Abstract
Gene therapy is a promising strategy for cerebrovascular diseases. Several genes that encode vasoactive products have been transferred via cerebrospinal fluid for the prevention of vasospasm after subarachnoid hemorrhage. Transfer of neuroprotective genes, including targeting of proinflammatory mediators, is a current strategy of gene therapy for ischemic stroke. Stimulation of growth of collateral vessels, stabilization of atherosclerotic plaques, inhibition of thrombosis, and prevention of restenosis are important objectives of gene therapy for coronary and limb arteries, but application of these approaches to carotid and intracranial arteries has received little attention. Several fundamental advances, including development of safer vectors, are needed before gene therapy achieves an important role in the treatment of cerebrovascular disease and stroke.
Keywords: Adenovirus, gene transfer, ischemic stroke, vasospasm
Introduction
Seven years have passed since we reviewed the promise and problems associated with gene therapy for stroke and several cerebrovascular diseases (Heistad & Faraci, 1996) (Figure 1). Since then, a large number of studies have demonstrated feasibility of gene transfer to alter the cerebral circulation in experimental animals. Clinical application of this strategy to human disease states, however, has not yet begun, although clinical trials of gene therapy for other cardiovascular diseases, including coronary artery disease and limb ischemia, are promising (Baumgartner et al., 1998; Grines et al., 2002; Losordo et al., 2002; Makinen et al., 2002). Special challenges are presented by cerebral circulation, including the limited therapeutic time window for the treatment of acute stroke. In this review, we summarize progress in experimental gene therapy of cerebrovascular diseases in recent years and propose possible future applications to patients.
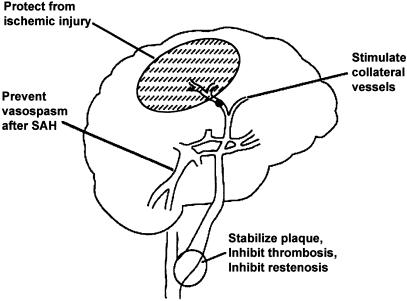
Figure 1.
Potential applications of gene therapy for cerebral vascular disease. Revised from Heistad and Faraci (1996).
Therapeutic framework
What to treat
We proposed previously (Heistad & Faraci, 1996) several possible targets for cerebral vascular gene therapy: (1) prevention of vasospasm after subarachnoid hemorrhage (SAH), (2) stimulation of growth of collateral blood vessels in the area at risk of ischemia, and (3) stabilization of atherosclerotic plaques, inhibition of thrombosis, and prevention of restenosis after angioplasty of the carotid and vertebrobasilar arteries. It is now clear that several transgenes are promising for the first target (Onoue et al., 1998; Stoodley et al., 2000; Toyoda et al., 2000c; Watanabe et al., 2002). For the latter two targets, clues about appropriate transgenes may emerge from experimental and clinical studies of coronary and limb circulations.
In the past few years, it has also become evident that gene transfer to brain parenchyma for cerebrovascular diseases holds considerable promise (Betz et al., 1995). Neuroprotection during acute stroke, for example, by inhibition of inflammatory mediators, may be useful (Yang et al., 1997; Ooboshi et al., 2002). Gene therapy for risk factors for atherosclerosis, including hypertension and diabetes mellitus, may also prove to be useful for indirect prevention of cerebrovascular diseases (Table 1).
Table 1.
Some approaches to cerebrovascular gene therapy
| Gene | Vector | Administration | References |
|---|---|---|---|
| Vasospasm after SAH | |||
| Endothelial NOS | Adenovirus | Cisterna magna | Onoue et al. (1998) |
| Stoodley et al. (2000) | |||
| Khurana et al. (2002) | |||
| ECSOD | Adenovirus | Cisterna magna | Watanabe et al. (2002) |
| Antisense preproendothelin-1 | Oligodeoxynucleotide | Cisterna magna | Onoda et al. (1996) |
| Ohkuma et al. (1999) | |||
| Prepro-CGRP | Adenovirus | Cisterna magna | Toyoda et al. (2000a, b, c) |
| Decoy of NFκB | Oligodeoxynucleotide | Cisterna magna | Ono et al. (1998) |
| Intracranial collateral circulation | |||
| Basic fibroblast growth factor | Adenovirus | Ventricle | Yukawa et al. (2000) |
| Hepatocyte growth factor | HVJ–liposome complex | Cisterna magna | Yoshimura et al. (2002) |
| Arterial disease (regression of atherosclerosis or restenosis) | |||
| Apolipoprotein E | Adenovirus | Systemic injection | Desurmont et al. (2000) |
| Anti-MCP-1 | HVJ–liposome complex | Femoral muscle | Egashira et al. (2000) |
| Mori et al. (2002) | |||
| Tissue factor pathway inhibitor | Adenovirus | Carotid artery | Atsuchi et al. (2001) |
| Hirudin | Adenovirus | Carotid artery | Rade et al. (1996) |
| Tissue plasminogen activator | Adenovirus | Femoral artery | Waugh et al. (1999) |
| Decoy of transcription factor E2F | Oligodeoxynucleotide | Carotid artery | Morishita et al. (1995) |
| Brain ischemia | |||
| Calbindin D28K | Herpes simplex virus | Striatum | Yenari et al. (2001a, b) |
| Glucose transporter | Plasmid | Striatum | Lawrence et al. (1996) |
| HSP72 | Herpes simplex virus | Striatum | Hoehn et al. (2001) |
| Interleukin-1 receptor antagonist | Adenovirus | Lateral ventricle | Yang et al. (1997) |
| Interleukin-10 | Adenovirus | Lateral ventricle | Ooboshi et al. (2002) |
| Transforming growth factor-β1 | Adenovirus | Lateral ventricle | Pang et al. (2001) |
| Glial cell line-derived neurotropic factor | Adenovirus | Cortex | Kitagawa et al. (1999) |
| Hepatocyte growth factor | HVJ–liposome complex | Cisterna magna | Hayashi et al. (2001) |
| bcl-2 | Adeno-associated virus | Hippocumpus | Shimazaki et al. (2000) |
| Neuronal apoptosis inhibitory protein | Adenovirus | Hippocumpus | Xu et al. (1997) |
| Cyclooxygenase-1 | Adenovirus | Lateral ventricle | Lin et al. (2002) |
| Redox-inducible antioxidant protein | Adenovirus | Caudate putamen | Yang et al. (2001) |
Where to treat
In the treatment of cerebral vascular disease, there are several important sites for gene transfer: cerebral blood vessels and brain parenchyma.
Since the first successful studies (Nabel et al., 1989; Lin et al., 1990) of gene transfer to blood vessels in vivo, the most common approach has been intraluminal delivery of vectors with transgenes. Using this approach, nitric oxide synthase (NOS) and other vasoactive transgene products have been expressed in vascular endothelium to alter vascular reactivity (Von der Leyen et al., 1995). The intraluminal approach, however, has important limitations for gene transfer to cerebral circulation. First, interruption of blood flow for several minutes, which is needed for effective gene delivery to cells in the vessel wall, would produce cerebral ischemia. Second, intraluminal gene transfer usually requires occlusion of a vessel and injection of the vector between ligatures, so that transfection is limited to only the small segment of the vessel into which the vector is instilled. Third, the intraluminal approach results in low efficiency of gene transfer beyond the endothelium, unless the endothelium is denuded or damaged.
An alternative approach is perivascular delivery of transgenes with vectors. Gene transfer to the intracranial circulation can be achieved by intracisternal injection of vectors into the cerebrospinal fluid (CSF) (Ooboshi et al., 1995; Christenson et al., 1998). Gene transfer to the extracranial carotid artery can also be accomplished by the injection of vectors into the periarterial sheath (Rios et al., 1995). Overexpression of vasoactive transgene products in the vascular adventitia, after perivascular gene transfer, alters nitric oxide (NO)-dependent vascular responses, which normally are endothelium dependent (Chen et al., 1997; Ooboshi et al., 1997b; Toyoda et al., 2000b). The perivascular approach has been used in human cerebral arteries ex vivo (Khurana et al., 2000; Gunnett et al., 2002).
