Abstract
The effects of flecainide and quinidine were studied on wild-type Kv4.2 channels (Kv4.2WT), channels with deletion of the N-terminal domain (N-del) and channels with mutations in the valine residues located at positions 402 and 404 in the presence (V[402,404]I) or in the absence (N-del/V[402,404]I) of the N-terminus.
The experiments were performed at 37°C on COS7 cells using the whole-cell configuration of the patch-clamp technique.
Flecainide and quinidine inhibited Kv4.2WT currents in a concentration-dependent manner (IC50=23.6±1.1 and 12.0±1.4 μMat +50 mV, respectively), similar to their potency for the rest of the constructs at the same voltage. In Kv4.2WT channels, flecainide- and quinidine-induced block increased as channel inactivation increased. In addition, the inhibition produced by quinidine, but not by flecainide, increased significantly at positive test potentials. Similar effects were observed in N-del channels. However, in V[402,404]I and N-del/V[402,404]I channels, the voltage dependence of block by both quinidine and flecainide was lost, without significant modifications in potency at +50 mV.
These results point to an important role for S6 valines at positions 402 and 404 in mediating voltage-dependent block by quinidine and flecainide.
Keywords: K+ currents, inactivation, patch clamp, antiarrhythmic drugs
Introduction
The voltage-dependent calcium-independent transient outward K+ current (Itol or Itof) plays a key role in cardiac repolarization of several species including humans (Nerbonne, 2000). This current determines the initial phase of repolarization and influences the balance of inward and outward ionic currents during the plateau phase, modulating the voltage and time dependence of the cardiac action potential (Nattel, 1999; Nerbonne, 2000). Considerable experimental evidence has now been provided documenting a role for α-subunits of the Kv4 subfamily in the generation of cardiac Itof (Nerbonne, 2000). Thus, Kv4.2 channels are highly expressed in rat heart (Barry et al., 1995) and Kv4.2 mRNA displays the same ventricular distribution as rat Itof, increasing from endocardial to epicardial layers (Dixon & McKinnon, 1994). These channels generate transient outward currents, that is, they display rapid activation and inactivation (Nerbonne, 2000). The molecular basis of the inactivation process in Kv4.2 channels remains unclear, supporting the hypothesis of a novel mechanism different from the N- and C-type shown for Shaker channels (Rasmusson et al., 1998).
N-terminal deletion of Kv4.1 (Jerng & Covarrubias, 1997) and Kv4.2 channels (Zhu et al., 1999; Bähring et al., 2001; Pourrier et al., 2000) slightly slows fast inactivation. A double mutation of valine residues located in the distal section of S6, a putative component of the inner mouth of the pore, to isoleucines (V[404,406]I in Kv4.1 and V[402,404]I in Kv4.2) shifts the voltage dependence and slows the time course of inactivation (Jerng et al., 1999; Pourrier et al., 2000), while suppressing the blocking effects of 4-AP (Jerng et al., 1999) both in the presence and absence of the N-terminal domain. Class I antiarrhythmic drugs characteristically block cardiac Na+ channels, but some of them also prolong action potential duration by blocking one or more K+ channels (Nattel, 1999). It has been shown that flecainide and quinidine inhibit Itof in native cells (Imaizumi & Giles, 1987; Slawsky & Castle, 1994; Wang et al., 1995) as well as in heterologous expression systems (Yeola & Snyders, 1997; Rolf et al., 2000). The effects of quinidine are consistent with open-channel block (Imaizumi & Giles, 1987; Wang et al., 1995), whereas flecainide seems to exhibit a higher affinity for the inactivated state (Wang et al., 1995).
Information about the molecular determinants of the blocking effects of flecainide and quinidine on Itof is limited. We are not aware of studies characterizing the effects of interventions altering Kv4.2 channel inactivation on flecainide or quinidine action. The present work was designed to investigate the effects of flecainide and quinidine on Kv4.2 currents transiently expressed in COS7 cells and to analyze whether the observed effects are affected by modifications altering channel inactivation. We studied the effects of flecainide and quinidine on Kv4.2 wild-type channels (Kv4.2WT), on channels with a deletion of the N-terminal domain (N-del) and on channels with mutations in the valine residues located in positions 402 and 404 in the presence (V[402,404]I) or absence of the N-terminal domain (N-del/V[402,404]I). The present results demonstrate that flecainide and quinidine block Kv4.2WT and all the studied constructs with the same potency at a positive voltage, but that the absence of the S6 valine residues located at positions 402 and 404 abolishes the voltage dependence of block. Preliminary results have been previously published in abstract form (Caballero et al., 2001).
Methods
Molecular biology
Wild-type rat Kv4.2 was cloned into pRc/CMV vector (Invitrogen Carlsbad, CA, USA) between HindIII and NotI sites. The N-terminal deletion (Δ2–151), the V(402,404) mutations and the combination of both (N-del/V[402,404]I) were generated as previously described (Pourrier et al., 2000) and then were cloned into pTarget vector (Promega). Wild-type and mutant channels were subsequently transfected into COS7 cells for electrophysiological studies.
Cell culture and transfection
Cell culture and transfection methods have been previously described (Pourrier et al., 2000). COS7 cells were cotransfected with the construct of interest (1 μg ml−1) and CD8 antigen (0.5 μg ml−1) expression plasmids using Lipofectamine (Gibco). After 48 h, cells were removed from the plates and incubated in bovine serum albumin (BSA) solution containing Dynabeads M450 conjugated to CD8 antibody (1 μl ml−1) (Dynal). Dynabead adhesion to the cell surface was used to select successfully transfected cells (Jurman et al., 1994).
