Abstract
In this study, we investigated whether serotonin could regulate the in vitro activity of phagocytosis through 5-hydroxytryptamine or serotonin (5-HT1A) receptors.
Mouse peritoneal macrophages were cultured with serotonin and the activity of phagocytosis was assessed by the uptake of zymosan and latex particles added to the culture media. Specific binding of [3H]8-OH-DPAT and immunohistochemistry using an affinity-purified anti-5-HT1A-receptor antibody were assayed in the macrophages. In addition, we took advantage of the availability of pharmacological inhibitors of nuclear factor-κB (NF-κB) to explore its role in the regulation of the 5-HT1A receptor.
Serotonin increased the in vitro activity of phagocytosis in a dose-dependent manner. The 5-HT1A receptor agonist (±)-8-hydroxy-2-(di-n-propyl-amino)-tetralin (R(+)-8-OH-DPAT) reproduced these effects. Serotonin- or R(+)-8-OH-DPAT-induced increases in phagocytosis were blocked by the 5-HT1A receptor antagonist WAY100635 and the NF-κB inhibitor pyrrolidinedithiocarbamate. Moreover, mouse peritoneal macrophages expressed specific binding sites for [3H]8-OH-DPAT when cultivated in the presence of zymosan or latex beads. Immunohistochemistry confirmed the expression of the 5-HT1A receptor protein in the macrophages.
These results show that serotonin can upregulate the activity of peritoneal macrophages through 5-HT1A receptors.
Keywords: Serotonin, 5-HT1A, phagocytosis, R(+)-8-OH-DPAT, WAY100635
Introduction
Serotonin (5-HT) is one of the most extensively studied neurotransmitters of the central nervous system and is also present in a variety of peripheral tissues including in constituents of the immune system (Iken et al., 1995; Mossner & Lesch, 1998). Stored in large amounts in peripheral tissues by mast cells, platelets, and synthesized by enterochromaftin cells of the gut mucosa, 5-HT modulates aspects of both adaptive and innate immunity. Evidence suggests that 5-HT modulates immune function through several 5-HT receptor subtypes including the 5-HT1A receptor (Mossner & Lesch, 1998). A G-protein-linked receptor, the 5-HT1A receptor is coupled primarily to adenylate cyclase and the second messenger cyclic adenosine monophosphate (cAMP) (Julius, 1991). 5-HT1A receptor agonists inhibit the intracellular formation of cAMP (Peroutka, 1993) and increased formation of cAMP is associated predominantly with suppression of adaptive and innate immunity (Bourne et al., 1974). 5-HT modulates adaptive immunity, in part, by operating through 5-HT1A receptors, which have been characterized on B and T lymphocytes (Aune et al., 1993; Iken et al., 1995; Abdouh et al., 2001). Exposure of B lymphocytes to 5-HT1A receptor antagonists abrogates 5-HT enhancement of lymphocyte proliferation to mitogens (Iken et al., 1995). Likewise, 5-HT1A receptor antagonists suppress T lymphocyte proliferation in response to both mitogenic and interleulkin (IL)-2 stimulation (Aune et al., 1994). Further, 5-HT1A receptor antagonists inhibit interferon-γ (INF-γ) production and antigen stimulated IL-2 production while inducing increased concentrations of cAMP in T lymphocytes (Aune et al., 1994).
At sites of inflammation, macrophages are exposed both to products released from activated lymphocytes and aggregated platelets (Jeynes et al., 1980; Pawlowski et al., 1983). Serotonin is one major product released from platelets upon aggregation at sites of inflammation. Previous literature shows that 5-HT negatively modulates IFN-γ-induced macrophage La expression (Sternberg et al., 1986) through a 5-HT receptor with some characteristics of the 5-HT2 type (Sternberg et al., 1987). Serotonin, acting through 5-HT1A receptors, also may modulate monocyte regulation of natural killer (NK) cells (Hellstrand & Hermodsson, 1990a,1990b; Hellstrand et al., 1993; Frank et al., 2001), which are an integral component of innate immunity (Herberman & Ortaldo, 1981).
Nevertheless, there are still many questions to be explained. First, the effects of serotonin agonists on the activity of phagocytes in the absence of cytokines or other type of immune cells have not been described. Second, despite many attempts have been made to define 5-HT receptors on phagocytes with binding experiments, these experiments did not clearly show 5-HT binding to classical 5-HT cell surface receptors (Eliseeva & Stefanovich, 1982; Roszman et al., 1984).
To further elucidate previous investigations, in the present report we conducted pharmacological studies in vitro examining the effects of the full 5-HT1A serotonin receptor agonist with high affinity for subtype 5-HT1A receptor (±)-8-hydroxy-2-(di-n-propyl-amino)-tetralin (R(+)-8-OH-DPAT) (Bjork et al., 1989; Cornfield et al., 1991) on the in vitro activity of phagocytosis of mouse peritoneal macrophages in the absence or presence of the highly selective 5-HT1A serotonin receptor antagonist (N-[2-(4-[2-mehoxyphenil]-1-piperazinyl)-ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY100635) (Fletcher et al., 1996). Binding experiments with unstimulated and zymosan- or latex-stimulated peritoneal macrophages using radiolabeled 8-OH-DPAT, as well as immunohistochemistry to evaluate the expression of the 5- HT1A receptor protein in the macrophages using an affinity-purified anti-5-HT1A were also performed. In addition, we took advantage of the availability of pharmacological inhibitors of nuclear factor-κB (NF-κB) to explore its role in the regulation of the 5-HT1A receptor.
