Abstract
α2-Adrenoceptor-mediated contractions in porcine blood vessels can be enhanced in the presence of the thromboxane-mimetic U46619, and forskolin. The aim of this study was to determine the role of U46619 in the enhanced contractions, and to determine whether signalling through the ERK–MAP kinase pathway is involved.
Responses to the α2-adrenoceptor agonist UK14304 (1 μM) were increased from 22±3% of the response to 60 mM KCl to 68±12% (n=8, mean±s.e.m.) in the presence of a low concentration of U46619 (<20% of the 60 mM KCl response).
Both the direct and the U46619-enhanced UK14304 responses were inhibited by 50 μM PD98059, an inhibitor of the ERK–MAP kinase pathway. UK14304-induced contractions were associated with an increase in ERK2 phosphorylation, indicating an increased activity. In the presence of U46619, there was an enhanced phosphorylation of ERK2. U46619 on its own had no effect on ERK phosphorylation.
Both the direct and enhanced UK14304 contractions were inhibited in the absence of extracellular calcium. These conditions also prevented the increase in ERK2 phosphorylation. This indicates a role for calcium influx in the enhanced contractions.
In conclusion, this study demonstrates that precontraction with the thromboxane-mimetic U46619 enhances α2-adrenoceptor-mediated vasoconstriction through the enhancement of the ERK–MAP kinase pathway, and influx of extracellular calcium.
Keywords: ERK, α2-adrenoceptors, thromboxane, vasoconstriction, calcium influx
Introduction
α2-Adrenoceptors are generally considered to be negatively coupled to adenylyl cyclase through a Gi-protein (Bylund et al., 1994). Activation of these receptors in blood vessels causes a reduction in intracellular adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP) production (Wright et al., 1995; Roberts et al., 1998). Studies in the porcine isolated ear artery have demonstrated that α2-adrenoceptor-mediated vasoconstriction can be enhanced by precontraction with the thromboxane-mimetic U46619, followed by relaxation with the adenylyl cyclase-stimulating agent forskolin (Roberts et al., 1998). At least part of this enhanced contraction is caused by the ability of α2-adrenoceptors to inhibit forskolin-stimulated cyclic AMP levels, thereby allowing the U46619-induced tone to return (Roberts et al., 1998). However, there is also evidence to indicate that α2-adrenoceptor-mediated vasoconstriction can be enhanced through a pathway that is independent of an inhibition of adenylyl cyclase. For example, preconstriction with U46619 followed by relaxation of the tissue with either sodium nitroprusside or dibutyryl cyclic AMP (a cyclic AMP analogue) also uncovers enhanced contractions to UK14304, independent of a reduction of cyclic AMP (adenylyl cyclase-independent pathway; Roberts et al., 1998). Similar results have been found in the porcine splenic artery (Roberts et al., 1999). The mechanism of the enhanced vasoconstriction through this adenylyl cyclase-independent pathway is unknown. One possibility is that it is the presence of U46619 that leads to the enhanced response, as is the case in the bovine pulmonary artery (Sweeney et al., 1995).
Recent studies have demonstrated that α2-adrenoceptor-mediated vasoconstriction in the porcine palmar lateral vein occurs through activation of the ERK–MAP kinase pathway, which is downstream of an influx of extracellular calcium (Roberts, 2001). U46619 is also able to stimulate the ERK–MAP kinase pathway in vascular smooth muscle cells, although whether this signalling pathway is involved in U46619-mediated vasoconstriction is not known (Grosser et al., 1997). Interestingly, in bovine coronary artery smooth muscle cells U46619 is able to enhance the mitogenic effect of platelet-derived growth factor through a mechanism that may involve the ERK signal transduction cascade (Grosser et al., 1997). As the ERK–MAP kinase signal transduction cascade is important in direct α2-adrenoceptor-mediated vasoconstriction, it is possible that U46619 enhances the α2-adrenoceptor-mediated responses by enhancing the signalling through this pathway. U46619-induced vasoconstriction is dependent upon influx of calcium, although it can also cause vasoconstriction in the absence of calcium, suggesting that it can cause contraction through calcium sensitisation (Han et al., 1995; Tosun et al., 1998). As α2-adrenoceptor-mediated vasoconstriction and ERK activation in the porcine palmar lateral vein are dependent upon influx of extracellular calcium it is possible that this part of the signalling pathway may also be involved in the enhancement by U46619. On the other hand, if U46619 enhances the α2-adrenoceptor-mediated vasoconstriction through increasing the sensitivity of the tissues to calcium, the enhanced response may not be sensitive to removal of extracellular calcium.