Gene transfer to brain parenchyma has been mainly achieved by direct administration of vectors with transgenes into the parenchyma, including the striatum and cortex (Davidson et al., 1993; Le Gal La Salle et al., 1993). The approach can be used to overexpress transgene products in several cell types, including neurons and astrocytes. After the injection of vectors into cerebral ventricles, transgene products are overexpressed in the leptomeninges and ependymal cells that line the ventricles (Akli et al., 1993; Bajocchi et al., 1993). Overexpressed genes in the leptomeninges and ependymal cells had therapeutic effects for the ischemic lesion in brain parenchyma (Betz et al., 1995).
In addition to local gene transfer, systemic administration of vectors may be an important approach if the transgene product is secretable protein. After intravenous injection of adenoviral vectors, viruses are cleared by the liver, and transgene products are mainly overexpressed in the liver and secreted into the systemic circulation (Drazan et al., 1995; Balague et al., 2000). Binding of the circulating transgene product to the cerebral or systemic vascular endothelium may require special targeting. For example, extracellular superoxide dismutase (ECSOD) contains a positively charged heparin-binding domain (HBD), which mediates binding of ECSOD to cells and the interstitium (Oury et al., 1996). Preliminary studies suggest that intravenous administration of an adenoviral vector encoding ECSOD produced overexpression of ECSOD in the aorta and carotid artery, reduced systemic vascular resistance, and reduced arterial pressure in an animal model of hypertension, but administration of an adenoviral vector encoding ECSOD with deletion of its HBD did not reduce arterial pressure (Chu et al., 2003).
Injection of vectors into skeletal muscle can also be used for expression of transgenes, and release of transgene products into the systemic circulation, to affect remote organs (Ueno et al., 2000). For example, attenuation of atherosclerosis in carotid and coronary arteries was accomplished after intramuscular injection of a vector that expresses soluble FLT-1 gene, which acts as a decoy to suppress the normal function of vascular endothelial growth factor (VEGF), or antimonocyte chemoattractant protein-1 (MCP-1) gene, a mechanism that is discussed later (Mori et al., 2002; Zhao et al., 2002).
How to treat
The principal goal of gene therapy is either to express a gene that is deficient or to overexpress a therapeutic gene. Selection of a vector is challenging. Naked plasmid DNA is effective as a delivery system in skeletal muscle, but naked DNA does not effectively pass into vascular or neuronal cells (Wolff et al., 1990). Viral vectors or carrier molecules (nonviral vectors) can augment the efficiency of gene transfer to cells.
Replication-deficient recombinant adenovirus is a widely used vector both in experimental gene transfer to cerebral blood vessels and in clinical cardiovascular trials of gene therapy (Isner et al., 2001). Adenoviral vectors can transduce efficiently both dividing and nondividing cells. The virus has room for fairly large cDNA inserts, and can be prepared in higher titers than most other viral vectors. Several studies reported successful adenovirus-mediated gene transfer to carotid arteries (Lee et al., 1993; Lemarchand et al., 1993) and intracranial arteries and perivascular tissue (Ooboshi et al., 1995; Christenson et al., 1998). Adenovirus-mediated vascular gene transfer has been performed in disease states, including hyperlipidemia and atherosclerosis (Rios et al., 1995; Kozarsky et al., 1996; Ooboshi et al., 1997a, 1998; Lund et al., 1998; Tangirala et al., 1999), diabetes (Lund et al., 2000; Zanetti et al., 2001), hypertension (Alexander et al., 1999; Gelband et al., 2000; Nakane et al., 2000; Phillips, 2001; Li et al., 2002), and after SAH (Muhonen et al., 1997).
An advantage of adenoviral vectors is that viral proteins facilitate some steps of gene transduction, including escape from the endosome and entry into the nucleus. A disadvantage of the virus, however, is that binding to vascular cells is limited by a paucity of adenoviral fiber receptor activity in vascular cells (Seth et al., 1994; Wickham et al., 1996). Approaches to overcoming this limitation include the use of complexes of adenovirus with cationic polymer or lipids (Toyoda et al., 1998, 2001), or the use of complexes of adenovirus with calcium phosphate precipitates (Toyoda et al., 2000a). These complexes are easy to prepare. For augmentation of adenovirus-mediated gene transfer to specific cells without widespread effects on other cells, tissue-specific ligands are useful (Wickham, 2000). Improvement of adenoviral vectors to deliver genes selectively, safely, and with little immune response is necessary before application of the vectors to human gene therapy becomes widespread (Thomas et al., 2001).
Viral vectors that have been used for vascular and neuronal gene transfer include adeno-associated virus, herpes simplex virus, retrovirus, and lentivirus (Hu & Pathak, 2000; Buchschacher and Wong-Staal, 2001; Kay et al., 2001; Yenari et al., 2001b; Lowenstein, 2002). Nonviral vectors, or carrier molecules, include cationic polymer and liposomes.
An alternative to transfer of genes that produce enzymes and other proteins is gene transfer using oligonucleotides that regulate transcription of endogenous genes and inhibit their expression. For example, antisense oligodeoxynucleotides are widely used (Simons et al., 1992). Double-stranded oligodeoxynucleotide cis-elements act as a decoy to bind transcription factors and block the expression of genes that require the transcription factor to be transcribed (Morishita et al., 1995). Small interfering RNA (siRNA) may emerge as a particularly promising strategy to inhibit specifically gene expression (Xia et al., 2002). These tools are potentially useful for gene transfer to cerebral circulation.
Advances toward cerebrovascular gene therapy
Vasospasm after SAH
Vasospasm is a catastrophic complication after SAH for which no effective prevention has yet been demonstrated, and gene therapy may hold great potential. Several features of vasospasm after SAH suggest that it may be amenable to gene therapy. First, vasospasm typically occurs several days after SAH, which allows sufficient time after SAH to administer the vector and for expression of a transgene product. Second, the period of risk of vasospasm is only 2–3 weeks at most, and thus prolonged expression of a transgene is not needed. Third, intracisternal administration of vectors allows expression of transgene products around vessels at the base of the brain, and a hematoma at the site of the SAH does not prevent access of the virus or its product to target blood vessels (Muhonen et al., 1997). Proliferation of adventitial fibroblasts and resultant fibrosis after SAH may alter transgene delivery to vascular adventitia (Onoue et al., 1998).
Several mechanisms may contribute to vasospasm after SAH, including (1) impaired endothelium-dependent vasorelaxation, (2) production of endothelium-derived contracting factors, including endothelin, and (3) impaired activity of potassium channels in cerebral blood vessels (Harder et al., 1987; Faraci & Heistad, 1998). To address the first mechanism, gene transfer in vivo of endothelial NOS improved NO-mediated relaxation in vitro of basilar arteries after experimental SAH (Onoue et al., 1998). Gene transfer of endothelial NOS, however, failed to protect against vasospasm in vivo in one study, presumably due in part to scavenging of NO by hemoglobin in CSF (Stoodley et al., 2000) and to reduced activity of soluble guanylate cyclase (Sobey et al., 1996). Another study reported partial attenuation of constriction of the basilar artery in vivo after SAH, using gene transfer of endothelial NOS before SAH (Khurana et al., 2002). Since the vector was administered 1 day before SAH, the approach as described would not be clinically useful.
Superoxide may contribute to vasospasm after SAH (Kajita et al., 1994; Shishido et al., 1994), and superoxide dismutase (SOD) is a candidate for the prevention of vasospasm after SAH. Transgenic mice that overexpress CuZnSOD or ECSOD have attenuated cerebral vasoconstriction after experimental SAH (Kamii et al., 1999; McGirt et al., 2002). We recently demonstrated a partial protective effect against vasospasm after SAH by injection into CSF of an adenovirus that expresses ECSOD (Nakane et al., 2001; Watanabe et al., 2003). Antisense preproendothelin-1 oligodeoxynucleotide, which reduces production of endothelin peptide, attenuates vasospasm after SAH following intracisternal injection of the antisense alone (Onoda et al., 1996) or together with tissue plasminogen activator to lyse the subarachnoid thrombi (Ohkuma et al., 1999).
