Abstract
In all, 27 compounds related chemically to phenanthrolines were investigated for their ability to stimulate electrogenic chloride secretion in murine colonic epithelia under short circuit current conditions. A total of 11 compounds, not previously examined, were shown to have substantial activity. Cumulative concentration – response relations were obtained in the presence of amiloride to inhibit electrogenic sodium absorption and Ba2+ to prevent potassium secretion.
Single- or double-ring substitutions in 1,10 phenanthroline (EC50=612 μM) produced only modest increases in affinity of between 0.7- and 2.1-fold.
Naphthalenes with either one or two ring nitrogens showed some activity but had very low affinity, yet some (e.g. isoquinoline, EC50=700 μM) were able to produce maximal responses.
Removal of one nitrogen from 1,10-phenanthroline or from 4,7-phenanthroline gave, respectively, 7,8-benzoquinoline and 5,6-benzoquinoline. These two were the most active compounds found, the EC50 for 5,6-benzoquinoline being 29 μM.
Most concentration – response curves were steep with Hill slopes of approximately 3. At high concentration, some compounds inhibited the response, producing a characteristic bell shape.
5,6- and 7,8-benzoquinoline may be useful starting points for the synthesis of more potent agents by the addition of ring substituents.
Keywords: CFTR (cystic fibrosis transmembrane conductance regulator); cystic fibrosis; chaperone; receptor trafficking 5,6-benzoquinoline
Introduction
This paper reports a search for agents that increase epithelial electrogenic chloride secretion. A total of 27 compounds, not previously examined, have been applied to murine colonic epithelia under short circuit current conditions, of which 11 proved to have substantial activity. In an earlier study, the effects of 1,10-phenanthroline were examined in detail using the same bioassay system (Duszyk et al., 2001). The choice of 1,10-phenanthroline for the earlier study followed from a suggestion that inhibition of the matrix metalloproteinase MMP-2 activates a chloride conductance (Duszyk et al., 1999). However, other phenanthrolines that did not inhibit MMP-2, such as 1,7- and 4,7-phenanthroline also stimulated chloride secretion. It was shown that 1,10-phenanthroline acted via coordinate actions on both an apical chloride conductance plus a basolateral potassium conductance. As cystic fibrosis transmembrane conductance regulator (CFTR) is agreed to be the only chloride conductance relevant to chloride secretion in the apical membrane of the mouse colon (Grubb & Boucher, 1999) it must be involved in the response to 1,10-phenanthroline. It was not shown whether the effect of the phenanthroline was direct or indirect upon CFTR. At present there is intense interest in compounds that activate CFTR since these may be useful in the treatment of cystic fibrosis (CF). The most common mutation in this disease is ΔF508 (70% of all CF alleles, Jentsch & Gunter, 1997). The protein ΔF508 CFTR is not normally trafficked to the cell membrane and is destroyed in the proteasome. Yet, if it can be coaxed into the membrane, for example by lowering the temperature (Denning et al., 1992), it acts as a chloride conductant, albeit with a shorter open time than wild-type CFTR (Drumm et al., 1991). Therefore, what is required is a pharmacological chaperone that can act in concert with the cellular chaperones, such as HSP70 and HSP90 (Fuller & Cuthbert, 2000), to ensure folding and delivery to the membrane. Therefore, it is logical to argue that agents that interact with the CFTR might act in this way. There is precedence for this view, for example, small nonpeptides that activate the vasopressin V2 receptor are able to rescue the mutant receptor, not normally trafficked, and assist its passage into the cell membrane (Morello et al., 2000). Similarly, Loo & Clarke (1997) have shown that inhibitors or substrates of P-glycoprotein, the multiple drug resistance (MDR) protein, are able to rescue the mutant form and promote normal trafficking to the membrane. The purpose here is to explore the type of chemical nucleus that is worthy of further study with a view of producing high-affinity ligands for CFTR. It is shown that the benzoquinoline nucleus is superior to the phenanthroline nucleus as a starting point for further chemical modification.