Solutions
Cells were perfused with Tyrode's solution containing (mM): NaCl 136, KCl 5.4, MgCl2 1, HEPES 5, NaH2PO4 0.3, CaCl2 1 and glucose 10 (pH 7.4, NaOH). The intracellular solution contained (mM): guanosine triphosphate (GTP) 1, K-Aspartate 110, KCl 20, MgCl2 1, ATP-Mg 5, HEPES 10, EGTA 5 and phosphocreatine 5 (pH 7.3, KOH). BSA solution contained (mM): NaCl 155, KCl 5, MgCl2 1, HEPES 10, CaCl2 3 and BSA 0.5% (pH was adjusted to 7.4 with NaOH). Flecainide and quinidine were dissolved in dimethyl sulphoxide (DMSO) to obtain 10 mM stock solutions.
Data acquisition and analysis
Currents were recorded using the whole-cell configuration of the patch-clamp technique. Glass pipettes had a tip resistance of 2–3 MΩ when filled with internal solution and immersed in external solution. Series resistance (Rs) averaged 5.0±0.4 MΩ (n=27), the capacitive time constant (τc) averaged 206.9±20.4 μs and membrane capacitance was 42.1±3.5 pF. Rs and τc were compensated (80–95%) and the voltage drop across the series resistance was <5 mV. Data were acquired and analysed using an Axopatch 200A amplifier and pCLAMP 6.0 software (Axon Instruments). All experiments were performed at 37°C. Current amplitude was measured as the difference between peak outward and end-pulse current. Block was fitted to the equation
where Y is the fractional block at concentration X, IC50 the concentration that produces half-maximal block and nH the Hill coefficient. The activation and inactivation curves were obtained as previously described (Jerng et al., 1999; Pourrier et al., 2003) and were fitted with the Boltzmann equation
where V1/2 is the voltage for half-maximum activation or inactivation, V the potential of the test (for activation) or conditioning (for inactivation) pulse and k is the slope factor.
The voltage dependence of block was fitted using the Woodhull formalism
where z, F, R and T have their usual meaning, δ represents the fractional electrical distance from the intracellular side of the membrane and KD* represents the dissociation constant at the reference potential (0 mV).
Data are expressed as the mean±s.e. Statistical comparisons were performed with ANOVA with a Newman–Keuls multiple-comparison post hoc test. P<0.05 was considered statistically significant.
Results
Effects of flecainide and quinidine on Kv4.2 currents
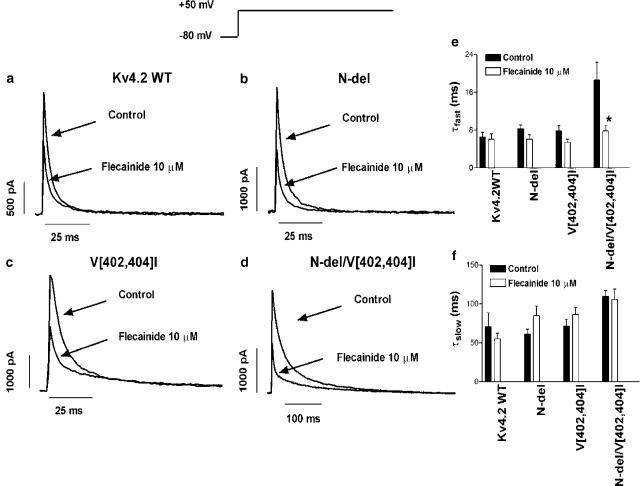
Figures 1 and 2 show typical recordings of Kv4.2WT, N-del, V[402,404]I and N-del/V[402,404]I currents during 500 ms pulses from −80 to +50 mV in the absence and in the presence of 10 μM flecainide and quinidine, respectively. Kv4.2WT currents rose rapidly to a peak (1341±255 pA, n=28) and inactivated rapidly. Two exponential components were required to describe the time course of inactivation (τfast=6.5±1.0 ms, τslow=70.9±17.7 ms, n=28). Flecainide (Figure 1a) decreased the current amplitude by 44.4±3.1% (n=7) but did not modify the time course of inactivation (τfast=6.1±1.1 ms and τslow=55.2±7.5 ms, n=5, P=NS, Figures 1e and f). Figure 1b shows the effects of flecainide on N-del currents. In this construct, rapid-phase inactivation was significantly slower than for Kv4.2WT (Table 1). In the presence of flecainide, inactivation kinetics were not significantly changed (Figures 1e and f) and current inhibition was similar to that for Kv4.2WT (46.1±6.9% at +50 mV, n=10, P=NS vs Kv4.2WT). For V[402,404]I, rapid-phase inactivation was also slower compared with Kv4.2WT (Figure 1c, Table 1). Flecainide decreased current amplitude by 46.5±8.4% (n=5, P=NS vs Kv4.2WT and N-del) and did not alter τfast (5.5±0.7 vs 7.8±1.2 ms, n=5, P=NS) or τslow (86.4±9.0 vs 71.2±9.2 ms, P=NS). In N-del/V[402,404]I (Figure 1d), both rapid- and slow-phase inactivation were slowed (Table 1). Flecainide accelerated fast-phase (τfast=7.8±1.1 ms, n=12 vs 21.6±2.1 ms control, P<0.05) but did not significantly modify slow-phase inactivation (τslow=105.8±13.3, P=NS, Figures 1e and f), while inhibiting the current by 51.2±3.7% at +50 mV (n=9, P=NS vs Kv4.2WT, N-del and V[402,404]I).
Figure 1.