Methods
Mice
Male mice (8 weeks old at the beginning of the experiment) of the BALB-c strain (Iffa-Credo, Barcelona, Spain) were used. They were housed, 7 days before experiments, four per cage in an aseptic chamber kept between 21 and 22°C and maintained on an alternating 12-h light–dark cycle. Sterilized food (Panlab Diet A.03, Barcelona, Spain) and water were given ad libitum. The University of Santiago de Compostela Review Committee for the use of Animal Subjects approved this experimental protocol.
Cells
Macrophages were collected by washing the peritoneal cavity with ice-cold phosphate-buffered saline solution (PBS, 0.1 M, pH 7.3). The peritoneal cells were washed twice with RPMI culture medium (Sigma, St Louis, MO, U.S.A.) by centrifugation (1000 rpm, 5 min, 0°C) and finally resuspended in an adequate volume of RPMI medium supplemented with 10% heat-inactivated fetal calf serum (FCS, Sigma).
Phagocytosis of zymosan A
Aliquots (1 ml) of a suspension of 1 × 106 nucleated cells ml−1 were seeded in 35 mm Petri dishes and incubated at 37°C in a humidified 5% CO2 and 95% air incubator. After 3 h, nonadherent cells were washed off. Fresh medium (1 ml) containing 10% FCS and 5 × 106 particles ml−1 of zymosan A (Sigma) and different concentrations of R(+)-8-OH-DPAT and or(+) WAY100635 was added. Incubation was continued for 3 h. Then media were removed and particle uptake was measured by light microscopy. In total, 100 cells were counted per plate in 10 microscopic fields randomly selected. Cells containing three or more zymosan particles were counted as phagocytic (Okimura et al., 1986). Assays were done in sixtuplicate.
Flow cytometry measurement of the phagocytosis of latex microparticles
Yellow–green carboxylate-modified latex beads (1 μm in diameter; Sigma) were incubated (40 min at 37°C) as a suspension of 1 × 109 beads ml−1, with 1.5 × 106 cells in a 24-well plate. At the end of the incubation, cells were harvested, washed twice, fixed in 400 μl of 5% formaldehyde in PBS, and fluorescence was read in FL-1 channel. Markers were set to calculate the percentage of cells that had phagocytosed >1 particle per cell and >5 particles per cell.
Immunohistochemistry analysis of 5-HT1A receptor protein
Cells (106) were mounted on 3-aminopropyl-triethoxysilane-coated slides and fixed 15 min in −20°C ethanol (96%) and washed twice for 5 min with PBS (50 mM, pH 7.4). Guinea-pig anti-5-HT1A polyclonal antibody (Chemicon International Inc., CA, U.S.A.) was used. The streptavidin–biotin complex (SABC) immunohistochemical method was used and the sections were consecutively incubated in (1) polyclonal anti-5-HT1A antibody at a dilution of 1 : 200 for 1 h at room temperature; (2) biotinylated antibodies to guinea-pig immunoglobulins (Multilink, Biogenex, San Ramon, CA, U.S.A.) at a dilution of 1 : 20 for 30 min; (3) 3% hydrogen peroxide in methanol for 10 min (Merck, Darmstadt, Germany) in order to block endogenous peroxidase; (4) streptavidin–biotin–peroxidase complex (Duet kit, Dakopatts, Copenhaguen, Denmark) prepared according to the protocol provided by the manufacturer, for 30 min; and (5) 3,3′-diamino-benzidine-tetrahydrochloride (DAB) solution prepared by dissolving one DAB-buffer tablet (Merck) in 10 ml of distilled water, for 10 min. Between steps, the sections were washed twice with PBS and after step 5, with distilled water. All dilutions were made in PBS. This buffer was added with 0.1% bovine serum albumin (Sigma) for dilution of the primary antibody (step 2). Counterstaining was done with hematoxylin.
Measurement of [3H]8-OH-DPAT binding
The presence of specific binding sites for 8-OH-DPAT on peritoneal cultured macrophages was investigated by measuring specific binding of [3H]8-OH-DPAT. Cells were prepared by incubating mouse peritoneal macrophages at 37°C during 3 h in the absence or presence of 30 mg ml−1 of zymosan A or latex beads.
The cells were then washed three times in RPMI. Resulting pellet was weighed and homogenized in fresh ice-cold 50 mM Tris-HCl pH 6.8 buffer using a polytron, followed by centrifugation at 36,500 × g for 15 min. The resulting pellets were resuspended in 50 mM Tris-HCl, pH 7.7 and homogenized prior to preincubation in the same buffer at 37°C for 30 min to remove endogenous 5-HT. The homogenates were then centrifuged at 36,500 × g for a further 15 min and the pellets resuspended at a concentration of 10 mg ml−1 in the final homogenization buffer (50 mM Tris-HCl, pH 7.7) containing CaCl2 (4 mM, ascorbic acid (0.1%) and pargyline (1 μM). The binding of [3H]8-OH-DPAT (Amersham, UK) in triplicate was determined using 10 ligand concentrations for [3H]8-OH-DPAT (0.25–12 nM). The binding at each concentration was measured in the presence and absence of 10 μM 5-HT (Sigma) in order to determine the nonspecific and total binding, respectively. Binding assays were carried in 50 mM Tris-HCl buffer containing CaCl2 (4 mM), ascorbic acid (0.1%) and pargyline (1 μM) at 37°C for 15 min. Assays were terminated by filtration through Whatman GF-B filters, using a M-24 cell harvester, washing three times with 4 ml of ice-cold Tris-HCl buffer and radioactivity determined by liquid scintillation spectrometry.
