Abstract
This study examined the effects of SR33805, a fantofarone derivative with reported strong Ca2+ -antagonistic properties, on the contractile properties of intact and skinned rat ventricular myocytes.
On intact cells loaded with the Ca2+-fluorescent indicator Indo-1, the application of low concentrations of SR33805 enhanced the amplitude of unloaded cell shortening and decreased the duration of cell shortening. Amplitude of the Ca2+ transient was also decreased.
These effects were accompanied with a shortening of the action potential and a dose-dependent blockade of L-type calcium current (IC50=2.4 × 10−8 M).
On skinned cardiac cells, the application of a low SR33805 concentration (10−8 M) induced a significant increase in maximal Ca2+-activated force at the two-tested sarcomere lengths (SLs), 1.9 and 2.3 μm.
The application of a larger dose of SR33805 (10−6–10−5 M) induced a significant leftward shift of the tension–pCa relation that accounts for Ca2+-sensitization of the myofilaments, particularly at 2.3 μm SL.
In conclusion, despite its strong Ca2+-antagonistic properties SR33805 increases cardiac cell contractile activity as a consequence of its Ca2+-sensitizing effects. These effects are attributable to both an increase in the maximal Ca2+-activated force and a length-dependent Ca2+-sensitization.
Keywords: Ca2+-antagonist, stretch, heart, contractile proteins, Frank–Starling relation
Introduction
SR33805 ([[N-[dimetoxy-3,4-phenethyl]-N-methyl-amino-propoxyl]-4-benzenesulfonyl]-2-isopropyl-3-methyl-1-indole) is structurally related to fantofarone and classified as a substituted indole. It has several distinct and important differences, and in vivo studies have shown that SR33805 possesses a different profile of activity in terms of contractile activity compared with fantofarone and other Ca2+ channel blockers (Chatelain et al., 1993). This compound binds to alpha1-subunit of the L-type Ca2+ channels with a high affinity (KD value=20 pM), but at a site different from the binding site of the classical Ca2+ channel blockers (Chatelain et al., 1994). It potently inhibits the Ca2+ channel in mouse cardiac myocytes in primary culture with IC50 ranging from 4.1 to 33 nM (Romey et al., 1994). In spite of this potent calcium inhibitor effect, Chatelain et al. (1993) reported that SR33805 had a weak negative inotropic effect on electrically evoked contraction in rabbit isolated atria. The IC50 of the negative inotropic effects on rabbit papillary muscles were 38.0 and 0.25 μM for SR33805 and verapamil, respectively. SR33805 was also shown to induce vasorelaxation of rat and rabbit basilar artery strips (Chatelain et al., 1993). A comparison of IC50 values for cardiac and smooth muscles indicates that SR33805 is highly selective to vascular tissue compared with other Ca2+ channel blockers such as verapamil, diltiazem or nifedipine. This led the authors to suggest that the effects of SR33805 depend mainly on membrane potential (Chatelain et al., 1993). However, it has been recently reported on porcine coronary artery that SR33805 reduces Ca2+ transient but increases Ca2+ sensitivity of the myofilaments by an increase of MLC phosphorylation such that force development resisted to the reduction by SR33805 of intracellular Ca2+ release (Ieiri et al., 2000). The concentration of SR33805 required to inhibit contraction was approximately 10 times higher than that required to inhibit [Ca2+]i elevation.
Myocardial contractility can be improved by increasing Ca2+ concentration or by increasing sensitivity of myofilaments to Ca2+ (Lee & Allen, 1997). The latter mechanism has been proposed to be mainly responsible for the Frank–Starling mechanism in which preload of the heart enhances contractile forces. Despite complex relations between ventricular pressure and myocyte tension as well as between volume and sarcomere length (SL), ultimately the volume-end systolic pressure relation depends on the number of strong force-generating crossbridges as SL changes. Thus, the developed tension by isolated cardiac preparations was found to increase in parallel with an increase in muscle length. The same is true at the single-cell level (Vannier et al., 1997). It is admitted that contractility of the failing heart is reduced at the organ and cellular levels. The volume or length sensitivity of the heart or cardiomyocytes, respectively, in cardiac diseases is more controversial from being absent (Schwinger et al., 1994) to reduced (Holubarsch et al., 1996; Anand et al., 1997). Thus, cell length is an important parameter to consider when strategies to restore contractility are investigated.
Besides these intrinsic properties, the responsiveness of contractile proteins can also be increased by Ca2+-sensitizing drugs. This new class of inotropic agents has the advantages of avoiding the problems associated with Ca2+ overloading such as arrhythmias by glycosides and catecholamines, and of enhancing force production without increasing energy utilization. However, most of these agents, including EMD57033 or CGP48506, that sensitize the myofilaments to Ca2+ by promoting the actin–myosin interaction, significantly reduce the length dependency (from 1.9 to 2.3 μm SL) of Ca2+ activation (Arteaga et al., 2000). This will attenuate the Frank–Starling mechanism and consequently these drugs will be less effective on diseased hearts.
In the present work, we investigated the effects of SR33805 on the contractile properties of intact and skinned rat cardiac myocytes. We found that a low concentration SR33805 increases contractile force and decreases cell shortening duration without affecting much the Ca2+ transient. This effect is attributable to an increase in maximal Ca2+ -activated force elicited in skinned cells. At higher concentrations, SR33805 had a clear Ca2+-sensitizing effect that occurred mainly at high SL.
