known. In the present study, we demonstrate that cacospongionolide B downregulates COX-2, iNOS and TNF-α protein or mRNA expression, blocking the production of the corresponding mediators in vitro in mouse peritoneal macrophages and also in vivo in the mouse air pouch model of inflammation. We also show that this marine metabolite is a potent inhibitor of the transcription factor NF-κB-DNA binding activity. This effect is parallelled by inhibition of NF-κB translocation into the nucleus, a likely consequence of decreased IκB-α phosphorylation and degradation.
Figure 1.
Chemical structure of cacospongionolide B.
Methods
Mouse peritoneal macrophages
Female CD-1 mice (Harlan, Spain) weighing 25–30 g were used to obtain highly purified peritoneal macrophages. Cells were harvested by peritoneal lavage 4 days after intraperitoneal injection of 1 ml 10% w v−1 thioglycollate broth and were resuspended in culture medium (120 mM NaCl, 4.7 mM KCl, 1.2 mM CaCl2 × 7·H2O, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, 1 mM L-arginine and 10 mM glucose), supplemented with 10% foetal bovine serum, 2 mM L-glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin at a concentration of 2 × 106 ml−1 and incubated in 96-well culture plate for 2 h at 37°C in a 5% CO2 atmosphere incubator. The non-adherent cells were removed by two washes with culture medium. Adherent macrophages were used to perform the following experiments. The mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan (Gross & Levi, 1992) was used to assess the possible cytotoxic effects of test compounds.
TNF-α, PGE2 and nitrite determination
Peritoneal macrophages (4 × 105/well) were coincubated with test compounds or vehicle at different concentrations and zymosan (0.1 mg ml−1) at 37°C. After centrifugation at 2000 × g for 10 min at 4°C, aliquots of the supernatants were used to measure different mediators. After 6 h of stimulation, supernatants were collected to measure TNF-α levels by time-resolved fluoroimmunoassay (Pennanen et al., 1995). In supernatants from 18 h zymosan-stimulated cells, PGE2 levels were determined by radioimmunoassay (Moroney et al., 1988) and nitrite production was quantified fluorometrically (Misko et al., 1993) in microtitre plates using a standard curve of sodium nitrite. In another series of experiments, to establish the possible inhibitory activity on iNOS or COX-2, cells were stimulated previously with zymosan for 18 h and then they were washed. Fresh medium supplemented with L-arginine (0.5 mM) and arachidonic acid (10 μM) was added for a 2 h incubation with test compound. Supernatants were collected for the measurement of nitrite and PGE2 accumulation for the last 2 h, as above.
Human monocytes
Human leukocytes were obtained from the citrated blood of healthy volunteers after sequential centrifugation as previously described (Bustos et al., 1995). The mononuclear cell interphase was obtained by Ficoll–Paque density gradient centrifugation. Cells were resuspended in RPMI 1640 supplemented with 10% foetal bovine serum (107 cells ml−1) and incubated in Petri dishes. Monocytes purified by 2-h adhesion were resuspended at a concentration of 2 × 106 ml−1 and cultured in 24-well culture plates. Cell viability was greater than 95% according to the Trypan blue exclusion test. Cells were preincubated with test compounds for 30 min and then stimulated with zymosan (0.1 mg ml−1). After 4 or 18 h incubation, supernatants were used to measure TNF-α or PGE2 levels, respectively, as above.
Western-blot analysis
Mouse peritoneal macrophages were cultured in 24-well culture plates at a concentration of 2 × 106 ml−1 with zymosan (0.1 mg ml−1) in the presence of test compounds or vehicle. After 18 h stimulation, medium was removed and cells were collected with lysis buffer (1% Triton X-100, 1% deoxycholic acid, 20 mM NaCl and 25 mM Tris, pH 7.4). After centrifugation at 10,000 × g for 10 min, supernatants were used for Western blot assay. Protein (25 μg) was loaded on 12.5% w v−1 SDS-PAGE and transferred onto PVDF membranes for 90 min at 125 mA. Membranes were blocked in PBS-Tween 20 containing 3% w v−1 unfatted milk and incubated with a polyclonal antibody (1/1000) anti-iNOS, anti-COX-2 or anti-β-actin, followed by incubation with peroxidase-conjugated goat anti-rabbit IgG (1/20,000). Cytoplasmic or nuclear extracts from peritoneal cells were used for Western blotting of proteins of the NF-κB pathway. Equal amounts of protein were loaded on 15% SDS-PAGE and transferred onto PVDF membranes. Membranes were blocked in PBS-Tween 20 containing 3% w v−1 unfatted milk and incubated with polyclonal antibodies against p65 or IκB-α (1/500). Antiphos pho-(Ser32) IκB-α antibody (1/750) was used according to the manufacturer's instructions, and incubation solution contained GST-IκB-α (1–317) (50 ng ml−1) (Castrillo et al., 2000). Finally, membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (1/20,000). The immunoreactive bands were visualized using an enhanced chemiluminescence system (ECL, Amersham Biosciences, Barcelona, Spain). β-Actin was used as internal control.