Effects of flecainide on Kv4.2 currents. Currents were elicited by 500 ms pulses from −80 to +50 mV in the absence and the presence of flecainide (10 μM) in Kv4.2WT (a), N-del (b), V[402,404]I (c) and N-del/V[402,404]I channels (d). (e, f) Inactivation τfast and τslow values (mean±s.e.m. of five to 12 experiments). *P<0.05 vs control.
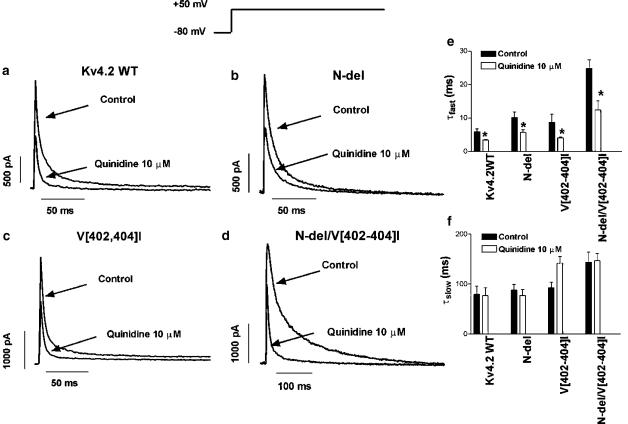
Figure 2.
Effects of quinidine on Kv4.2 currents. Currents were elicited by 500 ms pulses from −80 to +50 mV in the absence and the presence of quinidine (10 μM) in Kv4.2WT (a), N-del (b), V[402,404]I (c) and N-del/V[402,404]I channels (d). (e, f) Inactivation τfast and τslow values (mean±s.e.m. of seven to nine experiments). *P<0.05 vs control.
Table 1.
Inactivation kinetics and voltage dependence of the activation and inactivation on Kv4.2WT and N-del, V[402,404]I and N-del/V[402,404]I mutants
| Inactivation kinetics | Voltage dependence of activation | Voltage dependence of inactivation | ||||
|---|---|---|---|---|---|---|
| Construct | τf (ms) | τs (ms) | V1/2 (mV) | k (mV) | V1/2 (mV) | k (mV) |
| Kv4.2WT | 6.0±0.6 | 74.1±10.7 | −5.8±4.2 | 11.7±1.0 | −56.0±1.9 | 8.3±0.9 |
| N-del | 8.8±0.8** | 71.2±6.9 | −1.4±2.0 | 14.4±3.4 | −49.9±2.5 | 8.7±0.9 |
| V[402,404]I | 8.1±1.2* | 81.7±6.9 | 13.0±3.7** | 9.9±1.1 | −38.4±5.0** | 7.8±1.1 |
| N-del/V[402,404]I | 21.6±2.1*** | 130.2*** | −0.4±2.8 | 13.3±2.0 | −42.9±3.0** | 20.5±1.5*** |
P<0.05
P<0.01
P<0.001 vs Kv4.2WT.
Figure 2 shows the effects of 10 μM quinidine on Kv4.2 currents. Quinidine decreased Kv4.2WT current by 49.3±06.0% (n=8, Figure 2a) and accelerated fast-phase inactivation, decreasing τfast from 5.9±0.8 to 3.4±0.2 ms (n=7, P<0.05, Figure 2e). Quinidine did not modify slow-phase inactivation (τslow=76.6±16.0 vs 79.4±16.1, P=NS, Figure 2f). In N-del (Figure 2b), quinidine reduced the current by 45.0±2.6% (n=8, P=NS vs Kv4.2WT) and decreased τfast from 9.5±1.6 to 5.8±0.7 ms (n=8, P<0.05) but did not modify τslow (85.7±13.2 vs 86.1±13.5 ms, P=NS, Figures 2e and f). In V[402,404]I (Figure 2c), the drug decreased current amplitude by 40.4±8.3% (n=6, P=NS vs Kv4.2WT and N-del), accelerated fast-phase inactivation, decreasing τfast from 8.7±2.4 to 4.1±0.2 ms (n=5, P<0.05) and did not affect τslow (121.6±13.6 vs 92.7±10.9 ms, P=NS, Figures 2e and f). Figure 2d shows the effects of quinidine on N-del/V[402,404]I. In control conditions, τfast and τslow values were 24.8±2.6 (n=9, P<0.05 vs Kv4.2WT) and 143.6±20.6 ms (P=NS), respectively, and quinidine decreased τfast to 12.4±2.8 ms (P<0.01), without modifying τslow (146.5±15.1 ms, P=NS, Figures 2e and f).
Concentration-dependent effects of flecainide and quinidine on Kv4.2 currents
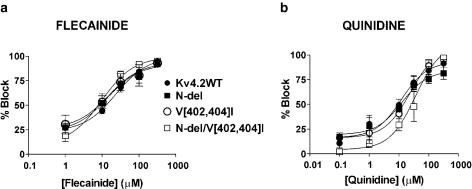
The concentration–response curves for flecainide and quinidine on Kv4.2WT, N-del, V[402,404]I and N-del/V[402,404]I currents at +50 mV are shown in Figure 3. The percentage of block at +50 mV for each construct was plotted as a function of the drug concentration and fitted to a dose–response relation (see Methods). Flecainide (a) and quinidine (b) clearly inhibited Kv4.2 currents in a concentration-dependent manner. The IC50 values were not significantly different, averaging 23±1.1 μM for Kv4.2WT (nH=1.1±0.1), 12.1±2.6 μM for N-del (nH=0.6±0.5), 14.5±1.9 μM for V[402,404]I (nH=0.7±0.5), and 11.1±1.0 μM for N-del/V[402,404]I (nH=1.4±0.08), respectively. For quinidine, the IC50 values averaged 12.2±2.2 μM for Kv4.2WT (nH=0.5±0.3), 12.3±1.5 μM for N-del (nH=0.95±0.4), 19.1±1.2 μM for V[402,404]I (nH=1.3±0.3), and 33.4±1.5 μM for N-del/V[402,404]I channels (nH=1.2±0.5) (P=NS for IC50 and nH among constructs). Overall, the results suggest that the potency of block by flecainide and quinidine was not significantly different at +50 mV among the various constructs studied.
