Abstract
The involvement of bradykinin (BK) receptors in the allergic inflammation associated with airway hyper-reactivity (AHR) was evaluated by means of the selective bradykinin B1 receptor (BKB1-R) antagonists R-715 (Ac-Lys-[D-βNal7, Ile8]desArg9-BK) and R-954 (Ac-Orn[Oic2, α-MePhe5, D-βNal7, Ile8]desArg9-BK) or the selective bradykinin B2 receptor (BKB2-R) antagonist HOE-140 (D-Arg0-Hyp3-Thi5-D-Tic7-Oic8-BK). Cellular migration and AHR were examined 24 h after the second ovalbumin (OA) challenge.
R-715 (10–500 μg kg−1) and R-954 (1–100 μg kg−1) injected intravenously (i.v.), 5 min prior to aerosol OA challenges, decreased by approximately 50% the induced lung eosinophilia in OA-sensitized mice but did not reduce AHR.
HOE-140 (1 μg kg−1) administered in the same manner, decreased mononuclear cell and eosinophil infiltration in the bronchoalveolar lavage fluid (BALF) of OA-sensitized mice. Moreover, treatment of OA-sensitized mice with HOE-140 (100 μg kg−1) completely abolished the AHR to carbachol.
The BKB1-R agonist desArg9-BK (DBK; 10–1000 μg kg−1) administered intratrachealy to normal mice had no effect on the basal cell counts recovered in BALF nor on the plasma extravasation, while the BKB2-R selective agonist BK (20 μg kg−1) stimulated mononuclear cell migration, neutrophilia and plasma extravasation in normal mouse lungs. Such effects were inhibited by HOE-140 (10 μg kg−1).
Our results suggest that the airway inflammatory response induced by antigen challenge in mice is mediated by stimulation of both BKB1-R and BKB2-R.
Keywords: Bradykinin, bradykinin B1 and B2 receptors, desArg9-bradykinin, Hoe-140, R-715, R-954, ovalbumin sensitization, bronchoalveolar lavage, airway hyperreactivity, leucocytes
Introduction
Several observations support a participatory role for kinins in the pathogenesis of inflammatory diseases including allergic airway disease (Fuller et al., 1987; Proud et al., 1988; Christiansen et al., 1992). Under pathophysiological stimuli, kinins (bradykinin; BK or kallidin) are produced from the cleavage of kininogens either by tissue or plasma proteolytic kallikreins. Kinin receptors are pharmacologically classified into B1 and B2 subtypes according to the relative potency of various BK agonists and antagonists (Regoli & Barabé, 1980). Molecular cloning of B1 and B2 receptors from a variety of species including humans, revealed that they belong to the family of G protein-coupled receptors (McEachern et al., 1991; Hess et al., 1996). The bradykinin B2 receptor (BKB2-R) is activated by BK and kallidin, while the bradykinin B1 receptor (BKB1-R) is selectively sensitive to kinin metabolites without the C-terminal arginine residue, desArg9-BK (DBK) and Lys-desArg9-BK. Whereas the BKB2-R is constitutively expressed and is believed to be responsible for most of kinin-mediated physiological functions and for the acute phase of inflammation, the BKB1-R – normally absent in tissues – is highly induced under many inflammatory conditions including experimental endotoxemia, rheumatoid arthritis, hyperalgesia, diabetes and in a model of Sephadex beads-induced lung inflammation in guinea-pigs (Regoli et al., 1977; Marceau et al., 1983; Farmer et al., 1991; Correa & Calixto, 1993; Chakir et al., 1995; Campos et al., 1996; Perron et al., 1999) and participates in the chronic phase of inflammation (Couture et al., 2001).
Experimental evidence supports a significant role for the BKB2-R in the pharmacological actions of kinins in airway inflammation (Burch et al., 1989; Christiansen et al., 1992). Increased levels of kinins have been detected in secretions from individuals with allergic rhinitis (Naclerio et al., 1985) and in the bronchoalveolar lavage fluid (BALF) of asthmatics (Christiansen et al., 1992). Symptomatic and physiological changes, which mimic naturally occurring rhinitis and asthma, are provoked by inhaled challenge with BK (Fuller et al., 1987; Proud et al., 1988). BK administration causes bronchoconstriction, microvascular leakage and mucus secretion in the airways of several animal species via BKB2-R (Herxheimer & Streseman, 1961; Bhoola et al., 1962). Inhalation of BK or Lys-BK provoked acute bronchoconstriction in humans (Polosa & Holgate, 1990). In addition, the selective BKB2-R antagonist HOE-140 improved pulmonary function in asthma subjects in a 4-week-treatment phase (Akbary et al., 1996), abolished hyper-responsiveness to histamine and reduced antigen-induced nasal eosinophilia in subjects with allergic rhinitis (Turner et al., 2001).
On the other hand, the physiological and pathophysiological functions of the selective BKB1-R agonist DBK, particularly at the airway level, remain still not well defined. The aim of the present study was to investigate, through the use of selective BKB1-R and BKB2-R antagonists, the contribution of BKB1-R in a murine model of allergic lung inflammation.
Methods
Animals
Male Balb/c mice, weighing 20–25 g (Charles River Laboratories, St-Constant, QC, Canada) were used. The mice were housed four by cage and maintained under conditions of standard lighting (alternating 12-h light/dark cycle), temperature (22±0.5°C) and humidity (60±10%) with food and water available ad libitum. All experiments were carried out in accordance with the ethical recommendations and guidelines of the Canadian Council on Animal Care (CCAC) and were approved by the Ethics Committee of the University of Sherbrooke.
Antigen sensitization
Mice were sensitized on days 0 and 5 by intraperitoneal (i.p.) injections of 8 μg ovalbumin (OA) adsorbed to 2 mg aluminium hydroxide; A1(OH)3 in saline (a total volume of 0.5 ml) according to the modified method of Kung et al. (1994). Control animals received equal volume of saline and A1(OH)3. On days 12 and 13, animals were challenged for 30 min with 0.5% (wv−1) OA solution (containing 0.8% antifoam B) in saline using an ultrasonic nebulizer (Model Spag-2, Montreal, PQ, Canada).
At 5 min before each of the two nebulizations, mice received intravenous (i.v.) injection of either R-715, R-954, HOE-140 or saline in the caudal vein in a volume of 100 μl. Animals were divided into the following groups: (i) sensitized group, treated with R-715 (10, 100 and 500 μg kg−1); (ii) sensitized group, treated with R-954 (1, 10 and 100 μg kg−1); (iii) sensitized group, treated with HOE-140 (1 μg kg−1); (iv) control group that was given saline; and (v, vi and vii) control groups that were given R-715, or R-954 or HOE-140, respectively. Bronchoalveolar lavage (BAL) or airway hyper-reactivity (AHR) measurements were performed 24 h after the second nebulization.