Electrophoretic mobility shift assay (EMSA)
Nuclear and cytosolic extracts from mouse peritoneal macrophages were prepared as described (López-Collazo et al., 1998). Protein was determined by the DC Bio-Rad protein reagent (Bio-Rad, CA, U.S.A.). The double-stranded oligonucleotide containing the consensus NF-κB sequence (Promega Corp., WI, U.S.A.) was end-labelled using T4 polynucleotide kinase (Amersham Pharmacia Biotech Europe GmbH, Spain) and [γ-32P]-ATP, followed by purification using G-25 microcolumns (Amersham Pharmacia Biotech Europe GmbH, Spain). Incubations were performed on ice with 6 μg of nuclear extract, 100,000 c.p.m. of labelled probe, 2 μg poly(dI-dC), 5% v v−1 glycerol, 1 mM EDTA, 5 mM MgCl2, 1 mM dithiothreitol, 100 mM NaCl and 10 mM Tris-HCl buffer (pH 8.0) for 15 min. To assess a direct interaction of cacospongionolide B with nuclear proteins, in another series of experiments, this compound or 15-deoxy-Δ12,14-PGJ2 was incubated for 10 min with nuclear extracts from zymosan-stimulated cells prior to the addition of the oligonucleotide probe. Complexes were analysed by nondenaturating 6% polyacrylamide gel electrophoresis in 0.5 × Tris-borate buffer followed by autoradiography of the dried gel.
Ribonuclease protection assay (RPA)
Total RNA was extracted using the Trizol® reagent (Life Technologies SA, Barcelona, Spain). The Riboquant™ multi-probe Rnase protection assay system was used according to the manufacturer's instructions with a mouse cytokine multiprobe template set (PharMingen, San Diego, CA, U.S.A.). Band intensities were quantitated using a laser densitometer.
Mouse air pouch
Female CD-1 mice (25–30 g) were anaesthetized with ethyl ether, and 10 ml of sterile air was injected into the subcutaneous tissue of the back. Three days later, 5 ml of sterile air was injected into the same cavity. At day six, 1 ml of 1% w v−1 zymosan in saline+vehicle (10 μl ethanol: control group) or 1 ml of 1% w v−1 zymosan in saline+cacospongionolide B or dexamethasone (dissolved in 10 μl of ethanol: treated groups) at the concentrations indicated in the results was administered into the air pouch. Another group received only 1 ml of saline+vehicle (saline group). At 2 or 12 h after administration, animals were killed by cervical dislocation and the exudate was collected with 1 ml of saline (Posadas et al., 2000). In the 12 h zymosan-injected air pouch, an additional dose of test compounds was administered into the air pouch 8 h after first administration. Leukocyte infiltration into the air pouch was measured using a Coulter counter. After centrifugation of exudates (1200 × g at 4°C for 10 min), supernatants were used to assay TNF-α, PGE2 and nitrite levels. The cell pellets from 12 h air pouches were used for iNOS and COX-2 Western blotting (Posadas et al., 2000). All studies were performed in accordance with the European Union regulations for the handling and use of laboratory animals. The protocols were approved by the institutional Animal Care and Use Commitee.
Materials
Cacospongionolide B was isolated from the sponge F. cavernosa following known procedures (De Rosa et al., 1995). [γ-32P]-ATP and [α-32P]-UTP were purchased from NEN Life Sciences Products, Inc. (Boston, MA, U.S.A.) and [5,6,8,11,12,14,15(n)-3H] PGE2 was from Amersham Biosciences (Barcelona, Spain). Anti-mouse TNF-α antibody was from Immunokontact (Frankfurt, Germany). iNOS and COX-2 specific polyclonal antisera and 15-deoxy-Δ12,14-PGJ2 were purchased from Cayman Chem. (Ann Arbor, MI, U.S.A.). Polyclonal antibodies against p65 and IκB-α, and GST–IκB-α (1–317) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, U.S.A.) and antiphospho-(Ser32) IκB-α antibody from New England Biolabs (Beverly, MA, U.S.A.). Z-Leu-Leu-Leu-CHO (MG-132) was from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA, U.S.A.). The peroxidase-conjugated IgG was purchased from Dako (Copenhagen, Denmark) and the rest of the reagents were from Sigma Chem. (St Louis, MO, U.S.A.).
Statistical analysis
The results are presented as mean±s.e. mean. IC50 values were calculated from at least four significant concentrations (n=6). The level of statistical significance was determined by analysis of variance (ANOVA) followed by Dunnett's t-test for multiple comparisons.
