Abstract
Sauchinone, a lignan isolated from Saururus chinensis (Saururaceae), is a diastereomeric lignan with cytoprotective and antioxidant activities in cultured hepatocytes. The effects of sauchinone on the inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α) and cyclooxygenase 2 (COX-2) gene expression and on the activation of transcription factors, nuclear factor-κB (NF-κB), CCAAT/enhancer-binding protein (C/EBP), activator protein-1 (AP-1) and cAMP-response element-binding protein (CREB) were determined in Raw264.7 cells as part of the studies on its anti-inflammatory effects.
Expression of the iNOS, TNF-α and COX-2 genes was assessed by Northern and Western blot analyses. NO production was monitored by chemiluminescence detection using a NO analyzer. To identify the transcriptional factors affected by sauchinone, the extents of NF-κB, C/EBP, AP-1 and CREB activation were measured. Activation of the transcription factors was monitored by gel mobility shift assay, whereas p65 and I-κBα were analyzed by immunocytochemical and immunoblot analyses.
Sauchinone inhibited the induction of iNOS, TNF-α and COX-2 by lipopolysaccharide (LPS) (IC50⩽10 μM) with suppression of the mRNAs.
Sauchinone (1–30 μM) inhibited LPS-inducible nuclear NF-κB activation and nuclear translocation of p65, which was accompanied by inhibition of I-κBα phosphorylation.
LPS-inducible increase in the intensity of C/EBP binding to its consensus sequence was also inhibited by sauchinone. The AP-1, but not CREB, DNA binding activity was weakly inhibited by sauchinone.
These results demonstrate that sauchinone inhibits LPS-inducible iNOS, TNF-α and COX-2 expression in macrophages through suppression of I-κBα phosphorylation and p65 nuclear translocation and of C/EBP and/or AP-1 activation, which may constitute anti-inflammatory effects of the lignan.
Keywords: Nuclear factor-κB, iNOS, TNF-α, COX-2, C/EBP
Introduction
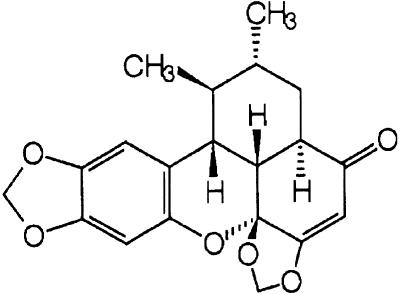
Saururus chinensis has been traditionally used for the treatment of hepatitis in Oriental folk medicine (Chung & Shin, 1990). The aqueous fraction of the Saururus herbs also induces humoral changes implicated with hypertension and symp-tomatically relieves edema (Chung & Shin, 1990). Diaste-reomeric lignans including sauchinone, sauchinone A and 1′-epi-sauchinone have been isolated from the n-hexane fraction of S. chinensis (Lour.) Baill. (Saururaceae). Sauchinone was identified as a biologically active lignan (Figure 1). Previous studies have shown that sauchinone protects hepatocytes against the injury induced by toxicants, as evidenced by both the inhibition of carbon tetrachloride-induced cell death and the restoration of cellular glutathione and antioxidant enzymes (Sung et al., 2000).
Figure 1.
A chemical structure of sauchinone.
Lipopolysaccharide (LPS) is an endotoxin, which induces septic shock syndrome and stimulates the production of inflammatory mediators such as NO, tumor necrosis factor-α (TNF-α), interleukins, prostanoids and leukotrienes (Hewett & Roth, 1993; Watson et al., 1999; Kubes & McCafferty, 2000). NO is a radical produced from L-arginine via nitric oxide synthase (NOS) and also an important cellular second messenger. NO plays a dual role as both a beneficial and detrimental molecule in the process of inflammation. Inducible NOS (iNOS) is capable of producing high output of NO during inflammation, whereas constitutively expressed NOS is physiologically critical. It has been proposed that iNOS-mediated high output production of NO causes cell injury through generation of potent reactive radicals such as peroxynitrite. Since NO is one of the major contributing factors during the inflammatory process, we first studied the effects of sauchinone on nuclear factor-κB (NF-κB)-mediated iNOS expression and NO production in macrophages exposed to LPS.
TNF-α is the principal mediator of the responses to LPS and may play a role in innate immune responses. High concentrations of LPS cause tissue injury and shock, in which TNF-α is one of the principal mediators. As part of the studies on sauchinone's effects against acute inflammation, we designed to study the effect of sauchinone on LPS-inducible TNF-α expression.
Cyclooxygenase 2 (COX-2) is induced by LPS, certain serum factors, cytokines and growth factors, and is a predominant cyclooxygenase at sites of inflammation. Development of COX-2 inhibitors represents a major advance in the therapy of inflammatory processes and their use includes prevention or treatment of disorders associated with the induction of this enzyme (e.g. colon cancer). In view of the observation that sauchinone has cytoprotective and antioxidant effects in cultured hepatocytes, we further evaluated the effect of sauchinone on LPS-inducible COX-2 gene expression in macrophages.
NF-κB, which is activated by the inflammatory responses during viral and bacterial infections (Grilli & Memo, 1999; Kim et al., 2000), is involved in the expression of iNOS and TNF-α genes (Watson et al., 1999). In addition to the potential role of NF-κB response element in the expression of COX-2, the regulatory region for the gene includes CCAAT/enhancer-binding protein (C/EBP) and cAMP-response element (CRE)/E-box elements. Activation of activator protein-1 (AP-1) consisting of Jun, Fos and Fra homodimers or heterodimers is associated with the cellular oxidative stress and the altered redox state, and the transcriptional factor regulates expression of the associated genes including iNOS and TNF-α (Dieter et al., 1999; Zhou et al., 2001). Since NF-κB, C/EBP, AP-1 and cyclic-AMP-response element-binding protein (CREB) are commonly or individually involved in the regulation of inflammatory genes, alterations in the activation of these transcription factors by sauchinone were determined as part of the mechanistic studies.
The effects of sauchinone on LPS-induced activation of NF-κB and degradation of I-κBα and iNOS gene expression were monitored by gel mobility shift assay and immunoblot analysis. The DNA binding activities of C/EBP, AP-1 and CREB were also monitored to identify the transcriptional factors affected by sauchinone in association with the suppression of TNF-α and COX-2. We found that activation of NF-κB, C/EBP and AP-1, but not that of CREB, was inhibited by sauchinone in parallel with the suppression of inflammatory gene expression.