Methods
Ventricular myocyte isolation
Cardiac ventricular cells were isolated from 200–250 g male Wistar rats as previously described (Cazorla et al., 1999). Briefly, rats were anesthetized by intraperitoneal injection of pentobarbital (200 mg 100 g−1). The heart was perfused retrogradely with a Ca2+-free Hanks-HEPES-buffered solution for 5 min at 37°C and then with an enzyme-containing solution for 20–30 min (see Solutions). The ventricles were gently dissociated with forceps in the same medium without enzyme. The cells were then filtered and further incubated for 15 min. Cells were washed several times in a solution containing 0.3 mM Ca2+. Finally, myocytes were kept in a medium containing 1 mM Ca2+ and 0.5% bovine serum albumin (BSA). After collagenase treatment all solutions contained protease inhibitors (PMSF, leupeptin and E-64 (trans-epoxysuccinyl-l-leucine-guanidobutylamide); see Cazorla et al., 2001).
Measurements of Ca2+ transient and cell shortening
To test the effects of SR33805 on Ca2+ movements and contractile activity, cells were loaded for 30 min at room temperature with Indo-1 AM (10 μM, Molecular Probes, Inc., Oregon, U.S.A.). Cells were field stimulated at a frequency of 0.4 Hz, and fluorescence was measured using a multiphotonic microscope (LSM510-LNO, Zeiss, Le Pecq, France, coupled to a 5 mW laser, Mira-Verdi from Coherent, Orsay, France). The fluorescence emitted at 405 and 480 nm, following femtosecond laser pulse excitation at 740 nm, were simultaneously recorded in the line-scan mode (1.9 ms per line). Their ratio (F405/F480) was used to determine the variation of intracellular Ca2+ concentration. Edges of the cell were measured on the fluorescent images, allowing for simultaneous determination of cell shortening. Both Ca2+ transient and cell shortening were analyzed by subroutines of IDL software (RSI, Paris, France).
Mechanical recording and SL measurement
Myocytes were skinned for 6 min at room temperature in a relaxing solution (pCa 9, see below) containing 0.3% vol/vol Triton X-100 and protease inhibitors (see above). Cells were then rinsed twice with the same solution without Triton X-100 and maintained at 4°C. The experimental apparatus and cell attachment procedure were similar to those described previously (Cazorla et al., 1999). Myocytes were observed through an inverted microscope (Diaphot, Nikon, Boulogne, France). Both ends of skinned myocytes were glued to a thin glass rod with optical glue (NOA 63, Norland products Inc., North Brunswick, NJ, U.S.A.) that was polymerized by UV illumination for 3 min. An SL was determined online at 50 Hz by using a fast Fourier transform algorithm (FFT) on the digitized striation images of the attached cell (Gannier et al., 1993). The system for recording force consisted of a piezoresistive strain gauge (model AE 801, SensoNor a.s., Horten, Norway) with a light, 1-mm diameter, 3-cm long glass rod. It was connected to an amplifier to yield a sensitivity of 140 mV μN−1 with a noise level below 0.2 μN. The first amplification stage was achieved by means of a low-noise transducer amplifier (model 1B32, Analog Devices, Boston, U.S.A.). Compliance of the strain gauge was 0.1 μm μN−1 and unloaded resonance frequency was 500 Hz (Vannier et al., 1997). Force measurements were low-pass filtered at 100 Hz and recorded on a thermal paper recorder (Dash IV, Astro-Med Inc., West Warwick, U.S.A.). The cell was positioned at the tip of a conical microcapillary that received the outlet of 10 microcapillaries connected to 5-ml syringes. Solutions were selected with clips allowing flowing or not of the different solutions (flow rate 200 μl min−1). Forces were normalized with the cell section calculated using the width of the cell measured on the video monitor and an estimated value for the thickness. To this aim, some myocytes (n=40) were loaded with membrane fluorescent indicator di-4-ANEPPS (5 μm for 5 min, Molecular Probes, Inc., Oregon, U.S.A.) to visualize the surface membrane and to measure cell width and thickness with a laser scanning confocal microscope (Zeiss LSM510). Cell thickness was measured by Z-axis scanning through the upper and lower surfaces of the cell at a central point. Thus, an averaged value for the ratio between the width (W) and the thickness (T) was determined (T=W*2/3).
Slack SL was determined for each cell before attachment and ranged between 1.82 and 1.9 μm. Cells were first maximally activated with a pCa 4.5 solution to test the quality of the attachment and recovery to baseline. Passive force was determined by stretching the cell using a stepper motor-driven micromanipulator (MP-285, Sutter instrument company, Novato, CA, U.S.A.) at a speed of 0.1 length s−1. The force–pCa relations were established on each cell at two SLs, 1.9 and 2.3 μm. For relative tension–pCa curves, active forces at submaximal activations were normalized to that produced at pCa 4.5 at the same SL. Force slightly diminished with the number of Ca2+ applications; the mean reduction for maximal force was 6.3±2.0% (n=28) during the establishment of two successive force–pCa curves. The relation between force and pCa was fitted to the following equation: force=[Ca2+]nH/(K+[Ca2+]nH, where nH is the Hill coefficient and pCa50, pCa for half-maximal activation, equals–(log K)/nH.
Electrophysiology
The electrophysiological experiments were performed using the whole-cell patch-clamp technique. An Axopatch 200A (Axon instruments, Burlingham, CA, U.S.A.) was used with patch pipettes of 2–3 MΩ resistance. Experiments were controlled and analyzed using pClamp 8.1 and Clampfit 8.1 software (Axon instruments), respectively. Action potential (AP) was recorded in current-clamp mode. The myocytes were superperfused with a Hanks-HEPES solution containing 1.8 mM CaCl2. APs were elicited by 0.2 ms current injection of suprathreshold intensity and recorded at 0.1 Hz. The L-type Ca2+ current (ICa,L) was elicited by depolarizing voltage steps (150 ms) from −80 to +10 mV at 0.1 Hz (for composition of external and internal solutions see below).
