Abstract
Bradykinin (BK) appears to play an important role in the development and maintenance of inflammation. Here, we assessed the role of the BK B2 receptor for the injuries that occur after ischemia and reperfusion (I/R) of the territory irrigated by the superior mesenteric artery.
Tissue (lung and duodenum) kallikrein activity increased after ischemia with greater enhancement after reperfusion. A selective inhibitor of tissue kallikrein, Phenylacetyl-Phe-Ser-Arg-N-(2,3-dinitrophenyl)-ethylenediamine (TKI, 0.001–10 mg ml−1), inhibited kallikrein activity in a concentration-dependent manner in vitro. In vivo, pretreatment with TKI (30 mg kg−1) prevented the extravasation of plasma and the recruitment of neutrophils.
Similarly, the bradykinin B2 receptor antagonists, HOE 140 (0.01–1.0 mg kg−1) or FR173657 (10.0 mg kg−1), inhibited reperfusion-induced increases in vascular permeability and the recruitment of neutrophils in the intestine and lungs.
In a model of more severe I/R injury, HOE 140 (1.0 mg kg−1) inhibited the increase in vascular permeability, neutrophil recruitment, haemorrhage and tissue pathology. Furthermore, HOE 140 significantly inhibited the elevations of TNF-α in tissue and serum and partially prevented lethality. This was associated with an increase in the concentrations of IL-10 in tissue and serum.
Thus, our results demonstrate that, following intestinal I/R injury, there is an increase in tissue kallikrein activity and activation of BK B2 receptors. B2 receptor activation is essential for the development of inflammatory tissue injury and lethality. These results contrast with those of others showing that BK mostly exerts a protective role during I/R injury.
Keywords: Ischemia and reperfusion, cytokines, TNF-α, bradykinin, neutrophils, shock
Introduction
Kinins are vasoactive proinflammatory peptides involved in numerous pathophysiological processes, including expression of adhesion molecules, leukocyte infiltration and formation of interendothelial gaps and protein extravasation in postcapillary venules (Ahluwalia & Perretti, 1996; Félétou et al., 1996; Saleh et al., 1997; Bandeira-Melo et al., 1999; Calixto et al., 2000). The actions of kinins may be direct or associated with the stimulation of secondary mediators of inflammation, including prostanoids, tachykinins, cytokines, mast-cell-derived products and nitric oxide (Marceau & Bachvarov, 1998; Calixto et al., 2000). These proinflammatory actions reinforce the idea that kinins are important in the development and maintenance of inflammatory and nociceptive processes (Marceau & Bachvarov, 1998; Calixto et al., 2000). Indeed, the activation of the kallikrein–kinin system in acute inflammation has long been recognized (reviewed by Calixto et al., 2000). Kinins are generated from kininogens following cleavage by plasma or tissue kallikreins and act on specific G protein-coupled receptors of which there are two clearly defined and cloned types: B1 and B2 receptors.
The results of a number of recent studies indicate that administration of bradykinin (BK) at comparatively low doses attenuates ischemia–reperfusion (I/R) injury. These protective effects appear to be secondary to the potent vasodilatory effects of BK. For example, intracoronary administration of BK reduces myocardial infarct size after reperfusion in canine, rabbit and pig hearts (Noda et al., 1992; Jin & Chen, 1998; Kositprapa et al., 2001; Shigematsu et al., 2001). Furthermore, I/R injury is also attenuated in preparations treated with angiotensin-converting enzyme inhibitors that act to prevent the degradation of endogenous BK by kininase II (Lazar et al., 2001; Whang et al., 2001). In addition, there are studies demonstrating that HOE 140, a specific B2 receptor antagonist, abolished the protective effect of BK during (I/R) injury (Li & Sato, 2001). The latter studies suggest that BK is released during I/R and acts to protect the tissue from injury. That BK exerts such powerful protective effects in I/R is surprising because of its well-known proinflammatory actions (Calixto et al., 2000).
We have previously demonstrated that neutrophil recruitment is an essential component of the local and remote inflammatory changes that occur after I/R of the superior mesenteric artery in rats (Souza et al., 2000a,2000b). Of note, in the latter model, there is a marked systemic inflammatory response that precedes the death of animals (Souza et al., 2000a). The aim of the present study was to assess the role of BK in mediating the reperfusion injury that follows ischemia of the superior mesenteric artery in rats. Initial experiments evaluated the dose-dependent effects of B2 receptor antagonists and the effects of a tissue kallikrein-specific inhibitor, phenylacetyl-Phe-Ser-Arg-N-(2,3-dinitrophenyl)-ethylenediamine (TKI) (Juliano et al., 1995; Emim et al., 2000), on a model of mild reperfusion-induced injury. We then investigated the effects of the B2 receptor antagonist HOE 140 in the model of more severe reperfusion-induced injury, with particular emphasis on the effects of this drug on lethality, systemic injuries and cytokine concentration in tissue and serum.
Methods
Animals
Male Wistar rats (200–220 g) obtained from the Bioscience unit of our Institution were housed in standard conditions and had free access to commercial chow and water. All procedures described here had prior approval from the local animal ethics committee.
I/R injury
Rats were anesthetized with urethane (140 mg kg−1, i.p. ) and laparotomy was performed. This procedure was sufficient to keep the animals under anesthesia until the end of the experiment. The superior mesenteric artery (SMA) was isolated and ischemia was induced by totally occluding the SMA for 30 or 120 min. After ischemia, reperfusion was initiated by removal of the occlusion. Animals made ischemic for 30 or 120 min were allowed to reperfuse for 30 (mild I/R) or 120 (severe I/R) min, respectively. The durations of I/R were based upon previous experiments (Souza et al., 2000a,2000b) and were optimal for mild and severe reperfusion injuries. Sham-operated animals or animals only made ischemic were used as controls for the reperfusion-induced injury. At the end of the experiment, animals were killed by cervical dislocation. To maintain the homogeneity within a particular group, inflammatory parameters were assessed only in animals that were alive after 120 min of reperfusion.
Initial experiments were carried out in the mild reperfusion injury model to examine the dose-dependent effects of BK B2 receptor antagonist (HOE 140, 0.01–1.0 mg kg−1). For comparison, a structure unrelated B2 receptor antagonist, FR173657 (10.0 mg kg−1, Asano et al., 1997) and TKI (30 mg kg−1, Emim et al., 2000) were also used. In these experiments, BK B2 receptor antagonists were administered i.v. just prior to the reperfusion of the SMA, while TKI was administered s.c. 40 min before reperfusion. We then tested the effects of the administration of HOE 140 (1.0 mg kg−1, i.v., just prior to reperfusion) in the more severe I/R model. None of the drugs used in the present study had any significant effects on basal parameters (data not shown) and to simplify the graphs presented, basal data obtained in vehicle- or drug-treated animals have been pooled for presentation.