Drugs
Serotonin, R(+)-8-OH-DPAT, and WAY100635 were purchased from Sigma. Drugs were dissolved in appropriate solvents and further diluted in RPMI 1640 at concentrations of 10−9–10−3 M just prior to use. Controls were made with solvent alone and were included in all experiments. To determine the potential role of the transcription factor NF-κB in 5-HT1A mRNA expression, macrophages were also incubated in the presence of 5–40 μMpyrrolidinedithiocarbamate (PDTC, Sigma) that acts as inhibitor of NF-κB activation.
Statistical analysis
Statistical analysis was performed using the analysis of variance (ANOVA) with grouping of means by Student–Newman–Keuls multiple range tests (SPSS, windows, 6.5.1). Differences were considered to be significant when the probability (P) value was <0.05.
Results
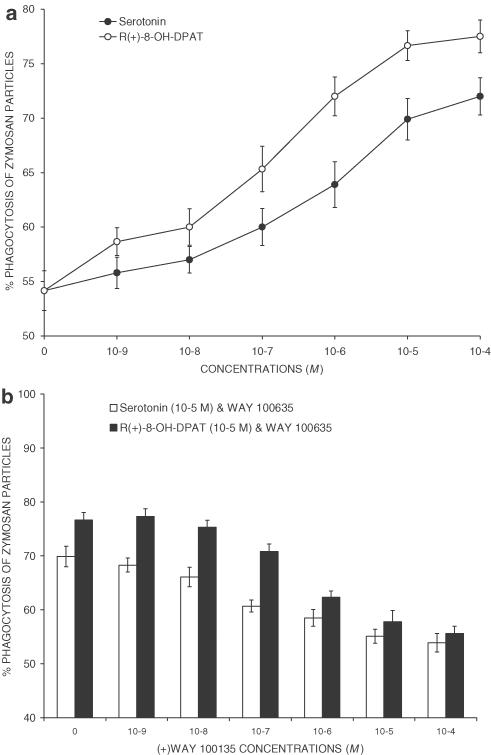
Serotonin increased in a dose-dependent manner the phagocytic activity of peritoneal macrophages (Figure 1a). Although there were no significant differences among the number of macrophages harvested in all experimental groups, the number of macrophages containing zymosan particles was higher when serotonin was added to the culture media. The effect of serotonin was apparent at 10−7 M and it reached a maximum at 10−5 M with about 20% increase of zymosan particle uptake. The half-maximally effective concentration (EC50) was 9 × 10−7 M. The selective 5-HT1A receptor agonist R(+)-8-OH-DPAT mimicked the increasing effect of 5-HT with an EC50 of 10−7 M. The maximally effective concentration was 10−5 M, yielding about 24% increase over control levels. To further determine the specificity of the upregulating effects of serotonin and R(+)-8-OH-DPAT on the activity of phagocytosis, competition studies were performed with the 5-HT1A receptor antagonist WAY100635 (Figure 1b). This drug strongly and dose-dependently inhibited serotonin- or R(+)-8-OH-DPAT-induced increase in the in vitro activity of phagocytosis.
Figure 1.
In vitro phagocytosis of zymosan particles. (a) Serotonin (open circles) and the selective 5-HT1A receptor agonist R(+)-8-OH-DPAT (filled circles) increased the in vitro activity of phagocytosis by peritoneal macrophages of BALB-c mice cultured in RPMI-1640 medium containing 5 × 106 zymosan particles for 30 min. Each point represents the mean±s.d. of six measures. The effect of serotonin was apparent at 10−7 M, it reached a maximum at 10−5 M with about 20% increase of zymosan particle uptake and the EC50 was 9 × 10−7 M. The selective 5-HT1A receptor agonist R(+)-8-OH-DPAT mimicked the increasing effect of 5-HT with an EC50 of 10−7 M, a maximally effective concentration of 10−5 M, yielding about 24% increase over control levels. Differences between control and 5-HT were significant (P<0.01) when administered at 10−7–10−4 M. Differences between control and R(+)-8-OH-DPAT were significant (P<0.01) when administered at 10−9–10−4 M. (b) Effects of the 5-HT1A receptor antagonist WAY100635 on serotonin- or R(+)-8-OH-DPAT-induced increase in the in vitro activity of phagocytosis by peritoneal macrophages. Differences between a single dose of agonist (10−5 M) and the combination agonist+antagonist (10−7–10−4 M) were significant (P<0.01).