Bronchoalveolar lavage
Bronchoalveolar cells were obtained from BAL of animals killed following an i.m. injection of 50 μl of ketamine/xylazine (87/13 mg kg−1). Briefly, the trachea was cannulated and the lungs were washed with 5 ml of phosphate-buffered saline (PBS) (KCl, Na2HPO4 and KH2PO4). The first 1 ml of BALF was collected and centrifuged (300 × g, 10 min, 4°C), and aliquots of the supernatant were removed and stored at −20°C for albumin measurement. Total cell count was carried out using a haemocytometer, and viability was assessed with the Trypan blue exclusion test. Cell differential analysis was performed after cytocentrifugation and staining with Wright – Giemsa solution.
Measurement of AHR
Bronchoconstriction was measured according to the method of Konzett & Rössler (1940) using a pressure transducer (Model P23ID; Statham Gould). Briefly, 24 h after the second nebulization, mice were anaesthetized with a ketamine/xylazine solution (80/10 mg kg−1, i.m.) and the trachea was cannulated and ventilated with a mouse ventilator (Model 687; Harvard) at a frequency of 140 breaths min−1 and at tidal volume of 4 ml kg−1. The carotid artery and jugular vein were cannulated for monitoring systemic blood pressure and for drugs injection, respectively. To eliminate spontaneous respiration, mice were treated with succinylcholine chloride (8 mg kg−1, s.c.). After a stabilization period of 15 min, the BKB1-R or BKB2-R antagonists were administered in the jugular vein: R-715 (10, 100 and 500 μg kg−1), R-954 (1, 10 and 100 μg kg−1) and HOE-140 (1 and 100 μg kg−1). After 5 min, an intravenous OA injection (1 mg kg−1) was administered to antigen-challenged and control mice. Following another 15 min stabilization period, pulmonary insufflation pressure (PIP; mmHg) was recorded for assessing bronchial reactivity to increasing doses of carbachol (1–400 μg kg−1; i.v. at 5 min intervals). Airway resistance and arterial blood pressure were monitored continuously during the experiments.
Intratracheal injections
Control nonsensitized mice were anaesthetized with ketamine/xylazine (26/4 mg kg−1, i.m.), then given intratracheal (i.t.) injection of the angiotensin-converting enzyme inhibitor, captopril (4 mg kg−1) in order to prevent the degradation of the different peptides. The BKB1-R or BKB2-R antagonists, R-715 (500 μg kg−1), R-954 (100 μg kg−1) or HOE-140 (10 μg kg−1) were administered i.t., 10 min after captopril, while the BKB1-R agonist, DBK (10–1000 μg kg−1) and the BKB2-R agonist BK (1, 20 μg kg−1) were injected i.t., 20 min following captopril. Control animals received an i.t. injection of captopril and/or saline. The BALF was collected for analysis of plasma leakage and cellular accumulation, 1 and 24 h following peptides administration.
Measurement of albumin in BALF
A colorimetric method using bromocresol green developed by Doumas et al. (1971) was used to measure albumin leakage in BALF. This method has been shown to be specific for the albumin and not for γ-globulin. In brief, 120 μl of the albumin solution was added to 80 μl of samples and the absorbance was determined spectrophotometrically at 595 nm (Titertek Multiskan Flow lab.). The amount of albumin in BALF of control and treated mice, expressed in mg ml−1, was calculated from a standard curve of bovine albumin (0–1 mg kg−1).
Chemicals
BK, DBK, R-715 (Ac-Lys-[D-βNal7, Ile8]desArg9-BK) and R-954 (Ac-Orn[Oic2, α-MePhe5, D-βNal7, Ile8]desArg9-BK) were synthesized by Dr Witold Neugebauer in the Institute of Pharmacology of Sherbrooke, School of Medicine, University of Sherbrooke, Canada. HOE-140 (D-Arg0-Hyp3-Thi5-D-Tic7-Oic8-BK), captopril, carbachol, succinylcholine chloride, ketamine hydrochloride, xylazine, ovalbumin (Grade II), antifoam B, bovine albumin (fraction V) and bromocresol green were purchased from Sigma Chem. (St Louis, MO, USA). PBS was purchased from Baxter Corporation (Toronto, ON, Canada), Aluminium hydroxide gel (Rehydragel) was purchased from Reheis Inc. (Berkley Heights, NJ, USA). Wright–Giemsa staining and Trypan blue were purchased from Fisher Scientific (Montreal, PQ, Canada).
Statistical analysis
Data are presented as means±s.e.m. Statistical analyses were performed using the Student's t-test for unpaired data or analysis of variance (ANOVA) followed by the ‘Student – Newman – Keuls Multiple Comparisons Test' using the Instat 3.0 software (GraphPad Software, San Diego, CA, U.S.A.). A probability (P) value less than 0.05 was considered significant.
Results
Induction of pulmonary leukocytes infiltration
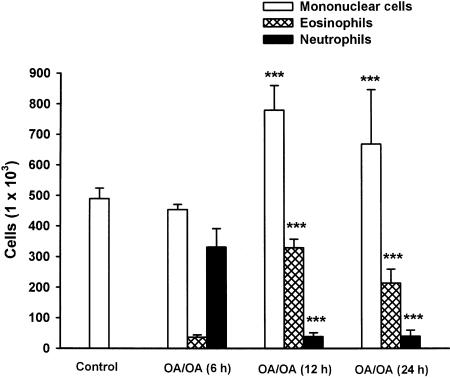
In the first series of experiments, the kinetics of inflammatory cell recruitment into the airway lumen – 6, 24 and 48 h after the second OA challenge – were studied. BALF of control mice contained 100% mononuclear phagocytes (macrophages and monocytes). As shown in Figure 1, the total cell number harvested in BALF increased by 1.7-fold (from 4.9±0.3 × 105 to 8.2±0.1 × 105 cells), 6 h after the second antigenic provocation. Wright – Giemsa staining demonstrated that this inflammatory infiltrate constituted of 40.3% neutrophils (3.3±0.6 × 105 cells), 4.5% eosinophils (0.4±0.1 × 105 cells) and 55.2% mononuclear cells (macrophages, monocytes and lymphocytes; 4.5±0.2 × 105 cells). However, 24 h after the second allergic provocation, we observed an inverse phenomenon: the neutrophil number decreased from 3.3±0.6 × 105 to 0.4±0.2 × 105 cells, while the eosinophil number increased and reached its maximum (from 0.4±0.1 × 105 to 3.3±0.3 × 105 cells). Mononuclear cells also increased from 4.5±0.2 × 105 to 7.8±0.8 × 105 cells. These increases remained significant 48 h following the second provocation (neutrophils 0.4±0.2 × 105, eosinophils 2.1±0.5 × 105 and mononuclear cells 6.7±1.8 × 105 cells) and started to decline within 72 h after the second provocation.