Methods
All experiments have been conducted on the isolated colonic epithelium from the mouse, used under voltage clamp conditions to measure short circuit current. Mice were killed by a slowly rising concentration of CO2 with the distal colon placed in Krebs Henseleit Solution (KHS). The colon was opened by a longitudinal incision, washed and pinned out on a wax block so that the muscle layers and some underlying tissue could be peeled away, leaving the mucosa. The epithelia were mounted in Ussing chambers with a window area of 20 mm2 and bathed on both sides with 20 ml KHS at 37°C and gassed with 95% O2/5% CO2. Up to four preparations were obtained from one colon. Transepithelial potential was monitored via two KHS-filled electrodes with their ends close to the tissue and led, via calomel cells to the input stage of a WPI DVC-1000 voltage clamp (Sarosota, FL, U.S.A.). The tissues were voltage clamped at zero potential (i.e. short circuited), the current passing via AgCl/KCl electrodes into the chambers at points distant from the tissue. Fluid resistance compensation was used throughout. The output from the voltage clamp was recorded using an ADInstruments PowerLab/8SP (NSW 2154, Australia) and displayed on a computer as short circuit current.
A standard protocol used in all experiments was as follows. Amiloride, 100 μM and Ba2+, 5 mM were added to the apical bathing solution in all experiments. This ensured that there was no electrogenic sodium absorption and that electrogenic potassium secretion was abolished or reduced to a minimum. All the compounds investigated in this study were added to both the apical and basolateral bathing solution. Most are very lipid soluble, so that addition to one face of the epithelium only would produce an undesirable and changing gradient during drug action. All compounds were dissolved in either alcohol or dimethyl sulphoxide (DMSO). From previous work, it was known that the maximal amount of alcohol used had no effect on the epithelium, but a study was made with DMSO. DMSO was added cumulatively up to 0.2% vv−1, greater than the maximal concentration used with the compounds. The maximal current change at 0.2% DMSO was −3.6±1.8 μA cm−2 (n=4) and in the presence of this concentration of DMSO forskolin, 10 μM was able to produce a current change of 219.9±30.8 μA cm−2 (n=4). The effect of DMSO on basal current was trivial and the responses to forskolin were in the normal range. Therefore, solvent effects do not appear to be important in this study.
Throughout, compounds have been added cumulatively and the peak short circuit current at each concentration noted. At the end of many experiments, furosemide (1 mM), was added to the basolateral bathing fluid to inhibit electrogenic chloride secretion. The compounds used in this study (5-nitro,5-chloro,5-methyl,4,7-dimethyl, 5,6-dimethyl 1,10 phenanthroline,7,8-, 5,6- and 3,4-benzoquinoline, cinnoline, benzo[c]cinnoline, cinnoline-4-carboxylate, quinoline, isoquinoline, quinazoline, quinoxaline, 2-phenylquinoline, 3-,5-and 6-aminoquinoline were all obtained from Sigma-Aldrich Chemie GmbH, Germany. Other compounds that proved inactive are listed elsewhere and were also obtained from Sigma-Aldrich. Furosemide was from Sigma and forskolin from Calbiochem. The composition of KHS was as follows (mM): NaCl 117, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaHCO3 24.8, KH2PO4 1.2 and glucose 11.1.
Results
Substitutions in 1,10-phenanthroline
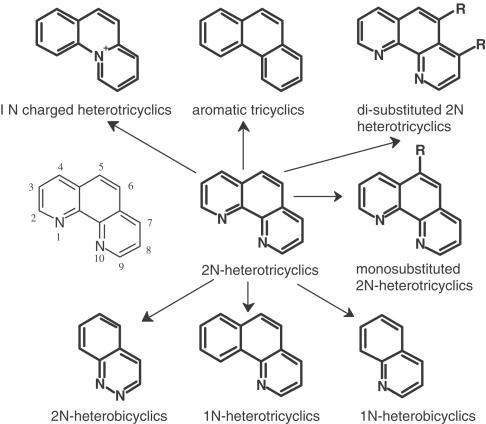
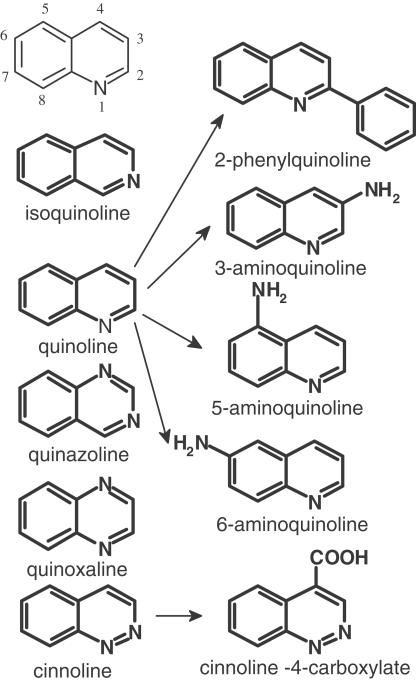
The compound most extensively investigated in the previous study (Duszyk et al., 2001) was 1,10-phenanthroline. Figure 1 shows the main chemical routes used for exploration of structures related to 1,10-phenanthroline, principally by ring substitutions in 1,10-phenanthroline or by removal of heterocyclic nitrogens. Finally, attempts were made to see if activity was retained with ring removal to give heterobicyclic compounds with either one or two nitrogens.