Methods
Reagents
Sauchinone, a diastereomeric lignan, was isolated from the n-hexane fraction of S. chinensis by successive silica gel chromatography and reverse-phase high-pressure liquid chromatography. The chemical structure was confirmed by a variety of spectroscopic analyses (Figure 1) (Sung & Kim, 2000; Sung et al., 2000). Alkaline phosphatase-conjugated goat anti-mouse and anti-goat IgGs were purchased from KPL (Gaithersburg, MD, U.S.A.). Anti-c-Rel (p65), anti-p50, anti-I-κBα, anti-C/EBP (α, β, δ, ɛ) and p300 antib-odies were supplied from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Antimurine iNOS antiserum was obtained from Transduction Laboratories (Lexington, KY, U.S.A.). [α-32P]dCTP (3000 mCi mmol−1) and [γ-32P]ATP (3000 mCi mmol−1) were obtained from Amersham (Arlington Heights, IL, U.S.A.). The consensus sequence of NF-κB, C/EBP, AP-1 and CREB, and random-prime and 5′-end labeling kits were supplied from Promega Co. (Madison, WI, U.S.A.). Most reagents for the molecular studies were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Cell culture
Raw264.7 cells, a murine macrophage cell line (American Type Culture Collection, Menassas, VA, U.S.A.), were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Raw264.7 cells were plated at a density of 2–3 × 106 ml−1 and preincubated for 24 h at 37°C. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. For all experiments, cells were grown to 80–90% confluency, and were subjected to no more than 20 cell passages. Raw264.7 cells were incubated with 1 μg ml−1 LPS (Escherichia coli 026:B6; Difco, Detroit, MI, U.S.A.) to activate NF-κB, C/EBP, AP-1 and CREB, and to stimulate the iNOS, COX-2 and TNF-α gene expression. Cells were incubated in the medium without 10% FBS for 12 h and then exposed to LPS or LPS+sauchinone for the indicated time periods (1–18 h). Sauchinone as dissolved in dimethylsulfoxide was added to the incubation medium 1 h prior to the addition of LPS. Dimethylsulfoxide (vehicle) alone was ineffective.
Assay of nitrite production
NO production was monitored by measuring the nitrite content in culture medium. This was performed by mixing the samples with Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine dihydrochloride and 2.5% phosphoric acid). Absorbance was measured at 540 nM after incubation for 10 min.
Northern blot analysis
Total RNA was isolated from Raw264.7 cells according to the modified method of Chomczynski & Sacchi (1987). Total RNA (20 μg) was resolved by electrophoresis in a 1% agarose gel containing 2.2 M formaldehyde and transferred to nitrocellulose paper by capillary transfer. The nitrocellulose paper was baked in a vacuum oven at 80°C for 2 h. Blots were incubated in hybridization buffer containing 50% deionized formamide, 0.1% sodium dodecyl sulfate (SDS), 200 μg ml−1 of sonicated salmon sperm DNA, 6 × standard saline/phosphate/EDTA (1 × standard saline/phosphate/EDTA contains 0.15 M NaCl, 10 mM NaH2PO4, and 1 mM Na2EDTA, pH 7.4), and 5 × Denhardt's solution [0.1% Ficoll, 0.1% polyvinylpyrrolidine and 0.1% bovine serum albumin (Pentex fraction V)] at 42°C for 1 h without probe. Specific cDNA probes for TNF-α and COX-2 genes were amplified by reverse transcription-polymerase chain reaction (RT–PCR) using the selective primers and cloned in a TA vector (Promega, Madison, WI, U.S.A.). The primers used are as follows, COX-2, sense primer: 5′-TCTCCAACCTCTCCTACTAC-3′, antisense primer: 5′-GCACGTAGTCTTCGATCACT-3′ (624 bp); and TNF-α, sense primer: 5′-TACTGAACTTCGGGGTGATTGGTCC-3′, antisense primer: 5′-CAGCCTTGTCCC-TTGAAGAGAA-CC-3′ (295 bp). Hybridization was performed at 42°C for 18 h with a heat-denatured probe, which was random-prime labeled with [α-32P]dCTP. Filters were washed in 2 × standard saline citrate (SSC) (1 × SSC contains 0.15 M NaCl and 0.015 M sodium citrate, pH 7.4) and 0.1% SDS for 10 min at room temperature twice and in 0.1 × SSC and 0.1% SDS for 10 min at room temperature twice. Filters were finally washed in the solution containing 0.1 × SSC and 0.1% SDS for 60 min at 60°C. The stripped membranes were hybridized with a labeled probe complementary to 18S rRNA to quantify the amount of RNA loaded onto the agarose gel and transferred to the nitrocellulose paper. Films were exposed at −70°C for 12–48 h using intensifying screens. Each data point represents mean±s.d. from independent measurements of three or four different experiments.
RT–PCR analysis
RT–PCR was performed with total RNA (0.5 μg) obtained from the liver using the selective primers [iNOS, sense primer: 5′-ATGTCCGAAGCAAACATCAC-3′, antisense primer: 5′-TAATGTCCAGGAAGTAGGTG-3′, 499 bp; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), sense primer: 5′-TCGTGGAGTCTACTGGCGT-3′, antisense primer: 5′-GCCTGCTTCACCACCTTCT-3′, 510 bp]. PCRs were conducted using the following conditions for 38 cycles: denaturation at 94°C for 0.5 min, annealing at 49°C for 0.5 min, and elongation at 72°C for 1 min. Band intensities of the amplified DNAs were compared after visualization on an UV transilluminator.