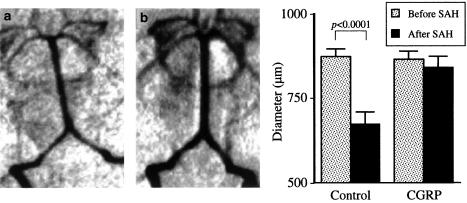
Calcitonin gene-related peptide (CGRP) is an extremely potent cerebral vasodilator, which may prove to be useful for the prevention of vasospasm after SAH. The peptide opens potassium channels, hyperpolarizes arterial muscle, and dilates arteries (Nelson et al., 1990; Kitazono et al., 1993). After SAH, CGRP is depleted from nerves to cerebral arteries (Nozaki et al., 1989a; Edvinsson et al., 1991). Intracisternal or systemic administration of exogenous CGRP increases cerebral arterial diameter in vivo after experimental SAH (Nozaki et al., 1989b; Toshima et al., 1992). Systemic administration of exogenous CGRP to patients with SAH also transiently reduced cerebral vasoconstriction and neurological deficits, but efficacy of intravenous CGRP in increasing cerebral blood flow was limited by its hypotensive effect (Johnston et al., 1990; Juul et al., 1994). Thus, we speculated that intracranial overexpression of CGRP for longer periods by a gene transfer technique might be effective for the prevention of vasospasm after SAH. We prepared a recombinant adenoviral vector encoding prepro-CGRP, and demonstrated that it modulates cerebrovascular tone after intracisternal gene transfer (Toyoda et al., 2000b). Treatment of rabbits with this vector after SAH prevented vasospasm (Toyoda et al., 2000c) (Figure 2). This was, to our knowledge, the first successful transfer of vasoactive genes in vivo for the prevention of vasospasm after SAH. The potential of this strategy was also demonstrated using a dog model of SAH in which vasoconstriction is greater than in rabbits, and thus more closely represents clinical vasospasm (Satoh et al., 2002).
Figure 2.
Gene transfer to prevent vasospasm after experimental SAH. Revised from Toyoda et al. (2000c). Arteriogram of the vertebrobasilar arteries in rabbits 2 days after experimental SAH. Rabbits were treated with vehicle (a) or adenovirus encoding CGRP gene (b).
Vasospasm after SAH may be related in part to an inflammatory vasculitis (Peterson et al., 1990; Aihara et al., 2001), and inhibition of inflammation by gene transfer appears to attenuate vasospasm (Ono et al., 1998). The transcription factor NFκB plays an essential role in the activation of inflammatory cytokines and adhesion molecules. Intracisternal administration of a decoy oligodeoxynucleotide of NFκB is reported to be useful for the prevention of vasospasm (Ono et al., 1998).
Collateral circulation
Stimulation of growth of collateral blood vessels may be useful for the preservation of cerebral circulation and prevention of cerebral infarction. Growth factors, including VEGF (Baumgartner et al., 1998; Rosengart et al., 1999), hepatocyte growth factor (HGF) (Hayashi et al., 1999), and basic fibroblast growth factor (bFGF) (Udelson et al., 2000), have been reported to have angiogenic effects in experimental ischemia of myocardium and limbs, and are being used for clinical trials of gene therapy. Gene delivery of these growth factors with vectors into CSF induced angiogenesis on the brain surface in animal models (Yukawa et al., 2000; Yoshimura et al., 2002). It is not yet clear whether this strategy will be useful in focal stimulation of angiogenesis to regions of the cerebrum that are at risk for infarction.
Arterial disease
Carotid endarterectomy and angioplasty with stenting are in wide use for the treatment of stenotic atherosclerotic lesions. An important goal is the prevention of progression of arterial stenosis. Regression of atherosclerotic lesions in mouse aorta has been documented after adenovirus-mediated gene transfer of human apolipoprotein E (Desurmont et al., 2000). MCP-1 recruits monocytes into the arterial wall and contributes to atherogenesis. Anti-MCP-1 gene transfer suppressed coronary and carotid hyperplasia after chronic inhibition of NOS (Egashira et al., 2000; Mori et al., 2002). Thrombosis and proliferativc changes after vascular injury were inhibited in the carotid artery by gene transfer of tissue factor pathway inhibitor (Atsuchi et al., 2001) and in other arteries by gene transfer of hirudin (Rade et al., 1996) and tissue plasminogen activator (Waugh et al., 1999).
After arterial bypass and angioplasty, restenosis is produced in part by intimal hyperplasia. Restenosis is inhibited by gene transfer of several inhibitors of cell cycle, intracellular signal transducers, transcription factors, cytokines, growth factors, NO, and Fas ligand (Kibbe et al., 2000). Among them, the transcription factor E2F transactivates cell cycle regulatory genes. Decoy oligodeoxynucleotides of E2F modulate gene expression and inhibit the proliferation of smooth muscle in the injured carotid artery in vivo (Morishita et al., 1995). Efficacy of the E2F decoy has been tested in clinical trials of restenosis after vascular bypass grafts (Mann et al., 1999) and after angioplasty (Morishita et al., 2001).
Ischemic stroke
Damage in the ischemic core starts very soon after the onset of stroke. Reduction in cerebral blood flow (CBF) reduces energy availability and, as a result, membrane ionic gradients fail rapidly, which result in excessive influx of calcium into cells within seconds to minutes after the onset of ischemia. Increased intracellular calcium causes release of excitotoxic neurotransmitters including glutamate within an hour, and thereby increases damage to neuronal cells. Cellular damage may be augmented during reperfusion, which implies that damage might be reduced by the prevention of reperfusion injury. The therapeutic time window appears to be (at most) only a few hours after the onset of stroke. Since expression of transgene products requires hours using current techniques of gene transfer, the initial damage after stroke is not a promising target for gene therapy, except when a direct effector (with no need of expression) including oligonucleotide is used.
In the peri-ischemic area, or ‘ischemic penumbra,' an inflammatory cascade has been characterized (Barone & Feuerstein, 1999) in the following order: gene expression of transcription factors (wave 1), heat-shock proteins (HSPs) (wave 2), cytokines, chemokines, adhesion molecules, and growth factors (wave 3), proteinases and proteinase inhibitors (wave 4), and delayed remodeling proteins (wave 5). The third wave, consisting of upregulation of many proinflammatory genes, begins about 1 h after stroke, peaks at about 12 h, and subsides after 2–5 days. The entire process is completed in about 2 weeks. The current strategy for gene therapy of stroke focuses on augmentation of neuroprotective genes or inhibition of genes that are believed to be harmful during the first 2 weeks, especially within about 5 days after the onset of stroke.
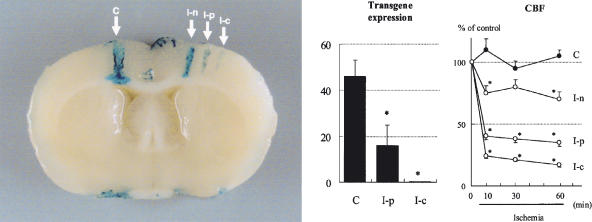
Protein synthesis begins to decrease when CBF declines to <50% of the resting value or about 30 mL/100 g per minute (Xie et al., 1989; Mies et al., 1993), which results in the inhibition of transcriptional and translational processes. Since most of the currently available viral and nonviral vectors use the expression mechanisms of host cells, a critical question about possible efficacy for gene therapy for brain ischemia is whether, despite cellular ischemia, effective transgene expression and protein synthesis can be expected in ischemic and peri-ischemic areas. After middle cerebral artery (MCA) occlusion and reperfusion, adenovirus-mediated transgene expression was observed to increase in the peri-ischemic area 7 days after ischemia/reperfusion to a level similar to that of the control, and diminished by 21 days (Abe et al., 1997). At 4 days after photochemical occlusion of the distal MCA and adenovirus-mediated gene transfer, transgene expression was minimal in the ischemic core, and moderate in the peri-ischemic area, where CBF decreased to about 40% (Ooboshi et al., 2001) (Figure 3). Thus, protein synthesis appears to be sufficient, at least in the peri-ischemic region, which suggests that gene transfer to ischemic penumbra may be a promising approach for the treatment of ischemic stroke.
Figure 3.
Adenovirus-mediated transgene expression in the ischemic brain. Revised from Ooboshi et al. (2001). (Left) X-gal staining of rat brain (coronal section) 4 days after brain ischemia and gene transfer of β-galactosidase. Arrows indicate sites of injection of vector. C, control (contralateral to ischemic side); I-n, nonischemic area; I-p, peri-ischemic area; I-c, ischemic core area. (Center) Quantitative analysis of transgene expression. *P<0.05 vs C. (Right) Changes in CBF. *P<0.05 vs C.