Solutions
For isolating the cells, three different solutions were used. (i) The Hanks-HEPES solution contained (mM): NaCl 117, KCl 5.7, NaHCO3 4.4, KH2PO4 1.5, MgCl2 1.7, HEPES 21, glucose 11, taurine 20, pH 7.2 adjusted with NaOH and bubbled with 100% O2. (ii) The enzyme solution had the same composition as the Hanks-HEPES solution, except that it contained 1.3 mg ml−1 collagenase type IV (Worthington, Freehold, NJ, U.S.A.) and 20 μM Ca2+. (iii) The two washing solutions had the same composition as the Hanks-HEPES solution, except that they contained the crossbridge inhibitor 2,3-butanedione monoxime (BDM, 15 mM), the protease inhibitor PMSF (0.5 mM) and 0.3 or 1 mM Ca2+. Intact cells were maintained for the experiments in a 1.8 mM Ca2+ solution without BDM.
Relaxing (pCa 9, solution A) and maximal activating solutions (pCa 4.5, solution B) were as reported by Ventura-Clapier et al. (1987). Briefly, the relaxing solution A contained (mM): phosphocreatine 12, K-acetate 78.4, Na-acetate 30.6, imidazole 30, EGTA 10, dithiothreitol 0.3, with pMg 2.5, pMgATP 2.5 and pCa 9; pH 7.1 adjusted with acetic acid. Solution B contained K-acetate 79.4 and pCa 4.5. Solutions of intermediate Ca2+ concentrations were obtained by mixing solutions A and B in appropriate proportions.
For AP measurement, pipettes were filled with a solution containing (mM): KCl 130, HEPES 25, MgATP 3, NaGTP 0.4 and EGTA 0.5. ICa,L was recorded using an external solution containing (mM): CaCl2 1.8, tetraethyl-ammonium chloride TEA-Cl 140, MgCl2 2, glucose 10, HEPES 10, pH adjusted to 7.4 with TEAOH and a pipette solution that contained (mM): CsCl 140, HEPES 10, EGTA 10, NaGTP 0.4, MgATP 3, pH adjusted to 7.2 with CsOH.
Experiments were performed at room temperature (25°C). SR33805 was a gift from Dr P. Gautier (Sanofi -Synthelabo, Montpellier, France).
Statistics
Data are presented as mean ±s.e.m. (n=number of cells studied). Significant differences were assigned to selected parameters using paired or unpaired Student's t-test or one-way ANOVA (as appropriate) with P< 0.05.
Results
Effect of SR33805 on Ca2+ transient and shortening of intact cardiomyocytes
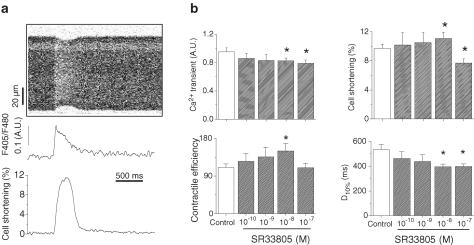
The effects of SR33805 were first investigated by measuring both the variations in intracellular Ca2+ concentration and shortening in isolated rat ventricular cardiomyocytes, using a multiphoton laser scanning microscope (Figure 1). In intact myocytes, the application of SR33805 (from 10−10 to 10−7 M) produced a concentration-dependent decrease in the amplitude of the Ca2+ transient. This effect was statistically significant at 10−8 and 10−7 M with a decrease of 14 and 17%, respectively. This result is in accordance with the Ca2+ channel blockade properties of SR33805 previously observed in mouse cardiomyocytes in culture (Romey et al., 1994) that we confirmed in freshly isolated adult ventricular myocytes (Figure 2b). Furthermore, the time course of the Ca2+ transient was hardly affected (not shown). Cell-shortening characteristics were also modified by SR33805. The amplitude of cell shortening was significantly reduced at 10−7M SR33805 by 18% compared with control conditions. However surprisingly, cell shortening increased on applying SR33805 from 10−10 to 10−8 M. The latter dose induced a significant increase in cell shortening by 14%. Consequently, contractile efficiency (ratio between cell shortening and Ca2+ transient amplitude) increased by 36% after treatment with 10−8 M SR33805. The duration of the cell shortening was significantly reduced particularly the duration at 10% of maximal shortening (Figure 1b).
Figure 1.
Effects of SR33805 on Ca2+ transient and cell shortening of rat cardiac myocytes. (a) Original recordings from top to bottom of a line scan image of an intact cardiomyocyte loaded with Indo-1, Ca2+ transient and variations in cell length, both estimated from this image. (b) Averaged data of Ca2+ transient, cell shortening, calculated contractile efficiency and duration at 10% maximal shortening (D10%) in control (n=26), and in the presence of 10−10–10−7 M (n=25) SR33805. *Indicates statistical difference between nontreated and SR33805-treated cells (P<0.05).
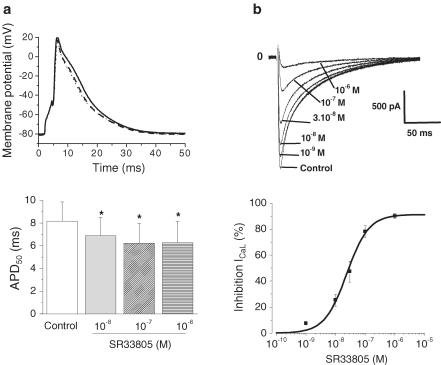
Figure 2.