The aim of this study was to determine the role of the thromboxane-mimetic U46619 in the enhancement of α2-adrenoceptor-mediated vasoconstriction in the porcine-isolated ear artery. Further aims were to determine whether the enhanced response is mediated through an increase in the ERK signal transduction cascade and to determine the role of calcium influx.
Methods
Isometric tension recordings
Porcine ears were obtained from a local abattoir and transported to the laboratory on ice. Ear arteries were dissected out and placed in Krebs–Henseleit buffer containing 2% Ficoll which had been pregassed with 95% O2/5% CO2, and stored overnight at 4°C. The following day, ear arteries were dissected into 5 mm ring segments and suspended in an isolated organ bath containing Krebs–Henseleit buffer maintained at 37°C and constantly gassed with 95% O2/5% CO2. The lower support was fixed and the upper support was connected to a force transducer (Lectromed, Letchworth, U.K.) linked to a PCLab data-acquisition system (AD Instruments Ltd., Hastings, U.K.) via an amplifier. After a 20 min equilibration period, tension was applied to the tissue, which was allowed to relax to a final resting tension of between 0.5 and 1 g wt. Before each experiment the tissues were contracted at least three times with 60 mM KCl, until the final two responses to KCl differed by less than 10%.
Effect of precontraction with U46619 on α2-adrenoceptor-mediated vasoconstriction
Concentration–response curves to the selective α2-adrenoceptor agonist UK14304 (1 nM–3 μM) were constructed in the absence or presence of U46619 (1–10 nM; contraction less than 20% of the 60 mM KCl response). Alternatively, tissues were exposed to a single, near-maximal concentration of UK 14304 (1 μM) in the absence or presence of U46619-induced tone. Contractions to UK 14304 were measured from the pre-UK14304 level of tone. In some experiments, 50 μM PD98059 was added 1 h prior to addition of UK14304 or U46619/UK14304. In the calcium-free experiments, the Krebs–Henseleit buffer was replaced with calcium-free Krebs–Henseleit buffer in which the calcium chloride had been replaced with 2 mM EGTA, 5 min before the addition of UK14304 or U46619/UK14304.
To determine the effect of precontraction with U46619, and relaxation with forskolin on α2-adrenoceptor-mediated vasoconstriction
In a separate set of experiments, tissues were precontracted with a low concentration of U46619 (<25% of the 60 mM KCl response), and then relaxed back to baseline with forskolin (0.1–0. 3 μM) prior to the addition of a single concentration of UK14304 (1 μM).
Western blotting
Western blotting for phosphorylated or total ERK was carried out as described previously (Roberts, 2001). Briefly, segments of the porcine ear artery were set up in tissue baths as above. Tissues were exposed to UK14304 (1 μM), U46619 (1–3 nM), or preconstricted with U46619 (1–3 nM, 10–20% of 60 mMKCl response) and treated with UK14304 (1 μM). Control tissues were not exposed to any compound (basal conditions). When the contractions reached a plateau, the segments were quickly removed from the tissue baths, and immediately frozen on dry ice. Frozen segments were then homogenised in ice cold buffer (80 mM sodium β-glycerophosphate, 20 mM imidazole [pH 7.0], 1 mM dithiothreitol, 1 mM sodium fluoride, 500 μM 4-(2-aminoethyl)benzenesulphonyl fluoride (AEBSF), 1 μM trans-epoxysuccinyl-L-leucylamide-(4-guanidino) butane (E-64), 10 μg ml-1 aprotonin, 1 μM leupeptin, 500 μM EDTA). Samples were diluted in 1 : 1 in Laemmli sample buffer, and heated at 95°C for 5 min. Equal amounts of protein from each sample were separated on 10% SDS–PAGE gels, and then transferred onto nitrocellulose membranes by Western blotting. Membranes were probed for phosphorylated or total ERK using appropriate antibodies (both Cell Signalling Technology, Beverly, MA, U.S.A.). Bands were detected by probing with a hydrogen peroxidase-conjugated secondary antibody, and visualised using the ECL system (Amersham Life Sciences, Little Chalfont, U.K.). Both phosphorylated and total ERK bands were analysed by densitometry using the BioRad molecular analyst software package.
Drugs
5-Bromo-6-[2-imidazolin-2-ylamine]-quinoxaline bitartrate (UK14304), (Pfizer, Sandwich, U.K.); (5Z, 9α, 11α, 13E, 15 (S) )-15-hydroxy-9 (11) methanoepoxyprosta-5,13-dien-1 oic acid (U46619), (Cayman Chemicals, Ann Arbor, MI, U.S.A.); 2-amino-3-methoxyflavone (PD98059), (Calbiochem, Nottingham, U.K.). All other compounds were obtained from Sigma, Poole, U.K.