Several experimental trials of gene therapy have been attempted for the treatment of poststroke disruption of ionic gradients, energy failure, and the inflammatory cascade. Calbindin D28K, which is an endogenous calcium-binding protein that inhibits excessive accumulation of intracellular calcium, attenuated brain damage by prestroke gene delivery (Yenari et al., 2001a). Prestroke administration of a glucose transporter gene also protected against ischemic brain injury presumably by increasing glucose uptake in neurons (Lawrence et al., 1996). HSP72 is neuroprotective, and is usually expressed within the first couple of hours and days after stroke. Gene transfer of HSP72 attenuated neuronal damage even when the vector was administered after the onset of stroke (Hoehn et al., 2001).
Proinflammatory genes during the third wave of the inflammatory cascade seem to be good candidates for gene therapy. Interleukin-1 receptor antagonist (Yang et al., 1997), interleukin-10 (Ooboshi et al., 2002), transforming growth factor-β1 (Pang et al., 2001), and neurotropic factors including glial cell line-derived neurotropic factor (Kitagawa et al., 1999) and HGF (Hayashi et al., 2001) all may have neuroprotective effects on brain ischemia and other mechanisms of neuronal damage. After transfer of these genes to brain parenchyma or subarachnoid space, ischemic brain injury was attenuated.
Gene transfer of antiapoptotic genes, including the proto-oncogene bcl-2 (Shimazaki et al., 2000), neuronal apoptosis inhibitory protein (Xu et al., 1997), and a redox-inducible antioxidant protein (Yang et al., 2001) are potentially useful for neuroprotection from ischemia. Gene transfer of cyclooxygenase-1 also is potentially useful for treating ischemic stroke, by augmenting synthesis of neuroprotective prostaglandins (Lin et al., 2002).
Future directions
Strategies that may lead to clinical use of cerebrovascular gene therapy include stimulation of growth of collateral vessels, and inhibition of atherogenesis, thrombogenesis, and restenosis for cervical arteries, because these approaches are already under study for coronary and limb arteries of patients. Since little information is available about the effects of these strategies on vascular morphology and function of the brain, application to intracranial arteries is unlikely in the near future. For example, based on the findings in peripheral vessels, a concern is that intracranial growth of collateral circulation might lead to vascular malformation and brain hemorrhage (Isner et al., 1996; Lee et al., 2000).
Protection against vasospasm is a very promising target for clinical use. Since experimental SAH in animals does not fully replicate vasospasm after SAH in humans, confirmation of findings in primates will be necessary.
Since there is currently no effective treatment to reduce significantly the size of strokes in humans, except possibly thrombolytic therapy, gene therapy for stroke is attractive. A serious limitation of several experimental gene therapy studies for brain ischemia, however, is that gene transfer was undertaken prior to stroke.
A critical concern for any gene therapy is safety of the vector. The death of Jesse Gelsinger during the adenovirus-mediated gene therapy trial (Teichler-Zallen et al., 2000) was a major setback for the area of research. The American Society of Gene Therapy (2002) announced that a child developed leukemia after gene therapy using a retroviral vector. It has been recognized that there is a potential risk of insertional mutagenesis when retroviruses are used for gene transfer. The risk of induction of malignancies by adenoviral gene transfer appears to be minimal, because the transgene remains episomal, not inserted into the chromosomes. Nevertheless, improvement of vectors is urgently needed.
It is not yet clear when gene therapy for cerebrovascular diseases will become clinically useful. It still appears likely that progress will be made in steps, and not in a great leap.
Acknowledgments
We thank Ms Arlinda LaRose for typing the manuscript, and Dr Frank Faraci for a critical review. Original studies by the authors were supported by the Department of Veterans Affairs; NIH Grants HL 16066, HL 62984, NS 24621, HL 14388; the Carver Research Program of Excellence; and the Wendy Hamilton Trust.
Abbreviations
- bFGF
basic fibroblast growth factor
- CBF
cerebral blood flow
- CGRP
calcitonin gene-related peptide
- CSF
cerebrospinal fluid
- HGF
hepatocyte growth factor
- HSP
heat-shock protein
- HVJ
hemagglutinating virus of Japan
- MCA
middle cerebral artery
- MCP-1
monocyte chemoattractant protein-1
- NO
nitric oxide
- NOS
nitric oxide synthase
- SAH
subarachnoid hemorrhage
- SOD
superoxide dismutase
- VEGF
vascular endothelial growth factor
References
- ABE K., SETOGUCHI Y., HAYASHI T., ITOYAMA Y. In vivo adenovirus-mediated gene transfer and the expression in ischemic and reperfused rat brain. Brain Res. 1997;763:191–201. doi: 10.1016/s0006-8993(97)00389-2. [DOI] [PubMed] [Google Scholar]
- AIHARA Y., KASUYA H., ONDA H., HORI T., TAKEDA J. Quantitative analysis of gene expressions related to inflammation in canine spastic artery after subarachnoid hemorrhage. Stroke. 2001;32:212–217. doi: 10.1161/01.str.32.1.212. [DOI] [PubMed] [Google Scholar]
- AKLI S., CAILLAUD C., VIGNE E., STRATFORD-PERRICAUDET L.D., POENARU L., PERRICAUDET M., KAHN A., PESCHANSKI M.R. Transfer of a foreign gene into the brain using adenovirus vectors. Nat. Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- ALEXANDER M.Y., BROSNAN M.J., HAMILTON C.A., DOWNIE P., DEVLIN A.M., DOWELL F., MARTIN W., PRENTICE H.M., O'BRIEN T., DOMINICZAK A.F. Gene transfer of endothelial nitric oxide synthase improves nitric oxide-dependent endothelial function in a hypertensive rat model. Cardiovasc. Res. 1999;43:798–807. doi: 10.1016/s0008-6363(99)00146-7. [DOI] [PubMed] [Google Scholar]
- American Society of Gene Therapy Serious adverse event in a clinical trial of gene therapy for the X-linked form of severe combined immune deficiency disease (XSCID) 2002ASGT Website: http://www.asgt.org
- ATSUCHI N., NISHIDA T., MARUTSUKA K., ASADA Y., KAMIKUBO Y., TAKESHITA A., UENO H. Combination of a brief irrigation with tissue factor pathway inhibitor (TFPI) and adenovirus-mediated local TFPI gene transfer additively reduces neointima formation in balloon-injured rabbit carotid arteries. Circulation. 2001;103:570–575. doi: 10.1161/01.cir.103.4.570. [DOI] [PubMed] [Google Scholar]
- BAJOCCHI G., FELDMAN S.H., CRYSTAL R.G., MASTRANGELI A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat. Genet. 1993;3:229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- BALAGUE C., ZHOU J., DAI Y., ALEMANY R., JOSEPHS S.F., ANDERSON G., HARIHARAN M., SETHI E., PROKOPENKO E., JAN H., LOU Y.C., HUBERT-LESLIE D., RUIZ L., ZHANG W.W. Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophiliac mice using a minimal adenoviral vector. Blood. 2000;95:820–828. [PubMed] [Google Scholar]