Effects of SR33805 on cell electrical activity of rat cardiac myocytes. (a) Superimposed APs elicited under current-clamp conditions in control conditions (solid line) and in the presence of 10−8 (dashed line) and 10−7 M (dotted line) SR33805 (upper panel). Pooled data of action potential duration estimated at 50% repolarization (APD50) in control and in the presence of SR33805 (lower panel). *P<0.05, n = 6. (b) Superimposed L-type Ca2+ current traces recordings elicited in a cell perfused with increasing SR33805 concentrations (upper panel). SR33805 induced a concentration-dependent blockade of ICaL as revealed by the dose–response curve (lower panel). The IC50 of the blockade of ICaL by SR33805 is 2.4 × 10−8 M (n=6 cells).
Effect of SR33805 on AP and calcium current of intact rat cardiomyocytes
To test whether the increase in contractile force could be in part attributable to a prolongation of the electrical activity, the effects of SR33805 were investigated on AP recorded on single cells under current-clamp conditions. SR33805 (10−8–10−6 M) slightly but significantly reduced the AP duration at half-repolarization (APD50). This effect on the repolarization phase was maximal at 10−7 M (24%, Figure 2a). This result could be related to L-type Ca2+-channel blockage previously observed by Romey et al. (1994) in mouse cardiac cells in primary culture. To test this hypothesis, we measured L-type Ca2+ current under voltage-clamp conditions on rat adult ventricular myocytes by applying a test potential to +10 mV from the holding of –80 mV. Cells were exposed to a fixed concentration of SR33805 until a steady-state effect on the Ca2+ current was reached within 2 min. The Ca2+ current decreased as SR33805 concentration was increased from 10−9 to 10−6 M. The dose–response curve for this inhibition is represented on Figure 2b (lower panel). The IC50 value of the blockade of ICaL by SR33805 was 2.4 × 10−8 M in rat ventricular cells, a value close to the one found in other cell types (Romey et al., 1994).
Effect of SR33805 on mechanical properties of skinned cardiomyocytes
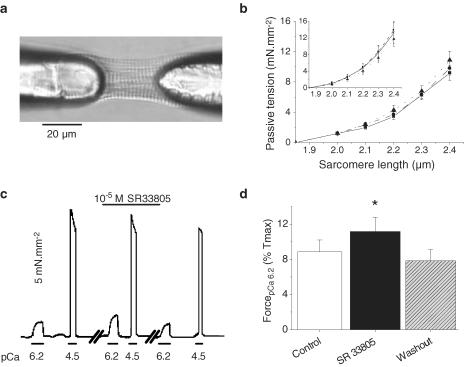
An increase in contractile activity while ICa is partially blocked could be because of a direct interaction of SR33805 on the contractile machinery like that observed in coronary artery (Ieiri et al., 2000). We first examined the general effects of SR33805 on cell stiffness, submaximal and maximal Ca2+ -activated tension as well as reversibility of the effects on skinned myocytes. SL and passive tension were measured in skinned myocytes by two protocols: (1) imposing a ramp using a stepper motor-driven micromanipulator at a speed of 0.1 length/s and (2) by manually stretching the cell with steps of 0.1 μm SL from resting SL to 2.45 μm SL and allowing for stretch relaxation. Figure 3b shows steady-state passive tension–SL relations in the absence and presence of SR33805. Values of passive tension developed at each investigated SL were similar to those previously observed (Cazorla et al., 2000; Yamasaki et al., 2002) and were unaffected by SR33805. When cells were submaximally activated (pCa 6.2) at 2.3 μm SL, active force was significantly higher in the presence of SR33805 (Figure 3c). This increase in force was fully reversible. Maximal Ca2+-activated force was rather decreased with successive applications as a consequence of the rundown generally observed and did not show marked change in the presence of the compound. Figure 3d summarizes the positive effects of 10−6 M SR33805 on the active tension elicited at pCa 6.2 relative to maximal Ca2+ -activated tension.
Figure 3.
Effects of SR33805 on passive and active forces in skinned cardiomyocytes. (a) Picture of a rat-skinned ventricular myocyte glued to glass needles and held at 1.9 μm SL. (b) Passive tension–SL relations established in the relaxing solution (pCa 9) by stretching the cells stepwise (in control solution (solid line) and during the successive applications of 10−7 (dashed line) and 10−5 M (dotted line) SR33805 (n=8)). Inset: stretching was induced by a controlled ramp at 0.1 length s−1 (n=8). (c) Original recordings of the tension elicited by a skinned cell maintained at 2.3 μm SL and submitted to stepwise superfusion of submaximal (pCa 6.2) and maximal (pCa 4.5) Ca2+-ativating solutions in the absence and in the presence of 10−5 M SR33805. The third series of recording obtained 3 min after washout demonstrates the recovery of the SR33805 effects. (d) Averaged contractile forces elicited at pCa 6.2 relative to maximal Ca2+ -activated forces in control conditions and in the presence of 10−5 M SR33805 on cells held at 2.3 μm SL (n=6). Washout was estimated at 3–5 min. *P<0.05.