Evaluation of changes in vascular permeability
The extravasation of Evans blue dye into the tissue was used as an index of increased vascular permeability (Souza et al., 2000b). Evans blue (20 mg kg−1) was administered i.v. (1 ml kg−1) via a femoral vein 2 min prior to reperfusion of the ischemic artery. At 30 (in the mild injury model) or 120 min (in the severe injury model) after reperfusion, fragments of the duodenum (10 cm) were cut open and allowed to dry in a Petri dish for 24 h at 37°C. The dry weight of the tissue was determined and Evans blue extracted using 3 ml of formamide (24 h at room temperature). The amount of Evans blue in the tissue was obtained by comparing the optical density (OD) of the extract with that of a standard Evans blue curve read at 620 nm in an ELISA plate reader. Results are presented as the amount of Evans blue in micrograms per 100 mg of tissue. The right ventricle was flushed with 20 ml of phosphate-buffered saline (PBS) to wash the intravascular Evans blue in the lungs. The left lung was then excised and used for Evans blue extraction. The right lung was used for the determination of myeloperoxidase (MPO) as described below.
MPO levels
The extent of neutrophil accumulation in the intestine and right lung tissue was measured by assaying MPO activity as previously described (Matos et al., 1999). Briefly, a fragment of the duodenum and the flushed right lungs of animals that had undergone I/R injury were removed and snap-frozen in liquid nitrogen. Upon thawing, the tissue (1 g of tissue per 19 ml of buffer) was homogenized in pH 4.7 buffer (0.1 M NaCl, 0.02 M NaPO4, 0.015 M NaEDTA), centrifuged at 260 × g for 10 min and the pellet underwent hypotonic lysis (15 ml of 0.2% NaCl solution followed 30 s later by the addition of an equal volume of a solution containing 1.6% NaCl and 5% glucose). After a further centrifugation, the pellet was then resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and rehomogenized. Aliquots (1 ml) of the suspension were transferred into 1.5 ml Eppendorf tubes followed by three freeze–thaw cycles using liquid nitrogen. These were then centrifuged for 15 min at 10,000 × g; the pellet was resuspended to 1 ml and samples of intestine and lung were diluted prior to the assay. MPO activity in the resuspended pellet was assayed by measuring the change in OD at 450 nM using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). Results were expressed as the total number of neutrophils by comparing the OD of tissue supernatant with that of rat peritoneal neutrophils processed in the same way. To this end, neutrophils were induced in the peritoneum of rats by injecting 3 ml of 5% casein. A standard curve of neutrophil numbers versus OD was obtained by processing purified neutrophils (>95% purity) as above and assaying for MPO activity.
Determination of the concentration of circulating leukocytes
The total number of circulating leukocytes and neutrophils was evaluated in blood samples obtained via a cannula in the femoral artery. Samples were collected prior to ischemia (time 0), 120 min after ischemia, and 30 and 120 min after reperfusion. The number of total circulating leukocytes was determined by counting leukocytes in a modified Neubauer chamber after staining with Turk's solution and differential counts by evaluating the percentage of each leukocyte on blood films stained with May–Grunwald–Giemsa.
Measurement of hemoglobin levels
The levels of hemoglobin in the tissues were used as an index of tissue hemorrhage. Tissues were carefully washed with excess saline to remove blood attached to the intestinal epithelia or serosa. No attempt was made to perfuse the vessels with saline as no obvious hyperemia was present. After washing, a sample of approximately 100 mg of duodenum was removed and homogenized in Drabkin's colour reagent according to instructions of the manufacturer (Analisa, Belo Horizonte, Brazil). The suspension was centrifuged for 15 min at 3000 × g and filtered using 0.2 μM filters. The resulting solution was read using an ELISA plate reader at 520 nM and compared with a standard curve of hemoglobin.
Measurement of cytokine levels in serum, intestine and lungs
TNF-α, IL-1β, IL-6 and IL-10 levels were measured in the serum, lung and intestine of animals using ELISA techniques as previously described (Hagan et al., 1993; Rees et al., 1999a,1999b; Francischi et al., 2000). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum samples were analyzed at a 1 : 3 dilution in PBS. Duodenum (100 mg) or lung of sham-operated and reperfused animals was homogenized in 1 ml of PBS (0.4 M NaCl and 10 mM NaPO4) containing antiproteinases (0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3000 × g and the supernatant immediately used for ELISA assays at a 1 : 5 dilution in PBS. ELISA plates (Nunc MaxiSorb) were coated with a sheep anti-rat TNF-α/IL-1β/IL-6 or IL-10 polyclonal antibodies (1–2 μg ml−1) overnight. The plates were washed thrice and then blocked with 1% bovine serum albumin. After a further wash, plates were incubated with samples or recombinant rat cytokine and incubated overnight. The biotinylated polyclonal antibodies were used at a 1 : 1000 to 1 : 2000 dilution and the assays had a sensitivity of 16 pg ml−1.
Evaluation of tissue kallikrein activity and effects of TKI
Kallikrein activity was determined as previously described (Chagas et al., 1992) by incubation of the samples with the specific chromogenic synthetic peptide DVal.Leu.Arg-pNA as substrate in the absence or presence of TKI. The specific activity was expressed as picomoles of the product liberated per minute per milligram of protein of the sample.
Histology
Sections of duodenum were obtained from the same areas of the small intestine from representative animals in each of the treatment groups. The tissue was fixed in 10% formalin, embedded in paraffin and 4 μM-thick sections obtained. The sections were then stained with hematoxylin and eosin and examined under a light microscope. Lungs were inflated with 2 ml of 10% phosphate-buffered formalin, removed from the animals and embedded and sectioned as above.
Drugs and reagents
The following drugs were obtained from Sigma (U.S.A.): urethane, Evans blue, hexadecyltrimethylammonium bromide and 3,3,5,5, tetramethyl-benzidine. HOE 140 was a gift from Dr Wirth (Hoechst, Frankfurt, Germany), FR173657 (Asano et al., 1997) from Dr Aromori (Fujisawa, Pharmaceutical Co., Japan). TKI was prepared as previously described by Juliano et al. (1995). HOE 140 was dissolved in saline just prior to use. TKI was dissolved in saline/Tween 80 (0.3%) and FR173657 in 5% DMSO. Experiments in drug-treated vehicles were performed in parallel with vehicle-treated controls. However, and because none of the drug vehicles described above had any effect on I/R injury, data on vehicle-treated animals were pooled for presentation.