- BARONE F.C., FEUERSTEIN G.Z. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J. Cereb. Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- BAUMGARTNER I., PIEEZEK A., MANOR O., BLAIR R., KEARNEY M., WALSH K., ISNER J.M. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- BETZ A.L., YANG G.Y., DAVIDSON B.L. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J. Cereb. Blood Flow Metab. 1995;15:547–551. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- BUCHSCHACHER G.L., WONG-STAAL F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2001;95:2499–2504. [PubMed] [Google Scholar]
- CHEN A.F.Y., JIANG S.W., CROTTY T.B., TSUTSUI M., SMITH L.A., O'BRIEN T., KATUSIC Z.S. Effects of in vivo adventitial expression of recombinant endothelial nitric oxide synthase gene in cerebral arteries. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12568–12573. doi: 10.1073/pnas.94.23.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSON S.D., LAKE K.D., OOBOSHI H., FARACI F.M., DAVIDSON B.L., HEISTAD D.D. Adenovirus-mediated gene transfer in vivo to cerebral blood vessels and perivascular tissue in mice. Stroke. 1998;29:1411–1416. doi: 10.1161/01.str.29.7.1411. [DOI] [PubMed] [Google Scholar]
- CHU Y., IIDA S., LUND D.D., WEISS R.M., DIBONA G.F., WATANABE Y., FARACI F.M., HEISTAD D.D. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ. Res. 2003;92:461–468. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- DAVIDSON B.L., ALLEN E.D., KOZARSKY K.F., WILSON J.M., ROESSLER B.J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat. Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- DESURMONT C., CAILLAUD J.M., EMMANUEL F., BENOIT P., FRUCHART J.C., CASTRO G., BRANELLEC D., HEARD J.M., DUVERGER N. Complete atherosclerosis regression after human ApoE gene transfer in ApoE-deficient/nude mice. Arterioscler. Thromb. Vasc. Biol. 2000;20:435–442. doi: 10.1161/01.atv.20.2.435. [DOI] [PubMed] [Google Scholar]
- DRAZAN K.E., CSETE M.E., DA SHAN X.D., BULLINGTON D., COTTLE G., BUSUTTIL R.W., SHAKED A. Hepatic function is preserved following liver-directed, adenovirus-mediated gene transfer. J. Surg. Res. 1995;59:299–304. doi: 10.1006/jsre.1995.1168. [DOI] [PubMed] [Google Scholar]
- EGASHIRA K., KOYANAGI M., KITAMOTO S., NI W., KATAOKA C., MORISHITA R., KANEDA Y., AKIYAMA C., NISHIDA K., SUEISHI K., TAKESHITA A. Anti-monocyte chemoattractant protein-1 gene therapy inhibits vascular remodeling in rats: blockade of MCP-1 activity after intramuscular transfer of a mutant gene inhibits vascular remodeling induced by chronic blockade of NO synthesis. FASEB J. 2000;14:1974–1978. doi: 10.1096/fj.00-0141com. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., EKMAN R., JANSEN I., MCCULLOH J., MORTENSEN A., UDDMAN R. Reduced levels of calcitonin gene-related peptide-like immunoreactivity in human brain vessels after subarachnoid haemorrhage. Neurosci. Lett. 1991;121:151–154. doi: 10.1016/0304-3940(91)90672-g. [DOI] [PubMed] [Google Scholar]
- FARACI F.M., HEISTAD D.D. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol. Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- GELBAND C.H., KATOVICH M.J., RAIZADA M.K. Current perspectives on the use of gene therapy for hypertension. Circ. Res. 2000;87:1118–1122. doi: 10.1161/01.res.87.12.1118. [DOI] [PubMed] [Google Scholar]
- GRINES C.L., WATKINS M.W., HELMER G., PENNY W., BRINKER J., MARMUR J.D., WEST A., RADE J.J., MARROTT P., HAMMOND H.K., ENGLER R.L. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- GUNNETT C.A., LUND D.D., HOWARD M.A., CHU Y., FARACI F.M., HEISTAD D.D. Gene transfer of inducible NO-synthase impairs relaxation in human and rabbit cerebral arteries. Stroke. 2002;33:2292–2296. doi: 10.1161/01.str.0000027427.86177.d4. [DOI] [PubMed] [Google Scholar]
- HARDER D.R., DERNBACH P., WATERS A. Possible cellular mechanism for cerebral vasospasm after experimental subarachnoid hemorrhage in the dog. J. Clin. Invest. 1987;80:875–880. doi: 10.1172/JCI113146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., MORISHITA R., NAKAGAMI H., YOSHIMURA S., HARA A., MATSUMOTO K., NAKAMURA T., OGIHARA T., KANEDA Y., SAKAI N. Gene therapy for preventing neuronal death using hepatocyte growth factor: in vivo gene transfer of HGF to subarachnoid space prevents delayed neuronal death in gerbil hippocampal CA1 neurons. Gene Ther. 2001;8:1167–1173. doi: 10.1038/sj.gt.3301498. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., MORISHITA R., NAKAMURA S., YAMAMOTO K., MORIGUCHI A., NAGANO T., TAIZI M., NOGUCHI H., MUTSUMOTO K., NAKAMURA T., HIGAKI J., OGIHARA T. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: down-regulation of HGF in response to hypoxia in vascular cells. Circulation. 1999;100:II301–II308. doi: 10.1161/circ.100.suppl_2.Ii-301. [DOI] [PubMed] [Google Scholar]
- HEISTAD D.D., FARACI F.M. Gene therapy for cerebral vascular disease. Stroke. 1996;27:1688–1693. doi: 10.1161/01.str.27.9.1688. [DOI] [PubMed] [Google Scholar]
- HOEHN B., RINGER T.M., XU L., GIFFARD R.G., SAPOLSKY R.M., STEINBERG G.K., YENARI M.A. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J. Cereb. Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- HU W.S., PATHAK V.K. Design of retroviral vectors and helper cells for gene therapy. Pharmacol. Rev. 2000;52:493–511. [PubMed] [Google Scholar]
- ISNER J.M., PIECZEK A., SCHAINFELD R., BLAIR R., HALEY L., ASAHARA T., ROSENFIELD K., RAZVI S., WALSH K., SYMES J. Clinical evidence of angiogenesis following arterial gene transfer of phVEGF165. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- ISNER J.M., VALE P.R., SYMES J.F., LOSORDO D.W. Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ. Res. 2001;89:389–400. doi: 10.1161/hh1701.096259. [DOI] [PubMed] [Google Scholar]
- JOHNSTON F.G., BELL B.A., ROBERTSON I.J., MILLER J.D., HALIBURN C., O'SHAUGHNESSY D., RIDDELL A.J., O'LAOIRE S.A. Effect of calcitonin-gene-related peptide on postoperative neurological deficits after subarachnoid haemorrhage. Lancet. 1990;335:869–872. doi: 10.1016/0140-6736(90)90473-i. [DOI] [PubMed] [Google Scholar]
- JUUL R., AAKHUS S., BJORNSTAD K., GISVOLD S.E., BRUBAKK A.O., EDVINSSON L. Calcitonin gene-related peptide (human a-CGRP) counteracts vasoconstriction in human subarachnoid haemorrhage. Neurosci. Lett. 1994;170:67–70. doi: 10.1016/0304-3940(94)90240-2. [DOI] [PubMed] [Google Scholar]
- KAJITA Y., SUZUKI Y., OYAMA H., TANAZAWA T., TAKAYASU M., SHIBUYA M., SUGITA K. Combined effect of L-arginine and superoxide dismutase on the spastic basilar artery after subarachnoid hemorrhage in dogs. J. Neurosurg. 1994;80:476–483. doi: 10.3171/jns.1994.80.3.0476. [DOI] [PubMed] [Google Scholar]
- KAMII H., KATO I., KINOUCHI H., CHAN P.H., EPSTEIN C.J., AKABANE A., OKAMOTO H., YOSHIMOTO T. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn-superoxide dismutase. Stroke. 1999;30:867–872. doi: 10.1161/01.str.30.4.867. [DOI] [PubMed] [Google Scholar]