Immunoblot analysis
Cells were lysed in the buffer containing 20 mM Tris·Cl (pH 7.5), 1% Triton X-100, 137 mM sodium chloride, 10% glycerol, 2 mM EDTA, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 1 mM phenylmethylsulfonylfluoride and 1 μg/ml leupeptin. Cell lysates were centrifuged at 10,000 × g for 10 min to remove debris. Expression of iNOS and COX-2 was immunochemically monitored in the lysate fraction of Raw264.7 cells using anti-mouse iNOS and COX-2 antibodies, respectively. Polyclonal anti-I-κBα antibody was used to assess I-κBα protein in cytosol. Polyclonal anti-C/EBPβ and C/EBPδ antibodies were used to assess C/EBPβ and C/EBPδ proteins in the nuclear fraction. The secondary antibodies were alkaline phosphatase-conjugated anti-mouse and anti-goat antibodies. The bands of iNOS and COX-2 proteins were visualized using 5-bromo-4-chloro-3-indolylphosphate and 4-nitroblue tetrazolium chloride, or ECL chemiluminescence detection kit.
Enzyme-linked immunosorbent assay (ELISA)
Raw264.7 cells were preincubated with 3–30 μM sauchinone for 1 h and then further incubated with LPS (1 μg ml−1) for 6 h. The level of TNF-α in the culture medium was measured by ELISA using anti-mouse TNF-α antibody and biotinylated secondary antibody (Endogen, Woburn, MA, U.S.A.).
Preparation of nuclear extracts
Nuclear extracts were prepared essentially according to Schreiber et al. (1990). Briefly, dishes were washed with ice-cold PBS, scraped and transferred to microtubes. Cells were allowed to swell by adding 100 μl of lysis buffer [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.5% Nonidet-P40, 1 mM dithiothreitol and 0.5 mM phenylmethylsulfonylfluoride]. Tubes were vortexed to disrupt cell membranes. The samples were incubated for 10 min on ice and centrifuged for 5 min at 4°C. Pellets containing crude nuclei were resuspended in 50 μl of the extraction buffer containing 20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol and 1 mM phenylmethylsulfonylfluoride, and then incubated for 30 min on ice. The samples were centrifuged at 15,800 × g for 10 min to obtain the supernatant containing nuclear extracts.
Gel retardation assay
A double-stranded DNA probe for the consensus sequence of NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′), C/EBP (5′-TGCAGATTGCGCAATCTGCA-3′), AP-1 (5′-CGCTT-GATGACTCAGCCGGAA-3′) and CREB (5′-AGAGATTG-CCTGACGTCAGAGAGCTAG-3′) were used for gel shift analyses after end labeling of the probes with [γ-32P]ATP and T4 polynucleotide kinase. The reaction mixtures contained 2 μl of 5 × binding buffer containing 20% glycerol, 5 mM MgCl2, 250 mM NaCl, 2.5 mM EDTA, 2.5 mM dithiothreitol, 0.25 mg ml−1 poly dI-dC and 50 mM Tris-Cl (pH 7.5), 2 μg of nuclear extracts and sterile water in a total volume of 10 μl. Reactions were initiated by the addition of 1 μl probe (106 cpm) following 10 min preincubation and continued for 20 min at room temperature. The specificity of protein binding to the DNA was confirmed by competition reactions, in which a 20-fold molar excess of unlabeled oligonucleotides was added to each reaction mixture before the addition of radiolabeled probe. In some experiments, the specificity of NF-κB binding to the DNA consensus sequence was confirmed by supershift analysis using specific antibodies directed against p65 or p50 (2 μg each). Also, the specificity of C/EBP binding to the DNA consensus sequence was confirmed by the inhibition of C/EBP binding and supershift using anti-C/EBPα, anti-C/EBPβ, anti-C/EBPδ, anti-C/EBPɛ or anti-p300 antibody. Samples were loaded onto 4% polyacrylamide gels at 140 V. The gels were removed, fixed and dried, followed by autoradiography.
Immunocytochemistry of p65
Standard immunocytochemical method was used to detect nuclear translocation of p65 subunit of NF-κB (Cho et al., 2002). Counter staining with propidium iodide (PI) verified the location and integrity of nuclei. After washing, stained cells were examined using a laser scanning confocal microscope.
Scanning densitometry
Scanning densitometry was performed with Image Scan & Analysis System (Alpha-Innotech Corporation, San Leandro, CA, U.S.A.). One-way analysis of variance (ANOVA) procedures were used to assess significant differences among treatment groups. For each significant effect of treatment, the Newman–Keuls test was used for comparisons of multiple group means. The criterion for statistical significance was set at P<0.01.
Results
Inhibition of LPS-inducible iNOS expression
The potential toxicity of sauchinone to Raw264.7 cells was assessed by MTT assay after 24 h incubation. Cell viability was not changed by the presence of sauchinone up to the concentration of 100 μM. Thus, 1–30 μM concentrations of sauchinone were chosen in subsequent experiments.
We determined whether sauchinone inhibited the induction of iNOS by LPS. NO production was monitored in Raw264.7 cells stimulated by LPS in the presence or absence of sauchinone for 18 h. LPS (1 μg ml−1) increased the level of nitrite and nitrate (i.e. NO) in culture medium by 20-fold as compared to control. Sauchinone (1–30 μM) significantly inhibited LPS-inducible NO production (i.e. 35–60%) (Figure 2a). Western blot analysis confirmed that LPS (1 μg ml−1, 18 h) induced iNOS protein and that sauchinone at the concentration of 1 μM markedly suppressed the induction of iNOS by LPS (Figure 2b). Expression of iNOS was >80% inhibited by sauchinone. Studies were extended to determine whether the expression of iNOS protein paralleled that of its mRNA. LPS maximally increased the iNOS mRNA at 6–12 h (Takahashi et al., 2000), which was confirmed by our previous study (Cho et al., 2002). RT–PCR analysis showed that sauchinone inhibited LPS-inducible increase in the iNOS mRNA (6 h) (Figure 2c).
Figure 2.
Effects of sauchinone (Sau) on the induction of iNOS by LPS. (a) Inhibition of NO production by Sau in Raw264.7 cells. Raw264.7 cells were treated with various concentrations of Sau dissolved in dimethylsulfoxide for 1 h prior to the addition of LPS (1 μg ml−1), and the cells were further incubated for 18 h. Control cells were incubated with vehicle alone. The concentrations of nitrite and nitrate in culture medium were monitored as described in the Methods section. (b) Inhibition of LPS-inducible iNOS protein expression by Sau. The level of iNOS protein was monitored 18 h after treatment of cells with LPS (1 μg ml−1) with or without Sau pretreatment (i.e. 1 h before LPS). The relative iNOS protein levels were measured by scanning densitometry of the band intensities. (c) The effects of Sau on the iNOS mRNA level in Raw264.7 cells stimulated with LPS (1 μg ml−1). RT–PCR was performed to determine iNOS mRNA in total RNA fractions (40 μg each) isolated from cells treated with LPS in the presence or absence of Sau. Cells were pretreated with Sau for 1 h followed by the addition of LPS, and the iNOS mRNA level was assessed 6 h after the addition of LPS. The amount of RNA loaded in each lane was confirmed by RT–PCR of GAPDH mRNA.