Statistical analysis
Results are shown as the mean±s.e.m. Per cent inhibition was calculated by subtracting the background levels of Evans blue extravasation or MPO (obtained in sham-operated animals) from control and treated animals. Differences were evaluated by using analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc analysis. Results with P<0.05 were considered significant. For survival curves, differences between groups at different time points were compared using Fisher's exact test and considered significant when P<0.05.
Results
Determination of kallikrein activity and effects of a TKI in a model of mild I/R injury
In animals only submitted to ischemia, there was a detectable increase of tissue kallikrein activity in the intestine or lungs when compared to sham-operated or control animals (Figure 1a, b). In the intestine, kallikrein activity increased and peaked at 15 min after reperfusion (Figure 1a). In the lungs, kallikrein activity was similar to postischemic levels at 15 min after reperfusion but greatly increased thereafter (Figure 1b).
Figure 1.
Kallikrein activity in the intestine (a) and lungs (b) following reperfusion of the ischemic superior mesenteric artery and inhibition by a specific TKI (c,d). In (a) and (b), kallikrein activity in intestinal and lung extracts were evaluated in control, sham-operated rats and at various times after ischemia and/or reperfusion of the superior mesenteric artery. In (c) and (d), the inhibitory effects of increasing concentrations of TKI (0.001–10 mg ml−1) were assessed on intestinal and lung kallikrein activity, respectively, following mild ischemia (30 min) and reperfusion (30 min) injury. Results are shown as kallikrein activity (mol of pNA min−1 mg−1 of protein) and are the mean±s.e.m. of five to six animals in each group. *P<0.01 when compared to sham-operated or control animals. In (c) and (d), there was significant inhibition in concentrations of TKI above 0.01 mg ml−1.
Next, we examined the effects of TKI, a specific inhibitor of tissue kallikrein (Juliano et al., 1995; Emim et al., 2000), on the kallikrein activity found after 30 min of ischemia and 30 min of reperfusion. TKI inhibited the activity found both in the intestine and lungs in a concentration-dependent manner (Figure 1c, d), with IC50 [95% confidence interval] values of 15.0 [4.4–50.8] and 12.2 [4.3–34.7] μg ml−1, respectively. We also assessed the effect of an inhibitor of serum kallikrein, soybean trypsin inhibitor (SBTI, 40 μg ml−1). SBTI, a plasma kallikrein inhibitor (Shori et al., 1992), had little effect (inhibition of 20% of the total activity) on the kallikrein activity detected in the lung or intestine after reperfusion injury (data not shown).
Overall, the results above clearly demonstrate the activation of the kallikrein–kinin system during I/R injury and the ability of TKI to abolish kallikrein activity. In order to investigate the potential functional contribution of the kallikrein–kinin system to the development of mild I/R injury, animals were treated with TKI. As seen in Figure 2, pretreatment with TKI prevented not only the extravasation of plasma, but also the recruitment of neutrophils that followed the reperfusion of the ischemic superior mesenteric artery (Figure 2).
Figure 2.
Dose-dependent effects of the treatment with TKI or BK B2 receptor antagonists on the increase in vascular permeability and recruitment of neutrophils in the intestine and lungs following mild ischemia (30 min) and reperfusion (30 min) injury of the superior mesenteric artery. Changes in vascular permeability in the (a) intestine and (c) lungs were assessed by evaluating the extravasation of Evans blue dye. Neutrophil recruitment in the (b) intestine and (d) lungs was assessed by evaluating tissue levels of MOP. BK B2 receptor antagonists HOE 140 (0.01–1.0 mg kg−1) or FR173657 (10 mg kg−1) or TKI (30 mg kg−1) were given i.v. 5 min prior to reperfusion. Control animals (I/R) received drug vehicle and results are pooled for presentation. Results are shown as micrograms of Evans blue or as number of neutrophils per 100 mg of tissue and are the mean±s.e.m. of at least five to six animals in each group. *for P<0.01 when compared to sham-operated animals and # for P<0.05 when compared to vehicle-treated animals submitted to I/R.
Dose-dependent effects of B2 receptor antagonists in a mild model of I/R injury
The experiments above suggest the local activation of kallikrein with potential release of BK and action on its receptors. The next series of experiments was then designed to investigate the putative role of the BK B2 receptor for reperfusion injury. As clearly observed in Figure 2, postischemic treatment of animals with the B2 receptor antagonist HOE 140 inhibited in a dose-dependent manner both the increase in vascular permeability and the recruitment of neutrophils in the intestine and lungs following reperfusion of the ischemic SMA. Of note, maximal inhibition occurred at the dose of 1.0 mg kg−1 of HOE 140 (Figure 2).
In order to confirm an important role for the B2 receptor, animals were treated with FR173657, a nonpeptide B2 receptor antagonist structurally distinct from HOE 140 (Asano et al., 1997). As seen with HOE 140, postischemic treatment with FR173657 effectively inhibited both the increase in vascular permeability and the recruitment of neutrophils following mild I/R injury (Figure 2).
Effects of a B2 receptor antagonist on the local, remote and systemic injuries in a model of severe I/R injury
The next series of experiments was carried out in a model of severe I/R injury where, in addition to the changes in vascular permeability and neutrophil accumulation, we could observe tissue haemorrhage, leucopoenia, increase in the levels of cytokine in tissue and blood and significant lethality (Souza et al., 2000a). As in the model of mild I/R injury, there was an increase of kallikrein activity in intestine and lungs after severe I/R injury when compared to sham-operated or control animals (Figure 1). Moreover, TKI was also capable of completely inhibiting (>95%) this tissue kallikrein (data not shown).
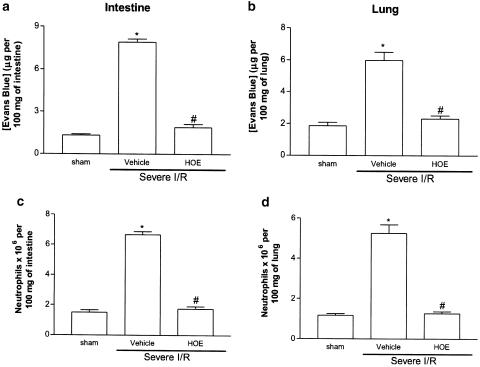
For the experiments evaluating the role of the B2 receptor during severe I/R injury, HOE 140 was used at a dose shown to be maximally inhibitory in the mild I/R injury model (1.0 mg kg−1). Postischemic treatment with HOE 140 virtually abolished the increase in vascular permeability and neutrophil recruitment in the intestine and in the lung following severe I/R injury (Figure 3). Treatment with HOE 140 also abolished the intestinal increase of hemoglobin, a marker of tissue hemorrhage (Figure 4).