Figure 3.
Concentration-dependent effects of flecainide and quinidine on Kv4.2 currents upon pulsing to +50 mV. Dose–response curves for flecainide (a) and quinidine (b) were fitted to the Hill equation to obtain the IC50 values in Kv4.2WT, N-del, V[402,404]I and N-del/V[402,404]I channels. Each point represents the mean ± s.e.m. of three to 10 experiments.
Voltage-dependent effects of flecainide and quinidine on Kv4.2 currents
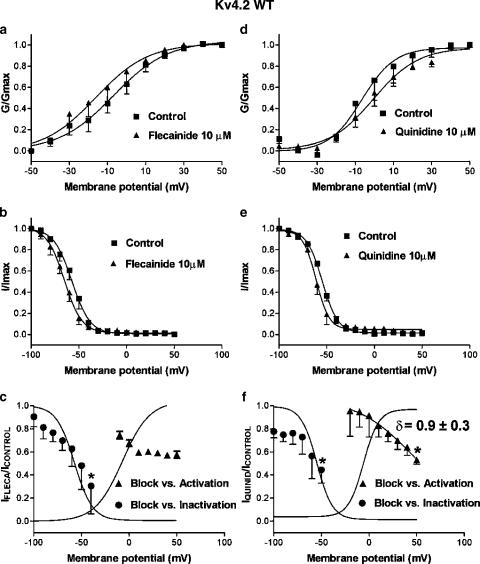
Flecainide (10 μM) significantly inhibited Kv4.2WT current at all potentials positive to −10 mV. Figure 4a shows the normalized conductance–voltage curves of Kv4.2WT channels in the absence and the presence of flecainide, with conductance (G) at each voltage normalized to the maximum conductance (Gmax) in each experiment. Boltzmann fitting yielded a mean midpoint (V1/2) of −6.9±5.7 mV, n=4 and slope factor (k) of 12.1±1.5 mV. Flecainide did not modify k (11.6±1.8 mV, n=4, P=NS) or V1/2 (−13.1±7.7 mV, P=NS). In Figure 4b, normalized-inactivation curves in the absence and presence of flecainide are shown. The current amplitude elicited by 500 ms test pulses to +60 mV was plotted as a function of conditioning pulse voltage and normalized to maximum current amplitude (Imax). In control, inactivation curves had V1/2 and k values of −57.1±3.4 and 8.4±1.5 mV, respectively, while in the presence of flecainide, these values were −66.0±4.0 and 8.9±1.4 mV (n=4, P=NS).
Figure 4.
Effects of flecainide (10 μM) (a–c) and quinidine (d–f) on Kv4.2WT. (a) Effects of flecainide on voltage dependence of channel activation. Conductance (G) was normalized to the maximal conductance (Gmax) and plotted against membrane potential. (b) Effects of flecainide on the voltage dependence of channel inactivation. Current amplitude was normalized to the maximum amplitude in each experiment (I/Imax) and plotted as a function of the voltage command of the prepulse. (c) Relations between block and the voltage dependence of channel inactivation and activation. Circles: flecainide-induced block of currents obtained with pulses to +60 mV after prepulses to potentials ranging between −100 and −40 mV as a function of conditioning-pulse voltage. *P<0.05 vs block after prepulses to −100 mV. Triangles: block of currents elicited with test pulses to voltages from −100 to +50 mV as a function of test-pulse voltage. Curves are best-fit Boltzmann relations to control activation and inactivation curves. Results are mean±s.e. of four experiments. (d) Conductance–voltage and (e) inactivation data obtained in the absence and presence of quinidine. Curves in (a), (b), (d) and (e) are best-fit Boltzmann relations. (f) Quinidine-induced blockade related to channel inactivation and activation. *P<0.05 vs blockade at −100 and −20 mV for inactivation and activation, respectively. Curves are Boltzmann fits of control activation and inactivation curves. Results are mean±s.e. from four experiments.
Figure 4c shows the relations between block and the voltage dependence of inactivation and activation of Kv4.2WT channels. To relate the voltage dependence of drug-induced block to the voltage dependence of Kv4.2WT inactivation, the ratio between the current amplitude obtained with pulses to +60 mV after prepulses from −100 to −40 mV in the presence vs absence of flecainide was plotted as a function of the voltage of the conditioning pulse. The blocking effect of flecainide increased at more positive prepulse potentials (9.6±8.7% at −100 mV vs 69.6±24.1% at −40 mV, n=4, P<0.05), that is, blockade became more pronounced as the fraction of inactivated channels increased. To relate the voltage dependence of block to Kv4.2WT channel activation, the ratio between the current amplitude during 500 ms pulses in the presence vs the absence of flecainide was plotted as a function of the test potential. The blocking effects of flecainide were apparent at potentials positive to −10 mV, increasing slightly but not significantly to 40.4±2.1% at +10 mV and did not change further at more positive potentials (42.6±3.1% at +50 mV, n=4).