Inhibition of LPS-inducible TNF-α expression
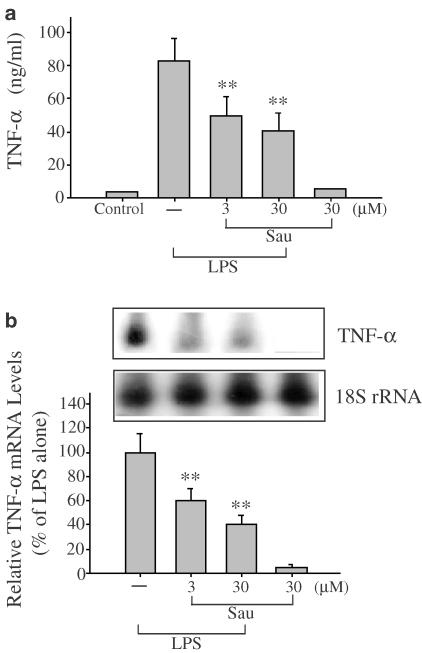
Production of TNF-α was measured in the medium of Raw264.7 cells cultured with LPS (1 μg ml−1) in the presence or absence of sauchinone for 6 h (Figure 3a). Sauchinone at the concentrations of 3 and 30 μM inhibited TNF-α production in LPS-treated cells by 40 and 50%, respectively. Northern blot analysis was used to verify whether the inhibition of TNF-α production by sauchinone accompanied suppression of TNF-α mRNA. Sauchinone also inhibited the increase in TNF-α mRNA by LPS (Figure 3b).
Figure 3.
The effect of sauchinone (Sau) on LPS-inducible TNF-α expression. (a) The level of TNF-α. Production of TNF-α was measured in the medium of Raw264.7 cells cultured with LPS (1 μg ml−1) in the presence or absence of Sau for 6 h. (b) The expression of TNF-α mRNA. TNF-α mRNA was monitored by Northern blot analysis in cells exposed to LPS with or without Sau for 3 h. The amount of RNA loaded in each lane was confirmed by rehybridization of the stripped membrane with a 32P-labeled probe complementary to 18S rRNA. Each bar represents the mean±s.d. from four separate experiments. One-way ANOVA was used for comparisons of multiple group means followed by Newman–Keuls test (significant as compared to LPS alone, **P<0.01).
Inhibition of LPS-inducible COX-2 expression
The expression of COX-2 protein was monitored in Raw264.7 cells exposed to LPS (1 μg ml−1, 12 h). Sauchinone (1–30 μM) effectively suppressed the induction of COX-2 by LPS (Figure 4a). LPS (1 μg ml−1, 6 h) also increased the COX-2 mRNA, which was >90% inhibited by the presence of sauchinone (Figure 4b). Hence, sauchinone was active in suppressing the expression of the genes implicated with inflammation.
Figure 4.
Inhibition of LPS-inducible COX-2 expression by sauchinone (Sau). (a) COX-2 protein expression. COX-2 expression was measured in Raw264.7 cells cultured with 1 μg ml−1 LPS with or without various concentrations of Sau for 12 h. (b) The level of COX-2 mRNA. COX-2 mRNA was assessed by Northern blot analysis in Raw264.7 cells exposed to LPS with or without Sau. The amount of RNA loaded in each lane was confirmed by rehybridization of the stripped membrane with a 32P-labeled probe complementary to 18S rRNA. Each bar represents the mean±s.d. from four separate experiments. One-way ANOVA was used for comparisons of multiple group means followed by Newman–Keuls test (significant as compared to LPS alone, **P<0.01).
Effect of sauchinone on LPS-inducible NF-κB activation
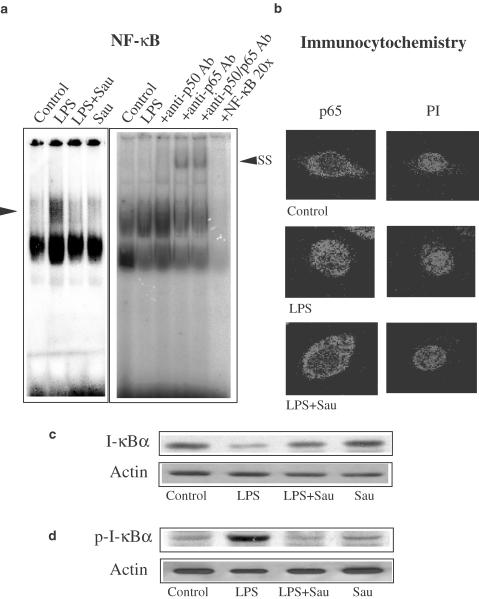
NF-κB is activated in cells challenged with LPS and other inflammatory stimuli and involved in the transcriptional activation of responsive genes (Baldwin, 1996). Gel shift analysis was conducted to determine whether sauchinone changed NF-κB DNA binding activity. Previous studies have shown that NF-κB was activated at 30 min–1 h after LPS treatment (Kim et al., 2000). LPS (1 μg ml−1, 1 h) increased the binding activity of nuclear extracts to the NF-κB DNA consensus sequence. Treatment of macrophages with sauchinone (3 μM) for 1 h prior to the addition of LPS significantly (<50%) inhibited LPS-inducible increase in the band intensity of NF-κB binding (Figure 5a, left). We chose the concentration of 3 μM for the gel shift analysis because a preliminary study showed that sauchinone notably inhibited NF-κB activation at the concentration. Addition of anti-p65 antibody to the reaction mixture obtained from LPS-treated cells caused supershift of the NF-κB DNA binding, whereas anti-p50 antibody weakly shifted the retarded band. The presence of both anti-p65 and anti-p50 antibodies also supershifted the NF-κB band (Figure 5a, right).