Figure 1.
Kinetics of inflammatory cell recruitment in the BALF of control and OA-sensitized Balb/c mice. Cells were harvested from control or OA-sensitized mice 6, 24 and 48 h following a second nebulization. Data are expressed as means±s.e.m. of 4–15 separate experiments. Values significantly different from OA/OA (6 h) at ***P<0.001.
Cellular infiltration in OA-sensitized mice
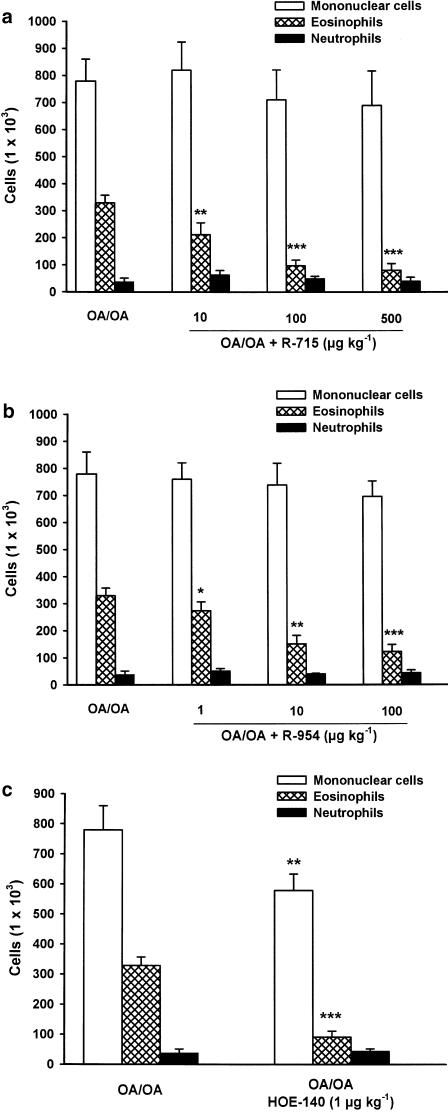
The effect of the selective BKB1-R antagonists R-715 and R-954 as well as the effect of the selective BKB2-R antagonist, HOE-140 on inflammatory cells recruitment in the lungs of OA-sensitized mice was measured. The i.v. administration of either of these two BKB1-R antagonists, 5 min before each nebulization, produced a dose-related decrease of polymorphonuclear cell influx in lung lavage fluid. R-715 at a dose of 10 μg kg−1 inhibited by 37% the eosinophil infiltration as compared with the cell numbers in OA-challenged mice treated with saline (from 3.3±0.3 × 105 to 2.1±0.3 × 105 cells). At the dose of 100 μg kg−1, the inhibition was reported as 69% (from 3.3±0.3 × 105 to 1.0±0.4 × 105 cells), while at the dose of 500 μg kg−1, it produced a 76% inhibition (from 3.3±0.3 × 105 to 0.8±0.2 × 105 cells) (P<0.001; Figure 2a). The more potent and stable analogue of R-715, R-954 was also administered i.v. in the same model. R-954 decreased the antigen-induced airway eosinophilia by 18% at a dose of 1 μg kg−1 (from 3.3±0.3 × 105 to 2.6±0.5 × 105 cells), by 54% at the dose of 10 μg kg−1 (from 3.3±0.3 × 105 to 1.5±0.3 × 105 cells) and by 64% at the dose of 100 μg kg−1 (from 3.3±0.3 × 105 to 1.2±0.4 × 105 cells) (P<0.001; Figure 2b).
Figure 2.
Effect of the BKB1-R antagonists, R-715 (a), R-954 (b) and the BKB2-R antagonist, HOE-140 (c) on OA-induced eosinophil accumulation in sensitized Balb/c mice. R-715 (10, 100 and 500 μg kg−1) or R-954 (1, 10 and 100 μg kg−1) or HOE-140 (1 μg kg−1) was injected i.v., 5 min, before each antigen provocation. Mononuclear cells, eosinophils and neutrophils were collected from BALF 24 h after the second provocation by aerosol. Data are expressed as means±s.e.m. of 5–14 separate experiments. Values significantly different from OA/OA injected with saline at *P<0.05, **P<0.01 and ***P<0.001, respectively.
The BKB2-R antagonist, HOE-140 (1 μg kg−1) injected intravenously before each antigenic provocation decreased the number of eosinophils in the BALF of OA-sensitized mice by 72% (from 3.3±0.3 × 105 to 0.9±0.2 × 105 cells) and the number of mononuclear cells by 26% (from 7.8±0.8 × 105 to 5.8±0.6 × 105 cells) compared with saline-treated animals (P<0.001; Figure 2c).
It is noteworthy that neither R-715 nor R-954 caused a significant alteration in the number of mononuclear cells and neutrophils. In addition, i.v. injections of R-715, R-954 or HOE-140 to control mice did not have any effect on basal cell levels (data not shown).
Airway hyper-reactivity in OA-sensitized mice
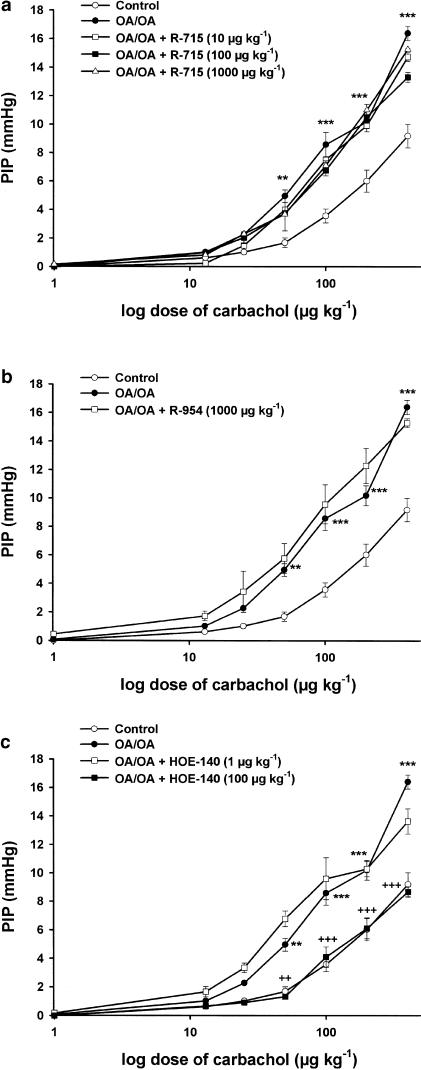
Carbachol (1–400 μg kg−1) administered i.v. to control and OA-challenged mice induced a dose-dependent increase of PIP that averaged 9.2±0.8 mmHg in control animals (n=8) and 16.4±0.5 mmHg in OA-challenged mice (n=10). As shown in Figure 3a and b, i.v. injection of the selective BKB1-R antagonists, R-715 and R-954 at doses that have been shown previously to antagonize OA-induced cellular infiltration, did not affect AHR to carbachol in OA-challenged animals. In contrast, the selective BKB2-R antagonist, HOE-140 (100 μg kg−1, i.v.) significantly reduced AHR from 16.3±0.5 to 8.6±0.4 mmHg (P<0.001; Figure 3c). All antagonists were administered i.v., 5 min before each OA nebulization and 5 min before OA injection (1 mg kg−1). The BKB1-R and BKB2-R antagonists had no effect on the PIP in control mice.