Figure 3.
Effects of the treatment with the BK B2 receptor antagonist HOE 140 was on the increase in vascular permeability and recruitment of neutrophils in the intestine and lung following severe ischemia (120 min) and reperfusion (120 min) injury of the superior mesenteric artery. Changes in vascular permeability in the (a) intestine and (b) lungs were assessed by evaluating the extravasation of Evans blue dye. Neutrophil recruitment in the (c) intestine and (d) lungs was assessed by evaluating tissue levels of MOP. HOE 140 (1.0 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received PBS. Results are shown as micrograms of Evans blue or as number of neutrophils per 100 mg of tissue and are the mean±s.e.m. of five to six animals in each group. *for P< 0.01 when compared to sham-operated animals and # for P<0.05 when compared to vehicle-treated animals submitted to I/R.
Figure 4.
Effects of the treatment with the BK B2 receptor antagonist HOE 140 on the hemorrhage observed in the intestine following severe ischemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. Tissue hemorrhage was assessed by evaluating tissue levels of hemoglobin. HOE 140 (1.0 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received PBS. Results are shown as μg hemoglobin per 100 mg of tissue and are the mean±s.e.m. of five to six animals in each group. *for P<0.01 when compared to sham-operated animals and # for P<0.01 when compared to vehicle-treated animals submitted to I/R.
When compared to sham-operated animals (Figure 5a), there was marked intestinal destruction and inflammation in animals submitted to severe I/R injury, as demonstrated by a striking loss of villi and crypts, submucosal hyperemia, tissue edema and neutrophil influx (Figure 5b). In rats treated with HOE 140, tissue architecture was significantly more preserved. However, there was still a degree of tissue damage, as assessed by the loss of tips and widening of villi and tissue edema (Figure 5c).
Figure 5.
Intestinal damage following severe ischemia (120 min) and reperfusion (120 min) of the territory irrigated by the superior mesenteric artery. Rats were sham-operated (a), or submitted to I/R injury in the presence of vehicle (b) or HOE 140 (c, 1 mg kg−1). Duodenum was fixed and processed for histological analysis after H&E staining. Original magnification, × 100.
We have previously shown an increase in the concentration of blood neutrophils during the ischemic period and a rapid drop in neutrophil levels once reperfusion occurs (Souza et al., 2000a). As HOE 140 was administered just prior to reperfusion, the concentration of circulating neutrophils at 120 min of ischemia was similar and markedly greater than sham-operated animals (sham, 0.9±0.2 neutrophils × 106 ml−1 of blood; 120 min after ischemia, 4.3±0.4 neutrophils; 120 min after in HOE 140-treated animals, 4.4±0.3; n=5–6). In vehicle-treated animals, reperfusion of the ischemic SMA induced a rapid fall of circulating neutrophils to levels observed in sham-operated animals (data not shown). Pretreatment with HOE 140 reversed by approximately 30% the rapid neutropaenia that occurred 120 min after reperfusion (sham, 0.8±0.1 neutrophils × 106 ml−1 of blood; 120 min after reperfusion, 0.2±0.03 neutrophils; 120 min after reperfusion in HOE 140-treated animals, 1.3±0.3; n=5–6, P<0.05).
The levels of proinflammatory cytokines–IL-1β, IL-6 and TNF-α–and the antiinflammatory cytokine IL-10 are markedly elevated in serum and tissues after severe I/R injury (Figure 6, Table 1) (Souza et al., 2000a). Postischeimic treatment with HOE 140 significantly inhibited the elevations of TNF-α in tissue and serum after severe I/R injury (Figure 6a,c,e). Of note, the magnitude of TNF-α inhibition was greater in tissue than in serum (Figure 6). Interestingly, pretreatment with HOE 140 was accompanied by an increase in the concentrations of IL-10 in the tissue and serum above that observed after severe I/R injury only (Figure 6b,d,f). Overall, pretreatment with HOE 140 prevented the increase in the concentrations of IL-6 in tissues and serum, whereas the drug had little effect on the concentrations of IL-1β in tissue (Table 1). The increase in serum concentrations of IL-1β was exacerbated by HOE 140 treatment (Table 1).
Figure 6.
Effects of the treatment with the BK B2 receptor antagonist HOE 140 on the concentrations of TNF-α and IL-10 in the intestine, lung and serum following severe ischemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. The concentration of TNF-α (a,c,e) and IL-10 (b,d,f) were assessed in the intestine (a,b), lung (c,d) and serum (e,f) by using specific ELISA. HOE 140 (1.0 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received PBS. Results are shown as pg TNF-α or IL-10 per ml of plasma or as pg TNF-α or IL-10 per 100 mg of tissue and are the mean±s.e.m. of five to six animals in each group. *for P<0.01 when compared to sham-operated animals and # for P<0.05 when compared to vehicle-treated animals submitted to I/R.
Table 1.
Effects of the treatment with the bradykinin B2 receptor antagonist HOE 140 on the concentrations of IL-1β and IL-6 in the intestine, lung and serum following severe ischemia (120 min) and reperfusion (120 min) of the superior mesenteric artery
| IL-1β | IL-6 | |||||
|---|---|---|---|---|---|---|
| Intestine | Lung | Serum | Intestine | Lung | Serum | |
| Sham | 118±15 | 512±42 | 22±3 | 6±3 | 12±4 | 211±11 |
| Vehicle | 127±10 | 1104±120* | 149±14* | 169±9* | 900±101* | 541±46* |
| HOE 140 | 145±18 | 1009±16* | 294±22*** | 84±7*** | 467±42*** | 392±30*** |
IL-1β and IL-6 were assessed by using specific ELISA. HOE 140 (1.0 mg kg−1) was given i.v. 5 min after reperfusion. Control animals submitted to I/R (vehicle) received PBS. Results are shown as pg cytokine per ml of serum or as pg cytokine per 100 mg of tissue and are the mean±s.e.m. of five to six animals in each group.
P<0.01 when compared to sham-operated animals and ** P<0.05 when compared to vehicle-treated animals submitted to I/R.