Figure 5.
Inhibition of LPS-inducible NF-κB activation and I-κBα phosphorylation by sauchinone (Sau). (a) Gel shift analysis of nuclear extracts using the consensus sequence of NF-κB. Nuclear extracts were isolated 1 h after LPS treatment (1 μg ml−1) of Raw264.7 cells, and were subjected to gel shift analysis. Each lane contained 5 μg of nuclear extracts. The specificity of NF-κB binding was confirmed by supershift analysis using the antibodies directed against p65 and/or p50 protein. The arrow (left) shows NF-κB complex and SS indicates supershift of the retarded NF-κB band. The specificity of NF-κB binding was also confirmed by the addition of an excess amount of free probe (20 ×). (b) Immunofluorescence subcellular localization of p65 protein. Raw264.7 cells were treated with 1 μg ml−1 of LPS for 1 h, and p65 protein localization was immunochemically detected using anti-p65 antibody. LPS caused p65 protein to migrate to the nucleus at 1 h. Sau (3 μM) prevented LPS-induced nuclear translocation of p65 protein. The same fields were counter stained with PI for location of nuclei. Results were confirmed by repeated experiments. (c) The effect of Sau on LPS-induced I-κBα degradation. The effect of LPS on I-κBα degradation was immunochemically assessed in Raw264.7 cells. Degradation of I-κBα protein was significantly inhibited by treatment of cells with 3 μM Sau for 1 h. Equal loading of proteins was verified by actin immunoblotting. (d) Western blot analysis of phosphorylated I-κBα. Representative immunoblot shows the inhibition of I-κBα phosphorylation by Sau in Raw264.7 cells. Phosphorylated I-κBα protein was monitored at 15 min. Equal loading of proteins was verified by actin immunoblotting. Results were confirmed by repeated experiments.
Since p65 was the major component of NF-κB activated by LPS in macrophages, we determined the translocation of p65 into the nucleus by immunocytochemistry (Figure 5b). Raw264.7 cells were incubated with LPS in the presence or absence of 3 μM sauchinone for 1 h, fixed and permeabilized. p65 protein was located predominantly in the cytoplasm of control cells. In contrast, p65 protein moved into the nucleus at 1 h after LPS treatment. The p65 protein was detected predominantly in the cytoplasm of cells exposed to LPS in combination with sauchinone, which verified that sauchinone inhibited nuclear localization of p65 protein. Nuclear integrity was confirmed by PI staining of the identical cells (Figure 5b).
Translocation of NF-κB to the nucleus is preceded by proteolytic degradation of I-κBα subunit. To assess whether sauchinone could directly affect I-κBα in macrophage cells, the level of I-κBα protein was immunochemically assessed in Raw264.7 cells incubated with or without sauchinone (Figure 5c). LPS (1 μg ml−1) reduced the I-κBα level at 30 min, resulting in 25% of control. Sauchinone significantly recovered the level of I-κBα protein (Figure 5c). Phosphorylation of I-κBα precedes degradation of I-κBα. Sauchinone inhibited LPS-inducible I-κBα phosphorylation (Figure 5d). Thus, sauchinone inhibited nuclear NF-κB binding through prevention of I-κBα phosphorylation and subsequent nuclear translocation of p65 protein.
Effect of sauchinone on LPS-inducible C/EBP activation
Expression of the COX-2 gene depends on the C/EBP element present in the upstream region of the gene (Thomas et al., 2000). To test whether suppression of COX-2 gene induction by sauchinone was mediated by inactivation of C/EBP, electrophoretic mobility shift for C/EBP binding activity was performed with the nuclear extracts of cells exposed to LPS in the presence or absence of sauchinone using a radiolabeled C/EBP consensus oligonucleotide. Treatment of cells with LPS for 3 h resulted in an increase in C/EBP binding compared to control. Sauchinone inhibited LPS-inducible C/EBP binding (Figure 6a, left). Addition of anti-C/EBPβ or anti-C/EBPδ antibody to the nuclear extracts obtained from LPS-treated cells caused supershift of the C/EBP DNA binding. Anti-C/EBPα, anti-C/EBPɛ or anti-p300 antibody failed to affect the mobility of the retarded band (Figure 6a, right).
Figure 6.
Activation of C/EBP transcription complex. (a) Gel shift analysis of C/EBP. Nuclear extracts were prepared from Raw264.7 cells cultured with LPS (1 μg ml−1) in the presence or absence of sauchinone (Sau, 3 μM) for 3 h. All lanes contained 5 μg of nuclear extracts and 5 ng of labeled C/EBP consensus sequence. The specificity of C/EBP binding was confirmed by supershift analysis using the antibodies directed against C/EBP (α, β, δ, ɛ) and p300 proteins. The arrow (left) shows C/EBP complex and SS indicates supershift of the retarded NF-κB band. The specificity of C/EBP binding was confirmed by the addition of an excess amount of free probe (20 ×). (b) The levels of C/EBPβ and δ proteins in the nuclear fraction. Sauchinone (3 μM) inhibited the increase in the level of nuclear C/EBPβ. The level of C/EBPδ in the nuclear fraction was not notably changed. Equal loading of proteins was verified by actin immunoblotting.
Next, we assessed whether the levels of nuclear C/EBPβ and C/EBPδ were changed by sauchinone in LPS-treated cells. The increase in nuclear C/EBPβ by LPS was completely inhibited by sauchinone (Figure 6b, upper). However, the level of C/EBPδ protein was not changed by the presence of sauchinone (Figure 6b, lower). These results verified that sauchinone inhibits the nuclear translocation of C/EBPβ and C/EBPβ binding to the consensus DNA oligonucleotide.
Effect of sauchinone on LPS-inducible AP-1 and CREB activation
Studies were extended to determine whether sauchinone affected the activation of AP-1. Gel shift retardation analysis revealed that nuclear AP-1 transcription complex was activated by LPS (1 h) in macrophages. The specificity of the DNA probe to LPS-activated AP-1 binding complex was supported by competition for binding to a radiolabeled AP-1 probe with an excess of unlabeled AP-1 oligonucleotide, and the band intensity of the migrating complex with the AP-1 consensus oligonucleotide was decreased by the specific antibodies against Jun D (Cho MK & Kim SG, unpublished data). Pretreatment of cells with 3 μM sauchinone weakly inhibited LPS-inducible AP-1 activation with a slight decrease in gel retardation (Figure 7a). It has been shown that mutation of the CRE site in the COX-2 gene abrogated COX-reporter activity and that expression of CREB substantially repressed LPS-dependent COX-reporter activity presumably through CRE site(s) (Wadleigh et al., 2000). We monitored the effect of sauchinone on LPS-inducible CREB binding activity. In cells exposed to LPS (6 h), sauchinone (3 μM) failed to change the band intensity of CREB DNA binding (Figure 7b).