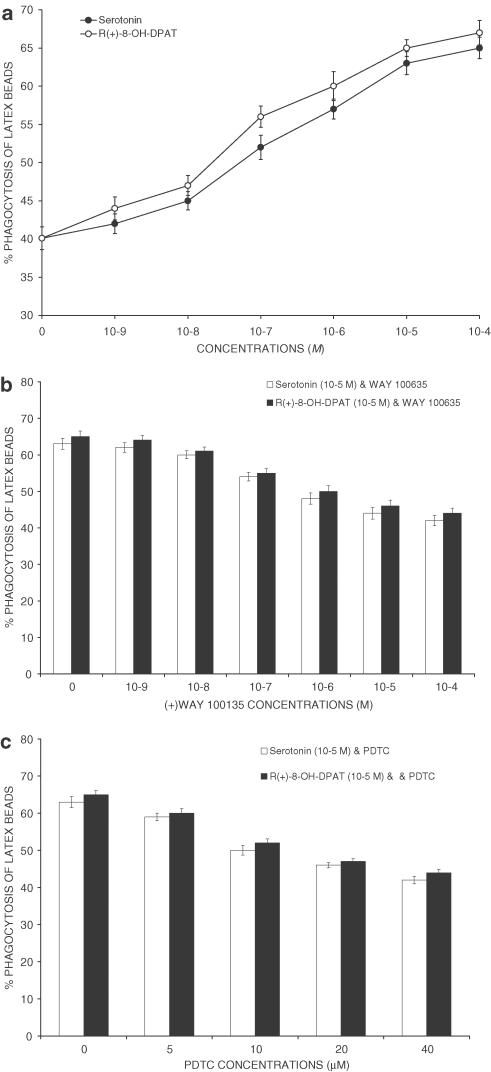
Flow cytometry measurement of the phagocytosis of latex microparticles was also performed. Both serotonin and R(+)-8-OH-DPAT increased the phagocytosis of latex microparticles in a dose-dependent manner (Figure 2a). The effect of serotonin was apparent at 10−7 M and it reached a maximum at 10−5 M with about 25% increase of latex particle uptake. The half-maximally effective concentration (EC50) was 2 × 10−7 M. The EC50 for R(+)-8-OH-DPAT was of 4 × 10−7 M and the maximally effective concentration was 10−5 M, yielding about 26% increase over control levels. WAY100635 also reduced the stimulatory effects of a single dose (10−5 M) of serotonin and R(+)-8-OH-DPAT on the activity of phagocytosis when administered at doses of 10−7–10−4 M (Figure 2b). The 5-HT1A receptor antagonist WAY100635 in the absence of agonist was devoid of any effect on the activity of phagocytosis both measured by the uptake of zymosan and latex microparticles (differences P>0.05). The NF-κB inhibitor PDTC was found to cause a dose-dependent inhibition of the activity of phagocytosis (Figure 2c) in the presence but not in the absence of serotonin or R(+)-8-OH-DPAT.
Figure 2.
Phagocytic ability of latex microparticles. Phagocytosis was determined by incubating 1.5 × 106 cells with of 1 × 109 beads ml−1 yellow–green carboxylate-modified latex beads (1 μm in diameter; Sigma) for 40 min at 37°C followed by two washes to remove uningested particles, and then analysis of fluorescence in the FL-1 channel. Markers were set to calculate the percentage of cells that had ingested >5 particles. Values are mean±s.d. (n=3). (a) Serotonin (open circles) and the selective 5-HT1A receptor agonist R(+)-8-OH-DPAT (filled circles) increased the activity of phagocytosis. The effect of serotonin was apparent at 10−7 M and it reached a maximum at 10−5 M (EC50=2 × 10−7 M). The EC50 for R(+)-8-OH-DPAT was of 4 × 10−7 M and the maximally effective concentration was 10−5 M, yielding about 26% increase over control levels. (b) Effects of the 5-HT1A receptor antagonist WAY100635 on serotonin- or R(+)-8-OH-DPAT-induced increase in the in vitro activity of phagocytosis by peritoneal macrophages. Differences between a single dose of agonist (10−5 M) and the combination agonist+antagonist (10−7–10−4 M) were significant (P<0.01). (c) The NF-κB inhibitor PDTC was found to cause a dose-dependent inhibition of the serotonin- and R(+)-8-OH-DPAT-induced increase of the activity of phagocytosis. The effect of PDTC was apparent at 5 μM.
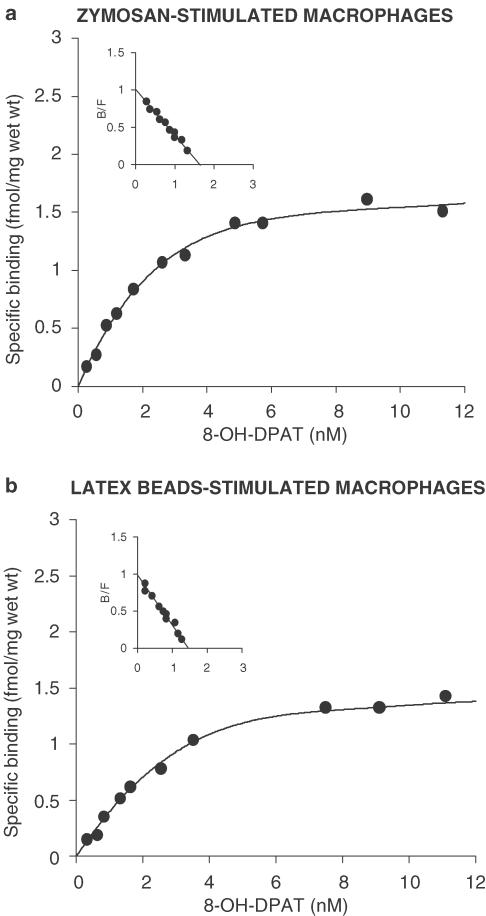
[3H]8-OH-DPAT showed high-affinity saturable binding in zymosan- and latex-beads-stimulated macrophages, but not in unstimulated ones. The mean binding curves and Scatchard transformations are shown in Figure 3. The maximum binding capacity (Bmax) of zymosan- and latex-stimulated macrophages was 1.58 and 1.38 fmol mg−1 protein, respectively. The affinity constant (Kd) for [3H]8-OH-DPAT was 1.7±0.14 and 1.5±0.08 nM when determined for zymosan- and latex-stimulated macrophages, respectively. No specific binding was detected in nonstimulated cells.