- KAY M.A., GLORIOSO J.C., NALDINI L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- KHURANA V.G., SMITH L.A., BAKER T.A., EGUCHI D., O'BRIEN T., KATUSIC Z.S. Protective vasomotor effects of in vivo recombinant endothelial nitric oxide synthase gene expression in a canine model of cerebral vasospasm. Stroke. 2002;33:782–789. doi: 10.1161/hs0302.103735. [DOI] [PubMed] [Google Scholar]
- KHURANA V.G., SMITH L.A., WEILER D.A., SPRINGETT M.J., PARISI J.E., MEYER F.B., MARSH W.R., O'BRIEN T., KATUSIC Z.S. Adenovirus-mediated gene transfer to human cerebral arteries. J. Cereb. Blood Flow Metab. 2000;20:1360–1371. doi: 10.1097/00004647-200009000-00011. [DOI] [PubMed] [Google Scholar]
- KIBBE M.R., BILLIAR T.R., TZENG E. Gene therapy for restenosis. Circ. Res. 2000;86:829–833. doi: 10.1161/01.res.86.8.829. [DOI] [PubMed] [Google Scholar]
- KITAGAWA H., SASAKI C., SAKAI K., MORI A., MITSUMOTO Y., MORI T., FUKUCHI Y., SETOGUCHI Y., ABE K. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents ischemic brain injury after transient middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 1999;19:1336–1344. doi: 10.1097/00004647-199912000-00007. [DOI] [PubMed] [Google Scholar]
- KITAZONO T., HEISTAD D.D., FARACI F.M. Role of ATP-sensitive K+ channels in CGRP-induced dilatation of basilar artery in vivo. Am. J. Physiol. 1993;265:H581–H585. doi: 10.1152/ajpheart.1993.265.2.H581. [DOI] [PubMed] [Google Scholar]
- KOZARSKY K.F., JOOSS K., DONAHEE M., STRAUSS J.F., WILSON J.M. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat. Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- LAWRENCE M.S., SUN G.H., KUNIS D.M., SAYDAM T.C., DASH R., HO D.Y., SAPOLSKY R.M., STEINBERG G.K. Overexpression of the glucose transporter gene with a herpes simplex viral vector protects striatal neurons against stroke. J. Cereb. Blood Flow Metab. 1996;16:181–185. doi: 10.1097/00004647-199603000-00001. [DOI] [PubMed] [Google Scholar]
- LEE R.J., SPRINGER M.L., BLANCO-ROSE W.E., SHAW R., URSELL P.C., BLAU H.M. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- LEE S.W., TRAPNELL B.C., RADE J.J., VIRMANI R., DICHEK D.A. In vivo adenoviral vector-mediated gene transfer into balloon-injured rat carotid arteries. Circ. Res. 1993;73:797–807. doi: 10.1161/01.res.73.5.797. [DOI] [PubMed] [Google Scholar]
- LE GAL LA SALLE G., ROBERT J.J., BERRARD S., RIDOUX V., STRATFORD-PERRICAUDET L.D., PERRICAUDET M., MALLET J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- LEMARCHAND P., JONES M., YAMADA I., CRYSTAL R.G. In vivo gene transfer and expression in normal uninjured blood vessels using replication-deficient recombinant adenovirus vectors. Circ. Res. 1993;72:1132–1138. doi: 10.1161/01.res.72.5.1132. [DOI] [PubMed] [Google Scholar]
- LI L., CROCKETT E., WANG D.H., GALLIGAN J.J., FINK G.D., CHEN A.F. Gene transfer of endothelial NO synthase and manganese superoxide dismutase on arterial vascular cell adhesion molecule-1 expression and superoxide production in deoxycorticosterone acetate-salt hypertension. Arterioscler. Thromb. Vasc. Biol. 2002;22:249–255. doi: 10.1161/hq0202.104124. [DOI] [PubMed] [Google Scholar]
- LIN H., LIN T.-N., CHEUNG W.-M., NIAN G.-M., TSENG P.-H., CHEN S.-F., CHEN J.-J., SHYUE S.-K., LIOU J.-Y., WU C.-W., WE K.K. Cyclooxygenase-1 and bicistronic cyclooxygenase-1/prostacyclin synthase gene transfer protect against ischemic cerebral infarction. Circulation. 2002;105:1962–1969. doi: 10.1161/01.cir.0000015365.49180.05. [DOI] [PubMed] [Google Scholar]
- LIN H., PARMACEK M.S., MORLE G, BOLLING S., LEIDEN J.M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- LOSORDO D.W., VALE P.R., HENDEL R.C., MILLIKEN C.E., FORTUIN F.D., CUMMINGS N., SCHATZ R.A., ASAHARA T., ISNER J.M., KUNTZ R.E. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN P.R. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- LUND D.D., FARACI F.M., MILLER F.J, JR, HEISTAD D.D. Gene transfer of endothelial nitric oxide synthase improves relaxation of carotid arteries from diabetic rabbits. Circulation. 2000;101:1027–1033. doi: 10.1161/01.cir.101.9.1027. [DOI] [PubMed] [Google Scholar]
- LUND D.D., FARACI F.M., OOBOSHI H., DAVIDSON B.L., HEISTAD D.D. Adenovirus-mediated gene transfer is augmented in basilar and carotid arteries of heritable hyperlipidemic rabbits. Stroke. 1998;29:120–125. doi: 10.1161/01.str.30.1.120. [DOI] [PubMed] [Google Scholar]
- MAKINEN K., MANNINEN H., HEDMAN M., MATSI P., MUSSALO H., ALHAVA E., YLA-HERTTUALA S. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol. Ther. 2002;6:127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- MANN M.J., WHITTEMORE A.D., DONALDSON M.C., BELKIN M., CONTE M.S., POLAK J.F., ORAV E.J., EHSAN A., DELL'ACQUA G., DZAU V.J. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomized, controlled trial. Lancet. 1999;354:1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- MCGIRT M.J., PARRA A., SHENG H., HIGUCHI Y., OURY T.D., LASKOWITZ D.T., PEARLSTEIN R.D., WARNER D.S. Attenuation of cerebral vasospasm after subarachnoid hemorrhage in mice overexpressing extracellular superoxide dismutase. Stroke. 2002;33:2317–2323. doi: 10.1161/01.str.0000027207.67639.1e. [DOI] [PubMed] [Google Scholar]
- MIES G., KOHNO K., HOSSMANN K.A. MK-801, a glutamate antagonist, lowers glow threshold for inhibition of protein synthesis after middle cerebral artery occlusion of rat. Neurosci. Lett. 1993;155:65–68. doi: 10.1016/0304-3940(93)90674-a. [DOI] [PubMed] [Google Scholar]
- MORI E., KOMORI K., YAMAOKA T., TANII M., KATAOKA C., TAKESHITA A., USUI M., EGASHIRA K., SUGIMACHI K. Essential role of monocyte chemoattractant protein-1 in development of restenotic changes (neointimal hyperplasia and constrictive remodeling) after balloon angioplasty in hypercholesterolemic rabbits. Circulation. 2002;105:2905–2910. doi: 10.1161/01.cir.0000018603.67989.71. [DOI] [PubMed] [Google Scholar]
- MORISHITA R., AOKI M., KANEDA Y. Decoy oligodeoxynucleotides as novel cardiovascular drugs for cardiovascular disease. Ann. N. Y. Acad. Sci. 2001;947:294–302. doi: 10.1111/j.1749-6632.2001.tb03950.x. [DOI] [PubMed] [Google Scholar]
- MORISHITA R., GIBBONS G.H., HORIUCHI M., ELLISON K.E., NAKAJIMA M., ZHANG L., KANEDA Y., OGIHARA T., DZAU V.J. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUHONEN M.G., OOBOSHI H., WELSH M.J., DAVIDSON B.L., HEISTAD D.D. Gene transfer to cerebral blood vessels after subarachnoid hemorrhage. Stroke. 1997;28:822–829. doi: 10.1161/01.str.28.4.822. [DOI] [PubMed] [Google Scholar]
- NABEL E.G., PLAUTZ G, BOYEE F.M., STANLEY J.C., NABEL G.J. Recombinant gene expression in vivo within endothelial cells of the arterial wall. Science. 1989;244:1342–1344. doi: 10.1126/science.2499928. [DOI] [PubMed] [Google Scholar]
- NAKANE H., CHU Y., FARACI F.M., OBERLEY L.W., HEISTAD D.D. Gene transfer of extracellular superoxide dismutase increases superoxide dismutase activity in cerebrospinal fluid. Stroke. 2001;32:184–189. doi: 10.1161/01.str.32.1.184. [DOI] [PubMed] [Google Scholar]