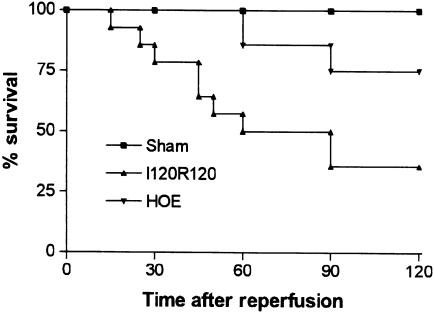
Our previous studies have shown that severe reperfusion injury is accompanied by significant TNF-α-dependent lethality, reaching 60% in most experiments (Souza et al., 2001). In the present series of experiments, 65% of animals were dead after 120 min of reperfusion (Figure 7). Treatment with HOE 140 partially prevented lethality (P<0.05) and approximately 75% of animals were alive at 120 min (Figure 7).
Figure 7.
Effects of the treatment with the BK B2 receptor antagonist HOE 140 on the lethality following severe I/R of the superior mesenteric artery. HOE 140 (1.0 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received PBS. Survival was monitored as indicated and animals were killed after 120 min. The lethality in HOE 140-treated animals was significantly different (P<0.05) from vehicle-treated rats.
Discussion
There is compelling evidence indicating that kinins are rapidly generated after tissue injury and they seem to modulate many of the changes observed during the inflammatory process, including vasodilatation, increase of vascular permeability, plasma extravasation, cell migration, pain and hyperalgesia (Marceau & Bachvarov, 1998; Calixto et al., 2000). The B2 receptors are by far the best characterized of the kinin receptors, being constitutively and widely expressed, throughout the central and peripheral nervous system, mediating many of the physiological and pathological effects of BK. We have recently described the reperfusion-induced inflammatory injury, which occurs following ischemia of the superior mesenteric artery in rats (Souza et al., 2000a,2000b,2001,2002a,2002b). In our model, the inflammatory injury is marked, affecting both local and remote organs and accompanied by systemic cardiovascular manifestations and lethality. As I/R injury is a process that occurs very rapidly, we reasoned that the participation of rapidly generated mediator systems (such as the kallikrein–kinin system) and constitutively expressed receptors (such as the B2 receptor) was likely. In the present work, we evaluated the activation of the kallikrein–kinin system and the effects of treatment with inhibitors of the system or antagonists of the receptor most likely to be activated following reperfusion of the ischemic superior mesenteric artery in rats.
Pathological activation of the kallikrein–kinin system from preformed substrates generally occurs during pathological situations characterized by inflammation (Calixto et al., 2000). In addition, there are studies demonstrating that the synthesis of kinin-active proteases is enhanced after trauma or inflammation (Cumming et al., 1994) and tissue kallikrein is also present and secreted from infiltrating leucocytes (Wu et al., 1993; Marceau & Bachvarov, 1998). In our model, the activity of tissue kallikrein increased in the duodenum and lung after ischemia. However, the activity of the enzyme enhanced further after reperfusion. These results suggest that preformed enzymes in tissue may have been activated during ischemia and further activated during the reperfusion process. As neutrophils infiltrate during reperfusion and are essential for tissue damage (e.g. Souza et al., 2000a), it is possible that kallikrein derived from these cells may contribute to the enhanced activity observed after reperfusion. However, further studies are necessary to demonstrate the latter possibility. Of note, there was little difference in the activity of tissue kallikrein between groups submitted to mild or severe I/R injury, suggesting that kallikrein activation was already maximal after short periods of I/R.
Experiments using TKI, a tissue kallikrein-specific inhibitor (Juliano et al., 1995; Emim et al., 2000), showed that the drug suppressed in a concentration-dependent manner the kallikrein activity extracted from tissues. On the other hand, SBTI, an inhibitor with greater selectivity for plasma kallikrein (Chagas et al., 1992), had little effect on the kallikrein activity extracted from tissues. More importantly than the detection of kallikrein activity, our results clearly demonstrate that inhibition of the enzyme in vivo with TKI is accompanied by marked inhibition of the injury that follows reperfusion of the superior mesenteric artery. Thus, in our system, there is activation of tissue kallikrein, which is functionally relevant for reperfusion-associated injury. As BK is the major mediator derived from the action of tissue kallikrein, we assessed the role of the constitutively expressed B2 receptor for the actions of the presumably released BK.
Initial experiments in a model of mild I/R injury clearly showed that the classic B2 receptor antagonist, HOE 140, inhibited both the local (intestine) and remote (lung) increase in vascular permeability and neutrophil accumulation, in a dose-dependent manner. The role of the B2 receptor in mediating injury was re-enforced by the inhibitory effects of a nonpeptide B2 receptor antagonist, FR 173657. The inhibitory effects of B2 receptor antagonists are in line with previous studies demonstrating the capacity of this class of drugs to suppress leucocyte recruitment and activation in acute models of inflammation (e.g. Abraham et al., 1991; Bandeira-Melo et al., 1999; Perron et al., 1999). As the local influx of neutrophils is a determinant in the development of reperfusion injury following ischemia (Souza et al., 2000a,2000b), the capacity of HOE 140 to modulate the recruitment of neutrophils may underlie most of the beneficial effects in this model of mild reperfusion-induced injury. Overall, the data above are consistent with the important role of the local activation of tissue kallikrein, release of BK in tissues and activation of B2 receptors for injury to occur.
In a model of more severe I/R injury, in addition to the vascular permeability and neutrophil influx, there are marked systemic alterations that include hypotension, elevated levels of proinflammatory cytokines, neutropenia and death (Souza et al., 2000a,2001). It was thus of interest to examine whether B2 receptor antagonists would also function in this model of more severe injury. We chose to use HOE 140 in these experiments because of the greater availability and more common usage of this drug in the literature. In the model of severe injury, pretreatment with HOE 140 markedly inhibited both the neutrophil accumulation and increase in vascular permeability. Not only was the site of injury (i.e. the intestine) protected, but there was also marked protection of the reperfusion injury to the lungs. The inhibition of neutrophil recruitment into tissue was reflected by the partial capacity of HOE 140 treatment to reverse the leucopenia observed during reperfusion. Moreover, HOE 140 greatly attenuated intestinal pathology, as attested by the decrease in hemorrhage and the amelioration of tissue histopathology.