Figure 1.
Illustrates the relation between phenanthrolines (centre, 2N-heterotricyclics) and related compounds, which have been examined for their ability to stimulate chloride secretion in murine colonic epithelia.
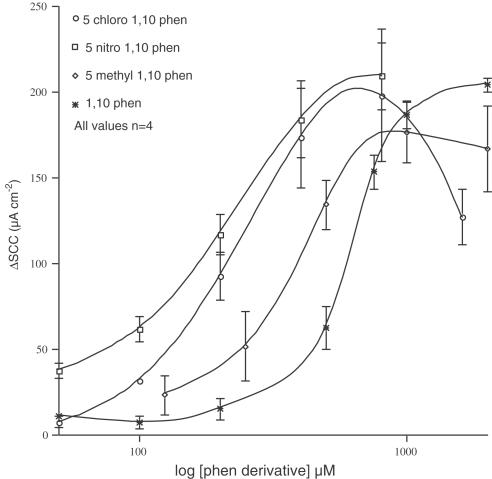
Three mono-substitutions on position 5 were examined, namely 5-nitro, 5-chloro and 5-methyl 1,10-phenanthroline. All the three compounds gave concentration – response curves to the left of that for the parent compound, although the maximal shift was only 2.1-fold (Figure 2). The EC50 values for 5-nitro, 5-chloro and 5-methyl compounds are 213, 209 and 368 μM, respectively, compared to 612 μM for 1,10-phenanthroline.
Figure 2.
Concentration – response curves for 5-chloro, 5-nitro and 5-methyl-1,10 phenanthroline in comparison to the relation for the parent compound, 1,10-phenanthroline, measured as short circuit current in mouse colonic epithelia. In this and all other figures, mean values, in μA cm−2, and standard errors of the mean are illustrated, with the number of observations shown on the figure itself. ΔSCC indicates the change in short circuit current.
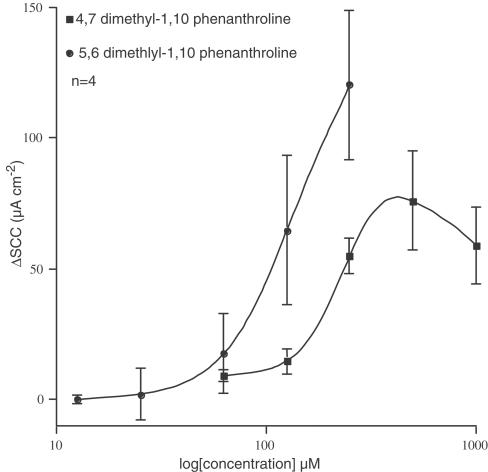
Two di-methyl-substituted 1,10-phenanthrolines were also examined, namely 4,7- and 5,6-dimethyl 1,10-phenanthroline (Figure 3). The 4,7-dimethyl compound proved to be as active as the nitro- and chloro-derivatives, with an EC50 of 195 μM; however, the maximum response was less, and the concentration – response relation showed a bell-shaped form. Solubility limited the upper concentration of 5,6-dimethyl-1,10-phenanthroline that could be used; so the maximal response was undetermined. However, it is clear that the potency of this compound is not sufficiently different from other phenanthrolines to warrant further exploration.
Figure 3.
Concentration – response curves for 4,7-dimethyl-1,10-phenanthroline and a partial curve for 5,6-dimethyl-1,10-phenanthroline. ΔSCC indicates the change in short circuit current caused by the compounds.