Results
Effect of cacospongionolide B on TNF-α, nitrite and PGE2 production in zymosan-stimulated mouse peritoneal macrophages
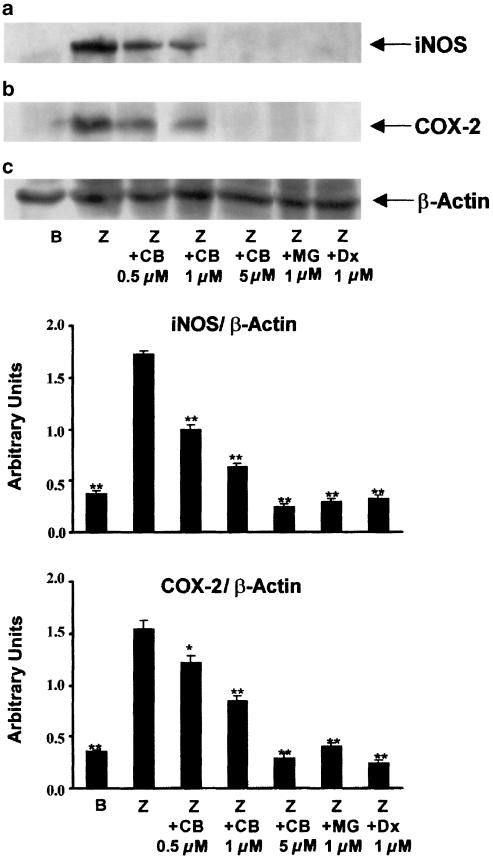
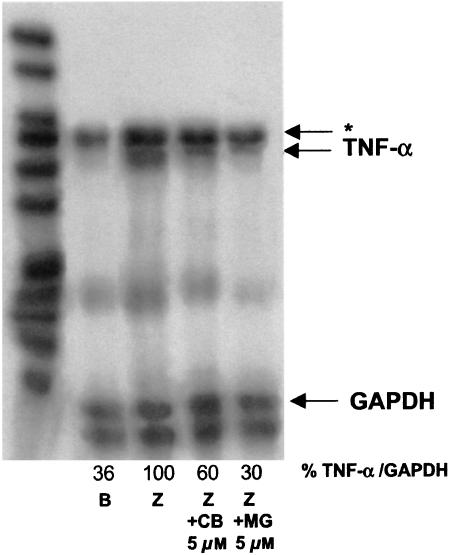
In preliminary experiments, we observed that zymosan (0.1 mg ml−1) induced the release of TNF-α in mouse peritoneal macrophages, with maximal levels 6 h after stimulation, whereas nitrite, as final product of iNOS activity, and PGE2 were detected around 18 h. As shown in Table 1, cacospongionolide B was able to reduce TNF-α, nitrite and PGE2 production in a concentration-dependent manner with IC50 values in the submicromolar range, whereas dexamethasone showed a higher potency. It is noteworthy that none of these compounds affected cell viability, as assessed by the mitochondrial reduction of MTT (data not shown). To determine if cacospongionolide B exerts inhibitory effects on iNOS and COX-2 activity in intact cells, cacospongionolide B was incubated at 10 μM with mouse peritoneal macrophages, after iNOS and COX-2 induction by 18 h of zymosan treatment. In this assay system, cacospongionolide B did not reduce nitrite or PGE2 production, indicating that this compound is neither an inhibitor of iNOS nor of COX-2 activities (data not shown). Moreover, Western blot analysis for iNOS and COX-2 proteins using zymosan-stimulated mouse peritoneal macrophages (Figure 2) showed clearly that cacospongionolide B, as well as the reference compounds MG-132 and dexamethasone, inhibited protein expression for both enzymes. To test whether the inhibition produced by cacospongionolide B on TNF-α levels could be related to effects on gene expression, we used RPA analysis. Our experiments indicated that cacospongionolide B treatment of mouse peritoneal macrophages caused a marked reduction in the mRNA expression of TNF-α induced by zymosan (Figure 3).
Table 1.
Effect of cacospongionolide B on TNF-α, nitrite and PGE2 generation in zymosan-stimulated mouse peritoneal macrophages
| TNF-α (IC50) | Nitrite (IC50) | PGE2 (IC50) | |
| Cacospongionolide B | 260 nM | 330 nM | 197 nM |
| Dexamethasone | 50 nM | 20 nM | 19 nM |
Data represent IC50 calculated for at least four significant concentrations (n=6). Measurement of TNF-α release was made 6 h after zymosan stimulation (nonstimulated cells=0.8±0.1 ng ml−1; zymosan-stimulated cells=5.5±0.4 ng ml−1). Nitrite (nonstimulated cells=86.2±6.0 ng ml−1; zymosan-stimulated cells=291.3±16.5 ng ml−1) and PGE2 (nonstimulated cells=3.0±0.5 ng ml−1; zymosan-stimulated cells=20.8±1.9 ng ml−1) levels were determined in 18 h zymosan-stimulated mouse peritoneal macrophages.
Figure 2.
Effect of cacospongionolide B on (a) iNOS and (b) COX-2 protein expression in mouse peritoneal macrophages. Cells were preincubated with drugs for 30 min and then stimulated with zymosan for 18 h. B: basal (nonstimulated cells); Z: zymosan; CB: cacospongionolide B; MG: MG-132; Dx: dexamethasone. After densitometric analysis, iNOS and COX-2 expression levels were normalized to β-actin and expressed as mean ± s.e. mean (n=3). **P<0.01; *P<0.05 with respect to Z.
Figure 3.
Effect of cacospongionolide B on TNF-α mRNA expression in mouse peritoneal macrophages. Cells were preincubated with drugs for 30 min and then stimulated with zymosan for 3 h. RNA was extracted and RPA was performed as described in Methods. B: basal (nonstimulated cells); Z: zymosan; CB: cacospongionolide B; MG: MG-132. Band intensities are expressed as percentages of the rate TNF-α/GAPDH with respect to the zymosan control. *This band is assigned to interleukin-6. The figure is representative of three similar experiments.