Figure 7.
The effect of sauchinone (Sau) on AP-1 and CREB binding activity. (a) AP-1 binding activity. Gel shift analysis was carried out with nuclear extracts from control cells or cells treated with LPS in the presence or absence of 3 μM Sau for 1 h. Each reaction contained 5 μg of nuclear extracts and 5 ng of radio-labeled AP-1 consensus sequence. Results were confirmed by repeated analyses. (b) Gel shift analysis of nuclear extracts using the consensus sequence of CREB. Nuclear extracts were isolated 6 h after LPS treatment in the presence or absence of Sau (3 μM). CREB binding activity was assessed as described in (a). Results were confirmed by repeated experiments.
Discussion
NO is a free radical generated from L-arginine by NOS. Increased expression of iNOS is associated with inflammatory responses and also with serious disorders such as septic shock and rheumatoid arthritis (Salerno et al., 2002). In view of the involvement of iNOS in inflammatory process, we monitored the iNOS gene expression in macrophages exposed to sauchinone, a biologically active lignan derived from S. chinensis. Northern and Western blot analyses revealed that sauchinone inhibited the induction of iNOS. Suppression of iNOS expression by sauchinone was in parallel with the comparable inhibition of NO production.
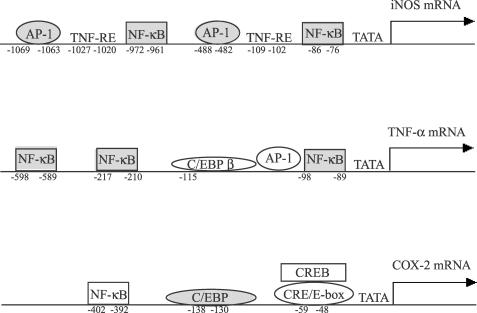
Both NF-κB and AP-1 binding sites have been identified on the murine iNOS promoter and play a role in LPS-mediated induction of iNOS in Raw264.7 cells (Figure 8). The present study demonstrated that sauchinone prevents activation of p65/NF-κB by LPS in macrophage cells and effectively inhibits nuclear translocation of p65. NF-κB is activated by oxidative stress as well as by inflammation. Activation of the NF-κB complex is also related with the cellular redox state (Hirota et al., 1999). The intracellular thiol level changes the expression of several genes following early activation of NF-κB (Parmentier et al., 2000). The activation of NF-κB can be blocked by thiol compounds such as N-acetylcysteine and cysteine (Shrivastava & Aggarwal, 1999). An additional study showed that N-acetylcysteine (1 μM, 18 h) failed to change the inhibitory effect of sauchinone on LPS-inducible iNOS expression. Hence, the inhibition of NF-κB activation by sauchinone may not be related with the change in the cellular redox state.
Figure 8.
The cis-acting response elements present in the promoter regions of the murine iNOS, TNF-α and COX-2 genes. The NF-κB and AP-1 binding sites on the iNOS promoter play a role in the induction of iNOS. Whereas the upstream promoter region of the TNF-α gene contains binding sites for the activators including NF-κB, C/EBPβ and c-Jun, that of the COX-2 gene comprises NF-κB, C/EBP and CREB binding sites.
The current study showed that phosphorylation of I-κBα (i.e. degradation of I-κBα), which is required for NF-κB activation was inhibited by sauchinone. Members of the protein tyrosine kinase family play roles in macrophage activation in response to LPS (Geng et al., 1993). Phosphorylation of I-κBα in cells stimulated by LPS is considered to be mediated with the NF-κB-inducing kinase and the subsequent I-κB kinase complexed with other proteins in the plasma membrane (Stancovski & Baltimore, 1997). This is supported by the observation that the inhibition of the NF-κB-inducing kinase and the subsequent I-κB kinase reduced the expression of iNOS by LPS in macrophages (Matusushima et al., 2001). NF-κB becomes activated by I-κBα degradation following phosphorylation of I-κBα at serine residues (Michael & Sanker, 1998). The cellular level of I-κBα was monitored to infer if sauchinone blocked degradation process of I-κBα. Phosphorylation of I-κBα, I-κBα degradation and nuclear translocation of p65 protein by LPS were all significantly abolished in cells pretreated with sauchinone. The recovery of I-κBα protein in Raw264.7 cells provided strong evidence that sauchinone inhibited activation of NF-κB as a consequence of the inhibition of I-κBα phosphorylation. This supports that sauchinone may inhibit the upstream cellular kinase(s), but not NF-κB binding to the DNA sequence. The pathways of NF-κB-inducing kinase and MEKK1 regulate the phosphorylation of I-κBα via IKK (Pan et al., 2000). It is likely that the inhibition of I-κBα phosphorylation by sauchinone is mediated with the suppression of IKK.
Additional experiments were carried out to assess whether sauchinone changed the nuclear binding activity of AP-1 in response to LPS. We also found that LPS increased the AP-1 binding activity in macrophages. Previous studies have shown that the AP-1 complex activated by LPS includes Jun family members including Jun D, c-Jun and Jun B (Granger et al., 2000). The migration of LPS-induced retarded AP-1 band appeared to be faster in the presence of sauchinone, indicating that one or more components involved in the activation of AP-1 complex were affected by the agent. It is highly likely that inhibition of LPS-induced AP-1 activation by sauchinone also contributes to the inhibition of iNOS expression. The activation of AP-1 is also involved in the induction of iNOS (Cho et al., 2002). In addition to the inhibition of the NF-κB activation by sauchinone, a slight decrease in the AP-1 activation in this study may also contribute to the inhibition of iNOS induction.