- NAKANE H., MILLER F.J., FARACI F.M., TOYODA K., HEISTAD D.D. Gene transfer of eNOS reduces angiotensin II-induced endothelial dysfunction. Hypertension. 2000;35:595–601. doi: 10.1161/01.hyp.35.2.595. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., HUANG Y., BRAYDEN J.E., HESCHELER J., STANDEN N.B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- NOZAKI K., KIKUCHI H., MIZUNO N. Changes of calcitonin gene-related peptide-like immunoreactivity in cerebrovascular nerve fibers in the dog after experimentally produced subarachnoid hemorrhage. Neurosci. Lett. 1989a;102:27–32. doi: 10.1016/0304-3940(89)90302-9. [DOI] [PubMed] [Google Scholar]
- NOZAKI K., UEMURA Y., OKAMOTO S., KIKUCHI H., MIZUNO N. Relaxant effect of calcitonin gene-related peptide on cerebral arterial spasm induced by experimental subarachnoid hemorrhage in dogs. J. Neurosurg. 1989b;71:558–564. doi: 10.3171/jns.1989.71.4.0558. [DOI] [PubMed] [Google Scholar]
- OHKUMA H., PARNEY I., MEGYESI J., GHAHARY A., FINDLAY J.M. Antisense preproendothelin-oligoDNA therapy for vasospasm in a canine model of subarachnoid hemorrhage. J. Neurosurg. 1999;90:1105–1114. doi: 10.3171/jns.1999.90.6.1105. [DOI] [PubMed] [Google Scholar]
- ONO S., DATE I., ONODA K., SHIOTA T., OHTOMO T., NINOMIYA, Y, ASARI S., MORISHITA R. Decoy administration of NF-κB into the subarachnoid space for cerebral angiopathy. Hum. Gene Ther. 1998;9:1003–1011. doi: 10.1089/hum.1998.9.7-1003. [DOI] [PubMed] [Google Scholar]
- ONODA K., ONO S., OGIHARA K., SHIOTA T., ASARI S., OHMOTO T., NINOMIYA Y. Inhibition of vascular contraction by intracisternal administration of preproendothelin-1 mRNA antisense oligoDNA in a rat experimental vasospasm model. J. Neurosurg. 1996;85:846–852. doi: 10.3171/jns.1996.85.5.0846. [DOI] [PubMed] [Google Scholar]
- ONOUE H., TSUTSUI M., SMITH I., STELTER A., O'BRIEN T., KATUSIC Z.S.Expression and function of recombinant endothelial nitric oxide synthase gene in canine basilar artery after experimental subarachnoid hemorrhage Stroke 1998291959–1965.discussion 1965–1966 [DOI] [PubMed] [Google Scholar]
- OOBOSHI H., CHU Y., RIOS C.D., FARACI F.M., DAVIDSON B.L., HEISTAD D.D. Augmented adenovirus-mediated gene transfer to atherosclerotic vessels. Arterioscler. Thromb. Vasc. Biol. 1997a;17:1786–1792. doi: 10.1161/01.atv.17.9.1786. [DOI] [PubMed] [Google Scholar]
- OOBOSHI H., CHU, Y, RIOS C.D., FARACI F.M., DAVIDSON B.L., HEISTAD D.D. Altered vascular function following adenovirus-mediated over-expression of endothelial nitric oxide synthase. Am. J. Physiol. (Heart Circ. Physiol.) 1997b;42:H265–H270. doi: 10.1152/ajpheart.1997.273.1.H265. [DOI] [PubMed] [Google Scholar]
- OOBOSHI H., IBAYASHI S., ARAKAWA S., KITAZONO T., YAO H., FUJISHIMA M., IIDA M. Postischemic gene transfer of IL10 protects against focal ischemia. Stroke. 2002;33:346. [Google Scholar]
- OOBOSHI H., IBAYASHI S., TAKADA J., YAO H., KITAZONO T., FUJISHIMA M. Adenovirus-mediated gene transfer to ischemic brain: ischemic flow threshold for transgene expression. Stroke. 2001;32:1043–1047. doi: 10.1161/01.str.32.4.1043. [DOI] [PubMed] [Google Scholar]
- OOBOSHI H., TOYODA K., FARACI F.M., LANG M.G., HEISTAD D.D. Improvement of relaxation in an atherosclerotic artery by gene transfer of endothelial nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 1998;18:1752–1758. doi: 10.1161/01.atv.18.11.1752. [DOI] [PubMed] [Google Scholar]
- OOBOSHI H., WELSH M.J., RIOS C.D., DAVIDSON B.L., HEISTAD D.D. Adenovirus-mediated gene transfer in vivo to cerebral blood vessels and perivascular tissue. Circ. Res. 1995;77:7–13. doi: 10.1161/01.res.77.1.7. [DOI] [PubMed] [Google Scholar]
- OURY T.D., DAY B.J., CRAPO J.D. Extracellular superoxide dismutase: a regulator of nitric oxide bioavailability. Lab. Invest. 1996;75:617–636. [PubMed] [Google Scholar]
- PANG L., YE W., CHE X.M., ROESSLER B.J., BETZ A.L., YANG G.Y. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- PETERSON J.W., KWUN B.D., HACKETT J.D., ZERVAS N.T. The role of inflammation in experimental cerebral vasospasm. J. Neurosurg. 1990;72:767–774. doi: 10.3171/jns.1990.72.5.0767. [DOI] [PubMed] [Google Scholar]
- PHILLIPS M.I. Gene therapy for hypertension: the preclinical data. Hypertension. 2001;38:543–548. doi: 10.1161/hy09t1.092927. [DOI] [PubMed] [Google Scholar]
- RADE J.J., SCHULICK A.H., VIRMANI R., DICHEK D.A. Local adenoviral-mediated expression of recombinant hirudin reduces neointima formation after arterial injury. Nat. Med. 1996;2:293–298. doi: 10.1038/nm0396-293. [DOI] [PubMed] [Google Scholar]
- RIOS C.D., OOBOSHI H., PIEGORS D., DAVIDSON B.L., HEISTAD D.D. Adenovirus-mediated gene transfer to normal and atherosclerotic arteries: a novel approach. Arterioscler. Thromb. Vasc. Biol. 1995;15:2241–2245. doi: 10.1161/01.atv.15.12.2241. [DOI] [PubMed] [Google Scholar]
- ROSENGART T.K., LEE L.Y., PATEL S.R., KLIGFIELD P.D., OKIN P.M., HACKETT N.R., ISOM O.W., CRYSTAL R.G. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann Surg. 1999;230:466–470. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATOH M., PERKINS E., KIMURA H., TANG J., CHU Y., HEISTAD D.D., ZHANG J.H. Post-treatment with adenovirus-mediated gene transfer of calcitonin gene-related peptide to reverse cerebral vasospasm in dogs. J. Neurosurg. 2002;97:136–142. doi: 10.3171/jns.2002.97.1.0136. [DOI] [PubMed] [Google Scholar]
- SETH P., ROSENFELD M., HIGGINBOTHAM J., CRYSTAL R.G. Mechanism of enhancement of DNA expression consequent to co-internalization of a replication-deficient adenovirus and unmodified plasmid DNA. J. Virol. 1994;68:933–940. doi: 10.1128/jvi.68.2.933-940.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMAZAKI K., URABE M., MONAHAN J., OZAWA K., KAWAI N. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 2000;7:1244–1249. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- SHISHIDO T., SUZUKI R., QIAN I., HIRAKAWA K. The role of superoxide anions in the pathogenesis of cerebral vasospasm. Stroke. 1994;25:864–868. doi: 10.1161/01.str.25.4.864. [DOI] [PubMed] [Google Scholar]
- SIMONS M., EDELMAN E.R., DEKEYSER J.-L., LANGER R., ROSENBERG R.D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- SOBEY C.G., HEISTAD D.D., FARACI F.M. Effect of subarachnoid hemorrhage on dilatation of rat basilar artery in vivo. Am. J. Physiol. 1996;271:H126–H132. doi: 10.1152/ajpheart.1996.271.1.H126. [DOI] [PubMed] [Google Scholar]
- STOODLEY M., WEIHL C.C., ZHANG Z.D., LIN G., JOHNS L.M., KOWALCZUK A., GHADGE, G, ROOS R.P., MACDONALD R.L.Effect of adenovirus-mediated nitric oxide synthase gene transfer on vasospasm after experimental sub-arachnoid hemorrhage Neurosurgery 2000461193–1203.discussion 1202–1203 [DOI] [PubMed] [Google Scholar]