Effect of cacospongionolide B on NF-κB activation
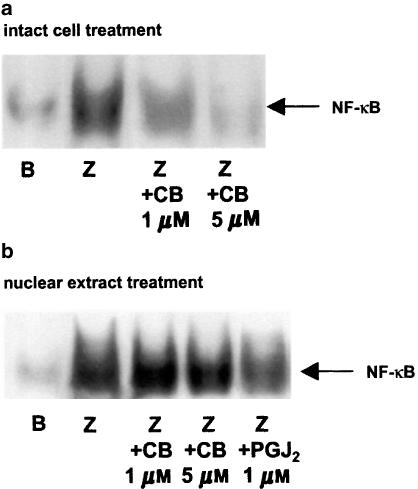
The effect of cacospongionolide B at 1 and 5 μM concentrations on NF-κB-DNA binding activity was analysed by EMSA. A low basal level of NF-κB-DNA binding activity was detected in nuclear proteins from unstimulated macrophages (Figure 4a). After 2 h of treatment with zymosan (0.1 mg ml−1), enhanced nuclear DNA binding was observed. Cacospongionolide B caused a concentration-dependent inhibitory effect when preincubated with cells before zymosan stimulation. In contrast, if cells were previously stimulated with zymosan and then nuclear extracts prepared and incubated with cacospongionolide B, we did not observe any variation in NF-κB-DNA binding by this compound (Figure 4b). In these experiments we used as reference 15-deoxy-Δ12,14-PGJ2, since this cyclopentenone PG is able to directly interact with nuclear proteins (Rossi et al., 2000).
Figure 4.
Effect of cacospongionolide B on NF-κB-DNA binding in nuclear extracts of mouse peritoneal macrophages. (a) Intact cell treatment. Cells were preincubated with cacospongionolide B for 30 min followed by zymosan stimulation for 2 h and then nuclear extracts were obtained as described in Methods. (b) Nuclear extract treatment. Cells were stimulated with zymosan for 2 h and then nuclear extracts were obtained as described in Methods. B: basal (nonstimulated cells); Z: zymosan. Cacospongionolide B (CB) or 15-deoxy-Δ12,14 -PGJ2 (PGJ2) was incubated with nuclear extracts for 10 min. The figures are representative of three similar experiments.
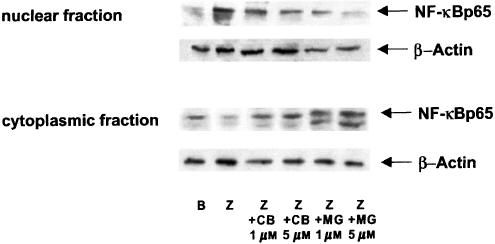
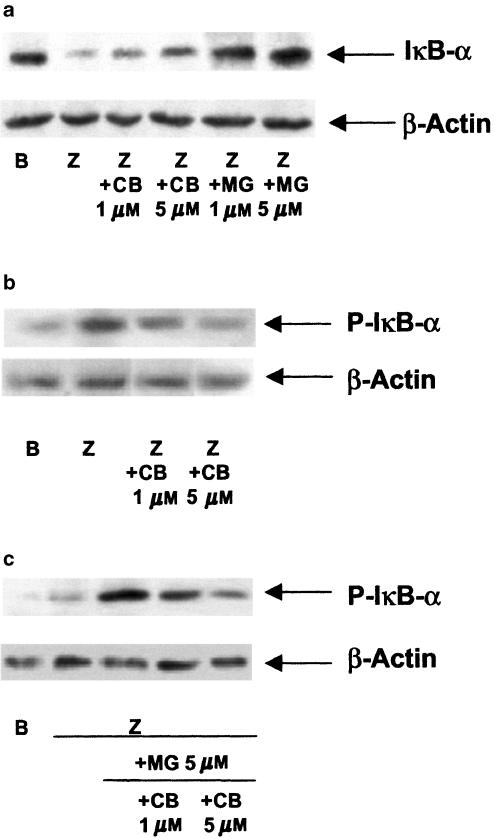
When p65 protein expression in nuclear and cytoplasmic extracts of mouse peritoneal macrophages was studied (Figure 5), we observed that zymosan induced the translocation of this protein into the nucleus, whereas treatment of stimulated cells with either cacospongionolide B or the proteasome inhibitor MG-132 retained p65 in the cytoplasm. The dependence of this effect on IκB-α degradation was followed by Western blotting. As shown in Figure 6a, unstimulated cells expressed a high basal level of this protein in the cytoplasm, whereas zymosan induced the proteolysis of IκB-α. Treatment of cells with either cacospongionolide B or MG-132 increased IκB-α expression in extracts from cells stimulated with zymosan. Phosphorylation of IκB-α on serines 32 and 36 is a step prior to its degradation (Karin, 1999; Abraham, 2000). The effect of cacospongionolide B or MG-132 on IκB-α serine 32 phosphorylation was studied in cytoplasmic extracts of mouse peritoneal macrophages using Western blot with a specific antibody. As shown in Figure 6b, cacospongionolide B reduced in a concentration-dependent manner the accumulation of P-IκB-α in zymosan-stimulated cells. Cacospongionolide B showed a different profile when compared with the proteasome inhibitor MG-132, which increased the expression of the phoshorylated form of IκB-α (Figure 6c), an effect also inhibited by this marine product. Our data suggest that cacospongionolide B does not inhibit the proteasome complex but it interferes with the phosphorylation of IκB-α.
Figure 5.
Effect of cacospongionolide B on p65 protein expression in nuclear and cytoplasmic extracts of mouse peritoneal macrophages. Cells were preincubated with drugs for 30 min before zymosan stimulation for 2 h and then nuclear and cytoplasmic extracts were prepared as described in Methods. B: basal (nonstimulated cells); Z: zymosan; CB: cacospongionolide B; MG: MG-132. The figures are representative of three similar experiments.
Figure 6.
Effect of cacospongionolide B on IκB-α degradation (a) and IκB-α serine 32 phosphorylation (b) and (c), in cytoplasmic extracts of mouse peritoneal macrophages. Cells were preincubated with cacospongionolide B or/and MG-132 for 30 min before zymosan stimulation for 60 min and then cytoplasmic extracts were prepared. B: basal (nonstimulated cells); Z: zymosan; CB: cacospongionolide B; MG: MG-132. The figures are representative of three similar experiments.