Phenanthrolines versus benzoquinolines
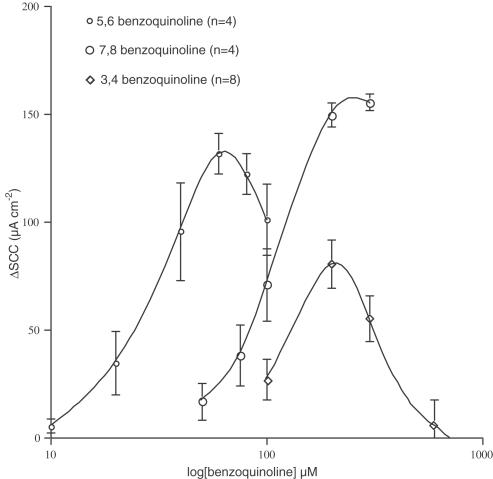
Removal of one nitrogen from phenanthrolines yields benzoquinolines. Removal of either nitrogen from 1,10-phenanthroline gives 7,8-benzoquinoline, while removal of either nitrogen from 4,7-phenanthroline yields 5,6-benzoquinoline. Both 1,10- and 4,7-phenanthroloine have been examined previously and gave EC50 values of 612±10.0 and 201±11.0 μM, respectively (Duszyk et al., 2001). To investigate the effect of nitrogen removal from phenanthrolines, concentration – response curves were obtained for 7,8-, 5,6- and 3,4-benzoquinoline. These are shown in Figure 4.
Figure 4.
Concentration – response curves for 7,8-, 5,6- and 3,4-benzoquinoline. ΔSCC indicates the change in short circuit current.
The apparent EC50 values were 133, 29 and 109 μM for 3,4-, 5,6- and 7,8- benzoquinoline, respectively, the first two more active than the associated phenanthroline compounds. Both 3,4- and 5,6-benzoquinoline showed well-defined bell-shaped curves, as did 4,7-dimethyl-1,10-phenanthroline and 5-chloro-1,10-phenanthroline. The responses to 3,4-benzoquinoline were significantly reversed at higher concentrations so that the maximal response failed to reach 100 μA cm−2. Although the responses to 5,6-benzoquinoline were similarly curtailed, a maximal response of 140 μA cm−2 was reached. The responses to 5,6-benzoquinoline represent a substantial improvement in affinity compared to 1,10-phenanthroline, being 20 times more active.
Heterotricyclics versus heterobicyclics
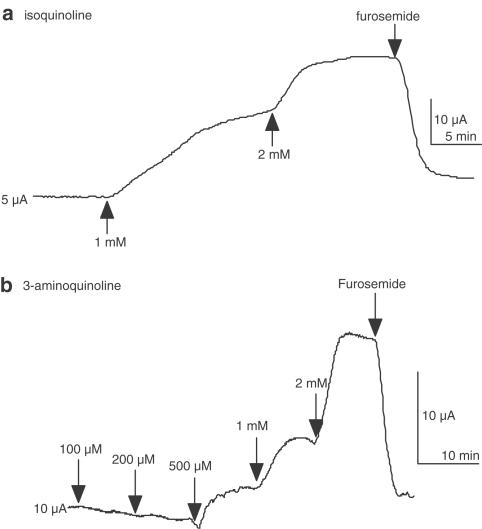
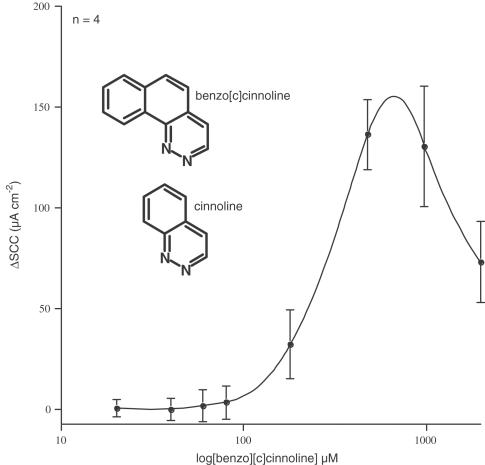
Five types of nitrogen containing heterobicyclics were investigated, represented by isoquinoline, quinoline, quinazoline, quinoxaline and cinnoline, as shown on the left-hand side of Figure 5. Table 1 shows the increase in short circuit current caused by these compounds listed at either 1 or 2 mM concentration. None of these compounds were potent, but isoquinoline and 3-aminoquinoline proved the most active. Figure 6 shows short circuit current responses to isoquinoline and to 3-aminoquinoline. Although high concentrations of these compounds were required to stimulate SCC, it is notable that virtually all the current increase is susceptible to the actions of furosemide, confirming that electrogenic chloride secretion has been stimulated. A complete concentration curve for isoquinoline is given later in Figure 8, indicating an EC50 value of around 700 μM, even less than the parent compound 1,10-phenanthroline. As both quinoline and isoquinoline showed activity it might be expected that cinnoline, a structure combining that of quinoline and isoquinoline, might be more active than either. Cinnoline proved to have very little activity (Table 1) whereas the closely related compound benzo[c] cinnoline had an EC50 of 290 μM (Figure 7). Note that benzo[c]cinnoline shows, in common with other compounds in the study, a bell-shaped concentration – response curve.