Figure 3.
Effect of the BKB1-R antagonists, R-715 (a), R-954 (b) and the BKB2-R antagonist, HOE-140 (c) on carbachol-induced increase in pulmonary insufflation pressure PIP in OA-sensitized Balb/c mice, 24 h after the antigen provocation. R-715 (10, 100 and 500 μg kg−1), R-954 (1, 10 and 100 μg kg−1) and HOE-140 (1 and 100 μg kg−1) were administered i.v., 5 min, before each OA nebulization and 5 min before OA injection (1 mg kg−1, i.v.). Bronchoconstriction was provoked by injection of increasing doses of carbachol (1–400 μg kg−1; i.v.), 15 min following the i.v. OA injection, at 5 min intervals, and the PIP (mmHg) was recorded. Data are expressed as means±s.e.m. of 4–18 observations. Values significantly different from control at **P<0.01 and ***P<0.001, respectively and values significantly different from OA/OA injected with saline at ++P<0.01 and +++P<0.001, respectively.
Cellular infiltration and bronchoalveolar permeability in normal mice
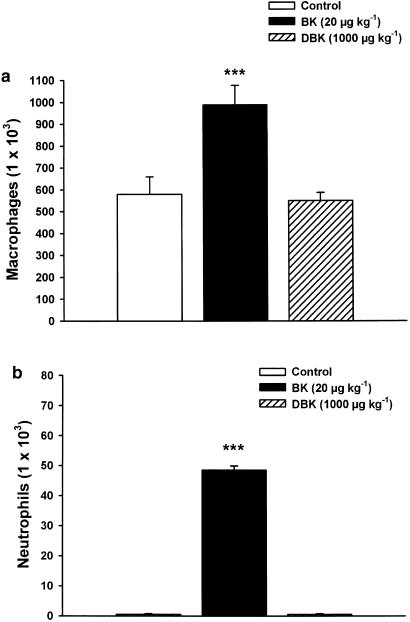
The i.t. injection of BK (20 μg kg−1) in the presence of captopril (4 mg kg−1) produced, 24 h later, a marked increase in macrophages/monocytes number harvested from BALF (1.7-fold; from 5.8±0.8 × 105 to 9.9±0.9 × 105 cells) (P<0.001; Figure 4a) and in neutrophils number (from 0.0 to 0.5±0.01 × 105 cells) (P<0.001; Figure 4b). Such effect was significantly reduced by prior treatment with the BKB2-R antagonist HOE-140 (10 μg kg−1), 10 min before BK administration (data not shown). In addition, the i.t. injection of BK caused a dose-dependent increase of albumin leakage in the BALF from normal nonsensitized mice compared to saline-treated controls. BK (1, 20, 100 μg kg−1) increased the levels of albumin measured, 1 h after BK injections, by 1.5-, 1.9- and 2.1-fold, respectively (P<0.001; Figure 5a). These increases were completely inhibited by preadministration of HOE-140 (10 μg kg−1) (Figure 5b).
Figure 4.
Effect of the BKB2-R agonist, BK and the BKB1-R agonist, DBK on macrophages (a) and neutrophils (b) infiltration in the BALF from normal nonsensitized Balb/c mice. BK (20 μg kg−1) or DBK (1000 μg kg−1) was administered i.t., 20 min after captopril (4 mg kg−1). The BALF was collected for analysis of cellular accumulation, 24 h following peptide injection. Data are expressed as means±s.e.m. of six observations. Values significantly different from control at ***P<0.001.
Figure 5.
Effect of the BKB2-R agonist, BK and the BKB1-R agonist, DBK (a) or the combined administration of BK and the BKB2-R antagonist, HOE-140 (b) on bronchoalveolar permeability in normal nonsensitized Balb/c mice. BK (1, 20, 100 μg kg−1), DBK (1000 μg kg−1) or HOE-140 (10 μg kg−1) was administered i.t., 20 min after captopril (4 mg kg−1). The BALF was collected for analysis of plasma leakage, 1 h following peptide injection. Data are expressed as means±s.e.m. of five observations. Values significantly different from control at **P<0.01 and ***P<0.001, respectively and values significantly different from BK at +++P<0.001.
On the other hand, the i.t. administration of DBK (10–1000 μg kg−1) had no chemotactic effect in the airways of normal mice (Figure 4). Furthermore, the i.t. instillation of DBK did not alter the basal protein levels in BALF of normal mice (Figure 5a). Finally, the BKB1-R antagonists, R-715 and R-954, had neither an effect on cellular infiltration nor on bronchoalveolar permeability in control nonsensitized mice (data not shown).
Discussion
Murine model of airway inflammation
A murine model of airway inflammation characterized by lung eosinophilia and AHR was used. We demonstrated that two antigen injections were sufficient to induce lung eosinophilia, but did not produce a bronchial hyper-reactivity. On the other hand, after an i.v. injection of OA (1 mg kg−1) to OA-sensitized mice, a significant increase of the bronchoconstrictor response to intravenous carbachol as well as an increase of blood pressure were observed. Our results also showed that a major lung infiltration of neutrophils (40%) and monocytes (55%) was noted 6 h after the second antigenic challenge and was followed by a marked increase of eosinophils (40%) and a decrease in the number of neutrophils (4%), 24 h later. The total number of mononuclear cells also increased after the induction of airway allergic inflammation. Previous studies demonstrated that the infiltration of neutrophils in tissues begins a few minutes after the administration of the inflammatory stimulus and decreases a few hours later with the increase in the number of monocytes, lymphocytes and eosinophils (Metzger et al., 1986; Frew & Kay, 1988). Experimental evidence suggests that neutrophils could also play an important role in the eosinophil recruitment in the lungs (Cook et al., 1988). Other studies showed that eosinophils and their products contribute to airway inflammation and to the development of AHR (Broide et al., 1991; Lefort et al., 1996). Taken together, it could be suggested that the neutrophilia was a nonspecific inflammatory response caused by the introduction of a foreign protein into the airways, whereas the eosinophil response was a specific immunological response to OA challenge.