Effect of cacospongionolide B on TNF-α, nitrite and PGE2 production in the zymosan-injected mouse air pouch
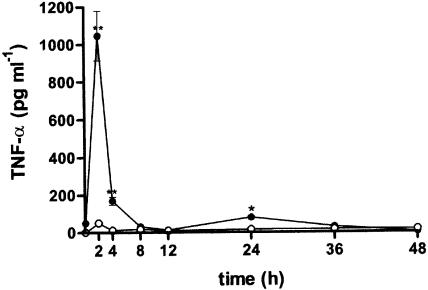
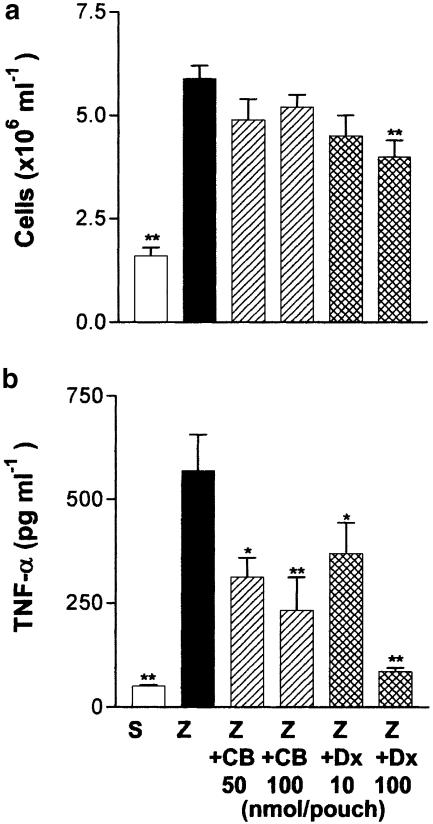
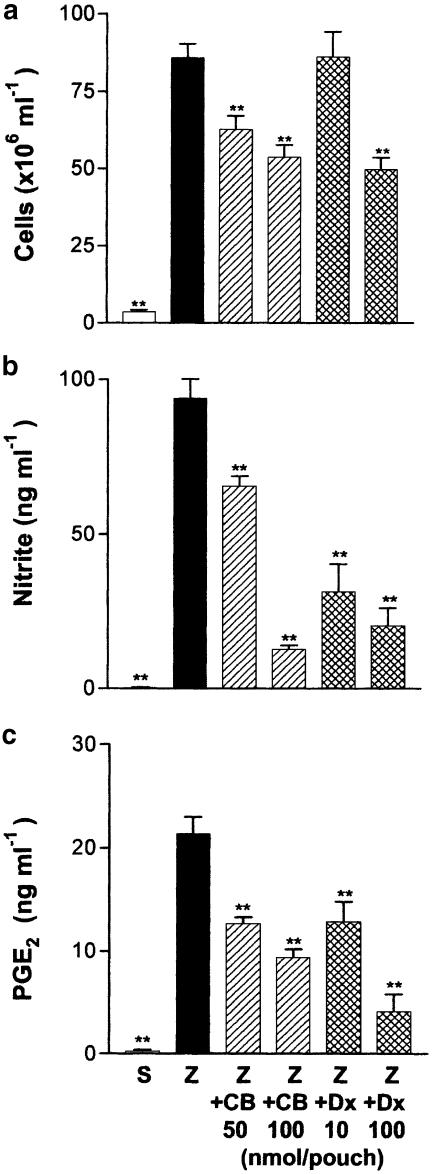
Zymosan injected into the 6-day-old mouse air pouch induced a rapid increase of TNF-α released into the cavity, which was accompanied by a rapid influx of leukocytes. Maximal production of TNF-α was detected at 2 h and decreased to basal levels at 8 h poststimulation (Figure 7). Saline-treated pouches contained barely detectable levels of TNF-α. We selected the time of maximal TNF-α production to study the effect of cacospongionolide on cytokine release in vivo. Cacospongionolide B reduced TNF-α production without affecting cellular infiltration significantly (Figure 8). Dexamethasone reduced cellular infiltration into the air pouch at the highest dose assayed and also reduced TNF-α levels in the exudates at both doses.
Figure 7.
Time course of TNF-α levels in exudates of the mouse air pouch. Each point is the mean±s.e. mean (n=6–12 animals). Closed symbols represent the values observed for zymosan-injected air pouches and open symbols for saline-injected air pouches. **P<0.01; *P<0.05 with respect to saline-injected air pouches.
Figure 8.
Effect of cacospongionolide B and dexamethasone on the 2 h zymosan-injected mouse air pouch. (a) Cell migration and (b) TNF-α levels in exudates. S: saline; Z: zymosan; Z+CB: zymosan-injected air pouch treated with cacospongionolide B; Z+Dx: zymosan-injected air pouch treated with dexamethasone. Results are the mean±s.e. mean, n=6–10. **P<0.01; *P<0.05 with respect to the zymosan control group.
Previous results have shown the participation of NO and PGE2 in the later phase of this experimental model (Posadas et al., 2000). In this regard, we selected 12 h of zymosan stimulation to determine the effect of cacospongionolide B and dexamethasone on these parameters. As shown in Figure 9, cacospongionolide B and dexamethasone reduced cellular infiltration, nitrite and PGE2 content in the air pouch at this time point.