Figure 5.
Structures of 1N- and 2N-heterobicyclics and some derivatives tested on mouse colon epithelia for ability to stimulate chloride secretion.
Table 1.
Chloride secretory activity in quinolines
| Compound | ΔSCC (μA cm−2) | [Concentration] (mM) |
|---|---|---|
| Quinoline | 37.3 | 1.0 |
| Isoquinoline | 101.2±3.9 | 1.0 |
| Quinazoline | 20.2±5.6 | 2.0 |
| Quinoxaline | 55.5±6.0 | 2.0 |
| Cinnoline | 11.1 | 1.0 |
| 2−Phenylquinoline | 0 | 1.0 |
| 3−Aminoquinoline | 92.8 | 2.0 |
| 5−Aminoquinoline | 24.9 | 2.0 |
| 6−Aminoquinoline | 45.5 | 2.0 |
| Cinnoline−4−carboxylate | 37.3 | 0.5 |
Figure 6.
Short circuit current recordings from two individual colonic epithelia (each 20 mm2) showing responses to isoquinoline (a) and to 3-aminoquinoline (b). Note that addition of furosemide, 1 mM, to the basolateral bathing solution inhibits the responses.
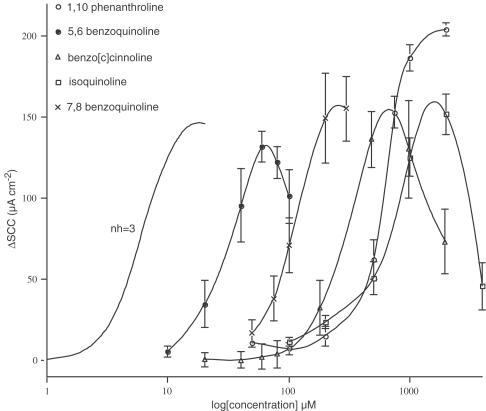
Figure 8.
Concentration – response curves for five agents with contrasting potencies. To the left is a theoretical curve with a Hill slope of 3, approximating to the slope of all the curves, except that to 1,10-phenanthroline that has a slope of 4.9. Note three of the compounds show reduced responses at the higher concentrations. All values are n=4 for the means and standard errors.
Figure 7.
Concentration – response curves to benzo[c]cinnoline showing a bell-shaped form measured as short circuit current responses. Cinnoline was virtually without activity (see Table 1).
Hill slopes and bell-shaped curves
Many of the compounds investigated showed bell-shaped concentration – response curves, which in some instances appear to limit the peak height. This effect can be present with both the most active and least active compounds. In Figure 8, a number of concentration – response curves showing bell-shaped relations are illustrated alongside the curve for 1,10-phenanthroline. The curve for 1,10-phenanthroline has a Hill slope of 4.9 (Duszyk et al., 2001), while the other compounds shown in Figure 8 gave relation that were less steep. Curve fitting according to any drug–receptor interaction scheme has not been attempted (see discussion), but the theoretical curve included in the figure indicates that the experimental slopes correspond to a Hill slope of around 3.
Other compounds
In all, 10 other compounds, not previously mentioned, were also tested for activity on the mouse colon. With two exceptions, they were either inactive or had only minor activity and were not explored further. They were 2-bromo-6-methyl phenanthridine, phenanthridinone, 2-phenyl-benzo[H] quinoline, 6-aminochrysene, 2′,2′ dipyridyl, 9-aminophenanthrene, phthalazine and 4-phenyl-pyrimidine. The two exceptions were 1-aminofluorene and 1-benzo[F] quinoline-2-yl-ethanone, the latter was particularly active but solubility limited the exploration of its maximal capability.
Discussion
The main purpose of this study was to investigate structure–activity relations in a series of chloride secretagogues chemically related to 1,10-phenanthroline. The parent compound 1,10-phenanthroline, while effective, has an EC50 of 600 μM and compounds with greater potency are desirable as starting points for further chemical modification.