On the other hand, correlations between the eosinophil number present in BALF and the intensity of AHR have not been demonstrated yet. Renz et al. (1992) showed that adjuvant-free OA-sensitization of Balb/c mice induced airway hyper-responsiveness to intravenous methacholine but without inflammatory cell infiltration in the lungs. Aerosolized LPS inhalation to guinea-pigs was also shown to cause neutrophil and macrophage airway infiltration, and an early development of AHR followed 48 h later by airway hyporeactiviry to histamine (Toward and Broadley, 2000). In addition, in the Brown Norway rat model of allergic airway inflammation, AHR was not apparent in sensitized animals after a single or multiple challenges although eosinophil influx was seen in the same animals (Underwood et al., 2002). However, in agreement with our findings, Schmidlin et al. (2002) showed that OA-sensitized and challenged mice stimulated the infiltration of leukocytes into BAL and induced AHR to inhaled methacholine. These observations underline the complexity of these two phenomena.
Cellular migration and AHR in OA-sensitized and control mice
In the present study, we demonstrated that the selective BKB1-R antagonists, R-715 and R-954, significantly decreased eosinophilia in BALF of antigen-challenged mice without affecting AHR. In contrast, the selective BKB2-R antagonist, HOE-140, significantly inhibited airway hyper-responsiveness, eosinophilia and mononuclear cell infiltrations in BALF of OA-sensitized mice. It is interesting to note that at a dose of 1 μg kg−1, HOE-140 decreased cellular infiltration but did not inhibit AHR; however, a dose of 100 μg kg−1 significantly inhibited AHR.
We also reported that the BKB2-R agonist, BK induced cell migration and a dose-dependent protein extravasation in BALF of normal animals and increased the number of macrophages and neutrophils. Such effects in mouse lungs were completely abolished by HOE-140. In contrast, the selective BKB1-R agonist DBK did not have a chemotactic effect nor produced a change of bronchoalveolar permeability in control mice. Neither R-715 nor R-954 had a significant effect on cellular infiltration and protein leakage in normal mice. These results provide evidence for the presence of functionally active BKB1-R in our model of pulmonary inflammation and for the implication of both subtypes of kinin receptors in the eosinophilia, but only the BKB2-R subtype appears to be involved in the mononuclear cell infiltrations and the AHR associated with the inflammatory process.
A number of studies demonstrated the implication of the BKB2-R in airway inflammation (Bhoola et al., 1962; Fuller et al., 1987; Proud et al., 1988; Burch et al., 1989; Christiansen et al., 1992; Farmer et al., 1992; Perron et al., 1999). BK induced proinflammatory effects and cellular infiltrations in a murine model of pleurisy (Saleh et al., 1997). In addition, it has been shown that BK induces bronchoconstriction in vivo by various cholinergic, nonadrenergic and noncholinergic mechanisms (Fuller et al., 1987; Sakamoto et al., 1993). BK also produced an increase in vascular permeability that appeared to be mediated through BKB2-R activation (Fuller et al., 1987; Ichinose & Barnes, 1990). These results were supported by further studies, which demonstrated that BKB2-R antagonists are able to inhibit the airway inflammation and prevent AHR in selected animal models (Soler et al., 1990; Farmer et al., 1992).
Although B1 receptors were shown to be expressed during inflammatory reactions, little is known about their role in the physiopathology of the asthma. Recently, Marsh & Hill (1994) and Menke et al. (1994) have demonstrated that the B1 receptors are expressed on bovine tracheal smooth muscle cells and human lung fibroblasts. In addition, several studies demonstrated that B1 receptors could be expressed on immunocompetent cells such as macrophages (Bhoola et al., 1992) and T-lymphocytes (McFadden & Vickers, 1989). The expression of B1 receptors was shown to be stimulated by various cytokines including interleukin-1β (in MH-S murine alveolar macrophages), interleukin-8 (in human lung fibroblast) and endothelium growth factors (EGF) (Deblois et al., 1988; Bastian et al., 1998; Tsukagoshi et al., 1999). Another recent study showed that the in vitro exposure of mouse trachea to methacholine caused a time-dependent expression of B1 receptors (Li et al., 1998). It was also observed by immunofluorescence that B1 receptor expression increased within pulmonary fibrous tissues and basement membrane of alveoli and capillaries during pathological modifications of interstitial lung disease associated with progressive systemic sclerosis (Nadar et al., 1996). Bhoola (1996) reported the first localization of BKB1-R on the basement membranes of bronchopulmonary cells and the surrounding fibrous stroma in transbronchial biopsies taken from patients with interstitial lung disease associated with progressive systemic sclerosis. In addition, Trevisani et al. (1999) provided evidence for in vitro expression of BKB1-R in the mouse trachea and urinary bladder. Christiansen et al. (2002) demonstrated the presence of functional BKB1-R in the airways during allergic inflammation and suggested that they participate in the regulation of gene expression. This was proved by the marked increase in the expression of BKB1-R mRNA in subjects with allergic rhinitis, while no significant difference was found in BKB2-R expression.
Further experimental evidence supports a role for the BKB1-R in airway inflammation. Goldstein & Wall (1984) showed that DBK stimulated collagen secretion and the proliferation of human lung fibroblasts. Farmer et al. (1992) showed that a BKB1-R antagonist, desArg9-[Leu8]-BK inhibited the lung neutrophilia in OA-sensitized guinea-pigs. Later, the chemotactic action of DBK in the mouse air pouch pretreated with IL-1β (Ahluwalia & Perretti, 1996) was reported. The group of Pesquero (1996) showed that polymorphonuclear leucocytes decreased by 65% in inflammed tissues from transgenic B1 knockout mice. Vianna & Calixto (1998) demonstrated that the intrathoracic administration of DBK in a mouse model of pleurisy induced plasma leakage and neutrophil accumulation in mouse pleura. Recent studies demonstrated that the B1 receptors are also involved in the release of inflammatory cytokines by human type II pneumocytes that are responsible for the modulation of lung inflammation (Koyama et al., 1998). A recent study conducted in our laboratory (Perron et al., 1999) strongly suggested the implication of B1 receptors in eosinophil recruitment in a model of lung inflammation induced by the intravenous injection of Sephadex beads in guinea-pigs.