Figure 9.
Effect of cacospongionolide B and dexamethasone on the 12 h zymosan-injected mouse air pouch. (a) Cell migration, (b) nitrite and (c) PGE2 (c) in exudates. S: saline; Z: zymosan; Z+CB: zymosan-injected air pouch treated with cacospongionolide B; Z+Dx: zymosan-injected air pouch treated with dexamethasone. Results are the mean±s.e. mean, n=6–10. **P<0.01 with respect to the zymosan control group.
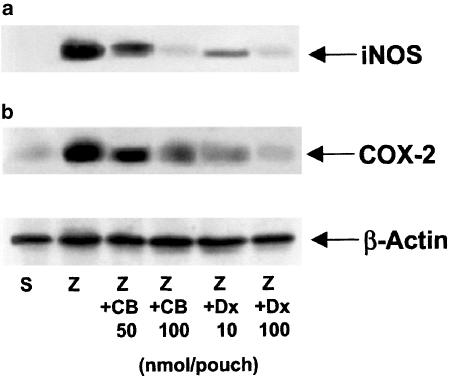
In the cytosolic fraction of cell pellets from 12 h zymosan-stimulated mouse air pouch, we detected high levels of a protein corresponding immunologically to a 130 kDa iNOS, which was not present in cell pellets from saline-treated animals. Western blot analysis of iNOS expression in the cytosolic fraction of cells from exudates of zymosan-injected (12 h) mouse air pouches treated with test compounds showed that cacospongionolide B and dexamethasone reduced the expression of this protein (Figure 10). In addition, a 70 kDa band immunologically detected as COX-2 was significantly decreased by both compounds, in the microsomal fraction of the same cells.
Figure 10.
Effect of cacospongionolide B on (a) iNOS and (b) COX-2 protein expression in cells from 12 h zymosan-injected mouse air pouch. S: saline; Z: zymosan; Z+CB: zymosan-injected air pouch treated with cacospongionolide B; Z+Dx: zymosan-injected air pouch treated with dexamethasone. The figures are representative of three similar experiments.
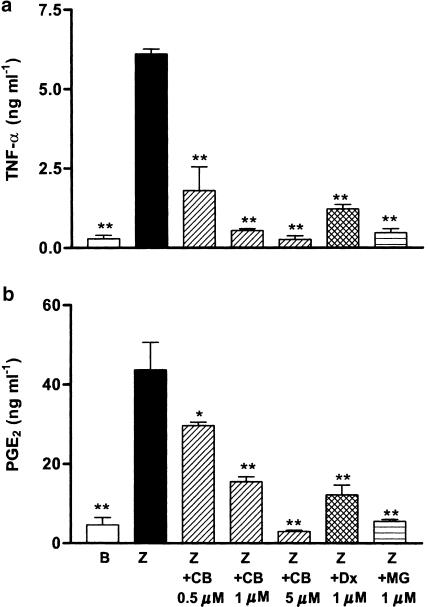
Effect of cacospongionolide B on TNF-α and PGE2 production in human monocytes
We have also shown that cacospongionolide B inhibits the production of inflammatory mediators in primary cells of human origin. As seen in Figure 11, this marine metabolite reduced in a concentration-dependent manner the levels of TNF-α and PGE2 in human monocytes stimulated with zymosan. The inhibition of TNF-α production at 1 μM was higher than that of dexamethasone.
Figure 11.
Effect of cacospongionolide B on (a) TNF-α and (b) PGE2 production in human monocytes. Cells were preincubated with test compounds for 30 min and then stimulated with zymosan. B: basal (nonstimulated cells); Z: zymosan; CB: cacospongionolide B; MG: MG-132; Dx: dexamethasone. Measurement of TNF-α release was made 6 h after zymosan stimulation and PGE2 levels were determined 18 h after zymosan. Results are the mean±s.e. mean, n=6. **P<0.01; *P<0.05 with respect to Z.
Discussion
Macrophage activation is a key component of immune and inflammatory responses. Stimuli such as bacterial products and fungal cell wall glucans stimulate macrophages leading to the induction of a variety of proteins relevant to the inflammatory process. Thus, zymosan induces in macrophages COX-2, iNOS and cytokines including TNF-α, which play a key role in the pathogenesis of inflammatory conditions such as rheumatoid arthritis (Foxwell et al., 2000). NF-κB is activated in macrophages by different stimuli to regulate the expression of genes encoding for cytokines and inducible enzymes (Pahl, 1999; Young et al., 2001). Cytokines can in turn activate NF-κB-dependent transcription, increasing the duration of chronic inflammation (Yamamoto & Gaynor, 2001). Our data show that cacospongionolide B inhibits in a concentration-dependent manner the zymosan-induced NF-κB-DNA binding activity. This effect does not involve a direct modification of NF-κB by cacospongionolide B, in contrast to 15-deoxy-Δ12,14-PGJ2, which contains a cyclopentenone ring able to interact with nucleophiles such as sulphydryls groups in cellular proteins (Rossi et al., 2000). Although cacospongionolide B contains a reactive α,β-unsaturated carbonyl group in the γ-hydroxybutenolide ring, this feature does not seem to be determinant for a direct modification of NF-κB proteins.