Quinidine significantly inhibited the current at potentials positive to −20 mV, did not modify the activation voltage (V1/2=−1.0±1.8 vs −3.8±4.7 mV, n=4, P=NS), but increased the k value for activation from 10.9±4.0 to 14.4±1.2 mV (P<0.05) (Figure 4d). Quinidine did not modify k (7.2±1.4 vs 7.5±0.6 mV) or V1/2 (−60.4±2.6 vs −54.5±1.1 mV, n=4, P=NS) of inactivation (Figure 4e). In contrast to flecainide, quinidine-induced block did not change in the presence of prepulses to potentials between −100 and −70 mV. However, the blockade increased significantly at more positive preconditioning potentials, reaching 55.5±11.5% at −50 mV (n=4, P<0.05 vs blockade at −100 mV). When quinidine's blocking effects during test pulses to different voltages were related to the voltage dependence of activation (Figure 4f) blockade was small at −20 mV (4.6±21.9%, n=4) and steeply increased as the voltage of the pulse became more positive, even for voltages at which channel opening reached saturation (between +20 and +50 mV, e.g. 47.1±3.7% at +50 mV, n=4, P<0.05). Applying the Woodhull equation to results at voltages with maximal activation (⩾10 mV), a fractional electrical distance (δ) of 0.9 was obtained for the quinidine binding site.
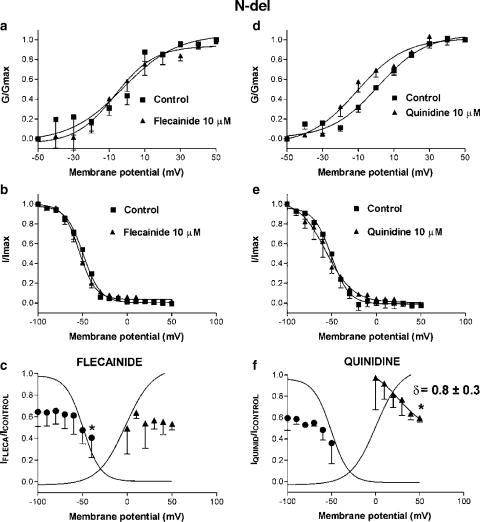
Figure 5 illustrates the voltage-dependent effects of flecainide and quinidine on N-del channels. The N-terminal deletion did not modify the voltage dependence of activation or inactivation as compared to Kv4.2WT (Table 1). Figures 5a and d show the activation curves obtained in control conditions and in the presence of flecainide and quinidine, respectively. Flecainide did not modify the voltage dependence of activation (V1/2=−1.6±5.2 vs −3.7±3.3 mV and k=15.1±7.4 vs 15.4±7.5, n=4, P=NS), whereas quinidine did not modify k (10.4±1.8 vs 13.7±1.7 mV, n=4, P=NS), but shifted V1/2 in the negative direction (−11.4±1.9 vs 0.7±1.7, P<0.05). Figures 5b and e show that, for this construct, neither flecainide (V1/2=−54.5±1.3 vs −50.5±4.4 mV, n=4, P=NS) nor quinidine (V1/2=−54.8±3.7 vs −49.2±3.0 mV, n=4, P=NS) modified the voltage dependence of inactivation. As for Kv4.2WT, flecainide-induced block did not significantly change in the range of channel activation (Figure 5c). Flecainide-induced block increased as the prepulse became more positive (e.g. 59.5±18.1% at −40 mV, n=4, P<0.05 vs 35.5±13.6% blockade at −100 mV). The voltage dependence of quinidine-induced block was not modified by the deletion of the N-terminus (Figure 5f). Blockade was more pronounced as the amount of activated (2.8±29.8% at 0 mV vs 40.4±3.7% at +50 mV, n=4) and inactivated (40.5±11.2% with prepulses to −100 mV vs 64.0±19.1% with prepulses to −50 mV) channels increased. The fractional electrical distance was not altered.
Figure 5.
Effects of flecainide (10 μM) and quinidine (10 μM) on voltage-dependent N-del current activation (a, d) and inactivation (b, e). (c, f) Flecainide- and quinidine-induced blockade related to channel inactivation and activation. *P<0.05 vs blockade at −100 mV for inactivation and 0 mV for activation. Curves in (c) and (f) are Boltzmann fits of control activation and inactivation. Results are mean±s.e. of four experiments.
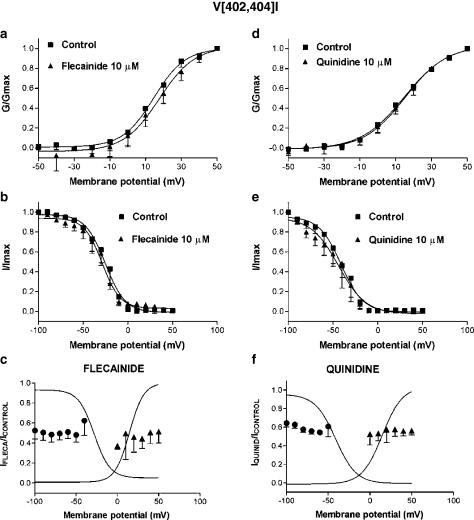
Figure 6 summarizes the voltage dependence of flecainide and quinidine effects on V[402,404]I. This mutation shifted the V1/2 of the activation curve in the positive direction (Table 1). Neither flecainide (V1/2=14.3±5.9 vs 13.3±5.6 mV, n=4, P=NS) nor quinidine (V1/2=15.2±2.9 vs 13.9±2.9 mV, n=4, P=NS) modified the voltage dependence of activation (Figures 6a and d). As previously described (Jerng et al., 1999; Pourrier et al., 2000), substitution of the valine residues located in positions 402 and 404 by isoleucines shifted the inactivation curve to more positive potentials (Table 1). Neither flecainide (V1/2=−36.6±8.1 vs −35.8±8.5 mV, n=4, P=NS) nor quinidine (V1/2=−47.0±9.7 vs −40.0±7.5 mV, n=4, P=NS) modified the voltage dependence of inactivation. In contrast to Kv4.2WT, drug-induced block of V[402,404]I showed no significant voltage dependence (Figures 6c and f). Thus, the blocking effects of both drugs were similar when prepulses to −100 mV (47.5±8.4 and 35.6±4.1% for flecainide and quinidine, respectively) or to −50 mV were applied (51.9±7.2 and 39.2±11.2%, n=4, P=NS). Similarly, the same amount of block was obtained when pulses to 0 mV (62.7±3.4 and 47.6±11.6% for flecainide and quinidine, respectively) and to +50 mV (48.5±11.6 and 43.7±4.7, n=4, P=NS) were applied.