Figure 3.
Specific binding characteristics of [3H]8-OH-DPAT in peritoneal macrophages. The inset shows a Scatchard plot derived from the specific binding data. The mean binding curves and Scatchard transformations are shown in the figure. The maximum binding capacity (Bmax, fmol mg−1) of zymosan- and latex-stimulated macrophages was 1.58 and 1.38, respectively. The affinity constant (Kd, nM) for [3H]8-OH-DPAT was 1.7±0.14 and 1.5±0.08 when determined for zymosan- and latex-stimulated macrophages, respectively. B=bound, F=free. Each sample was run in triplicate. No specific binding was detected in nonstimulated cells.
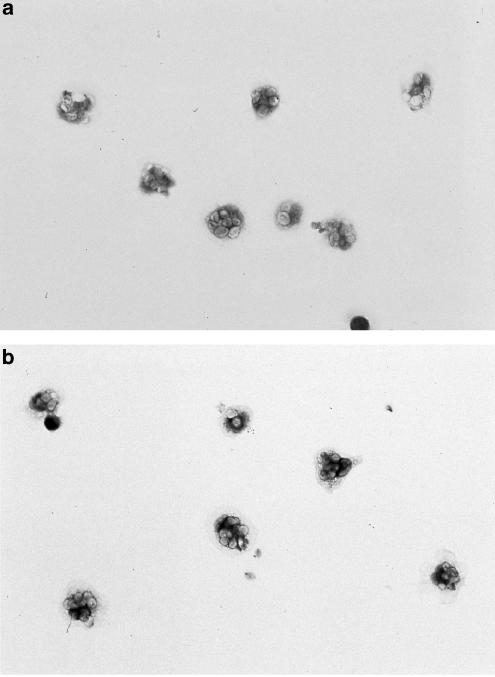
To visualize the localization of the 5-HT1A receptor immunoreactivity, unstimulated and zymosan-stimulated cells were permeabilized and incubated with the anti-5-HT1A antiserum whose binding was revealed by immunohistochemistry using the horseradish peroxidase system. Labeling with the anti-5-HT1A receptor antiserum yielded a little staining in the unstimulated cells (Figure 4a), while labeling of zymosan-stimulated cells (Figure 4b) showed a marked and uniform staining. Labeling with the preimmune serum manifested no detectable signal in unstimulated and stimulated macrophages. PDTC (10−4 M) was also found to block 5-HT1A receptor induction in zymosan- and latex-stimulated macrophages both in [3H]8-OH-DPAT binding and immunohistochemistry analysis of 5-HT1A receptor protein.
Figure 4.
5-HT1A protein expression in peritoneal macrophages as detected by immunohistochemistry. To visualize the localization of the 5-HT1A receptor immunoreactivity, unstimulated and zymosan-stimulated cells were permeabilized and incubated with the anti-5-HT1A antiserum whose binding was revealed by immunohistochemistry using the horseradish peroxidase system. Labeling with the anti-5-HT1A receptor antiserum yielded a little staining in the unstimulated cells (4a), while labeling of zymosan-stimulated cells (4b) showed a marked and uniform staining. Labeling with the preimmune serum manifested no detectable signal in unstimulated and stimulated macrophages.
Discussion
Our results show that serotonin and the selective 5-HT1A receptor agonist R(+)-8-OH-DPAT increase the in vitro activity of phagocytosis, and the 5-HT1A receptor antagonist WAY100635 blocks the effects of 5-HT or R(+)-8-OH-DPAT. Radioligand binding assays and immunohistochemistry analysis also point in the direction of the presence of 5-HT1A receptors in macrophages.
Previous studies reported evidence about the regulatory effects of serotonin on phagocytes. A combined application of phenthyrine and cyclophosphane together with serotonin helps re-establish the macrophages function in tumor-bearing animals and to heighten the antineoplastic activity of these compounds (Pereverzeva, 1978). Muramyl peptides and serotonin interact at specific binding sites on macrophages and enhance superoxide release (Silverman et al., 1985). An active, 5-HT uptake system similar in affinity to that described in platelets uptake system appears to be confined to macrophages (Jackson et al., 1988). Further, macrophages rapidly metabolize 5-HT to its 5-hydroxyindole acetic acid metabolite. Specific uptake of serotonin by macrophages may thus constitute an important mechanism whereby this amine is able to regulate immune function. Tokmakov et al. (1991) reported that serotonin activates the monocyte capacity of phagocytizing the opsonized sheep erythrocytes. Polanski et al. (1995) demonstrated that serotonin acts as a muramyl peptide-like agonist by increasing phagocytosis of tubercle bacilli by murine peritoneal macrophages, and as a partial agonist–antagonist in the induction of messenger ribonucleic acid (mRNA) for tumor necrosis factor.
Alveolar macrophages release neutrophil chemotactic activity and monocyte chemotactic activity in response to serotonin and may modulate the inflammatory cell recruitment into the lung (Nomura et al., 2001) differing from peritoneal macrophages in biogenic amine content: peritoneal macrophages contain smaller amounts of histamine and serotonin. When activated, alveolar macrophages release biogenic amines, whereas in peritoneal macrophages, stimulators of macrophage activity induce the increase in amine content. Therefore, the morphofunctional heterogeneity between peritoneal and alveolar macrophages in their biogenic amine content seems to depend on the presence or absence in the microenvironment of mast cells (Bochkarev & Kupriianov, 1995). In our previous studies, we also observed that the specific serotonin reuptake inhibitors (SRIs) fluoxetine and nefazodone reversed the stress-induced suppression of in vivo and in vitro phagocytic activity in mice in a dose-dependent manner (Freire-Garabal et al., 2000), but did not affect the activity of phagocytosis in unstressed animals.