One of the key intracellular events for NF-κB activation is the proteolytic cleavage of a complex of NF-κB bound to IκB-α. In unstimulated cells, NF-κB is present as an inactive heterodimer of p50/p65 subunits bound to the NF-κB inhibitor protein IκB. Upon stimulation, IκB becomes phosphorylated leading to its degradation in an ubiquitin-dependent process. Activation of IκB kinase results in phosphorylation and rapid degradation of IκB (Karin, 1999; Abraham, 2000). We have observed in murine peri-toneal macrophages that zymosan enhances IκB-α serine 32 phosphorylation and subsequent degradation, leading to NF-κB release and translocation to the nucleus. We have also presented evidence that cacospongionolide B impairs serine 32 phosphorylation of IκB-α, increasing the expression of this protein in the cytoplasm and thus preventing p65 nuclear translocation.
In animal studies, zymosan exposure induces an inflammatory response where iNOS and COX-2 play an important role resulting in the production of NO and PGE2 (Posadas et al., 2000). We have shown in the present work the participation of TNF-α in this response. It is noteworthy that cacospongionolide B inhibits TNF-α and PGE2 production in human monocytes. Our data indicate that this marine metabolite suppresses zymosan-induced NO and PGE2 production in vitro and in vivo. These effects are correlated with downregulation of iNOS and COX-2 expression, without affecting the catalytic activity of both proinflammatory enzymes. Our results also suggest that inhibition of TNF-α mRNA expression by cacospongionolide B could play a role in the observed inhibitory effects on this cytokine.
This marine compound is an inhibitor of NF-κB-DNA binding with a potency higher than drugs previously reported, such as cyclolinteinone, a sesterterpene chemically related to cacospongionolide B (D'Acquisto et al., 2000). It is interesting to note that other inhibitors of phospholipase A2 have also been reported to block the activation of NF-κB (Thommesen et al., 1998; Scholz-Pedretti et al., 2000).
The inhibition of the NF-κB pathway is a therapeutic target in chronic inflammatory disorders and cancer (Yamamoto & Gaynor, 2001). Interestingly, cacospongionolide B by oral route exerts inhibitory effects in acute and chronic models of inflammation (García Pastor et al., 1999). In comparison with known anti-inflammatory agents, this marine compound may offer some advantages. Selective regulation of COX-2 by cacospongionolide B could be beneficial to avoid side effects of classical nonsteroidal anti-inflammatory drugs. In this respect, it is interesting to note that this marine compound is not a COX-1 inhibitor and lacks gastrointestinal toxicity after administration to rats (García Pastor et al., 1999). The occurrence of important side effects also limits the therapeutic use of glucocorticoids, potent anti-inflammatory agents regulating gene expression. However, the molecular mechanisms underlying the pharmacological effects of these drugs are quite complex and include direct regulation of gene expression and control of several signalling pathways such as activator protein-1 and NF-κB (Vanden Berghe et al., 1999).
We have shown in the present study that cacospongionolide B may represent an alternative approach to find new agents that modulate iNOS, COX-2 and TNF-α. The ability of this marine compound to control NF-κB-dependent gene expression and regulate cellular functions may have a potential therapeutic application to management of inflammatory conditions.
Acknowledgments
This work was supported by Grant SAF-2001 2639 (Ministerio de Ciencia y Tecnología, Spain).
Abbreviations
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- EMSA
electrophoretic mobility-shift assay
- IκB
NF-κB inhibitory protein
- INOS
inducible nitric oxide synthase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor-κB
- NO
Nitric oxide
- PGE2
prostaglandin E2
- RPA
ribonuclease protection assay
- TNF-α
tumour necrosis factor-α
References
- ABRAHAM E. NF-kappaB activation. Crit. Care Med. 2000;28:N100–N104. doi: 10.1097/00003246-200004001-00012. [DOI] [PubMed] [Google Scholar]
- ARIAS-NEGRETE S., KELLER K., CHADEE K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem. Biophys. Res. Commun. 1995;208:582–589. doi: 10.1006/bbrc.1995.1378. [DOI] [PubMed] [Google Scholar]
- BUSTOS G., FERRÁNDIZ M.L., SANZ M.J., PAYÁ M., ALCARAZ M.J. A study of the novel anti-inflammatory agent florifenine. Topical anti-inflammatory activity and influence on arachidonic acid metabolism and neutrophil functions. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;351:298–304. doi: 10.1007/BF00233250. [DOI] [PubMed] [Google Scholar]
- CASTRILLO A., DIAZ-GUERRA M.J., HORTELANO S., MARTIN-SANZ P., BOSCA L. Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol. Cell Biol. 2000;20:1692–1698. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLANCY R.M., AMIN A.R., ABRAMSON S.B. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998;41:1141–1151. doi: 10.1002/1529-0131(199807)41:7<1141::AID-ART2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- D'ACQUISTO F., LANZOTTI V., CARNUCCIO R.Cyclolinteinone, a sesterterpene from sponge Cacospongia linteiformis, prevents inducible nitric oxide synthase and inducible cyclo-oxygenase protein expression by blocking nuclear factor-kappaB activation in J774 macrophages Biochem. J. 2000346793–798.Part 3 [PMC free article] [PubMed] [Google Scholar]