Figure 6.
Effects of flecainide (10 μM) and quinidine (10 μM) on voltage-dependent V[402,404]I current activation (a and d) and inactivation (b, e). (c, f) Flecainide- and quinidine-induced blockade related to channel inactivation and activation. The curves are Boltzmann fits of control activation and inactivation curves. Results are mean±s.e. of four experiments.
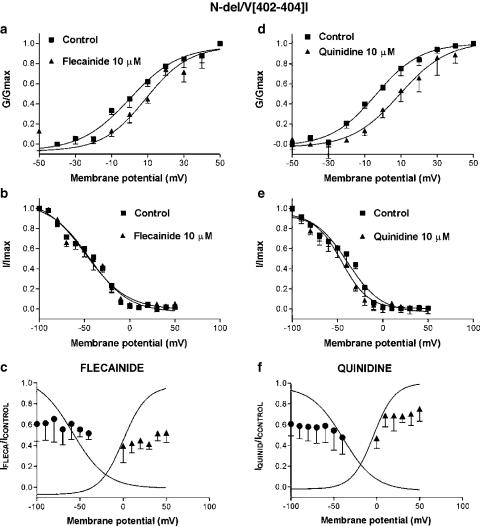
Figure 7 shows the effects of flecainide and quinidine on N-del/V[402,404]I. For this construct, the V1/2 of the activation curve was not significantly altered (Table 1). Both drugs shifted the activation V1/2 to slightly more positive potentials (by 4.3±3.5 mV for flecainide, n=4, P=NS and 16.2±3.3 mV for quinidine, n=4, P<0.05, Figures 7a and d), without modifying k (9.8±0.8 vs 10.2±1.8 mV for flecainide and 15.6±4.1 vs 12.3±0.7 mV for quinidine, P=NS). The inactivation voltage dependence of N-del/V[402,404]I was shifted positively (Table 1). Neither flecainide nor quinidine modified the voltage dependence of channel inactivation (Figures 7b and e).
Figure 7.
Effects of flecainide (10 μM) and quinidine (10 μM) on voltage-dependent N-del/V[402,404]I current activation (a, d) and inactivation (b, e). (c, f) Flecainide- and quinidine-induced blockade related to channel inactivation and activation. The curves are Boltzmann fits of control activation and inactivation curves. Results are mean±s.e. of four experiments.
As for V[402,404]I, drug-induced blockade did not increase as the fraction of inactivated channels increased (Figures 7c and f). Thus, flecainide inhibited the current by 39.2±16.7 and 44.6±7.0% at −100 and −50 mV, respectively (n=4, P=NS) and quinidine by 39.3±11.4 and 52.3±16.0%, respectively (n=4, P=NS). Similarly, there was no statistically significant change in block with either flecainide or quinidine over the activation range of the current.
Discussion
In this study, we found that: (1) flecainide and quinidine inhibit Kv4.2 currents in a concentration- and voltage-dependent manner, but with significant differences between their voltage-dependent blocking properties, (2) deletion of the N-terminus does not modify qualitatively any of the drug effects and (3) valine to isoleucine mutations at positions 402 and 404 eliminate voltage dependence of flecainide and quinidine block.
Comparison with previous studies of the effects of quinidine and flecainide on Ito-like currents
We found that flecainide and quinidine inhibit Kv4.2WT currents at +50 mV with IC50's of 23.6 and 12 μM, respectively. These values are consistent with previous reports in native cells and heterologous expression systems. In rat ventricular myocytes, flecainide and quinidine inhibit Ito with IC50's of 3.7 and 3.9 μM, respectively (Slawsky & Castle, 1994). In human atrial myocytes, 10 μM flecainide and quinidine inhibit Ito by 40 and 60%, respectively (Wang et al. 1995), while in Ltk− cells they inhibit Kv4.2 currents by ∼50% (Yeola & Snyders, 1997). Quinidine consistently accelerates inactivation (Slawsky & Castle, 1994; Wang et al., 1995; Yeola & Snyders, 1997), and block increases over the activation voltage range (Wang et al., 1995) but is not increased by depolarizing prepulses (Wang et al., 1995), suggesting open-state block. The state dependence of flecainide-induced block of Itof has been more controversial. Flecainide acceleration of inactivation suggests open-channel block (Slawsky & Castle, 1994). However, in human atrium, flecainide block is enhanced by depolarizing prepulses, does not show use dependence and does not increase with channel opening, suggesting a preferential inactivated-state drug interaction (Wang et al., 1995).
In our study quinidine, but not flecainide, accelerated the inactivation kinetics of Kv4.2WT channels, suggesting preferential open-channel block for quinidine but not flecainide. Quinidine, but not flecainide, block increased over the activation voltage range, again consistent with open-state block for quinidine only. At more positive potentials, when the channels were fully open, only quinidine-induced block increased significantly. For both flecainide and quinidine, the intensity of block increased with stronger depolarizing prepulses, compatible with inactivated-state block.