The object has been achieved since 5,6-benzoquinoline shows a 20-fold improvement in affinity compared to 1,10-phenanthroline. Both 5,6-benzoquinoline (EC50=29 μM) and 7,8-benzoquinoline (EC50=109 μM) are ‘naked' compounds and the consequences of substitution to enhance activity further are yet to be explored.
First, a comment should be made about the test system used to assay the agents described here. The presence of amiloride in the apical bathing solution means that stimulation of electrogenic sodium absorption is not possible, which otherwise might be confused with electrogenic chloride secretion. Finally, the sensitivity of the responses to all the compounds tested to furosemide is confirmation that SCC is a measure of chloride secretion. Sustained chloride secretion, as produced by these agents, requires coordinate action at both the apical and basolateral membranes of epithelial cells. Thus, both activation of an apical chloride conductance and hyperpolarisation of the basolateral membrane are necessary, the latter to maintain an appropriate electrochemical gradient for chloride transport (Devor et al., 1996a). For example, mouse colonic epithelia from cystic fibrosis mice fail to respond to 1,10-phenanthroline because of the lack of CFTR (Duszyk et al., 2001). It is generally agreed that CFTR is the only effective chloride conductance in the apical membrane of murine colonic epithelium (Grubb & Boucher, 1999). We showed for 1,10-phenanthroline that basolateral K+-channels as well as apical chloride conductance were activated. Although detailed studies on the present series of compounds have not been carried out, their ability to maintain chloride secretion suggests they too have dual actions. Indeed, this has been demonstrated for 7,8-benzoquinoline, using a different epithelial test system, Calu-3 epithelia (data unpublished). The characteristics of 1,10-phenanthroline actions are similar to those of the benzimidazolone, 1-ethyl-2-benzimidazolinone (EBIO) (Devor et al. 1996a, 1996b; Cuthbert et al., 1999; Duszyk et al., 2001) causing both activation of basolateral K+-conductances and an apical Cl−conductance. In a recent study, Singh et al. (2001) examined 30 benzimidazolone derivatives and showed that 5,6-dichloro EBIO had an EC50 value of 25 μM for chloride secretion in T84 monolayers, representing a 24-fold increase in potency on EBIO. Thus, 5,6-benzoquinoline has a potency comparable to 5,6-dichloro EBIO and, as pointed out earlier, is a ‘naked' unsubstituted molecule.
The variety of structures that have been shown here to support electrogenic chloride secretion in mouse colon epithelium has been informative. Most importantly, the dominance of the phenanthrene nucleus is striking. It was known from the earlier study that nitrogen substitution in the phenanthrene rings was important, since phenanthrene itself was inactive (Duszyk et al., 2001). Further, the presence of a quaternary nitrogen did not confer activity on the phenanthrene nucleus (Figure 1, see also Duszyk et al., 2001), which was surprising in view of the activity present in a number of benzo[c]quinolinizinium compounds. These compounds are of great interest since they appear to cause trafficking of CFTR, as well as channel activation, an important characteristic for a pharmacological chaperone for CFTR. However, while the benzo[c]quinolinizinium compounds are interesting, their potency is low as stimulants of epithelial chloride secretion (Becq et al., 1999; Dormer et al., 2001). Either mono- or di-substitutions onto 1,10-phenanthroline led to modest increases in potency, but insufficient to suggest this was a valuable approach. Both mono- and di-nitrogen-substituted naphthalenes showed some activity but with very low potency and generally limited solubility prevented the estimation of EC50 values. A good example to illustrate the difference between nitrogen-substituted phenanthrenes and naphthalenes was provided by cinnoline and benzo[c]cinnoline, the former being virtually inactive.