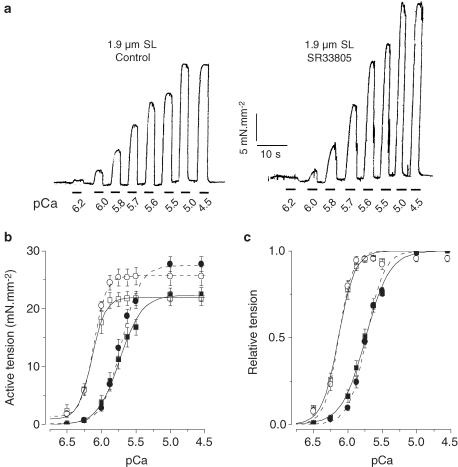
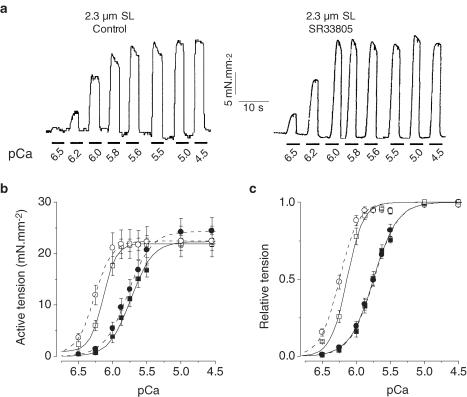
To investigate more specifically the effects of SR33805 on the contractile properties, a series of experiments was carried out using skinned myocytes at two controlled SL to obtain the following informations: (1) changes in tension development, (2) changes in myofilaments Ca2+ sensitivity, (3) alterations in myofilaments cooperativity as determined by Hill coefficients and (4) the effect on SL-dependent Ca2+ activation. In these experiments, the effects of SR33805 were checked on cardiomyocytes held at either 1.9 or 2.3 μm SL by applying for short periods the activating solution at various [Ca2+] in the absence or presence of SR33805. To determine active force, passive force before activation was subtracted from total force during Ca2+ -activation. To account for run-down of force during supramaximal activation and to allow comparison between groups (with or without drug) only two full tension–pCa relations were established firstly at 1.9 μm SL and then at 2.3 μm SL. Figure 4a illustrates two series of tension recordings elicited in skinned cardiomyocytes held at 1.9 μm SL in control solution and in the presence of 10−8 M SR33805. In the presence of the drug, force was increased only at the lowest pCa's. In control conditions, the averaged active tension–pCa relations demonstrate a characteristic leftward shift when stretching from 1.9 to 2.3 μm SL (Cazorla et al., 1999; 2001) (Figure 4b). As already reported, the maximal Ca2+ -activated forces were similar at both SL, while the Hill coefficient, nH, increased with SL. The pCa50 increased from 5.74±0.03 to 6.13±0.03 pCa units with lengthening. In the presence of 10−8 M SR33805, both active tension–pCa relations, established at 1.9 and 2.3 μm SL, exhibited a marked increase in maximal tension by 37 and 29%, respectively (Table 1). The relative tension–pCa curves were nearly superimposed (Figure 4c).
Figure 4.
Effects of a low SR33805 concentration on myofilament Ca2+ sensitivity. (a) Original tracings of contractile activity elicited by applying stepwise solutions of increasing Ca2+ concentration on a skinned cell held at 1.9 μm in control and in the presence of 10−8 M SR33805. (b) Active tension–pCa relations established at 1.9 (closed symbols) and 2.3 μm SL (open symbols) on control cells (square symbols, solid lines, n=6) and on cells (circle symbols, dash lines, n=7) in the presence of 10−8 M SR33805. (c) Relative tension–pCa of the data shown in (b). See Table 1 for contractile parameters.
Table 1.
Contractile parameters of skinned cardiac cells in control conditions (n=6) and in the presence of 10−8 M (n=7) or 10−5 M SR33805 (n=8), at 1.9 and 2.3 μm SL
| Control | 10−8 M SR33805 | 10–5 M SR33805 | ||||
|---|---|---|---|---|---|---|
| 1.9 μm | 2.3 μm | 1.9 μm | 2.3 μm | 1.9 μm | 2.3 μm | |
| Tmax | 22.0±1.2 | 21.0±1.3 | 27.8±1.3* | 25.6±1.6* | 24.4±2.6 | 22.7±3.0 |
| nH | 2.6±0.2 | 4.1±0.3 | 3.1±0.1 | 4.4±0.2 | 2.6±0.1 | 3.5±0.4 |
| pCa50 | 5.74±0.03 | 6.13±0.03 | 5.72±0.02 | 6.14±0.02 | 5.75±0.03 | 6.25±0.02* |
| delta pCa50 | 0.39±0.02 | 0.41±0.01 | 0.48±0.02* | |||
Tmax is the maximal active tension in mN0 mm−2.
Indicates a significant statistical difference between the control cells versus the cells treated with SR33805 at the same SL (P<0.05).
A high dose of SR33805 (10−5 M) had no significant effect on the maximal Ca2+ -activated tensions at both SL (Figure 5). The tension–pCa relation was unaffected at short SL, while pCa50 increased at 2.3 μm SL from 6.13±0.03 to 6.25±0.02 pCa units demonstrating an increase in myofilament Ca2+ sensitivity (Table 1).
Figure 5.
Effects of a high SR33805 concentration on myofilament Ca2+ sensitivity. (a) Original tracings of contractile activity elicited by applying stepwise solutions of increasing Ca2+ concentration on a skinned cell held at 2.3 μm in control and in the presence of 10−5 M SR33805. (b) Active tension–pCa relations established at 1.9 (closed symbols) and 2.3 μm (open symbols) SL on cells in control (square symbols, solid lines, n=6) and on cells (circle symbols, dash lines, n=9) in the presence of 10−5 M SR33805. (c) Relative tension–pCa of the data shown in b; see Table 1 for contractile parameters.
The Hill coefficients were not significantly different in the presence of SR33805. Despite the lack of statistical differences Hill coefficients tended to increase in the presence of low concentration of SR33805 at both SL and to decrease at long SL with 10−5 M SR33805 (Table 1). These changes in molecular cooperativity could influence at least in part the Ca2+ sensitivity of the contractile machinery.