Statistics
Contractile responses were expressed as a percentage of the response to 60 mM KCl, and the results expressed as mean±s.e.m. Bands obtained by immunoblotting were analysed by densitometry. Multiple comparisons between treatment groups were performed using analysis of the variance (ANOVA) followed by a Bonferroni test. All other statistical evaluations were carried out using a two-tailed Student's paired or unpaired t-test for normally distributed data, or a Wilcoxon signed rank test for paired, nonparametric data.
Results
Contractions to UK14304 after precontraction with a low concentration of U46619 and relaxation with forskolin
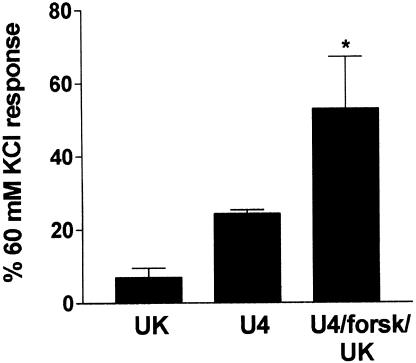
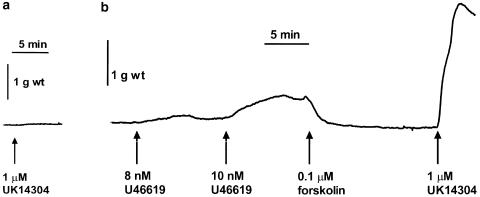
In this set of experiments, the response to a single concentration of UK14304 (1 μM) was 7±3% of the response to 60 mM KCl (mean±s.e.m., n=7, see Figure 1). Tissues were precontracted with a low concentration of U46619 (1–10 nM) to give an average response of 24±1% of the response to 60 mM KCl (n=7). Tissues were then relaxed back to baseline with forskolin (0.1–0.3 μM). Subsequent addition of 1 μM UK14304 produced a contraction which was on average two-fold greater than the response to U46619 in the same tissue (53±14%, P<0.05. Wilcoxon signed rank test, n=7; see Figure 1). Figure 2 shows a typical trace recording of a contractile response from a segment of porcine ear artery precontracted with U46619, and relaxed back to baseline with forskolin prior to addition of 1 μM UK14304.
Figure 1.
Bar chart showing contractions to 1 μM UK14304 in segments of porcine ear artery in the absence (UK), or after precontraction with U46619 (<25% of the 60 mM KCl response) followed by relaxation back to baseline with forskolin (0.1–0.3 μM) (U4/forsk/UK). Also shown is the average precontraction obtained with U46619 prior to addition of forskolin (U4). Responses are expressed as a percentage of the response to 60 mM KCl, and are mean±s.e.m. of seven experiments. * Indicates significant difference from 1 μM UK14304 alone (P<0.05, Wilcoxon signed rank test).
Figure 2.
Typical trace recordings showing the contractile response of segments of porcine ear artery from the same animal to 1 μM UK14304 alone (a), or after precontraction with a low concentration of U46619 (to <25% of the 60 mM KCl response), followed by relaxation back to baseline with forskolin (b).
U46619 enhances contractions to UK14304
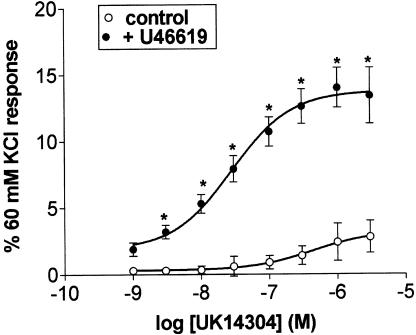
UK14304 (1 nM–3 μM) alone produced only a small contractile response in the porcine isolated ear artery (maximum response 3±1% of 60 mM KCl response, n=7; see Figure 3). Precontraction of the tissues with a low concentration of U46619 (average response 23±4% of 60 mM KCl response), without relaxation with forskolin, resulted in a five-fold enhancement of the maximum response to UK14304 (the response to UK14304 is the response in the presence of UK14304 minus the tone induced by U46619; 17±1%, n=7; see Figure 3). Responses to a single concentration of UK14304 (1 μM) were also enhanced. In this case, the response to 1 μM UK 14304 on its own was 22±3%. In the presence of U46619 (contraction to <20% of the 60 mM KCl response) this was increased to 68±12% (n=8, P<0.01, Student's two-tailed, unpaired t-test). It is interesting to note that the responses to a single concentration of UK14304 either in the presence or absence of U46619 were greater than those obtained in the concentration–response curves. On the other hand, the degree of enhancement was similar. As precontraction with U46619 on its own was able to enhance the response to UK14304, all subsequent experiments were carried out in the absence or presence of U46619, without relaxation with forskolin.