It is becoming clear that a prominent role could be attributed to the BKB1-R in pulmonary inflammation. First, it is known that BKB1-R, selectively activated by BKB1-R agonists, is normally absent or of little activity under normal physiological conditions (Couture et al., 2001), whereas BKB1-R agonists are effective in pathological conditions as allergic airway diseases. Secondly, in inflammatory conditions, the chronic activation of the inducible BKB1-R is likely to be amplified by the accumulation of DBK, the metabolite resulting from the degradation of BK, at the site of inflammation (Marceau et al., 1998; Marceau & Bachvarov, 1998). This can be attributed in part to the upregulation of carboxypeptidase M (kininase I, the enzyme responsible for the metabolism of BK to DBK), which would increase the endogenous level of DBK as observed in pig aorta infused with lipopolysaccharide (Schremmer-Danninger et al., 1998). DBK is able to stimulate the production of inflammatory mediators such as prostaglandins E2 and I2 (PGE2, PGI2), platelet activating factor (PAF) by endothelial cells, interleukin 1 (IL-1) and tumour necrosis factor-alpha (TNF-α) by macrophages (Toda et al., 1987; D'Orleans-Juste et al., 1989; Bhoola et al., 1992).
In conclusion, our results showed that both BKB1-R and BKB2-R play a significant role in the development of the allergic inflammatory responses in our experimental model of pulmonary inflammation in Balb/c mice. We showed that the activation of the BKB2-R is amplified in allergic inflammation, which demonstrates an important role for BKB2-R in maintaining bronchial inflammation induced by OA. In addition, our data also indicate that the BKB1-R, that is absent in control animals, is expressed in OA-sensitized mice and is involved in the evolution of allergic reactions. The ability of BKB1-R and BKB2-R antagonists to inhibit the eosinophilia and/or AHR induced by OA sensitization in mouse lungs suggests a pivotal role for endogenous kinins, BK and DBK in the initiation and maintenance of allergic airway inflammation.
Acknowledgments
We acknowledge the Canadian Medical Research Council (MRC) for financial support, Dr Witold Neugebauer and Dr Domenico Regoli (Institute of Pharmacology of Sherbrooke, School of Medicine, University of Sherbrooke, Canada) for the synthesis and supply of peptides and Ms Solange Cloutier for technical assistance.
Abbreviations
- AHR
airway hyper-reactivity
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- BK
bradykinin
- BKB1-R
bradykinin B1 receptor
- BKB2-R
bradykinin B2 receptor
- DBK
desArg9-bradykinin
- HOE-140
D-Arg0-Hyp3-Thi5-D-Tic7-Oic8-BK
- i.m.
intramuscular
- i.p.
intraperitoneal
- i.t.
intratracheal
- OA
ovalbumin
- PBS
phosphate-buffered saline
- PIP
pulmonary insufflation pressure
- R-715
Ac-Lys-[D-βNal7,Ile8]desArg9-BK
- R-954
Ac-Orn[Oic2,α-MePhe5,D-βNal7,Ile8]desArg9-BK
References
- AHLUWALIA A., PERRETTI M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1βin vivo in the mouse. J. Immunol. 1996;156:269–274. [PubMed] [Google Scholar]
- AKBARY A.M., WIRTH K.J., SCHOLKENS B.A. Efficacy and tolerability of Icatibant (Hoe 140) in patients with moderately severe chronic bronchial asthma. Immunopharmacology. 1996;33:238–242. doi: 10.1016/0162-3109(96)00065-3. [DOI] [PubMed] [Google Scholar]
- BASTIAN S., PAQUET J.L., ROBERT C., CREMERS B., LOILLIER B., LARRIVEE J.F., BACHAROV D.R., MARCEAU F., PRUNEAU D. Interleukin 8 (IL-8) induces the expression of kinin B1 receptor in human lung fibroblasts. Biochem. Biophys. Res. Commun. 1998;30:750–755. doi: 10.1006/bbrc.1998.9848. [DOI] [PubMed] [Google Scholar]
- BHOOLA K.D. Translocation of the neutrophil kinin moiety and changes in the regulation of kinin receptors in inflammation. Immunopharmacology. 1996;33:247–256. doi: 10.1016/0162-3109(96)00067-7. [DOI] [PubMed] [Google Scholar]
- BHOOLA K.D., COLLIER H.O.J., SCHACHTER M., SHORLEY P.G. Actions of some peptides on bronchial muscle. Br. J. Pharmacol. 1962;19:190–197. doi: 10.1111/j.1476-5381.1962.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHOOLA K.D., FIGUEROA C.D., WORTHY K. Bioregulation of kinins: kallikreins, kininogens and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- BROIDE D.H., GLEICH G.J., CUOMO A.J., COBURN D.A., FEDERMAN E.C., SCHWARTZ L.B., WASSERMAN S.I. Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthma airway. J. Allergy Clin. Immunol. 1991;88:637–648. doi: 10.1016/0091-6749(91)90158-k. [DOI] [PubMed] [Google Scholar]
- BURCH R.M., CONNOR J.R., TIFFANY C.W. The kallikrein–kininogen–kinin system in chronic inflammation. Agents Action. 1989;27:258–260. doi: 10.1007/BF01972790. [DOI] [PubMed] [Google Scholar]
- CAMPOS M., SOUZA G., CALIXTO J.B. Upregulation of B1 receptor mediating desArg9-BK-induced rat paw oedema by systemic treatment with bacterial endotoxin. Br. J. Pharmacol. 1996;117:793–798. doi: 10.1111/j.1476-5381.1996.tb15262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKIR M., REGOLI D., SIROIS P., GOBEIL F., PLANTE G.E. Hypersensibilité du récepteur B1 de la bradykinine au niveau de la veine porte de rat diabétique. Méd/Sci. 1995;11 Suppl. 2:15. [Google Scholar]
- CHRISTIANSEN S.C., EDDLESTON J., WOESSNER K.M., CHAMBERS S.S., YE R., PAN Z.K., ZURAW B.L. Up-regulation of functional kinin B1 receptors in allergic airway inflammation. J. Immunol. 2002;169:2054–2060. doi: 10.4049/jimmunol.169.4.2054. [DOI] [PubMed] [Google Scholar]
- CHRISTIANSEN S.C., PROUD D., SARNOFF R.B., JUERGENSEN U., COCHRANE C.G., ZURAN B.L. Elevation of tissue kallikrein and kinin in the airways of asthmatic subjects after endobronchial allergen challenge. Am. Rev. Respir. Dis. 1992;145:900–905. doi: 10.1164/ajrccm/145.4_Pt_1.900. [DOI] [PubMed] [Google Scholar]