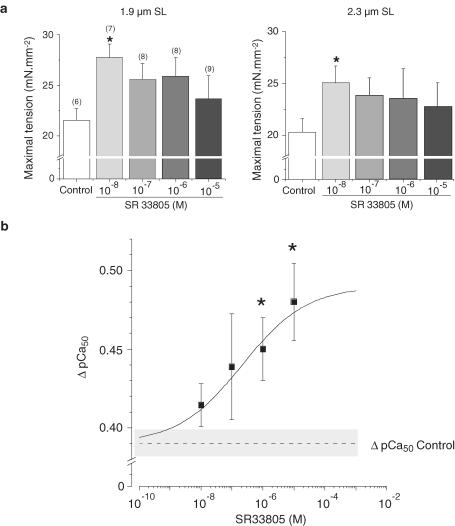
Figure 6 summarizes the effects of different concentrations of SR33805 at the two SL on the maximal Ca2+-actived tension and on the length dependence of myofilaments Ca2+ -sensitization. At both lengths, cells in the presence of SR33805 tended to develop more maximal force. However, this result was significant only at a concentration of 10−8 M (Figure 6a), the concentration at which cell shortening and contractile efficiency in intact cells were the highest (see Figure 1). A few cells (n=3) investigated in the presence of 10−9 M SR33805 demonstrated a weak positive inotropic effect at both lengths, while 10−10 SR33805 applied previously had been without effect (data not shown). Besides the length-dependent delta pCa50 (difference between the pCa50 values obtained at 1.9 and 2.3 μm SL) was 0.39 pCa units for the control cells. This value was increased by SR33805 and reached 0.48 pCa units with 10−5 M SR33805 (Figure 6b). The EC50 of this effect was 2.1 × 10−7 M. Thus, there was a significant increase in the length-dependent Ca2+ activation with increasing SR33805 concentrations together with a lessening of the SR33805-dependent increase in maximal Ca2+-activated tension.
Figure 6.
Summary of the effects of SR33805 on maximal Ca2+ -activated force and on the length-dependent Ca2+ sensitization of the myofilaments. (a) SR33805 significantly increased the maximal Ca2+-activated force at both 1.9 and 2.3 μm at the lowest investigated concentration. (b) The length-dependent Ca2+ sensitization, delta pCa50, difference between the pCa50 values at 1.9 and 2.3 μm SL increased with the SR33805 concentration. Data were fitted according to a logistic dose–response function (y=A2+(A1−A2)/(1+(x/x0)p), with x0 being the EC50, A1 and A2 the minimal and maximal delta pCa50 respectively, solid line). Dashed line and gray band represent mean delta pCa50 and its s.e.m. measured in control conditions, respectively. x0=centre, p=power.
Discussion
To fulfill cardiac needs, positive inotropism can be achieved by increasing the amount of Ca2+ ions available to the contractile proteins or by sensitizing these proteins to Ca2+. The main finding of this work is that at low concentration SR33805 increases cell contraction and shortens its duration. These effects occur despite a reduction of Ca2+ current and Ca2+ transient, which were expected from its known Ca2+-antagonistic properties, and are associated with a reduction of the AP duration. The positive inotropic effect is thus attributable to Ca2+ sensitization of myofilaments. When analyzed on skinned cardiomyocytes, this Ca2+-sensitizing effect results in most part from an increase in maximal Ca2+ -activated force at low SR33805 concentration and at higher drug concentration from a leftward shift of the tension–pCa relation that occurs mostly at the longer SL.
The present study revealed that SR33805 is both a Ca2+ channel blocker (Figure 2b) and a myofilament Ca2+ sensitizer (Figure 6b). It is interesting to note that in patch-clamp experiments SR33805 decreases greatly ICa−L (by 80% at 10−7 M) while in field-stimulated cell, Ca2+ transient was decreased by about 20% with 10−7 M SR33805. These parameters are indicators of Ca2+ movements at different stages of the excitation–contraction coupling. This result suggests either that ICa and Ca2+ transient are not strictly correlated or that the excitation–contraction gain (ratio between Ca2+ transient and ICa) could be changed by SR33805. More experiments are needed to investigate the potential role of SR33805 on excitation–contraction coupling.
Ideally, Ca2+ -sensitizing agents were developed to augment myocardial contractility without increasing Ca2+ transients with no or only minimal increments in myocardial O2-consumption (Lee & Allen, 1997). Their mechanisms of action are quite variable. Some compounds, such as pimobendan and levosimendan, act by increasing Ca2+ binding to troponin C (TnC) without affecting maximal Ca2+ -activated force; they do have some phosphodiesterase (PDE) inhibitory properties as well. Other agents, such as MCI 154 and EMD 57033, do not modify Ca2+ binding but rather induce Ca2+ sensitization and increase maximal force. Their mechanism of action is unclear and was attributed to a facilitation of the nearest-neighbor interaction in which crossbridge binding enhances cooperatively activation of near-neighbor crossbridges (Solaro et al., 1993) or to a decrease in the probability of the transition from the force-generating strong-binding states to the weak-binding states (Vannier et al., 1997). This is accompanied by a reduction in tension cost, that is, more force produced by unit of energy consumed (Popping et al., 1996). In skinned cardiomyocytes, low concentrations of SR33805 increase the maximal Ca2+ -activated force without significantly altering the Ca2+ sensitivity of the myofilaments. Ca2+ sensitivity is enhanced only at higher concentrations, at which the effect on maximal force is less. Altogether these effects are rather similar to those reported for EMD 57033 and MCI 154 although a detailed analysis of changes in crossbridge kinetics has not been attempted in this work.