Figure 3.
Log concentration–response curves to UK14304 in segments of porcine ear artery in the absence (control) or presence of U46619. Responses are expressed as a percentage of the 60 mM KCl response and are mean±s.e.m. of eight experiments. *Indicates significant difference from control responses (P<0.01, ANOVA followed by Bonferroni test).
Effect of PD98059 on UK14304-mediated contractions
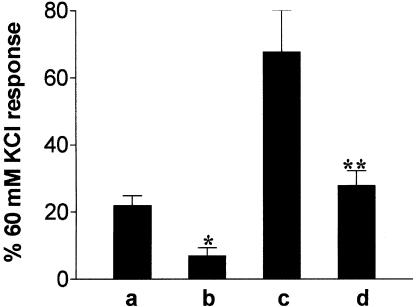
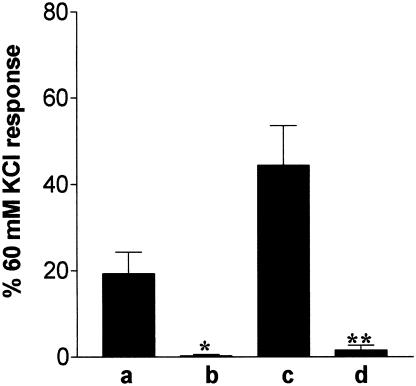
Preincubation with 50 μM PD98059 (1 h) caused a significant reduction in the contraction induced by 1 μM UK14304 on its own (Figure 4). PD98059 also caused a significant reduction in the response to 1 μM UK14304 in the presence of U46619 (Figure 4). The degree of tone induced by U46619 was the same in both control (18±2% of 60 mM KCl response, n=6) and tissues exposed to PD98059 (18±1% of 60 mM KCl response obtained prior to addition of PD98059, n=6). However, the concentration of U46619 required to induce this degree of tone was higher in tissues exposed to PD98059 (10–40 nM compared to 1–3 nM in the absence of PD98059).
Figure 4.
Bar chart showing contractions to 1 μM UK14304 in segments of porcine ear artery under the following conditions: (a, b) 1 μM UK14304 alone, (c, d) 1 μM UK14304 after precontraction with U46619 (<20% of the 60 mM KCl response). The responses shown in (b) and (d) were obtained in the presence of 50 μM PD98059. Responses are expressed as a percentage of the response to 60 mM KCl, and are mean±s.e.m. of six experiments. *Indicates significant difference from 1 μM UK14304, P<0.05, Student's two-tailed, unpaired t-test. **Indicates significant difference from UK14304 response in the presence of U46619, P<0.01, Student's two-tailed, unpaired t-test.
Effect of removal of extracellular calcium on UK14304-mediated contractions
Removal of extracellular calcium virtually abolished the contraction induced by 1 μM UK14304 on its own (Figure 5). The U46619-enhanced UK14304 response was also significantly reduced by the removal of extracellular calcium (Figure 5). As with the PD98059 experiments, the degree of tone induced by U46619 was the same in both control (15±2%, n=9) and tissues exposed to calcium-free buffer (15+4%, n=9, expressed as a percentage of the 60 mM KCl response obtained before extracellular calcium was removed). However, the concentration of U46619 required to induce this degree of tone was higher in the absence of calcium (10–40 nM), compared to 1–4 nM in the presence of calcium.
Figure 5.
Bar chart showing contractions to 1 μM UK14304 in segments of porcine ear artery under the following conditions: (a, b) 1 μM UK14304 alone, (c, d) 1 μM UK14304 after pre-contraction with U46619 (<20% of the 60 mM KCl response). The responses shown in (b) and (d) were obtained in the absence of extracellular calcium. Responses are expressed as a percentage of the response to 60 mM KCl, and are means±s.e.m. of nine experiments. * Indicates significant difference from 1 μM UK14304, P<0.01, Student's two-tailed, unpaired t-test. **Indicates significant difference from UK14304 response in the presence of U46619, P<0.01, Student's two-tailed, unpaired t-test.