In this present study, [3H]8-OH-DPAT specifically binds to macrophages when cultured in the presence of zymosan or latex beads. Previous attempts to define 5-HT receptors on macrophages with binding experiments using radiolabeled 5-HT and 5-HT antagonists (Eliseeva & Stefanovich, 1982; Roszman et al., 1984) have suffered from the relatively crude cell populations studied, and none clearly showed 5-HT binding to classical 5-HT cell surface receptors on macrophages.
5-HT receptors and 5-HT transporter systems are described on immune cells, but immune responses to 5-HT may attain in the extracellular space in physiological conditions and under pathological circumstances such as inflammation, thrombosis, and ischemia. At these 5-HT concentrations, functions for 5-HT emerge. These include T-cell and NK-cell activation, delayed-type hypersensitivity responses, production of chemotactic factors, and natural immunity delivered by macrophages (Mossner & Lesch, 1998). In this regard, studies by Essman (1985) and Olson et al. (1974) showed that 5-HT at the physiologic concentrations released from platelets at sites of inflammation, (10−8–10−6 M) had no effect on macrophage function. However, at these concentrations 5-HT negatively modulated IFN-γ-induced macrophage La expression (Sternberg et al., 1986) through a 5-HT receptor with some characteristics of the 5-HT2 type (Sternberg et al., 1987).
Hellstrand & Hermodsson (1990a) postulated that 5-HT1A receptors on monocytes mediate 5-HT enhancement of NK-cell activity by abrogating monocyte suppression of NK-cell function. Further, Hellstrand & Hermodsson (1993) contended that the reactive oxygen species H2O2 is a mediator, released by monocytes, which suppresses NK cell activity and may be modulated by 5-HT. The results provided by Frank et al. (2001) support this hypothesis by demonstrating that antagonism of the 5-HT1A receptor exacerbates monocyte suppression of NK-cell activity and that H2O2 may mediate the observed monocyte inhibition of NK-cell activity.
In our experiment, [3H]8-OH-DPAT binding was observed in macrophages cultured in the presence but not in the absence of zymosan A or latex beads. Macrophages can take up small particulate matter by surrounding the particle with extrusions of the cell membrane. In culture systems, a whole range of different particles from small latex beads to polymer beads have been tested. In this experiment, we used both zymosan and latex particles in order to stimulate phagocytosis. Zymosan A is prepared from yeast cell wall and consists of protein–carbohydrate complexes. Experimentally, zymosan is used to induce sterile inflammation. In macrophages, zymosan-induced responses include the induction of proinflammatory cytokines, arachidonate mobization, protein phosphorylation, and inositol phosphate formation. Zymosan A also raises cyclin D2 levels suggesting a role for the latter in macrophage activation besides proliferation. Latex beads are also commonly used to determine phagocytosis thanks to easy macrophage recognition of this particles as phagocytic. In our assays, no significant differences on [3H]8-OH-DPAT binding between zymosan- and latex-stimulated macrophages were observed. This suggests that this phagocytosis stimulation, in the absence of other type of immune cells or cytokines may induce, increase, or activate macrophage surface 5-HT1A receptors.
Our studies further elucidate the possible mechanism of 5-HT1A receptor induction or action. In previous studies in B and T cells, NFκB was implicated in induction of 5-HT1A receptors, and a similar mechanism may occur in macrophages (Abdouh et al., 2001). NF-κB is a ubiquitous and inducible transcription factor involved in many immune and inflammatory responses, including activation and proliferation of B and T lymphocytes stimulated by mitogens such as LPS, PHA, and PMA (Abdouh et al., 2001). NF-κB is mainly composed of p50 and p65 subunits, which are normally retained in the cytosol of nonstimulated cells by inhibitory molecules, IκB. In response to stimuli, IκB are rapidly phosphorylated and degraded, allowing translocation of NF-κB complexes into the nucleus and activation of NF-κB elements. In this report, we observed that the NF-κB inhibitor PDTC (5–40 μM) caused a dose-dependent inhibition of the 5-HT1A-induced increase of the activity of phagocytosis. Thus, we hypothesize that zymosam- or latex stimulation may increase nuclear translocation of NF-κB to enhance transcription of the 5-HT1A receptor gene in macrophages.
In summary, the present evidence further implicates the 5-HT1A receptor as a component of serotonergic immunomodulation and pharmacological interventions aimed at the 5-HT1A receptor may thus have significant immunological consequences. Recently, 5-HT1A receptor antagonists, in combination with selective serotonin reuptake inhibitors, have been used to treat major depressive disorders (Thase et al., 1998). However, adverse impacts of 5-HT1A receptor antagonist treatment on immune function have not been examined. The preponderance of evidence indicates that 5-HT1A receptor antagonists potently suppress aspects of cell mediated and humoral immunity in vitro, thus suggesting that investigations into the immunologic effects of pharmacological treatment with 5-HT1A receptor antagonists may be warranted (Frank et al., 2001).