Multiple studies of the length-dependent behavior of intact cardiac muscles, isolated cardiomyocytes and various types of skinned preparations have been performed. However, the detailed molecular interactions that adjust molecular motor function to myocyte length remain unclear. According to Fuchs & Wang (1996), both Ca2+ sensitivity and Ca2+ binding of cardiac myofilaments correlated more closely with change in interfilament spacing than with change in SL. In favor of this view, measurements of radioactive Ca2+ binding to cTnC in skinned bovine cardiac fibers show that the length dependence of the Ca2+–cTnC interaction was based on a length dependence of strong-binding actin–myosin interactions rather than length itself (Fuchs & Smith, 2001). In more recent studies, Cazorla et al. (1999; 2001) reported in single-skinned cardiomyocytes that the Ca2+ sensitivity is correlated with passive tension rather than SL with a major role for titin that accounts for most of the elasticity of the striated fibers. It is interesting to note that the Ca2+-sensitizing effect of SR33805 is length-dependent with very weak effects on cells held near slack length and with main effect at optimal SL. The positively length-dependent Ca2+-sensitizing effect of SR33805 suggests that the Frank–Starling relation is steeper in the presence of the drug. This is contrary to the blunting of the length-dependent Ca2+ activation of force in myofilaments reported for EMD57033, CGP-48506 and calmodizilium (Arteaga et al., 2000). The latter drug sensitizes the myofilaments at 1.9 μm SL and desensitizes them at 2.3 μm. This stresses out the need to study Ca2+ sensitizers at various controlled SLs and not only on cell shortening in order to evaluate the effects of these drugs on the Frank–Starling mechanism. Besides the Ca2+ -sensitizing effect per se, such an increase in length dependence induced by SR33805 could be anticipated to be favorable to rescue contraction of diseased hearts particularly in relation with myocardium dilatation. The length-dependent increase in Ca2+ activation induced by SR33805 cannot be attributed to changes in lateral spacing nor in diastolic tension since the effects occur without changes in passive tension of skinned cells (Figure 3b). It would be interesting to check whether the Ca2+ -sensitizing effects are accompanied by increase in the phosphorylation of the myosin light chains as reported in smooth muscle (Ieiri et al., 2000).
The benefit of using Ca2+ sensitizers during heart failure could be limited by a worsening of diastolic relaxation associated with an increase in diastolic force impairing ventricular filling. Thus, EMD 57033 has been shown to increase time to 80% relaxation and diastolic force especially in failing human myocardium (Hajjar et al., 1997) possibly because of the major leftward shift of the tension–pCa curves that occur at both 1.9 and 2.3 μm SL (Arteaga et al., 2000). Rather in the presence of SR 33805, the amplitude and more clearly the duration of cell shortening of intact myocytes were accelerated (Figure 1b).
Another limitation for using Ca2+ sensitizers is their potentiality to inhibit PDE activity that increases cAMP. Mimicking beta-adrenergic stimulation would induce: (1) proarrhythmic effects and (2) desensitization of Ca2+ activation through TnI phosphorylation. The latter was observed in particular with pimobendan (Lee et al., 1989) and recently with levosimendan in human failing myocardium (Brixius et al., 2002). SR33805 that demonstrates poor inhibition of the calmodulin-dependent PDE (IC50=2.10−7 M, Dr D. Nisato, personal communication) should be devoid of this proarrhythmic effect. This is in accordance with the negative chronotropic effect reported on rabbit isolated atrial preparations (Chatelain et al., 1993) and with the lack of change of Ca2+ sensitivity at 1.9 μm SL in our study.
In conclusion, the poor negative inotropic effects of this highly potent Ca2+-channel antagonist initially reported (Chatelain et al., 1993) is related to the fact that SR33805 demonstrates myofilaments Ca2+ sensitization. The Ca2+-sensitizing effects result mostly from an increase in maximal Ca2+ -activated tension with some increase in nH such that the minute sensitization at the lowest Ca2+ concentration would not significantly affect diastolic relaxation and resting tension. At high SR33805 concentration, the Ca2+ -sensitizing effect increases with SL. This drug has interesting Ca2+-sensitizing characteristics in control cardiac tissue that requires more investigations in order to evaluate if it is a good candidate to support contractility in failing heart.
Acknowledgments
This work was supported in part by ‘Fondation pour la Recherche Médicale Fondation de france' and ‘Fondation Cino del Luca'. Thanks are due to Pierre Fontaneau for help with IDL subroutines.