Effect of precontraction with U46619 on ERK activation
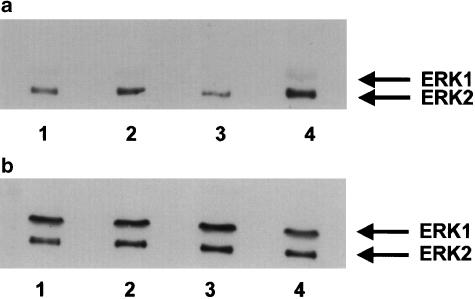
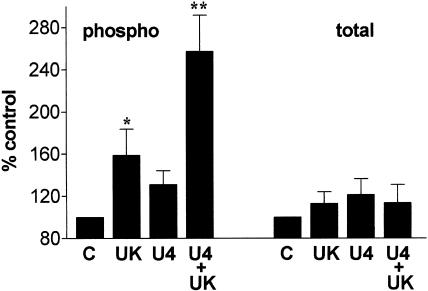
Levels of phosphorylated and total ERK were measured by Western blotting. In tissues contracted with 1 μM UK14304 on its own there was a significant increase in levels of phosphorylated ERK2 over control levels. Levels of total ERK were unaffected, indicating an increase in ERK2 activity (see Figure 6 and Figure 7). There was also an apparent increase in the level of ERK1 phosphorylation (Figure 6 and Figure 7). However, as levels of ERK1 phosphorylation were often below the level of accurate measurement by densitometry, we have concentrated on the effects on ERK2 phosphorylation. Levels of phosphorylated ERK2 were significantly enhanced in tissues precontracted with U46619, and exposed to 1 μM UK14304 compared to those just exposed to 1 μM UK14304 (Figure 6 and Figure 7). Contracting the tissues with U46619 to 10–20% of the 60 mM KCl response had no measurable effect on ERK2 phosphorylation (Figure 6 and Figure 7).
Figure 6.
Representative immunoblot of porcine ear artery proteins separated by SDS–PAGE, transferred onto nitrocellulose membranes, and incubated with a primary antibody against phosphorylated ERK1/2 or total ERK1/2 (b). Segments of porcine ear artery were set up in a tissue bath and exposed to the following conditions: no UK14304 (basal conditions, 1), 1 μM UK 14304 (2), contracted to 20% of the 60 mM KCl response with U46619 (3), or precontracted with U46619, and exposed to 1 μM UK14304 (4). Tissues were rapidly frozen, homogenised and then subjected to SDS–PAGE.
Figure 7.
Bar charts showing changes in phosphorylated (phospho) and total ERK2 in segments of porcine ear artery contracted with 1 μM UK14304 (UK), U46619 (contracted to <20% of the 60 mM KCl response, U4), or precontracted with U46619 and exposed to 1 μM UK14304 (U4+UK). Control tissues (C) were not exposed to any agent (basal conditions). Responses are expressed as a percentage of control values, and are means±s.e.m. of eight experiments. *Indicates significant difference from control, P<0.05, Student's two-tailed, paired t-test (direct comparison of densitometric values). **Indicates significant difference from UK14304 responses and U46619 responses (P<0.05, ANOVA, followed by Bonferroni test).
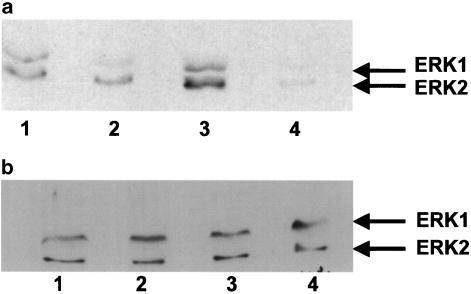
Removal of extracellular calcium caused a 16±5% (n=4) inhibition of ERK2 phosphorylation in tissues exposed to 1 μM UK14304 alone (see Figure 8), although this was not statistically significant (P=0.07, Student's two-tailed, paired t-test). In tissues precontracted with U46619 and then exposed to 1 μM UK14304, removal of extracellular calcium caused a 32±7% (n=4) inhibition of ERK2 phosphorylation (Figure 8, P<0.01, Student's two-tailed, paired t-test). There was no effect on levels of total ERK2.
Figure 8.
Representative immunoblot of porcine ear artery proteins separated by SDS–PAGE, transferred onto nitrocellulose membranes, and incubated with a primary antibody against phosphorylated ERK1/2 (a) or total ERK1/2 (b). Segments of porcine ear artery were set up in a tissue bath and exposed to the following conditions: 1 μM UK14304 (1), 1 μM UK14304 in calcium-free Krebs–Henseleit buffer (2), precontracted with U46619, and exposed to 1 μM UK14304 (3), or precontracted with U46619, and exposed to 1 μM UK14304 in calcium-free Krebs–Henseleit buffer (4). Tissues were rapidly frozen, homogenised, and then subjected to SDS–PAGE.