Novel aspects and potential significance
Antiarrhythmic drugs inhibit Ito and this action is believed to contribute to their antiarrhythmic properties (Imaizumi & Giles, 1987; Duan et al., 1992; Slawsky & Castle, 1994; Wang et al., 1995; Feng et al., 1997; Nattel, 1999; Sánchez-Chapula, 1999). They are also well known to produce time- and voltage-dependent Ito block (Imaizumi & Giles, 1987; Slawsky & Castle, 1994; Wang et al., 1995; Sánchez-Chapula, 1999). Voltage-dependent block is generally interpreted as indicating state-dependent action (Snyders et al., 1992; Wang et al., 1995). Block that increases with depolarizing prepulses over the inactivation voltage range is often interpreted as reflecting preferential inactivated-state interaction (Wang et al., 1995), whereas block that increases over the voltage range of activation is generally interpreted as open-channel block (Snyders et al., 1992; Wang et al., 1995).
The present study is, to our knowledge, the first detailed study of quinidine and flecainide block of Kv4.2 currents. In addition, it is the first of which we are aware to report on structural factors affecting voltage-dependent Kv4.2 block. Although both mutations involving V[402,404]I slowed inactivation and affected the voltage-dependence of inactivation, both processes remained qualitatively intact. N-del/V[402,404]I slowed inactivation much more than V[402,404]I and V[402,404]I produced a depolarizing shift of activation voltage dependence, which was not altered by N-del/V[402,404]I. In addition, neither mutation significantly altered drug effects on the voltage dependence of activation or inactivation or on inactivation kinetics. Nevertheless, the very clear voltage dependencies observed when positions 402 and 404 were occupied by valines were lost upon mutation to isoleucine, suggesting that voltage-dependent behaviour is not because of preferential drug binding to open or inactivated channel states per se, but to a voltage-dependent interaction involving the terminal S6 valine moieties. The 402 and 404 valines may play a critical role in determining the response of the drug–receptor conformation to changing voltage fields. It has been previously described that the replacement of the Kv1.4 sequence in the cytoplasmic half of S6 with that of Kv4.2 converted the open-state 4AP block to rested-state block (Tseng, 1999), pointing to a key role of this region in the drug–channel interaction. We have observed that the V[402,404]I mutation alone is sufficient to produce this behaviour (Caballero et al., 2001), consistent with a role of S6 valines in determining the voltage dependence of the conformation of the drug binding site.
Kv4 channel inactivation induced by prepulses at negative potentials may be mechanistically different from inactivation at positive potentials upon depolarization (Jerng et al., 1999). Kv4.2 channels can inactivate from the open state at strongly depolarized voltages or directly from closed states at more negative potentials (Bähring et al., 2001). We therefore cannot rule out the possibility that the S6 valine mutations, which shift the inactivation curve in the positive direction, increase closed-state inactivation and thereby alter drug-induced block, particularly upon depolarizing prepulses. The effect of V402 and 404 on prepulse-related voltage-dependent drug-induced block does not necessarily imply that V402 and 404 play a role in drug interactions during open-inactivated state transitions. Indeed, the effects of quinidine on inactivation rate at +50 mV were unaltered by the V[402,404]I mutations.
Quinidine's block increased with more positive test pulses beyond the voltage range for full activation (Figures 4f and 5f). Such behaviour has generally been interpreted as indicating a blocking site in the voltage field, allowing for an estimation of the dielectric distance of the presumed binding site (Snyders et al., 1992; Wang et al., 1995; Yeola et al., 1996). We used the Woodhull formalism to calculate the electrical distance of the quinidine blocking site in Kv4.2WT and N-del, and estimated these are at a site exposed to ≈90% of the transmembrane voltage gradient (considering that drug access is from the inner mouth of the pore, Figure 4f and Figure 6f). The voltage-dependent behaviour was eliminated by the V[402,404]I mutations suggesting either that a dramatic change in the blocking site caused by the mutation (altered binding site) or that the voltage-dependent behaviour of quinidine block at positive potentials is not due to a voltage effect on ionized drug molecules per se but due to a voltage-dependent rearrangement of the drug binding site requiring the presence of the S6 valines (effects on access to or within the binding site). Further studies are clearly needed to clarify the mechanisms underlying voltage-dependent drug block of Kv4.2.
Acknowledgments
The Kv4.2 clone was kindly provided by Dr Jeanne Nerbonne. We thank Chantal St Cyr for excellent technical assistance and Diane Campeau for fine secretarial work. This work was supported by the Canadian Institutes of Health Research and by the Quebec Heart and Stroke Foundation. Ricardo Caballero was a fellow of the Spanish Ministery of Education (SAF96/ 0042).