BK acting via B2 receptors may induce nuclear factor-κB (NF-κB) activation in various cell types (Pan et al., 1998) and, consequently, induce expression of proinflammatory cytokines (Valen et al., 2001). In the model of more severe injury, there is a marked local and systemic release of proinflammatory cytokines, including TNF-α, IL-6 and IL-1β (Souza et al., 2001). Of these cytokines, TNF-α appears to play a major pathophysiological role in the model, as its inhibition prevents tissue injury and lethality that follows reperfusion (Souza et al., 2001). Interestingly, we have previously shown that the local influx of neutrophils is an important player in the cascade of events leading to tissue, but not systemic, TNF-α production. On the other hand, the initial tissue release of TNF-α, possibly mast-cell-derived, is essential for neutrophil influx to occur. An amplification circuit is thus installed in which neutrophil influx facilitates TNF-α production and TNF-α production facilitates neutrophil influx (Souza et al., 2000a,2001). Blockade of B2 receptors is accompanied by virtual abolishment of the increase in concentration of TNF-α in tissues of reperfused animals. Thus, the shown ability of B2 receptor antagonists to modulate both neutrophil influx and TNF-α production could be contributing to the beneficial effects of these drugs in the system. In contrast to the ability of HOE 140 to abolish the increase in tissue concentrations of TNF-α, the B2 receptor antagonist only partially prevented the increase in the concentration of this cytokine in serum. As systemic concentrations of TNF-α appear to be the best correlate of lethality in our system (Souza et al., 2001), the latter results are consistent with the ability of B2 receptor antagonists to prevent lethality only partially.
In agreement with the literature (Cuzzocrea et al., 1999; Souza et al., 2001; Yamamoto et al., 2001), the concentrations of IL-1β and IL-6 in tissue and serum were elevated following intestinal I/R injury (with the exception of the lack of elevation of IL-1β in the intestine). Pretreatment with HOE 140 effectively decreased IL-6 concentration in tissue and serum. The ability of B2 receptor antagonists to inhibit IL-6 production in our system is consistent with the findings of Koyama et al. (1998), who demonstrated that A549 cells stimulated with BK released several proinflammatory cytokines, including IL-6, and contributed to lung inflammation. Thus, the inhibition of IL-6 may be an additional beneficial effect of B2 receptor blockade. In contrast to its inhibitory effect on IL-6 production, HOE 140 had little effect on the increases in the concentration of IL-1β in tissues and actually enhanced the systemic concentrations of IL-1β. The latter was a surprising result as IL-1β has been shown to play a pathophysiological role during I/R injury in several situations (e.g. Hoshida et al., 2000; Yamamoto et al., 2001). At present, we are investigating the role of IL-1β in our model.
One important additional finding was the ability of the pretreatment with the B2 receptor antagonist to enhance the concentrations of IL-10 in tissues following severe reperfusion-associated injury. As IL-10 is an important modulator of the injury and lethality, which follows I/R (our own data, Frangogiannis et al., 2000; Zingarelli et al., 2001), it is possible that the increase in IL-10 levels observed may play a role in the beneficial effects of the B2 receptor antagonist in the severe injury model. The mechanisms by which the blockade of BK receptors enhances IL-10 production in the system are not known but clearly merit further investigation.
Thus, our results demonstrate that following intestinal ischemia and reperfusion injury there is an increase in tissue kallikrein activity and activation of B2 receptors. B2 receptor activation markedly contributes to the cascade of events leading to the local, remote and systemic inflammatory changes that follow intestinal reperfusion injury. In addition to activating leucocytes to migrate directly, BK may also act on leucocytes and endothelial cells to release proinflammatory mediators, such as cytokines and PAF (e.g. Koyama et al., 1998; Shigematsu et al., 1999; Calixto et al., 2000; Paegelow et al., 2002). Moreover, BK acting on B2 receptors may activate sensory nerves to release neuropeptides and potentiate the inflammatory response further (Averbeck & Reeh, 2001; Madeddu et al., 2001; Rawlingson et al., 2001). As activation of PAF and tachykinin NK1 receptors contribute to the inflammatory injuries observed in our system (Souza et al., 2000, 2002a), it is likely that B2 receptor activation may trigger a series of events eventually leading to enhanced local mediator production and inflammation. Finally, some authors have proposed that B2 receptor activation may take place at the beginning of the inflammatory process followed by desensitization of B2 receptors and cytokine-mediated induction of B1 receptors (Campos & Calixto, 1995; Phagoo et al., 1999). Of note, previous studies have demonstrated that myocardial I/R induced the expression of functional B1-receptors, which may have a deleterious role during I/R injury (Mazenot et al., 2001; Lagneux et al., 2002). Whether the proinflammatory cytokines produced in our system are sufficient to induce B1 receptor upregulation and whether the latter receptor has any functional role during intestinal I/R injury merit further investigation. Altogether, our results contrast with those of others (e.g. Noda et al., 1992; Jin et al., 1998; Kositprapa et al., 2001; Shigematsu et al., 2001) showing that BK release and action is mostly protective during I/R injury. Thus, while in the heart, the B2-dependent vasodilatory effects of BK may be sufficient to facilitate reperfusion and salvage of live tissue, in other tissues, such as in the gut, the proinflammatory actions of BK may predominate, especially after severe injury. Altogether, these results suggest that B2 receptor antagonists may be useful adjunct therapy for the treatment of the severe inflammatory injuries that follow ischemia and reperfusion of the superior mesenteric artery.