Abbreviations
- MLC
myosin light chain
- PDE
phosphodiesterase
- SL
sarcomere length
- TnC
troponin C
- TnI
troponin I
References
- ANAND S., LIU D., CHUGH S., PRAHASH A., GUPTA S., JOHN R., POPESCU F., CHANDRASHEKHAR Y. Isolated myocyte contractile function is normal in postinfarct remodeled rat heart with systolic dysfunction. Circulation. 1997;96:3974–3984. doi: 10.1161/01.cir.96.11.3974. [DOI] [PubMed] [Google Scholar]
- ARTEAGA G.M., PALMITER K.A., LEIDEN J.M., SOLARO R.J. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J. Physiol. 2000;526:541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIXIUS K., REICKE S., SCHWINGER R.H. Beneficial effects of the Ca(2+) sensitizer levosimendan in human myocardium. Am. J. Physiol. 2002;282:H131–H137. doi: 10.1152/ajpheart.2002.282.1.H131. [DOI] [PubMed] [Google Scholar]
- CAZORLA O., FREIBURG A., HELMES M., CENTNER T., MCNABB M., WU Y., TROMBITAS K., LABEIT S., GRANZIER H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ. Res. 2000;86:59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- CAZORLA O., VASSORT G., GARNIER D., LE GUENNEC J.Y. Length modulation of active force in rat cardiac myocytes: is titin the sensor. J. Mol. Cell Cardiol. 1999;31:1215–1227. doi: 10.1006/jmcc.1999.0954. [DOI] [PubMed] [Google Scholar]
- CAZORLA O., WU Y., IRVING T.C., GRANZIER H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ. Res. 2001;88:1028–1035. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- CHATELAIN P., CLINET M., POLSTER P., CHRISTOPHE B., MANNING A.S. In vitro characterization of a novel Ca2+ entry blocker: SR 33805. Eur. J. Pharmacol. 1993;246:181–193. doi: 10.1016/0922-4106(93)90030-d. [DOI] [PubMed] [Google Scholar]
- CHATELAIN P., DEWINKELEER P., BEAUFORT P., MEYSMANS L., CLINET M. Characterization of the binding of [3H]SR 33805 to the slow Ca2+ channel in rat heart sarcolemmal membrane. Eur. J. Pharmacol. 1994;267:151–160. doi: 10.1016/0922-4106(94)90166-x. [DOI] [PubMed] [Google Scholar]
- FUCHS F., SMITH S.H. Calcium, cross-bridges, and the Frank-Starling relationship. News. Physiol. Sci. 2001;16:5–10. doi: 10.1152/physiologyonline.2001.16.1.5. [DOI] [PubMed] [Google Scholar]
- FUCHS F., WANG Y.P. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J. Mol. Cell Cardiol. 1996;28:1375–1383. doi: 10.1006/jmcc.1996.0129. [DOI] [PubMed] [Google Scholar]
- GANNIER F., BERNENGO J.C., JACQUEMOND V., GARNIER D. Measurements of sarcomere dynamics simultaneously with auxotonic force in isolated cardiac cells. IEEE Trans. Biomed. Eng. 1993;40:1226–1232. doi: 10.1109/10.250578. [DOI] [PubMed] [Google Scholar]
- HAJJAR R.J., SCHMIDT U., HELM P., GWATHMEY J.K. Ca++ sensitizers impair cardiac relaxation in failing human myocardium. J. Pharmacol. Exp. Ther. 1997;280:247–254. [PubMed] [Google Scholar]
- HOLUBARSCH C., RUF T., GOLDSTEIN D.J., ASHTON R.C., NICKL W., PIESKE B., PIOCH K., LUDEMANN J., WIESNER S., HASENFUSS G., POSIVAL H., JUST H., BURKHOFF D. Existence of the Frank–Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation. 1996;94:683–689. doi: 10.1161/01.cir.94.4.683. [DOI] [PubMed] [Google Scholar]
- IEIRI S., HIRANO K., NISHIMURA J., SUITA S., KANAIDE H. Alteration of the [Ca(2+)](i)–force relationship during the vasorelaxation induced by a Ca(2+) channel blocker SR33805 in the porcine coronary artery. Br. J. Pharmacol. 2000;131:1597–1606. doi: 10.1038/sj.bjp.0703721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE J.A., ALLEN D.G. Calcium sensitisers: mechanisms of action and potential usefulness as inotropes. Cardiovas. Res. 1997;36:10–20. doi: 10.1016/s0008-6363(97)00174-0. [DOI] [PubMed] [Google Scholar]
- LEE J.A., RUEGG J.C., ALLEN D.G. Effects of pimobendan, a novel inotropic agent, on intracellular calcium and tension in isolated ferret ventricular muscle. Clin. Sci. (London) 1989;76:609–618. doi: 10.1042/cs0760609. [DOI] [PubMed] [Google Scholar]
- POPPING S., MRUCK S., FISCHER Y., KULSCH D., IONESCU I., KAMMERMEIER H., ROSE H. Economy of contraction of cardiomyocytes as influenced by different positive inotropic interventions. Am. J. Physiol. 1996;271:H357–364. doi: 10.1152/ajpheart.1996.271.1.H357. [DOI] [PubMed] [Google Scholar]
- ROMEY G., BOIS P., LAZDUNSKI M. Effects of two chemically related new Ca2+ channel antagonists, SR33557 (fantofarone) and SR33805, on the L-type cardiac channel. Eur. J. Pharmacol. 1994;263:101–105. doi: 10.1016/0014-2999(94)90529-0. [DOI] [PubMed] [Google Scholar]
- SCHWINGER R.H., BOHM M., KOCH A., SCHMIDT U., MORANO I., EISSNER H.J., UBERFUHR P., REICHART B., ERDMANN E. The failing human heart is unable to use the Frank-Starling mechanism. Circ. Res. 1994;74:959–969. doi: 10.1161/01.res.74.5.959. [DOI] [PubMed] [Google Scholar]
- SOLARO R.J., GAMBASSI G., WARSHAW D., KELLER M., SPURGEON H., BEIER N., LAKATTA E. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ. Res. 1993;73:981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- VANNIER C., LAKOMKINE V., VASSORT G. Tension response of the cardiotonic agent (+)-EMD-57033 at the single cell level. Am. J. Physiol. 1997;272:C1586–C1593. doi: 10.1152/ajpcell.1997.272.5.C1586. [DOI] [PubMed] [Google Scholar]
- VENTURA-CLAPIER R., MEKHFI H., VASSORT G. Role of creatine kinase in force development in chemically skinned rat cardiac muscle. J. Gen. Physiol. 1987;89:815–837. doi: 10.1085/jgp.89.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMASAKI R., WU Y., MCNABB M., GREASER M., LABEIT S., GRANZIER H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ. Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]