Abbreviations
- ANOVA
analysis of variance
- Bmax
maximum binding capacity
- cAMP
cyclic adenosine monophosphate
- DAB
3,3′-diamino-benzidine-tetrahydrochloride
- EC50
half-maximally effective concentration
- FCS
fetal calf serum
- 5–HT
5-hydroxytryptamine or serotonin
- IL-2
interleulkin-2
- INF-γ
interferon-gamma
- Kd
affinity constant
- mRNA
messenger ribonucleic acid
- NF-κB
nuclear factor-kappa B
- NK
natural killer
- PBS
phosphate-buffered saline solution
- PDTC
pyrrolidinedithiocarbamate
- R(+)-8-OH-DPAT
(±)-8-hydroxy-2-(di-n-propyl-amino)-tetralin
- SABC
streptavidin–biotin complex
- WAY100635
(N-[2-(4-[2-mehoxyphenil]-1-piperazinyl)-ethyl]-N-2-pyridinylcyclohexanecarboxamide)
References
- ABDOUH M., STORRING J.M., RIAD M, , PAQUETTE Y., ALBERT P.R., DROBETSKY E., KOUASSI E. Transcriptional mechanisms for induction of 5-HT1A receptor mRNA and protein in activated B and T lymphocytes. J. Biol. Chem. 2001;276:4382–4388. doi: 10.1074/jbc.M004559200. [DOI] [PubMed] [Google Scholar]
- AUNE T.M., GOLDEN H.W., MCGRATH K.M. Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. J. Immunol. 1994;153:489–498. [PubMed] [Google Scholar]
- AUNE T.M., MCGRATH K.M., SARR T., BOMBARA M.P., KELLEY K.A. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell pro-liferation by 5-hydroxytryptamine. J. Immunol. 1993;151:1175–1183. [PubMed] [Google Scholar]
- BJORK L., HOOK B.B., NELSON D.L., ANDEN N.E., HACKSELL U. Resolved N,N-dialkylated 2-amino-8-hydroxytetralins: stereoselective interactions with 5-HT1A receptors in the brain. J. Med. Chem. 1989;32:779–783. doi: 10.1021/jm00124a009. [DOI] [PubMed] [Google Scholar]
- BOCHKAREV V.A., KUPRIIANOV V.S. The morphofunctional heterogeneity between peritoneal and alveolar macrophages in their biogenic amine content: the effect of their presence or absence in the microenvironment of mast cells. Tsitol. Genet. 1995;29:68–72. [PubMed] [Google Scholar]
- BOURNE H.R., LICHTENSTEIN L.M., MELMON K.L., HENNEY C.S., WEINSTEIN Y., SHEARER G.M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974;184:19–27. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- CORNFIELD L.J., LAMBERT G., ARVIDSSON L.E., MELLIN C., VALLGARDA J., HACKSELL U., NELSON D.L. Intrinsic activity of enantiomers of 8-hydroxy-2-(di-n-propylamino)tetralin and its analogs at 5-hydroxytryptamine1A receptors that are negatively coupled to adenylate cyclase. Mol. Pharmacol. 1991;39:780–787. [PubMed] [Google Scholar]
- ELISEEVA L.S., STEFANOVICH L.E. Specific binding of serotonin by blood leukocytes and peritoneal cells in the mouse. Biokhimiia. 1982;47:810–813. [PubMed] [Google Scholar]
- ESSMAN E.J. Serotonin receptors on pulmonary alveolar macrophages. Ric. Clin. Lab. 1985;15:19–24. doi: 10.1007/BF03029157. [DOI] [PubMed] [Google Scholar]
- FLETCHER A., FORSTER E.A., BILL D.J., BROWN G., CLIFFE I.A., HARTLEY J.E., JONES D.E., MCLENACHAN A., STANHOPE K.J., CRITCHLEY D.J., CHILDS K.J., MIDDLEFELL V.C., LANFUMEY L., CORRADETTI R., LAPORTE A.M., GOZLAN H., HAMON M., DOURISH C.T. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- FRANK M.G., JOHNSON D.R., HENDRICKS S.E., WIESELER-FRANK J.L. Monocyte 5-HT1A receptors mediate pindobind suppression of natural killer cell activity: modulation by catalase. Int. Immunopharmacol. 2001;1:247–253. doi: 10.1016/s1567-5769(00)00015-1. [DOI] [PubMed] [Google Scholar]
- FREIRE-GARABAL M., VARELA M., RIVEIRO P., BALBOA J., LIÑARES D., MAÑÁ P., MAYÁN J.M., REY-MÉNDEZ M., NÚÑEZ M.J. Effects of nefazodone on the immune system. Eur. Neuropsychopharmacol. 2000;10:255–264. doi: 10.1016/s0924-977x(00)00080-8. [DOI] [PubMed] [Google Scholar]
- HELLSTRAND K., CZERKINSKY C., RICKSTEN A., JANSSON B., ASEA A., KYLEFJORD H., HERMODSSON S. Role of serotonin in the regulation of interferon-γ production by human natural killer cells. J. Interferon Res. 1993;13:33–38. doi: 10.1089/jir.1993.13.33. [DOI] [PubMed] [Google Scholar]
- HELLSTRAND K., HERMODSSON S. Monocyte-mediated suppression of IL-2-induced NK-cell activation. Regulation by 5-HT1A-type serotonin receptors. Scand. J. Immunol. 1990a;32:183–192. doi: 10.1111/j.1365-3083.1990.tb02908.x. [DOI] [PubMed] [Google Scholar]
- HELLSTRAND K., HERMODSSON S. Enhancement of natural killer cell cytotoxicity by serotonin: role of non-T/CD10+NK cells, accessory monocytes and 5-HT1A receptors. Cell Immunol. 1990b;127:199–214. doi: 10.1016/0008-8749(90)90125-b. [DOI] [PubMed] [Google Scholar]