Discussion
We have previously shown that α2-adrenoceptor-mediated vasoconstriction in the porcine ear artery can be enhanced by precontraction with a large concentration of the thromboxane-mimetic U46619, followed by relaxation with the cyclic AMP-elevating compound forskolin, prior to addition of the α2-adrenoceptor agonist (Roberts et al., 1998). This enhanced contraction seems to be because of the inhibition of the forskolin-stimulated cyclic AMP response, and hence a return of the U46619-induced contraction (Roberts et al., 1998). However, another pathway also seems to be involved in the enhanced response. For example, in this present study some tissues were precontracted with a low concentration of U46619 (<25% of the KCl response), and then relaxed back to baseline with forskolin. The subsequent, enhanced α2-adrenoceptor-mediated response was double the response obtained with U46619. If α2-adrenoceptor activation only causes a reversal of the forskolin-mediated relaxation by reducing the forskolin-stimulated cyclic AMP levels, then the maximum response that would be expected would be that obtained by the U46619-induced precontraction. As the α2-adrenoceptor-mediated response was greater, there must be another mechanism involved. This led us to believe that the presence of U46619 itself was inducing an enhanced response. Indeed, precontraction with a low concentration of U46619 (10–20% of the KCl response), without relaxation with forskolin, does lead to an enhancement of the α2-adrenoceptor-mediated vasoconstriction in the porcine isolated ear artery (this paper).
As noted in the Results section, the maximum response to UK14304 alone in the response curve was only around 2–3% of the 60 mM KCl response, whereas the response to a single concentration of UK14304 was around 15–20%. This difference is probably because of a low sensitivity to UK14304 in the tissues in which the response curves were carried out, rather than it being the result of receptor desensitisation. Previous concentration–response curves to UK14304 in the ear artery have produced maximum responses closer to those seen with a single concentration of UK14304 (Roberts et al., 1998). The differences observed are probably the result of using a mixed population of pigs reared on different farms.
We have previously demonstrated that α2-adrenoceptor-mediated vasoconstriction in the porcine palmar lateral vein is dependent upon activation of the ERK–MAP kinase pathway (Roberts, 2001). This in turn is dependent upon an influx of extracellular calcium possibly through non-L-type calcium channels (Roberts, 2001; Roberts, 2003). Therefore, it was of interest to us to determine whether U46619 was enhancing the α2-adrenoceptor response in the porcine ear artery through an enhancement of the ERK–MAP kinase pathway. The α2-adrenoceptor agonist UK14304 on its own caused a small contraction of the porcine ear artery, and this was inhibited by PD98059, an inhibitor of the ERK signal transduction cascade (Alessi et al., 1995). Furthermore, there was an increase in the levels of phosphorylated ERK2 in tissues contracted with UK14304, with no change in the level of total ERK2. ERK–MAP kinases are activated by the dual phosphorylation of Thr and Tyr residues by the upstream kinase MEK (Cobb & Goldsmith, 1995). Therefore, an increase in phosphorylation at these sites, with no change in the level of total ERK, indicates that there was an increase in ERK2 activity. Taken together, these data demonstrate that, as in the porcine palmar lateral vein, direct α2-adrenoceptor-mediated vasoconstriction in the porcine ear artery is dependent upon activation of the ERK – MAP kinase pathway.
Precontraction with U46619 on its own had no significant effect on ERK2 phosphorylation levels, suggesting that, at these concentrations at least, it has no effect on ERK activity. On the other hand, we observed that a greater concentration of U46619 was required to induce a low level of tone (10–20% of the 60 mM KCl response) in tissues preincubated with PD98059 than in control tissues, suggesting that there may be a low-level stimulation of ERK activity that could not be detected by Western blotting. Previous studies have shown that thromboxane receptor activation stimulates ERK activation in a number of cell types grown in culture, including bovine coronary artery smooth muscle cells (Grosser et al., 1997; Gao et al., 2001; Miggin & Kinsella, 2001). However, further experiments are required to determine the role of ERK activation in U46619-induced vasoconstriction. An alternative explanation for the apparent inhibition of U46619-induced contractions by PD98059, without any detectable increase in ERK1/2 activity, could be that U46619-induced contractions are dependent upon MEK5/ERK5 activation. Recent studies have demonstrated that PD98059 can inhibit MEK5 as well as MEK1/2 (Kamakura et al., 1999), although there is no evidence to suggest that MEK5/ERK5 play a role in vasoconstriction. Further work is required to determine whether this is the case.