In the original study (Duszyk et al., 2001), both 1,10- and 4,7-phenanthroline were shown to be active. Removal of either nitrogen from either of these molecules gives the two benzoquinolines, 5,6- and 7,8-benzoquinoline, of which 5,6-benzoquinoline was the most potent. 7,8- and 5,6-benzoquinoline are the focus for further efforts to increase potency by ring substitution. Both have shown potencies identical to those in the mouse colon on two other epithelial tissues of human origin, namely colonic biopsy material and Calu-3 cell monolayers (data unpublished). The pKa values for 5,6- and 7,8-benzoquinoline are, respectively, 5.15 and 4.25, indicating that at physiological pH values they are completely unprotonated, suggesting these compounds differ in their mechanisms from the charged benzo[c]quinolinizinium compounds. The effects of 3,4-benzoquinoline are worth further comment. This compound shows a most marked inhibition of SCC as the concentration increases. This feature is also shown, but to a lesser extent, by other compounds used in this study (Figures 7,8). Other compounds known to activate CFTR also demonstrate both activating and inhibitory properties, that is inhibition at high concentration following activation at lower concentrations, such as with the flavonoids (Illek & Fischer, 1998; Illek et al., 2000). Many of the compounds examined in this study have Hill slopes close to 3. As stated earlier, no attempt has been made to fit the concentration – response curves to a theoretical drug receptor model. Hill slopes greater than one imply cooperativity, usually applied to the interaction of more than one drug molecule with its receptor. A different form of cooperativity probably applies here. It is known that agents that increase apical chloride conductance, such as NS004 (Devor et al., 1996a), have little effect on chloride secretion since the electrical gradient from the cell across the apical membrane is insufficient. However, hyperpolarisation of the cell by increasing the basolateral potassium conductance provides the driving force for chloride exit. Both 1,10-phenanthroline (Duzsyk et al., 2001) and EBIO (Cuthbert et al., 1999) have these dual actions. Consequently, the concentration – response relations will have, at least, two components that may have quite different EC50 values, to say nothing of any synergism between them. There is, however, evidence that CFTR combines as dimers in the cell membrane to create the chloride channel (Zerhusen et al., 1999; Schillers et al., 2001). If so, a more classical type of cooperativity may be involved where channel opening only occurs if sites in both units are occupied.
It is of interest to contemplate the sort of conditions where anion secretagogues, particularly for chloride and bicarbonate, may find application. Chronic obstructive pulmonary disease and CF are two examples where ways of promoting mucociliary clearance are of interest. Of course, in the absence of CFTR, as in CF such compounds would be of little use, unless they too acted as pharmacological chaperones. The benzoquinolines, as well as the benzimidazolones, have the two essential features for anion secretagogues: they increase apical chloride conductance and activate a basolateral potassium conductance. If further chemical modification can introduce a substantial potency increase, there is a possibility that such agents would interact with the mutant protein during the folding process and act as pharmacological chaperones. High-affinity ligands for the ADH receptor protein have been shown to rescue mutant receptors from degradation in the proteasome, resulting in their delivery to the cell surface where they are available for activation (Morello et al., 2000).
Acknowledgments
I am grateful to the Cystic Fibrosis Trust and the Leverhulme Trust for support, and to Dr D. Horwell for useful suggestions.
Abbreviations
- ADH
antidiuretic hormone
- CFTR
cystic fibrosis transmembrane conductance regulator
- DMSO
dimethyl sulphoxide
- EBIO
1-ethyl-2- benzimidazolinone
- EC50
concentration producing 50% of the maximal response
- HSP70/90
heat shock protein 70/90
- KHS
Krebs Henseleit Solution
- MDR
multiple drug resistance
References
- BECQ F., METTEY Y., GRAY M.A., GASLIETTA L.J.V., DORMER R.L., MERTEN M., METAYE T., CHAPPE V., MARIVINGT-MOUNIR C., ZEGARRA-MORAN O., TARRAN R., BULTEA L., DERAND R., PEREIRA M.M.C., MCPHERSON M.A., ROGIER C., JOFFRE M., ARGENT B.E., SARROUILHE D., KAMMOUNI M., FIGARELLA C., VERRIER B., GOLA M., VIERFOND J.M. Development of substituted benzo[c]quinolizinium compounds as novel activators of the cystic fibrosis chloride channel. J. Biol. Chem. 1999;274:27415–27425. doi: 10.1074/jbc.274.39.27415. [DOI] [PubMed] [Google Scholar]