- TANGIRALA R.K., TSUKAMOTO K., CHUN S.H., USHER D., PURE E., RADER D.J. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- TEICHLER-ZALLEN D. US gene therapy in crisis. Trends Genet. 2000;16:272–275. doi: 10.1016/s0168-9525(00)02025-4. [DOI] [PubMed] [Google Scholar]
- THOMAS C.E., BIRKETT D., ANOZIE I., CASTRO M.G., LOWENSTEIN P.R. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- TOSHIMA M., KASSELL N.F., TANAKA Y., DOUGHERTY D.A. Effect of intracisternal and intravenous calcitonin gene-related peptide on experimental cerebral vasospasm in rabbits. Acta Neurochir. 1992;119:134–138. doi: 10.1007/BF01541797. [DOI] [PubMed] [Google Scholar]
- TOYODA K., ANDRESEN J.J., ZABNER J., FARACI F.M., HEISTAD D.D. Calcium phosphate precipitates augment adenovirus-mediated gene transfer to blood vessels in vitro and in vivo. Gene Ther. 2000a;7:1284–1291. doi: 10.1038/sj.gt.3301214. [DOI] [PubMed] [Google Scholar]
- TOYODA K., FARACI F.M., RUSSO A.F., DAVIDSON B.L., HEISTAD D.D. Gene transfer of calcitonin gene-related peptide to cerebral arteries. Am. J. Physiol. 2000b;278:H586–H594. doi: 10.1152/ajpheart.2000.278.2.H586. [DOI] [PubMed] [Google Scholar]
- TOYODA K., FARACI F.M., WATANABE Y., UEDA T., ANDRESEN J.J., CHU Y., OTAKE S., HEISTAD D.D. Gene transfer of calcitonin gene-related peptide prevents vasoconstriction after subarachnoid hemorrhage. Circ. Res. 2000c;87:818–824. doi: 10.1161/01.res.87.9.818. [DOI] [PubMed] [Google Scholar]
- TOYODA K., NAKANE H., HEISTAD D.D. Cationic polymer and lipids augment adenovirus-mediated gene transfer to cerebral arteries in vivo. J. Cereb. Blood Flow Metab. 2001;21:1125–1131. doi: 10.1097/00004647-200109000-00010. [DOI] [PubMed] [Google Scholar]
- TOYODA K., OOBOSHI H., CHU Y., FASBENDER A., DAVIDSON B.L., WELSH M.J., HEISTAD D.D. Cationic polymer and lipids enhance adenovirus-mediated gene transfer to carotid artery. Stroke. 1998;29:2181–2188. doi: 10.1161/01.str.29.10.2181. [DOI] [PubMed] [Google Scholar]
- UDELSON J.E., DILSIZIAN V., LAHAM R.J., CHRONOS N., VANSANT J., BLAIS M., GALT J.R., PIKE M., YOSHIZAWA C., SIMONS M. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 improves stress and rest myocardial perfusion abnormalities in patients with severe symptomatic chronic coronary artery disease. Circulation. 2000;102:1605–1610. doi: 10.1161/01.cir.102.14.1605. [DOI] [PubMed] [Google Scholar]
- UENO H., SAKAMOTO T., NAKAMURA T., QI Z., ASTUCHI N., TAKESHITA A., SHIMIZU K., OHASHI H. A soluble transforming growth factor beta receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum. Gene Ther. 2000;11:33–42. doi: 10.1089/10430340050016139. [DOI] [PubMed] [Google Scholar]
- VON DER LEYEN H.E., GIBBONS G.H., MORISHITA R., LEWIS N.P., ZHANG L., NAKAJIMA M., KANEDA Y., COOKE J.P., DZAU V.J. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE Y., CHU Y., ANDRESEN J.J., NAKANE H., FARACI F.M., HEISTAD D.D. Gene transfer of extracellular superoxide dismutase reduces vasospasm following subarachnoid hemorrhage. Stroke. 2003;34:434–440. doi: 10.1161/01.str.0000051586.96022.37. [DOI] [PubMed] [Google Scholar]
- WAUGH J.M., KATTASH M., LI J., YUKSEL E., KUO M.D., LUSSIER M., WEINFELD A.B., SAXENA R., RABINOVSKY E.D., THUNG S., WOO S.L., SHENAQ S.M. Gene therapy to promote thromboresistance: local overexpression of tissue plasminogen activator to prevent arterial thrombosis in an in vivo rabbit model. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1065–1070. doi: 10.1073/pnas.96.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKHAM T.J. Targeting adenovirus. Gene Ther. 2000;7:110–114. doi: 10.1038/sj.gt.3301115. [DOI] [PubMed] [Google Scholar]
- WICKHAM T.J., SEGAL D.M., ROELVINK P.W., CARRION M.E., LIZONOVA A., LEE G.M., KOVESDI I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J. Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF J.A., MALONE R.W., WILLIAMS P., CHONG W., ACSADI G., JANI A., FEIGNER P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- XIA H., MAO Q., PAULSON L.P., DAVIDSON B.L. siRNA-mediated gene silencing in vitro and in vivo. Nat. Biotech. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- XIE Y., MIES G., HOSSMANN K.A. Ischemic threshold of brain protein synthesis after unilateral carotid artery occlusion in gerbils. Stroke. 1989;20:620–626. doi: 10.1161/01.str.20.5.620. [DOI] [PubMed] [Google Scholar]
- XU D.G., CROCKER S.J., DOUCET J.-P., ST-JEAN M., TAMAI K., HAKIM A.M., IKEDA J.-E., LISTEN P., THOMPSON C.S., KORNELUK R.G., MACKENZIE A., ROBERTSON G.S. Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nat. Med. 1997;3:997–1004. doi: 10.1038/nm0997-997. [DOI] [PubMed] [Google Scholar]
- YANG G.-Y., PANG L., GE H.-I., TAN M., YE W., LIU X.-H., HUANG F.-P., WU D.-C., CHE X.-M., SONG Y., WEN R., SUN Y. Attenuation of ischemia-induced mouse brain injury by SAG, a redox-inducible antioxidant protein. J. Cereb. Blood Flow Metab. 2001;21:722–733. doi: 10.1097/00004647-200106000-00010. [DOI] [PubMed] [Google Scholar]
- YANG G-Y., ZHAO Y.-J., DAVIDSON B.L., BETZ A.L. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751:181–188. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- YENARI M.A., DUMAS T.C., SAPOLSKY R.M., STEINBERG G.K. Gene therapy for treatment of cerebral ischemia using defective herpes simplex viral vectors. Ann. N. Y. Acad. Sci. 2001a;939:340–357. doi: 10.1111/j.1749-6632.2001.tb03643.x. [DOI] [PubMed] [Google Scholar]
- YENARI M.A., MINAMI M., SUN G.H., MEIER T.J., KUNIS D.M., MCLAUGHLIN J.R., HO D.Y., SAPOLSKY R.M., STEINBERG G.K. Calbindin D28K overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke. 2001b;32:1028–1035. doi: 10.1161/01.str.32.4.1028. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA S., MORISHITA R., HAYASHI K., KOKUZAWA J., AOKI M., MATSUMOTO K., NAKAMURA T., OGIHARA T., SAKAI N., KANEDA Y. Gene transfer of hepatocyte growth factor to subarachnoid space in cerebral hypoperfusion model. Hypertension. 2002;39:1028–1034. doi: 10.1161/01.hyp.0000017553.67732.e1. [DOI] [PubMed] [Google Scholar]
- YUKAWA H., TAKAHASHI J.C., MIYATAKE S.-I., SAIKI M., MATSUOKA N., AKIMOTO M., YANAMOTO H., NAGATA I., KIKUCHI H., HASHIMOTO N. Adenoviral gene transfer of basic fibroblast growth factor promotes angiogenesis in rat brain. Gene Ther. 2000;7:942–949. doi: 10.1038/sj.gt.3301182. [DOI] [PubMed] [Google Scholar]
- ZANETTI M., SATO J., KATUSIE Z.S., O'BRIEN T. Gene transfer of superoxide dismutase isoforms reverses endothelial dysfunction in diabetic rabbit aorta. Am. J. Physiol. (Heart Circ. Physiol.) 2001;280:H2516–H2523. doi: 10.1152/ajpheart.2001.280.6.H2516. [DOI] [PubMed] [Google Scholar]
- ZHAO Q., EGASHIRA K., INOUE S., USUI M., KITAMOTO S., NI W., ISHIBASHI M., HIASA, KI K., ICHIKI T., SHIBUYA M., TAKESHITA A. Vascular endothelial growth factor is necessary in the development of arteriosclerosis by recruiting/activating monocytes in a rat model of long-term inhibition of nitric oxide synthesis. Circulation. 2002;105:1110–1115. doi: 10.1161/hc0902.104718. [DOI] [PubMed] [Google Scholar]