Acknowledgments
This work received financial support from Fundação Amparo a pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
Abbreviations
- BK
bradykinin
- I/R
ischemia and reperfusion
- MPO
myeloperoxidase
- SMA
superior mesenteric artery
- TKI
phenylacetyl-Phe-Ser-Arg-N-(2,4-dinitrophenyl)-ethylenediamine
References
- ABRAHAM W.M., BURCH R.M., FARMER S.G., SIELCZAK M.W., AHMED A., CORTES A. A bradykinin antagonist modifies allergen-induced mediator release and late bronchial responses in sheep. Am. Rev. Respir. Dis. 1991;143:787–796. doi: 10.1164/ajrccm/143.4_Pt_1.787. [DOI] [PubMed] [Google Scholar]
- AHLUWALIA A., PERRETTI M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1 beta in vivo in the mouse. J. Immunol. 1996;156:269–274. [PubMed] [Google Scholar]
- ASANO M., INAMURA N., HATORI C., SAWAI H., FUJIWARA T., KATAYAMA A., KAYAKIRI H., SATOH S., ABE Y., INOUE T., SAWADA Y., NAKAHARA K., OKU T., OKUHARA M. The identification of an orally active, nonpeptide bradykinin B2 receptor antagonist, FR173657. Br. J. Pharmacol. 1997;120:617–624. doi: 10.1038/sj.bjp.0700955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVERBECK B., REEH P.W. Interactions of inflammatory mediators stimulating release of calcitonin gene-related peptide, substance P and prostaglandin E(2) from isolated rat skin. Neuropharmacology. 2001;40:416–423. doi: 10.1016/s0028-3908(00)00171-4. [DOI] [PubMed] [Google Scholar]
- BANDEIRA-MELO C., CALHEIROS A.S., SILVA P.M., CORDEIRO R.S., TEIXEIRA M.M., MARTINS M.A. Suppressive effect of distinct bradykinin B2 receptor antagonist on allergen-evoked exudation and leukocyte infiltration in sensitized rats. Br. J. Pharmacol. 1999;127:315–320. doi: 10.1038/sj.bjp.0702536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., CALIXTO J.B. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br. J. Pharmacol. 1995;114:1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAGAS J.R., HIRATA I.Y., JULIANO M.A., XIONG W., WANG C., CHAO J., JULIANO L., PRADO E. Substrates specificities of tissues kallikrein and T-kininogenase: their possible role in kininogen processing. Biochemistry. 1992;31:4969–4974. doi: 10.1021/bi00136a008. [DOI] [PubMed] [Google Scholar]
- CUMMING A.D., WALSH T., WOJTACHA D., FLEMING S., THOMSON D., JENKINS D.A. Expression of tissue kallikrein in human kidney. Clin. Sci. 1994;87:5–11. doi: 10.1042/cs0870005. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., DE SARRO G., COSTANTINO G., CILIBERTO G., MAZZON E., DE SARRO A., CAPUTI A.P. IL-6 knock-out mice exhibit resistance to splanchnic artery occlusion shock. J. Leukoc. Biol. 1999;66:471–480. doi: 10.1002/jlb.66.3.471. [DOI] [PubMed] [Google Scholar]
- EMIM J.A.S., SOUCCAR C., DE A. CASTRO M.S., GODINHO R.O., CEZARI M.H., JULIANO L., LAPA A.J. Evidence for activation of the tissue kallikrein–kinin system in nociceptive transmission and inflammatory responses of mice using a specific enzyme inhibitor. Br. J. Pharmacol. 2000;130:1099–1107. doi: 10.1038/sj.bjp.0703362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FÉLÉTOU M., BONNARDEL E., CANET E. Bradykinin and changes in microvascular permeability in the hamster cheek pouch: role of nitric oxide. Br. J. Pharmacol. 1996;118:1371–1376. doi: 10.1111/j.1476-5381.1996.tb15547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCISCHI J.N., YOKORO C.M., CUNHA F.Q., TAFURI W.L., TEIXEIRA M.M. Effects of the PDE4 inhibitor rolipram in a rat model of arthritis. Eur. J. Pharmacol. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- FRANGOGIANNIS N.G., MENDOZA L.H., LINDSEY M.L., BALLANTYNE C.M., MICHAEL L.H., SMITH C.W., ENTMAN M.L. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J. Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- HAGAN P., POOLE S., BRISTOW A.F. Endotoxin-stimulated production of rat hypothalamic interleukin-1 beta in vivo and in vitro, measured by specific immunoradiometric assay. J. Mol. Endocrinol. 1993;11:31–36. doi: 10.1677/jme.0.0110031. [DOI] [PubMed] [Google Scholar]
- HOSHIDA S., YAMASHITA N., OTSU K., HORI M. FR167653, a cytokine-suppressive agent, reduces myocardial ischemia–reperfusion injury in rats. Cytokines Cell Mol. Ther. 2000;6:165–170. doi: 10.1080/mccm.6.4.165.170. [DOI] [PubMed] [Google Scholar]
- JIN Z.Q., CHEN X. Bradykinin mediates myocardial ischaemic preconditioning against free radical injury in guinea pig isolated heart. Clin. Exp. Pharmacol. Physiol. 1998;25:932–935. doi: 10.1111/j.1440-1681.1998.tb02346.x. [DOI] [PubMed] [Google Scholar]
- JULIANO L., PORTARO F.C.V., CHAGAS J.R., HIRATA I.Y., JULIANO M.A., BIZETO L., ANTUNES E., DE NUCCI G., PRADO E.S. Design of inhibitors for tissue kallikreins based on their extended binding site specificities. Peptides. 1995;1994:885–886. [Google Scholar]
- KOSITPRAPA C., OCKAILI R.A., KUKREJA R.C. Bradykinin B2 receptor is involved in the late phase of preconditioning in rabbit heart. J. Mol. Cell Cardiol. 2001;33:1355–1362. doi: 10.1006/jmcc.2000.1396. [DOI] [PubMed] [Google Scholar]
- KOYAMA S., SATO E., NOMURA H., KUBO K., MIURA M., YAMASHITA T., NAGAI S., IZUMI T. Bradykinin stimulates type II alveolar cells to release neutrophil and monocyte chemotactic activity and inflammatory cytokines. Am. J. Pathol. 1998;153:1885–1893. doi: 10.1016/S0002-9440(10)65702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGNEUX C., BADER M., PESQUERO J.B., DEMENGE P., RIBUOT C. Detrimental implication of B1 receptors in myocardial ischemia: evidence from pharmacological blockade and gene knockout mice. Int. Immunopharmacol. 2002;2:815–822. doi: 10.1016/s1567-5769(02)00022-x. [DOI] [PubMed] [Google Scholar]
- LAZAR H.L., BAO Y., RIVERS S., COLTON T., BERNARD S.A. High tissue affinity angiotensin-converting enzyme inhibitors improve endothelial function and reduce infarct size. Ann. Thorac. Surg. 2001;72:548–554. doi: 10.1016/s0003-4975(01)02779-5. [DOI] [PubMed] [Google Scholar]
- LI Y., SATO T. Dual signaling via protein kinase C and phosphatidylinositol 3′-kinase/Akt contributes to bradykinin B2 receptor-induced cardioprotection in guinea pig hearts. J. Mol. Cell Cardiol. 2001;33:2047–2053. doi: 10.1006/jmcc.2001.1455. [DOI] [PubMed] [Google Scholar]