- COOK R.M., MUSGROVE N.R.J., SMITH H. Relationship between neutrophil infiltration and tissue eosinophilia in the rat. Int. Arch. Allergy Appl. Immunol. 1988;87:105–108. doi: 10.1159/000234658. [DOI] [PubMed] [Google Scholar]
- CORREA C.R., CALIXTO J.B. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br. J. Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTURE R., HARRISSON M., VIANNA R.M., CLOUTIER F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- DEBLOIS D., BOUTHILLIER J., MARCEAU F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to desArg9-bradykinin. Br. J. Pharmacol. 1988;93:969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ORLEANS-JUSTE P., DENUCCI G., VANE J.R. Kinins act on B1 and B2 receptors to release conjointly endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells. Br. J. Pharmacol. 1989;96:920–926. doi: 10.1111/j.1476-5381.1989.tb11903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUMAS B.T., WATSON W.A., BIGGS H.G. Albumin standards and the measurement of serum albumin with bromocresol green. Clin. Chim. Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- FARMER S.G., MCMILLAN B.A., MEEKER S.N., BURCH R.M. Induction of vascular smooth muscle bradykinin B1 receptors in vivo during antigen arthritis. Agents Actions. 1991;34:191–193. doi: 10.1007/BF01993275. [DOI] [PubMed] [Google Scholar]
- FARMER S.G., WILKINS D.E., MEEKER S.A., SEEDS E.A., PAGE C.P. Effects of bradykinin receptor antagonists on antigen-induced respiratory distress, airway hyperresponsiveness and eosinophilia in guinea-pigs. Br. J. Pharmacol. 1992;107:653–659. doi: 10.1111/j.1476-5381.1992.tb14502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREW A.J., KAY A.B. The relationship between infiltrating CD4+lymphocytes, activated eosinophils, and the magnitude of the allergen-induced late phase cutaneous reaction in man. J. Immunol. 1988;141:4158–4172. [PubMed] [Google Scholar]
- FULLER R.W., DIXON C.M.S., CUSS F.M., BARNES P.J. Bradykinin-induced bronchoconstriction in humans. Mode of action. Am. Rev. Respir. Dis. 1987;135:176–180. doi: 10.1164/arrd.1987.135.1.176. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN R.H., WALL M. Activation of protein formation and cell division by bradykinin and desArg9-bradykinin. J. Biol. Chem. 1984;259:9263–9268. [PubMed] [Google Scholar]
- HERXHEIMER H., STRESEMAN E. The effect of bradykinin aerosol in guinea-pigs and in man. J. Physiol. 1961;158:38–39. [Google Scholar]
- HESS J.F., DERRICK A.W., MACNEIL T., BORKOWSKI J.A. The agonist selectivity of a mouse B1 bradykinin receptor differs from human and rabbit B1 receptors. Immunopharmacology. 1996;33:1–8. doi: 10.1016/0162-3109(96)00074-4. [DOI] [PubMed] [Google Scholar]
- ICHINOSE M., BARNES P.J. Bradykinin-induced airway microvascular leakage and bronchoconstriction are mediated via a bradykinin B2-receptor. Am. Rev. Respir. Dis. 1990;142:1104–1107. doi: 10.1164/ajrccm/142.5.1104. [DOI] [PubMed] [Google Scholar]
- KONZETT H., RÖSSLER R. Versuchonordnung zu untersuchungen an der bronchial musculatur. Naunyn-Schmeidbergs Arch. Pharmacol. 1940;195:71–75. [Google Scholar]
- KOYAMA S., SATO E., NOMURA H., KUBO K., MIURA M., YAMASHITA T., NAGAI S., IZUMI T. Bradykinin stimulates type II alveolar cells to release neutrophil and monocyte chemotactic activity and inflammatory cytokines. Am. J. Pathol. 1998;153:1885–1893. doi: 10.1016/S0002-9440(10)65702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNG T.T., JONES H., ADAMS G.K., UMLAND S.P., KREUTNER W., EGAN R.W., CHAPMAN R.W., WATNICK A.S. Characterization of a murine model of allergic pulmonary inflammation. Int. Arch. Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- LEFORT J., BACHELET C.M., LEDUC D., VARGAFTIG B.B. Effect of antigen provocation of IL-5 transgenic mice on eosinophil mobilization and bronchial hyperresponsiveness. J. Allergy Clin. Immunol. 1996;97:788–799. doi: 10.1016/s0091-6749(96)80157-6. [DOI] [PubMed] [Google Scholar]
- LI L., VAALI K., PAAKKARI I., VAPAATALO H. Involvement of bradykinin B1 and B2 receptors in relaxation of mouse isolated trachea. Br. J. Pharmacol. 1998;123:1337–1342. doi: 10.1038/sj.bjp.0701741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCEAU F., BACHVAROV D.R. Kinin receptors. Clin. Rev. Allergy Immunol. 1998;16:385–401. doi: 10.1007/BF02737658. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MARCEAU F., LUSSIER A., REGOLI D., GIROUD J.P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen. Pharmacol. 1983;14:209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- MARSH K.A., HILL S.J. DesArg9-bradykinin-induced increases in intracellular calcium ion concentration in single bovine tracheal smooth muscle cells. Br. J. Pharmacol. 1994;112:934–938. doi: 10.1111/j.1476-5381.1994.tb13170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCEACHERN A.E., SHELTON E.R., BHAKTA S., OBERNOLTE R., BACH C., ZUPPAN P., FUJISAKI J., ALDRICH R.W., JARNAGIN K. Expression cloning of rat B2 bradykinin receptor. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFADDEN R.G., VICKERS K.E. Bradykinin augments the in vitro migration of nonsensitized lymphocytes. Clin. Invest. Med. 1989;12:247–253. [PubMed] [Google Scholar]
- MENKE J.G., BORKOWSKI J.A., BIERLO K.K., MACNEIL T., DERRICK A.W., SCHNECK K.A., RANSOM R.W., STRADER C.D., LINEMEYER D.L., HESS J.F. Expression cloning of a human B1 bradykinin receptor. J. Biol. Chem. 1994;269:21583–21586. [PubMed] [Google Scholar]