TNF-α is a toxic cytokine, which is involved in inflammation and other pathological processes such as rheumatoid arthritis and infections. Also, macrophages are the principal source of TNF-α. TNF-α promoter–reporter assay revealed that activation of NF-κB largely contributes to the induction of TNF-α expression among the NF-κB (κB3), C/EBPβ and c-Jun binding sites present in the 120 bp promoter region of human TNF-α gene (Figure 8) (Liu et al., 2000). In the activation of NF-κB, the cellular NF-κB p65 subunit was identified as a dominant transcription factor responsible for the induction of the TNF-α gene (Liu et al., 2000). In the present study, the activation of the band retarded as a complex of p65 and p50 was inhibited by sauchinone, which was consistent with blockage of the nuclear translocation of p65 subunit. Hence, the inhibitory effect of sauchinone on TNF-α expression may also result from the inhibition of NF-κB activation and of I-κBα phosphorylation. It has been reported that neither c-Jun nor C/EBP activation affected the expression of TNF-α gene (Liu et al., 2000). Hence, it is unlikely that the inhibition of AP-1 and C/EBP activation by sauchinone contribute to that of TNF-α expression. We found that the extent of TNF-α suppression by sauchinone was less than that of iNOS expression. This reflects that only the inhibition of NF-κB by sauchinone contributes to TNF-α expression.
Macrophages secrete inflammatory mediators including lipid metabolites (e.g. prostaglandins (PGs)) and other cytokines. COX-2 catalyzes the inducible production of PGs, which clearly represents an important step in the inflammatory process (Wadleigh et al., 2000). The production of PGs by LPS in macrophages is primarily because of the transcriptional activation of the COX-2 gene (Lee et al., 1992; Reddy & Herschman, 1994). The cis-acting elements identified on the promoter region of murine COX-2 include NF-κB, C/EBP and CREB (Figure 8) (Caivano et al., 2000). Although the NF-κB binding site is present in the regulatory region of COX-2 gene, the putative NF-κB is not required for the induction of COX-2 by LPS in murine macrophages, as shown by dominant-negative inhibition of NF-κB and COX-2-reporter gene activity (Wadleigh et al., 2000). More importantly, the C/EBP element is believed to play a critical role in the induction of COX-2 in macrophages. In particular, activation of C/EBPβ leads to the induction of COX-2 (Thomas et al., 2000; Wadleigh et al., 2000). If C/EBPβ is inactivated, the expression of COX-2 in response to LPS is impaired (Gorgoni et al., 2001). In the present study, we found that C/EBPβ, constitutively expressed in Raw264.7 cells, was increased after treatment of cells with LPS and the band intensity of LPS-inducible C/EBP binding returned to that of control by treatment of cells with sauchinone. The complex consisting of C/EBPβ homodimer is primarily involved in the activation of C/EBP response element in macrophage cells exposed to LPS (Granger et al., 2000). Hence, the suppression of COX-2 by sauchinone may result from its inhibition of LPS-inducible activation of C/EBPβ. We also observed that the LPS-inducible C/EBP protein complex binding to the C/EBP DNA consensus sequence comprised C/EBPδ, but not C/EBPα and C/EBPɛ. However, the level of nuclear C/EBPδ was not increased by LPS in the present study.
Activation of CREB alone was not sufficient to stimulate COX-2 expression (Caivano et al., 2000), although the initial phase of COX-2 expression by LPS involved CREB (Caivano et al., 2001). In addition, the expression of CREB repressed LPS-dependent COX-reporter activity. Since sauchinone does not change CREB binding activity, the inhibition of COX-2 gene expression by sauchinone is unlikely associated with CREB.
In summary, sauchinone inhibits LPS-inducible iNOS, TNF-α and COX-2 expression in murine macrophages through suppression of I-κBα phosphorylation and p65 nuclear translocation and of C/EBP and/or AP-1 activation. Inhibition of iNOS, TNF-α and COX-2 expression in macrophages may constitute anti-inflammatory effects of the lignan. The observation that the transcription factors NF-κB, C/EBP and AP-1 are all affected by sauchinone supports the possibility that the upstream enzymes responsible for the activation of the transcription factors be inhibited by the compound.
Acknowledgments
This research was supported by a grant (PF2-4) from Plant Diversity Research Center of the 21st Frontier Research Program funded by Ministry of Science and Technology of Korean Government.
Abbreviations
- CREB
cAMP-response element-binding protein
- COX-2
cyclooxygenase 2
- FBS
fetal bovine serum
- iNOS
inducible nitric oxide synthase
- I-κB
Inhibitor-κB
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- PI
propidium iodide
- SDS
sodium dodecyl sulfate
- SSC
standard saline citrate
- TNF-α
tumor necrosis factor-α
References
- BALDWIN A.S., JR The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- CAIVANO M., GORGONI B., COHEN P., POLI V. The induction of cyclooxygenase-2 mRNA in macrophages is biphasic and requires both CCAAT enhancer-binding protein beta (C/EBPβ) and C/EBPδ transcription factors. J. Biol. Chem. 2001;276:48693–48701. doi: 10.1074/jbc.M108282200. [DOI] [PubMed] [Google Scholar]
- CHO M.K., PARK J.W., JANG Y.P., KIM Y.C., KIM S.G. Potent inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by dibenzylbutyrolactone lignans through inhibition of I-κBα phosphorylation and of p65 nuclear translocation in macrophages. Int. Immunopharmacol. 2002;2:105–116. doi: 10.1016/s1567-5769(01)00153-9. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CHUNG B.S., SHIN M.G. Dictionary of Korean Folk Medicine. Seoul, Korea: Young Lim Sa; 1990. pp. 813–814. [Google Scholar]