- MADEDDU P., EMANUELI C., BONARIA SALIS M., FRANCA MILIA A., STACCA T., CARTA L., PINNA A., DEIANA M., GASPA L. Role of calcitonin gene-related peptide and kinins in post-ischemic intestinal reperfusion. Peptides. 2001;22:915–922. doi: 10.1016/s0196-9781(01)00417-x. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., BACHVAROV D.R. Kinin receptors. Clin. Rev. Allergy Immunol. 1998;16:385–401. doi: 10.1007/BF02737658. [DOI] [PubMed] [Google Scholar]
- MATOS I.M., SOUZA D.G., SEABRA D.G., FREIRE-MAIA L., TEIXEIRA M.M. Effects of tachykinin NK1 or PAF receptor blockade on the lung injury induced by scorpion venom in rats. Eur. J. Pharmacol. 1999;376:293–300. doi: 10.1016/s0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- MAZENOT C., LOUFRANI L., HENRION D., RIBUOT C., MULLER-ESTERL W., GODIN-RIBUOT D. Endothelial kinin B(1)-receptors are induced by myocardial ischaemia–reperfusion in the rabbit. J. Physiol. 2001;530:69–78. doi: 10.1111/j.1469-7793.2001.0069m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NODA K., SASAGURI M., IDEISHI M., IKEDA M., ARAKAWA K. Cardioprotection of ACE inhibitor in ischemic heart is not dependent on the local angiotensin II formation. Agents Actions Suppl. 1992;38:217–227. [PubMed] [Google Scholar]
- PAEGELOW I., TRZECZAK S., BOCKMANN S., VIETINGHOFF G. Migratory responses of polymorphonuclear leukocytes to kinin peptides. Pharmacology. 2002;66:153–161. doi: 10.1159/000063797. [DOI] [PubMed] [Google Scholar]
- PAN Z.K., YE R.D., CHRISTIANSEN S.C., JAGELS M.A., BOKOCH G.M., ZURAW B.L. Role of the Rho GTPase in bradykinin-stimulated nuclear factor-kappaB activation and IL-1beta gene expression in cultured human epithelial cells. J. Immunol. 1998;160:3038–3045. [PubMed] [Google Scholar]
- PERRON M.S., GOBEIL F., JR, PELLETIER S., REGOLI D., SIROIS P. Involvement of bradykinin B1 and B2 receptors in pulmonary leukocyte accumulation induced by Sephadex beads in guinea pigs. Eur. J. Pharmacol. 1999;37:683–689. doi: 10.1016/s0014-2999(99)00348-9. [DOI] [PubMed] [Google Scholar]
- PHAGOO S.B., POOLE S., LEEB-LUNDBERG L.M. Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1beta shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblasts. Mol. Pharmacol. 1999;56:325–333. doi: 10.1124/mol.56.2.325. [DOI] [PubMed] [Google Scholar]
- RAWLINGSON A., GERARD N.P., BRAIN S.D. Interactive contribution of NK(1) and kinin receptors to the acute inflammatory oedema observed in response to noxious heat stimulation: studies in NK(1) receptor knockout mice. Br. J. Pharmacol. 2001;134:1805–1813. doi: 10.1038/sj.bjp.0704436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES G.S., BALL C., WARD H.L., GEE C.K., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat IL-6: expression in recombinant Escherichia coli, purification and development of a novel ELISA. Cytokine. 1999a;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- REES G.S., GEE C.K., WARD H.L., BALL C., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat tumour necrosis factor-alpha: expression in recombinant Pichia pastoris, purification, characterization and development of a novel ELISA. Eur. Cytokine Netw. 1999b;10:383–392. [PubMed] [Google Scholar]
- SALEH T.S., CALIXTO J.B., MEDEIROS Y.S. Pro-inflammatory effects induced by bradykinin in a murine model of pleurisy. Eur. J. Pharmacol. 1997;331:43–52. doi: 10.1016/s0014-2999(97)01005-4. [DOI] [PubMed] [Google Scholar]
- SHIGEMATSU S., ISHIDA S., GUTE D.C., KORTHUIS R.J. Concentration-dependent effects of bradykinin on leukocyte recruitment and venular hemodynamics in rat mesentery. Am. J. Physiol. 1999;277:H152–H160. doi: 10.1152/ajpheart.1999.277.1.H152. [DOI] [PubMed] [Google Scholar]
- SHIGEMATSU S., ISHIDA S., GUTE D.C., KORTHUIS R.J. Postischemic anti-inflammatory effects of bradykinin preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H441–H454. doi: 10.1152/ajpheart.2001.280.1.H441. [DOI] [PubMed] [Google Scholar]
- SHORI D.K., PROCTOR G.B., CHAO J., CHAN K.M., GARRET J.R. New specific assays for tonin and tissue kallikrein activities in rat submandibular glands. Biochem. Pharmacol. 1992;43:1209–1217. doi: 10.1016/0006-2952(92)90494-4. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S.P., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2000a;131:1800–1888. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br. J. Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000b;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., MENDONÇA V.A., DE-A-CASTRO M.S., POOLE S., TEIXEIRA M.M. Role of tachykinin NK receptors on the local and remote injuries following ischaemia and reperfusion of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2002a;135:303–312. doi: 10.1038/sj.bjp.0704464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., PINHO V., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effect of a BLT receptor antagonist in a model of severe ischemia and reperfusion injury in the rat. Eur. J. Pharmacol. 2002b;440:61–69. doi: 10.1016/s0014-2999(02)01313-4. [DOI] [PubMed] [Google Scholar]
- VALEN G., VAN Z.Q., HANSSON G.K. Nuclear factor kappa-B and the heart. J. Am. Cell Cardiol. 2001;38:307–314. doi: 10.1016/s0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- WHANG J., RAMASAMY R., DIZON J.M., BERGMANN S.R. Enalaprilat attenuates ischemic rises in intracellular sodium in the isolated rat heart via the bradykinin receptor. J. Cardiovasc. Magn. Reson. 2001;3:27–34. doi: 10.1081/jcmr-100001834. [DOI] [PubMed] [Google Scholar]
- WU N.Z., KLITZMAN B., ROSNER G., NEEDHAM D., DEWHIRST M.W. Measurement of material extravasation in microvascular networks using fluorescence video-microscopy. Microvasc. Res. 1993;46:231–253. doi: 10.1006/mvre.1993.1049. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO S., TANABE M., WAKABAYASHI G., SHIMAZU M., MATSUMOTO K., KITAJIMA M. The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia–reperfusion injury of the rat small intestine. J. Surg. Res. 2001;99:134–141. doi: 10.1006/jsre.2001.6106. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., YANG Z., HAKE P.W., DENENBERG A., WONG H.R. Absence of endogenous interleukin-10 enhances early stress response during post-ischaemic injury in mice intestine. Gut. 2001;48:610–622. doi: 10.1136/gut.48.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]