- DE ROSA S., CRISPINO A., DE GIULIO A., IODICE C., PRONZATO R., ZAVODNIK N. Cacospongionolide B, a new sesterterpene from the sponge Fasciospongia cavernosa. J. Nat. Products. 1995;58:1776–1780. doi: 10.1021/np50125a024. [DOI] [PubMed] [Google Scholar]
- DEAKIN A.M., PAYNE A.N., WHITTLE B.J., MONCADA S. The modulation of IL-6 and TNF-alpha release by nitric oxide following stimulation of J774 cells with LPS and IFN-gamma. Cytokine. 1995;7:408–416. doi: 10.1006/cyto.1995.0056. [DOI] [PubMed] [Google Scholar]
- DROUET C., SHAKHOV A.N., JONGENEEL C.V. Enhancers and transcription factors controlling the inducibility of the tumor necrosis factor-alpha promoter in primary macrophages. J. Immunol. 1991;147:1694–1700. [PubMed] [Google Scholar]
- FOXWELL B.M., BONDESON J., BRENNAN F., FELDMANN M. Adenoviral transgene delivery provides an approach to identifying important molecular processes in inflammation: evidence for heterogenecity in the requirement for NFkappaB in tumour necrosis factor production. Ann., Rheum. Dis. 2000;59 Suppl 1:I54–I59. doi: 10.1136/ard.59.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCÍA PASTOR P., DE ROSA S., DE GIULIO A., PAYÁ M., ALCARAZ M.J. Modulation of acute and chronic inflammatory processes by cacospongionolide B, a novel inhibitor of human synovial phospholipase A2. Br. J. Pharmacol. 1999;126:301–311. doi: 10.1038/sj.bjp.0702302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S.S., LEVI R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J. Biol. Chem. 1992;267:25722–25729. [PubMed] [Google Scholar]
- HANDEL M.L., MCMORROW L.B., GRAVALLESE E.M. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- KARIN M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J. Biol. Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- KOCH A.E. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41:951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- LÓPEZ-COLLAZO E., HORTELANO S., ROJAS A., BOSCÁ L. Triggering of peritoneal macrophages with IFN-α/β attenuates the expression of inducible nitric oxide synthase through a decrease in NF-κB activation. J. Immunol. 1998;160:2889–2895. [PubMed] [Google Scholar]
- MAINI R.N., TAYLOR P.C. Anti-cytokine therapy for rheumatoid arthritis. Annu. Rev. Med. 2000;51:207–229. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., SCHILLING R.J., SALVEMINI D., MOORE W.M., CURRIE M.G. A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- MIYASAKA N., HIRATA Y. Nitric oxide and inflammatory arthritides. Life Sci. 1997;61:2073–2081. doi: 10.1016/s0024-3205(97)00585-7. [DOI] [PubMed] [Google Scholar]
- MORONEY M.A., ALCARAZ M.J., FORDER R.A., CAREY F., HOULT J.R.S. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J. Pharm. Pharmacol. 1988;40:787–792. doi: 10.1111/j.2042-7158.1988.tb05173.x. [DOI] [PubMed] [Google Scholar]
- PAHL H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- PENNANEN N., LAPINJOKI S., PALANDER A., URTTI A., MONKKONEN J. Macrophage-like RAW 264 cell line and time-resolved fluoroimmunoassay (TRFIA) as tools in screening drug effects on cytokine secretion. Int. J. Immunopharmacol. 1995;17:475–480. doi: 10.1016/0192-0561(95)00030-6. [DOI] [PubMed] [Google Scholar]
- POSADAS I., TERENCIO M.C., GUILLÉN I., FERRÁNDIZ M.L., COLOMA J., PAYÁ M., ALCARAZ M.J. Co-regulation between cyclo-oxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;361:98–106. doi: 10.1007/s002109900150. [DOI] [PubMed] [Google Scholar]
- ROSSI A., KAPAHI P., NATOLI G., TAKAHASHI T., CHEN Y., KARIN M., SANTORO M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- SCHOLZ-PEDRETTI K., EBERHARDT W., RUPPRECHT G., BECK K.F., SPITZER S., PFEILSCHIFTER J., KASZKIN M. Inhibition of NfkappaB-mediated pro-inflammatory gene expression in rat mesangial cells by the enolized 1,3-dioxane-4,6-dione-5-carboxamide, CGP-43182. Br. J. Pharmacol. 2000;130:1183–1190. doi: 10.1038/sj.bjp.0703419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMMESEN L., SJURSEN W., GASVIK K., HANSSEN W., BREKKE O.L., SKATTEBOL L., HOLMEIDE A.K., ESPEVIK T., JOHANSEN B., LAEGREID A. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J. Immunol. 1998;161:3421–3430. [PubMed] [Google Scholar]
- VANDEN BERGHE W., FRANCESCONI E., DE BOSSCHER K., RESCHE-RIGON M., HAEGEMAN G. Dissociated glucocorticoids with anti-inflammatory potential repress interleukin-6 gene expression by a nuclear factor-kappaB-dependent mechanism. Mol. Pharmacol. 1999;56:797–806. [PubMed] [Google Scholar]
- VANE J.R., BOTTING R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:2S–8S. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- VASSALLI P. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- VLAHOPOULOS S., BOLDOGH I., CASOLA A., BRASIER A.R. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–1889. [PubMed] [Google Scholar]
- WALLACE J.L., BAK A., MCKNIGHT W., ASFAHA S., SHARKEY K.A., MACNAUGHTON W.K. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y., GAYNOR R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG S.H., YE J., FRAZER D.G., SHI X., CASTRANOVA V. Molecular mechanism of tumor necrosis factor-alpha production in 1->3-beta-glucan (zymosan)-activated macrophages. J. Biol. Chem. 2001;276:20781–20787. doi: 10.1074/jbc.M101111200. [DOI] [PubMed] [Google Scholar]