- DIETER P., HEMPEL U., KAMIONKA S., KOLADA A., MALESSA B., FITZKE E., TRAN T. Prostaglandin E2 affects differently the release of inflammatory mediators from resident macrophages by LPS and muramyl tripeptides. Mediators Inflamm. 1999;8:295–303. doi: 10.1080/09629359990306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENG Y., ZHANG B., LOTZ M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J. Immunol. 1993;151:6692–6700. [PubMed] [Google Scholar]
- GORGONI B., CAIVANO M., ARIZMENDI C., POLI V. The transcription factor C/EBPβ is essential for inducible expression of the Cox-2 gene in macrophages but not in fibroblasts. J. Biol. Chem. 2001;276:40769–40777. doi: 10.1074/jbc.M106865200. [DOI] [PubMed] [Google Scholar]
- GRANGER R.L., HUGHES T.R., RAMJI D.P. Gene, stimulus and cell-type specific regulation of activator protein-1 in mesangial cells by lipopolysaccharide and cytokines. Biochim. Biophys. Acta. 2000;1492:100–107. doi: 10.1016/s0167-4781(00)00089-0. [DOI] [PubMed] [Google Scholar]
- GRILLI M., MEMO M. Nuclear factor-κB/Rel proteins. Biochem. Pharmacol. 1999;57:1–7. doi: 10.1016/s0006-2952(98)00214-7. [DOI] [PubMed] [Google Scholar]
- HEWETT J.A., ROTH R.A. Hepatic and extrahepatic pathobiology of bacterial lipopolysaccharides. Pharmacol. Rev. 1993;45:381–509. [PubMed] [Google Scholar]
- HIROTA K., MURATA M., SACHI Y., NAKAMURA H., TAKEUCHI J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-κB. J. Biol. Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- KIM S.G., KIM H.J., CHOI S.H., RYU J.Y. Inhibition of lipopolysaccharide-induced I-κB degradation and tumor necrosis factor-α expression by dimethyl-4,4′-dimethoxy-5,6,5′,6′-dimethylene dioxybiphenyl-2,2′-dicarboxylate (DDB): minor role in hepatic detoxifying enzyme expression. Liver. 2000;20:319–329. doi: 10.1034/j.1600-0676.2000.020004319.x. [DOI] [PubMed] [Google Scholar]
- KUBES P., MCCAFFERTY D.M. Nitric oxide and intestinal inflammation. Am. J. Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- LEE S.H., SOYOOLA E., CHANMUGAM P., HART S., SUN W., ZHONG H., LIOU S., SIMMONS D., HWANG D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- LIU H., SIDIROPOULOS P., SONG G., PAGLIARI L.J., BIRRER M.J., STEIN B., ANRATHER J., POPE R.M. TNF-α gene expression in macrophages: regulation by NF-κB is independent of c-Jun or C/EBPβ. J. Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- MATUSUSHIMA A., KAISHO T., RENNERT P.D., NAKANO H., KUROSAWA K. Essential role of nuclear factor(NF)-κB-inducing kinase and inhibitor of κB (I-κB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAEL J.M., SANKER G. Signal transduction through NF-κB. Immunol. Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- PAN M.H., LIN-SHIAU S.Y., LIN J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through downregulation of IκB kinase and NFκB activation in macrophages. Biochem. Pharmacol. 2000;60:1665–1676. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- PARMENTIER M., HIRANI N., RAHMAN I., DONALDSON K., MACNEE W. Regulation of lipopolysaccharide-mediated interleukin-1β release by N-acetylcysteine in THP-1 cells. Eur. Respir. J. 2000;16:933–939. doi: 10.1183/09031936.00.16593300. [DOI] [PubMed] [Google Scholar]
- REDDY S.T., HERSCHMAN H.R. Ligand-induced prostaglandin synthase requires expression of the TIS10/PGS-2 prostaglandin synthase gene in murine fibroblast and macrophages. J. Biol. Chem. 1994;269:15473–15480. [PubMed] [Google Scholar]
- SALERNO L., SORRENTI V., DI GIACOMO C., ROMEO G., SIRACUSA M.A. Progress in the development of selective nitric oxide synthase inhibitors. Curr. Pharm. Des. 2002;8:177–200. doi: 10.2174/1381612023396375. [DOI] [PubMed] [Google Scholar]
- SCHREIBER E., HARSHMAN K., KEMLER I., MALIPIERO U., SCHAFFNER W. Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res. 1990;18:5495–5503. doi: 10.1093/nar/18.18.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHRIVASTAVA A., AGGARWAL B.B. Antioxidants differentially regulate activation of nuclear factor-κB, activator protein-1, c-Jun amino-terminal kinases, and apoptosis induced by tumor necrosis factor: evidence that JNK and NF-κB activation are not linked to apoptosis. Antioxid. Redox. Signal. 1999;1:181–191. doi: 10.1089/ars.1999.1.2-181. [DOI] [PubMed] [Google Scholar]
- STANCOVSKI I., BALTIMORE D. NF-κB activation: the IκB kinase revealed. Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- SUNG S.H., KIM Y.C. Hepatoprotective diastereomeric lignans from Saururus chinensis herb. J. Nat. Prod. 2000;63:1019–1021. doi: 10.1021/np990499e. [DOI] [PubMed] [Google Scholar]
- SUNG S.H., LEE E.J., CHO J.H., KIM H.S., KIM Y.C. Sauchinone, a lignan from Saururus chinensis, attenuates CCl4-induced toxicity in primary cultures of rat hepatocytes. Biol. Pharm. Bull. 2000;23:666–668. doi: 10.1248/bpb.23.666. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI F., TAKAHASHI K., MAEDA K., TOMINAGA S., FUKUCHI Y. Osteopontin is induced by nitric oxide in Raw264.7 cells. IUBMB Life. 2000;49:217–221. doi: 10.1080/713803614. [DOI] [PubMed] [Google Scholar]
- THOMAS B., BERENBAUM F., HUMBERT L., BIAN H., BEREZIAT G., CROFFORD L., OLIVIER J.L. Critical role of C/EBPδ and C/EBPβ factors in the stimulation of the cyclooxygenase-2 gene transcription by interleukin-1β in articular chondrocytes. Eur. J. Biochem. 2000;267:6798–6809. doi: 10.1046/j.1432-1033.2000.01778.x. [DOI] [PubMed] [Google Scholar]
- WADLEIGH D.J., REDDDY S.T., KOPP E., GHOSH S., HERSCHMAN H.R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated Raw264.7 macrophages. J. Biol. Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- WATSON W.H., ZHAO Y., CHAWLA R.K. S-adenosylmethionine attenuates the lipopolysaccharide-induced expression of the gene for the tumour necrosis factor-α. Biochem. J. 1999;342:21–25. [PMC free article] [PubMed] [Google Scholar]
- ZHOU L.Z., JOHNSON A.P., RANDO T.A. NF-κB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radical Biol. Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]