- CUTHBERT A.W., HICKMAN M.E., THORN P., MACVINISH L.J. Activation of Ca2+- and cAMP-sensitive K+ channels in murine colonic epithelia by 1-ethyl-2-benzimidazolone. Am. J. Physiol. 1999;277:C111–C120. doi: 10.1152/ajpcell.1999.277.1.C111. [DOI] [PubMed] [Google Scholar]
- DENNING G.M., ANDERSON M.P., AMARA J.F., MARSHALL J., SMITH A.E., WELSH M.J. Processing of the mutant cystic fibrosis conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- DEVOR D.C., SINGH A.K., BRIDGES R.J., FRIZZELL R.A. Modulation of Cl− secretion by benimidazolones. II. Coordinate regulation of apical GCl and basolateral GK. Am. J. Physiol. 1996a;271:L785–L795. doi: 10.1152/ajplung.1996.271.5.L785. [DOI] [PubMed] [Google Scholar]
- DEVOR D.C., SINGH A.K., FRIZZELL R.A., BRIDGES R.J. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am. J. Physiol. 1996b;271:L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- DORMER R.L., DERAND R., MCNEILLY C.N., METTEY Y., AAAAAAABULTEAU-PIGNOUX L., VIERFOND J.M., GRAY J.M., GALIETA L.J.V., MORRIS M.R., PEREIRA M.M.C., DOULL I.J.M., BECQ F., MCPHERSON M.A. Correction of delta F508CFTR activity with benzo[c]quinolizinium compounds through facilitation of its processing in cystic fibrosis airway cells. J. Cell Sci. 2001;114:4073–4081. doi: 10.1242/jcs.114.22.4073. [DOI] [PubMed] [Google Scholar]
- DRUMM M.L., WILKINSON D.J., SMIT L.S., WORRELL R.T., STRONG T.V., FRIZZELL R.J., DAWSON D.C., COLLINS F.S. Chloride conductance expressed by ΔF508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254:1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- DUSZYK M., MACVINISH L.J., CUTHBERT A.W. Phenanthrolines – a new class of CFTR chloride channel openers. Br. J. Pharmacol. 2001;134:853–864. doi: 10.1038/sj.bjp.0704328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUSZYK M., SHU Y., SAWICKI G., RADOMSKI A., MAN S.F.P., RADOMSKI M.W. Inhibition of matrix metalloproteinase MMP-2 activates chloride current in human airway epithelial cells. Can. J. Physiol. Pharmacol. 1999;77:529–535. [PubMed] [Google Scholar]
- FULLER W., CUTHBERT A.W. Post-translational disruption of the ΔF508 CFTR-molecular chaperone complex with geldanomycin stabilises ΔF508 CFTR in the rabbit reticulocyte lysate. J. Biol. Chem. 2000;275:37462–37468. doi: 10.1074/jbc.M006278200. [DOI] [PubMed] [Google Scholar]
- GRUBB B.R., BOUCHER R.C. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol. Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- ILLEK B., FISCHER H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am. J. Physiol. 1998;275:L902–L910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- ILLEK B., LIZARZABURU M.E., LEE V., NANTZ M.H., KURTH M.J., FISCHER H. Structural determinants for activation of and block of CFTR-mediated chloride currents by apigenin. Am. J. Physiol. 2000;279:C1838–C1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- JENTSCH T.J., GUNTER W. Chloride channels: an emerging molecular picture. Bioessays. 1997;19:117–126. doi: 10.1002/bies.950190206. [DOI] [PubMed] [Google Scholar]
- LOO T.W., CLARKE D.M. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J. Biol. Chem. 1997;272:709–712. doi: 10.1074/jbc.272.2.709. [DOI] [PubMed] [Google Scholar]
- MORELLO J.-P., SALAPOUR A., LAPERRIERE A., BERNIER V., ARTHUS M.-F., LONERGAN M., PETAGA-REPO U., ANGERS S., MORIN D., BICHET D.G., BOUVIER M. Pharmacological chaperones rescue cell surface expression and function in misfolded V2 vasopressin receptor mutants. J. Clin. Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILLERS H., DANKER T., MADEJA M., OBERLEITHNER H. Plasma membrane protein clusters appear in CFTR expressing Xenopus laevis oocytes after cAMP stimulation. J. Membrane. Biol. 2001;180:205–212. doi: 10.1007/s002320010071. [DOI] [PubMed] [Google Scholar]
- SINGH S., SYME C.A., SINGH A.K., DEVOR D.C., BRIDGES R.J. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J. Pharm. Exp. Ther. 2001;296:600–611. [PubMed] [Google Scholar]
- ZERHUSEN B., ZHAO J., XIE J., DAVIS P.B., MA J. A single conductance pore for chloride ions formed by two cystic fibrosis transmembrane conductance regulator molecules. J. Biol. Chem. 1999;274:7627–7630. doi: 10.1074/jbc.274.12.7627. [DOI] [PubMed] [Google Scholar]