In tissues precontracted with U46619, α2-adrenoceptor-mediated ERK2 phosphorylation was significantly enhanced, with no effect on total ERK2 levels, indicating an enhanced activation. Preincubation with PD98059 caused a 50% reduction in the U46619-enhanced contraction indicating that the enhanced ERK signalling response is involved in the enhanced α2-adrenoceptor-mediated vasoconstriction. The effect of PD98059 is more than just an inhibition of the direct α2-adrenoceptor-mediated vasoconstriction as the size of the inhibition of the UK14304 response in the presence of U46619 with PD98059 was greater than the direct α2-adrenoceptor response. Furthermore, as U46619 did not produce a detectable increase in ERK2 phosphorylation, the enhanced ERK2 activation is not just an additive effect of the U46619 response on top of the direct α2-adrenoceptor response.
One possible role for the ERK–MAP kinase signal transduction cascade is that it could increase the sensitivity of the contractile proteins to calcium thus allowing a concentration of UK14304 which would not normally cause a response in the tissue, to cause a contraction. In the presence of U46619, this pathway is enhanced resulting in a further increase in the sensitivity of the contractile proteins to calcium, and hence allowing a larger contraction without increasing calcium levels further. A number of studies have demonstrated that calcium sensitisation of the contractile proteins does appear to play a role in U46619-induced contractions in other blood vessels (Crichton et al., 1993; Jiang et al., 1994; Tosun et al., 1998). However, U46619-mediated contractions also appear to be dependent, to a certain extent, on influx of extracellular calcium (Han et al., 1995; Tosun et al., 1998). This also seems to be the case in the porcine ear artery in that greater concentrations of U46619 were required to cause a contraction in the absence of calcium compared to in the presence of calcium (10–40 nM in the absence of calcium, compared to 1–3 nM in the presence of calcium). On the other hand, at these higher concentrations U46619 could actually cause a contraction, suggesting that calcium sensitisation may play a role in the thromboxane receptor-mediated vasoconstriction in the porcine ear artery. Evidence against calcium sensitisation as a mechanism by which U46619 enhances the α2-adrenoceptor-mediated vasoconstriction comes from the experiments in which tissues were exposed to calcium-free Krebs–Henseleit buffer and precontracted with U46619 (10–20% of the 60 mM KCl response). Under these conditions the contractile responses to UK14304 were nearly abolished. The activation of ERK2 (enhanced response) was also dependent upon extracellular calcium. This suggests that U46619 may be enhancing calcium influx rather than sensitising the contractile proteins to low concentrations of calcium.
What is not clear from this, or any other study, is how precontraction with U46619 leads to an enhanced α2-adrenoceptor-mediated vasoconstriction. The data from this study indicate that, although enhancement of ERK activation is involved, the effect of U46619 is actually upstream of ERK as the enhanced response is highly dependent on calcium influx. Calcium influx is upstream of α2-adrenoceptor-mediated ERK activation as removal of extracellular calcium inhibits ERK phosphorylation (this study and Roberts, 2001). This also rules out calcium sensitisation as a mechanism. Future studies will be concentrated on determining the site of U46619-induced enhancement. However, in order to understand the mechanism of enhancement, the signalling pathways stimulated by U46619 in blood vessels need to be clarified.
In conclusion, α2-adrenoceptor-mediated responses in the porcine ear artery are potentiated by precontraction with a low concentration of U46619. This enhanced response is associated with an increased signalling through the ERK–MAP kinase pathway. The enhanced responses are also dependent upon influx of extracellular calcium. This study sheds some light on the signalling pathway involved in the enhancement of α2-adrenoceptor-mediated vasoconstriction, although the exact mechanism of enhancement remains to be clarified.
Acknowledgments
We thank the Wellcome Trust for financial support, and G.Woods and Sons Ltd. abattoir (Clipstone, Nottinghamshire) for supply of tissue.