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulphoxide
- G
conductance
- GTP
guanosine triphosphate
- Ip
current amplitude
- k
the slope factor of the activation/inactivation curve
- Kv4.2WT
wild-type Kv4.2 channels
- N-del
Kv4.2 channels with a deletion in the N-terminus
- N-del/V[402,404]I
channels with the N-terminal deletion and the valine mutations
- nH
Hill coefficient
- τfast
time constant of the fast phase of inactivation
- τslow
time constant of the slow phase of inactivation
- V[402,404]I
channels with valines at positions 402 and 404 mutated to isoleucines
- V1/2
the midpoint of the activation/inactivation curve
- Vc
voltage command
- Vr
reversal potential of the current
References
- BÄHRING R., BOLAND L.M., VARGHESE A., GEBAUER M., PONGS O. Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels. J. Physiol. 2001;535:65–81. doi: 10.1111/j.1469-7793.2001.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY D.M., TRIMMER J.S., MERLIE J.P., NERBONNE J.M. Differential expression of voltage-gated K+ channel subunits in adult rat heart. Relation to functional K+ channels. Circ. Res. 1995;77:361–369. doi: 10.1161/01.res.77.2.361. [DOI] [PubMed] [Google Scholar]
- CABALLERO R., POURRIER M., SCHRAM G., DELPON E., TAMARGO J., NATTEL S. A critical role for S6 valines in the pharmacological properties of transient outward current Ito carried by Kv4.2 subunits. Circulation. 2001;104 Suppl. II:217. [Google Scholar]
- DIXON J.E., MCKINNON D. Quantitative analysis of mRNA expression in atrial and ventricular muscle of rats. Circ. Res. 1994;75:252–260. doi: 10.1161/01.res.75.2.252. [DOI] [PubMed] [Google Scholar]
- DUAN D., FERMINI B., NATTEL S. Potassium channel blocking properties of propafenone in rabbit atrial myocytes. J. Pharmacol. Exp. Ther. 1992;264:1113–1123. [PubMed] [Google Scholar]
- FENG J., WANG Z., LI G.R., NATTEL S. Effects of class III antiarrhythmic drugs on transient outward and ultra-rapid delayed rectifier currents in human atrial myocytes. J. Pharmacol. Exp. Ther. 1997;281:384–392. [PubMed] [Google Scholar]
- IMAIZUMI Y., GILES W.R. Quinidine-induced inhibition of transient outward current in cardiac muscle. Am. J. Physiol. 1987;253:H704–H708. doi: 10.1152/ajpheart.1987.253.3.H704. [DOI] [PubMed] [Google Scholar]
- JERNG H.H., COVARRUBIAS M. K+ channel inactivation mediated by the concerted action of the cytoplasmic N- and C-terminal domains. Biophys. J. 1997;72:163–174. doi: 10.1016/S0006-3495(97)78655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERNG H.H., SHAHIDULLAH M., COVARRUBIAS M. Inactivation gating of Kv4 potassium channels. Molecular interactions involving the inner vestibule of the pore. J. Gen. Physiol. 1999;113:641–659. doi: 10.1085/jgp.113.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURMAN M.E., BOLAND L.M., LIU Y., YELLEN G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- NATTEL S. The molecular and ionic specificity of antiarrhythmic drug actions. J. Cardiovasc. Electrophysiol. 1999;10:272–282. doi: 10.1111/j.1540-8167.1999.tb00673.x. [DOI] [PubMed] [Google Scholar]
- NERBONNE J.M. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J. Physiol. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POURRIER M., CABALLERO R., SCHRAM G., WANG Z., NATTEL S.Molecular correlates of rapid inactivation of Kv4.2 potassium channels Pflugers Arch. Eur. J. Physiol. 2000(submitted)
- RASMUSSON R.L., MORALES M.J., WANG S., LIU S., CAMPBELL D.L., BRAHMAJOTHI M.V., STRAUSS H.C. Inactivation of voltage-gated cardiac K+ channels. Circ. Res. 1998;82:739–750. doi: 10.1161/01.res.82.7.739. [DOI] [PubMed] [Google Scholar]
- ROLF S., HAVERKAMP W., BORGGREFE M., MUHOFF U., ECKARDT L., MERGENTHALER J., SNYDERS D.J., PONGS O., SPECKMANN E.J., BREITHARDT G., MADEJA M. Effects of antiarrhythmic drugs on cloned cardiac voltage-gated potassium channels expressed in Xenopus oocytes. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:22–31. doi: 10.1007/s002100000257. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ-CHAPULA J.A. Mechanism of transient outward K+ channel block by disopyramide. J. Pharmacol. Exp. Ther. 1999;290:515–523. [PubMed] [Google Scholar]
- SLAWSKY M.T., CASTLE N.A. K+ channel blocking actions of flecainide compared with those of propafenone and quinidine in adult rat ventricular myocytes. J. Pharmacol. Exp. Ther. 1994;269:66–74. [PubMed] [Google Scholar]
- SNYDERS D.J., KNOTH K.M., ROBERDS S.L., TAMKUN M.M. Time–voltage- and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol. Pharmacol. 1992;41:322–330. [PubMed] [Google Scholar]
- TSENG G.N. Different state dependencies of 4-aminopyridine binding to rKv1.4 and rKv4.2: role of the cytoplasmic halves of the fifth and sixth transmembrane segments. J. Pharmacol. Exp. Ther. 1999;290:569–577. [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J. Pharmacol. Exp. Ther. 1995;272:184–196. [PubMed] [Google Scholar]
- YEOLA S.W., RICH T., UEBELE V., TAMKUN M.M., SNYDERS D.J. Molecular analysis of a binding site for quinidine in a human cardiac delayed rectifier K+ channel. Role of S6 in antiarrhythmic drug binding. Circ. Res. 1996;78:1105–1114. doi: 10.1161/01.res.78.6.1105. [DOI] [PubMed] [Google Scholar]
- YEOLA S.W., SNYDERS D.J. Electrophysiological and pharmacological correspondence between Kv4.2 current and rat cardiac transient outward current. Cardiovasc. Res. 1997;33:540–547. doi: 10.1016/s0008-6363(96)00221-0. [DOI] [PubMed] [Google Scholar]
- ZHU X.R., WULF A., SCHWARZ M., ISBRANDT D., PONGS O. Characterization of human Kv4.2 mediating a rapidly-inactivating transient voltage-sensitive K+ current. Receptors Channels. 1999;6:387–400. [PubMed] [Google Scholar]