- METZGER W.J., RICHERSON H.B., WORDEN K., MONICK M., HUNNINGHAKE G.W. Bronchoalveolar lavage of allergic asthmatic patients following allergen bronchoprovocation. Chest. 1986;89:477–483. doi: 10.1378/chest.89.4.477. [DOI] [PubMed] [Google Scholar]
- NACLERIO R.M., PROUD D., TOGIAS A.G., ADKINSON N.F., JR, MEYERS D.A., KAGEY-SOBOTKA A., PLAUT M., NORMAN P.S., LICHTENSTEIN L.M. Inflammatory mediators in late antigen-induced rhinitis. N. Engl. J. Med. 1985;313:65–70. doi: 10.1056/NEJM198507113130201. [DOI] [PubMed] [Google Scholar]
- NADAR R., DERRICK A., NAIDOO S., NAIDOO Y., HESS F., BHOOLA K. Immunoreactive B1 receptors in human transbronchial tissue. Immunopharmacology. 1996;33:317–320. doi: 10.1016/0162-3109(96)00053-7. [DOI] [PubMed] [Google Scholar]
- PERRON M.S., GOBEIL F., JR, PELLETIER S., REGOLI D., SIROIS P. Involvement of bradykinin B1 and B2 receptors in pulmonary leucocyte accumulation induced by Sephadex beads in guinea pigs. Eur. J. Pharmacol. 1999;376:83–89. doi: 10.1016/s0014-2999(99)00348-9. [DOI] [PubMed] [Google Scholar]
- PESQUERO J.B., PESQUERO J.L., OLIVEIRA S.M., ROSCHER A.A., METZGER R., GANTER D., BADER M. Molecular cloning and functional characterization of a mouse bradykinin B1 receptor gene. Biochem. Biophys. Res. Commun. 1996;220:219–225. doi: 10.1006/bbrc.1996.0384. [DOI] [PubMed] [Google Scholar]
- POLOSA R., HOLGATE S.T. Comparative airway response to inhaled bradykinin, kallidin, and desArg9-bradykinin in normal and asthmatic subjects. Am. Rev. Resp.Dis. 1990;142:1367–1371. doi: 10.1164/ajrccm/142.6_Pt_1.1367. [DOI] [PubMed] [Google Scholar]
- PROUD D., REYNOLDS C.J., LACAPRA S., KAGEY-SOBOTKA A., LICHTENSTEIN L.M., NACLERIO R.M. Nasal provocation with bradykinin induces symptoms of rhinitis and a sore throat. Am. Rev. Respir. Dis. 1988;137:613–616. doi: 10.1164/ajrccm/137.3.613. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J., PARK W.K. Receptors for bradykinin in rabbit aortae. Can. J. Physiol. Pharmacol. 1977;55:855–867. doi: 10.1139/y77-115. [DOI] [PubMed] [Google Scholar]
- RENZ H., SMITH H.R., HENSON J.E., RAY B.S., IRVIN C.G., GELFAND E.W. Aerosolized antigen exposure without adjuvent causes increased IgE production and increased airway responsiveness in the mouse. J. Allergy Clin. Immunol. 1992;89:1127–1138. doi: 10.1016/0091-6749(92)90296-e. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO T., TSUKAGOSHI H., BARNES P.J., CHUNG K.F. Role played by NK2 receptor and cyclooxygenase activation in bradykinin B2 receptor mediated-airway effects in guinea pigs. Agents Actions. 1993;39:111–117. doi: 10.1007/BF01998962. [DOI] [PubMed] [Google Scholar]
- SALEH T.S., CALIXTO J.B., MEDEIROS Y.S. Pro-inflammatory effects induced by bradykinin in a murine model of pleurisy. Eur. J. Pharmacol. 1997;331:43–52. doi: 10.1016/s0014-2999(97)01005-4. [DOI] [PubMed] [Google Scholar]
- SCHMIDLIN F., AMADESI S., DABBAGH K., LEWIS D.E., KNOTT P., BUNNETT N.W., GATER P.R., GEPPETTI P., BERTRAND C., STEVENS M.E. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J. Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- SCHREMMER-DANNINGER E., OFFNER A., SIEBECK M., ROSCHER A.A. B1 bradykinin receptors and carboxypeptidase M are both upregulated in the aorta of pigs after LPS infusion. Biochem. Biophys. Res. Commun. 1998;243:246–252. doi: 10.1006/bbrc.1997.7999. [DOI] [PubMed] [Google Scholar]
- SOLER M., SIELCZAK M., ABRAHAM W.M. A bradykinin antagonist blocks antigen-induced airway hyperresponsiveness and inflammation in sheep. Pulm. Pharmacol. 1990;3:9–15. doi: 10.1016/0952-0600(90)90003-2. [DOI] [PubMed] [Google Scholar]
- TODA N., BIAN K., AKIBA T., OKAMURA T. Heterogeneity in mechanisms of bradykinin action in canine isolated blood vessels. Eur. J. Pharmacol. 1987;135:321–329. doi: 10.1016/0014-2999(87)90681-9. [DOI] [PubMed] [Google Scholar]
- TOWARD T.J., BROADLEY K.J. Airway reactivity, inflammatory cell influx and nitric oxide in guinea-pig airways after lipopolysaccharide inhalation. Br. J. Pharmacol. 2000;131:271–281. doi: 10.1038/sj.bjp.0703589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVISANI M., SCHMIDLIN F., TOGNETTO M., NIJKAMP F.P., GIES J.P., FROSSARD N., AMADESI S., FOLKERTS G., GEPPETTI P. Evidence for in vitro expression of B1 receptor in the mouse trachea and urinary bladder. Br. J. Pharmacol. 1999;126:1293–1300. doi: 10.1038/sj.bjp.0702410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUKAGOSHI H., SHIMIZU Y., HORIE T., FUKABORI Y., IWAMAE S., HISADA T., ISHIZUKA T., IIZUKA K., DOBASHI K., MORI M. Regulation by interleukin-1beta of gene expression of bradykinin B1 receptor in MH-S murine alveolar macrophage cell line. Biochem. Biophys. Res. Commun. 1999;7:476–482. doi: 10.1006/bbrc.1999.0798. [DOI] [PubMed] [Google Scholar]
- TURNER P., DEAR J., SCADDING G., FOREMAN J.C. Role of kinins in seasonal allergic rhinitis: icatibant, a bradykinin B2 receptor antagonist, abolishes the hyperresponsiveness and nasal eosinophilia induced by antigen. J. Allergy Clin. Immunol. 2001;107:105–113. doi: 10.1067/mai.2001.111145. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD S.L., HADDAD EL-B., BIRRELL M.A., MCCLUSKIE K., PECORARO M., DABROWSKI D., WEBBER S.E., FOSTER M.L., BELVISI M.G. Functional characteri-zation and biomarker identification in the Brown Norway model of allergic airway inflammation. Br. J. Pharmacol. 2002;137:263–275. doi: 10.1038/sj.bjp.0704865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIANNA R.M., CALIXTO J.B. Characterization of the receptor and the mechanisms underlying the inflammatory response induced by desArg9-BK in mouse pleurisy. Br. J. Pharmacol. 1998;123:281–291. doi: 10.1038/sj.bjp.0701590. [DOI] [PMC free article] [PubMed] [Google Scholar]