- HELLSTRAND K., HERMODSSON S. Serotonergic 5-HT1A receptors regulate a cell contact-mediated interaction between natural killer cells and monocytes. Scand. J. Immunol. 1993;37:7–18. doi: 10.1111/j.1365-3083.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- HERBERMAN R., ORTALDO J. Natural killer cells: their role in defenses against disease. Science. 1981;214:24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- IKEN K., CHENG S., FARGIN A., GOULET A.C., KOUASSI E. Serotonin upregulates mitogen-stimulated B lymphocyte proliferation through 5-HT1A receptors. Cell. Immunol. 1995;163:1–9. doi: 10.1006/cimm.1995.1092. [DOI] [PubMed] [Google Scholar]
- JACKSON J.C., WALKER R.F., BROOKS W.H., ROSZMAN T.L. Specific uptake of serotonin by murine macrophages. Life Sci. 1988;142:1641–1650. doi: 10.1016/0024-3205(88)90443-2. [DOI] [PubMed] [Google Scholar]
- JEYNES B.J., ISSEKUTZ A.C., ISSEKUTZ T.B., MOVAT H.Z. Quantitation of platelets in the microcirculation. Measurement of indium-111 in microthrombi induced in rabbits by inflammatory lesions and related phenomena. Proc. Soc. Exp. Biol. Med. 1980;165:445–452. doi: 10.3181/00379727-165-41002. [DOI] [PubMed] [Google Scholar]
- JULIUS D. Molecular biology of serotonin receptors. Annu. Rev. Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- MOSSNER R., LESCH K.P. Role of serotonin in the immune system and neuroimmune interactions. Brain Behav. Immun. 1998;12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- NOMURA H., SATO E., KOYAMA S., HANIUDA M., KUBO K., NAGAI S., IZUMI T. Histamine stimulates alveolar macrophages to release neutrophil and monocyte chemotactic activity. J. Lab. Clin. Med. 2001;138:226–235. doi: 10.1067/mlc.2001.117988. [DOI] [PubMed] [Google Scholar]
- OLSON P.S., LJUNGQVIST U.B., BERGENTZ S.E. Analysis of platelet, red cell and fibrin content in experimental arterial and venous thrombi. Thromb. Res. 1974;5:1. doi: 10.1016/0049-3848(74)90106-6. [DOI] [PubMed] [Google Scholar]
- OKIMURA T., OGAWA M., YAMAUCHI T. Stress and immune responses III. Effect of restraint stress on delayed type hypersensitivity (DTH) response, natural killer (NK) activity and phagocytosis in mice. Jpn. J. Pharmacol. 1986;41:229–235. doi: 10.1254/jjp.41.229. [DOI] [PubMed] [Google Scholar]
- PAWLOWSKI N.A., KAPLAN G., HAMILL A.L., COHN Z.A., SCOTT W.A. Arachidonic acid metabolism by human monocytes. Studies with platelet-depleted cultures. J. Exp. Med. 1983;158:393–412. doi: 10.1084/jem.158.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREVERZEVA E.R. The influence of cyclophosphane and phenthyrine and their combination with zymosan and serotonin on the functional features distinguishing peritoneal macrophages in mice. Farmakol. Toksikol. 1978;41:422–427. [PubMed] [Google Scholar]
- PEROUTKA S.J. 5-hydroxytryptamine receptors. J. Neurochem. 1993;60:408–416. doi: 10.1111/j.1471-4159.1993.tb03166.x. [DOI] [PubMed] [Google Scholar]
- POLANSKI M., VERMEULEN M.W., WU J., KARNOVSKY M.L. Muramyl dipeptide mimicry in the regulation of murine macrophage activation by serotonin. Int. J. Immunopharmacol. 1995;17:225–232. doi: 10.1016/0192-0561(94)00097-8. [DOI] [PubMed] [Google Scholar]
- ROSZMAN T., SPARKS D.L., SLEVIN J.T., MARKESBERY W.R., JACKSON J.C., CROSS R.J. The presence of serotonin receptors on murine lymphocytes and macrophages. Soc. Neurosci. 1984;10:726. [Google Scholar]
- SILVERMAN D.H., WU H., KARNOVSKY M.L. Muramyl peptides and serotonin interact at specific binding sites on macrophages and enhance superoxide release. Biochem. Biophys. Res. Commun. 1985;131:1160–1167. doi: 10.1016/0006-291x(85)90212-8. [DOI] [PubMed] [Google Scholar]
- STERNBERG E.M., TRIAL J., PARKER C.W. Effect of serotonin on murine macrophages: suppression of la expression by serotonin and its reversal by 5-HT2 serotonergic receptor antagonists. J. Immunol. 1986;137:276–282. [PubMed] [Google Scholar]
- STERNBERG E.M., WEGNER H.J., LEUNG M.K., PARKER C.W. Effect of serotonin (5-HT) and other monoamines on murine macrophages: modulation of interferon-gamma induced phagocytosis. J. Immunol. 1987;138:4360–4365. [PubMed] [Google Scholar]
- THASE M.E., HOWLAND R.H., FRIEDMAN E.S. Treating antidepressant nonresponders with augmentation strategies: an overview. J. Clin. Psychiatry. 1998;59:5–12. [PubMed] [Google Scholar]
- TOKMAKOV A.A., KYKHOVA M.P., KOSHKINA O.V., VASILEV V.I. The effect of serotonin on the functional activity of monocytes. Tsitologiia. 1991;33:81–87. [PubMed] [Google Scholar]