Abbreviations
- AEBSF
4-(2-aminoethyl)benzenesulphonyl fluoride
- ANOVA
analysis of the variance
- cyclic AMP
adenosine 3′: 5′-cyclic monophosphate
- E-64
trans-epoxysuccinyl-L-leucylamide-(4-guanidino) butane
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N, N, N′, N′-tetraacetic acid
- ERK
extracellular signal-regulated kinase
- MAP kinase
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- TBS-T
Tris-buffered saline containing 0.1% Tween-20
References
- ALESSI D.R., CUENDA A., COHEN P., DUDLEY D.T., SALTIEL A.R. PD 098059 is a specific inhibitor of the acitvation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- BYLUND D.B., EIKENBURG D.C., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLINOFF P.B., RUFFOLO R.R., TRENDELENBURG U. International Union of Pharmacology nomenclature on adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- COBB M.H., GOLDSMITH E.J. How MAP Kinases are regulated. J Biol. Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- CRICHTON C.A., TEMPLETON A.G.B., MCGRATH J.C., SMITH G.L. Thromboxane A2 analogue, U-46619, potentiates calcium-activated force in human umbilical artery. Am. J. Physiol. 1993;264:H1878–H1883. doi: 10.1152/ajpheart.1993.264.6.H1878. [DOI] [PubMed] [Google Scholar]
- GAO Y., TANG S., ZHOU S., WARE A. The thromboxane A2 receptor activates mitogen-activated protein kinase via protein kinase C-dependent Gi coupling and Src-dependent phosphorylation of the epidermal growth factor receptor. J. Pharmacol Exp. Therap. 2001;296:426–433. [PubMed] [Google Scholar]
- GROSSER T., ZUCKER T.-P., WEBER A.-A., SCHULTE K., SACHINIDIS A., VETTER H., SCHROR K. Thromboxane A2 induces cell signaling but requires platelet-derived growth factor to act as a mitogen. Eur. J. Pharmacol. 1997;319:327–332. doi: 10.1016/s0014-2999(96)00860-6. [DOI] [PubMed] [Google Scholar]
- HAN S.Z., KARAKI H., OUCHI Y., AKISHITA M., ORIMO H. 17-Beta-estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A(2) in porcine coronary artery. Circulation. 1995;91:2619–2626. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- JIANG M.J., CHAN C-F., CHANG Y-L. Intracellular calcium and myosin light chain phosphorylation during U46619-activated vascular contraction. Life Sci. 1994;54:2005–2013. doi: 10.1016/0024-3205(94)90135-x. [DOI] [PubMed] [Google Scholar]
- KAMAKURA S., MORIGUCHI T., NISHIDA E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- MIGGIN S.M., KINSELLA B.T. Thromboxane A2 receptor mediated activation of the mitogen activated protein kinase cascades in human uterine smooth muscle cells. Biochim. Biophys. Acta. 2001;1539:147–162. doi: 10.1016/s0167-4889(01)00103-3. [DOI] [PubMed] [Google Scholar]
- ROBERTS R.E. Role of the extracellular regulated kinase (Erk) signal transduction cascade in α2 adrenoceptor-mediated vasoconstriction in porcine palmar lateral vein. Br. J. Pharmacol. 2001;133:859–866. doi: 10.1038/sj.bjp.0704149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS R.E. α2 adrenoceptor-mediated vasoconstriction in porcine palmar lateral vein: role of phosphatidylinositol 3-kinase and EGF receptor transactivation. Br. J. Pharmacol. 2003;138:107–116. doi: 10.1038/sj.bjp.0705014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS R.E., KENDALL D.A., WILSON V.G. α2-Adrenoceptor and NPY receptor-mediated contractions of porcine isolated blood vessels: evidence for involvement of the vascular endothelium. Br. J. Pharmacol. 1999;128:1705–1712. doi: 10.1038/sj.bjp.0702979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS R.E., TOMLINSON A.E., KENDALL D.A., WILSON V.G. α2-Adrenoceptor-mediated contractions of the porcine isolated ear artery: evidence for a cyclic AMP-dependent and a cyclic AMP-independent mechanism. Br. J. Pharmacol. 1998;124:1107–1114. doi: 10.1038/sj.bjp.0701935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEENEY G., TEMPLETON A., CLAYTON R.A., BAIRD M., SHERIDAN S., JOHNSTON E.D., MACLEAN M.R. Contractile responses to sumatriptan in isolated bovine pulmonary artery rings: relationship to tone and cyclic nucleotide levels. J. Cardiovasc. Pharmacol. 1995;26:751–760. doi: 10.1097/00005344-199511000-00012. [DOI] [PubMed] [Google Scholar]
- TOSUN M., PAUL R.J., RAPOPORT R.M. Role of extracellular Ca++ influx via L-type and non-L-type Ca++ channels in thromboxane A2 receptor-mediated contraction in rat aorta. J. Pharmacol. Exp. Therap. 1998;284:921–928. [PubMed] [Google Scholar]
- WRIGHT I.K., HARLING R., KENDALL D.A., WILSON V.G. Examination of the role of inhibition of cyclic AMP in α2-adrenoceptor mediated contractions of the porcine isolated palmar lateral vein. Br. J. Pharmacol. 1995;114:157–165. doi: 10.1111/j.1476-5381.1995.tb14920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]